Abstract
Background
Ion transport in the renal proximal tubule (RPT), which is increased in essential hypertension, is regulated by numerous hormones and humoral factors, including insulin and dopamine. Activation of dopamine receptor inhibits sodium reabsorption, whereas activation of insulin receptor increases sodium reabsorption in RPTs, and hyperinsulinemic animals and patients have defective renal dopaminergic system. We presume that there is an inhibition of D4 receptor on insulin receptor expression and effect, and the regulation is lost in spontaneously hypertensive rats (SHRs).
Methods and Results
Insulin receptor expression was determined by immunoblotting, and Na+‐K+‐ATPase activity was detected in both Wistar‐Kyoto (WKY) and SHR RPT cells. Stimulation of D4 receptor with PD168077 decreased expression of insulin receptors, which was blocked in the presence of the calcium‐channel blocker, nicardipine (10−6 mol/L per 24 hours), in cell culture medium without calcium or in the presence of inositol 1,4,5‐trisphosphate (IP3) receptor blocker (2‐aminoethyl diphenylborinate [2‐ADB]; 10−6 mol/L per 24 hours), indicating that extracellular calcium entry and calcium release from the endoplasmic reticulum were involved in the signal pathway. Stimulation of the insulin receptor stimulated Na+‐K+‐ATPase activity, whereas pretreatment with PD168077 for 24 hours decreased the inhibitory effects of insulin receptor on Na+‐K+‐ATPase activity in WKY cells. However, in SHR cells, inhibition of D4 receptor on insulin receptor expression and effect were lost.
Conclusions
Activation of D4 receptor inhibits insulin receptor expression in RPT cells from WKY rats. The aberrant inhibition of D4 receptor on insulin receptor expression and effect might be involved in the pathogenesis of essential hypertension.
Keywords: dopamine receptor, hypertension, insulin, renal proximal tubule cells
Subject Categories: High Blood Pressure, Basic Science Research, Hypertension
Introduction
Essential hypertension affects 25% of the adult population.1 Ion transport in the renal proximal tubule (RPT) is increased in essential hypertension, which is regulated by numerous hormones and humoral factors, including insulin and dopamine.2, 3 Activation of dopamine receptor inhibits sodium reabsorption, whereas activation of insulin receptor increases sodium reabsorption in RPTs.3, 4 Insulin has various effects. Besides its action on glucose metabolism, insulin affects blood pressure by regulation of vascular smooth muscle cellular proliferation, as well as renal salt and water excretion.4, 5, 6, 7 Diabetes mellitus is associated with sodium and water retention.8 There are evidences indicating that insulin, through stimulation of Na+/H+ exchange and Na+‐K+‐ATPase activities directly, increases volume absorption in renal proximal tubules.6 Dopamine is an endogenous catecholamine that modulates many cellular activities, including blood pressure and transmembrane ion transport.3 Dopamine receptors are classified into the D1‐like and D2‐like subtypes based on their structure and pharmacology. D1‐like receptors, comprised of D1 and D5 receptors, stimulate adenylyl cyclase activity, whereas D2‐like receptors, comprised of D2, D3, and D4 receptors, inhibit adenylyl cyclase activity, and regulate/modulate activity of several ion channels.3 Activation of D1‐like or D2‐like receptor induces natriuresis and diuresis.9
Increasing pieces of evidence show interactions between insulin and dopamine receptors.10, 11, 12, 13 Hyperinsulinemic animals and patients have a defective renal dopaminergic system10; activation of dopamine receptor ameliorates insulin levels in obese humans and improves peripheral insulin sensitivity and renal function in streptozotocin (STZ)‐induced type 2 diabetic rats.11, 12 Because both D4 receptor and insulin receptor exist in the RPTs,13 we presume that activation of D4 receptor inhibits insulin receptor expression and effect, which is aberrant in hypertensive states. In order to test the above hypotheses, we studied D4 and insulin receptor interaction in immortalized RPT cells from Wistar‐Kyoto (WKY) rats and spontaneously hypertensive rats (SHRs). These RPT cells behave similarly to freshly obtained RPT cells, at least with regard to dopamine receptors and responses to G‐protein stimulation.14, 15
Methods
Cell Culture and Sample Preparation
Immortalized RPT cells from microdissected Sl segments of proximal tubules of 4‐ to 8‐week‐old WKY and SHRs were cultured at 37°C in a 95% air/5% CO2 atmosphere in DMEM/F‐12 with transferrin (5 μg/mL), insulin (5 μg/mL), epidermal growth factor (10 ng/mL), dexamethasone (4 μg/mL), and FBS 5% on a 100‐mm Petri dish.16, 17 All animals in this study were handled in accord with the Guide for the Care and Use of Laboratory Animals of the Nation.
Cells were made quiescent by incubation for 2 hours in media without FBS before addition of reagents. Cells (80% confluence) were extracted in ice‐cold lysis buffer, sonicated, kept on ice for 1 hour, and centrifuged at 16 000g for 30 minutes. All samples were stored at −70°C until use.
Immunoblotting
Insulin receptor antibody is polyconal rabbit antihuman antipeptide (Santa Cruz Biotechnology, Santa Cruz, CA).18 Goat polyclonal p‐insulin Rβ antibody (Santa Cruz Biotechnology) was used as the phosphorylated insulin receptor antibody. RPT cells were treated with vehicle (dH2O), a D4 receptor agonist (PD168077; Tocris Cookson Ltd, Bristol, UK),19 or ABT724 (2‐[(4‐pyridin‐2‐ylpiperazin‐1‐yl)methyl]‐1H‐benzimidazole trihydrochloride; Sigma‐Aldrich, St. Louis, MO) or a D4 receptor antagonist20 (L‐745870 [Tocris] or L750667 [Sigma‐Aldrich]) at the indicated concentrations and times as described previously.21 Transblots were probed with insulin receptor antibody (1:400). The amount of protein transferred onto membranes was determined by immunoblotting for α‐actin.
Measurement of Intracellular Calcium Concentration
Twenty‐four hours before the experiments, cells were harvested and seeded into 7.5‐cm2 Petri dishes (Falcon, Franklin Lakes, NJ). Cells were loaded with the calcium indicator, Fura‐2AM (5 μmol/L), in HEPES buffered saline. Changes in intracellular calcium ([Ca2+]) in individual cells were measured using an Aquacosmos system with band‐pass filters for 340 and 380 nm. [Ca2+]i was calculated from the Fura‐2 fluorescence ratio (F340/F380) using linear regression between adjacent points on a calibration curve generated by measuring F340/F380 in at least 7 calibration solutions containing [Ca2+] between 0 and 854 nmol/L. Ca2+ concentration in individual groups were measured as previously described.22
Determination of the Second Messenger(s) Involved in Regulation of D4 Receptor on Insulin Receptor Expression in WKY Cells
To determine the second messenger(s) involved in the regulation of D4 on insulin receptor expression in WKY cells, several inhibitors or agonists were used: protein kinase C (PKC) inhibitor (PKC inhibitor 19–31, 10−6 mol/L)23 protein kinase A (PKA) inhibitor (PKA inhibitor 14–22, 10−6 mol/L)24 PKC activator (PMA, 10−7 mol/L)25 PKA activator (Sp‐cAMPs, 10−7 mol/L)26 calcium‐channel blocker (nicardipine, 10−6 mol/L)21 and calcium‐channel agonist (BAY‐K8644, 10−6 mol/L).27 Those reagents were added into the cell incubation medium 15 minutes before PD168077 treatment.
PKC inhibitor 19 to 31, PMA, Sp‐cAMPs, nicardipine, and BAY‐K8644 were purchased from Sigma‐Aldrich. PKA inhibitor 14 to 22 was purchased from Calbiochem Company (Darmstadt, Germany).
Real‐Time Polymerase Chain Reaction Detecting System of Insulin Receptors
A total of 2 to 3 μg of total RNA extracted from WKY and SHR cells were used to synthesize cDNA, which served as the template for amplification of receptor and β‐actin (as a housekeeping gene). For β‐actin, the forward primer was 5′‐GTGGGTATGGGTCAGAAGGA‐3′ and the reverse primer was 5′‐AGCGCGTAACCCTCATAGAT‐3′ (GeneBank Accession No.: NM031144). For insulin receptor, the forward primer was 5′‐TTCAGGAAGACCTTCGAGGATTACCTGCAC‐3′ and the reverse primer was 5′‐AGGCCAGAGATGACAAGTGACTCCTTGTT‐3′ (GeneBank Accession No.: X57764). Amplification was performed with the following conditions: denaturation at 95°C for 3 minutes, followed by 35 cycles at 95°C for 10 seconds and 60°C for 30 seconds. At the end of each run, a melting curve analysis was performed from 65°C to 95°C to monitor primer dimers or nonspecific product formation. Reactions were performed in triplicate. Insulin receptor mRNA expression was normalized for β‐actin mRNA.18
siRNA
siRNA against D4 receptor mRNA and its control scrambled RNA were synthesized and purified with reverse‐phase high‐performance liquid chromatography as 25‐mer phosphoro‐thioate‐modified oligodeoxynucleotides (D4 receptor siRNA sequence 5′‐AAGGACCUCAAUGAAUAUGAAGAUAdTdT‐3′; scrambled RNA sequence: 5′‐TGACGATAAGAACAATAACdTdT‐3′). Cells were grown in 6‐well plates until 60% confluence, and 50 nmol/L of siRNA or control RNA were mixed with 6 μL of Oligofectamine in Opti‐MEM medium (Invitrogen Life Technologies, Carlsbad, CA) and incubated for 24 hours, then switched to growth medium and incubated for another 24 hours. Cells were collected and processed for reverse‐transcription polymerase chain reaction for D4 receptor to determine the efficiency of siRNA‐induced D4 receptor gene silencing.
Na+‐K+‐ATPase Activity Assay
Na+‐K+‐ATPase activity was determined as the rate of inorganic phosphate released in the presence or absence of ouabain.28, 29 Rat RPT cells were treated with vehicle (dH2O) and D4 receptor agonist (PD168077) at the indicated concentrations and durations of incubation. To prepare membranes for Na+‐K+‐ATPase activity assay, RPT cells cultured in 21‐cm2 plastic culture dishes were collected and centrifuged at 3000g for 10 minutes. Cells were then placed on ice and lysed in 2 mL of lysis buffer (1 mmol/L of NaHCO3, 2 mmol/L of CaCl2 and 5 mmol/L of MgCl2). Cellular lysates were centrifuged at 3000g for 2 minutes to remove intact cells, debris, and nuclei. The resulting supernatant was suspended in an equal volume of 1 mol/L of sodium iodide, and the mixture was centrifuged at 48 000g for 25 minutes. The pellet (membrane fraction) was washed 2 times and suspended in 10 mmol/L of Tris containing 1 mmol/L of EDTA (pH 7.4). Protein concentrations were determined by Bradford assay (Bio‐Rad Laboratories, Hercules, CA) and adjusted to 1 mg/mL. Membranes were stored at −70°C until further use.
To measure Na+‐K+‐ATPase activity, 100‐μL aliquots of membrane fraction were added to an 800‐μL reaction mixture (75 mmol/L of NaCl, 5 mmol/L of KCl, 5 mmol/L of MgCl2, 6 mmol/L of sodium azide, 1 mmol/L of Na4EGTA, 37.5 mmol/L of imidazole, 75 mmol/L of Tris HCl, and 30 mmol/L of histidine; pH 7.4) with or without 1 mmol/L of ouabain (final volume=1 mL) and preincubated for 5 minutes in a water bath at 37°C. Reactions were initiated by adding Tris‐ATP (4 mmol/L) and terminated after 15 minutes of incubation at 37°C by adding 50 μL of 50% trichloracetate. For determination of ouabain‐insensitive ATPase activity, NaCl and KCl were omitted from the reaction mixtures containing ouabain. To quantify the amount of phosphate produced, 1 mL of coloring reagent (10% ammonium molybdate in 10 N of sulfuric acid+ferrous sulfate) was added to the reaction mixture. The mixture was then mixed thoroughly and centrifuged at 3000g for 10 minutes. Formation of phosphomolybdate was determined spectrophotometrically at 740 nm, against a standard curve prepared from K2HPO4. Na+‐K+‐ATPase activity was estimated as the difference between total and ouabain‐insensitive ATPase activity and expressed as nmol phosphate released per mg protein per minute.
To eliminate the function of proteases and phosphatases, protease inhibitors (1 mmol/L of phenylmethylsulfonyl fluoride, 10 μg/mL of each leupeptin and aprotinin) and a phosphatase inhibitor (50 μmol/L of sodium orthovanadate) were added in all solutions used after drug/vehicle incubations.
Statistical Analysis
Data are expressed as mean±SEM. Comparison within groups was made by repeated‐measures ANOVA (or paired t test when only 2 groups were compared), and comparison among groups (or t test when only 2 groups were compared) was made by factorial ANOVA with Holm‐Sidak test. A value of P<0.05 was considered significant.
Results
Activation of D4 Receptor Decreases Insulin Receptor Expression in WKY RPT Cells
Stimulation of D4 receptor with a D4 agonist, PD168077, decreased insulin receptor expression in a concentration‐dependent (10−9–10−5 mol/L) and time‐dependent (2–30 hours) manner in WKY RPT cells. The inhibitory effect was evident at 10−8 mol/L, noted as early as 8 hours, and maintained for at least 30 hours (Figure 1A and 1B).
Figure 1.
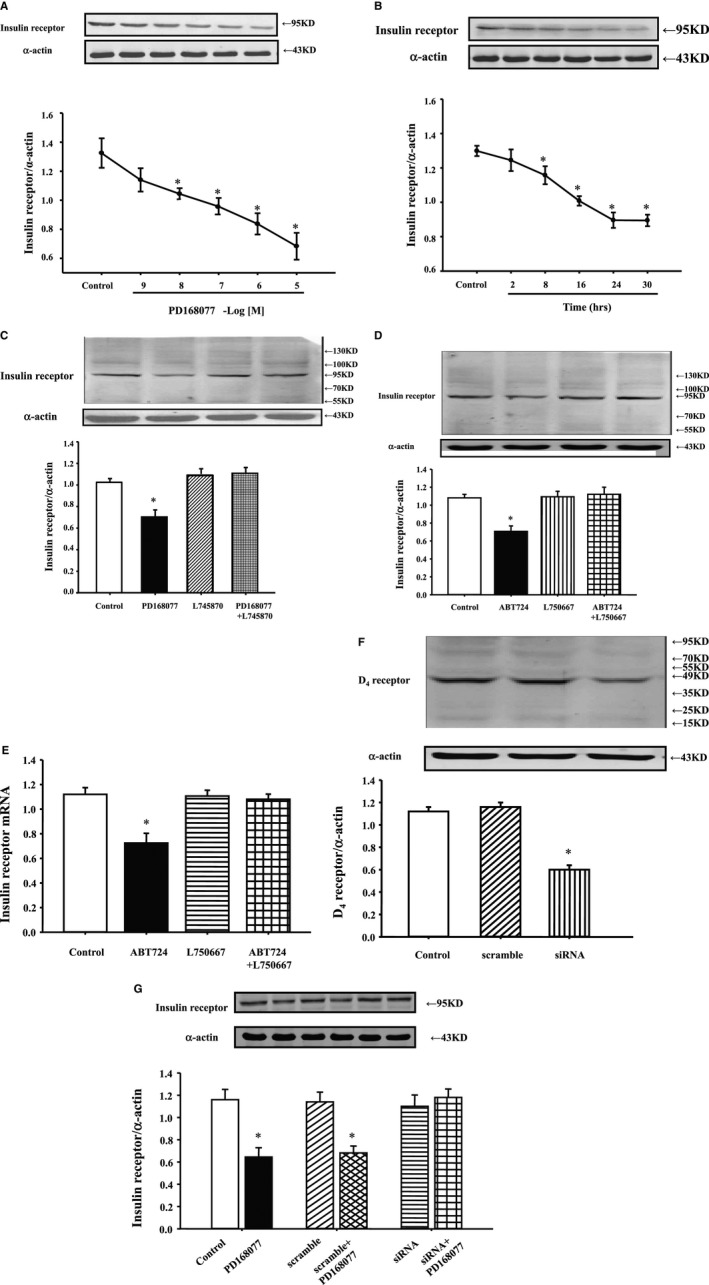
Effect of D4 receptor on insulin receptor expression in Wistar‐Kyoto (WKY) renal proximal tubule (RPT) cells. A, Concentration response of insulin receptor protein expression in WKY cells treated with different concentrations of the D4 receptor agonist, PD168077, for 24 hours. Results are expressed as the ratio of insulin receptor and α‐actin densities (n=6; *P<0.05 vs control). B, Time course of insulin receptor protein expression in WKY cells treated with D4 receptor agonist, PD168077 (10−7 mol/L) for different periods. Results are expressed as the ratio of insulin receptor and α‐actin densities (n=6; *P<0.05 vs control). C, Effect of a D4 receptor agonist (PD168077) and a D4 receptor antagonist (L745870) on insulin receptor expression in WKY cells. Cells were incubated with the indicated reagents (PD168077, 10−7 mol/L; L745870, 10−6 mol/L) for 24 hours. Results are expressed as the ratio of insulin receptor and α‐actin densities (n=9; *P<0.05 vs others). D and E, Effect of a D4 receptor agonist (ABT724) and a D4 receptor antagonist (L750667) on insulin receptor expression in WKY cells. Cells were incubated with the indicated reagents (ABT724, 10−7 mol/L; L750667, 10−6 mol/L) for 24 hours. Results are expressed as the ratio of insulin receptor and α‐actin densities (n=7; *P<0.05 vs others). F and G, Effect of D4 receptor siRNA on regulation of D4 receptor on insulin receptor expression in WKY cells. WKY cells were incubated with D4 receptor siRNA (50 nm, 24 hours), D4 receptor expressions were determined by immunoblotting (F, n=5; *P<0.05 vs others). Cells were incubated with the indicated reagents (PD168077, 10−7 mol/L) for 24 hours with or without the presence of D4 receptor siRNA. Insulin receptor expressions were determined by immunoblotting. Results are expressed as the ratio of insulin receptor and α‐actin densities (G, n=8; *P<0.05 vs others).
The specificity of PD168077 as a D4 receptor agonist was also determined by studying the effect of the D4 receptor antagonist, L745870. Consistent with the study shown in Figure 1A and 1B, PD168077 (10−7 mol/L per 24 hours), decreased insulin receptor expression. The D4 receptor antagonist, L745870 (10−6 mol/L), by itself, had no effect on insulin receptor expression, but reversed the inhibitory effect of PD168077 on insulin receptor expression (Figure 1C).
To further confirm the regulation of D4 receptor on insulin receptor expression, we used another D4 receptor agonist (ABT724) and antagonist (L750667). Consistent with Figure 1A through 1C, the inhibitory effect of ABT724 (10−7 mol/L per 24 hours) was blocked by L750667 (10−6 mol/L per 24 hours) (Figure 1D and 1E). We also checked the regulation of D4 receptor on insulin expression in the presence of D4 receptor siRNA. Transfection with the D4 receptor siRNA on WKY cells (50 nm, 24 hours) significantly decreased D4 receptor expression and blocked the inhibitory effect PD168077 (10−7 mol/L per 24 hours) on insulin receptor mRNA and protein expressions in WKY cells (Figure 1F and 1G).
It is reported that phosphorylation of insulin receptor will increase its function in RPT cells from both WKY and SHRs; it resulted that PD168077 (10−7 mol/L per 24 hours) inhibited phosphorylation of insulin receptor in WKY cells, not in SHR cells (Figure 2A and 2B). Morevoer, the D4 receptor antagonist, L745870 (10−6 mol/L), by itself, had no effect on insulin receptor phosphorylation, but reversed the inhibition of PD168077 on insulin receptor phosphorylation in WKY cells (Figure 2A).
Figure 2.
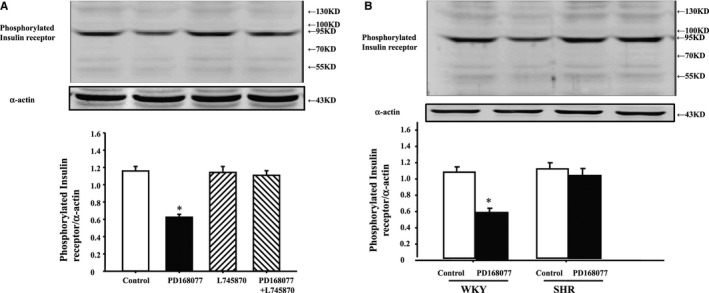
Effect of D4 receptor on insulin receptor phosphorylation in Wistar‐Kyoto (WKY) renal proximal tubule (RPT) cells. A, Effect of a D4 receptor agonist (PD168077) and a D4 receptor antagonist (L745870) on insulin receptor phosphorylation in WKY cells. Cells were incubated with the indicated reagents (PD168077, 10−7 mol/L; L745870, 10−6 mol/L) for 15 minutes. Results are expressed as the ratio of phosphorylated insulin receptor and α‐actin densities (n=9; *P<0.05 vs control). B, Effect of a D4 receptor agonist (PD168077, 10−7 mol/L per 15 minutes) on insulin receptor phosphorylation in WKY and SHR RPT cells (n=9; *P<0.05 vs control). SHR indicates spontaneously hypertensive rat.
Extracellular and Intracellular Calcium Mediates the Inhibitory Effect of D4 Receptor on Insulin Receptor Expression in RPT Cells
To investigate mechanisms of D4 receptor induced downregulation of insulin receptor, WKY RPT cells were treated with different agonists or antagonists. Treatment with the calcium‐channel blocker, nicardipine (10−6 mol/L), or withdrawl of calcium from cell culture meidium blocked the inhibitory effect of D4 receptor on insulin receptor expression (Figure 3A and 3B), indicating that extracellular calcium influx was involved in the signal transduction pathway. To check whether intracellular calcium release from the endoplasmic reticulum was also involved in the mechanisms, we used inositol 1,4,5‐trisphosphate (IP3) receptor blocker (2‐aminoethyl diphenyl borate [2‐ADB]; 10−6 mol/L per 24 hours); the inhibitory effect of D4 receptor on insulin receptor expression was abolished in the presence of IP3 blocker (Figure 3C), indicating that both extracellular calcium entry and calcium release from the endoplasmic reticulum were involved in the signal pathway. To further confirm the role of calcium channel and IP3 receptor, we used calcium‐channel activator BAY‐K8644 (10−6 mol/L per 24 hours; Figure 3D‐I) or IP3 (10−6 mol/L per 24 hours; Figure 3D‐II); it resulted that BAY‐K8644 or IP3 decreased insulin receptor expression in WKY RPT cells. The effect of PD168077 (10−7 mol/L per 15 minutes) stimulation on intracellular calcium concentration in SHR and WKY RPT cells were further studied (Figure 3E and 3F). We found that intracellular calcium concentration between SHR and WKY RPT cells have no significant difference in the basal level. PD168077 (10−7 mol/L per 15 minutes) increased intracellular calcium in WKY RPT cells, but not in SHRs. This effect was blocked by the presence of nicardipine (10−6 mol/L per 15 minutes) or 2‐ADB (10−6 mol/L per 15 minutes). We also evaluated the involvement of other key cell‐signaling proteins with the use of a PKA inhibitor (PKA inhibitor 14–22, 10−6 mol/L), PKC activator (PMA, 10−7 mol/L), and PKA activator (Sp‐cAMPs, 10−7 mol/L). None of these reagents was able to block the inhibitory effect of insulin on D5 receptor expression (data not shown).
Figure 3.
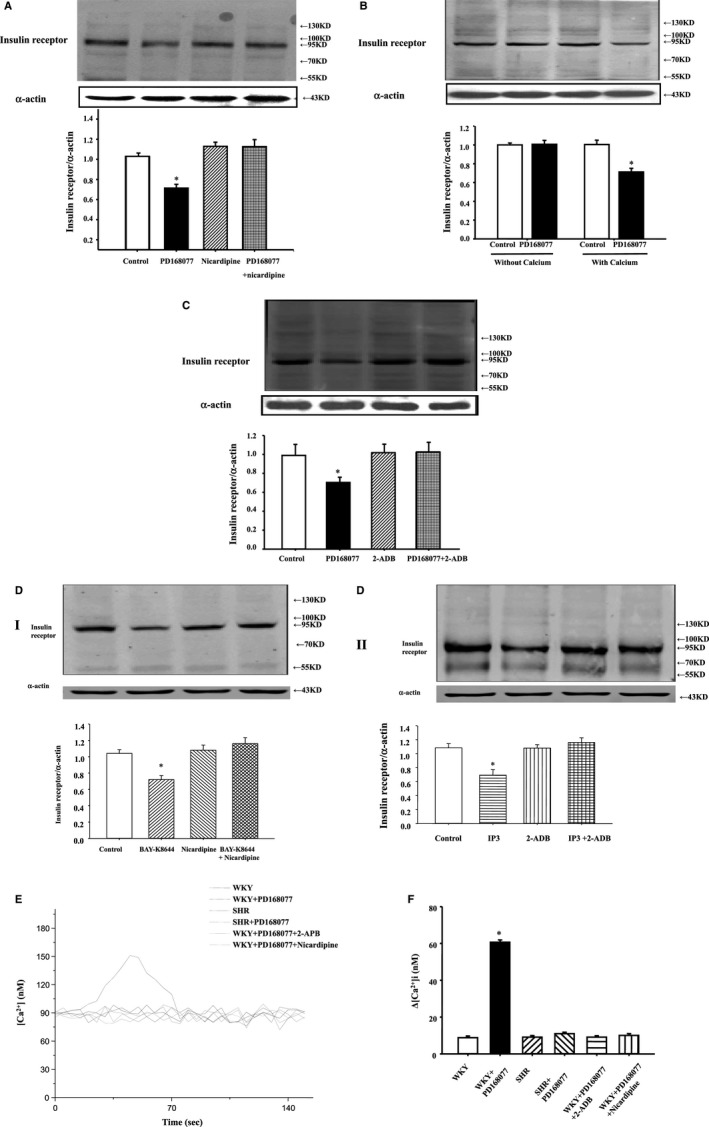
Extracellular and intracelluar calcium mediates the inhibitory effect of D4 receptor on insulin receptor expression in renal proximal tubule (RPT) cells. A, Effect of the D4 receptor agonist, PD168077 (10−7 mol/L per 24 hours), and the calcium‐channel blocker, nicardipine (10−6 mol/L per 24 hours), on insulin receptor protein in Wistar‐Kyoto (WKY) RPT cells. Cells were incubated with the indicated reagents. Results are expressed as the ratio of insulin receptor and α‐actin densities (n=6; *P<0.05 vs others). B, Effect of the D4 receptor agonist, PD168077 (10−7 mol/L per 24 hours), on insulin receptor expression in cell culture medium with or without calcium in WKY RPT cells. Results are expressed as the ratio of insulin receptor and α‐actin densities (n=6; *P<0.05 vs others). C, Effect of the D4 receptor agonist, PD168077 (10−7 mol/L per 24 hours), and inositol 1,4,5‐trisphosphate (IP3) receptor blocker (2‐aminoethoxydiphenyl borate [2‐ADB]; 10−6 mol/L per 24 hours) on insulin receptor protein in WKY RPT cells. Cells were incubated with the indicated reagents. Results are expressed as the ratio of insulin receptor and α‐actin densities (n=9; *P<0.05 vs others). D, Effect of calcium‐channel activator BAY‐K8644 (10−6 mol/L per 24 hours) (I) or IP3 (10−6 mol/L per 24 hours) (II) on insulin receptor protein expression in WKY RPT cells. Cells were incubated with the indicated reagents. Results are expressed as the ratio of insulin receptor and α‐actin densities (n=9; *P<0.05 vs others). E and F, Effect of the D4 receptor agonist, PD168077, on intracellular calcium concentration in the presence or absence of pharmacological agents in WKY and spontaneously hypertensive rats (SHRs) cells. Intracellular calcium concentration of RPT cells with or without PD168077 (10−7 mol/L per 15 minutes) treatment were determined by laser confocal microscopy. The stimulatory effect of PD168077 on intracellular calcium concentration was also tested in the presence of nicardipine (10−6 mol/L per 15 minutes) or 2‐ADB (10−6 mol/L per 15 minutes). Representative tracings are shown (E) and graphs of the data are shown (F) (Δ[Ca2+]i shows the difference in calcium concentration between 0 and 60 seconds). (n=6; *P<0.05 vs others).
D4 Receptors Decrease Insulin Receptor Expression in WKY RPT Cells, not in SHR RPT Cells
To investigate whether there is a different regulation between WKY and SHRs, RPT cells from WKY and SHRs were treated with PD168077 (10−7 mol/L) for 24 hours; consistent with the results in Figure 1A through 1C, activation of D4 receptor decreased insulin receptor expression in WKY cells, but not in SHR cells (Figure 4A). We also investigated insulin receptor mRNA levels; consistent with protein results, activation of D4 receptor with PD168077 (10−7 mol/L per 24 hours) inhibited insulin receptor mRNA expression in WKY RPT cells, not in SHR cells (Figure 4B). In the presence of the calcium‐channel blocker, nicardipine (10−6 mol/L per 24 hours; Figure 4C‐I), or IP3 receptor blocker (2‐; 10−6 mol/L per 24 hours; Figure 4C‐II), dose regulation of D4 receptor on insulin receptor mRNA expression was lost.
Figure 4.
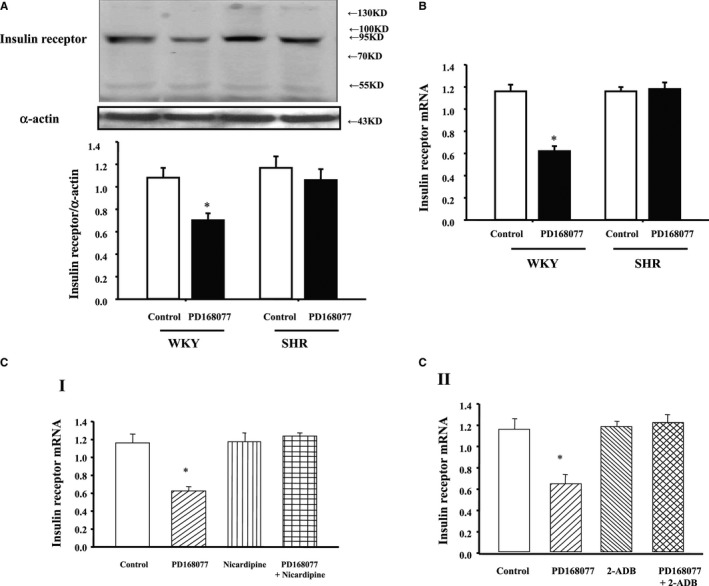
Differential effects of D4 receptor on insulin receptor expression in renal proximal tubule (RPT) cells from Wistar‐Kyoto (WKY) and spontaneously hypertensive rats (SHRs). A, Differential effects of the D4 receptor agonist, PD168077 (10−7 mol/L per 24 hours), on insulin receptor protein expression in RPT cells from WKY and SHRs. Cells were incubated at the indicated time points and concentrations. Results are expressed as the ratio of insulin receptor and α‐actin densities (n=6; *P<0.05 vs control). B, Differential effects of the D4 receptor agonist, PD168077 (10−7 mol/L per 24 hours), on insulin receptor mRNA expression in RPT cells from WKY and SHRs. Cells were incubated at indicated time points and concentrations. Results are expressed as the ratio of insulin receptor and β‐actin densities (n=9; *P<0.05 vs control). C, Effect of the D4 receptor agonist, PD168077 (10−7 mol/L per 24 hours), in the presence of calcium‐channel blocker (nicardipine, 10−6 mol/L per 24 hours) (I) or inositol 1,4,5‐trisphosphate (IP3) receptor blocker (2‐ADB; 10−6 mol/L per 24 hours) (II) on insulin receptor mRNA expression in WKY RPT cells. Cells were incubated with the indicated reagents. Real‐time quantitative polymerase chain reaction data were analyzed using the comparative CT method (n=9; *P<0.05 vs others). CT indicates threshold cycle.
Pretreatment With D4 Receptor Agonist Decreases the Stimulatory Effect of Insulin Receptor on Na+‐K+‐ATPase Activity in WKY RPT Cells, not in SHR RPT Cells
Effects of the D4 agonist, PD168077 (10−7 mol/L per 15 minutes), on Na+‐K+‐ATPase activity in WKY and SHR RPT cells were explored. Activity of Na+‐K+‐ATPase was higher in SHR than WKY RPT cells in the basal level. PD168077 inhibited Na+‐K+‐ATPase activity in WKY RPT cells, but not in SHR RPT cells (Figure 5A). The inhibitory effect was disappeared 2 hours after washing with PBS (Figure 5B).
Figure 5.
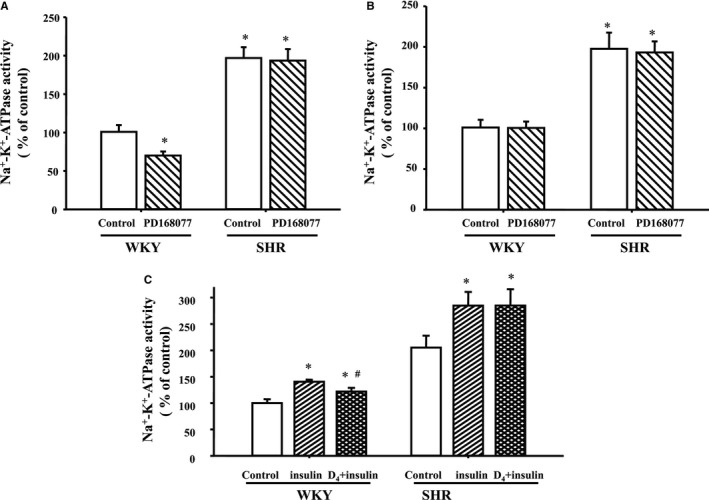
Effect of pretreatment with D4 receptor on the stimulatory effect of insulin receptor on Na+‐K+‐ATPase activity in Wistar‐Kyoto (WKY) and spontaneously hypertensive rats (SHRs) cells. A, Renal proximal tubule (RPT) cells from WKY and SHRs were treated with PD168077 (10−7 mol/L) or vehicle (dH2O) for 24 hours. Na+‐K+‐ATPase activity were investigated in each group. Results are expressed as percent change of control (*P<0.05 vs control; # P<0.05 vs insulin; n=7). B, RPT cells from WKY and SHRs were treated with PD168077 (10−7 mol/L) or vehicle (dH 2O) for 24 hours. Then, cells were washed 3 times (15 min/times) with serum‐free culture medium to remove all the added PD168077 and kept in serum‐free culture medium for 2 hours before Na+‐K+‐ATPase activity was investigated (*P<0.05 vs control; # P<0.05 vs insulin; n=8). C, Cells were pretreated with PD168077 (10−7 mol/L) or vehicle (dH2O) for 24 hours. After PD168077 pretreatment, cells were washed 3 times (15 min/times) with serum‐free culture medium to remove all the added PD168077, kept in serum‐free culture medium for 2 hours, and then treated with insulin (10−7 mol/L) for 15 minutes. Results are expressed as percent change of control (*P<0.05 vs control; # P<0.05 vs insulin; n=8).
To investigate the physiological significance of D4 receptor on insulin receptor expression, effects of insulin on Na+‐K+‐ATPase activity were determined in RPT cells from WKY and SHR cells. Insulin (10−7 mol/L per 15 minutes) increased Na+‐K+‐ATPase activity, which was reduced by pretreatment with the D4 receptor agonist, PD168077 (10−7 mol/L per 24 hours), in WKY cells. However, in SHR cells, pretreatment with PD168077 had no effect on the insulin‐mediated inhibitory effect on Na+‐K+‐ATPase activity in SHR cells (Figure 5C).
Discussion
Insulin receptor exists in RPTs; activation of insulin receptor induces antinatriuresis, which is supported by numerous previous studies. Early studies by Atchley et al. demonstrate that discontinuation of an insulin infusion to diabetic patients results in a brisk natriuresis.30 Miller and Bogdonoff show that infusion of insulin into normal subjects results in a reduction in urinary sodium excretion.31 Consistent with those studies, our present study found that insulin increases Na+‐K+‐ATPase activity in WKY cells and SHR cells.
Dopamine receptor subtypes are expressed in specific segments of the mammalian kidney. The D4 receptor has abundant protein expression in the proximal tubule.3, 13 Previous studies show interactions between insulin and dopamine receptors. Hyperinsulinemic animals and patients with noninsulin‐dependent diabetes have a defective renal dopaminergic system.32 In obese Zucker rats, a model of type 2 diabetes, renal D1 receptor are downregulated and dopamine fails to produce diuresis and natriuresis; treatment with insulin sensitizer rosiglitazone restores renal D1 receptor effect.33 Chronic exposure of cells to insulin causes both reduction in D1 receptor abundance and its uncoupling from G proteins and diminishes the inhibitory effect of dopamine on Na+‐K+‐ATPase activity in hyperinsulinemic rats.34 There are also evidences that activation of dopamine receptor influences insulin effect. Activation of D2 receptor with bromocriptine simultaneously ameliorates insulin levels in obese humans.11 Fenoldopam treatment improves peripheral insulin sensitivity and renal effect in STZ‐induced type 2 diabetic rats.12 Therefore, we hypothesize that D4 receptor, along with insulin receptor in RPTs, might affect insulin receptor expression and effect. This hypothesis is confirmed in this study. Stimulation of D4 receptor inhibits insulin receptor expression in WKY cells, but not in SHRs. The inhibitory effect of D4 receptor on insulin receptor expression has physiological significance. Stimulation with insulin increases Na+‐K+‐ATPase activities in WKY cells. Pretreatment with D4 receptor for 24 hours reduces insulin receptor expression and insulin‐mediated Na+‐K+‐ATPase activity in WKY cells.
Insulin receptor functions as a tyrosin protein kinase. Phosphorylation of insulin receptor results in a progressive activation of its intrinsic kinase activity and actually plays an important role in regulating the function of the receptor. It is suggested that phosphorylation of the receptor is a major step in the regulation of its function that is related to kinase activity.35, 36, 37 We found that stimulation of D4 receptor inhibits insulin receptor phosphorylation in WKY cells, but not in SHRs. It is in accord with the inhibition of D4 receptor on effect of insulin receptor.
The mechanism of the decrease in insulin receptors caused by D4 receptors is not clear. Ca2+ is suggested to be critical for expression of insulin receptor. DNA sequence analysis indicated that the promoter region of the gene contains several GGGCGG sequences that may be binding sites for the transcription factor, Sp1 (specificity protein 1).38, 39 Sp1 could be upregulated by high Ca2+ treatment.40 Furthermore, Ca2+ could stimulate phosphorylation of Sp1 and augment the activity of the gene promoter in an Sp1‐binding–dependent manner.41 Others also showed that the interactive role of cAMP regulatory element‐binding protein and Sp1 is regulated by calcium.42 As a D2‐like receptor subtype, activation of D4 receptor increases intracellular calcium concentration.3 The present study showed that the inhibitory effect of D4 receptor on insulin receptor expression is blocked by a calcium‐channel blocker, withdrawal of calcium in the cell culture medium, or in the presence of IP3 receptor blocker, which blocks the calcium release from endoplasmic reticulum, indicating that calcium mobilization, including extracellular calcium influx and intracellular calcium release from the endoplasmic reticulum, is involved in the signaling pathway. Besides insulin receptor protein expression, our present study found that activation of D4 receptor decreases insulin receptor mRNA expression in WKY cells. However, in the presence of a calcium‐channel blocker or IP3 receptor blocker, the stimulatory effect of D4 receptor on insulin receptor mRNA and protein expression is blocked. It indicates that the regulation may occur at the transcriptional/posttranscriptional level. The detailed mechanisms remain to be elucidated in the future.
The pathogenesis of essential hypertension is complex; both genetic and environmental factors are involved. In hypertensive states, sodium reabsorption in RPTs is increased,3 which is regulated by numerous hormones and humoral factors, including insulin and dopamine. In this study, we found that activation of D4 receptor reduces insulin receptor expression and insulin‐mediated sodium reabsorption by inhibition of Na+‐K+‐ATPase activity in the basolateral membranes of RPTs. It might indicate that D4 receptor is a counterbalance against insulin receptor. However, inhibition of D4 receptor on insulin receptor expression and effect is lost in SHRs, which might be involved in the pathogenesis of essential hypertension.
Conclusions
In summary, we have demonstrated that activation of D4 receptor, by calcium mobilization from extracellular calcium influx and intracellular calcium release from the endoplasmic reticulum, decreases insulin receptor expression and insulin‐mediated stimulatory effect on Na+‐K+‐ATPase activity in WKY cells. However, in SHR cells, inhibition of D4 receptor on insulin receptor expression and effect is lost, suggesting that aberrant inhibition of D4 receptor on insulin receptor expression and effect might be involved in the pathogenesis of essential hypertension.
Sources of Funding
These studies were supported by grants from the National Science Foundation of China (31430043, 81300197), a National International Technology Special Grant (2014DFA31070), and the National Basic Research Program of China (2012CB517801).
Disclosures
None.
(J Am Heart Assoc. 2016;5:e002448 doi: 10.1161/JAHA.115.002448)
References
- 1. Rosendorff C. Hypertension and coronary artery disease: a summary of the American Heart Association scientific statement. J Clin Hypertens (Greenwich). 2007;9:790–795. [DOI] [PubMed] [Google Scholar]
- 2. McDonough AA, Biemesderfer D. Does membrane trafficking play a role in regulating the sodium/hydrogen exchanger isoform 3 in the proximal tubule? Curr Opin Nephrol Hypertens. 2003;12:533–541. [DOI] [PubMed] [Google Scholar]
- 3. Hussain T, Lokhandwala MF. Renal dopamine receptors and hypertension. Exp Biol Med (Maywood). 2003;228:134–142. [DOI] [PubMed] [Google Scholar]
- 4. Sowers JR. Insulin resistance and hypertension. Am J Physiol Heart Circ Physiol. 2004;286:H1597–H1602. [DOI] [PubMed] [Google Scholar]
- 5. Wei Y, Chen K, Whaley‐Connell AT, Stump CS, Ibdah JA, Sowers JR. Skeletal muscle insulin resistance: role of inflammatory cytokines and reactive oxygen species. Am J Physiol Regul Integr Comp Physiol. 2008;294:R673–R680. [DOI] [PubMed] [Google Scholar]
- 6. Baum M. Insulin stimulates volume absorption in the rabbit proximal convoluted tubule. J Clin Invest. 1987;79:1104–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zeng C, Han Y, Huang H, Yu C, Ren H, Shi W, He D, Huang L, Yang C, Wang X, Zhou L, Jose PA. D1‐like receptors inhibit insulin‐induced vascular smooth muscle cell proliferation via down‐regulation of insulin receptor expression. J Hypertens. 2009;27:1033–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Siddiqui AH, Hussain T. Impaired angiotensin II AT1 receptor effect and enhanced Na, K‐ATPase affinity for sodium in proximal tubule of streptozotocin‐treated diabetic rats. Clin Exp Hypertens. 2007;29:435–444. [DOI] [PubMed] [Google Scholar]
- 9. Ladines CA, Zeng C, Asico LD, Sun X, Pocchiari F, Semeraro C, Pisegna J, Wank S, Yamaguchi I, Eisner GM, Jose PA. Impaired renal D1‐like and D2‐like dopamine receptor interaction in the spontaneously hypertensive rat. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1071–R1078. [DOI] [PubMed] [Google Scholar]
- 10. Tsuchida H, Imai G, Shima Y, Satoh T, Owada S. Mechanism of sodium load‐induced hypertension in non‐insulin dependent diabetes mellitus model rats: defective dopaminergic system to inhibit Na‐K‐ATPase activity in renal epithelial cells. Hypertens Res. 2001;24:127–135. [DOI] [PubMed] [Google Scholar]
- 11. Kok P, Roelfsema F, Frölich M, van Pelt J, Stokkel MP, Meinders AE, Pijl H. Activation of dopamine D2 receptors simultaneously ameliorates various metabolic features of obese women. Am J Physiol Endocrinol Metab. 2006;291:E1038–E1043. [DOI] [PubMed] [Google Scholar]
- 12. Umrani DN, Goyal RK. Fenoldopam treatment improves peripheral insulin sensitivity and renal effect in STZ‐induced type 2 diabetic rats. Clin Exp Hypertens. 2003;25:221–233. [DOI] [PubMed] [Google Scholar]
- 13. Shin Y, Kumar U, Patel Y, Patel SC, Sidhu A. Differential expression of D2‐like dopamine receptors in the kidney of the spontaneously hypertensive rat. J Hypertens. 2003;21:199–207. [DOI] [PubMed] [Google Scholar]
- 14. Parenti A, Cui XL, Hopfer U, Ziche M, Douglas JG. Activation of MAPKs in proximal tubule cells from spontaneously hypertensive and control Wistar‐Kyoto rats. Hypertension. 2000;35:1160–1166. [DOI] [PubMed] [Google Scholar]
- 15. Xu J, Li XX, Albrecht FE, Hopfer U, Carey RM, Jose PA. D1 receptor, Gsα, and Na+/H+ exchanger interactions in the kidney in hypertension. Hypertension. 2000;36:395–399. [DOI] [PubMed] [Google Scholar]
- 16. Woost PG, Orosz DE, Jin W, Frisa PS, Jacobberger JW, Douglas JG, Hopfer U. Immortalization and characterization of proximal tubule cells derived from kidneys of spontaneously hypertensive and normotensive rats. Kidney Int. 1996;50:125–134. [DOI] [PubMed] [Google Scholar]
- 17. Zeng C, Liu Y, Wang Z, He D, Huang L, Yu P, Zheng S, Jones JE, Asico LD, Hopfer U, Eisner GM, Felder RA, Jose PA. Activation of D3 dopamine receptor decreases angiotensin II type 1 receptor expression in rat renal proximal tubule cells. Circ Res. 2006;99:494–500. [DOI] [PubMed] [Google Scholar]
- 18. Elchebly M, Payette P, Michaliszyn E, Cromlish W, Collins S, Loy AL, Normandin D, Cheng A, Himms‐Hagen J, Chan CC, Ramachandran C, Gresser MJ, Tremblay ML, Kennedy BP. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase‐1B gene. Science. 1999;283:1544–1548. [DOI] [PubMed] [Google Scholar]
- 19. Gu Z, Yan Z. Bidirectional regulation of Ca2+/calmodulin‐dependent protein kinase II activity by dopamine D4 receptors in prefrontal cortex. Mol Pharmacol. 2004;66:948–955. [DOI] [PubMed] [Google Scholar]
- 20. Tanaka K, Okada Y, Kanno T, Otomo A, Yanagisawa Y, Shouguchi‐Miyata J, Suga E, Kohiki E, Onoe K, Osuga H, Aoki M, Hadano S, Itoyama Y, Ikeda JE. A dopamine receptor antagonist L‐745,870 suppresses microglia activation in spinal cord and mitigates the progression in ALS model mice. Exp Neurol. 2008;211:378–386. [DOI] [PubMed] [Google Scholar]
- 21. Chen K, Deng K, Wang X, Wang Z, Zheng S, Ren H, He D, Han Y, Asico LD, Jose PA, Zeng C. Activation of D4 dopamine receptor decreases angiotensin II type 1 receptor expression in rat renal proximal tubule cells. Hypertension. 2015;65:153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Splettstoesser F, Florea AM, Büsselberg D. IP(3) receptor antagonist, 2‐APB, attenuates cisplatin induced Ca2+‐influx in HeLa‐S3 cells and prevents activation of calpain and induction of apoptosis. Br J Pharmacol. 2007;151:1176–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Johnson EA, Oldfield S, Braksator E, Gonzalez‐Cuello A, Couch D, Hall KJ, Mundell SJ, Bailey CP, Kelly E, Henderson G. Agonist‐selective mechanisms of mu‐opioid receptor desensitization in human embryonic kidney 293 cells. Mol Pharmacol. 2006;70:676–685. [DOI] [PubMed] [Google Scholar]
- 24. Bobalova J, Mutafova‐Yambolieva VN. Activation of the adenylyl cyclase/protein kinase A pathway facilitates neural release of beta‐nicotinamide adenine dinucleotide in canine mesenteric artery. Eur J Pharmacol. 2006;536:128–132. [DOI] [PubMed] [Google Scholar]
- 25. Lei J, Mariash CN, Bhargava M, Wattenberg EV, Ingbar DH. T3 increases Na‐K‐ATPase activity via a MAPK/ERK1/2‐dependent pathway in rat adult alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2008;294:L749–L754. [DOI] [PubMed] [Google Scholar]
- 26. Scroggs RS. Up‐regulation of low‐threshold tetrodotoxin‐resistant Na+ current via activation of a cyclic AMP/protein kinase A pathway in nociceptor‐like rat dorsal root ganglion cells. Neuroscience. 2011;186:13–20. [DOI] [PubMed] [Google Scholar]
- 27. Inui T, Mori Y, Watanabe M, Takamaki A, Yamaji J, Sohma Y, Yoshida R, Takenaka H, Kubota T. Physiological role of L‐type Ca2+ channels in marginal cells in the stria vascularis of guinea pigs. J Physiol Sci. 2007;57:287–298. [DOI] [PubMed] [Google Scholar]
- 28. Liu Y, Yang J, Ren H, He D, Pascua A, Armando MI, Yang C, Zhou L, Felder RA, Jose PA, Zeng C. Inhibitory effect of ETB receptor on Na+‐K+ ATPase ATPase activity by extracellular Ca2+ entry and Ca2+ release from the endoplasmic reticulum in renal proximal tubule cells. Hypertens Res. 2009;32:846–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Silva E, Gomes P, Soares‐da‐Silva P. Overexpression of Na+/K+‐ATPase parallels the increase in sodium transport and potassium recycling in an in vitro model of proximal tubule cellular ageing. J Membr Biol. 2006;212:163–175. [DOI] [PubMed] [Google Scholar]
- 30. Atchley DW, Loeb RF, Richards DW, Benedict EM, Driscoll ME. On diabetic acidosis: a detailed study of electrolyte balances following the withdrawal and reestablishment of insulin therapy. J Clin Invest. 1933;12:297–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Millar JH, Bogdonoff MD. Antidiuresis associated with administration of insulin. J Appl Physiol. 1954;6:509–512. [DOI] [PubMed] [Google Scholar]
- 32. Segers O, Anckaert E, Gerlo E, Dupont AG, Somers G. Dopamine‐sodium relationship in type 2 diabetic patients. Diabetes Res Clin Pract. 1996;34:89–98. [DOI] [PubMed] [Google Scholar]
- 33. Banday AA, Hussain T, Lokhandwala MF. Renal dopamine D1 receptor dyseffect is acquired and not inherited in obese Zucker rats. Am J Physiol Renal Physiol. 2004;287:F109–F116. [DOI] [PubMed] [Google Scholar]
- 34. Banday AA, Asghar M, Hussain T, Lokhandwala MF. Dopamine‐mediated inhibition of renal Na. K‐ATPase is reduced by insulin. Hypertension. 2003;41:1353–1358. [DOI] [PubMed] [Google Scholar]
- 35. Rosen OM, Herrera R, Olowe Y, Petruzzelli LM, Cobb MH. Phosphorylation activates the insulin receptor tyrosine protein kinase. Proc Natl Acad Sci USA. 1983;80:3237–3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yu KT, Czech MP. Tyrosine phosphorylation of the insulin receptor beta subunit activates the receptor‐associated tyrosine kinase activity. J Biol Chem. 1984;259:5277–5286. [PubMed] [Google Scholar]
- 37. Czech MP. The nature and regulation of the insulin receptor: structure and function. Annu Rev Physiol. 1985;47:357–381. [DOI] [PubMed] [Google Scholar]
- 38. Mamula PW, Wong KY, Maddux BA, McDonald AR, Goldfine ID. Sequence and analysis of promoter region of human insulin‐receptor gene. Diabetes. 1988;37:1241–1246. [DOI] [PubMed] [Google Scholar]
- 39. McKeon C. Transcriptional regulation of the insulin receptor gene promoter. Adv Exp Med Biol. 1993;343:79–89. [DOI] [PubMed] [Google Scholar]
- 40. Apt D, Watts RM, Suske G, Bernard HU. High Sp1/Sp3 ratios in epithelial cells during epithelial differentiation and cellular transformation correlate with the activation of the HPV‐16 promoter. Virology. 1996;224:281–291. [DOI] [PubMed] [Google Scholar]
- 41. You HL, Eng HL, Hsu SF, Chen CM, Ye TC, Liao WT, Huang MY, Baer R, Cheng JT. A PKC‐Sp1 signaling pathway induces early differentiation of human keratinocytes through upregulation of TSG101. Cell Signal. 2007;19:1201–1211. [DOI] [PubMed] [Google Scholar]
- 42. Matlhagela K, Borsick M, Rajkhowa T, Taub M. Identification of a prostaglandin‐responsive element in the Na, K‐ATPase beta 1 promoter that is regulated by cAMP and Ca2+. Evidence for an interactive role of cAMP regulatory element‐binding protein and Sp1. J Biol Chem. 2005;280:334–346. [DOI] [PubMed] [Google Scholar]


