Significance
Lysine succinylation is a recently discovered protein posttranslational modification and SIRT5 is an efficient desuccinylase. Although many mammalian proteins have recently been found to be regulated by lysine succinylation and SIRT5, the physiological significance of succinylation and SIRT5 remains unknown. Here we report that protein lysine succinylation predominantly accumulates in the heart when Sirt5 is deleted. Sirt5-deficient mice exhibit defective fatty acid metabolism, decreased ATP production, and hypertrophic cardiomyopathy. Our data suggest that regulating heart metabolism and function is a major physiological role of lysine succinylation and SIRT5.
Keywords: sirtuin, lysine succinylation, fatty acid metabolism, desuccinylation, hypertrophic cardiomyopathy
Abstract
Cellular metabolites, such as acyl-CoA, can modify proteins, leading to protein posttranslational modifications (PTMs). One such PTM is lysine succinylation, which is regulated by sirtuin 5 (SIRT5). Although numerous proteins are modified by lysine succinylation, the physiological significance of lysine succinylation and SIRT5 remains elusive. Here, by profiling acyl-CoA molecules in various mouse tissues, we have discovered that different tissues have different acyl-CoA profiles and that succinyl-CoA is the most abundant acyl-CoA molecule in the heart. This interesting observation has prompted us to examine protein lysine succinylation in different mouse tissues in the presence and absence of SIRT5. Protein lysine succinylation predominantly accumulates in the heart when Sirt5 is deleted. Using proteomic studies, we have identified many cardiac proteins regulated by SIRT5. Our data suggest that ECHA, a protein involved in fatty acid oxidation, is a major enzyme that is regulated by SIRT5 and affects heart function. Sirt5 knockout (KO) mice have lower ECHA activity, increased long-chain acyl-CoAs, and decreased ATP in the heart under fasting conditions. Sirt5 KO mice develop hypertrophic cardiomyopathy, as evident from the increased heart weight relative to body weight, as well as reduced shortening and ejection fractions. These findings establish that regulating heart metabolism and function is a major physiological function of lysine succinylation and SIRT5.
Protein posttranslational modifications (PTMs) contribute toward the functional diversity of proteomes through regulating their activity, stability, and cellular localization. Many novel PTMs have been identified recently that result from enzymatic or nonenzymatic reactions with metabolites (1–5). Lysine, being the most frequently posttranslationally modified amino acid, has become the target of various PTMs such as acetylation, methylation, propionylation, butyrylation, crotonylation, succinylation, malonylation, glutarylation, long-chain fatty acylation, ubiquitination, and 2-hydroxyisobutyrylation (1, 3–9). Unlike lysine acetylation, lysine succinylation is a relatively new PTM and the succinyl donor is presumably succinyl-CoA. Acetylation on lysine neutralizes the positive charge of lysine side chain and is known to affect the structure and function of chromatin (10) as well as cellular metabolism (11). However, succinylation on lysine undergoes a complete charge reversal by changing a positively charged side chain to a negatively charged one. Regarding the change in charge, lysine succinylation is similar to phosphorylation, producing a two-unit charge shift in the modified residues. So, it can be anticipated that lysine succinylation would have a significant role in metabolic pathways, as was previously found for acetylation or phosphorylation.
Sirtuins are an evolutionarily conserved family of NAD-dependent lysine deacylases. Among the seven mammalian sirtuins (SIRT1–7), SIRT3–5 are located in mitochondria (12, 13). Unlike SIRT3, both SIRT4 and SIRT5 have very weak deacetylase activities (14). SIRT5 possesses unique enzymatic activity on hydrolyzing negatively charged lysine modifications such as lysine succinylation, malonylation, and glutarylation (1, 4, 8). The presence of two positively charged amino acids, Tyr102 and Arg105, in the active site of SIRT5 explained its preference for negatively charged acyl groups such as succinyllysine (1). Although proteomic studies (15–19) in mouse liver and skeletal muscle have identified hundreds of potential desuccinylation substrates of SIRT5 and several of these have been biochemically confirmed, the physiological significance of SIRT5 and lysine succinylation remains unclear. Deletion of Sirt5 in mice produced only subtle phenotypes that seemed normal under basal conditions (20, 21) despite increased serum ammonium levels (22). We thus set out to obtain crucial information that would help to reveal the function of lysine succinylation and SIRT5.
Acetyl-CoA and succinyl-CoA are important intracellular metabolites involved in diverse metabolic pathways including the TCA cycle. Differences in metabolism could lead to a differential distribution of acyl-CoAs across different tissues. In many recently discovered PTMs, the lysine side chains of proteins react with acyl-CoAs through their ε-amino groups. Thus, the distribution of acyl-CoA may significantly affect the PTMs. Herein, we have conducted a metabolomics study to first profile acyl-CoAs in various murine tissues and found that different tissues have very different acyl-CoA profiles. This has led us to examine protein lysine succinylation across different tissues. Protein lysine succinylation predominantly accumulates in the heart when Sirt5 is deleted. We have identified many desuccinylation substrates of SIRT5 using proteomics, among which ECHA, a protein involved in fatty acid oxidation, is a major substrate in the heart. SIRT5 activates ECHA via desuccinylation and, as a result, Sirt5-deficient mice exhibit defective fatty acid metabolism and decreased ATP production. Sirt5 knockout (KO) mice exhibit both reduced shortening fraction and ejection fraction, implying a reduced cardiac function. Taken together, these findings reveal that a major physiological role of lysine succinylation and SIRT5 is to regulate heart metabolism and function.
Results
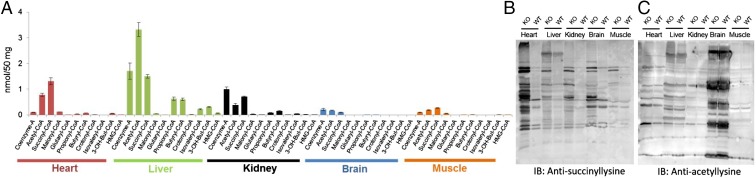
Different Mouse Tissues Have Unique Acyl-CoA Profiles.
To obtain information that would help reveal the function of lysine succinylation and SIRT5, we profiled acyl-CoA concentrations, including succinyl-CoA (the presumed donor of succinyl for lysine succinylation), in major mouse organs such as liver, heart, kidney, brain, and muscle. This targeted metabolomics study conducted on acyl-CoAs from wild type (WT) mouse tissues revealed that different tissues have unique acyl-CoA profiles. For example, succinyl-CoA is the most abundant acyl-CoA in the heart. In the liver, the absolute concentration of succinyl-CoA is similar to that in the heart, but acetyl-CoA and free CoA are more abundant than succinyl-CoA (Fig. 1A). This interesting acyl-CoA profile suggested that different tissues might have differential patterns of protein lysine succinylation and prompted us to examine succinylation in different mouse tissues.
Fig. 1.
Protein lysine succinylation occurs to the greatest extent in the heart. (A) Profiling of short-chain CoAs among different tissues from Sirt5 WT mice using LC-MS/MS (mean ± SEM, n = 3 mice). (B and C) Western blot of different tissue lysates (25 μg each) against (B) antisuccinyllysine antibody and (C) against antiacetyllysine antibody. Sirt5 KO heart has the highest succinylation level. Coomassie-stained gels (loading control) are shown in Fig. S2 D and E.
Protein Lysine Succinylation Predominantly Occurs in the Heart of Sirt5 KO Mice.
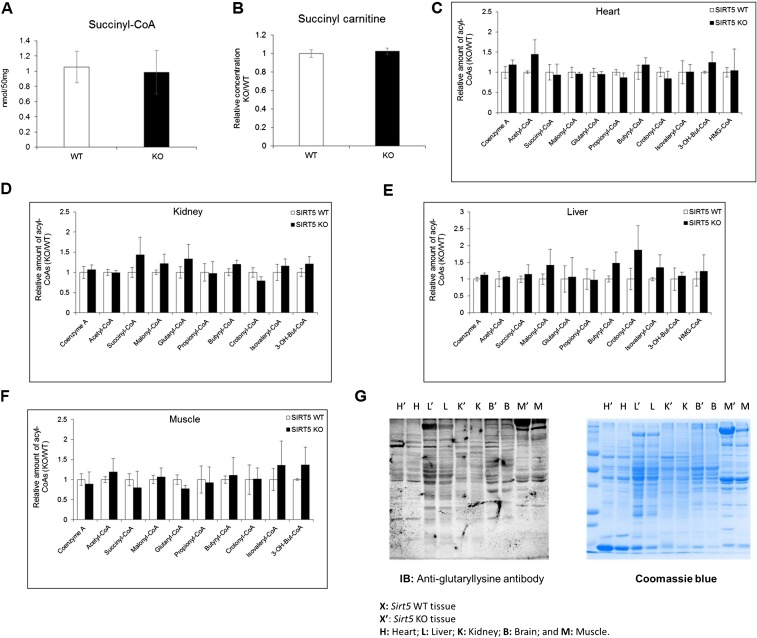
We next investigated the protein lysine succinylation and acetylation status in different tissues from Sirt5 WT and KO mice. Western blot analysis for succinyllysine demonstrated that although the level of succinylation increased in all tissues when Sirt5 was knocked out, it increased most dramatically in the heart (Fig. 1B). Importantly, concentrations of succinyl-CoA and succinyl-carnitine were comparable in the Sirt5 WT and KO mice’s hearts (Fig. S1 A and B), implicating that the observed hypersuccinylation was generated by the deficiency of SIRT5 and not by increased succinyl donors. Levels of most of the short-chain acyl-CoAs remained unaltered in Sirt5 WT and KO mice tissues (Fig. S1 C–F). Western blot analysis for acetyllysine showed no significant changes in acetylation in Sirt5 WT and KO tissues (Fig. 1C). The data suggested that among the mouse tissues tested, the desuccinylase activity of SIRT5 might play a very important role in the heart. Consistent with this hypothesis, among the different mouse tissues tested, the heart had the highest SIRT5 protein level (Fig. S2). Very recently SIRT5 is found to possess deglutarylation activity in addition to its known deacetylation, demalonylation, and desuccinylation activity (8). In general, glutaryl-CoA concentration is much lower compared with acetyl-CoA and succinyl-CoA. It is highest in the liver tissue among the tissues studied and hence, as expected, we found that the changes in protein lysine glutarylation level were rather small in all tissues examined when Sirt5 was knocked out (Fig. S1G). These results suggested that although SIRT5 can remove several different negatively charged acyl lysine modifications, the major acyl group removed in vivo is likely succinyl.
Fig. S1.
(A) Concentration of succinyl-CoA in mouse hearts from WT and Sirt5 KO animals. (B) Relative level of succinyl-carnitine in mouse hearts from WT and Sirt5 KO animals. (C–F) Relative level of short-chain acyl-CoAs in mouse heart, kidney, liver, and muscle from WT and Sirt5 KO animals after 30 min of endurance exercise. The metabolomics data are provided as mean ± SEM, n = 3 per genotype. (G) Western blot of different tissue lysates (25 μg each) from Sirt5 KO (designated by the prime sign) and WT mouse tissues against antiglutaryllysine antibody. B, brain; H, heart; K, kidney; L, liver; M, muscle.
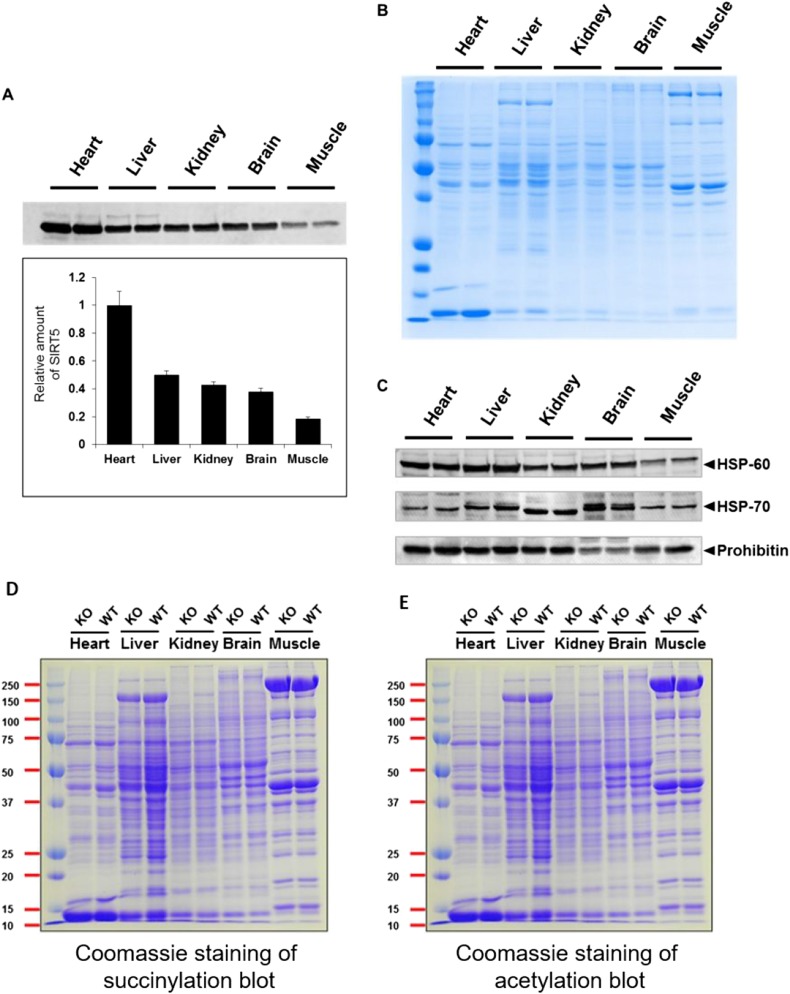
Fig. S2.
(A) SIRT5 Western blot for different tissue lysates (25 μg each) from Sirt5 WT mouse tissues showing that the heart has the highest amount of SIRT5. The experiment was done with tissues from two different mice (duplicate). (B) Coomassie-stained gel showing equal loading of total protein. (C) Prohibitin, HSP-60, and HSP-70 were used as a loading control. Because the readings with different markers were not consistent (e.g., in the brain lysate the Hsp70 level was very high but prohibitin level was very low), we used the total protein amount to normalize the results in A. (D and E) Coomassie-stained gels (loading control) for Western blot of different tissue lysates (25 μg each) against antisuccinyllysine antibody (D) and antiacetyllysine antibody (E).
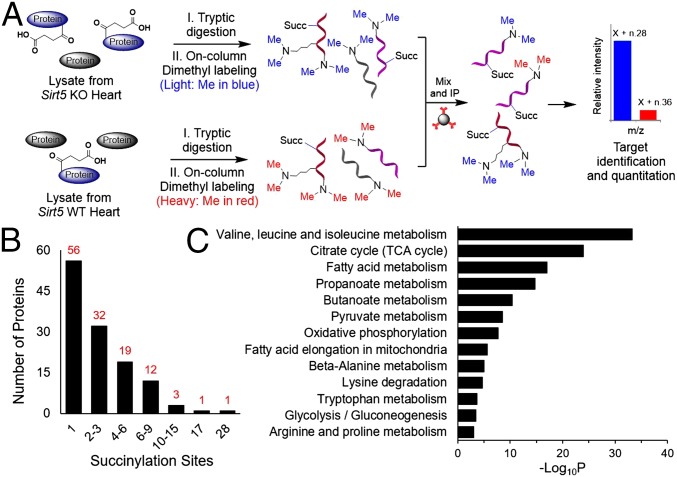
Quantitative Proteomics on Lysine Succinylation from Sirt5 WT and KO Heart.
We next sought to identify proteins that are succinylated and regulated by SIRT5 in mouse heart. We used a proteomics approach involving reductive dimethylation (23) of the tryptic peptides followed by the enrichment of the succinylated peptides for identification by mass spectrometry (MS) (Fig. 2A). Tryptic peptides from equal amounts of total lysate of Sirt5 WT and KO mice heart tissues were separately labeled with heavy and light dimethyl groups, respectively. The labeled peptides were then mixed and succinylated peptides were enriched using an antisuccinyllysine polyclonal antibody. Nano liquid chromatography (LC)-MS/MS analysis was then carried out to compare the abundance of succinylated peptides in Sirt5 WT and KO samples. MS analysis revealed 124 succinylated proteins that are potentially regulated by SIRT5. Among all of the identified succinylated proteins, more than 75% were mitochondrial proteins (Dataset S1). More than 90% of succinylation sites showed increased abundance in Sirt5 KO heart with an average KO/WT ratio of 8.37 and a median of 1.64 (Dataset S2). Significantly, over 25% of the sites showed over threefold greater abundance in Sirt5 KO heart (Dataset S2).
Fig. 2.
Workflow of the dimethyl-labeling strategy for the succinylome analysis. (A) One milligram of total protein from Sirt5 KO and WT heart was separately digested with trypsin and labeled with light and heavy dimethyl groups, respectively. The isotopically labeled peptides were mixed together and immunoprecipitated with antisuccinyllysine antibody. Succinyl-lysine peptides were then analyzed by nano LC-MS/MS. (B) Distribution of number of lysine succinylation sites per protein. (C) Metabolic pathways enriched with lysine succinylated proteins.
To gain insight into how lysine succinylation and SIRT5 might affect mitochondrial metabolic networks, we performed pathway enrichment analysis using DAVID bioinformatics resources (24, 25). Consistent with earlier reports, a number of metabolic pathways including branched-chain amino acids metabolism, the TCA cycle, fatty acid metabolism, propanoate metabolism, oxidative phosphorylation, pyruvate metabolism, and ATP synthesis are significantly enriched among the SIRT5 desuccinylation targets (Fig. 2C) (16, 18, 19). It is possible that the regulation of all these metabolic enzymes collectively contributes to the biological function of SIRT5 and lysine succinylation in the heart. Nevertheless, to gain a better understanding of the physiological roles for SIRT5, we sought to identify the pathway that is significantly affected in the heart when Sirt5 is knocked out and that can play important roles in regulating heart function.
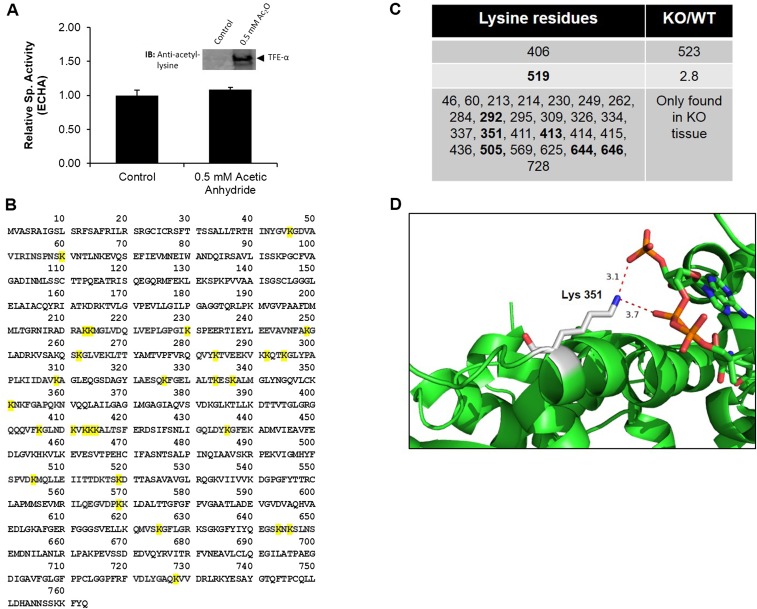
One feature that caught our attention was that the number of succinylation sites per protein varied significantly (from 1 to 28) depending on the protein (Fig. 2B). ECHA was identified to have the most succinylation sites (at 28 Lys residues) in the Sirt5 KO heart. Among the 66 lysine residues of ECHA, 28 were succinylated and the majority of succinylated residues (26 out of 28) were only found in Sirt5 KO heart, indicative of ECHA being a target of SIRT5. We focused on ECHA for biochemical validations for two considerations. First, we examined several other desuccinylation targets of SIRT5 (e.g., citrate synthase and ATP synthase) and found that the activities of these targets were not significantly affected by SIRT5 in the heart. Second, ECHA is most abundant in the heart compared with other tissues. ECHA is the α-subnit of mitochondrial trifunctional enzyme, which is important for fatty acid β-oxidation and has three distinct activities: enoyl-CoA hydratase (ECH), 3-hydroxyacyl-CoA dehydrogenase (HACD), and 3-ketoacyl-CoA thiolase (KCAT) (26). The α-subunit (ECHA) has the ECH and HACD activities whereas the β-subunit (ECHB) harbors the KACT activity.
SIRT5 Activates ECHA by Desuccinylation.
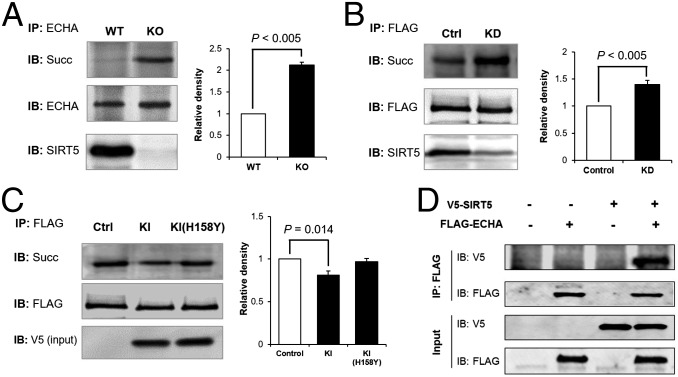
To confirm that ECHA was indeed hypersuccinylated in SIRT5-deficient mice, we immunoprecipitated ECHA from Sirt5 WT and KO mouse heart and analyzed the succinylation level by Western blot using antisuccinyllysine antibody. ECHA was highly succinylated in the absence of SIRT5 (Fig. 3A). Similarly, Flag-tagged mouse ECHA (Flag-ECHA) in Sirt5 knockdown (KD) HEK-293T cells was hypersuccinylated compared with ECHA from control KD cells (Fig. 3B). When Flag-ECHA was cotransfected with an expression vector for either SIRT5, or its catalytic mutant SIRT5-H158Y, into HEK-293T cells, ECHA succinylation level was decreased when coexpressed with SIRT5, but not with SIRT5-H158Y (Fig. 3C). Additionally, when coexpressed, Flag-ECHA was able to immunoprecipitate V5-tagged SIRT5, suggesting that ECHA and SIRT5 interact with each other (Fig. 3D).
Fig. 3.
Lack of SIRT5 leads to hypersuccinylation on ECHA. (A) ECHA was immunoprecipitated from Sirt5 WT and KO mouse heart using ECHA-specific antibody. Sirt5 KO mouse heart had increased succinylation on endogenous ECHA. (B) Flag-ECHA expressed in HEK-293T Sirt5 KD cells showed increased succinylation compared with Flag-ECHA from control KD cells. (C) Overexpression of WT SIRT5, but not catalytically inactive SIRT5-H158Y, decreased the succinylation level of ECHA. Quantitative representation of relative density of succinylation (mean ± SEM, n = 3) is shown for A–C. (D) Flag-ECHA and V5-tagged SIRT5 were co-overexpressed in HEK-293T cells. Immunoprecipitation of Flag-ECHA pulled down V5-tagged SIRT5.
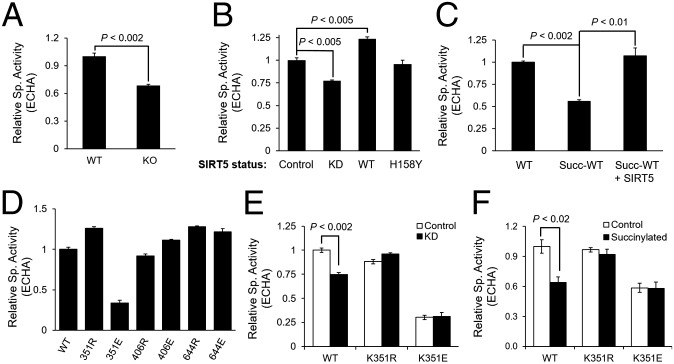
Next we aimed to determine whether the succinylation of ECHA modulates its enzymatic activity. The combined ECH and HACD activities of ECHA were measured by monitoring the formation of NADH from NAD at 340 nm (27) using 2-(E)-decenoyl-CoA as a substrate. ECHA from Sirt5 KO heart showed a 32% decrease in activity compared with that from Sirt5 WT heart, suggesting that ECHA succinylation down-regulates its activity (Fig. 4A). Similarly, ECHA purified from Sirt5 KD HEK-293T cells showed a lower activity than that from the control cells (Fig. 4B). Coexpression of ECHA with SIRT5 decreased ECHA succinylation (Fig. 3C) and led to a 24% increase in enzymatic activity (Fig. 4B). Coexpression of ECHA with SIRT5-H158Y did not change ECHA succinylation (Fig. 3C) or increase its activity (Fig. 4B). We also purified recombinant mouse trifunctional protein complex (ECHA and ECHB) from Escherichia coli to test the consequence of succinylation on its activity in vitro. We first treated the recombinant ECHA and ECHB complex with succinyl-CoA for 10 min at 27 °C to prompt nonenzymatic succinylation, and then the reaction mixture was further incubated with or without SIRT5 for 10 min at 27 °C. Nonenzymatically succinylated ECHA and ECHB complex showed a 40% reduction in activity, but upon SIRT5 treatment the activity was restored (Fig. 4C). To evaluate whether lysine acetylation also regulates ECHA activity, we performed a chemical acetylation of ECHA and ECHB complex by incubating it with acetic anhydride and checked its activity. As shown in Fig. S3A, we did not observe any change in ECHA activity after it was acetylated. This result further demonstrates that ECHA activity is regulated by succinylation and SIRT5-catalyzed desuccinylation.
Fig. 4.
SIRT5 increases ECHA activity by desuccinylation. (A) ECHA activity was higher in Sirt5 WT mouse hearts than in Sirt5 KO mice. (B) Flag-ECHA expressed in HEK-293T control, Sirt5 KD, and SIRT5 (WT or H158Y) overexpressing cells showed activities consistent with the hypothesis that SIRT5 increases ECHA activity by desuccinylation. (C) Recombinant ECHA and ECHB (coexpressed and purified in E. coli) could be nonenzymatically succinylated, which decreased the ECHA activity. Incubation with SIRT5 and NAD restored ECHA activity. (D) K351 is the only residue that decreases ECHA activity when mutated to E. (E) Neither K351R nor K351E show any change in activity when purified from HEK-293T control or Sirt5 KD cells. (F) Unlike WT, K351R and K351E mutant ECHA does not lose any additional activity when incubated with succinyl-CoA. Data shown as mean ± SEM, n = 3.
Fig. S3.
(A) Recombinant ECHA and ECHB complex was nonenzymatically acetylated with 0.5 mM acetic anhydride for 15 min at room temperature. Data are shown as mean ± SEM, n = 3. ECHA did not show any significant change in activity after chemical acetylation. (Inset) Western blot against antiacetyllysine antibody shows acetylation on ECHA after treating with 0.5 mM acetic anhydride. (B) Succinylated lysine residues of ECHA identified from our proteomics analysis are highlighted in yellow. (C) Fold change of succinyl-lysine–containing peptides (KO/WT) calculated from the peak area of the peptides. (D) The interactions between Lys351 ε-N (denoted by blue) and the negatively charged CoA phosphate groups (oxygen is denoted by red) are shown in the X-ray crystal structure of M. tuberculosis ECHA in complex with free CoA bound at the hydratase active sites (PDB ID code 4B3J). The figure was generated using PyMOL. Sequence alignment of ECHA showed Lys351 in mice is aligned with Ala-312 in M. tuberculosis. Hence, Ala-312 in ECHA of M. tuberculosis was mutated to Lys using PyMOL.
Lys351 Is the Major Succinylation Site of ECHA That Regulates Its Activity.
To elucidate which of the 28 succinyllysine residues (Fig. S3 B and C) identified on mouse ECHA down-regulates its enzymatic activity, we examined the crystal structure of a homologous ECHA from Mycobacterium tuberculosis in complex with free CoA bound at the ECH active site (28). The crystal structure shows that several lysine residues (K351, K406, and K644) targeted by SIRT5 are present at the interface between ECHA and ECHB or are close to the bound CoA in the ECH site. For example, K351 is very close to the bound CoA (the distance between the ε-N of K351 and the phosphate of CoA is less than 4 Å) and hence the succinylation on K351 could disrupt the interaction between ECHA and CoA (Fig. S3D). To test the effect of succinylation of these lysine residues (K351, K406, and K644) on ECHA activity, we expressed Flag-tagged ECHA WT, K-to-R mutants (mimicking the desuccinylated state) or K-to-E mutants (mimicking the negatively charged succinyllysine modification) in HEK-293T and carried out enzymatic activity assays after immunoprecipitation. Whereas all of the K-to-R mutants maintained basal activity, only K351E showed a significant loss (more than 70%) in ECHA enzymatic activity compared with the WT (Fig. 4D). These data identify Lys351 as a critical lysine residue for the regulation of ECHA enzymatic activity.
We further checked the enzymatic activity of K351R and K351E mutants immunopurified from HEK-293T control and Sirt5 KD cells. Whereas WT ECHA from Sirt5 KD cells showed a decrease in activity compared with that from control KD cells, neither K351R nor K351E showed any difference in enzymatic activity in control and Sirt5 KD cells (Fig. 4E). We also showed that upon chemical succinylation with succinyl-CoA, K351R and K351E ECHA did not lose additional activity (Fig. 4F). Thus, our mutational data suggest that SIRT5 regulates ECHA enzymatic activity mainly through desuccinylation of Lys351.
Absence of SIRT5 Resulted in Reduced Fatty Acid Oxidation and Accumulation of Long-Chain Fatty Acyl-CoAs.
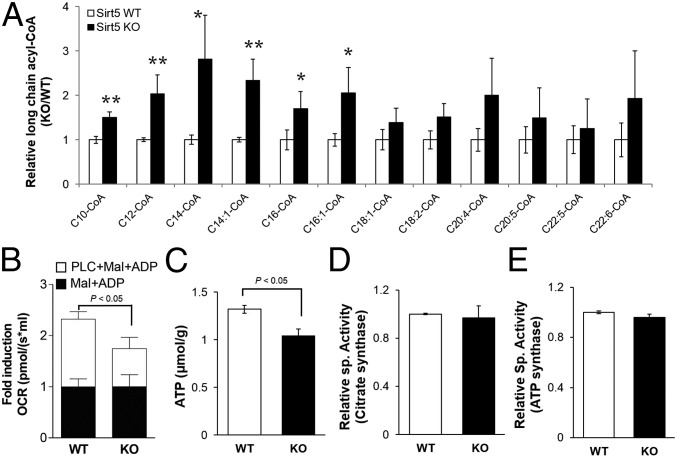
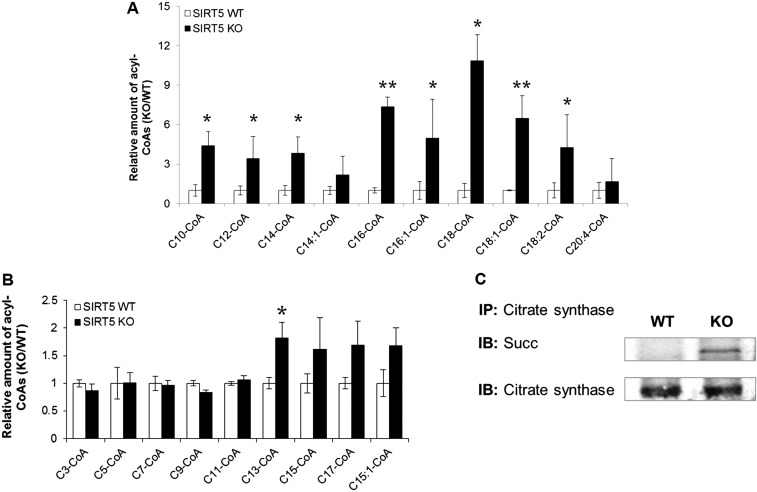
We wanted to determine the consequence of decreased ECHA enzymatic activity due to succinylation on the level of long-chain fatty acyl-CoAs in the heart. Defective ECHA would significantly slow down the β-oxidation of long-chain fatty acids, leading to accumulation of long-chain acyl-CoAs. Indeed, after 30 min of endurance exercise, long-chain acyl-CoA levels were elevated in the KO heart compared with the WT (Fig. 5A). Significant accumulation of long-chain acyl-CoAs in the heart was also observed in Sirt5 KO mice that were fasted for 24 h (Fig. S4A). Sirt5 KO heart also showed an accumulation of odd-chain fatty acyl-CoA with a chain length higher than 11 but not the shorter ones (Fig. S4B). The data suggest that the loss in ECHA activity in Sirt5 KO heart also slows down the odd-chain fatty acid oxidation. Endurance exercise or fasting forces the mice to use β-oxidation to get the necessary energy and therefore requires optimal ECHA activity. Our data suggest that succinylation impairs fatty acid oxidation through down-regulation of ECHA activity. Hence, SIRT5 is important to maintain efficient fatty acid oxidation in the heart during energy-demanding situations such as fasting and exercise. In addition, fatty acid oxidation, measured in permeabilized heart tissues from Sirt5 WT and KO mice, was clearly reduced in Sirt5 KO mice (Fig. 5B). Our findings are in agreement with the previous report of reduced fatty acid oxidation and accumulation of acylcarnitines in Sirt5 KO liver and muscles (18).
Fig. 5.
SIRT5 deficiency leads to accumulation of long-chain CoAs and decreased cardiac ATP levels. (A) Relative levels of long-chain CoA thioesters in Sirt5 KO hearts compared with WT (after 30 min of exercise, **P < 0.05, *P < 0.1). (B) Normalized fatty acid oxidation was significantly reduced in permeabilized Sirt5 KO heart tissue. Mitochondrial respiration in response to palmitoyl-l-carnitine (PLC) was monitored. Malate (2 mM) and ADP (2.5 mM) were used as a pretreatment. (C) Cardiac ATP levels were measured in Sirt5 WT and KO mice after 24 h of fasting. (D) Enzymatic activity of citrate synthase was measured in heart extracts from Sirt5 WT and KO mice. (E) Complex V activities were measured from Sirt5 WT and KO mice heart mitochondria. All data shown as mean ± SEM, n = 3 per genotype.
Fig. S4.
(A) Relative level of long-chain CoA thioesters in Sirt5 KO hearts compared with WT after 24 h of fasting (mean ± SEM, n = 3 per genotype, **P < 0.05, *P < 0.1). (B) Relative level of odd-chain acyl-CoAs in Sirt5 KO hearts compared with WT after 30 min of exercise (mean ± SEM, n = 3 per genotype, *P < 0.1). (C) Citrate synthase from Sirt5 KO mouse heart had increased succinylation.
Lack of SIRT5 Results in Lower Cardiac ATP Levels.
To further test the hypothesis that the regulation of ECHA by SIRT5 is important for cardiac energy production, we measured ATP levels in Sirt5 WT and KO hearts. As would be expected in the case of defective fatty acid oxidation, we observed more than 20% reduction in ATP in Sirt5 KO heart compared with WT (Fig. 5C). Other metabolic enzymes regulated by SIRT5 might also contribute to the decreased ATP production in Sirt5 KO hearts. For example, citrate synthase controls the flow of acetyl-CoA into the TCA cycle and any loss in its activity might also contribute to the observed decrease of fatty acid oxidation. To test this possibility, we measured the enzymatic activity of citrate synthase from Sirt5 WT and KO heart lysates and found that WT and KO heart had comparable citrate synthase activities despite citrate synthase being hypersuccinylated in KO heart (Fig. 5D and Fig. S4C). Another likely candidate for reduced ATP level in Sirt5 KO heart is ATP synthase, which was found to be hypersuccinylated in the proteomics study. However, we did not observe any significant change in ATP synthase (complex V) activity in Sirt5 WT and KO heart (Fig. 5E). These data support the conclusion that the observed succinylation-induced fatty acid oxidation deficit is mainly driven by the regulation of ECHA.
SIRT5 KO Mice Exhibit Reduced Cardiac Function and Develop Hypertrophic Cardiomyopathy with Aging.
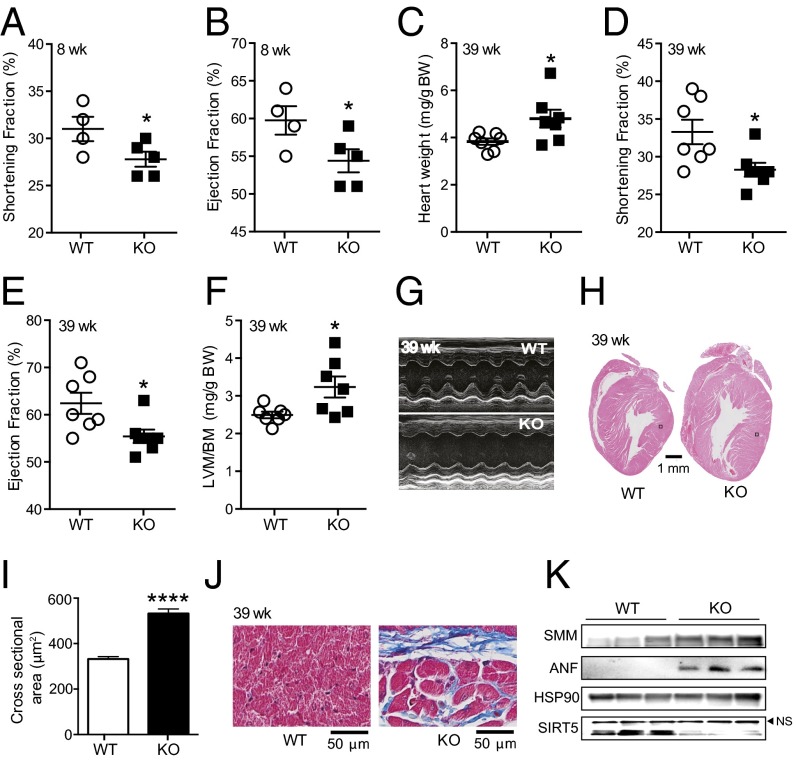
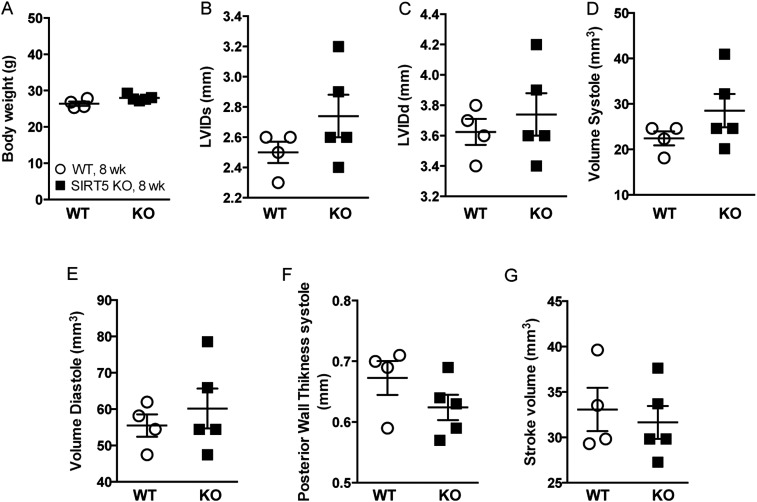
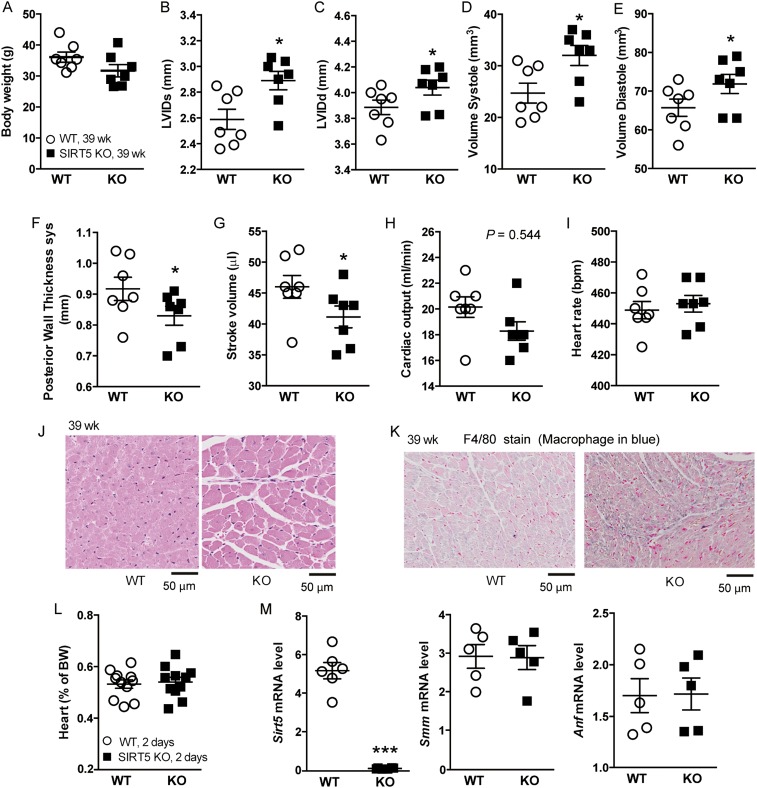
The heart has a very rapid and dynamic rate of ATP consumption (29), and hence a constant supply of ATP is necessary to keep the heart working properly. Lower cardiac ATP content might decrease the ability of Sirt5 KO mice to effectively convert the chemical energy to contractile work (30). Fatty acid is a major energy source used to sustain contractile function in the heart, and thus a decrease in fatty acid metabolism might result in heart dysfunction. To further explore cardiac function in Sirt5 WT and KO mice, we performed echocardiography on mice at 8 weeks of age after overnight fasting. Both the shortening fraction and ejection fraction were reduced in young adult Sirt5 KO mice, indicating reduced cardiac function in the absence of SIRT5 (Fig. 6 A and B and Fig. S5). To see whether the cardiac phenotype becomes more prominent upon aging, we also recorded echocardiographic parameters in 39-week-old mice. The older Sirt5 KO mice showed hallmarks of hypertrophic cardiomyopathy, such as significantly increased heart weight (normalized to body weight) and left ventricular mass (normalized to body weight) along with reduced shortening fraction and ejection fraction (Fig. 6 C–G and Fig. S6 A–I). Hematoxylin and eosin (H&E) staining and quantification of cardiomyocyte cross-sectional area shows evidence of cardiac hypertrophy in the Sirt5 KO mice (Fig. 6 H and I and Fig. S6J). Furthermore, there was evidence of fibrosis (Masson’s trichrome staining, Fig. 6J) and macrophage infiltration (F4/80 staining, Fig. S6K) in Sirt5 KO hearts. In addition, well-known markers for cardiomyopathy, such as smooth muscle myosin (SMM) and atrial natriuretic peptide (ANP) levels (Fig. 6K), were robustly induced in Sirt5 KO hearts. Finally, to rule out the possibility that the hypertrophic cardiomyopathy in Sirt5 KO mice was caused by a developmental defect, we monitored the hearts of neonatal Sirt5 WT and KO pups. In Sirt5 KO pups, heart weight was normal (Fig. S6L). Moreover, we failed to discover an induction of the transcript levels of the cardiomyopathy markers Smm and Anp in the hearts of Sirt5 KO pups at 2 days of age (Fig. S6M). Thus, the hypertrophic cardiomyopathy in Sirt5 KO mice was not caused by a developmental defect.
Fig. 6.
SIRT5 deficiency causes hypertrophic cardiomyopathy. (A and B) Shortening fraction (A) and ejection fraction (B) were reduced in Sirt5 KO mice (n = 4 and 5 for Sirt5 WT and KO respectively, 8-week-old males). (C) Normalized heart weight of Sirt5 WT and KO male mice (n = 7 per genotype). (D–F) The shortening fraction (D), and ejection fraction (E) were significantly reduced whereas left ventricular mass to body mass (LVM/BM, F) was significantly increased in hearts of Sirt5 KO mice. (G) Representative M-mode images of echocardiography showing cardiac dysfunction in Sirt5 KO mice. (H and I) H&E staining of heart cross-sections (H) and quantification of cardiomyocytes cross-sectional areas (n = 100 per genotype, I) showing cardiac hypertrophy in the Sirt5 KO mice. The two black boxes in H indicate the localization of the images that are shown in larger magnification in Fig. S6J. (J) Masson’s trichrome stain in cross-sections of the heart showing increased fibrosis in Sirt5 KO hearts. (K) Evaluation of SMM, ANP, HSP90, and SIRT5 protein levels in Sirt5 WT and KO mouse hearts. Experiments in C–J were performed with hearts of 39-week-old male mice. All graphs shown as mean ± SEM, *P < 0.05, ****P < 0.0001.
Fig. S5.
(A) Body weight of Sirt5 WT and KO male mice (n = 4–5 per genotype, 8-wk-old males). (B) Left ventricular internal dimension in systole (LVIDs), (C) left ventricular internal dimension in diastole (LVIDd), (D) volume in systole, (E) volume in systole, (F) posterior wall thickness, and (G) stroke volume did not altered significantly in Sirt5 KO male mice. All graphs are shown as mean ± SEM.
Fig. S6.
(A) Body weight of Sirt5 WT and KO male mice (n = 7 per genotype, 39-wk-old males). (B–H) Left ventricular internal dimension in systole (LVIDs, B), left ventricular internal dimension in diastole (LVIDd, C), and volume in systole (D) and diastole (E) were significantly increased whereas posterior wall thickness (F), stroke volume (G), and cardiac output (H) were reduced in Sirt5 KO male mice. (I) Heart rate did not show clear differences between the genotypes. (J) Representative zoomed-in (black boxes in Fig. 6H) image of H&E staining of heart cross-sections (39-wk-old males). (K) F4/80 staining in cross-sectional heart showing increased macrophage infiltration in Sirt5 KO heart (39-wk-old males). (L) Normalized heart weight of WT and Sirt5 KO pups (n = 11 per genotypes, 2-d-old pups) does not show any difference. (M) mRNA levels of Sirt5, Smm, and Anf as markers of cardiac dysfunction were evaluated by qRT-PCR (n = 5 per genotype, 2-d-old pups). All graphs are shown as mean ± SEM, ***P < 0.001, *P < 0.05.
Discussion
Sirtuins were originally thought to be NAD-dependent protein lysine deacetylases (31). The lack of efficient deacetylation activity for SIRT4–7 prompted studies that led to the discovery that SIRT5 is an efficient desuccinylase, demalonylase (1, 17) and deglutarylase (8). This finding also opened up directions to discover novel activities for other sirtuins (7, 9). Finding the desuccinylase and demalonylase activity of SIRT5 also led to the identification of lysine succinylation and malonylation as common PTMs (1, 4, 16–19, 32). Proteomic studies have identified about 1,000 proteins that are succinylated and regulated by SIRT5 (16, 18, 19). Despite these studies, the biological significance of lysine succinylation and SIRT5 remains unclear. Sirt5 KO mice only display subtle changes in physiology and seem normal under basal conditions regardless of elevated ammonia levels (22). In our study, we used targeted metabolomics to profile acyl-CoA distributions in different tissues. Interestingly, the metabolomics data show that succinyl-CoA is the most abundant short-chain acyl-CoA in the mouse heart. The heart needs a constant energy supply to sustain the mechanical pumping and thus may need to optimize metabolism to favor the TCA cycle and oxidative phosphorylation, which may lead to a higher succinyl-CoA concentration. Regardless of what exactly causes this interesting acyl-CoA profile, the results we obtained suggest that different tissues have very different metabolism and that it is worthwhile to examine the function of acylation in different tissues.
The unique acyl-CoA profiling results then led us to examine succinylation in different mouse tissues with and without SIRT5. Interestingly, succinylation increases most dramatically in the heart when Sirt5 is deleted, which suggests that SIRT5 may have important functions in the heart. Consistent with this, SIRT5 level is higher in the heart than in other mouse tissues tested. Sirt5 KO mice exhibit a reduced cardiac function and display signs of cardiomyopathy upon aging. Thus, protein succinylation and SIRT5 exert important roles in cardiac function. To understand the molecular mechanism underlying the function of succinylation and SIRT5 in the heart, we have identified over a hundred proteins with increased lysine succinylation in Sirt5 KO heart using semiquantitative proteomics. Among the proteins we identified, ECHA has the highest number of lysine succinylation sites. SIRT5 activates ECHA by desuccinylating it. Consistent with ECHA being inhibited by succinylation, Sirt5 KO hearts have compromised long-chain fatty acid oxidation along with a decreased ATP levels. We believe that the inhibition of the fatty acid oxidation pathway is the major contributor to the reduced ATP production because several lines of evidence suggest that neither the TCA cycle nor ATP synthase is inhibited by Sirt5 deletion.
Succinylation is a widespread PTM and affects major metabolic pathways including amino acid metabolism, the TCA cycle, fatty acid metabolism, oxidative phosphorylation, urea cycle, ketogenesis, and so on. SIRT5 can either activate or repress enzymatic activity via desuccinylation. SIRT5 is reported (18) to up-regulate hepatic ketogenesis through activation of 3-hydroxy-3-methylglutaryl-CoA synthase 2. There are reduced fatty acid oxidation and accumulation of medium- and long-chain acylcarnitines in Sirt5-deficient mouse liver and muscle, but the underlying mechanism was not investigated. In our current study, we show that SIRT5 positively modulates fatty acid oxidation in the mouse heart. In addition, we demonstrate that succinylation impairs fatty acid oxidation through down-regulation of ECHA activity. Altogether, this suggests that SIRT5 plays a critical role in regulating fatty acid metabolism in multiple tissues.
Although many of the substrate proteins that we identified here were also previously reported (16, 18, 19), the uniqueness of the current study is that for the first time to our knowledge we have connected lysine succinylation and SIRT5 to an important physiological function (i.e., the regulation of heart metabolism and function). This function likely underlies the impaired performance in an endurance run test reported earlier (21). In recent years, significant advances have been made on the role of fatty acid metabolism defects in the pathogenesis of cardiomyopathy. Fatty acid oxidation provides most of the energy required by the heart. In 1939, Herrmann and Decherd (33) proposed the energy-starvation hypothesis, which stated that deprivation of cardiac energy could lead to heart failure. Cardiomyopathy can occur in a broad range of pathological conditions. It is well documented that defects or disorders of fatty acid metabolism often lead to cardiomyopathy (34–36). Cardiomyopathy is a major symptom of inborn errors in fatty acid metabolism, such as malonyl-CoA decarboxylase deficiency, carnitine palmitoyl transferase 2 deficiency, medium-chain acyl-CoA dehydrogenase deficiency, and mitochondrial trifunctional protein (MTP) deficiency (37). A mutation of the Hadha gene, which encodes ECHA protein, leads to MTP deficiency with cardiac symptoms (38). In the present study, we have established that SIRT5 deficiency leads to decreased ECHA activity and deficiency in cardiac energy metabolism, and ultimately cardiomyopathy. Very recently, Boylston et al. (39) have showed that Sirt5 KO mice are more susceptible to ischemia-reperfusion injury compared with WT.
Connecting lysine succinylation and SIRT5 to heart function was made possible by the targeted metabolomics analysis of acyl-CoA concentrations in various mouse tissues. In the last decade, many novel PTMs have been reported, including propionylation, butyrylation, crotonylation, glutarylation (8), long-chain fatty acylation (7, 9), and 2-hydroxyisobutyrylation (6). All these PTMs likely result from reactions with cellular metabolites either via specific acyltransferases or via nonenzymatic pathways, similar to lysine succinylation (40, 41). Although our studies with ECHA suggest that chemical succinylation and SIRT5-catalyzed desuccinylation in vitro is able to recapitulate the effects of succinylation and desuccinylation on ECHA in vivo, protein-catalyzed succinylation cannot be completely ruled out. Regardless of the enzymatic or nonenzymatic nature of lysine acylation, our study here suggests that combining metabolomics of acyl-CoAs and proteomic identification of substrate proteins in different tissues is useful to understand the functions of these newly identified PTMs.
Materials and Methods
Full details are provided in SI Materials and Methods. In vitro chemical succinylation was achieved by incubating 50 nM recombinant ECHA and ECHB complex with 3 mM succinyl-CoA in a mixture of 100 mM Hepes, pH 7.4, 100 mM KCl, 10% (vol/vol) glycerol, 1 mM free CoA, and 1 mM NAD at 27 °C for 20 min. The activity of succinylated ECHA was measured by adding 100 μM 2-(E)-decenoyl-CoA into the above reaction mixture and monitoring formation of NADH at 340 nm. In a separate reaction, 50 nM ECHA and ECHB complex was chemically succinylated as described above for 10 min and then 0.5 μM SIRT5 was added to that reaction mixture and incubated for an additional 10 min. Then, 100 μM 2-(E)-decenoyl-CoA was added and activity was measured similarly to determine whether SIRT5 can recover the activity of ECHA. Student’s t test was used for statistical analysis.
All animal experiments were approved by the veterinary ethics committee of the canton of Vaud, Switzerland (permit ID 2444) and Cornell Institutional Animal Care and Use Committee protocol 2011-0098.
SI Materials and Methods
Reagents.
Mouse monoclonal anti-Flag M2 antibody conjugated with horseradish peroxidase, anti-Flag M2 affinity gel, d6-acetic anhydride, acyl-CoA standards, ammonium formate, and 2-2(pyridyl)ethyl silica gel were purchased from Sigma. The rabbit pan-specific antiacetyllysine was purchased from ImmuneChem Pharmaceuticals, Inc. Rabbit pan-specific antisuccinyllysine (PTM-401) and antiglutaryllysine (PTM-1151) antibodies were purchased from PTM Biolab, Inc., respectively. The mouse monoclonal antibody against V5 tag (46-0705) was purchased from Life Technologies. The ECHA antibody (sc-292195), protein A/G PLUS-agarose (sc-2003), citrate synthase antibody (sc-390693), and goat anti-rabbit/mouse IgG conjugated with HRP (sc-2004) were purchased from Santa Cruz Biotechnology. The SIRT5 rabbit monoclonal antibody (8782) was purchased from Cell Signaling. The 2-(E)-decenoic acid was purchased from MP Biomedicals. Octyl-β-d-glucopyranoside was purchased from Chem-Impex International, Inc. Ni-NTA agarose was purchased from Qiagen.
Synthesis of d3-Acetyl-CoA As the Internal Standard.
d3-Acetyl-CoA was synthesized according to the method reported by Dils and Carey (42). Briefly, 15 mg of CoA was dissolved in ice-cooled 2 mL of 0.1 M KHCO3. d6-Acetic anhydride (40 μL) was added into the mixture and incubated at 4 °C. After 30 min, the reaction mixture was acidified with 0.1 M HCl to adjust the pH to ∼2. No free CoA was detected in the crude reaction mixture based on LC-MS analysis. The crude reaction mixture was further purified by reverse-phase HPLC using Beckman Coulter System Gold 125p Solvent Module and 168 detector with a TARGA C18 column (250 × 20 mm i.d., 10 μm; Higgins Analytical, Inc.) monitoring at 215 and 260 nm. For the purification, solvent A was 5 mM ammonium acetate (pH 5.3), solvent B was 40% acetonitrile in water, and the following gradient was used: a linear gradient of 0–20% solvent B over 5 min, then a linear gradient of 20–50% solvent B over 25 min, and finally 50% solvent B for 5 min before equilibrating the column back to 0% solvent B over 10 min at a flow rate of 8 mL/min. The purified fractions were then lyophilized and analyzed by LC-MS. Calculated m/z of d3-acetyl-CoA for C23H36D3N7O17P3S ([M+H]+) was 813.60, found 813.33.
LC-MS analysis was performed on a Shimadzu HPLC LC20-AD and Thermo Scientific LCQ Fleet with a Sprite TARGA C18 column (40 × 2.1 mm i.d., 5 μm; Higgins Analytical, Inc.) monitoring at 215 (detector 1 channel A) and 260 nm (detector 1 channel B) with positive/negative mode for mass detection. Solvents used for LC-MS analysis were water with 0.1% acetic acid (solvent A) and acetonitrile with 0.1% acetic acid (solvent B). Acyl-CoA was eluted at a flow rate of 0.3 mL/min with 0% solvent B for 2 min, followed by a linear gradient of 0–10% solvent B over 2 min, followed by a linear gradient of 10–100% solvent B over 5 min, and finally 100% solvent B for 2 min before equilibrating the column back to 0% solvent B over 4 min.
Preparation of Acyl-CoA Standards.
One hundred micromolar stock solution for the standard acyl-CoA esters in Milli-Q water with 100 mM ammonium formate, pH 5.0, was made by measuring the absorbance at 260 nm (ε = 16,400 mM−1⋅cm−1). To prepare standard curves, equal concentrations of standard acyl-CoAs of interest were mixed, creating a master mix, and we performed twofold serial dilutions in Milli-Q water with 100 mM ammonium formate, pH 5.0. d3-Acetyl-CoA was used as the internal standard.
Extraction of Short-Chain Acyl-CoAs from Different Tissues.
All of the extraction solutions, buffers, and solvents used for the CoA extraction were precooled on ice. Powder frozen mice tissues (∼50 mg), spiked with 0.6 nmol of d3-acetyl-CoA as internal standard, were homogenized with 3 mL of methanol/water (1:1) containing 5% acetic acid (extraction buffer) using a Dounce homogenizer (25 strokes) on ice. The tissue homogenates were centrifuged at 20,000 × g for 15 min at 4 °C. The clear supernatant was loaded on a 3-mL ion exchange cartridge packed with 100 mg of 2-2(pyridyl)ethyl silica gel. The cartridge had been preactivated with 3 mL of methanol and then with 3 mL of extraction buffer. The ion exchange resin was washed with 2 mL of extraction buffer to remove unbound metabolites. The acyl-CoAs trapped on the silica gel cartridge were eluted with 2 mL of methanol/250 mM ammonium formate (4:1). The combined effluent was dried with nitrogen gas and stored at −80 °C until LC-MS analysis.
Extraction of Long-Chain Acyl-CoAs from Heart Tissues.
All of the extraction solutions and solvents used for the CoA extraction were precooled on ice. Powder frozen heart tissues (∼20 mg) were homogenized with 0.5 mL of methanol/water 4:1 using an electronic mixer for 30 s on ice. The tissue homogenates were centrifuged at 20,000 × g for 15 min at 4 °C. The clear supernatant was dried in a SpeedVac. The dried residue was stored at −80 °C until LC-MS analysis.
Absolute Quantitation of ATP.
ATP content in Sirt5 WT and KO heart tissues was measured using a colorimetric assay following the protocol of the ATP assay kit (Abcam).
Sample Reconstitution for LC-MS Analysis.
Dried tissue extract was dissolved into either water with 50 mM ammonium acetate (pH 6.8, short-chain acyl-CoA) or 50 mM ammonium acetate with 20% (vol/vol) acetonitrile (pH 6.8, long-chain acyl-CoA). Samples were centrifuged at 20,000 × g at 4 °C for 3 min and the supernatant was transferred to LC vials. The injection volume was 12 µL.
HPLC Method.
An Ultimate 3000 UHPLC (Dionex) was coupled to a Q Exactive mass spectrometer (QE-MS; Thermo Scientific) for metabolite separation and detection. For acyl-CoA analysis, a reverse-phase liquid chromatography method was used. A Luna C18 column (100 × 2.0 mm i.d., 3 µm; Phenomenex) was used. For short-chain acyl-CoA, the solvent A was water with 5 mM ammonium acetate (pH 6.8), and solvent B was methanol. The linear gradient was as follows: 0 min, 2% solvent B; 1.5 min, 2% solvent B; 3 min, 15% solvent B; 5.5 min, 95% solvent B; 14.5 min, 95% solvent B; 15 min, 2% solvent B; and 20 min, 2% solvent B. For long-chain acyl-CoA, solvent A was water with 10 mM ammonium acetate (pH 8.5, adjusted with 10 N ammonium hydroxide), and solvent B was acetonitrile. The linear gradient was as follows: 0 min, 20% solvent B; 1.5 min, 20% solvent B; 5 min, 95% solvent B; 14.5 min, 95% solvent B; 15 min, 20% solvent B; and 20 min, 20% solvent B. The column temperature was room temperature.
MS Method.
The QE-MS was equipped with a heated electrospray ionization (HESI) probe, and the relevant parameters were as follows: heater temperature, 120 °C; sheath gas, 30 psi; auxiliary gas, 10 psi; sweep gas, 3 psi; and spray voltage, 3.6 kV for positive mode. The capillary temperature was set at 320 °C, and the S-lens was 55. A full scan range was set at 300–1,500 (m/z). The resolution was set at 70,000 (at m/z 200). The maximum injection time was 200 ms. The automated gain control was targeted at 3 × 106 ions.
Data Analysis.
Raw data collected from LC-QE-MS were processed on Thermo Scientific software Sieve 2.0. Peak alignment and detection were performed according to manufacturer protocols. For a targeted metabolomics analysis, a frameseed including acyl-CoA metabolites that had been previously validated was used for targeted metabolites analysis with data collected in positive mode; m/z width was set at 8 ppm.
Dimethyl Labeling of Heart Protein Extracts.
Hearts were collected from Sirt5 WT and KO male mice at 12 wk of age. The frozen powdered heart tissues were homogenized and lysed using lysis buffer (50 mM Tris⋅HCl, pH 8.0, 150 mM NaCl, 5 mM nicotinamide, 1% Nonidet P-40, 10% glycerol, and 1% vol/vol protease inhibitor mixture). The lysate was centrifuged at 20,000 × g for 20 min at 4 °C to remove the cell debris. Protein concentration of the supernatant was measured using the Bradford method. One milligram of total protein was taken from Sirt5 WT and KO samples for tryptic digestion. The protein mixtures were denatured and reduced with 6 M guanidine hydrochloride, 10 mM DTT in 50 mM triethylammonium bicarbonate, pH 8.0, for 1 h at room temperature. Next, cysteine residues were alkylated by 50 mM iodoacetamide in darkness at room temperature for 1 h followed by quenching with 1 M DTT (final concentration 50 mM) for 1 h at room temperature. After proteolytic digestion by trypsin (with trypsin: protein 1:20 wt/wt) for 16 h at 37 °C, the peptides from Sirt5 WT and KO samples were separately labeled with heavy and light dimethyl groups, respectively, using an on-column labeling procedure (23). The isotopically labeled peptides were mixed together and lyophilized to powder.
Enrichment of Lysine-Succinylated Peptides.
The antisuccinyllysine antibody (40 μg) was immobilized on 40 μL 25% suspension of protein A/G PLUS-agarose beads by incubating at 4 °C for 6 h. The supernatant was discarded and the beads were washed three times with NETN buffer (50 mM Tris⋅HCl, pH 8.0, 100 mM NaCl, 1 mM EDTA, and 0.5% Nonidet P-40). The lyophilized tryptic peptides obtained above were redissolved in 500 μL NETN buffer and incubated with the antisuccinyllysine antibody immobilized protein A/G PLUS-agarose beads at 4 °C for overnight with gentle shaking. The beads were washed three times with 1 mL of NETN buffer and three times with ETN (50 mM Tris⋅HCl, pH 8.0, 100 mM NaCl, and 1 mM EDTA). The bound peptides were eluted by washing three times with 100 μL of 1% trifluoroacetic acid. The elutions were combined, lyophilized, and cleaned up with C18 ZipTips (Millipore Corp.) according to the manufacturer’s instructions before nano LC-MS/MS analysis.
Identification and Quantitation of Dimethyl-Labeled Lysine-Succinylated Peptides by Nano LC-MS/MS Analysis.
The tryptic digest was reconstituted in 50 µL of 2% acetonitrile with 0.5% formic acid (FA) and about 100–200 ng of tryptic digest were injected for nano LC-ESI-MS/MS analysis, which was carried out on a LTQ-Orbitrap Elite mass spectrometer (Thermo-Fisher Scientific) equipped with a CorConneX nano ion source device (CorSolutions LLC). The Orbitrap was interfaced with a Dionex UltiMate3000RSLCnano HPLC system (Thermo/Dionex). The tryptic peptide samples (5 µL) were injected onto a PepMap C18 trap column-nano Viper (5 µm, 100 µm × 2 cm; Thermo/Dionex) at 20 µL/min flow rate for on-line desalting and then separated on a PepMap C18 reverse-phase nano column (3 µm, 75 µm × 15 cm; Thermo/Dionex) that was installed in the “plug and play” device with a 10-µm spray emitter (NewObjective). As described previously (43), the peptides were eluted with a 120-min gradient of 5–38% acetonitrile in 0.1% FA at a flow rate of 300 nL/min, followed by a 5-min ramping to 90% acetonitrile in 0.1% FA and a 7-min hold at 90% acetonitrile in 0.1% FA. The column was reequilibrated with 2% acetonitrile in 0.1% FA for 25 min before the next run. The Orbitrap Elite was operated in positive ion mode with nano spray voltage set at 1.5 kV and source temperature at 250 °C. Instrument calibration was performed using Ultramark 1621 for both Fourier transform (FT) mass analyzer and LTQ mass analyzer along with an additional background ion signal at m/z 445.120025 used as the lock mass for FT mass analyzer.
The instrument was operated in data-dependent acquisition with FT-FT mode using the FT mass analyzer for one survey MS scan of precursor ions followed by MS/MS scans of the top 15 most intense peaks with multiple charged ions above a threshold ion count of 10,000 in a higher-energy collisional dissociation (HCD)-based FT mass analyzer with normalized collision energy of 35%. MS survey scans were set at a resolution of 60,000 (FWHM at m/z 400) for the mass range of m/z 375–1,800 and MS/MS scans at 15,000 resolution for the mass range m/z 100–2,000. Dynamic exclusion parameters were set at repeat count 1 with a 30-s repeat duration, exclusion list size of 500, 60-s exclusion duration, and ±10 ppm exclusion mass width. HCD parameters were set at the following values: isolation width 2.0 m/z, normalized collision energy 35%, activation Q at 0.25, and activation time 0.1 ms. All data were acquired using Xcalibur 2.2 operation software (Thermo-Fisher Scientific).
Data Analysis.
All MS and MS/MS raw spectra were processed using Proteome Discoverer 1.4 (PD1.4; Thermo). The mouse RefSeq sequence database was downloaded on April 12, 2012, from NCBInr and used for database searches. The database search was performed with two-missed cleavage site by trypsin allowed. The peptide tolerance was set to 10 ppm and MS/MS tolerance was set to 0.1 Da for HCD. A fixed carbamidomethyl modification of cysteine and light dimethyl (+28.031 Da) and heavy dimethyl (+36.076 Da) for any N-terminus was set along with the following variable modifications: methionine oxidation, deamidation on asparagines/glutamine residues, succinylation, and both light/heavy dimethyl on lysine residue. Data filtering parameters were as follows: (i) ≤ 1% FDR, (ii) the peptide spectrum matches (PSMs) with confidence at least high, (iii) the PSMs with delta Cn better than 0.15, and (iv) mass precision 2 ≤ ppm. All MS/MS spectra for identified succinylated peptides with marginal scores from initial database searching were manually inspected and validated using both PD 1.4 and Xcalibur 2.2 software.
For relatively quantitative analysis of succinylated peptides between high/heavy samples, the peak areas of detected precursor ions at each specific m/z corresponding to the succinylated peptides were generated from the precursor ion-based methyl-duplex algorithm in PD 1.4. The peak area of some important succinylated peptides in raw data files was manually inspected using Xcalibur 2.2 software with mass tolerance at 5 ppm and mass precision at 4 decimal.
Cell Culture and Transfection.
HEK 293T cells were cultured in complete DMEM containing glucose and l-glutamine (Invitrogen) supplemented with 10% heat-inactivated FBS (Invitrogen). Cells were transfected using lipofection method (Invitrogen). To make the cDNA library, total RNA was isolated from mouse embryonic fibroblasts (MEFs) using the RNeasy mini kit (Qiagen) according to the manufacturer’s instructions. The isolated total RNA was used for the cDNA synthesis using RT-PCR. Flag-tagged ECHA was PCR-amplified from MEFs cDNA using TAATGAGCTCATGGTGGCGTCCCGGGCG (forward primer) and TAATCTCGAGTCAC TTATCGTCGTCATCCTTGTAATCCTGGTAGAACTTCTTGCTAGAGTTGTTAGC (reverse primer) and cloned into SacI/XhoI restriction sites of a pCMV vector.
Construction of Plasmids for ECHA Mutants.
ECHA lysine-to-arginine and lysine-to-glutamate mutants in a pCMV vector were constructed using a quick-change site-mutagenesis strategy with the primers listed below:
ECHA K351R:
Forward primer: CAGGTCCTGTGCAAGAGGAATAAATTTGGAGCA
Reverse primer: CTTGCACAGGACCTGGCCGTTATAAAGCCCCAT
ECHA K351E:
Forward primer: CAGGTCCTGTGCAAGGAGAATAAATTTGGAGCA
Reverse primer: CTTGCACAGGACCTGGCCGTTATAAAGCCCCAT
ECHA K406R:
Forward primer: CAGCAGCAAGTGTTCAGGGGGCTGAACGACAAGGTGAAGAAGAAA GCTCTCACATCA
Reverse primer: GAACACTTGCTGCTGTCCCCGGCCCAGCCCCGT
ECHA K406E:
Forward primer: CAGCAGCAAGTGTTCGAGGGGCTGAACGACAAGGTGAAGAAGAAA GCTCTCACATCA
Reverse primer: GAACACTTGCTGCTGTCCCCGGCCCAGCCCCGT
ECHA K644R:
Forward primer: TATCAGGAGGGCTCAAGGAATAAGAGTTTGAATTCTGAA
Reverse primer: TGAGCCCTCCTGATAGATGTAAAAGCCCTTCCC
ECHA K644E:
Forward primer: TATCAGGAGGGCTCAGAGAATAAGAGTTTGAATTCTGAA
Reverse primer: TGAGCCCTCCTGATAGATGTAAAAGCCCTTCCC
Generation of Sirt5 Stable KD Cell Lines.
Sirt5 shRNA lentiviral plasmids in pLKO.1-puro vector were purchased from Sigma. Sirt5 shRNA 1 (TRCN0000018544) CCGGGAGTCCAATTTGTCCAGCTTTCTCGAGAAAGCTGGACAAATTGGACTCTTTTT and shRNA 2 (TRCN0000018545) CCGGGCTACGAACAGATTCAGGTTTCTCGAGAAACC TGAATCTGTTCGTAGCTTTTT were used. After cotransfection of Sirt5 shRNA plasmid, pCMV-ΔR8.2, and pMD2.G into HEK 293T cells, the medium was collected to infect HEK 293T cells for different experimental purposes. The Sirt5 KD cells were selected using 1.5 mg/mL puromycin in DMEM complete medium containing glucose and l-glutamine (Invitrogen) supplemented with 10% heat-inactivated FBS (Invitrogen). Cells infected with lentivirus containing control shRNA plasmid were carried out similarly. Knockdown by shRNA 1 was more efficient and data shown in the main text were from Sirt5 KD cells with shRNA 1.
Synthesis of 2-(E)-Decenoyl-CoA.
The 2-(E)-decenoic acid (8.5 mg, 0.05 mmol) and PyBOP (51.2 mg, 0.1 mmol) were dissolved in 200 µL THF. CoA (39 mg, 0.05 mmol) and K2CO3 (28 mg, 0.2 mmol) were dissolved in 200 µL of H2O. The two solutions were mixed and incubated for 2 h at room temperature with occasional vortexing. After that, the reaction mixture was diluted with 2 mL 1:1 water/MeOH. To purify the 2-(E)-decenoyl-CoA, preparative HPLC was performed on a Beckman Coulter System Gold 125p Solvent Module and 168 detector with a TARGA C18 column (250 × 20 mm, 10 μm; Higgins Analytical, Inc.) monitoring at 215 and 260 nm. Solvents for HPLC were water with 0.1% trifluoroacetic acid (solvent A) and acetonitrile with 0.1% TFA (solvent B). Acyl-CoA was eluted at a flow rate of 8 mL/min with a linear gradient of 0–100% solvent B over 20 min, and finally 100% solvent B for 10 min before equilibrating the column back to 0% solvent B over 10 min. The purified fractions were then lyophilized and analyzed by LC-MS. The calculated m/z for 2-(E)-decenoyl-CoA C31H51N7O17P3S ([M-H]−) was 918.76, found 919.11.
Activity Assay of ECHA.
As the substrate for the activity assay of ECHA, 2-(E)-decenoyl-CoA was used. The combined 2-enoyl-CoA hydratase and 3-hydroxyacyl CoA dehydrogenase activities were measured by monitoring the formation of NADH at 340 nm (absorption coefficient 6,220 M−1⋅cm−1) as described previously (27). The reaction mixture contained 100 mM Tris⋅HCl (pH 9.0), 100 mM KCl, 100 μg/mL BSA, 1 mM free CoA, 120 μM NAD, and 30 μM 2-(E)-decenoyl-CoA substrate. The reaction was initiated by the addition of 4 μg of total lysate from heart or the purified Flag-tagged ECHA from one 10-cm tissue culture plate (∼8 × 106 cells) HEK 293T cells to the reaction mixture. The increasing absorbance at 340 nm was monitored for 10 min using a UV-visible spectrophotometer (Cary 50 UV-visible spectrophotometer; Varian).
Expression and Purification of ECHA and ECHB from E. coli.
ECHA and ECHB were PCR-amplified from mouse embryonic fibroblasts cDNA. The following primers were used: TAATCATATGGTGGCGTCCCGGGCG (forward primer for Flag-tagged ECHA), TAATCTCGAGTCACTTATCGTCGTCATCCTTGTAATCCTGGTAGAACTTCTTGCTAGAGTTGTTAGC (reverse primer for Flag-tagged ECHA), TAATGAATTCATGACTACC ATCTTGACTTCCACTTTTAGAAAC (forward primer for His-tagged ECHB), and TAATGTCGACTCATTTGGGGTAAGCTTCCACAATCATAGC (reverse primer for His-tagged ECHB). ECHA and ECHB were cloned into NdeI/XhoI and EcoRI/SalI restriction sites of pET-Duet and pET28a, respectively. Sequence-confirmed plasmids were cotransformed and expressed in BL-21 Rosetta cells. Cells were cultured at 37 °C in 4 L of LB media with 100 μg/mL ampicillin, 50 μg/mL kanamycin, and 20 μg/mL chloramphenicol. At an OD600 of 0.7, 300 μM isopropyl β-d-1-thiogalactopyranoside was added to induce expression and cells were further incubated at 23 °C overnight. Cells were harvested at 11,325 × g for 8 min and the cell pellet was stored at −80 °C until use. Cells were thawed and then suspended in 100 mM Hepes, pH 8.0, and 1 mM PMSF and then lysed using an EmulsiFlex-C3 cell disruptor (Avestin, Inc.). To the lysate was added octyl β-d-glucopyranoside to a final concentration of 0.8% (wt/vol) and the mixture was incubated for 20 min at 4 °C before centrifuging at 48,384 × g for 30 min using a Beckman Coulter refrigerated floor centrifuge. The soluble fraction was loaded onto a column containing an Ni-NTA agarose. The column was then washed with wash buffer (100 mM Hepes, pH 8.0, 30 mM imidazole, and 0.8% octyl β-d-glucopyranoside) and then a linear gradient of 50–500 mM imidazole in wash buffer was used to elute the protein complex. Fractions containing relatively pure (determined by SDS/PAGE analysis) ECHA and ECHB complex were collected, buffer-exchanged [100 mM Hepes, pH 8.0, 0.8% octyl β-d-glucopyranoside, and 10% (vol/vol) glycerol], concentrated, and stored at −80 °C. All mutants of ECHA were purified similarly.
In Vitro Chemical Succinylation of ECHA and ECHB Complex.
In vitro chemical succinylation of ECHA and ECHB complex was achieved by incubating 50 nM E. coli purified recombinant complex with 3 mM succinyl-CoA in a mixture of 100 mM Hepes, pH 7.4, 100 mM KCl, 10% (vol/vol) glycerol, 1 mM CoA, and 1 mM NAD at 27 °C for 20 min. It should be noted that any primary amine containing buffers (such as Tris buffer) will interfere with the chemical succinylation process. Because succinyl-CoA will also react with these amines they affect the succinylation efficiency as well as reproducibility.
Activity Assay of Citrate Synthase.
Citrate synthase activity was measured as described previously (44). The reaction mixture contained 100 mM Tris⋅HCl (pH 8.0), 0.17 mM oxaloacetate, and 0.2 mM acetyl-CoA. The reaction was initiated by the addition of 4 μg of total lysate from heart to the reaction mixture. The decreasing absorbance at 232 nm due to the cleavage of the thioesters bond was monitored for 5 min using a UV-visible spectrophotometer (Cary 50 UV-visible spectrophotometer; Varian).
Activity Assay of ATP Synthase (Complex V).
ATP synthase (complex V) activity was measured by linking the ATPase activity to NADH oxidation via the conversion of phosphoenolpyruvate to pyruvate by pyruvate kinase followed by pyruvate to lactate by lactate dehydrogenase. The assay was performed using a commercial kit from Cayman (MitoCheck Complex V Activity Assay Kit, 701000) according to the manufacturer’s instructions. The reaction buffer was supplemented with 5 mM KCN. The reaction was started by adding 1 μg mitochondrial suspension isolated from WT and Sirt5 KO mouse heart. Oligomycin-sensitive activity was determined with 10 μg/mL oligomycin. Complex V activity was calculated by using the extinction coefficient 6.22 mM−1⋅cm−1.
Western Blot Analysis.
Tissues were lysed in lysis buffer (50 mM Tris⋅HCl, pH 8.0, 150 mM NaCl, 1 mM EDTA, 1% Nonidet P-40, 5 mM nicotinamide, and protease inhibitor mixture). Protein extracts were separated by 12% SDS/PAGE and transferred onto PVDF membranes. The membrane was blocked using 5% BSA or milk in TBST (25 mM Tris⋅HCl, pH 7.4, 150 mM NaCl, and 0.1% Tween-20), incubated with antibodies in 5% BSA or milk in TBST, and developed in ECL Plus Western blotting detection reagents (GE Healthcare). The chemiluminescence was recorded by a Storm 860 Imager (Amersham Biosciences) and analyzed with ImageQuant TL v2005.
Echocardiography Analysis.
Echocardiography was performed with a Vevo 2100 system (Visualsonics) using male mice as described (45). Briefly, mice that were fasted overnight were anesthetized with isoflurane (4–5% for induction and 1.5–2.5% for maintenance) and their chest hair was removed with depilatory cream. The mice were then placed in a supine position on the Vevo mouse platform, where the paws were taped to ECG electrodes. Respiration rate, heart rate, and body temperature were continuously monitored. Warmed echo gel was gently applied on the hairless chest and the mouse hearts were imaged with a 40-MHz Echo probe. After echography, the residual gel was removed and Bepanthene cream was applied to the nude skin to prevent skin irritation. After the procedure mice were returned to their home cage.
Cardiac Output Measurement.
Although cardiac output is the sum of aortic and coronary flow, aortic flow determines the majority of cardiac output, and hence we calculated cardiac output by extrapolating the aortic flow from the analysis of the left ventricle (LV) trace (46–48). First, the LV trace analysis confirmed the reduced shortening fraction (calculated with Eq. S1) that we measured during echocardiography. Therefore, we extrapolated cardiac output (aortic flow) through LV trace analysis (Eqs. S2 and S3).
Here are the equations:
| [S1] |
| [S2] |
| [S3] |
| [S4] |
LV trace analysis using these formulas revealed a reduced cardiac output of Sirt5 KO mice compared with the WT (P = 0.544, Fig. S6H).
Immunohistochemistry.
H&E, Masson’s trichrome, and F4/80 staining was performed as described previously (45, 49, 50). Briefly, tissue sections were obtained from Formalin-fixed heart slices. H&E, Masson’s trichrome, and F4/80 stains were done using 8-µm tissue slices. Fibrosis (accumulation of collagen) in heart was assessed by the blue color on Masson’s trichrome staining. Antibodies against F4/80 (Abcam) were applied for immunohistochemistry to detect macrophages. F4/80 staining was visualized with HRP-conjugated goat anti-rat antibodies (Jackson Immunoresearch) and DAB substrate (Sigma-Aldrich). Images were obtained using a slide scanner (VS120-L100; Olympus Life Sciences) and analyzed with ImageJ (imagej.nih.gov/ij/).
Fatty Acid Oxidation Measurements.
Fatty acid oxidation in permeabilized heart tissues (39-week-old male WT and Sirt5 KO mice) was monitored as described in previous reports (51, 52). Briefly, mitochondrial respiration in response to palmitoyl-l-carnitine (50 μM) was measured with an Oxygraph-2k instrumens (Oroboros Instruments). Malate (2 mM) and ADP (2.5 mM) were used as a pretreatment. Substrate concentrations were chosen through preliminary experiments.
Supplementary Material
Acknowledgments
This work is supported in part by NIH Grants R01 GM098596, R01 CA163255, T32GM008500, R00 CA168997, R01 CA193256, R21 CA201963, and R01 AG043930; École Polytechnique Fédérale de Lausanne; Swiss National Science Foundation Grant 31003A-140780; and NIH Shared Instrument Grant 1S10RR025449-01. J.A. is the Nestlé Chair in Energy Metabolism.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1519858113/-/DCSupplemental.
References
- 1.Du J, et al. Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science. 2011;334(6057):806–809. doi: 10.1126/science.1207861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin H, Su X, He B. Protein lysine acylation and cysteine succination by intermediates of energy metabolism. ACS Chem Biol. 2012;7(6):947–960. doi: 10.1021/cb3001793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan M, et al. Identification of 67 histone marks and histone lysine crotonylation as a new type of histone modification. Cell. 2011;146(6):1016–1028. doi: 10.1016/j.cell.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Z, et al. Identification of lysine succinylation as a new post-translational modification. Nat Chem Biol. 2011;7(1):58–63. doi: 10.1038/nchembio.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, et al. Lysine propionylation and butyrylation are novel post-translational modifications in histones. Mol Cell Proteomics. 2007;6(5):812–819. doi: 10.1074/mcp.M700021-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dai L, et al. Lysine 2-hydroxyisobutyrylation is a widely distributed active histone mark. Nat Chem Biol. 2014;10(5):365–370. doi: 10.1038/nchembio.1497. [DOI] [PubMed] [Google Scholar]
- 7.Jiang H, et al. SIRT6 regulates TNF-α secretion through hydrolysis of long-chain fatty acyl lysine. Nature. 2013;496(7443):110–113. doi: 10.1038/nature12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan M, et al. Lysine glutarylation is a protein posttranslational modification regulated by SIRT5. Cell Metab. 2014;19(4):605–617. doi: 10.1016/j.cmet.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu AY, et al. Plasmodium falciparum Sir2A preferentially hydrolyzes medium and long chain fatty acyl lysine. ACS Chem Biol. 2012;7(1):155–159. doi: 10.1021/cb200230x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang X-J, Seto E. Lysine acetylation: Codified crosstalk with other posttranslational modifications. Mol Cell. 2008;31(4):449–461. doi: 10.1016/j.molcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao S, et al. Regulation of cellular metabolism by protein lysine acetylation. Science. 2010;327(5968):1000–1004. doi: 10.1126/science.1179689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pirinen E, Lo Sasso G, Auwerx J. Mitochondrial sirtuins and metabolic homeostasis. Best Pract Res Clin Endocrinol Metab. 2012;26(6):759–770. doi: 10.1016/j.beem.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhong L, Mostoslavsky R. Fine tuning our cellular factories: Sirtuins in mitochondrial biology. Cell Metab. 2011;13(6):621–626. doi: 10.1016/j.cmet.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rauh D, et al. An acetylome peptide microarray reveals specificities and deacetylation substrates for all human sirtuin isoforms. Nat Commun. 2013;4:2327. doi: 10.1038/ncomms3327. [DOI] [PubMed] [Google Scholar]
- 15.Lin Z-F, et al. SIRT5 desuccinylates and activates SOD1 to eliminate ROS. Biochem Biophys Res Commun. 2013;441(1):191–195. doi: 10.1016/j.bbrc.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 16.Park J, et al. SIRT5-mediated lysine desuccinylation impacts diverse metabolic pathways. Mol Cell. 2013;50(6):919–930. doi: 10.1016/j.molcel.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peng C, et al. The first identification of lysine malonylation substrates and its regulatory enzyme. Mol Cell Proteomics. 2011;10(12):012658. doi: 10.1074/mcp.M111.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rardin MJ, et al. SIRT5 regulates the mitochondrial lysine succinylome and metabolic networks. Cell Metab. 2013;18(6):920–933. doi: 10.1016/j.cmet.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weinert BT, et al. Lysine succinylation is a frequently occurring modification in prokaryotes and eukaryotes and extensively overlaps with acetylation. Cell Reports. 2013;4(4):842–851. doi: 10.1016/j.celrep.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 20.Lombard DB, et al. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol. 2007;27(24):8807–8814. doi: 10.1128/MCB.01636-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu J, et al. Metabolic characterization of a Sirt5 deficient mouse model. Sci Rep. 2013;3:2806. doi: 10.1038/srep02806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakagawa T, Lomb DJ, Haigis MC, Guarente L. SIRT5 Deacetylates carbamoyl phosphate synthetase 1 and regulates the urea cycle. Cell. 2009;137(3):560–570. doi: 10.1016/j.cell.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boersema PJ, Raijmakers R, Lemeer S, Mohammed S, Heck AJR. Multiplex peptide stable isotope dimethyl labeling for quantitative proteomics. Nat Protoc. 2009;4(4):484–494. doi: 10.1038/nprot.2009.21. [DOI] [PubMed] [Google Scholar]
- 24.Huang W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 25.Huang W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37(1):1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uchida Y, Izai K, Orii T, Hashimoto T. Novel fatty acid beta-oxidation enzymes in rat liver mitochondria. II. Purification and properties of enoyl-coenzyme A (CoA) hydratase/3-hydroxyacyl-CoA dehydrogenase/3-ketoacyl-CoA thiolase trifunctional protein. J Biol Chem. 1992;267(2):1034–1041. [PubMed] [Google Scholar]
- 27.Fong JC, Schulz H. Short-chain and long-chain enoyl-CoA hydratases from pig heart muscle. Methods Enzymol. 1981;71(Pt C):390–398. doi: 10.1016/0076-6879(81)71049-8. [DOI] [PubMed] [Google Scholar]
- 28.Venkatesan R, Wierenga RK. Structure of mycobacterial β-oxidation trifunctional enzyme reveals its altered assembly and putative substrate channeling pathway. ACS Chem Biol. 2013;8(5):1063–1073. doi: 10.1021/cb400007k. [DOI] [PubMed] [Google Scholar]
- 29.Ingwall JS. ATP and the Heart. Kluwer; Boston: 2001. [Google Scholar]
- 30.Katz AM. Metabolism of the failing heart. Cardioscience. 1993;4(4):199–203. [PubMed] [Google Scholar]
- 31.Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403(6771):795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- 32.Nishida Y, et al. SIRT5 regulates both cytosolic and mitochondrial protein malonylation with glycolysis as a major target. Mol Cell. 2015;59(2):321–332. doi: 10.1016/j.molcel.2015.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herrmann G, Decherd JGM. The chemical nature of heart failure. Ann Intern Med. 1939;12(8):1233–1244. [Google Scholar]
- 34.Antozzi C, Zeviani M. Cardiomyopathies in disorders of oxidative metabolism. Cardiovasc Res. 1997;35(2):184–199. doi: 10.1016/s0008-6363(97)00141-7. [DOI] [PubMed] [Google Scholar]
- 35.Guertl B, Noehammer C, Hoefler G. Metabolic cardiomyopathies. Int J Exp Pathol. 2000;81(6):349–372. doi: 10.1046/j.1365-2613.2000.00186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neubauer S. The failing heart--an engine out of fuel. N Engl J Med. 2007;356(11):1140–1151. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- 37.den Boer MEJ, et al. Mitochondrial trifunctional protein deficiency: A severe fatty acid oxidation disorder with cardiac and neurologic involvement. J Pediatr. 2003;142(6):684–689. doi: 10.1067/mpd.2003.231. [DOI] [PubMed] [Google Scholar]
- 38.Brackett JC, et al. Two alpha subunit donor splice site mutations cause human trifunctional protein deficiency. J Clin Invest. 1995;95(5):2076–2082. doi: 10.1172/JCI117894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boylston JA, et al. Characterization of the cardiac succinylome and its role in ischemia-reperfusion injury. J Mol Cell Cardiol. 2015;88:73–81. doi: 10.1016/j.yjmcc.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gibson GE, et al. Alpha-ketoglutarate dehydrogenase complex-dependent succinylation of proteins in neurons and neuronal cell lines. J Neurochem. 2015;134(1):86–96. doi: 10.1111/jnc.13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wagner GR, Payne RM. Widespread and enzyme-independent Nε-acetylation and Nε-succinylation of proteins in the chemical conditions of the mitochondrial matrix. J Biol Chem. 2013;288(40):29036–29045. doi: 10.1074/jbc.M113.486753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dils R, Carey EM. Fatty acid synthase from rabbit mammary gland. Methods Enzymol. 1975;35:74–83. doi: 10.1016/0076-6879(75)35140-9. [DOI] [PubMed] [Google Scholar]
- 43.Yang Y, et al. Evaluation of different multidimensional LC-MS/MS pipelines for isobaric tags for relative and absolute quantitation (iTRAQ)-based proteomic analysis of potato tubers in response to cold storage. J Proteome Res. 2011;10(10):4647–4660. doi: 10.1021/pr200455s. [DOI] [PubMed] [Google Scholar]
- 44.Srere PA, Kosicki GW. The purification of citrate-condensing enzyme. J Biol Chem. 1961;236(10):2557–2559. [PubMed] [Google Scholar]
- 45.Ryu D, et al. A SIRT7-dependent acetylation switch of GABPβ1 controls mitochondrial function. Cell Metab. 2014;20(5):856–869. doi: 10.1016/j.cmet.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 46.Madonna R, Wu H, Shelat H, Geng YJ. CD1d-associated expression of NF-kB and cardiac dysfunction in diabetic and obese mice. Int J Immunopathol Pharmacol. 2013;26(1):59–73. doi: 10.1177/039463201302600106. [DOI] [PubMed] [Google Scholar]
- 47.Longhurst JC, Kelly AR, Gonyea WJ, Mitchell JH. Cardiovascular responses to static exercise in distance runners and weight lifters. J Appl Physiol. 1980;49(4):676–683. doi: 10.1152/jappl.1980.49.4.676. [DOI] [PubMed] [Google Scholar]
- 48.Kessler KM. Ejection fraction derived by M-mode echocardiography: A table and comments. Cathet Cardiovasc Diagn. 1979;5(3):295–299. doi: 10.1002/ccd.1810050312. [DOI] [PubMed] [Google Scholar]
- 49.Gariani K, et al. Eliciting the mitochondrial unfolded protein response via NAD repletion reverses fatty liver disease. Hepatology. 2015 doi: 10.1002/hep.28245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perino A, et al. TGR5 reduces macrophage migration through mTOR-induced C/EBPβ differential translation. J Clin Invest. 2014;124(12):5424–5436. doi: 10.1172/JCI76289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boyle KE, Zheng D, Anderson EJ, Neufer PD, Houmard JA. Mitochondrial lipid oxidation is impaired in cultured myotubes from obese humans. Int J Obes. 2012;36(8):1025–1031. doi: 10.1038/ijo.2011.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lemieux H, Semsroth S, Antretter H, Höfer D, Gnaiger E. Mitochondrial respiratory control and early defects of oxidative phosphorylation in the failing human heart. Int J Biochem Cell Biol. 2011;43(12):1729–1738. doi: 10.1016/j.biocel.2011.08.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.