Abstract
Background
The presence of a myocardial bridge (MB) has been shown to promote atherosclerotic plaque formation proximal to the MB, presumably because of hemodynamic disturbances provoked by retrograde blood flow toward this segment in cardiac systole. We aimed to determine the anatomic and functional properties of an MB related to the extent of atherosclerosis assessed by intravascular ultrasound.
Methods and Results
We enrolled 100 patients with angina but no significant obstructive coronary artery disease who had an intravascular ultrasound–detected MB in the left anterior descending artery (median age 54 years, 36% male). The MB was identified with intravascular ultrasound by the presence of an echolucent band (halo). Anatomically, the MB length was 22±13 mm, and halo thickness was 0.7±0.6 mm. Functionally, systolic arterial compression was 23±12%. The maximum plaque burden up to 20 mm proximal to the MB entrance was significantly greater than the maximum plaque burden within the MB segment. Among the intravascular ultrasound–defined MB properties, arterial compression was the sole MB parameter that demonstrated a significant positive correlation with maximum plaque burden up to 20 mm proximal to the MB entrance (r=0.254, P=0.011 overall; r=0.545, P<0.001 low coronary risk). In multivariate analysis, adjusting for clinical characteristics and coronary risk factors, arterial compression was independently associated with maximum plaque burden up to 20 mm proximal to the MB entrance.
Conclusions
In patients with an MB in the left anterior descending artery, the percentage of arterial compression is related directly to the burden of atherosclerotic plaque located proximally to the MB, particularly in patients who otherwise have low coronary risk. This may prove helpful in identifying high‐risk MB patients.
Keywords: angina, angiography, atherosclerosis, intravascular ultrasound, myocardial bridge
Subject Categories: Angiography, Ultrasound, Diagnostic Testing
Introduction
A myocardial bridge (MB) is a common anatomic variant that predominantly involves the mid‐ or distal coronary segments of the left anterior descending artery (LAD).1, 2, 3 An MB has traditionally been considered a benign condition because contraction of the bridging muscles alters blood flow within the underlying LAD during systole, whereas coronary flow in the LAD occurs predominantly in diastole. A number of studies, however, have indicated that an MB can lead to significant clinical symptoms, arrhythmia, or adverse cardiac events (ie, myocardial ischemia,4, 5 myocardial infarction,6 and sudden death7) in a subset of patients.
In addition to the reduced blood flow reserve and decreased blood perfusion derived from delayed arterial relaxation in diastole, progression of atherosclerotic plaque frequently reported in the segment proximal to the MB has been proposed as another potential mechanism of poor clinical outcomes in a subset of MB patients.6, 8, 9, 10 Consequently, accurate identification of high‐risk MB patients susceptible to this complication may not only provide prognostic information but also help determine the indication and strategy for therapeutic approaches.
To date, several autopsy reports and multidetector computed tomography studies have indicated that the anatomic properties of an MB, such as its length and thickness (depth), play decisive roles as regulators of atherosclerosis in the LAD segment proximal to the MB.11 Considering its mechanisms involving abnormal hemodynamics, it may be hypothesized that functional or dynamic properties of the MB may be more directly associated with the atherosclerotic plaque formation in this segment. Among several clinical diagnostic tools, intravascular ultrasound (IVUS) has the unique capability to provide both anatomic and functional information about an MB. This study aimed to determine anatomic and functional properties of an MB related to the extent of atherosclerosis assessed by IVUS in a clinical setting.
Methods
Participants
All patients were recruited at Stanford University Medical Center between March 2005 and July 2012. We included patients with angina and a clinical suspicion of coronary artery disease who were referred for coronary angiography and were found to have an MB in the LAD, identified by IVUS. Exclusion criteria were significant coronary artery stenosis ≥50% by visual estimation, acute coronary syndrome, prior coronary artery bypass grafting or percutaneous coronary intervention, and heart transplantation. Patient characteristics were obtained using hospital chart reviews at the time of the procedure, including clinical cardiovascular risk factors, such as hypertension (medically treated only), hyperlipidemia (medically treated or total cholesterol >240 mg/dL), diabetes mellitus (controlled by diet, oral agent, or insulin), and current smoking. This study was approved by the institutional review board at Stanford University, and written informed consent was obtained from all patients before study entry.
Cardiac Catheterization Procedure
Cardiac catheterization was performed in a standard fashion, and 200 μg of intracoronary nitroglycerin was administered prior to angiography and IVUS imaging. IVUS image acquisition was performed during an automated pullback at 0.5 mm/s using a 40‐MHz mechanical IVUS system (Atlantis SR Pro2; Boston Scientific Corp), which was placed as far distally as safely possible in the LAD. All images were stored on DVD for offline analysis.
Image Analysis
All images were analyzed at an independent core laboratory (Cardiovascular Core Analysis Laboratory, Stanford University). Quantitative coronary angiography was performed using a validated computer‐assisted method (QAngio XA7.3; Medis Medical Imaging Systems Inc). Measurements of interest included reference vessel diameter, minimum lumen diameter, and percentage diameter stenosis at tunneled coronary segments at end‐diastole and ‐systole after administration of intracoronary nitroglycerin. The quantitative coronary angiography boundaries (entrance and exit) of the tunneled segments were confirmed by corresponding IVUS images of the MB segments.
All IVUS images were reviewed frame by frame using validated IVUS analysis software (echoPlaque 3.0.60; Indec Systems Inc). According to our previous IVUS report with histological validation, an MB was defined as an echolucent band (halo) partially surrounding the artery (Figure 1).5, 12, 13, 14 Anatomic IVUS assessment of the MB included length of the MB segment, halo thickness, location of the MB, and the presence of branches arising within the MB segment. The MB length was measured from the first proximal appearance of the halo (MB entrance) to its distal end (MB exit), and the halo thickness was measured at the thickest part above the artery during systole (Figure 1B). The location of the MB was assessed as a distance from the LAD orifice to the MB entrance. Functional IVUS assessment of the MB included arterial compression, which was calculated as a decrease in external elastic membrane cross‐sectional area (CSA) at systole standardized by external elastic membrane CSA at end‐diastole, expressed as a percentage. Atherosclerotic plaque was assessed by calculating plaque burden as (external elastic membrane CSA−lumen CSA/external elastic membrane CSA)×100. Maximum plaque burden was obtained within the MB segment (Max PBMB) and in the proximal reference (Max PBProx), defined as a 20‐mm nontunneled segment immediately proximal to the MB entrance (Figure 1A). In addition, plaque types were categorized into fibrous, soft, calcified, or mixed, according to the “American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies.”15 The reproducibility of IVUS measurement of arterial wall compression was verified using randomly selected patients (10% of study participants). For the assessment of inter‐ and intraobserver variability, IVUS measurements were performed by 2 independent investigators who were blinded to each other's results, and then measurements were repeated at least 1 month later by 1 investigator who was blinded to his initial results.
Figure 1.
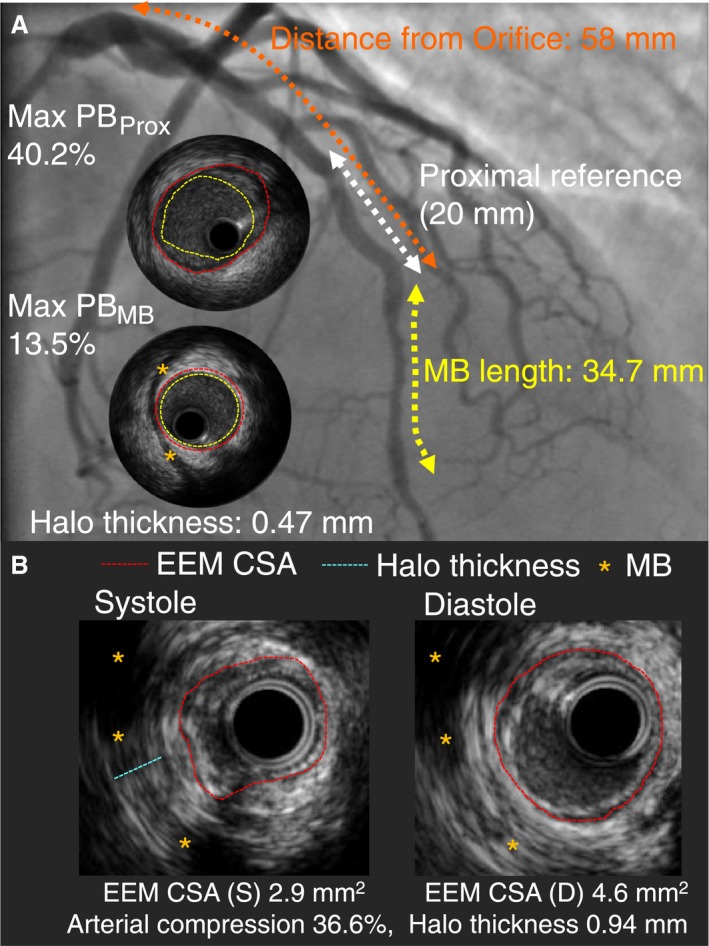
Intravascular ultrasound assessment of myocardial bridging. An MB was defined as an echolucent band (halo: orange asterisk) partially (or completely) surrounding the artery. The proximal reference was defined as a 20‐mm segment immediately proximal to the MB entrance. Max PB was measured in the proximal reference (Max PB Prox) and in the MB segment (Max PB MB). The location of the bridge was also measured, defined by the length from the orifice of the left anterior descending artery to the entrance of the MB (A). Within the MB segment, EEM‐CSA (red dotted line), arterial compression, and halo thickness (blue dotted line) were measured (B). CSA indicates cross‐sectional area; EEM, external elastic membrane; Max PBMB, maximum plaque burden with the myocardial bridge segment; Max PBProx, maximum plaque burden within a 20‐mm segment immediately proximal to the myocardial bridge entrance; MB, myocardial bridge.
Statistical Analysis
Statistical analysis was performed using StatView 5.0 (SAS Institute). Categorical variables are presented as percentages, and continuous variables are reported as mean±SD. The paired Student t test was used to differentiate 2 sets of data with normal distribution. A P value of <0.05 was considered statistically significant. Bonferroni correction was used to adjust a P value of significance for multiple comparisons between arterial compression and 2 anatomic MB parameters (a P value of <0.025 was considered statistically significant). Similarly, a P value of significance was adjusted for multiple comparisons between plaque burden and 4 MB parameters (a P value of <0.0125 was considered statistically significant). The Pearson correlation coefficients were calculated in simple linear regression analysis. Clinical risk factors and MB properties with P<0.20 found in simple regression analysis were inserted into multivariate linear regression analysis.
Results
Patient Demographics
From 185 patients with angina in the absence of obstructive coronary artery disease, a LAD MB was identified in 100 patients who fulfilled the inclusion and exclusion criteria of this study. Table 1 summarizes the clinical characteristics of the enrolled participants. The median age was 54 years, and 36% were men. Hyperlipidemia was present in 67% of the patients, the majority of whom were adequately controlled by statin therapy. More than half of the patients had hypertension (or were on an antihypertensive medication), whereas only 15% had diabetes, although all cardiac medications were routinely withdrawn ≥48 hours before the catheterization.
Table 1.
Clinical Characteristics
| Variable | n=100 |
|---|---|
| Patient characteristics | |
| Age, y | 53±13 |
| Body mass index | 28.9±7.2 |
| Male, n (%) | 36 (36.0) |
| Risk factors | |
| Hypertension, n (%) | 53 (53.0) |
| Diabetes mellitus, n (%) | 15 (15.0) |
| Hyperlipidemia, n (%) | 67 (67.0) |
| Current smoker, n (%) | 3 (3.0) |
| Lipid profile | |
| Total cholesterol, mg/dL | 169.6±37.4 |
| LDL cholesterol, mg/dL | 100.9±31.1 |
| HDL cholesterol, mg/dL | 49.6±13.9 |
| TG, mg/dL | 92.3±50.2 |
| Medications | |
| Aspirin, n (%) | 59 (59.0) |
| Clopidogrel, n (%) | 8 (8.0) |
| β‐blockers, n (%) | 41 (41.0) |
| ACEI/ARB, n (%) | 14 (14.0) |
| Diuretics, n (%) | 17 (17.0) |
| Statins, n (%) | 59 (59.0) |
| Nitrates, n (%) | 28 (28.0) |
Continuous variables are expressed as mean±SD. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; TG, triglyceride.
Anatomic and Functional Properties of Myocardial Bridging
Quantitative coronary angiography and IVUS findings of myocardial bridging analyzed by the core laboratory are summarized in Table 2. By quantitative coronary angiography, the minimum lumen diameter of the tunneled segment was 1.66±0.37 mm in diastole, and the percentage diameter stenosis increased from 25.7±13.0% to 42.0±17.8% during the cardiac cycle. When angiographically detectable MBs were defined as angiographic arterial compression of >20%, >30%, and >40%, the detection rates of MB by angiography were 42%, 23%, and 16%, respectively. By IVUS, the tunneled segment measured 21.6±13.4 mm in axial length, and the halo thickness was 0.66±0.58 mm, ranging from 0.11 to 3.17 mm. The MB entrance was located 37.7±12.7 mm distally to the orifice of the LAD. From diastole to systole, external elastic membrane CSA decreased from 7.0±2.8 to 5.4±2.4 mm2, and calculated arterial compression ranged from 0.4% to 50.7% (Figure 2). Intra‐ and interobserver differences for IVUS‐defined arterial compression were 0.3±3.1% and 2.2±5.7%, respectively. The degree of arterial compression had a weak but significant positive correlation to both MB length (r=0.288, P=0.004) and halo thickness (r=0.295, P=0.003; with the Bonferroni correction, a P value of <0.025 was considered statistically significant).
Table 2.
Angiographic and IVUS Findings
| Variable | n=100 |
|---|---|
| QCA results at MB segment | |
| RVD (D), mm | 2.28±0.57 (1.33–4.32) |
| RVD (S), mm | 2.40±0.50 (0.93–4.71) |
| MLD (D), mm | 1.66±0.37 (0.95–3.23) |
| MLD (S), mm | 1.37±0.42 (0.01–2.48) |
| %DS (D), % | 25.7±13.0 (0.00–58.89) |
| %DS (S), % | 42.0±17.8 (3.77–99.78) |
| IVUS results | |
| Bridge length, mm | 21.6±13.4 (4.7–76.9) |
| Arterial compression, % | 22.9±12.1 (0.4–50.7) |
| EEM CSA (D), mm2 | 7.0±2.8 (2.8–15.1) |
| EEM CSA (S), mm2 | 5.4±2.4 (2.1–12.7) |
| Halo thickness, mm | 0.66±0.58 (0.11–3.17) |
| Distance from LAD ostium, mm | 37.7±12.7 (5.3–77.5) |
| Presence of branches within MB, n (%) | 81 (81.0) |
| Max PBProx, % | 38.6±15.1 (11.3–76.9) |
| Max PBMB, % | 21.7±7.0 (10.1–45.3) |
| Plaque type (fibrous/soft/calcified/mixed), % | 49/33/10/8 |
Continuous variables are expressed as mean±SD (range). %DS indicates percentage diameter stenosis; CSA, cross‐sectional area; D, diastole; EEM, external elastic membrane; IVUS, intravascular ultrasound; LAD, left anterior descending artery; Max PBMB, maximum plaque burden with the myocardial bridge segment; Max PBProx, maximum plaque burden within a 20‐mm segment immediately proximal to the myocardial bridge entrance; MB, myocardial bridge; MLD, minimum lumen diameter; QCA, quantitative coronary angiography; RVD, reference vessel diameter; S, systole.
Figure 2.
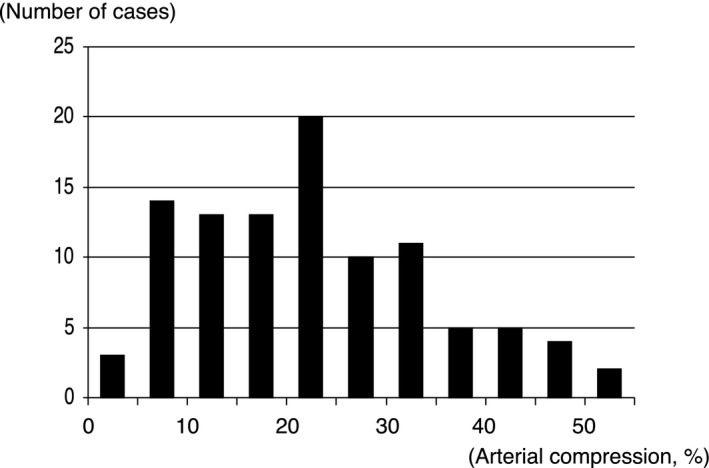
Histogram of arterial compression by myocardial bridging. Systolic arterial compression was 22.9±12.1%, ranging from 0.4% to 50.7%.
Atherosclerotic Plaque Related to Myocardial Bridging
The tunneled arterial segment under the MB was relatively spared from plaque, whereas the proximal segment adjacent to the MB showed a significantly larger amount of plaque, ranging from 11.3% to 76.9% (Max PBProx 38.6±15.1% versus Max PBMB 21.7±7.0%, P<0.001) (Figure 3). Although no anatomic or functional MB properties were related to the plaque burden within the MB, the degree of arterial compression showed a weak but significant positive correlation with the proximal plaque burden (Max PBProx r=0.254, P=0.011; with the Bonferroni correction, a P value of <0.0125 was considered statistically significant) (Figure 4). In contrast, the degree of angiographic “milking” by quantitative coronary angiography was not related to the proximal plaque burden by either correlation or categorical analysis. With respect to the plaque characteristics, fibrous and soft plaques were the dominant plaque types in the proximal segment, but no relationship was observed between plaque type and the degree of arterial compression.
Figure 3.
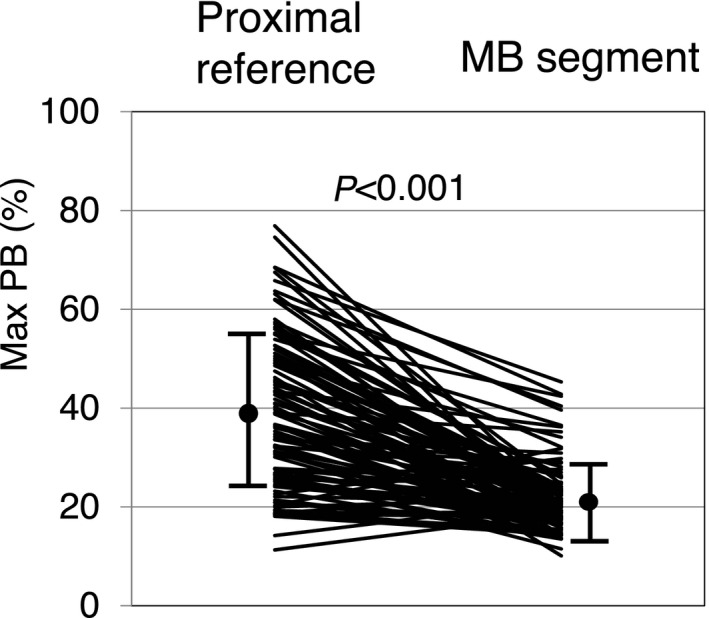
Maximum plaque burden in the proximal reference vs myocardial bridge segment. In the majority of the enrolled patients, the proximal reference segment adjacent to the MB showed a significantly larger amount of plaque, ranging up to 76.9%, compared with the MB segment (Max PB Prox 38.6±15.1% vs Max PB MB 21.7±7.0%, P<0.001). Max PBMB indicates maximum plaque burden with the myocardial bridge segment; Max PBProx, maximum plaque burden within a 20‐mm segment immediately proximal to the myocardial bridge entrance; MB, myocardial bridge.
Figure 4.
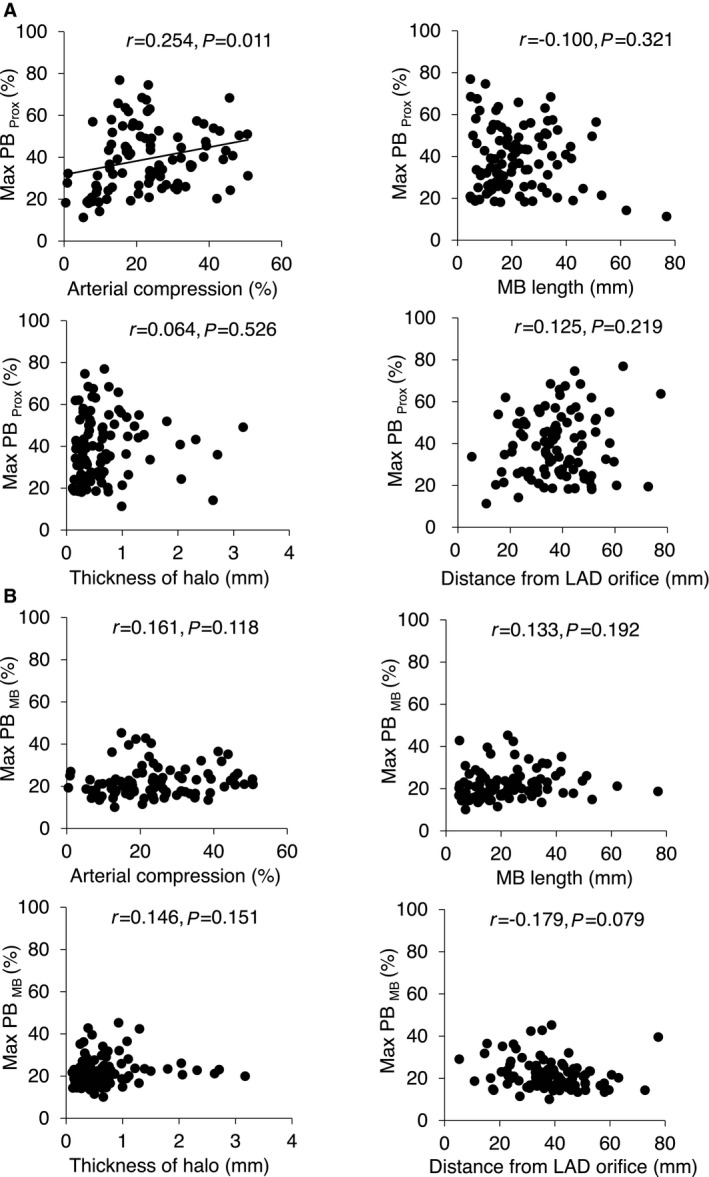
Correlations of anatomic and functional MB properties with maximum plaque burden at the proximal reference and the myocardial bridge segment. At the proximal reference (A), arterial compression showed a weak but significant positive correlation with maximum plaque burden. In contrast, no correlation was observed in anatomic properties of an MB, including MB length, thickness of halo, and the location of the MB based on its distance from the LAD orifice. At the MB segment (B), maximum plaque burden showed no association with any MB properties. LAD indicates left anterior descending artery; Max PBMB, maximum plaque burden with the myocardial bridge segment; Max PBProx, maximum plaque burden within a 20‐mm segment immediately proximal to the myocardial bridge entrance; MB, myocardial bridge.
Determinants of Atherosclerotic Plaque in the Proximal Segment
Table 3 shows the results of univariate and multivariate analyses looking for determinants of the maximum plaque burden in the adjacent proximal segment, including patient demographics, clinical characteristics such as baseline medications, and angiographic and IVUS imaging variables. The univariate analysis identified age, male sex, hyperlipidemia, and arterial compression as significant variables related to Max PBProx. The multivariate analysis confirmed systolic arterial compression as an independent determinant of Max PBProx, whereas other univariate variables were also independently associated with Max PBProx. Consequently, when focusing on younger patients (age <54 years as the median value) with ≤1 coronary risk factor, arterial compression and Max PBProx demonstrated stronger correlation (r=0.545, P<0.001) than in the overall population (Figure 5).
Table 3.
Determinants of Maximum Plaque Burden in the Proximally Adjacent Segment
| Variable | Simple Regression Analysis | Multiple Regression Analysis | ||||
|---|---|---|---|---|---|---|
| r | 95% CI | P Value | β | 95% CI | P Value | |
| Age | 0.424 | 0.28 to 0.69 | <0.001 | 0.290 | 0.12 to 0.54 | 0.003 |
| Male | 0.328 | 4.38 to 16.33 | <0.001 | 0.230 | 1.72 to 12.77 | 0.011 |
| Hyperlipidemia | 0.384 | 6.41 to 18.33 | <0.001 | 0.221 | 1.25 to 12.96 | 0.018 |
| Hypertension | 0.155 | −1.30 to 10.72 | 0.123 | 0.075 | −3.26 to 7.80 | 0.418 |
| Current smoker | −0.065 | −23.51 to 12.02 | 0.523 | — | — | — |
| Diabetes mellitus | 0.114 | −3.61 to 13.29 | 0.258 | — | — | — |
| Arterial compression | 0.254 | 0.08 to 0.56 | 0.011 | 0.192 | 0.02 to 0.46 | 0.031 |
| Length from LAD orifice | 0.125 | −0.09 to 0.39 | 0.219 | — | — | — |
| MB length | −0.100 | −0.34 to 0.11 | 0.321 | — | — | — |
| Halo thickness | 0.064 | −3.56 to 6.93 | 0.526 | — | — | — |
R 2=0.343, P<0.001 for multivariate model. LAD indicates left anterior coronary artery; MB, myocardial bridge.
Figure 5.
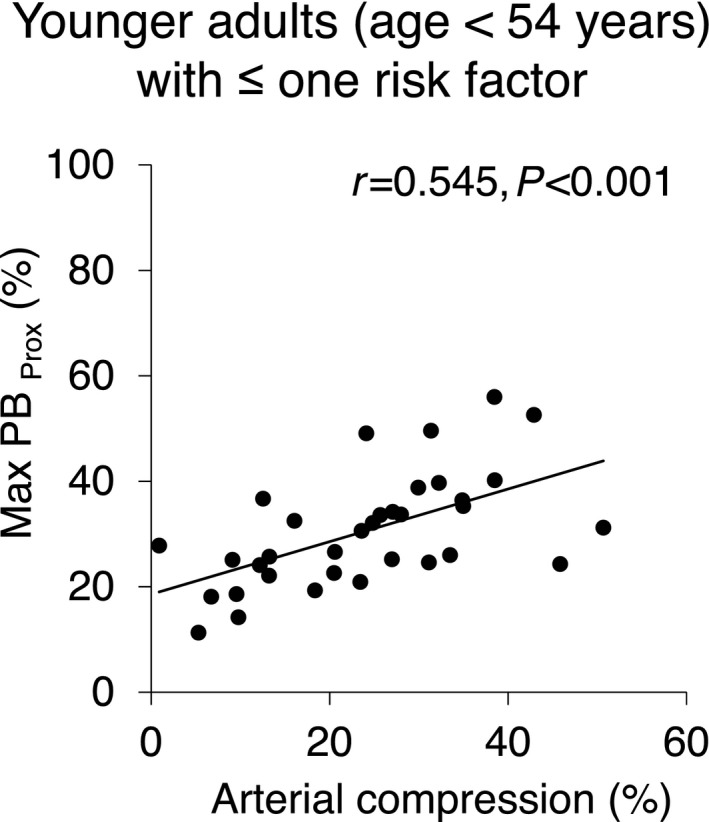
The correlation between arterial compression and Max PB Prox in younger adults with ≤1 coronary risk factor. The analysis using the adjusted (low coronary risk) population demonstrates a moderately strong correlation between the 2 variables, unmasking the effect of arterial compression on the proximal plaque formation. Max PBProx indicates maximum plaque burden within a 20‐mm segment immediately proximal to the myocardial bridge entrance.
Discussion
The present study adds to and extends the current literature regarding atherosclerotic plaque in the presence of an MB by demonstrating that (1) arterial compression, more than MB length, depth (halo thickness), or location, is correlated with the burden of atherosclerotic plaque proximal to the MB and (2) this is particularly true for patients with low coronary risk who otherwise are not expected to manifest a large plaque burden. This latter finding may help identify those patients for whom MB confers atherosclerotic risk beyond traditional cardiac risk factors.
Effects of Myocardial Bridging on Atherosclerotic Plaque Formation
Similar to previous autopsy,1, 16 angiographic,5 and multidetector computed tomography17 reports, we found a greater plaque burden in the LAD segment proximal to the MB than within the tunneled LAD segment. This preferential location of atherosclerotic plaque in relation to the MB is attributed partially to hemodynamic disturbances caused by MB muscle contraction. In particular, the distribution of wall shear stress in relation to the tunneled segment may play a role because lower sheer stress has been postulated to predispose patients to enhanced lipid transfer across the endothelium and to more atherosclerosis. A report using a computational fluid dynamics model of the LAD in a patient with a symptomatic MB revealed areas of relatively low wall shear stress proximal and distal to the MB and high wall sheer stress within the tunneled segment.18 Likewise, a case–control study of MB patients also demonstrated that the wall shear rate—the velocity gradient perpendicular to the wall, which relates to the wall shear stress by the factor of blood viscosity—was lower in the segment proximal to the MB than within the tunneled segment.19
Other potential mechanisms for proximal plaque formation include abnormal flow profiles at this segment, such as oscillatory flow reversal with or without significant collision with antegrade coronary flow. A number of studies have indicated that arterial segments in which blood flow departs from a laminar unidirectional pattern are prone to the development of atherosclerotic plaques.6, 8, 9, 10 Another study using a catheter‐based pressure measurement system demonstrated that the intracoronary pressure within the LAD segment proximal to the MB was significantly higher than the aortic blood pressure, suggesting potential augmentation of the intimal injury at this segment.9 Finally, solid mechanical forces that result from the motion and deformation of the coronary tree and myocardial material properties may lead to a heterogeneous stress field at this segment.6, 9, 20, 21
Anatomic Versus Functional Properties of Myocardial Bridging
Although previous studies have focused on the conventional anatomic properties of an MB with regard to proximal atherosclerotic plaque formation, the potential hemodynamic mechanisms discussed led us to believe that functional or dynamic properties of an MB may be associated more directly with the burden of proximal atherosclerotic plaque. To the best of our knowledge, the present study is the first to test this hypothesis in clinical patients by investigating the relationship between the degree of arterial compression and the amount of atherosclerotic plaque accumulated at the segment proximal to the MB.
The positive correlation of these 2 variables found in the present study supports the proposed mechanisms for the accelerated proximal plaque formation. As expected, however, other classic cardiac risk factors were also independently related to the amount of plaque in the LAD, presumably accounting for wide scatter of the correlation in the unadjusted overall population. The analysis of an adjusted population with low coronary risk unmasked the direct effect of arterial compression on proximal plaque formation by demonstrating a moderately strong correlation between the 2 variables.
Unlike previous studies, the conventional anatomic MB properties, such as axial MB length, halo thickness, and the distance from the LAD orifice, were not significantly related to the amount of proximal plaque in the current study. In addition to the limited statistical power of the current study, this may be related in part to the relatively large variability in correlations between the anatomic and functional MB properties found in our study population. Indeed, patients with a long or thick MB did not necessarily show strong arterial compression that could cause significant hemodynamic disturbances. In contrast, some patients who had only a short or thin MB demonstrated a considerable degree of arterial compression, presumably depending on the variable function and anatomic direction of bridging muscle cells, as well as the 3‐dimensional geography of the tunneled artery and the surrounding cardiac structures. Systolic arterial compression, for example, is known to occur even without an MB when the coronary segment is located deep within the interventricular groove.22 Possible independent effects of anatomic factors have yet to be investigated; however, adding the direct assessment of arterial compression, rather than simply using the anatomic MB properties alone as surrogate markers, may be beneficial to better estimate the severity of hemodynamic disturbances caused by MB.
Clinical Implications
Although most patients with an MB are presumed to be asymptomatic, MB has been shown to cause myocardial ischemia, conduction disturbances, and myocardial infarction, possibly leading to sudden cardiac death in some cases.4, 5, 6, 7 These adverse effects likely result from 2 distinct mechanisms: (1) accelerated plaque formation in the segment proximal to the MB, as investigated in the current study, and (2) alteration in the effective blood perfusion of the affected myocardial territory owing to delayed arterial relaxation in diastole or the Venturi effect on the septal branches that can cause functional ischemia of the septal wall.4 Furthermore, external systolic compression and endothelial dysfunction frequently observed in the tunneled artery could stimulate coronary vasospasm and platelet aggregation resulting in acute coronary syndrome in certain clinical settings.16, 21, 23, 24
Because these mechanisms are all linked to hemodynamic abnormalities caused by arterial compression, it is reasonable to add functional assessment to the conventional diagnosis of an MB. The combined assessment of MB anatomy and degree of arterial compression with consequences for proximal plaque burden, as performed with IVUS in the present study, may enhance our understanding of this common coronary variant and provide us with prognostic information in given patients to guide the indication and strategy for treatment. Particularly important is that the percentage of arterial compression is notably correlated with the burden of proximal plaque in a population with low coronary risk. It may be that such patients need more aggressive primary prevention than we would typically offer through current cardiac risk prediction models. The quantitative assessment of arterial compression may also be useful in evaluating the results of pharmacological (ß‐blockers)25 or surgical (unroofing of the intramural coronary with myotomy)26 interventions, although a noninvasive method of making such an assessment would be preferred. Unfortunately, angiography is also not sufficient; we and others5, 20 have shown that the majority of MBs are angiographically silent. We also note that the present study assessed the degree of arterial compression only at a resting state in the setting of cardiac catheterization. Exercise or pharmacological stress might help estimate the maximum physiological impact of a given MB.4
Another clinically relevant question regards the stability of the atherosclerotic plaque located proximally to the MB. A recent autopsy series of 150 patients with LAD‐related infarction (with or without MB) and 100 normal hearts with MB suggested that plaques in the LAD proximal to the MB are prone to rupture, resulting in myocardial infarction at a younger age.27 Although the present study demonstrated noncalcified plaques in the majority of the enrolled patients, detailed assessment of plaque vulnerability using advanced tissue characterization techniques, including radiofrequency IVUS analysis,28, 29 or hybrid IVUS with near‐infrared diffuse reflectance spectroscopy30 may be informative. Optical coherence tomography is also commercially available to provide detailed tissue‐type information, such as thin‐cap fibroatheroma31 or plaque erosion.32 Nevertheless, its limited signal penetration and the need for contrast flush with ultrafast pullback preclude the direct visualization of an MB beyond the artery and, more important, the functional assessment of arterial compression under baseline conditions. Further studies with long‐term clinical follow‐up are needed to address the clinical impact of MB‐induced arterial compression on atherosclerotic plaque vulnerability.
Limitations
Several limitations should be noted in this study. First, this study was used a single time point with a relatively small sample size. In particular, the sample size was not large enough to deny the possible correlations between anatomic MB properties and proximal atherosclerotic plaque burden. Second, the rate of MB appears higher in the current study compared with past angiographic reports (prevalence 0.4–16%) in the literature.20 This might be attributed to the higher image resolution of IVUS and its ability to directly visualize MB muscle or may suggest that MB itself is a substantial contributor to anginal symptoms without obstructive coronary artery disease. Of note, this was a retrospective analysis of a selected group of patients with angina in the absence of obstructive coronary artery disease who were recruited in a nonconsecutive fashion; therefore, the study was not designed to determine the exact prevalence of MB within this population or within the general population at large. Third, all IVUS measurements were obtained in the resting state. There could be unaccounted‐for variability in the measurements based on blood pressure or heart rate, and the measurements tell us little about the influence of these anatomic and functional properties on proximal plaque during states of exertion, such as exercise. Moreover, the current study design did not allow us to evaluate possible drug effects on arterial compression because all cardiac medications were routinely withdrawn ≥48 hours before the cardiac catheterization. Although none of the baseline medications, including the use of β‐blockers, was related to the plaque burden in the current study, this issue is notable and needs to be addressed in subsequent investigations with dedicated study designs. Fourth, plaque types were assessed by conventional gray‐scale IVUS with no advanced tissue characterization techniques. Fifth, although the majority of the current study participants had a sufficient MB length for IVUS evaluation, measuring arterial compression by IVUS may be difficult in patients with a short MB. In addition, the percentage of arterial compression may be underestimated by the presence of the IVUS catheter in small vessels or segments with extreme compression. Lastly, measurements were not compensated for transducer movement during the cardiac cycle. This issue is common and nearly inevitable for all catheter‐based coronary diagnostics and can compromise the precision of measurements, although it is unlikely that the catheter motion itself could artificially create the correlation observed in the current study.
Conclusion
In patients with a LAD MB, the percentage of arterial compression correlates with the burden of atherosclerotic plaques located proximal to the MB. This is particularly true in patients who are otherwise at low risk for developing coronary atherosclerosis. In addition to the conventional anatomic assessment, a functional assessment may provide further information with regard to the clinical import of an MB in certain patient subsets.
Disclosures
None.
Acknowledgments
The authors thank Heidi N. Bonneau, RN, MS, CCA for her expert review of the manuscript.
(J Am Heart Assoc. 2016;5:e001735 doi: 10.1161/JAHA.114.001735)
References
- 1. Geiringer E. The mural coronary. Am Heart J. 1951;41:359–368. [DOI] [PubMed] [Google Scholar]
- 2. Noble J, Bourassa MG, Petitclerc R, Dyrda I. Myocardial bridging and milking effect of the left anterior descending coronary artery: normal variant or obstruction? Am J Cardiol. 1976;37:993–999. [DOI] [PubMed] [Google Scholar]
- 3. Ishimori T, Raizner AE, Chahine RA, Awdeh M, Luchi RJ. Myocardial bridges in man: clinical correlations and angiographic accentuation with nitroglycerin. Cathet Cardiovasc Diagn. 1977;3:59–65. [DOI] [PubMed] [Google Scholar]
- 4. Lin S, Tremmel JA, Yamada R, Rogers IS, Yong CM, Turcott R, McConnell MV, Dash R, Schnittger I. A novel stress echocardiography pattern for myocardial bridge with invasive structural and hemodynamic correlation. J Am Heart Assoc. 2013;2:e000097 doi: 10.1161/JAHA.113.000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tsujita K, Maehara A, Mintz GS, Doi H, Kubo T, Castellanos C, Liu J, Yang J, Oviedo C, Franklin‐Bond T, Dasgupta N, Biro S, Dani L, Dangas GD, Mehran R, Kirtane AJ, Lansky AJ, Kreps EM, Collins MB, Stone GW, Moses JW, Leon MB. Comparison of angiographic and intravascular ultrasonic detection of myocardial bridging of the left anterior descending coronary artery. Am J Cardiol. 2008;102:1608–1613. [DOI] [PubMed] [Google Scholar]
- 6. Ishikawa Y, Akasaka Y, Suzuki K, Fujiwara M, Ogawa T, Yamazaki K, Niino H, Tanaka M, Ogata K, Morinaga S, Ebihara Y, Kawahara Y, Sugiura H, Takimoto T, Komatsu A, Shinagawa T, Taki K, Satoh H, Yamada K, Yanagida‐Iida M, Shimokawa R, Shimada K, Nishimura C, Ito K, Ishii T. Anatomic properties of myocardial bridge predisposing to myocardial infarction. Circulation. 2009;120:376–383. [DOI] [PubMed] [Google Scholar]
- 7. Desseigne P, Tabib A, Loire R. [Myocardial bridging on the left anterior descending coronary artery and sudden death. Apropos of 19 cases with autopsy]. Arch Mal Coeur Vaiss. 1991;84:511–516. [PubMed] [Google Scholar]
- 8. Kramer JR, Kitazume H, Proudfit WL, Sones FM Jr. Clinical significance of isolated coronary bridges: benign and frequent condition involving the left anterior descending artery. Am Heart J. 1982;103:283–288. [DOI] [PubMed] [Google Scholar]
- 9. Ge J, Erbel R, Gorge G, Haude M, Meyer J. High wall shear stress proximal to myocardial bridging and atherosclerosis: intracoronary ultrasound and pressure measurements. Br Heart J. 1995;73:462–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim JW, Seo HS, Na JO, Suh SY, Choi CU, Kim EJ, Rha SW, Park CG, Oh DJ. Myocardial bridging is related to endothelial dysfunction but not to plaque as assessed by intracoronary ultrasound. Heart. 2008;94:765–769. [DOI] [PubMed] [Google Scholar]
- 11. Iuchi A, Ishikawa Y, Akishima‐Fukasawa Y, Fukuzawa R, Akasaka Y, Ishii T. Association of variance in anatomical elements of myocardial bridge with coronary atherosclerosis. Atherosclerosis. 2013;227:153–158. [DOI] [PubMed] [Google Scholar]
- 12. Ge J, Erbel R, Rupprecht HJ, Koch L, Kearney P, Gorge G, Haude M, Meyer J. Comparison of intravascular ultrasound and angiography in the assessment of myocardial bridging. Circulation. 1994;89:1725–1732. [DOI] [PubMed] [Google Scholar]
- 13. Ge J, Jeremias A, Rupp A, Abels M, Baumgart D, Liu F, Haude M, Gorge G, von Birgelen C, Sack S, Erbel R. New signs characteristic of myocardial bridging demonstrated by intracoronary ultrasound and Doppler. Eur Heart J. 1999;20:1707–1716. [DOI] [PubMed] [Google Scholar]
- 14. Yamada R, Turcott R, Connolly AJ, Ikeno F, McConnell MV, Schnittger I, Fitzgerald P, Honda Y. Histological characteristics of myocardial bridge with an ultrasonic echolucent band: a comparison between intravascular ultrasound and histology. Circ J. 2013;78:502–504. [DOI] [PubMed] [Google Scholar]
- 15. Mintz GS, Nissen SE, Anderson WD, Bailey SR, Erbel R, Fitzgerald PJ, Pinto FJ, Rosenfield K, Siegel RJ, Tuzcu EM, Yock PG. American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS). A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2001;37:1478–1492. [DOI] [PubMed] [Google Scholar]
- 16. Ishii T, Hosoda Y, Osaka T, Imai T, Shimada H, Takami A, Yamada H. The significance of myocardial bridge upon atherosclerosis in the left anterior descending coronary artery. J Pathol. 1986;148:279–291. [DOI] [PubMed] [Google Scholar]
- 17. Bayrak F, Degertekin M, Eroglu E, Guneysu T, Sevinc D, Gemici G, Mutlu B, Aytaclar S. Evaluation of myocardial bridges with 64‐slice computed tomography coronary angiography. Acta Cardiol. 2009;64:341–346. [DOI] [PubMed] [Google Scholar]
- 18. Liu H, Yamaguchi T. Computer modeling of fluid dynamics related to a myocardial bridge in a coronary artery. Biorheology. 1999;36:373–390. [PubMed] [Google Scholar]
- 19. Herrmann J, Higano ST, Lenon RJ, Rihal CS, Lerman A. Myocardial bridging is associated with alteration in coronary vasoreactivity. Eur Heart J. 2004;25:2134–2142. [DOI] [PubMed] [Google Scholar]
- 20. Ishikawa Y, Kawawa Y, Kohda E, Shimada K, Ishii T. Significance of the anatomical properties of a myocardial bridge in coronary heart disease. Circ J. 2011;75:1559–1566. [DOI] [PubMed] [Google Scholar]
- 21. Cheng C, Tempel D, van Haperen R, van der Baan A, Grosveld F, Daemen MJ, Krams R, de Crom R. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation. 2006;113:2744–2753. [DOI] [PubMed] [Google Scholar]
- 22. Kim PJ, Hur G, Kim SY, Namgung J, Hong SW, Kim YH, Lee WR. Frequency of myocardial bridges and dynamic compression of epicardial coronary arteries: a comparison between computed tomography and invasive coronary angiography. Circulation. 2009;119:1408–1416. [DOI] [PubMed] [Google Scholar]
- 23. Ishii T, Asuwa N, Masuda S, Ishikawa Y, Kiguchi H, Shimada K. Atherosclerosis suppression in the left anterior descending coronary artery by the presence of a myocardial bridge: an ultrastructural study. Mod Pathol. 1991;4:424–431. [PubMed] [Google Scholar]
- 24. Ishii T, Asuwa N, Masuda S, Ishikawa Y. The effects of a myocardial bridge on coronary atherosclerosis and ischaemia. J Pathol. 1998;185:4–9. [DOI] [PubMed] [Google Scholar]
- 25. Inaba S, Okayama H, Higashi H, Nishimura K, Inoue K, Ogimoto A, Higaki J. Usefulness of transthoracic Doppler echocardiography for noninvasive assessment of coronary blood flow in a patient with symptomatic myocardial bridging. Eur J Echocardiogr. 2011;12:E15. [DOI] [PubMed] [Google Scholar]
- 26. Berry JF, von Mering GO, Schmalfuss C, Hill JA, Kerensky RA. Systolic compression of the left anterior descending coronary artery: a case series, review of the literature, and therapeutic options including stenting. Catheter Cardiovasc Interv. 2002;56:58–63. [DOI] [PubMed] [Google Scholar]
- 27. Ishikawa Y, Akasaka Y, Akishima‐Fukasawa Y, Iuchi A, Suzuki K, Uno M, Abe E, Yang Y, Li CP, Mukai K, Niino H, Tanaka M, Kawahara Y, Sugiura H, Shinagawa T, Morinaga S, Ogata K, Onuma J, Yanagida‐Iida M, Taki K, Komatsu A, Satoh H, Yamada K, Shimokawa R, Shibuya K, Takahashi K, Ishii T. Histopathologic profiles of coronary atherosclerosis by myocardial bridge underlying myocardial infarction. Atherosclerosis. 2013;226:118–123. [DOI] [PubMed] [Google Scholar]
- 28. Miyamoto Y, Okura H, Kume T, Kawamoto T, Neishi Y, Hayashida A, Yamada R, Imai K, Saito K, Yoshida K. Plaque characteristics of thin‐cap fibroatheroma evaluated by OCT and IVUS. JACC Cardiovasc Imaging. 2011;4:638–646. [DOI] [PubMed] [Google Scholar]
- 29. Yamada R, Okura H, Kume T, Neishi Y, Kawamoto T, Miyamoto Y, Imai K, Saito K, Hayashida A, Yoshida K. A comparison between 40 MHz intravascular ultrasound iMap imaging system and integrated backscatter intravascular ultrasound. J Cardiol. 2013;61:149–154. [DOI] [PubMed] [Google Scholar]
- 30. Gardner CM, Tan H, Hull EL, Lisauskas JB, Sum ST, Meese TM, Jiang C, Madden SP, Caplan JD, Burke AP, Virmani R, Goldstein J, Muller JE. Detection of lipid core coronary plaques in autopsy specimens with a novel catheter‐based near‐infrared spectroscopy system. JACC Cardiovasc Imaging. 2008;1:638–648. [DOI] [PubMed] [Google Scholar]
- 31. Kume T, Akasaka T, Kawamoto T, Okura H, Watanabe N, Toyota E, Neishi Y, Sukmawan R, Sadahira Y, Yoshida K. Measurement of the thickness of the fibrous cap by optical coherence tomography. Am Heart J. 2006;152:755.e1–755.e4. [DOI] [PubMed] [Google Scholar]
- 32. Kubo T, Imanishi T, Takarada S, Kuroi A, Ueno S, Yamano T, Tanimoto T, Matsuo Y, Masho T, Kitabata H, Tsuda K, Tomobuchi Y, Akasaka T. Assessment of culprit lesion morphology in acute myocardial infarction: ability of optical coherence tomography compared with intravascular ultrasound and coronary angioscopy. J Am Coll Cardiol. 2007;50:933–939. [DOI] [PubMed] [Google Scholar]


