Abstract
Background:
Gross physiological perturbations necessitating the Intensive Care Unit (ICU) admission might exacerbate the already existing or initiate bothersome symptoms among cancer patients. There is a lack of conclusive evidence concerning the symptomatic experience among this subgroup of cancer patients particularly so in the Indian population. The aim of this prospective observational study was to elucidate the symptom prevalence and overall symptomatic distress among critically ill cancer patients at the time of admission to a medical ICU.
Methods:
We prospectively evaluated 110 consecutive cancer patients at the time of admission to our medical ICU for the presence and intensity of symptoms using a modified Edmonton Symptom Assessment Scale (ESAS). The patients/caregivers were also enquired regarding the most bothersome symptom in the past 1 week and the presence of “symptom associated sleep disturbance.” The primary outcome was the prevalence of patients with moderate (ESAS ≥ 40) and severe (ESAS ≥ 70) symptomatic distress.
Results:
The average age was 52.49 years with 75.45% of the respondents in the economically productive age group (21–60 years). Carcinoma breast (19.35%) and lung (14.58%) were the most common cancers among females and males, respectively. 87.27% and 60% of the patients had advanced cancer and multi-organ dysfunction, respectively. About 76.36% patients were able to complete ESAS either by themselves or with caregiver's assistance within first 24 h of ICU admission. The mean ESAS distress score was 48.04 (0–81) with 72.72% of the patients having moderate-severe symptomatic distress. Loss of appetite (92.73%) and nausea (54.55%) were the most common and the least common reported symptoms, respectively. Pain was the most common and “most distressing symptom” reported by 40% of patients with 64.55% patients reporting one or more symptoms severe enough to interfere with their sleep.
Conclusion:
ESAS is a user-friendly cognitive aid to make the healthcare team cognizant of the symptom existence and overall symptomatic burden among cancer patients with gross physiological perturbations. The high prevalence of moderate-severe symptom distress requires the concomitant provision of palliative and intensive care among this group of cancer patients.
Keywords: Cancer, Critically ill, Distress, Edmonton Symptom Assessment Scale, Intensive Care Unit, Palliative care, Symptoms
INTRODUCTION
Cancer patients may require Intensive Care Unit (ICU) admission anytime during the protracted course of their illness and treatments. Physiological perturbations due to tumor burden, antineoplastic therapies, and immunological suppression leading to infections often culminating in sepsis usually act as the heralding cause for the above. The precipitants necessitating ICU admission such as acute respiratory failure and sepsis might exacerbate the already existing or initiate severe symptomatic distress among these patients.
Traditionally, intensive and palliative care have been considered mutually exclusive: The first being focused upon recovery from life-threatening acute illness utilizing sophisticated organ support and invasive monitoring while the other implying an overall encompassing team based approach to “patients and their families facing the problems associated with life-threatening illness, through the prevention and relief of suffering by means of early identification and impeccable assessment and treatment of pain and other problems such as physical, psychosocial, and spiritual.”[1] While palliation addresses life quality improvement by symptomatic support, critical care is purely objective and disease oriented; survival is addressed by emphasis upon vital signs and digital parameters. More and more studies mostly emerging from the West are unanimously contradicting this traditionally held concept.[2]
High symptom burden is known to exist among cancer and critically ill patients separately.[3,4,5] Extrapolating from above the magnitude of the problem is expected to be bigger among critically ill cancer patients; however, convincing data in Indian population are lacking. While the relevance of palliative care as an indispensable component of holistic patient care and translating into best achievable quality of care in critically ill patients is increasingly being recognized in the West, is still in the infancy stages in India.[6] Extending palliation while simultaneously administering intensive care requires continuous scrutiny and collation of patient symptomatology utilizing efficient tools. Assessment constitutes the foundation/initial step for recognizing the existence and magnitude of any given problem. It has also been recognized as one of the key components for improving palliative care delivery in ICU.[7] Such an assessment of symptoms in this particular group of cancer patients at the time of ICU admission will help the ICU staff in establishing a baseline, decide upon the goals of care, patient's priorities and is more apt in oncocritical care with high prevalence of symptomatic distress than the typical trigger criteria used in other ICU's.[8] Realizing the existence of symptoms and their effective management is important as they not only affect the quality of life but also the outcome of these patients. The present prospective study was therefore conducted with the aim to assess the presence and severity of various symptoms in the critically ill cancer patients at the time of admission to an ICU.
METHODS
Patient recruitment and exclusion criteria
After obtaining ethical approval from the Institutional Review Board and written informed consent consecutive cancer patients getting admitted to our medical ICU were included in this prospective observational study. The inclusion criteria included (1) Adult patient ≥18 years age, (2) patients diagnosed with cancer, (3) patients aware of their diagnosis, and (4) patients/relatives giving written informed consent for inclusion in the study. Patients/relatives not giving written informed consent for inclusion in the study were excluded from the study.
Tools and data collection
Self-report/caregiver/caregiver assisted assessment of patient's symptoms using “Edmonton Symptom Assessment Scale (ESAS)” was conducted at the time of ICU admission (earliest possible within first 24 h). The “0 (no) – 10 (worst possible)” numerical rating scale (NRS) version of ESAS was used to elucidate the intensity of various symptoms in the past 1 week. The ESAS measures the presence and the intensity of nine common (anxiety, appetite, depression, drowsiness, nausea, pain, shortness of breath, tiredness, and wellbeing) and one patient specific symptom.[9] We modified ESAS by asking the patients to include all the other bothersome symptoms they were suffering from rather than limiting them to one (patient specific symptom). The patients/caregivers were also enquired about: What is the most distressing symptom in the past 1 week? Whether any physical symptom interfered with their sleep? The reason for inability to self-report the symptoms was also noted. The patient and the caregivers were readily provided help if they had any difficulty in understanding any of the component of ESAS and a note of the same was also made in the records. Only completed ESAS forms were included in the analysis.
Patients with loco-regional progression, unresectable cancer, distant metastasis, on palliative therapy and those in whom anticancer treatment was no longer feasible were considered to have advanced malignancy. Physiological derangement involving ≥3 vital systems was considered as multisystem involvement (PaO2/FiO2 < 300, Glasgow Coma Scale <13, use of vasopressors/inotropes, platelet count <100,000, acute increase in serum creatine >2 mg/dl, acute increase in total bilirubin >2 mg/dl).[10] The grading of symptom severity (ESAS score) followed was: NRS 0: None, NRS 1–3: Mild, NRS: 4–6: Moderate, and NRS ≥7: Severe.[9,11] A patient record form to record the demographic and disease characteristics such as age, sex, primary cancer site, stage, sites of metastasis, time since diagnosis, and reason for admission to ICU were also developed and filled by an independent physician or nurse unaware of the nature of the study and the ESAS findings.
Our primary outcomes were mean cumulative ESAS score, prevalence of patients with ESAS score ≥40 (moderate-severe) and ≥70 (severe). Our secondary outcomes were the most common symptom among the cohort and the prevalence of patients with at least one severe symptom (NRS ≥ 7). The variables were tabulated and analyzed using Microsoft excel. The data are presented as mean (standard deviation) or number of patients (%). Chi-square test was used to check for the level of significance wherever appropriate and a P < 0.05 was considered as significant.
RESULTS
A total of 121 consecutive patients getting admitted to the medical ICU and giving written informed consent were included in the study. Out of them, 11 patients were excluded at the time of statistical analysis because of incomplete assessments submitted by the patient/caregiver. The average duration of ICU stay was 5.26 days (1–63 days). The most common reasons for ICU admission were acute respiratory failure (40.37%), sepsis (11.93%), altered consciousness (20.18%), bleeding (5.5%), and seizures (4.59%).
Demographics
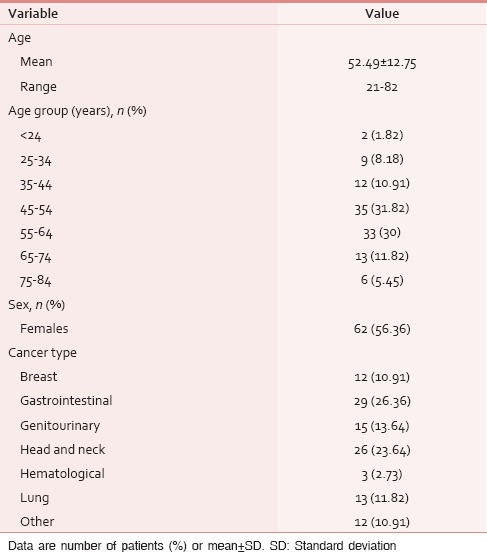
The demographic and clinical characteristics of the study population are depicted in Table 1. The average age was 52.49 years with 75.45% of the respondents in the economically productive age group (21–60 years). The four most common cancers, i.e., gastrointestinal, head and neck, genitourinary, and lung in that order represented 75.46% of the total study population. The most common site of primary malignancy among females were gastrointestinal (16/62, 25.81%), genitourinary and breast (12/62, 19.35% each) whereas among males were head and neck (15/48, 31.25%) followed by gastrointestinal (13/48, 27.08%). Carcinoma breast (12/62, 19.35%) and lung (7/48, 14.58%) were the most common cancers among females and males, respectively. About 96 (87.27%) and 66 (60%) patients had advanced cancer and multi-organ dysfunction, respectively.
Table 1.
Demographics
Symptom score
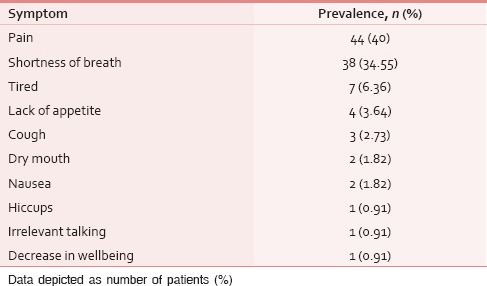
The ESAS assessment was completed by 84 (76.36%) patients either themselves or with the assistance of caregiver within first 24 h of ICU admission. However, in 26 (23.64%) patients, the symptom assessment was done completely by the caregivers. The reasons for the patient's inability to participate in symptom assessment have been depicted in Figure 1. Tables 2 and 3 demonstrate the prevalence of various symptoms by their severity and diagnosis specific prevalence of various symptoms, respectively. Loss of appetite (92.73%) and nausea (54.55%) were the most common and the least common reported symptoms, respectively. The mean number of symptoms was 6.927 ± 2.01. The various patient specific symptoms reported by the patients and their prevalence are shown in Table 4. Dry mouth was the most common patient specific symptom, and 16 (14.55%) patients reported more than 1 patient specific symptom. 57 (51.81%) of the respondents did not report any patient specific symptom. The overall symptomatic distress in terms of mean cumulative ESAS score is depicted in Table 5. The prevalence of moderate-severe symptomatic distress was found to be independent of age, sex, and staging as depicted in Table 6. Likewise, the prevalence of symptoms such as depression and anxiety was found to be unaffected by the staging of the malignancy being 63.54% versus 64.29% for depression and 68.75% versus 71.43% for anxiety in patients with advanced and early malignancy, respectively. Approximately, 70% of the patients had an ESAS distress score ≥40 indicating a moderate-severe symptomatic distress. About 71 (64.55%) patients reported symptoms to be interfering their sleep during the last 1 week. The prevalence of various “most distressing symptoms” reported by the patient is shown in Table 7.
Figure 1.
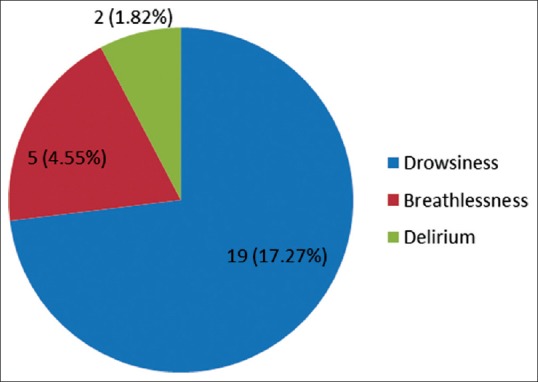
Reason for patient's inability to participate in symptom assessment
Table 2.
Prevalence and severity of various symptoms
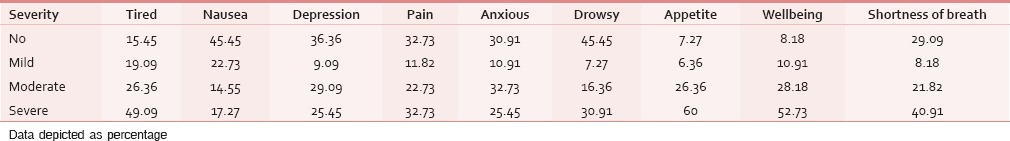
Table 3.
Diagnosis specific prevalence of various symptoms
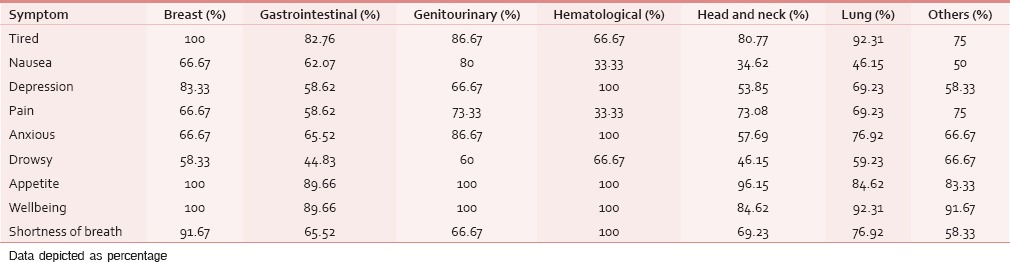
Table 4.
Prevalence of patient specific symptoms
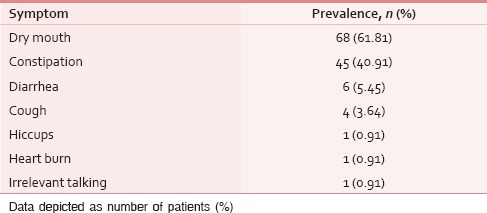
Table 5.
Severity of cumulative symptomatic distress
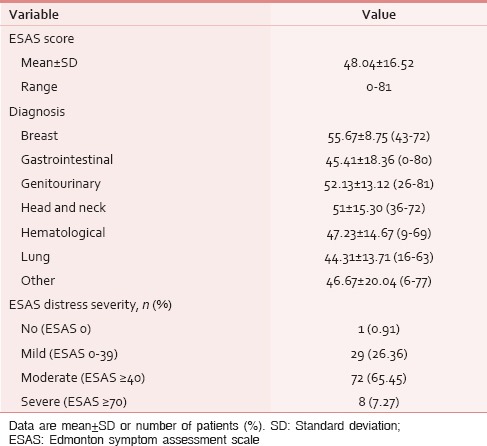
Table 6.
Distribution of patients with moderatesevere symptomatic distress
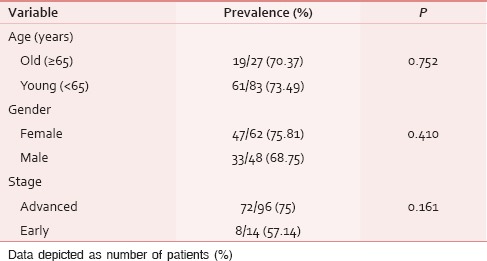
Table 7.
Most distressing symptom
DISCUSSION
This is the first study to evaluate the symptom burden among critically ill cancer patients in India. The frequent suffering of patients among this cohort from various symptoms and the lack of available evidence particularly in the Indian population prompted us to accomplish this endeavor in the form of formal research. Assessment constitutes the foundation step to identify the magnitude of the problem, and assessment focused palliative care research also acts as an effective medium toward the sensitization of fellow colleagues.
Ongoing progress in clinical oncology has led to the favorable shift in the duration of postdiagnosis survivorship.[12] The cumulative long-term toxicities with infections and organ failures becoming predictor of survival in chronic cancer patients, the reluctance of intensivists to admit such patients to ICU is going into oblivion.[12,13,14] A high percentage of our study group had multiple organ dysfunction (60%) with approximately 40% and 12% having acute respiratory failure and sepsis, respectively. Admission to an ICU results in increased suffering and pain for the patient and compounds psychological havoc upon caregivers with already existing long-term psychological distress. Approximately, 75% of the study population was in the economically productive age group. Lal et al. while studying the frequency of symptoms among Indian patients referred to a pain and palliative care clinic also found a similar preponderance of patients belonging to the economically productive age group.[15] Their young age and financial loss due to ongoing treatments, ICU admission, and loss of job or unpaid leaves add to the psychological distress of the patient and caregivers alike. The majority of patients (87.27%) getting admitted had advanced cancer which is in accordance with similar higher prevalence of advanced cancer (82%) observed by other authors among hospitalized cancer patients.[16]
The mean cumulative distress score in our study was 48.04 with 72.72% of the cohort having moderate-severe symptomatic distress (ESAS distress score ≥40). The symptoms tend to cluster in palliative care and assessment and treatment aimed at symptom cluster have been found to provide greater therapeutic benefit, patient function, and posttherapeutic outcomes. The mean number of symptoms in our study was 6.927 which were in accordance with those found by other authors (5.1–7.8).[15,16,17] Loss of appetite (92.63%), decreased sense of wellbeing (91.82%), and tiredness (84.55%) were the most common symptoms in the present study. Al-Shahri et al. also reported tiredness (79.8%) and loss of appetite (71.9%) to be the most common symptoms but at a lower frequency among cancer patients in an outpatient palliative care clinic. Similar higher prevalence of fatigue (80–88.4%) and anorexia (67%) have been reported among cancer inpatients outside the ICU setting by other authors.[16,18] Ongoing physiological perturbations, poor performance status, comorbidities such as sepsis and acute respiratory failure precipitating ICU admission might be responsible for higher prevalence of symptoms observed in our study.
Pain, shortness of breath, and tiredness were the most common “most distressing symptoms” reported by 40%, 34.55%, and 6.36% of the patients. Similar findings by Alshemmari et al. (pain 16–31%, dyspnea 24%, and weakness/fatigue 11–25%) not only validates our findings but also points toward the universal existence of these distressing symptoms among hospitalized cancer patients be in ICU or otherwise.[16] A “patient centered palliative care approach” should specifically assess the presence and severity of “most troublesome symptom” as they are likely to be missed in about one-third of the cases if not specifically asked for.[19]
Any attempt and tool for symptom assessment in patients with gross physiological perturbations should be short and relevant so as to ensure continued compliance. The ESAS is such an assessment tool that focuses upon the distressing symptoms impairing the quality of life of a patient.[10] The validity and reliability of ESAS have been studied and established throughout the cancer care continuum and in different patient populations including “critically ill patients in ICU”.[20,21,22] The higher scores on ESAS have been correlated with higher disease burden and the cumulative “ESAS Symptom Distress Score” can be utilized to evaluate the efficacy of symptom management strategies on overall disease burden.[21,23] An association between the severity of symptoms and level of interference with daily activities has also been postulated.[24] We set out arbitrary cut-off points to “ESAS distress score” and classified the patients into having mild, moderate or severe symptomatic distress. Such an approach might help in anchoring the response to symptom oriented interventions to these levels (mild, moderate, severe) and monitor the response to them, i.e. improvement from severe to moderate or moderate to mild distress.[21] However, any cut-offs with respect to symptom severity is arbitrary neglecting the individual thresholds, sensitivities, and other factors which confound the patient's experience of symptoms.
Approximately, 55% and 33% of the patients in our study had nausea and moderate-severe nausea. Our results are in accordance with the similar incidence reported by other authors.[25,26,27] Nausea was described as the most bothersome symptom by only two patients. Seow et al. while prospectively addressing various symptoms in advanced cancer patients reported nausea as the least bothersome symptom.[28] A number of other researches have also pointed out that nausea might not be as common and bothersome as previously speculated.[29] The highest prevalence of nausea was among genitourinary, breast, and gastrointestinal cancers a finding confirmed by previous studies.[30]
Unlike intensivists, the management goals of a patient aware of his diagnosis of advanced cancer might be “symptom rather than prognosis oriented.” However, the patients might be reluctant in bothering the “very busy intensivists” with symptoms such as anorexia, dry mouth, anxiety, or depression. It is imperative to enquire from all the cancer patients for various distressing symptoms at the time of admission itself before the ongoing physiological perturbations takes a toll upon their cognition so as to establish common mutually acceptable goals of care. In our study, 76% of the patients were able to communicate their symptomatic distress, its severity, the significance imparted to each, and their interference with their sleep. Simultaneous and ongoing provision of intensive and palliative care to critically ill cancer patients will help in supplementing not only “days to life” but also life to days. Also of equal importance is continued follow-up and management of symptoms after planned discharges so as to ensure continued optimum quality of life of these patients.
Limitation
The clinical heterogeneity of the study population and the small sample size of individual malignancies are the main drawback of our study. The main theme of our research was to elucidate the prevalence of symptoms in a group of critically ill cancer patients not restricting to any particular primary site. Such a diagnosis specific symptomatic research in this particular subgroup of cancer patients is a step forward.
CONCLUSION
ESAS is user friendly and acts as a great cognitive aid in making both the primary as well as the critical care team cognizant of the presence of various symptom and overall symptomatic distress among this group of critically ill cancer patients. The high symptom prevalence among this group of cancer population is an indirect measure of inadequate palliative care services available in the community as a whole. Assessing the palliative care needs of the patients at every point of contact with the healthcare facility be it outpatient, day care, inpatient, or ICU admissions will ensure not only wider coverage but also continued holistic care.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.WHO Definition of Palliative Care. [Last accessed on 2015 Sep 11]. Available from: http://www.who.int/cancer/palliative/definition/en .
- 2.Gay EB, Weiss SP, Nelson JE. Integrating palliative care with intensive care for critically ill patients with lung cancer. Ann Intensive Care. 2012;2:3. doi: 10.1186/2110-5820-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teunissen SC, Wesker W, Kruitwagen C, de Haes HC, Voest EE, de Graeff A. Symptom prevalence in patients with incurable cancer: A systematic review. J Pain Symptom Manage. 2007;34:94–104. doi: 10.1016/j.jpainsymman.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 4.Barbera L, Seow H, Howell D, Sutradhar R, Earle C, Liu Y, et al. Symptom burden and performance status in a population-based cohort of ambulatory cancer patients. Cancer. 2010;116:5767–76. doi: 10.1002/cncr.25681. [DOI] [PubMed] [Google Scholar]
- 5.Nelson JE, Meier DE, Oei EJ, Nierman DM, Senzel RS, Manfredi PL, et al. Self-reported symptom experience of critically ill cancer patients receiving intensive care. Crit Care Med. 2001;29:277–82. doi: 10.1097/00003246-200102000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Nelson JE, Bassett R, Boss RD, Brasel KJ, Campbell ML, Cortez TB, et al. Models for structuring a clinical initiative to enhance palliative care in the intensive care unit: A report from the IPAL-ICU Project (Improving Palliative Care in the ICU) Crit Care Med. 2010;38:1765–72. doi: 10.1097/CCM.0b013e3181e8ad23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nelson JE, Campbell ML, Cortez TB, Curtis JR, Lustbader DR, Mosenthal AC, et al. Organizing an ICU Palliative Care Initiative: A Technical Assistance Monograph from the IPAL-ICU Project. [Last accessed on 2015 Sep 11]. Available from: http://www.who.capc.org/ipal-icu/monographs-and-publications/ipal-icu-organizingan-icu-palliativecare-initiative.pdf.
- 8.Norton SA, Hogan LA, Holloway RG. Proactive palliative care in the medical intensive care unit: Effects on length of stay for selected high-risk patients. Crit Care Med. 2007;35:1530–5. doi: 10.1097/01.CCM.0000266533.06543.0C. [DOI] [PubMed] [Google Scholar]
- 9.Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K. The Edmonton symptom assessment system (ESAS): A simple method for the assessment of palliative care patients. J Palliat Care. 1991;7:6–9. [PubMed] [Google Scholar]
- 10.Hua MS, Li G, Blinderman CD, Wunsch H. Estimates of the need for palliative care consultation across united states intensive care units using a trigger-based model. Am J Respir Crit Care Med. 2014;189:428–36. doi: 10.1164/rccm.201307-1229OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Selby D, Cascella A, Gardiner K, Do R, Moravan V, Myers J, et al. A single set of numerical cutpoints to define moderate and severe symptoms for the Edmonton symptom assessment system. J Pain Symptom Manage. 2010;39:241–9. doi: 10.1016/j.jpainsymman.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 12.von Bergwelt-Baildon M, Hallek MJ, Shimabukuro-Vornhagen AA, Kochanek M. CCC meets ICU: Redefining the role of critical care of cancer patients. BMC Cancer. 2010;10:612. doi: 10.1186/1471-2407-10-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blot F, Guiguet M, Nitenberg G, Leclercq B, Gachot B, Escudier B. Prognostic factors for neutropenic patients in an intensive care unit: Respective roles of underlying malignancies and acute organ failures. Eur J Cancer. 1997;33:1031–7. doi: 10.1016/s0959-8049(97)00042-7. [DOI] [PubMed] [Google Scholar]
- 14.Garrouste-Orgeas M, Boumendil A, Pateron D, Aergerter P, Somme D, Simon T, et al. Selection of intensive care unit admission criteria for patients aged 80 years and over and compliance of emergency and intensive care unit physicians with the selected criteria: An observational, multicenter, prospective study. Crit Care Med. 2009;37:2919–28. doi: 10.1097/ccm.0b013e3181b019f0. [DOI] [PubMed] [Google Scholar]
- 15.Lal M, Raheja S, Kale S, Das N, Gogia AR, Bhowmik KT. An experience with 156 patients attending a newly organized pain and palliative care clinic in a tertiary hospital. Indian J Cancer. 2012;49:293–7. doi: 10.4103/0019-509X.104491. [DOI] [PubMed] [Google Scholar]
- 16.Alshemmari S, Ezzat H, Samir Z, Sajnani K, Alsirafy S. Symptom burden in hospitalized patients with cancer in Kuwait and the need for palliative care. Am J Hosp Palliat Care. 2010;27:446–9. doi: 10.1177/1049909110362438. [DOI] [PubMed] [Google Scholar]
- 17.Al-Shahri MZ, Eldali AM, Al-Zahrani O. Nonpain symptoms of new and follow-up cancer patients attending a palliative care outpatient clinic in Saudi Arabia. Indian J Palliat Care. 2012;18:98–102. doi: 10.4103/0973-1075.100822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghosn M, Boutros C, Geara S, Kattan J, Nasr F, Chahine G. Experience with palliative care in patients with advanced cancer at a tertiary care hospital in a developing country. Support Care Cancer. 2011;19:573–5. doi: 10.1007/s00520-010-1056-x. [DOI] [PubMed] [Google Scholar]
- 19.Hoekstra J, Vernooij-Dassen MJ, de Vos R, Bindels PJ. The added value of assessing the 'most troublesome' symptom among patients with cancer in the palliative phase. Patient Educ Couns. 2007;65:223–9. doi: 10.1016/j.pec.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Nekolaichuk C, Watanabe S, Beaumont C. The Edmonton symptom assessment system: A 15-year retrospective review of validation studies (1991--2006) Palliat Med. 2008;22:111–22. doi: 10.1177/0269216307087659. [DOI] [PubMed] [Google Scholar]
- 21.Cheifetz O, Packham TL, Macdermid JC. Rasch analysis of the Edmonton symptom assessment system and research implications. Curr Oncol. 2014;21:e186–94. doi: 10.3747/co.21.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richardson LA, Jones GW. A review of the reliability and validity of the Edmonton symptom assessment system. Curr Oncol. 2009;16:55. doi: 10.3747/co.v16i1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Modonesi C, Scarpi E, Maltoni M, Derni S, Fabbri L, Martini F, et al. Impact of palliative care unit admission on symptom control evaluated by the Edmonton symptom assessment system. J Pain Symptom Manage. 2005;30:367–73. doi: 10.1016/j.jpainsymman.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Jeon S, Given CW, Sikorskii A, Given B. Do interference-based cut-points differentiate mild, moderate, and severe levels of 16 cancer-related symptoms over time? J Pain Symptom Manage. 2009;37:220–32. doi: 10.1016/j.jpainsymman.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reuben DB, Mor V. Nausea and vomiting in terminal cancer patients. Arch Intern Med. 1986;146:2021–3. [PubMed] [Google Scholar]
- 26.Coyle N, Adelhardt J, Foley KM, Portenoy RK. Character of terminal illness in the advanced cancer patient: Pain and other symptoms during the last four weeks of life. J Pain Symptom Manage. 1990;5:83–93. doi: 10.1016/s0885-3924(05)80021-1. [DOI] [PubMed] [Google Scholar]
- 27.Bruera E, Neumann C, Brenneis C, Quan H. Frequency of symptom distress and poor prognostic indicators in palliative cancer patients admitted to a tertiary palliative care unit, hospices, and acute care hospitals. J Palliat Care. 2000;16:16–21. [PubMed] [Google Scholar]
- 28.Seow H, Barbera L, Sutradhar R, Howell D, Dudgeon D, Atzema C, et al. Trajectory of performance status and symptom scores for patients with cancer during the last six months of life. J Clin Oncol. 2011;29:1151–8. doi: 10.1200/JCO.2010.30.7173. [DOI] [PubMed] [Google Scholar]
- 29.Sigurdardottir KR, Haugen DF. Prevalence of distressing symptoms in hospitalised patients on medical wards: A cross-sectional study. BMC Palliat Care. 2008;7:16. doi: 10.1186/1472-684X-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vainio A, Auvinen A. Prevalence of symptoms among patients with advanced cancer: An international collaborative study. Symptom prevalence group. J Pain Symptom Manage. 1996;12:3–10. doi: 10.1016/0885-3924(96)00042-5. [DOI] [PubMed] [Google Scholar]


