Abstract
Background
Transradial percutaneous coronary intervention (PCI) has been increasingly adopted in clinical practice, given its potential advantages over transfemoral intervention; however, the impact of different access strategies on costs and clinical outcomes remains poorly defined, especially in the developing world.
Methods and Results
Using data from a consecutive cohort of 5306 patients undergoing PCI in China in 2010, we compared total hospital costs and in‐hospital outcomes for transradial intervention (TRI) and transfemoral intervention. Patients receiving TRI (n=4696, 88.5%) were slightly younger (mean age 57.4 versus 59.5 years), less often women (21.6% versus 33.1%), more likely to undergo PCI for single‐vessel disease, and less likely to undergo PCI for triple‐vessel or left main diseases. The unadjusted total hospital costs were 57 900 Chinese yuan (¥57 900; equivalent to 9190 US dollars [$9190]) for TRI and ¥67 418 ($10,701) for transfemoral intervention. After adjusting for all observed patient and procedural characteristics using the propensity score inverse probability weighting method, TRI was associated with a lower total cost (adjusted difference ¥8081 [$1283]). More than 80% of the cost difference was related to lower PCI‐related costs (adjusted difference −¥5162 [−$819]), which were likely driven by exclusive use of vascular closure devices in transfemoral intervention, and lower hospitalization costs (−¥1399 [−$222]). Patients receiving TRI had shorter length of stay and were less likely to experience major adverse cardiac events or post‐PCI bleeding. These differences were consistent among clinically relevant subgroups with acute myocardial infarction, acute coronary syndrome, and stable angina.
Conclusions
Among patients undergoing PCI, TRI was associated with lower cost and favorable clinical outcomes compared with transfemoral intervention.
Keywords: coronary artery disease, cost, health services research, interventional cardiology, outcomes research, percutaneous coronary intervention
Subject Categories: Complications, Quality and Outcomes, Health Services, Percutaneous Coronary Intervention
Introduction
Percutaneous coronary intervention (PCI) plays a pivotal role in the treatment of coronary artery disease. Randomized clinical trials and observational studies demonstrated fewer periprocedural complications, shorter length of stay, and better patient satisfaction associated with transradial intervention (TRI) relative to transfemoral intervention (TFI).1, 2, 3, 4, 5, 6, 7, 8, 9 Based on this evidence, the current US and European guidelines recommend TRI in patients at high risk of bleeding to decrease access site complications.10, 11 Despite these recommendations, relatively few economic evaluations have been done for TRI and TFI. Although some studies have suggested cost savings associated with TRI, these studies are relatively small and were conducted mainly in leading centers in the United States.12, 13, 14 Given the differences in health care delivery systems and practice patterns, it remains unclear whether these cost advantages are generalizable to other countries, especially those in the developing world.
Although used less often in contemporary US practice, TRI is more commonly used in Europe and Asian countries.6, 7, 8 Despite widespread adoption, the impact of different access strategies on treatment costs and outcomes remain poorly defined. Using data from the Fuwai PCI database, a single‐center registry from the largest heart center in the People's Republic of China, we compared total hospital costs and in‐hospital outcomes for TRI and TFI.
Methods
Study Population
Our study population consisted of a consecutive cohort of 6068 patients undergoing PCI between January 1 and December 31, 2010, at Fuwai Hospital in Beijing, China. We excluded patients who underwent >1 PCI procedure during the index hospitalization (n=294), who presented with cardiogenic shock (n=21) or chronic total occlusion (n=136), who participated in clinical trials (n=118), who had missing information on access site (n=1) or cost data (n=138), or who received a preprocedural intra‐aortic balloon pump (n=54). After these exclusions, our study population consisted of 5306 patients, of whom 4696 had TRIs and 610 had TFIs.
Hospital Costs and Clinical Outcomes
The primary outcome was total hospital costs, defined as the total cost of an in‐hospital stay from the day of admission through discharge. The cost data were obtained from the hospital accounting system and divided into the following categories: PCI‐related costs; medication costs; examination/laboratory costs; and hospitalization costs inclusive of bed, physician fee, and nursing services. Patients with total hospital costs >99th percentile were winsorized to minimize the influence of outliers (n=53).
Clinical outcomes included post‐PCI bleeding events, major adverse cardiac events (MACE), and length of stay. Post‐PCI bleeding events were evaluated according to the Bleeding Academic Research Consortium (BARC) criteira.15 Briefly, BARC bleeding is classified into the following hierarchical categories characterizing the severity of the bleeding event: type 0, indicating no bleeding; type 1, indicating bleeding that is not actionable; type 2, indicating overt actionable bleeding that does not fit the criteria for type 3, 4, or 5 but that requires nonsurgical medical intervention by a health care professional, leading to hospitalization or an increased level of care or prompting evaluation; type 3, indicating clinical, laboratory, and/or imaging evidence of bleeding, with specific health care provider responses; type 4, indicating coronary artery bypass grafting–related bleeding; and type 5, indicating fatal bleeding. For the purpose of this study, we reported BARC ≥2 and ≥3 bleeding events. MACE was a composite of death, myocardial infarction, revascularization, or BARC ≥3 bleeding during the index hospitalization. Post‐PCI bleeding (BARC ≥2) and MACE were validated by medical record review.
Statistical Analysis
Means, standard deviations, frequencies, and percentages were used to describe the distribution of continuous and categorical variables between TRI and TFI. Baseline characteristics were compared using standardized differences, calculated as the difference in means or proportions divided by a pooled estimate of the standard deviation. Compared with traditional significance testing such as t tests and chi‐square tests, standardized differences are not influenced by sample size and are commonly used as balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity score analysis.16 An absolute standardized difference >10 indicates significant imbalance of a baseline covariate, whereas a smaller value supports the balance assumption between treatment groups.
Unlike randomized clinical trials, the decision to treat in real‐world practice is often based on prognostic factors rather than randomization; therefore, the estimation of cost and clinical outcomes for TRI or TFI might be confounded as a result of treatment selection. We used an inverse probability weighting (IPW) approach to control for potential bias introduced by treatment selection. The IPW is an extension of the propensity score method to summarize the conditional probability of assignment for a treatment.17, 18, 19 Using the IPW method, the weights are the inverse probability of access location (TRI or TFI) derived from a treatment selection logistic regression model with TRI as the dependent variable and the all observed confounders as the independent variables. Variables included age; sex; prior history of PCI; coronary artery bypass grafting; myocardial infarction; stroke; diabetic mellitus; hypertension; hyperlipidemia; vital signs; creatinine; clinical diagnosis (ST‐segment elevation myocardial infarction [STEMI], non‐STEMI, unstable or stable angina); emergent PCI; left ventricular ejection fraction; 1‐, 2‐, or 3‐vessel disease; left main disease; ostium lesion; bifurcation lesion; number of stents used; lesion type (de novo, intrastent, restenosis); stent type (drug‐eluting stent, bare metal stent); arterial sheath size; and domestic versus imported stents (due to cost difference). Use of intravascular ultrasound and a vascular closure device was not included in the propensity score model because intravascular ultrasound occurred after the decision to approach the patient with a radial or femoral strategy and the vascular closure device was used exclusively for TFI.
After the weights were obtained, estimation of TRI versus TFI effect was derived by solving the IPW estimating equations. The adjusted relationships between TRI versus TFI and each outcome were then estimated using IPW regression models (generalized linear model with log link for cost and length of stay and logistic regression model for clinical outcomes) weighted with access location as the independent variable. This method has the theoretical advantage of allowing adjustment for more confounders and produces less biased estimates to assess comparative effectiveness using observational data.18 Both hospital costs and clinical outcomes were analyzed according to intention to treat. The generalized estimating equations method was used to account for clustering by physician. Significance tests and confidence intervals for each estimate were based on robust standard errors. Pre‐ and post‐IPW balances of the covariates between TRI and TFI were assessed using standardized difference. Sensitivity analyses were performed in clinically relevant subgroups (acute myocardial infarction [STEMI and non‐STEMI], acute coronary syndrome [STEMI, non‐STEMI, and unstable angina], and stable angina).
All P values were 2‐sided with <0.05 considered statistically significant. All statistical analyses were performed using SAS 9.3 (SAS Institute). The institutional review board of Fuwai Hospital, Peking Union Medical College, approved the study and waived the requirement for informed consent.
Results
Among 5306 patients who were eligible for the analysis, TRI was performed in 4696 (88.3%). Only 3 patients converted from radial to femoral access. Tables 1 and 2 compare baseline demographic, clinical, and procedural characteristics for TRI and TFI. Patients receiving TRI were slightly younger (mean age 57.4 versus 59.5 years); were less often women (21.6% versus 33.1%); had lower prevalence of prior myocardial infarction, PCI, and coronary artery bypass grafting; and were more likely to present with STEMI (all standardized differences >10). There were no differences in clinical diagnosis in terms of non‐STEMI, unstable angina, or stable angina between the 2 groups. A 6‐French sheath was used for the majority of patients in both groups. TRI patients were more likely to undergo PCI for single‐vessel lesions but less likely to undergo PCI for triple‐vessel lesions, left main disease, or ostial lesions. Less intravascular ultrasound was used in TRI; however, there were no differences in emergent PCI; number of treated lesions; and use of drug‐eluting versus bare metal stents, domestic versus imported stents, or total stents used between TRI and TFI cohorts. Vascular closure devices (3000 Chinese yuan [¥3000], equivalent to 476 US dollars [$476]) were used in two‐thirds of TFI patients. In contrast, all TRIs used manual compression or a radial compression device that cost <¥300 [<$48]. The distribution of the propensity scores of the 2 groups are shown in the Figure 1. After IPW adjustment, TRI and TFI cohorts were well balanced for all observed patient, clinical, and procedural characteristics, with an absolute standardized difference <10 (Figure 2).
Table 1.
Baseline Characteristics of the Study Population
| Characteristics | Transradial Intervention, n=4696 (%) | Transfemoral Intervention, n=610 (%) | Standardized Difference |
|---|---|---|---|
| Age, mean±SD, y | 57.4±10.0 | 59.5±10.9 | 20.3 |
| Female | 1014 (21.6) | 202 (33.1) | 26.1 |
| Medical history | |||
| Prior myocardial infarction | 815 (17.4) | 142 (23.3) | 14.8 |
| Prior coronary artery bypass grafting | 22 (0.5) | 52 (8.5) | 39.6 |
| Prior percutaneous coronary intervention | 720 (15.3) | 160 (26.2) | 27.1 |
| Stroke | 278 (5.9) | 50 (8.2) | 8.9 |
| Diabetic mellitus | 1211 (25.8) | 173 (28.4) | 5.8 |
| Hypertension | 2952 (62.9) | 393 (64.4) | 3.3 |
| Hyperlipidemia | 2913 (62.0) | 389 (63.8) | 3.6 |
| Diagnosis | |||
| ST‐segment elevation myocardial infarction | 787 (16.8) | 75 (12.3) | 12.7 |
| Non–ST‐segment elevation myocardial infarction | 205 (4.4) | 27 (4.4) | 0.3 |
| Unstable angina | 2268 (48.3) | 307 (50.3) | 4.1 |
| Stable angina | 1264 (26.9) | 173 (28.4) | 3.2 |
| Vital signs | |||
| Heart rate, mean±SD, bpm | 69 (10) | 68 (10) | 9.0 |
| Systolic blood pressure, mean±SD, mm Hg | 128 (17) | 127 (18) | 3.0 |
| Diastolic blood pressure, mean±SD, mm Hg | 79 (20) | 78 (10) | 10.8 |
| Tests at admission | |||
| Left ventricular ejection fraction, mean±SD, % | 62.4±7.3 | 61.7±7.6 | 9.1 |
| Creatinine, mean±SD, μmol/L | 81.9±18.6 | 82.4±21.2 | 2.2 |
Table 2.
Procedural Characteristics of the Study Population
| Characteristics | Transradial Intervention, n=4696 (%) | Transfemoral Intervention, n=610 (%) | Standardized Difference |
|---|---|---|---|
| Emergent percutaneous coronary intervention | 100 (2.1) | 11 (1.8) | 2.3 |
| Artery sheath size, French | 36.2 | ||
| Mean (SD) | 6.0±0.3 | 6.1±0.3 | |
| Median (interquartile range) | 6 (6–6) | 6 (6–6) | |
| Diseased coronary vessels | |||
| Single vessel | 1346 (30.3) | 118 (23.0) | 16.5 |
| Double vessel | 1489 (33.5) | 185 (36.1) | 5.4 |
| Triple vessel | 1599 (36.0) | 210 (40.9) | 10.2 |
| Left main disease | 226 (5.1) | 42 (8.2) | 12.5 |
| Lesion type | |||
| De novo | 5903 (97.9) | 761 (95.4) | 14.4 |
| Intrastent | 93 (1.5) | 27 (3.4) | 11.9 |
| Restenosis | 31 (0.5) | 10 (1.3) | 7.9 |
| Lesion location | |||
| Ostial lesion | 763 (12.5) | 141 (17.5) | 14.1 |
| Bifurcation lesion | 2028 (33.1) | 287 (35.4) | 4.8 |
| Number of treated lesion | 7.7 | ||
| Mean±SD | 1.4±0.6 | 1.4±0.7 | |
| Median (interquartile range) | 1 (1–2) | 1 (1–2) | |
| Number of stents used | 7.8 | ||
| Mean±SD | 1.8±1.0 | 1.9±1.0 | |
| Median (interquartile range) | 2 (1–2) | 2 (1–2) | |
| Drug‐eluting stent vs bare metal stent | 4.3 | ||
| Drug‐eluting stent only | 4685 (99.8) | 607 (99.5) | |
| Drug‐eluting and bare metal stents | 11 (0.2) | 3 (0.5) | |
| Domestic vs imported stents | 7.8 | ||
| Domestic only | 2400 (51.1) | 329 (53.9) | |
| Imported only | 2178 (46.4) | 267 (43.8) | |
| Domestic and imported stents | 118 (2.5) | 14 (2.3) | |
| Intravascular ultrasound | 141 (2.3) | 39 (4.9) | 13.8 |
| Vascular closure devices | 0 (0) | 409 (67.0) | 201.4 |
Figure 1.
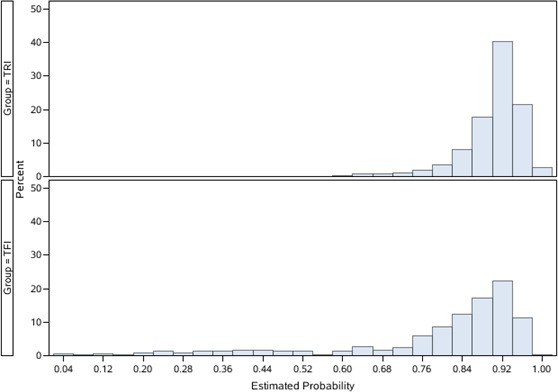
Distribution of propensity scores for transradial intervention (TRI) and transfemoral intervention (TFI).
Figure 2.
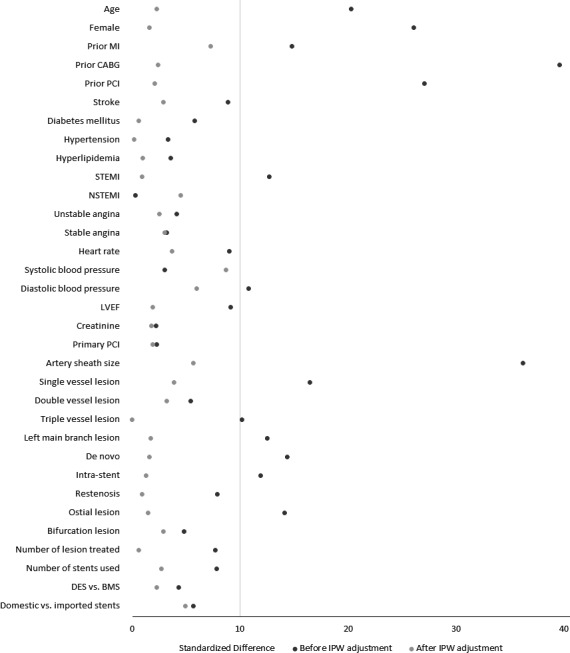
Baseline and procedural characteristics between transradial and transfemoral intervention before and after IPW adjustment. A standardized difference >10 indicates significant imbalance between 2 treatment groups. BMS indicates bare metal stent; CABG, coronary artery bypass grafting; DES, drug‐eluting stent; IPW, inverse probability weighting; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NSTEMI, non–ST‐segment elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST‐segment elevation myocardial infarction.
Hospital Costs and Clinical Outcomes
The average total hospital costs were ¥57 900 ($9190) for TRI and ¥67 418 ($10 701) for TFI (unadjusted cost difference −¥9518 [−$1511]) (Table 3). After IPW adjustment, TRI was associated with lower total hospital cost (adjusted difference ¥8081 [$1283]). More than 80% of the cost differences were due to lower PCI‐related costs (¥43 883 versus ¥50 016, adjusted difference −¥5162 [−$819]) and lower hospitalization costs (¥4557 versus ¥6263, adjusted difference −¥1399 [−$222]) for TRI. The differences in PCI‐related costs were partially driven by the exclusive use of vascular closure devices in TFI (67.0% in TFI and 0% in TRI). Medication costs (adjusted difference −¥692 [−$110]) and examination/laboratory costs (−¥917 [$146]) were also lower for TRI compared with TFI.
Table 3.
Hospital Costs and Clinical Outcomes Between Transradial and Transfemoral Intervention
| Transradial Intervention, n=4696 (95% CI) | Transfemoral Intervention, n=610 (95% CI) | Unadjusted Difference (95% CI) | Adjusted Difference (95% CI) | P Value | |
|---|---|---|---|---|---|
| Hospital costs, ¥ | |||||
| Total costs | 57 900 (54 833–61 138) | 67 418 (62 734–72 464) | −9518 (−11 448 to −7522) | −8081 (−9902 to −6201) | <0.001 |
| Percutaneous coronary intervention costs | 43 883 (41 539–46 359) | 50 015 (46 877–53 364) | −6133 (−7653 to −4559) | −5162 (−6843 to −3415) | <0.001 |
| Hospitalization costs | 4558 (4070–5103) | 6263 (5455–7191) | −1706 (−2017 to −1371) | −1399 (−1717 to −1058) | <0.001 |
| Medication costs | 4635 (4431–4848) | 5546 (4973–6185) | −911 (−1241 to −555) | −692 (−992 to −372) | <0.001 |
| Examination/laboratory costs | 4918 (4498–5377) | 5783 (5243–6379) | −865 (−1089 to −630) | −917 (−1146 to −676) | <0.001 |
| Length of stay, day | 6.1 (5.6–6.7) | 8.4 (7.4–9.6) | −2.3 (−2.7 to −1.8) | −1.6 (−2.1 to −1.2) | <0.001 |
| Major adverse cardiac eventsa | 83 (1.8) | 24 (3.9) | 0.44 (0.33–0.58)b | 0.49 (0.38–0.63)b | <0.001 |
| Bleeding Academic Research Consortium ≥2 | 250 (5.3) | 69 (11.3) | 0.44 (0.35–0.56)b | 0.42 (0.32–0.56)b | <0.001 |
| Bleeding Academic Research Consortium ≥3 | 30 (0.6) | 13 (2.1) | 0.30 (0.17–0.53)b | 0.31 (0.16–0.60)b | <0.001 |
One US dollar is approximately equivalent to 6.3 Chinese yuan.
Composite of death, myocardial infarction, revascularization, or Bleeding Academic Research Consortium ≥3 bleeding.
Odds ratio.
The average length of stay was 6.1 and 8.4 days for TRI and TFI, respectively. After adjustment, the length of stay was 1.6 days shorter for TRI compared with TFI. The unadjusted incidence of MACE was 1.8% for TRI and 3.9% for TFI. The unadjusted post‐PCI bleeding rates were also lower in the TRI cohort (BARC ≥2: 5.3% versus 11.3%; BARC ≥3: 0.6% versus 2.1%). After IPW adjustment, patients receiving TRI were less likely to experience MACE (adjusted odds ratio 0.49, 95% CI 0.38–0.63) or post‐PCI bleeding events (BARC ≥2: odds ratio 0.42, 95% CI 0.32–0.56; BARC ≥3: odds ratio 0.31, 95% CI 0.16–0.60) than TFI patients.
Subgroup Analyses
Of 5306 patients, 1094 underwent PCI for STEMI or non‐STEMI, 3669 underwent PCI for ACS, and 1437 underwent PCI for stable angina. Subgroup analyses by indication found similar trends of lower total hospital costs, shorter length of stay, and fewer MACE and post‐PCI bleeding events in each subgroup, although the differences in MACE and BARC ≥2 or ≥3 bleeding events were not statistically significant in patients with acute myocardial infarction or stable angina, likely due to smaller sample size within each subgroup (Table 4).
Table 4.
Hospital Costs and Clinical Outcomes Between Transradial and Transfemoral Intervention: Subgroup Analyses by Acute Myocardial Infarction, Acute Coronary Syndrome, and Stable Angina
| Transradial Intervention (95% CI) | Transfemoral Intervention (95% CI) | Unadjusted Difference (95% CI) | Adjusted Difference (95% CI) | P Value | |
|---|---|---|---|---|---|
| Acute myocardial infarction | n=992 | n=102 | |||
| Total costs, ¥ | 59 858 (57 120–62 728) | 72 308 (67 466–77 498) | −12 450 (−15 777 to −8927) | −8147 (−11 401 to −4708) | <0.001 |
| Length of stay, day | 7.5 (6.9–8.0) | 9.8 (8.5–11.2) | −2.3 (−3.1 to −1.5) | −1.3 (−2.1 to −0.3) | 0.01 |
| Major adverse cardiac events† | 18 (1.8) | 3 (2.9) | 0.61 (0.26–1.42)a | 0.87 (0.36–2.09)a | 0.75 |
| BARC ≥2 | 62 (6.3) | 19 (18.6) | 0.29 (0.17–0.51)a | 0.33 (0.17–0.63)a | <0.001 |
| BARC ≥3 | 12 (1.2) | 3 (2.9) | 0.40 (0.15–1.09)a | 0.57 (0.20–1.60)a | 0.28 |
| Acute coronary syndrome | n=3260 | n=409 | |||
| Total costs, ¥ | 57 585 (54 779–60 534) | 67 246 (63 082–71 684) | −9661 (−11 531 to −7727) | −8197 (−10 795 to −5477) | <0.001 |
| Length of stay, day | 6.3 (5.8–6.8) | 8.7 (7.7–9.9) | −2.4 (−2.9 to −1.9) | −1.8 (−2.3 to −1.4) | <0.001 |
| Major adverse cardiac events† | 61 (1.9) | 17 (4.2) | 0.44 (0.28–0.70)a | 0.50 (0.35–0.71)a | <0.001 |
| BARC ≥2 | 176 (5.4) | 47 (11.5) | 0.44 (0.34–0.56)a | 0.44 (0.34–0.58)a | <0.001 |
| BARC ≥3 | 24 (0.7) | 8 (2.0) | 0.37 (0.20–0.71)a | 0.38 (0.19–0.76)a | 0.007 |
| Stable angina | n=1264 | n=173 | |||
| Total costs, ¥ | 58 682 (54 962–62 654) | 66 746 (60 536–73 593) | −8064 (−11 575 to −4329) | −4829 (−8249 to −1200) | 0.01 |
| Length of stay, day | 5.9 (5.2–6.5) | 8.0 (6.9–9.1) | −2.1 (−2.6 to −1.6) | −1.2 (−1.7 to −0.7) | <0.001 |
| Major adverse cardiac events† | 20 (1.6) | 6 (3.5) | 0.45 (0.26–0.77)a | 0.62 (0.36–1.08)a | 0.09 |
| BARC ≥2 | 67 (5.3) | 20 (11.6) | 0.43 (0.17–1.06)a | 0.42 (0.13–1.41)a | 0.16 |
| BARC ≥3 | 5 (0.4) | 4 (2.3) | 0.17 (0.06–0.47)a | 0.29 (0.09–0.91)a | 0.03 |
One US dollar is approximately equivalent to 6.3 Chinese yuan. BARC indicates Bleeding Academic Research Consortium.†Composite of death, myocardial infarction, revascularization, or BARC ≥3 bleeding.
Odds ratio.
Discussion
In this large contemporary observational study of TRI versus TFI in China, we found that use of TRI was associated with lower total hospital costs compared with TFI. The majority of these cost differences were driven by reduced costs for PCI (¥5162 [$819]) and hospitalization (¥1399 [$222]). In addition, TRI was associated with a significant reduction in length of stay, MACE, and post‐PCI bleeding events. Although TRI was used more frequently among patients assessed by the risk measures as having lower risk, these differences remained statistically significant after accounting for all observed differences in propensity score IPW analysis and subgroup analyses by PCI indications. Collectively, these findings provide empirical support for the current guideline recommendations for the radial approach and add economic evidence to promote wider adoption of TRI in community practice.10, 11
A growing body of literature supports the use of TRI over TFI to reduce access site–related bleeding and complications in PCI1, 2, 3, 4, 5, 6, 8; however, only a few studies have investigated the economic consequences of TRI versus TFI. In a single‐center randomized study of 142 patients undergoing coronary stenting, Mann et al showed that TRI was associated with a significant cost reduction of ≈$3000 per patient or a 15% reduction of the total hospital charge.20 Another study comparing TRI versus TFI closed with an arterial suture device found that the cost of TRI was substantially less than that of TRI because of lower supply costs and fewer access complications.21 More recently, a multicenter cost analysis of TRI and TFI showed that TRI was associated with an average cost savings >$800 per patient, mainly driven by reduced length of stay with a minimal contribution from procedural costs.12 Despite cost advantages demonstrated in previous research, all of these studies were limited to relatively small samples that included <1500 patients receiving TRI. Importantly, these analyses were conducted in leading centers in the United States, where TFI is the dominant approach for PCI. Furthermore, given the differences in health care systems and practice patterns, which may have influenced economic comparisons, it remains unclear whether these cost savings are generalizable to other countries, especially those in the developing world.
Our study represents a large single‐center experience of TRI and TFI in China. Similar to US studies, we found that TRI was associated with significantly lower total hospital costs than TFI. Of an average ¥8081 ($1283) cost difference, >60% was attributable to reduced PCI costs (¥5162 [$819]). Although we do not have access to itemized bills for each procedure's supplies and equipment, the PCI‐related cost differences are unlikely caused by arterial sheath or number of stents used because patients had similar size and number of stents placed in both groups. It is possible that observed cost differences simply reflect patient risk profiles; however, these differences persisted after controlling for all observed patient and clinical characteristics in the propensity score IPW model. Furthermore, use of intravascular ultrasound was infrequent in both cohorts, albeit numerically greater in TFI, and is unlikely to be the major cause of observed PCI costs differences. Nevertheless, we observed that a vascular closure device was used in 67% of TFI patients, costing ¥3000 ($476) more than manual compression or a radial compression device. Consequently, lower hospital costs for TRI may be mainly driven by the differential use of a vascular closure device between intervention types.
In addition, we found an average difference of ¥1399 ($222) in hospitalization costs, ¥917 ($146) in examination/laboratory costs, and ¥692 ($110) in medication costs. These differences might be attributable to longer length of stay (an average of 1.6 days longer) and/or higher rates of bleeding complications associated with TFI. Although the radial approach accounts for the majority of PCIs in China, nearly 50 000 TFIs are performed each year. We estimate that ¥200 million ($32 million) could be saved if 50% of TFI patients switched to TRI. Because Chinese health care system reform will likely transition from the traditional fee‐for‐service model to the bundled payment system, wider adoption of TFI would provide a financial incentive to physicians and hospital systems while reducing total costs for the society.
Several issues must be considered in interpreting the results of our study. First, this study was a retrospective observational analysis, and treatment selection may bias our results. We used the propensity score IPW method to control for potential selection bias and found balance among all observed covariates. Nevertheless, there might be other unmeasured confounders that, with adjustment, would have influenced the outcomes comparison. Second, our cost analysis was based on the hospital accounting system from the hospital perspective. Because of lack of data on insurance and out‐of‐pocket costs, we were unable to perform a cost comparison from the societal or patient perspective. In addition, there may be variation in accounting that we were unable to measure. Nevertheless, fee for service is the dominant reimbursement method in China, and similar to most Chinese public hospitals, the price is set by the local and/or central government. As such, a cost analysis from the hospital perspective better reflects actual costs and utilization during hospitalization. Third, we were unable to assess outpatient costs prior to the index hospitalization. If TRI patients systematically underwent more preoperative evaluation or laboratory tests before PCI, the total in‐hospital costs associated with TRI would have been underestimated. Nevertheless, it is the interventional cardiologist rather than the referring physician who determines the access approach. As such, there is no prior reason to believe that the referring physician would differentially order more tests for one group versus another prior to the procedure. Fourth, our analysis included data from only a single center in China. The benefit of TRI over TFI likely depends on physician's expertise in the radial technique. Furthermore, the extensive use of a vascular closure device in local practice can markedly affect the results. Consequently, the generalizability of our findings to inexperienced physicians or other centers remains to be established.
In conclusion, this study represents a large single‐center experience of radial versus femoral access for PCI in patients with coronary artery disease in China. Compared with TFI, TRI was associated with lower MACE, fewer bleeding events, shorter length of day, and lower total hospital costs. The majority of the cost differences in TRI were driven by reduced costs for PCI costs and hospitalization and could be related to differential use of a vascular closure device, reduced length of stay, or bleeding complications. Wider adoption of TRI in interventional practice represents an opportunity to improve patient outcomes and to reduce costs to society.
Disclosures
None.
(J Am Heart Assoc. 2016;5:e002684 doi: 10.1161/JAHA.115.002684)
References
- 1. Brueck M, Bandorski D, Kramer W, Wieczorek M, Höltgen R, Tillmanns H. A, randomized comparison of transradial versus transfemoral approach for coronary angiography and angioplasty. JACC Cardiovasc Interv. 2009;2:1047–1054. [DOI] [PubMed] [Google Scholar]
- 2. Pristipino C, Trani C, Nazzaro MS, Berni A, Patti G, Patrizi R, Pironi B, Mazzarotto P, Gioffrè G, Biondi‐Zoccai GGL, Richichi G. Major improvement of percutaneous cardiovascular procedure outcomes with radial artery catheterisation: results from the PREVAIL study. Heart. 2009;95:476–482. [DOI] [PubMed] [Google Scholar]
- 3. Yang Y‐J, Kandzari DE, Gao Z, Xu B, Chen J‐L, Qiao S‐B, Li J‐J, Qin X‐W, Yao M, Wu Y‐J, Yuan J‐Q, Chen J, Liu H‐B, Dai J, Chen T, Wang Y, Li W, Gao R‐L. Transradial versus transfemoral method of percutaneous coronary revascularization for unprotected left main coronary artery disease: comparison of procedural and late‐term outcomes. JACC Cardiovasc Interv. 2010;3:1035–1042. [DOI] [PubMed] [Google Scholar]
- 4. Jolly SS, Yusuf S, Cairns J, Niemelä K, Xavier D, Widimsky P, Budaj A, Niemelä M, Valentin V, Lewis BS, Avezum A, Steg PG, Rao SV, Gao P, Afzal R, Joyner CD, Chrolavicius S, Mehta SR. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): a randomised, parallel group, multicentre trial. Lancet. 2011;377:1409–1420. [DOI] [PubMed] [Google Scholar]
- 5. Hamon M, Rasmussen LH, Manoukian SV, Cequier A, Lincoff MA, Rupprecht HJ, Gersh BJ, Mann T, Bertrand ME, Mehran R, Stone GW. Choice of arterial access site and outcomes in patients with acute coronary syndromes managed with an early invasive strategy: the ACUITY trial. EuroIntervention. 2009;5:115–120. [DOI] [PubMed] [Google Scholar]
- 6. Rao SV, Cohen MG, Kandzari DE, Bertrand OF, Gilchrist IC. The transradial approach to percutaneous coronary intervention: historical perspective, current concepts, and future directions. J Am Coll Cardiol. 2010;55:2187–2195. [DOI] [PubMed] [Google Scholar]
- 7. Baklanov DV, Kaltenbach LA, Marso SP, Subherwal SS, Feldman DN, Garratt KN, Curtis JP, Messenger JC, Rao SV. The prevalence and outcomes of transradial percutaneous coronary intervention for ST‐segment elevation myocardial infarction: analysis from the National Cardiovascular Data Registry (2007 to 2011). J Am Coll Cardiol. 2013;61:420–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Feldman DN, Swaminathan RV, Kaltenbach LA, Baklanov DV, Kim LK, Wong SC, Minutello RM, Messenger JC, Moussa I, Garratt KN, Piana RN, Hillegass WB, Cohen MG, Gilchrist IC, Rao SV. Adoption of radial access and comparison of outcomes to femoral access in percutaneous coronary intervention: an updated report from the National Cardiovascular Data Registry (2007–2012). Circulation. 2013;127:2295–2306. [DOI] [PubMed] [Google Scholar]
- 9. Agostoni P, Biondi‐Zoccai GGL, De Benedictis ML, Rigattieri S, Turri M, Anselmi M, Vassanelli C, Zardini P, Louvard Y, Hamon M. Radial versus femoral approach for percutaneous coronary diagnostic and interventional procedures: systematic overview and meta‐analysis of randomized trials. J Am Coll Cardiol. 2004;44:349–356. [DOI] [PubMed] [Google Scholar]
- 10. Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, Khot UN, Lange RA, Mauri L, Mehran R, Moussa ID, Mukherjee D, Nallamothu BK, Ting HH. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol. 2011;58:e44–e122. [DOI] [PubMed] [Google Scholar]
- 11. Kolh P, Windecker S, Alfonso F, Collet JP, Cremer J, Falk V, Filippatos G, Hamm C, Head SJ, Juni P, Kappetein AP, Kastrati A, Knuuti J, Landmesser U, Laufer G, Neumann FJ, Richter DJ, Schauerte P, Sousa Uva M, Stefanini GG, Taggart DP, Torracca L, Valgimigli M, Wijns W, Witkowski A; European Society of Cardiology Committee for Practice G , Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S; Committee ECG , Sousa Uva M, Achenbach S, Pepper J, Anyanwu A, Badimon L, Bauersachs J, Baumbach A, Beygui F, Bonaros N, De Carlo M, Deaton C, Dobrev D, Dunning J, Eeckhout E, Gielen S, Hasdai D, Kirchhof P, Luckraz H, Mahrholdt H, Montalescot G, Paparella D, Rastan AJ, Sanmartin M, Sergeant P, Silber S, Tamargo J, ten Berg J, Thiele H, van Geuns RJ, Wagner HO, Wassmann S, Wendler O, Zamorano JL; Task Force on Myocardial Revascularization of the European Society of C, the European Association for Cardio‐Thoracic S and European Association of Percutaneous Cardiovascular I . 2014 ESC/EACTS guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio‐Thoracic Surgery (EACTS). Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur J Cardiothorac Surg. 2014;46:517–592. [DOI] [PubMed] [Google Scholar]
- 12. Amin AP, House JA, Safley DM, Chhatriwalla AK, Giersiefen H, Bremer A, Hamon M, Baklanov DV, Aluko A, Wohns D, Mathias DW, Applegate RA, Cohen DJ, Marso SP. Costs of transradial percutaneous coronary intervention. JACC Cardiovasc Interv. 2013;6:827–834. [DOI] [PubMed] [Google Scholar]
- 13. Cooper CJ, El‐Shiekh RA, Cohen DJ, Blaesing L, Burket MW, Basu A, Moore JA. Effect of transradial access on quality of life and cost of cardiac catheterization: a randomized comparison. Am Heart J. 1999;138:430–436. [DOI] [PubMed] [Google Scholar]
- 14. Roussanov O, Wilson SJ, Henley K, Estacio G, Hill J, Dogan B, Henley WF, Jarmukli N. Cost‐effectiveness of the radial versus femoral artery approach to diagnostic cardiac catheterization. J Invasive Cardiol. 2007;19:349–353. [PubMed] [Google Scholar]
- 15. Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Menon V, Nikolsky E, Serebruany V, Valgimigli M, Vranckx P, Taggart D, Sabik JF, Cutlip DE, Krucoff MW, Ohman EM, Steg PG, White H. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736–2747. [DOI] [PubMed] [Google Scholar]
- 16. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity‐score matched samples. Stat Med. 2009;28:3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 18. Curtis LH, Hammill BG, Eisenstein EL, Kramer JM, Anstrom KJ. Using inverse probability‐weighted estimators in comparative effectiveness analyses with observational databases. Med Care. 2007;45:S103–S107 doi: 10.1097/MLR.0b013e31806518ac. [DOI] [PubMed] [Google Scholar]
- 19. Austin PC. Optimal caliper widths for propensity‐score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10:150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mann T, Cubeddu G, Bowen J, Schneider JE, Arrowood M, Newman WN, Zellinger MJ, Rose GC. Stenting in acute coronary syndromes: a comparison of radial versus femoral access sites. J Am Coll Cardiol. 1998;32:572–576. [DOI] [PubMed] [Google Scholar]
- 21. Mann T, Cowper PA, Peterson ED, Cubeddu G, Bowen J, Giron L, Cantor WJ, Newman WN, Schneider JE, Jobe RL, Zellinger MJ, Rose GC. Transradial coronary stenting: comparison with femoral access closed with an arterial suture device. Catheter Cardiovasc Interv. 2000;49:150–156. [DOI] [PubMed] [Google Scholar]


