Abstract
Objective:
To monitor pain intensity, pain symptoms, and medication use in elderly with dementia.
Methods:
Nursing home residents above 65 years of age, diagnosed with dementia, and showing pain symptoms were included in the study. The patients’ mental status was monitored through a mini-mental state examination score and observations of pain symptoms using Part 1 of the Mobilization-Observation-Behaviour-Intensity-Dementia-2 (MOBID-2) pain scale. Community pharmacists reviewed the patients’ medication use, and the prescriptions were compared with guidelines for treatment of geriatric patients. Alterations to the patients’ medicine use were forwarded to the general practitioners.
Findings:
Sixty-one nursing home residents diagnosed with dementia were identified, 15 of these fulfilled the inclusion criteria, and 12 agreed to participate in the study. The mean age was 87 years of age (range: 77-96), and 42% of the residents were males. The patients’ overall pain intensity was 83% for observations on the numeric pain rating scale (NRS) >0 and 67% for NRS ≥3. Most painful were the situations in which the residents were to mobilize their legs, turn around to both sides of the bed, and when sitting on the bed. The medication reviews identified a total of 95 individual prescriptions, and 33% of these were for nervous system medications, followed by medicines for the treatment of alimentary tract and metabolism disorders (31% of total). Eleven prescriptions for pain medicine were identified; the majority of these were for paracetamol and opioids. Seventeen proposals to patients’ medication use were suggested, but the general practitioners accepted only 6% of these.
Conclusion:
This study indicates that the MOBID-2 pain scale in combination with medication reviews can be used as a tool for optimization of patients’ medication use. However, we recommend the conduction of a larger-scale study in multiple settings, to validate our results and the generalizability of the findings.
Keywords: Dementia, Denmark, elderly, medication review, nursing home residents, pain assessment, pain intensity, pain symptoms
INTRODUCTION
Dementia is a clinical syndrome which gradually leads to disturbances in the cognitive functions, and over time, patients will lose ability to express basic conditions and feelings such as pain and abstract thinking.[1,2] Persistent pain in the musculoskeletal system is a well-known problem in elderly, and the condition is associated with a progressive decrease in functional, mental and social capacity.[3,4] Furthermore, insufficient treatment of pain in dementia can lead to agitation, anxiety and externalizing behavioral.[5] Assessment of pain in dementia is challenging, as the patients may not be able to verbally express pain, or use less obvious indicators such as aggression and or agitation.[6,7] In elderly with a normal cognitive function, a numeric pain scale ranging can be used in pain assessment, but not in dementia, due to the uncertainty of the reliability of the patients’ ratings.[8] Therefore, previous studies examining pain assessment in cognitively impaired elderly patients have systematically eliminated noncommunicative and demented patients from participating in the trials.[9] The lack of proper pain assessment methods in dementia can results in underestimation of the pain prevalence, and lead to inadequate medical treatment.[6,7,8,9] The Mobilization-Observation-Behaviour-Intensity-Dementia-2 (MOBID-2) pain scale has been developed for pain assessment in elderly with dementia.[10] The scale is an extended two-part version of the nurse-administered MOBID pain scale, and the scale is structured around observations of the patients’ actual pain signs through simple physical exercises, and based on these observations an estimation of the patients’ overall pain score can be made.[10,11,12,13] The scale is developed for assessment of pain behavior occurring from the muscles and skeleton (Part 1) and internal organs (Part 2).[10] Hence, the scale has several limitations, as it can be difficult for the assessor to distinguishing between acute and chronic pain, and identify whether the pain symptoms are related to muscle pain, or to behavioral disturbances due to the patients’ dementia disease and other comorbidities.[11] Pharmacological treatment of pain in dementia is difficult, as the existence of multiple chronic diseases can lead to polypharmacy and the occurrence of serious adverse drug reactions.[6,14,15] Empirical studies have demonstrated that pharmaceutical interventions can contribute to the identification and prevention of drug-related problems in elderly.[16,17,18,19,20]
The aim of this study was to monitor pain intensity, pain symptoms, and medication use in elderly nursing home residents with dementia. The study was initiated as part of the project “medicine, pain and dementia” which were established in 2014 by the Health Department at Sonderborg Municipality, Denmark, to optimize the pain treatment of local citizens with dementia.
METHODS
The study was conducted in a Danish nursing home from February to June 2015. We included all residents’ living in the nursing home above 65 years of age diagnosed with dementia, and for whom the staff rated that they showed pain symptoms. The nursing home staffs’ pain ratings were based on their personal observations of the elderlies’ daily verbal expressions and/or facial signs over a period of 2 weeks. The residents who showed pain symptoms every day or second day was included in the study. Terminal patients and nursing home residents, evaluated by the nursing home staff as not having pain, were excluded from this study. Information about the patients’ dementia status was not available since this information was stored in the patients’ medical records. Verbal and written informed and presumed consent was obtained in direct conversation with the patient and his/her legal guardian, usually a family member, after explaining the aims of the study and its protocol. As required by Danish law the study was approved by the Data Protection Agency and the Danish Medicines Agency. All individual patient data are presented anonymously.
The included nursing home residents mental status were examined with the mini-mental state examination (MMSE) score.[21,22] The MMSE testing was conducted by questioning the residents whether they wanted to assist the interviewer, an experienced nurse, with a special task in the residential home. The MMSE test was carried out in the residents’ apartment or the common area without interrupting elements. If the citizens’ response time to the proposed questions was too long, the interviewer asked, whether the person was able to respond or not. If the resident did not respond the proposed question, it was skipped, and the next question introduced. During the introduction to the MMSE test, it was expressed to the interviewer on the instruction sheet, which he/she was not allowed to help the resident with the answers. Part 1 of the MOBID-2 pain scale was used for monitoring of pain intensity and symptoms in the individuals.[10,11] Trained nursing home staff assistants carried out the MOBID-2 exercises with the included patients. The first author, MMT, rated the patients’ reactions toward the exercises on the numeric scale range ranging from 0 to 10, where “10” is the worst possible pain, and “0” is no observed pain. In cases where the elderly could not complete the MOBID-2 exercises by themselves, they were assisted by the nursing home staff. If the patient expressed pain in connection with performing an exercise it was terminated immediately. The exercises were carried out anytime between 9 and 12 am, and the patients’ “facial expressions,” “cry of pain,” and “ward off reactions” were observed and rated on the numeric rating scale (NRS). The five exercises carried out in relation to the MOBID-2 pain scale was: (1) Open and close hands; (2) stretching of the arms over the head; (3) bending of ankles, knees, hips and thereafter stretching them again; (4) turn around to both sides of the bed; and (5) sitting up on the bed. The exercises were carried out with one arm or leg at a time. All exercises were performed twice for each included patient with at least 1-week break. To secure consistency between the pain intensity and symptoms, the same nurse repeated the MOBID-2 exercises for each patient, and the observations of the patients’ pain intensity were made by MMT. Physicians with a specialty within geriatrics and clinical pharmacology trained the nurses who carried out the MMSE and MOBID-2 testing of the included residents. The physicians’ pretraining of the included nurses and MMT was conducted in another nursing home including residents diagnosed with dementia as test persons.
Medication review was conducted for all included residents in collaboration with local community pharmacies, nursing home staff and MMT. Information about the patients’ general health status, sex, age, height, weight, present clinical symptoms, diagnoses, and relevant laboratory data were collected from the patients general practitioner, the nursing home record, and if possible also from the patient itself. The information was collected and recorded by the nursing home staff and entered into special data sheets that were forwarded to the pharmacists. During the medication review process national recommendation lists for conduction of medication reviews, as well as guidelines for prescribing of medicines in geriatric patients, product information, and other relevant information was consulted.[23,24] Information about the residents’ actual medication use was retrieved from the following sources: The national personal electronic medicine (PEM) profile, general practitioners health records, and data present in the nursing home and the pharmacy's record. In Denmark, data on individual medicine use has been collected in PEM, and in this register, all information about the citizens’ prescribed medicine and purchases of medicine through private pharmacies has been recorded.[25] The PEM provided information about citizens’ medicine use over the previous 2 years. The PEM is available through the Internet, and, therefore, it can be accessed in both the nursing home and in private pharmacies. Information about patients’ use of over-the-counter medicine and complementary medicine was collected from the nurse, the patient, and/or relatives. The results of the medication review were discussed with the nurse, and after the finalization of the medication reviews, the pharmacists’ alterations to the patient's medication use, were forwarded to the general practitioners. The physicians’ accept rate of the alterations were later recorded. In cases where the general practitioner did not respond to the letter from the pharmacist, and no follow-up was made due to the limited time resources. In this article, the identified medications were categorized and presented according to therapeutic groups.[26]
For each included nursing home resident, a datasheet, in which all information about health status, medication use, MMSE score, and MOBID-2 pain scale observations was made. The pharmacists’ suggestions for changes in medication use were also together noted with the physicians’ acceptance rate. Hence, according to the MOBID-2 pain scale, the change in pain intensity must be NRS ≥ 3 to safeguard against measurement errors,[13] and, therefore, the pain observations were grouped into two categories: NRS >0 and NRS ≥3. The mean and standard deviation of inferred pain intensity scores were calculated for each MOBID-2 item, as well as an overall pain score. All analyses were performed with SPSS Statistics version 13.0 (IBM, Denmark).
RESULTS
Of 61 nursing home residents diagnosed with dementia, 15 fulfilled the inclusion criteria, and of these, 12 residents agreed by themselves or through a relative, to participate in the study. Table 1 displays the characteristics of the 12 included residents. On average, the included patients were 87 years of age (range: 77-96), and 42% of these were male. A total number of 53 different medical diagnoses were reported for the included patients. The average number of diagnoses per patient was 4.4 (range 2-8), and the majority of these were mental and cardiovascular diseases. The residents’ average MMSE scores were 9.6 (range 0-21) [Table 1]. Table 2 displays the residents’ pain intensity and symptoms after the first and second application of the MOBID-2 pain scale. During the first application of the scale, all patients showed pain symptoms (NRS > 0), and 58.3% of patients had a pain score ≥ 3. In the second round, 83.3% of patients reported pain symptoms >0, and 67% of patients reported a pain score ≥3. When applying the MOBID-2 pain scale for the 1st time, the highest pain intensity were found for exercises involving arms (4.5), legs (5.4), turnover (5.1), and sitting up on the bedside (5). For the second round, the most painful situations were sitting up on the bedside (3.8) followed by arms and legs (3.7). Table 3 displays the overall pain intensity and the MMSE scores for all included patients. From the table, it can be concluded, that no direct relationship between MMSE scores and MOBID-2 pain evaluations was found. Table 4 displays the included residents’ identified medication use. Each person regularly used 7.8 different medications (range 3-14) corresponding to a total of 95 different pharmaceutical products for all 12 included patients. The largest share of drugs prescribed was nervous system medications (33% of total), followed by drugs from anatomical therapeutic chemical Group A (alimentary tract and metabolism) (31% of total). A total of 11 prescriptions for pain medicine were located. Of these, 7 were for paracetamol, 1 for fentanyl, 1 for buprenorphine, and 2 for tramadol. Six patients had access to rescue medicine, tramadol (n = 2), and paracetamol (n = 4). Seventeen different changes to the residents’ medicine use were suggested by the pharmacists, corresponding to an average of 1.42 proposals per resident [Table 5]. Only 6% of the proposals were later accepted.
Table 1.
Characteristics of participants (n=12)
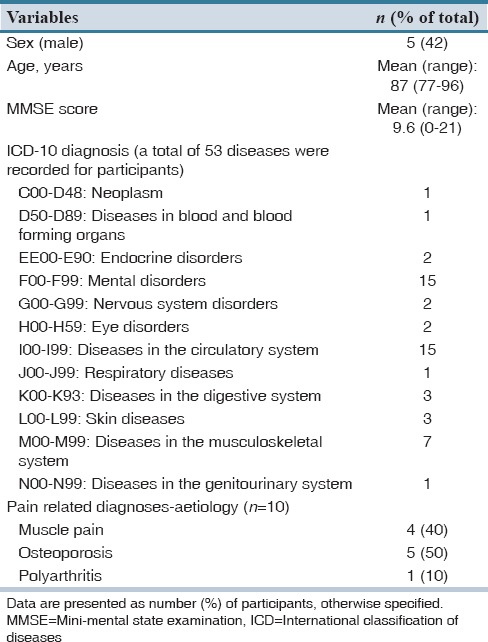
Table 2.
Pain prevalence (presented as percentage of patients) by Mobilization-Observation-Behaviour-Intensity-Dementia-2 Part 1 items, based on average test data
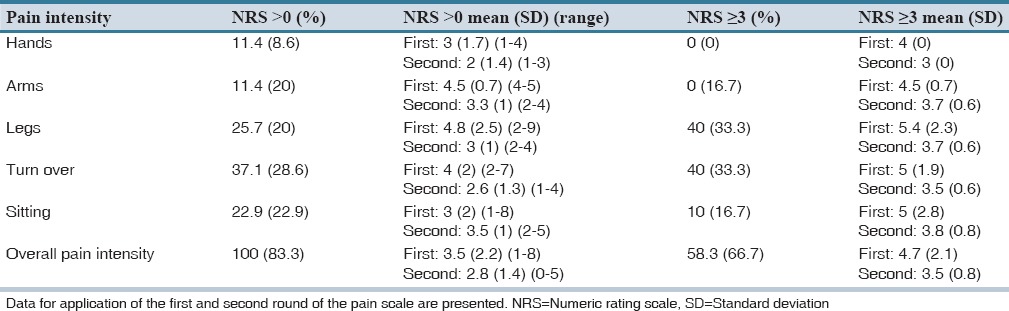
Table 3.
Distribution of pain intensity (overall Mobilization-Observation-Behaviour-Intensity-Dementia-2 pain scale) and cognitive function (mini-mental state examination score) for each included patient
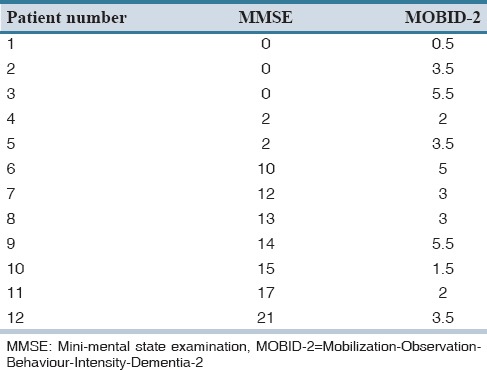
Table 4.
Distribution of number prescribed medications by therapeutic groups and patients
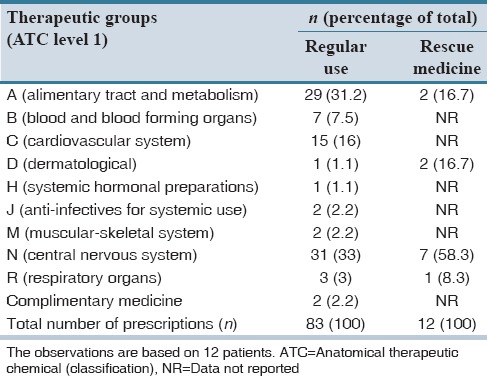
Table 5.
Distribution of proposals of medication alterations by category

DISCUSSION
This is the first study, which has combined pain monitoring of elderly with medication reviews with the purpose to optimize the patients’ medical treatment. The measured pain intensity and symptoms were in line with results found in another empirical study measuring pain in 77 patients diagnosed with dementia.[11] The pharmacists identified several proposals for changes in the patient's medication use, but only few of these changes were related to patients’ use of pain medicine. This finding could indicate that the residents were poorly diagnosed and managed for their pain therapy, and therefore only few recommendations related to patients’ use of pain medicine were made. The majority of pharmacists’ alterations were proposed due to lack of information about the indication for use. Lack of information about the actual indication for prescribed medicine is a common problem in dementia, and this finding has also been identified in other studies.[16,17,18,19,20] Physicians accept rate of the pharmacist’ proposals were surprisingly low. In a literature review, the physicians’ average acceptance rate of pharmaceutical interventions was estimated as being 69% (range 39-100%).[27] The low response rate could probably be explained by forgetfulness, lack of time, and/or limited prestudy communication between the general practitioners, the community pharmacists, and the nursing home. Hence, community pharmacists are, compared to hospital pharmacists, not specially trained in conducting medication reviews in patients with complicated diseases such as dementia, and therefore, many of the proposals may have been of minor clinical significance, and therefore not accepted, or even considered by the general practitioner.[28] On the other hand, the general practitioners may have not been educated in prescribing of medicines in dementia, and therefore, the patients’ medication use could have been more qualified.
In this study, only part one of the MOBID-2 pain scale was applied, and therefore, the measured pain intensity and symptoms only represents individual observations of the nursing home staff and MMT, and not pain symptoms from the organs. During the exercises, the residents’ could have made strange facial expressions related to the physical difficulties in carrying out the exercises and not because of pain, which may have overestimated the patients’ pain intensity. Another weakness of this study was that we did not have an individual graduation of the dementia status of the included residents. However, no direct link between the MMSE and MOBID-2 pain scale can be found. Therefore, a person with a low MMSE score can have difficulties in expressing pain and vice versa. With the MOBID-2 pain scale the distinction between acute and chronic pain is difficult, and therefore, pain monitoring in elderly with a light degree of dementia could preferably be replaced or supplemented by other pain scales, for example, the visual analog scale. Another limitation to this study is the low sample size, however problems with recruiting this patient type for clinical research were also observed in similar studies.[9,14,16] The prevalence of dementia in Denmark is approximately 7% among 65-84 years old, and, therefore, the study sample was not representative of the disease prevalence.[29] The study was designed as a pilot study including only one nursing home, and therefore, it was not the aim of the study to include a sample size matching the disease prevalence. Considering the number of items in MMSE scale and in MOBID-2, the included sample size was not sufficient to detect clinically minimal important differences of cognitive scales and or the pain scale. In addition, the testing of the residents was done by nursing home staff with minor experiences in using the scales, and, therefore, the validity of the individual MMSE and MOBID-2 ratings was further biased.
This study indicates that the MOBID-2 pain scale in combination with medication reviews can be used as a tool for optimization of patients’ medication use. However, we recommend the conduction of a larger-scale study in multiple settings, to validate our results and generalizability of the findings.
AUTHORS’ CONTRIBUTION
MMT and LA designed the study, analyzed data and wrote the first version of the manuscript. MMT and MGW carried out the data sampling and handling. All authors saw and approved the final version of the manuscript. No sources of funding were used to assist in the preparation of this study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to thank the staff at Moelleparken nursing home, Sonderborg for assistance with the data collection, and pharmacists from local pharmacies in Graasten and Sonderborg for assistance with the medication reviews. Finally, we also thank Ph.D. (Pharm.) Trine Andresen and Professor, DMSci, Professor Anette Bygum for scientific support during the project period, and for critical reviews of previous versions of this manuscript.
REFERENCES
- 1.Achterberg WP, Pieper MJ, van Dalen-Kok AH, de Waal MW, Husebo BS, Lautenbacher S, et al. Pain management in patients with dementia. Clin Interv Aging. 2013;8:1471–82. doi: 10.2147/CIA.S36739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hadjistavropoulos T, Herr K, Prkachin KM, Craig KD, Gibson SJ, Lukas A, et al. Pain assessment in elderly adults with dementia. Lancet Neurol. 2014;13:1216–27. doi: 10.1016/S1474-4422(14)70103-6. [DOI] [PubMed] [Google Scholar]
- 3.Reynolds KS, Hanson LC, DeVellis RF, Henderson M, Steinhauser KE. Disparities in pain management between cognitively intact and cognitively impaired nursing home residents. J Pain Symptom Manage. 2008;35:388–96. doi: 10.1016/j.jpainsymman.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Weiner D, Peterson B, Keefe F. Chronic pain-associated behaviors in the nursing home: Resident versus caregiver perceptions. Pain. 1999;80:577–88. doi: 10.1016/S0304-3959(98)00249-8. [DOI] [PubMed] [Google Scholar]
- 5.Nygaard HA, Jarland M. Are nursing home patients with dementia diagnosis at increased risk for inadequate pain treatment? Int J Geriatr Psychiatry. 2005;20:730–7. doi: 10.1002/gps.1350. [DOI] [PubMed] [Google Scholar]
- 6.Ferrell B, Argoff C, Epplin J. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc. 2009;57:1331–46. doi: 10.1111/j.1532-5415.2009.02376.x. [DOI] [PubMed] [Google Scholar]
- 7.Corbett A, Husebo B, Malcangio M, Staniland A, Cohen-Mansfield J, Aarsland D, et al. Assessment and treatment of pain in people with dementia. Nat Rev Neurol. 2012;8:264–74. doi: 10.1038/nrneurol.2012.53. [DOI] [PubMed] [Google Scholar]
- 8.Lichtner V, Dowding D, Esterhuizen P, Closs SJ, Long AF, Corbett A, et al. Pain assessment for people with dementia: A systematic review of systematic reviews of pain assessment tools. BMC Geriatr. 2014;14:138. doi: 10.1186/1471-2318-14-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inelmen EM, Mosele M, Sergi G, Toffanello ED, Coin A, Manzato E. Chronic pain in the elderly with advanced dementia. Are we doing our best for their suffering? Aging Clin Exp Res. 2012;24:207–12. doi: 10.3275/8020. [DOI] [PubMed] [Google Scholar]
- 10.Husebo BS, Strand LI, Moe-Nilssen R, Husebo SB, Snow AL, Ljunggren AE. Mobilization-Observation- Behavior-Intensity-Dementia Pain Scale (MOBID): Development and validation of a nurse-administered pain assessment tool for use in dementia. J Pain Symptom Manage. 2007;34:67–80. doi: 10.1016/j.jpainsymman.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 11.Husebo BS, Strand LI, Moe-Nilssen R, Husebo SB, Ljunggren AE. Pain behaviour and pain intensity in older persons with severe dementia: Reliability of the MOBID Pain Scale by video uptake. Scand J Caring Sci. 2009;23:180–9. doi: 10.1111/j.1471-6712.2008.00606.x. [DOI] [PubMed] [Google Scholar]
- 12.Husebo BS, Strand LI, Moe-Nilssen R, Husebo SB, Ljunggren AE. Pain in older persons with severe dementia. Psychometric properties of the Mobilization-Observation-Behaviour-Intensity-Dementia (MOBID-2) Pain Scale in a clinical setting. Scand J Caring Sci. 2010;24:380–91. doi: 10.1111/j.1471-6712.2009.00710.x. [DOI] [PubMed] [Google Scholar]
- 13.Husebo BS, Ostelo R, Strand LI. The MOBID-2 Pain Scale: Reliability and responsiveness to pain in patients with dementia. Eur J Pain (London, England) 2014;18:1–12. doi: 10.1002/ejp.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reeve E, Bell JS, Hilmer SN. Barriers to optimising prescribing and deprescribing in older adults with dementia: A narrative review. Curr Clin Pharmacol. 2015;10:168–77. doi: 10.2174/157488471003150820150330. [DOI] [PubMed] [Google Scholar]
- 15.Spinewine A, Fialová D, Byrne S. The role of the pharmacist in optimizing pharmacotherapy in older people. Drugs Aging. 2012;29:495–510. doi: 10.2165/11631720-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 16.Silva C, Ramalho C, Luz I, Monteiro J, Fresco P. Drug-related problems in institutionalized, polymedicated elderly patients: Opportunities for pharmacist intervention. Int J Clin Pharm. 2015;37:327–34. doi: 10.1007/s11096-014-0063-2. [DOI] [PubMed] [Google Scholar]
- 17.Wouters H, Quik EH, Boersma F, Nygård P, Bosman J, Böttger WM, et al. Discontinuing inappropriate medication in nursing home residents (DIM-NHR Study): Protocol of a cluster randomised controlled trial. BMJ Open. 2014;4:e006082. doi: 10.1136/bmjopen-2014-006082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanlon JT, Weinberger M, Samsa GP, Schmader KE, Uttech KM, Lewis IK, et al. A randomized, controlled trial of a clinical pharmacist intervention to improve inappropriate prescribing in elderly outpatients with polypharmacy. Am J Med. 1996;100:428–37. doi: 10.1016/S0002-9343(97)89519-8. [DOI] [PubMed] [Google Scholar]
- 19.Hanlon JT, Lindblad CI, Gray SL. Can clinical pharmacy services have a positive impact on drug-related problems and health outcomes in community-based older adults? Am J Geriatr Pharmacother. 2004;2:3–13. doi: 10.1016/s1543-5946(04)90002-5. [DOI] [PubMed] [Google Scholar]
- 20.Furniss L, Burns A, Craig SK, Scobie S, Cooke J, Faragher B. Effects of a pharmacist's medication review in nursing homes. Randomised controlled trial. Br J Psychiatry. 2000;176:563–7. doi: 10.1192/bjp.176.6.563. [DOI] [PubMed] [Google Scholar]
- 21.Burns A, Karim S, Morris J, Byrne J. The conversational mini-mental state examination: Estimating cognitive impairment. Aging Ment Health. 2010;14:692–4. doi: 10.1080/13607860902845566. [DOI] [PubMed] [Google Scholar]
- 22.Kørner EA, Lauritzen L, Nilsson FM, Wang A, Christensen P, Lolk A. Mini mental state examination. Validation of new Danish version. Ugeskr Laeger. 2008;170:745–9. [PubMed] [Google Scholar]
- 23.Danish National Recommendation List. [Last accessed on 2015 Nov 16]. Available from: http://www.irf.dk/dk/rekommandationsliste/oversigt/IRF .
- 24.Makris UE, Abrams RC, Gurland B, Reid MC. Management of persistent pain in the older patient: A clinical review. JAMA. 2014;312:825–36. doi: 10.1001/jama.2014.9405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henriksen JP, Noerregaard S, Buck TC, Aagaard L. Medication histories by pharmacy technicians and physicians in an emergency department. Int J Clin Pharm. 2015;37:1121–7. doi: 10.1007/s11096-015-0172-6. [DOI] [PubMed] [Google Scholar]
- 26.Drug Statistics Methodology. ATC/DDD Index, WHO Collaborating Centre for Drug Statistics, Norway. 2015. [Last accessed on 2015 Nov 16]. Available from: http://www.whocc.no/atc_ddd_index/
- 27.Graabaek T, Kjeldsen LJ. Medication reviews by clinical pharmacists at hospitals lead to improved patient outcomes: A systematic review. Basic Clin Pharmacol Toxicol. 2013;112:359–73. doi: 10.1111/bcpt.12062. [DOI] [PubMed] [Google Scholar]
- 28.Barry HE, Parsons C, Passmore AP, Hughes CM. Community pharmacists and people with dementia: A cross-sectional survey exploring experiences, attitudes, and knowledge of pain and its management. Int J Geriatr Psychiatry. 2013;28:1077–85. doi: 10.1002/gps.3931. [DOI] [PubMed] [Google Scholar]
- 29.Andersen K, Nielsen H, Lolk A, Andersen J, Becker I, Kragh-Sørensen P. Incidence of very mild to severe dementia and Alzheimer's disease in Denmark: The Odense Study. Neurology. 1999;52:85–90. doi: 10.1212/wnl.52.1.85. [DOI] [PubMed] [Google Scholar]


