Abstract
Introduction
Cystic renal cell carcinoma (cystic RCC) is thought to carry an improved prognosis relative to clear cell RCC (CCRCC); however, this is based on small case series. We used a population-based tumor registry to compare clinicopathologic features and cancer-specific mortality (CSM) of cystic RCC with those of CCRCC.
Materials and methods
The Surveillance, Epidemiology, and End Results database was queried for all patients diagnosed and treated for cystic RCC and CCRCC between 2001 and 2010. Clinical and pathologic factors were compared using t tests and chi-square tests as appropriate. Kaplan-Meier survival analysis compared CSM differences between cystic RCC and CCRCC.
Results
A total of 678 patients with cystic RCC and 46,677 with CCRCC were identified. The mean follow-up duration was 52 and 40 months, respectively. When compared with CCRCC patients, those with cystic RCC were younger (mean age 58 vs. 61 y, P < 0.001), more commonly black (22% vs. 9%, P < 0.001), and female (45% vs. 41%, P = 0.02). Cystic RCCs were more commonly T1a tumors (66% vs. 55%, P < 0.001), well differentiated (33% vs. 16%, P < 0.001), and smaller (mean size 3.8 vs. 4.5 cm, P < 0.001). Cystic RCC was associated with a reduction in CSM when compared with CCRCC (P = 0.002). In a subset analysis, this reduction in CSM was seen only for those with T1b/T2 tumors (P = 0.01) but not for those with T1a RCCs lesions (P = 0.31).
Conclusions
We report the largest series of cystic RCC and corroborate the findings of improved CSM when compared with CCRCC for larger tumors; however, no difference was noted in smaller tumors, suggesting that tumor biology becomes more relevant to prognosis with increasing size. These data may suggest a role for active surveillance in appropriately selected patients with small, cystic renal masses.
Keywords: Renal cell carcinoma, Cystic RCC, Clear cell RCC, Survival
1. Introduction
It is estimated that up 15% of renal cell carcinomas (RCCs) may have a cystic component [1] that can be benign or malignant in nature (e.g., simple cyst vs. cystic degeneration with necrosis). Postulated mechanisms for cyst formation include intrinsic multilocular growth, intrinsic unilocular growth, cystic necrosis, or growth from the wall of a preexisting simple cyst [1].
A distinct subset of RCC, described as “cystic RCC,” accounts for <5% of all renal cell carcinomas [2]. The World Health Organization defines this as a “tumor composed of numerous cysts, the septa of which contain small groups of clear cells indistinguishable from grade I clear cell carcinoma” [3]. On gross examination, these tumors are typically well circumscribed, composed entirely of cysts, and separated from adjacent parenchyma by a fibrous wall [2–5]. Microscopically, the cysts are generally lined by epithelial cells, single-celled or multilayered, with some forming papillary structures [3,5]. In the existing literature, the cystic component required for a cystic RCC diagnosis has varied from 75% to 90% among studies by different authors [4,6–11].
Overall, RCC remains a surgical disease with variant histology that is predictive of survival [12]. Cystic RCC is considered to have a favorable prognosis when compared with that of clear cell RCC (CCRCC); however, this perception is based on small retrospective series [4,6–11,13,14], which may not represent the true natural history. To further clarify the prognosis of cystic RCC, we accessed the population-based Surveillance, Epidemiology, and End Results (SEER) database to delineate the clinicopathologic features of cystic RCC as well as compare cancer-specific mortality (CSM) between patients with cystic and CCRCC.
2. Materials and methods
2.1. Data source
Cases were identified from the SEER database, which comprises 17 cancer registries and accounts for approximately 26% of the U.S. population. We queried cancer incidence and survival data and restricted the analysis to the years 2001 to 2010, as reporting of cystic RCC was minimal before 2001.
2.2. Study population
Cases were identified using International Classification of Diseases for Oncology (third edition, ICD-O-3) site codes for the kidney (C649). Cystic RCC cases were identified by the ICD-O-3 histology code (8316). The comparison CCRCC cohort was identified using ICD-O-3 histology codes 8310 (Clear Cell Adenocarcinoma NOS) and 8312 (Clear Cell Adenocarcinoma, and Renal Cell Carcinoma). We limited the analysis to those with organ-confined RCC (pT1a/b, pT2, N0/x, and M0/x) to limit misclassification bias of larger tumors (e.g., CCRCC with cystic degeneration). We also limited the analysis to those undergoing surgical excision with radical or partial nephrectomy to ensure pathologic confirmation of histology rather than rely on radiographic and biopsy diagnoses.
2.3. Data collection and coding
Demographic data included patient age, sex, and race. Age was categorized in 10-year increments ranging from younger than 50 to greater than 80 years old. Race was categorized as white, black, or other. Surgery (partial or complete nephrectomy) and year of treatment were also recorded. Pathologic data include tumor size, pathologic T category classification, and tumor grade (well differentiated, moderate, poorly/undifferentiated, or missing). Fuhrman grade, chemotherapy, immunotherapy, and comorbidity data are not available in the SEER database. CSM stratified by pT1a vs. pT1b/T2 tumors was calculated from date of diagnosis to date of death due to kidney cancer. Patients were censored at the date of last follow-up if alive or a non–kidney cancer–related death had occurred. Pathologic staging was based on the TNM classification from the American Joint Committee on Cancer (Chicago, IL), seventh edition.
2.4. Statistical analysis
Demographic and pathologic data comparing cystic RCC with CCRCC are presented using t tests for continuous variables and chi-square tests for categorical variables as appropriate. Unadjusted survival experience between cystic RCC and CCRCC was compared with Kaplan-Meier curves using log-rank tests. As there was a low overall event rate, multivariate analysis was not performed. All statistical analyses were conducted using Stata, version 13 (Stata, Inc., College Station, TX).
3. Results
Demographic and pathologic data are shown in Table 1. A total of 678 patients were identified as having cystic RCC and 46,677 patients had CCRCC. The mean follow-up duration was 52 and 40 months, respectively. There were 1,760 deaths (3.8%) due to CCRCC and 12 deaths (1.8%) due to cystic RCC. Patients with cystic RCC were younger and were more commonly black and female. Patients with cystic RCC were also more likely to present with lower stage disease, well-differentiated tumors, and to receive nephron-sparing surgery. There were no differences in tumor laterality between the 2 groups.
Table 1.
Demographic and pathologic data for pT1/T2 cases of clear cell and cystic renal cell carcinoma
| Clear cell RCC, N = 46,677 (%) |
Cystic RCC, N = 678 (%) |
P value | |
|---|---|---|---|
| Age, y | |||
| <50 | 9,413 (20) | 208 (31) | <0.001 |
| 51–59 | 12,025 (26) | 157 (23) | |
| 60–69 | 12,798 (27) | 161 (24) | |
| 70–79 | 9,547 (20) | 119 (18) | |
| >80 | 2,894 (6) | 33 (5) | |
| Age, mean (SD), y | 61 (14) | 58 (13) | <0.001 |
| Race | |||
| White | 39,535 (85) | 495 (74) | <0.001 |
| Black | 4,245 (9) | 150 (22) | |
| Other | 2,547 (6) | 26 (4) | |
| Sex | |||
| Male | 27,679 (59) | 372 (55) | 0.02 |
| Female | 18,998 (41) | 306 (45) | |
| Pathologic category | |||
| pT1a | 25,644 (55) | 446 (66) | <0.001 |
| pT1b | 14,232 (31) | 134 (20) | |
| pT2 | 6,801 (15) | 98 (15) | |
| Tumor size, mean (SD), cm | 4.5 (2.7) | 3.8 (2.9) | <0.001 |
| Tumor grade | |||
| Well differentiated | 7,257 (16) | 222 (33) | <0.001 |
| Moderately differentiated | 22,762 (49) | 219 (32) | |
| Poorly differentiated | 9,342 (20) | 53 (8) | |
| Missing | 7,316 (16) | 184 (27) | |
| Surgical intervention | |||
| Partial nephrectomy | 13,276 (28) | 272 (40) | <0.001 |
| Radical nephrectomy | 33,401 (72) | 406 (60) |
All numbers within parentheses are percentages unless otherwise noted. Percentages may not add up to 100% because of missing data. The P values calculated from 2-sided t tests for continuous variables and chi-square tests for categorical variables as appropriate.
Kaplan-Meier survival curves for cystic RCC vs. CCRCC are shown in Fig. 1. Using the log-rank test, survival was significantly better for patients with cystic RCC when compared with CCRCC patients (P = 0.002). In Fig. 2, Kaplan-Meier survival curves stratified by stage category (T1a vs. T1b/T2) are shown. We found a nonsignificant reduction in CSM in patients with T1a tumors (P = 0.31); however, in patients with T1b/T2 tumors, cystic RCC histology predicted a significant reduction in CSM (P = 0.01).
Fig. 1.
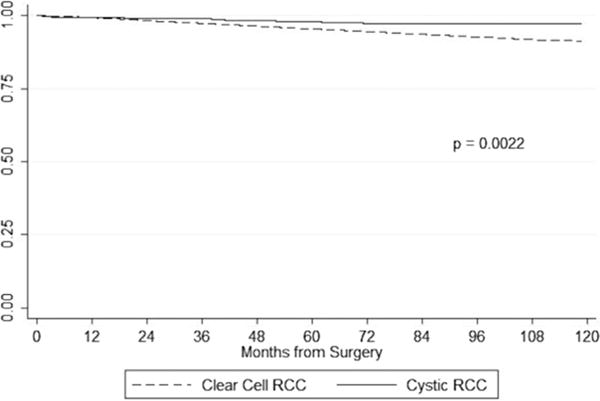
Kaplan-Meier cancer-specific survival curves for cystic and CCRCC. P value calculated from the log-rank test.
Fig. 2.
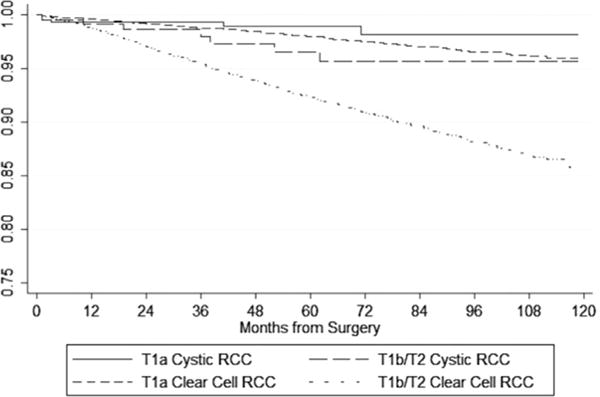
Kaplan-Meier cancer-specific survival curves for cystic and CCRCC by stage.
4. Discussion
In this study, we report the largest series of patients with cystic RCC to date and describe the clinical features and survival experience compared with CCRCC. We confirm the findings from small case series of improved survival in cystic RCC, although this appears to be driven by the survival benefit in larger (pT1b or T2) tumors specifically.
Cystic RCC is an uncommon variant of RCC accounting for <5% of all RCCs [2]. The knowledge of its presenting characteristics and outcomes is derived from small case series, all with fewer than 100 patients and most with fewer than 30 cases (Table 2). In this population-based series, we report on almost 700 cases of cystic RCC, finding that patients were more commonly younger, women, and black when compared with those with CCRCC.
Table 2.
Representative reference cohorts evaluating cystic renal cell carcinoma
| Bielsa et al. [13] | Koga et al. [8] | Onishi et al. [7] | Nassir et al. [9] | Han et al. [6] | Webster et al. [10] | Donin et al. [14] | Current cohort | |
|---|---|---|---|---|---|---|---|---|
| Year published | 1998 | 2000 | 2001 | 2002 | 2003 | 2007 | 2015 | 2015 |
| # Patients | 25 | 21 | 27 | 12 | 18 | 85 | 61 | 678 |
| Age, mean, y | – | 54 | 52 | 59 | 58 | – | 64 | 58 |
| Male (%) | 96 | 76 | 96 | 33 | 61 | 64 | 64 | 55 |
| Pathologic T1 (%) | 88 | 71 | 89 | 100 | 83 | 89 | 88 | 86 |
| 5-y DSS (%) | 83 | 100a | 88.6 | – | 82b | 100 | 100 | 98 |
The bold values were used to separate the current cohort from the historical literature.
All numbers are displayed as percentages unless otherwise noted. –, Data not described. DSS = disease-specific survival.
Survival analysis includes patients with cystic necrosis (DSS = 80%), a known poor prognostic indicator, and patients without cystic necrosis (DSS = 100%).
Authors calculated a 4-year DSS.
These demographic differences may relate to underlying tumor biology. In a review of patients with renal masses <7 cm, Snyder et al. [15] found female sex to be the only significant variable associated with benign histology. Furthermore, in a study of younger patients (younger than 45 y, mean age = 37 y), Eggener et al. [16] found that women were significantly more likely to have non-CCRCC histology or a benign tumor. This is consistent with our data in that female sex was more common in patients with cystic RCC. There are minimal available data on the relationship between race and cystic RCC in existing literature. However, it is well described that black race is associated with younger age at presentation and higher incidence and mortality in CCRCC when compared with other races [17]. Despite the higher proportion of patients with cystic RCC who were black at presentation, we still found a decrease in CSM when compared with that of patients with CCRCC.
Our finding of excellent cancer-specific survival among patients with cystic RCC corroborates other reports (Table 2). Interestingly, for the smaller T1a tumors, CSM was not statistically different between the 2 histologic subgroups (P = 0.31). However, with larger tumors (pT1b/T2), patients with cystic RCC experienced improved survival (P = 0.01). This suggests that patients treated surgically in the early stages of both cystic RCC and CCRCC have excellent survival, but as tumor size increases, tumor biology may become more important to overall prognosis. Although increasing tumor size is an established risk factor for metastases [18] and CSM in CCRCC [18–21], metastases in cystic RCC are exceedingly rare [6–11,13,14,22,23], consistent with a more benign natural history. Given the established importance of nephron-sparing surgery to decrease the risk of chronic kidney disease [24,25], improved insight into tumor biology may therefore support nephron-sparing surgery in well-selected patients with larger cystic masses. Furthermore, the excellent survival observed with cystic RCC may represent a clinical scenario where active surveillance of cystic masses is appropriate, with several authors describing preoperative imaging features suggestive of cystic RCC with correspondingly benign tumor histology and behavior [1,11,22]. Considering the growing literature on active surveillance for small renal masses [26,27], these data can inform selection criteria for vigilant observation, particularly in those with significant comorbidity.
Limitations to this analysis include those inherent to the SEER database, including lack of comorbidity, chemotherapy, or immunotherapy data and the retrospective nature of the study. Importantly, the rate of cystic RCC diagnoses was similar throughout the years studied, indicating internal consistency within the dataset. There is variability in the existing literature regarding cyst predominance required for diagnosis (75% [4,6–9], 80% [14], or 90% [28]), and as there is no central pathologic review in the SEER database, this may lead to disease misclassification. Furthermore, cystic predominance is key to this benign histology and excellent survival, and although this is most often defined by authors as > 75%, this cut off can be debated and “any” cystic component may not provide the same prognostic benefit. Therefore, caution when interpreting preoperative imaging and subsequent counseling of patients is required. We attempted to correct for this possibility by focusing on smaller tumors (to exclude misdiagnosis of cystic degeneration in larger CCRCC tumors) and limited the analysis to those undergoing surgical excision to ensure pathologic confirmation of histology rather than rely on radiographic and biopsy diagnoses. Finally, give the described low event rate in this population, we were unable to perform meaningful multivariate analyses which may have provided further prognostic insight.
5. Conclusion
Cystic RCC commonly presents at a lower stage and grade with improved cancer-specific survival when compared with solid CCRCC. Demographic factors including younger age, female sex, and black race are also associated with cystic RCC. We found improved cancer-specific survival when compared with CCRCC for larger tumors; however, no difference was noted in smaller tumors, suggesting that tumor biology becomes more relevant to prognosis with increasing size. These data may suggest a role for active surveillance in appropriately selected patients with cystic renal masses.
References
- 1.Hartman DS, Davis CJ, Jr, Johns T, Goldman SM. Cystic renal cell carcinoma. Urology. 1986;28:145–53. doi: 10.1016/0090-4295(86)90109-3. [DOI] [PubMed] [Google Scholar]
- 2.Eble JN, Bonsib SM. Extensively cystic renal neoplasms: cystic nephroma, cystic partially differentiated nephroblastoma, multilocular cystic renal cell carcinoma, and cystic hamartoma of renal pelvis. Semin Diagn Pathol. 1998;15:2–20. [PubMed] [Google Scholar]
- 3.Eble JN, Sauter G, Epstein JI, Sesterhenn IA. Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs. World Health Organization; Lyon, France: 2004. [Google Scholar]
- 4.Corica FA, Iczkowski KA, Cheng L, et al. Cystic renal cell carcinoma is cured by resection: a study of 24 cases with long-term followup. J Urol. 1999;161:408–11. doi: 10.1016/s0022-5347(01)61903-7. [DOI] [PubMed] [Google Scholar]
- 5.Halat SK, MacLennan GT. Multilocular cystic renal cell carcinoma. J Urol. 2007;177:343. doi: 10.1016/j.juro.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 6.Han KR, Janzen NK, McWhorter VC, et al. Cystic renal cell carcinoma: biology and clinical behavior. Urol Oncol. 2004;22:410–4. doi: 10.1016/S1078-1439(03)00173-X. [DOI] [PubMed] [Google Scholar]
- 7.Onishi T, Oishi Y, Goto H, Tomita M, Abe K, Sugaya S. Cyst-associated renal cell carcinoma: clinicopathologic characteristics and evaluation of prognosis in 27 cases. Int J Urol. 2001;8:268–74. doi: 10.1046/j.1442-2042.2001.00298.x. [DOI] [PubMed] [Google Scholar]
- 8.Koga S, Nishikido M, Hayashi T, Matsuya F, Saito Y, Kanetake H. Outcome of surgery in cystic renal cell carcinoma. Urology. 2000;56:67–70. doi: 10.1016/s0090-4295(00)00540-9. [DOI] [PubMed] [Google Scholar]
- 9.Nassir A, Jollimore J, Gupta R, Bell D, Norman R. Multilocular cystic renal cell carcinoma: a series of 12 cases and review of the literature. Urology. 2002;60:421–7. doi: 10.1016/s0090-4295(02)01742-9. [DOI] [PubMed] [Google Scholar]
- 10.Webster WS, Thompson RH, Cheville JC, Lohse CM, Blute ML, Leibovich BC. Surgical resection provides excellent outcomes for patients with cystic clear cell renal cell carcinoma. Urology. 2007;70:900–4. doi: 10.1016/j.urology.2007.05.029. [discussion 904] [DOI] [PubMed] [Google Scholar]
- 11.Jhaveri K, Gupta P, Elmi A, et al. Cystic renal cell carcinomas: do they grow, metastasize, or recur? Am J Roentgenol. 2013;201:W292–W296. doi: 10.2214/AJR.12.9414. [DOI] [PubMed] [Google Scholar]
- 12.Lee JH, Choi JW, Kim YS. The value of histologic subtyping on outcomes of clear cell and papillary renal cell carcinomas: a meta-analysis. Urology. 2010;76:889–94. doi: 10.1016/j.urology.2010.01.039. [DOI] [PubMed] [Google Scholar]
- 13.Bielsa O, Lloreta J, Gelabert-Mas A. Cystic renal cell carcinoma: pathological features, survival and implications for treatment. Br J Urol. 1998;82:16–20. doi: 10.1046/j.1464-410x.1998.00689.x. [DOI] [PubMed] [Google Scholar]
- 14.Donin NM, Mohan S, Pham H, et al. Clinicopathologic outcomes of cystic renal cell carcinoma. Clin Genitourin Cancer. 2015;13:67–70. doi: 10.1016/j.clgc.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 15.Snyder ME, Bach A, Kattan MW, Raj GV, Reuter VE, Russo P. Incidence of benign lesions for clinically localized renal masses smaller than 7 cm in radiological diameter: influence of sex. J Urol. 2006;176:2391–5. doi: 10.1016/j.juro.2006.08.013. [discussion 2395–6] [DOI] [PubMed] [Google Scholar]
- 16.Eggener SE, Rubenstein JN, Smith ND, et al. Renal tumors in young adults. J Urol. 2004;171:106–10. doi: 10.1097/01.ju.0000099028.95679.52. [DOI] [PubMed] [Google Scholar]
- 17.Stafford HS, Saltzstein SL, Shimasaki S, Sanders C, Downs TM, Sadler GR. Racial/ethnic and gender disparities in renal cell carcinoma incidence and survival. J Urol. 2008;179:1704–8. doi: 10.1016/j.juro.2008.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ingimarsson JP, Sigurdsson MI, Hardarson S, et al. The impact of tumour size on the probability of synchronous metastasis and survival in renal cell carcinoma patients: a population-based study. BMC Urol. 2014;14:72. doi: 10.1186/1471-2490-14-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheville JC, Blute ML, Zincke H, Lohse CM, Weaver AL. Stage pT1 conventional (clear cell) renal cell carcinmoa: pathological features associated with cancer specific survival. J Urol. 2001;166(2):453–6. doi: 10.1016/s0022-5347(05)65962-9. [DOI] [PubMed] [Google Scholar]
- 20.Klatte T, Patard JJ, Goel RH, et al. Prognostic impact of tumor size on pT2 renal cell carcinoma: an international multicenter experience. J Urol. 2007;178:35–40. doi: 10.1016/j.juro.2007.03.046. [discussion 40] [DOI] [PubMed] [Google Scholar]
- 21.Lam JS, Klatte T, Patard JJ, et al. Prognostic relevance of tumour size in T3a renal cell carcinoma: a multicentre experience. Eur Urol. 2007;52:155–62. doi: 10.1016/j.eururo.2007.01.106. [DOI] [PubMed] [Google Scholar]
- 22.Hindman NM, Bosniak MA, Rosenkrantz AB, Lee-Felker S, Melamed J. Multilocular cystic renal cell carcinoma: comparison of imaging and pathologic findings. Am J Roentgenol. 2012;198:W20–6. doi: 10.2214/AJR.11.6762. [DOI] [PubMed] [Google Scholar]
- 23.Huber J, Winkler A, Jakobi H, et al. Preoperative decision making for renal cell carcinoma: cystic morphology in cross-sectional imaging might predict lower malignant potential. Urol Oncol. 2014;32:37, e1–6. doi: 10.1016/j.urolonc.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 24.Huang WC, Levey AS, Serio AM, et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol. 2006;7:735–40. doi: 10.1016/S1470-2045(06)70803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barlow LJ, Korets R, Laudano M, Benson M, McKiernan J. Predicting renal functional outcomes after surgery for renal cortical tumours: a multifactorial analysis. BJU Int. 2010;106:489–92. doi: 10.1111/j.1464-410X.2009.09147.x. [DOI] [PubMed] [Google Scholar]
- 26.Pierorazio PM, Johnson MH, Ball MW, et al. Five-year analysis of a multi-institutional prospective clinical trial of delayed Intervention and Surveillance for Small Renal Masses: the DISSRM registry. Eur Urol. 2015 doi: 10.1016/j.eururo.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Smaldone MC, Corcoran AT, Uzzo RG. Active surveillance of small renal masses. Nat Rev Urol. 2013;10:266–74. doi: 10.1038/nrurol.2013.62. [DOI] [PubMed] [Google Scholar]
- 28.Murad T, Komaiko W, Oyasu R, Bauer K. Multilocular cystic renal cell carcinoma. Am J Clin Pathol. 1991;95:633–7. doi: 10.1093/ajcp/95.5.633. [DOI] [PubMed] [Google Scholar]


