Abstract
Objective:
Effects of ascorbic acid on hemodynamic parameters of septic shock were evaluated in nonsurgical critically ill patients in limited previous studies. In this study, the effect of high-dose ascorbic acid on vasopressor drug requirement was evaluated in surgical critically ill patients with septic shock.
Methods:
Patients with septic shock who required a vasopressor drug to maintain mean arterial pressure >65 mmHg were assigned to receive either 25 mg/kg intravenous ascorbic acid every 6 h or matching placebo for 72 h. Vasopressor dose and duration were considered as the primary outcomes. Duration of Intensive Care Unit (ICU) stay and 28-day mortality has been defined as secondary outcomes.
Findings:
During the study period, 28 patients (14 in each group) completed the trial. Mean dose of norepinephrine during the study period (7.44 ± 3.65 vs. 13.79 ± 6.48 mcg/min, P = 0.004) and duration of norepinephrine administration (49.64 ± 25.67 vs. 71.57 ± 1.60 h, P = 0.007) were significantly lower in the ascorbic acid than the placebo group. No statistically significant difference was detected between the groups regarding the length of ICU stay. However, 28-day mortality was significantly lower in the ascorbic acid than the placebo group (14.28% vs. 64.28%, respectively; P = 0.009).
Conclusion:
High-dose ascorbic acid may be considered as an effective and safe adjuvant therapy in surgical critically ill patients with septic shock. The most effective dose of ascorbic acid and the best time for its administration should be determined in future studies.
Keywords: Ascorbic acid, norepinephrine, sepsis, shock, vasopressor
INTRODUCTION
Sepsis is a life-threatening systemic inflammatory response that can result in multi-organ dysfunction and intractable hypotension.[1] In critically ill patients, severe sepsis and septic shock are known as the main causes of mortality.[2] Adequate fluid resuscitation, infection control, cardiovascular and respiratory supports are recommended for the management of severe sepsis and septic shock.[3] Septic shock usually does not response to intravenous fluid resuscitation and most patients need a vasopressor drug administration.[1,4,5] Concomitant vasodilatation and reduced vascular response to the vasopressors have been detected in septic shock.[6,7] Several mechanisms such as adrenal insufficiency, endothelial damage, oxidative stress, and depletion of catecholamine storage are proposed for vascular hyporesponsiveness to vasopressors in critically ill patients with septic shock.[8,9,10,11]
Ascorbic acid (Vitamin C) is an essential nutrient with potent antioxidant properties.[12,13] Ascorbic acid deficiency defined as serum ascorbic acid level <23 μmol/L has been reported in critically ill patients with acute respiratory infections and sepsis.[14,15,16] High-dose ascorbic acid improved edema and respiratory function in critically ill patients with severe burn injury and decreased organ failure and duration of Intensive Care Unit (ICU) stay in patients after major surgery.[17,18]
Effects of ascorbic acid on hemodynamic parameters were evaluated in nonsurgical critically ill patients in limited previous studies. High-dose ascorbic acid in patients with burn injury decreased fluid and vasopressors’ requirements. Also, in patients with severe sepsis, high-dose intravenous ascorbic acid improved hemodynamic parameters.[15,17,19,20] Potential benefit of ascorbic acid in surgical critically ill patients with diagnosis of severe sepsis or septic shock has not yet been evaluated. In this study, the effects of high-dose ascorbic acid on vasopressor requirement were evaluated in surgical critically ill patients with septic shock.
METHODS
This double-blinded randomized clinical trial was conducted in an ICU of Imam Khomeini Hospital, a referral general hospital affiliated to Tehran University of Medical Sciences, Tehran, Iran. This ICU has 18 beds and most of the beds are usually allocated for postoperative patients. The researchers, ICU nurses, and physicians were blinded regarding the intervention and the product (ascorbic acid or placebo) was prepared by Hospital Pharmacy Department. During the study period, from September 2014 to January 2016, 28 adult (18-65-year-old) surgical critically ill patients with diagnosis of septic shock who needed a vasopressor drug to maintain mean arterial pressure (MAP) >65 mmHg despite adequate fluid resuscitation were recruited. The sample size of the study was considered based on the ICU patients’ turn-over and number of patients who were hospitalized with a diagnosis of septic shock during the same period in previous years.
Septic shock was defined based on the Surviving Sepsis Campaign and patients with the following criteria: (1) Presence of a systemic inflammatory response: (fever: T >38°C or hypothermia: T <36°C, heart rate >90 beats/min, leukocytosis or leukopenia (white blood cell >12,000/μL and <4000/μL, respectively, or >10% band forms), (2) suspected or proven infection, (3) presence of sepsis-induced organ dysfunction (arterial hypoxemia [PaO2 /FiO2 <300], systolic blood pressure [SBP] <90 mmHg, or SBP decrease >40 mmHg unexplained by other causes), urine output <0.5 ml/kg/h for >6 h despite fluid resuscitation, platelet count <100,000/μL, acutely developing coagulopathy (international normalized ratio >1.5), serum bilirubin concentration >2 mg/dL were considered for enrollment[3] Concomitant use of other antioxidants (such as Vitamin E, selenium, and N-acetylcysteine), corticosteroids administration, any contraindication for high-dose ascorbic acid including bilateral ureteric obstruction, chronic hemodialysis, iron overload, oxalate stone formers,[21] hemochromatosis, and glucose-6-phosphate dehydrogenase deficiency[22] were defined as exclusions.
Ethical Committee of the hospital approved the study protocol and the patients or one of their first-degree family members signed the study's informed consent form.
Treatment of septic shock in the ICU was based on the Surviving Sepsis Campaign recommendations.[3] Fluid resuscitation strategy was the administration of a crystalloid solution (0.9% saline) to maintain central venous pressure >12 mmHg. In patients who had MAP <65 mmHg despite adequate fluid administration for 6 h, a vasopressor drug (norepinephrine) was started. Norepinephrine was started with a dose of 5 mcg/min and was titrated based on the patient's hemodynamic status, up to 30 mcg/min. Antibiotic regimens were guided based on the hospital recommendations. Stress ulcer prophylaxis (pantoprazole 40 mg daily as intravenous injection) and deep venous thrombosis prophylaxis (heparin 5000 IU every 8 h as subcutaneous injection) were considered for all patients. Analgosedation (fentanyl as continuous intravenous infusion) was the sedation protocol in our center.
The patients were included in ascorbic acid or placebo group according to the permuted block randomization. The randomization scheme consisted of seven blocks and each block contained four patients in random order. Patients in the ascorbic acid group received 25 mg/kg intravenous ascorbic acid every 6 h for 72 h. The dose and duration of intervention were selected based on the literature review.[15,17,19,20] Each dose of ascorbic acid was diluted in 50 ml of dextrose 5% solution and was administered as intravenous infusion over 30 min. Patients in the placebo group received 50 ml of dextrose 5% solution as intravenous infusion over 30 min.
The patients’ demographic data (including age, sex, baseline diseases, and causes of ICU admission) were extracted from their medical charts, and the patients’ clinical characteristics (such as vital signs and hemodynamic parameters) were monitored as daily interval. Laboratory data such as serum electrolyte concentrations, blood urea nitrogen (BUN), and serum creatinine levels were extracted from the patients’ medical charts. The patients’ Acute Physiology and Chronic Health Evaluation II (APACHE II) and sequential organ failure assessment (SOFA) scores were calculated at the time of recruitment.
Change in the hemodynamic parameters (MAP and pulse rate), oxygenation status (respiratory rate, oxygen saturation, duration of mechanical ventilation, pH, and PaO2 /FiO2 ratio), fluid intake, renal function (BUN, serum creatinine, and urine output), and serum electrolytes during the study period were compared between the groups.
Vasopressor dose and duration were evaluated on a daily basis for 3 days and were considered as the primary outcomes. Duration of ICU stay and 28-day mortality has been defined as secondary outcomes.
During the study period, patients were followed for any ascorbic acid-related adverse effects including nausea, vomiting, abdominal pain, hematuria, flushing, and significant arterial blood pressure change.
Data were analyzed using SPSS software version 17 (SPSS Inc., Chicago, USA). The Kolmogorov-Smirnov test was used to assess the normal distribution of continuous variables. Normally distributed continuous data were expressed as mean ± standard deviation. Categorical variables were reported as percentages. Chi-square or Fisher's exact test was used for comparing the categorical variables between the groups. Continuous variables were compared by the independent t-test. Mortality rate was compared between the groups using Chi-square test. Changes in the hemodynamic, oxygenation, and electrolyte parameters during the study period were assessed using one-way analysis of variance with repeated measure. P < 0.05 was defined as statistically significant.
RESULTS
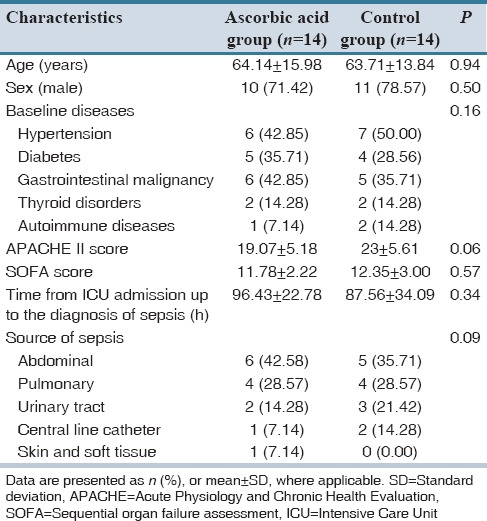
During the study period, 28 patients (14 in each group) were recruited. No patient was excluded during 3-day intervention. Demographic and clinical characteristics of the recruited patients are shown in Table 1. From the participants, 71.42% and 78.57% in the ascorbic acid and control groups were male, respectively. Included subjects were admitted to the ICU following gastrointestinal surgeries. No statistically significant difference was detected between the groups in terms of demographic data and clinical characteristics. Severity of illnesses based on the SOFA and APACHE II scores was comparable between the groups at the time of enrollment.
Table 1.
Demographic data of the patients
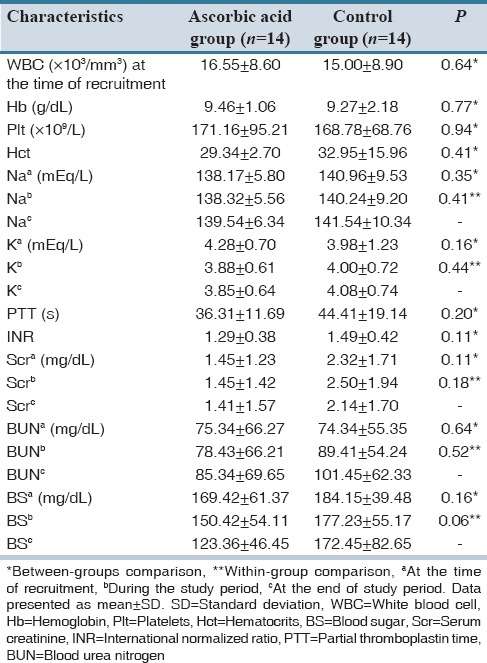
Hemodynamic parameters and laboratory tests did not differ significantly between the groups. In addition, changes in these parameters during the study period were not statistically significant. Data regarding these parameters are shown in [Tables 2 and 3].
Table 2.
Hemodynamic parameters and oxygenation status of the patients
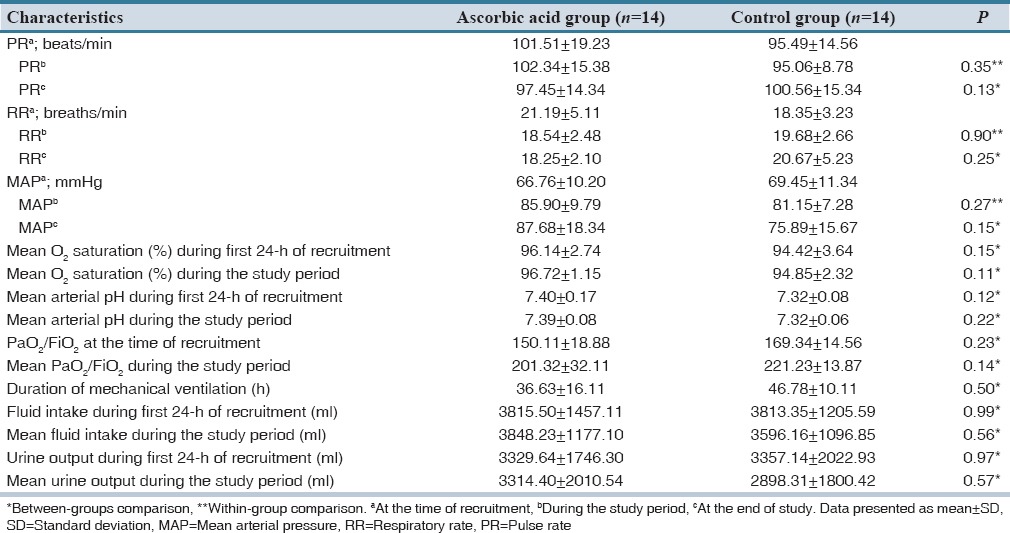
Table 3.
Laboratory parameters of the patients
Mean dose of vasopressor (norepinephrine) during the study period (7.44 ± 3.65 vs. 13.79 ± 6.48 mcg/min, P = 0.004), mean dose of norepinephrine in the first 24 h of enrollment (6.51 ± 3.53 vs. 12.58 ± 5.99 mcg/min, P = 0.003), total dose of norepinephrine in the first 24 h (156.42 ± 84.81 vs. 302.14 ± 143.85 mcg, P = 0.003), and duration of receiving norepinephrine (49.64 ± 25.67 vs. 71.57 ± 1.60 h, P = 0.007) were significantly lower in the ascorbic acid than the placebo group [Table 4].
Table 4.
Primary and secondary outcomes of the study in ascorbic and placebo groups
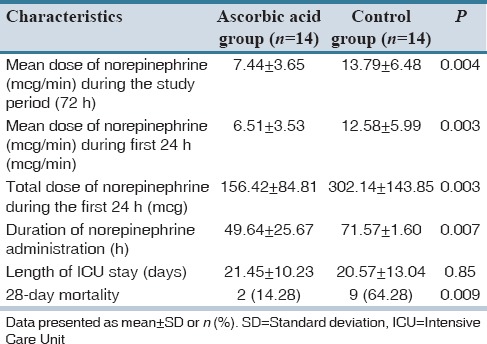
No statistically significant difference was detected between the groups regarding the length of ICU stay. However, the 28-day mortality was significantly lower in the ascorbic acid than the placebo group (14.28% vs. 64.28%, respectively; P = 0.009).
No ascorbic acid-related adverse events were identified in the treatment group during the study.
DISCUSSION
In this study, administration of high-dose ascorbic acid significantly decreased the requirement for vasopressor's dose and duration in surgical critically ill patients with septic shock. Several mechanisms including anti-oxidant, anti-inflammatory, nitric oxide (NO) synthase inhibitory, reversing vascular hyporesponsiveness to vasopressors, increasing catecholamines and cortisol synthesis in adrenal medulla, and improving vascular endothelium integrity properties may justified the role of ascorbic acid in septic shock.[8,9,10,11,15,18,23,24]
Severe ascorbic acid deficiency (serum ascorbate level <27 μmol/L) was reported in critically ill patients.[15,16,25,26,27] Up to 3000 mg/day, ascorbic acid was needed to normalize the plasma level of ascorbic acid (68 μmol/L) in these patients.[28] Ascorbic acid deficiency was not prevented by the use of parenteral nutrition containing ascorbic acid in critically ill patients and most patients required high dose of this nutrient.[29,30,31,32]
Catecholamines (dopamine, norepinephrine, and epinephrine) are produced by sympathetic nervous system and adrenal medulla.[33] Brain and adrenal glands have the highest levels of ascorbic acid in the body.[34] In catecholamine synthesis pathway, ascorbic acid is an essential cofactor for the copper-containing enzyme; dopamine β-hydroxylase.[35,36] This enzyme has a critical role in norepinephrine synthesis from dopamine. Ascorbic acid deficiency was associated with decreased level of norepinephrine, especially in the adrenal glands.[37,38,39] Insufficiency in adrenal hormone synthesis has also been observed in critically ill patients.[40,41]
Vasopressin is a peptide hormone that is synthesized form a preprohormone in the hypothalamus and stored in the posterior pituitary gland.[42] It is secreted following blood volume and arterial pressure dropping or plasma osmolality raising, attaches to vascular smooth muscle cells (AVPR1a), and kidney-collecting ducts (AVPR2), receptors, and consequently induces vasoconstriction and water retention.[43] A significant increase in the vasopressin level was detected during the initial phase of septic shock. However, in the later phase, it decreased dramatically.[6,44] The decrease in circulating vasopressin level is due to the depletion of pituitary storage and its synthesis.[7] Peptidylglycine α-amidating monooxygenase, a copper-containing enzyme with ascorbic acid cofactor, is essential for endogenous synthesis of vasopressin.[45] Decreasing activity of this enzyme during sepsis period may limit vasopressin synthesis.[7,44,46] Correlation between ascorbic acid deficiency and vasopressin insufficiency has been reported in critically ill patients.[26] Administration of ascorbic acid enhanced the circulating level of vasopressin.[47]
Septic shock is characterized by decreased peripheral vascular resistance and impaired blood and oxygen supply to the body's vital organs. A combination of vasodilatation and pronounced decrease in vascular response to vasopressor including high-dose norepinephrine often results in resistant hypotension.[6,46] Overproduction of NO appears to be an important mechanism in septic shock, and inhibition of NO synthase might provide a novel therapeutic approach. In 1991, Petros et al.[8] assessed the effects of NO synthase inhibitors in two patients with life-threatening and unresponsive septic shock. NG monomethyl-L-arginine (L-NMMA) caused dose-dependent increase in blood pressure and systemic vascular resistance in both patients, and a similar effect was observed in the second patient after treatment with NG-nitro-L-arginine methyl ester. In another study, L-NMMA increased vascular tone and raised blood pressure in 12 patients with septic shock.[9] Furthermore, methylene blue as a guanylate cyclase and NO synthase inhibitor increased arterial blood pressure and improved cardiac function in septic patients.[10,11]
Intravenous ascorbic acid improved cardiovascular functioning and decreased requirement for catecholamine administration in a patient with septic shock.[25] In another case with typical symptoms of scurvy and fluid-resistant severe orthostatic hypotension, intravenous ascorbic acid improved the patients’ hemodynamic status within 24 h after its administration.
Inflammatory cascades and release of inflammatory cytokines are from known pathways in the pathogenesis of sepsis.[26] Administration of high-dose intravenous ascorbic acid to severely affected patients with burn injury decreased the fluid and vasopressors requirements.[17,48] In a recent phase I, randomized controlled trial in 24 patients with severe sepsis, intravenous infusions of high-dose ascorbic acid (200 mg/kg/24 h) caused rapid reduction in SOFA score and improvement in hemodynamic parameters. Vascular endothelial repair following reduction in the proinflammatory biomarkers including C-reactive protein and procalcitonin as well as reduction of thrombomodulin level was suggested for ascorbic acid.[15,49]
Anti-oxidant property may be responsible for some beneficial effects of ascorbic acid in severe sepsis and septic shock. Early administration of antioxidants; α-tocopherol, 1000 IU every 8 h and ascorbic acid 1000 mg intravenously in 100 ml D5W every 8 h for a short duration, reduced the incidence of organ failure and ICU length of stay in critically ill surgical patients.[18] Administration of Vitamin C augmented the inotropic response to dobutamine in human subjects who were selected for elective diagnostic heart catheterization. Redox-regulated pathways of adrenergic system and ventricular contractility may be responsible for this effect of ascorbic acid.[23]
Corticosteroids have an important position in preventing beta adrenergic receptors’ down-regulation and maintaining vasopressors’ responsiveness of the vascular bed in septic shock.[11] Cortisol-sparing effect of ascorbic acid has been reported. Vitamin C significantly prevented etomidate-induced adrenal insufficiency and serum cortisol depletion.[24]
Ascorbic acid is a safe anti-oxidant nutrient and no any considerable adverse event has been reported yet, even after high-dose administration. In Nathens et al. study, critically ill surgical patients tolerated ascorbic acid, 1 g every 8 h for 28 days, without any adverse effect.[18] In addition, no adverse event was detected in critically ill patients with burn injury who received ascorbic acid with a dose of 66 mg/kg/h for 24 h.[17] Aggressive repletion of plasma ascorbic acid level was safe in patients with severe sepsis. No patient with the low (50 mg/kg/24 h) or high (200 mg/kg/24 h) dose of ascorbic acid experienced any identifiable ascorbic acid-related adverse event.[15] Similar to previous studies, we did not detect any ascorbic acid-related adverse events in the included patients.
Fluid resuscitation, vasopressor therapy, and ventilation supports are cornerstones of severe sepsis and septic shock management. Although oxygenation status, duration of mechanical ventilation, and hemodynamic parameters were comparable between the two groups in our study, for targeting these points, patients in the placebo group needed higher dose and longer duration of vasopressor therapy.
Although the results of our study are promising, the study suffers from some limitations. Small sample size, short period of intervention, and no assessment of the patients’ serum ascorbate baseline level are the major limitations of our study. Further randomized controlled trials with sufficient sample size and assessment of baseline serum ascorbate level, serum antioxidant capacity, and proinflammatory cytokines may be considered in future studies.
Our results suggested that high-dose of ascorbic acid (25 mg/kg intravenously every 6 h for 72 h) with its probable anti-oxidant, anti-inflammatory, cortisol sparing, NO synthase inhibitory and increasing catecholamines synthesis in the brain, and adrenal medulla properties may be considered as an effective and safe adjuvant therapy in critically ill surgical patients with septic shock. However, as limited evidences are now available, the most effective dose of ascorbic acid and the best time for its administration should be determined in future studies.
AUTHORS’ CONTRIBUTION
MH: Data gathering and primary drafting of the manuscript, MM: Clinical evaluation of the patients, MR: Controlling of the patients’ medications and hemodynamic assessment, HK: Designing of the study and editing the manuscript.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
This study was the result of a Pharm.D student thesis. The authors expressed sincere gratitude to all the nursing staff of general ICU of Imam Khomeini Hospital for their kind support.
REFERENCES
- 1.Remick DG. Pathophysiology of sepsis. Am J Pathol. 2007;170:1435–44. doi: 10.2353/ajpath.2007.060872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: A trend analysis from 1993 to 2003. Crit Care Med. 2007;35:1244–50. doi: 10.1097/01.CCM.0000261890.41311.E9. [DOI] [PubMed] [Google Scholar]
- 3.Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:840–51. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 5.Vasu TS, Cavallazzi R, Hirani A, Kaplan G, Leiby B, Marik PE. Norepinephrine or dopamine for septic shock: Systematic review of randomized clinical trials. J Intensive Care Med. 2012;27:172–8. doi: 10.1177/0885066610396312. [DOI] [PubMed] [Google Scholar]
- 6.Landry DW, Levin HR, Gallant EM, Seo S, D’Alessandro D, Oz MC, et al. Vasopressin pressor hypersensitivity in vasodilatory septic shock. Crit Care Med. 1997;25:1279–82. doi: 10.1097/00003246-199708000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Sharshar T, Carlier R, Blanchard A, Feydy A, Gray F, Paillard M, et al. Depletion of neurohypophyseal content of vasopressin in septic shock. Crit Care Med. 2002;30:497–500. doi: 10.1097/00003246-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Petros A, Bennett D, Vallance P. Effect of nitric oxide synthase inhibitors on hypotension in patients with septic shock. Lancet. 1991;338:1557–8. doi: 10.1016/0140-6736(91)92376-d. [DOI] [PubMed] [Google Scholar]
- 9.Petros A, Lamb G, Leone A, Moncada S, Bennett D, Vallance P. Effects of a nitric oxide synthase inhibitor in humans with septic shock. Cardiovasc Res. 1994;28:34–9. doi: 10.1093/cvr/28.1.34. [DOI] [PubMed] [Google Scholar]
- 10.Preiser JC, Lejeune P, Roman A, Carlier E, De Backer D, Leeman M, et al. Methylene blue administration in septic shock: A clinical trial. Crit Care Med. 1995;23:259–64. doi: 10.1097/00003246-199502000-00010. [DOI] [PubMed] [Google Scholar]
- 11.O’Brien A, Clapp L, Singer M. Terlipressin for norepinephrine-resistant septic shock. Lancet. 2002;359:1209–10. doi: 10.1016/S0140-6736(02)08225-9. [DOI] [PubMed] [Google Scholar]
- 12.Carr A, Frei B. Does Vitamin C act as a pro-oxidant under physiological conditions? FASEB J. 1999;13:1007–24. doi: 10.1096/fasebj.13.9.1007. [DOI] [PubMed] [Google Scholar]
- 13.Carr AC, Frei B. Toward a new recommended dietary allowance for Vitamin C based on antioxidant and health effects in humans. Am J Clin Nutr. 1999;69:1086–107. doi: 10.1093/ajcn/69.6.1086. [DOI] [PubMed] [Google Scholar]
- 14.Hunt C, Chakravorty NK, Annan G, Habibzadeh N, Schorah CJ. The clinical effects of Vitamin C supplementation in elderly hospitalised patients with acute respiratory infections. Int J Vitam Nutr Res. 1994;64:212–9. [PubMed] [Google Scholar]
- 15.Fowler AA, 3rd, Syed AA, Knowlson S, Sculthorpe R, Farthing D, DeWilde C, et al. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J Transl Med. 2014;12:32. doi: 10.1186/1479-5876-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schorah CJ, Downing C, Piripitsi A, Gallivan L, Al-Hazaa AH, Sanderson MJ, et al. Total Vitamin C, ascorbic acid, and dehydroascorbic acid concentrations in plasma of critically ill patients. Am J Clin Nutr. 1996;63:760–5. doi: 10.1093/ajcn/63.5.760. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka H, Matsuda T, Miyagantani Y, Yukioka T, Matsuda H, Shimazaki S. Reduction of resuscitation fluid volumes in severely burned patients using ascorbic acid administration: A randomized, prospective study. Arch Surg. 2000;135:326–31. doi: 10.1001/archsurg.135.3.326. [DOI] [PubMed] [Google Scholar]
- 18.Nathens AB, Neff MJ, Jurkovich GJ, Klotz P, Farver K, Ruzinski JT, et al. Randomized, prospective trial of antioxidant supplementation in critically ill surgical patients. Ann Surg. 2002;236:814–22. doi: 10.1097/00000658-200212000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson JX. Evaluation of Vitamin C for adjuvant sepsis therapy. Antioxid Redox Signal. 2013;19:2129–40. doi: 10.1089/ars.2013.5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oudemans-van Straaten HM, Spoelstra-de Man A, de Waard MC. Vitamin C revisited. Crit Care Med. 2014;18:460. doi: 10.1186/s13054-014-0460-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riordan HD, Hunninghake RB, Riordan NH, Jackson JJ, Meng X, Taylor P, et al. Intravenous ascorbic acid: Protocol for its application and use. P R Health Sci J. 2003;22:287–90. [PubMed] [Google Scholar]
- 22.Hoffer LJ, Levine M, Assouline S, Melnychuk D, Padayatty SJ, Rosadiuk K, et al. Phase I clinical trial of i.v. ascorbic acid in advanced malignancy. Ann Oncol. 2008;19:1969–74. doi: 10.1093/annonc/mdn377. [DOI] [PubMed] [Google Scholar]
- 23.Mak S, Newton GE. Vitamin C augments the inotropic response to dobutamine in humans with normal left ventricular function. Circulation. 2001;103:826–30. doi: 10.1161/01.cir.103.6.826. [DOI] [PubMed] [Google Scholar]
- 24.Nooraee N, Fathi M, Edalat L, Behnaz F, Mohajerani SA, Dabbagh A. Effect of Vitamin C on serum cortisol reduction after etomidate induction of anesthesia. J Cell Mol Anesth. 2015;1:28–33. [Google Scholar]
- 25.Kieffer P, Thannberger P, Wilhelm JM, Kieffer C, Schneider F. Multiple organ dysfunction dramatically improving with the infusion of Vitamin C: More support for the persistence of scurvy in our “welfare” society. Intensive Care Med. 2001;27:448. doi: 10.1007/s001340000830. [DOI] [PubMed] [Google Scholar]
- 26.Zipursky JS, Alhashemi A, Juurlink D. A rare presentation of an ancient disease: Scurvy presenting as orthostatic hypotension. BMJ Case Rep 2014. 2014:pii: Bcr2013201982. doi: 10.1136/bcr-2013-201982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holley AD, Osland E, Barnes J, Krishnan A, Fraser JF. Scurvy: Historically a plague of the sailor that remains a consideration in the modern intensive care unit. Intern Med J. 2011;41:283–5. doi: 10.1111/j.1445-5994.2010.02413.x. [DOI] [PubMed] [Google Scholar]
- 28.Long CL, Maull KI, Krishnan RS, Laws HL, Geiger JW, Borghesi L, et al. Ascorbic acid dynamics in the seriously ill and injured. J Surg Res. 2003;109:144–8. doi: 10.1016/s0022-4804(02)00083-5. [DOI] [PubMed] [Google Scholar]
- 29.Fukushima R, Yamazaki E. Vitamin C requirement in surgical patients. Curr Opin Clin Nutr Metab Care. 2010;13:669–76. doi: 10.1097/MCO.0b013e32833e05bc. [DOI] [PubMed] [Google Scholar]
- 30.Crandon JH, Landau B, Mikal S, Balmanno J, Jefferson M, Mahoney N. Ascorbic acid economy in surgical patients as indicated by blood ascorbic acid levels. N Engl J Med. 1958;258:105–13. doi: 10.1056/NEJM195801162580301. [DOI] [PubMed] [Google Scholar]
- 31.Kirkemo AK, Burt ME, Brennan MF. Serum Vitamin level maintenance in cancer patients on total parenteral nutrition. Am J Clin Nutr. 1982;35:1003–9. doi: 10.1093/ajcn/35.5.1003. [DOI] [PubMed] [Google Scholar]
- 32.Lowry SF, Goodgame JT, Jr, Maher MM, Brennan MF. Parenteral Vitamin requirements during intravenous feeding. Am J Clin Nutr. 1978;31:2149–58. doi: 10.1093/ajcn/31.12.2149. [DOI] [PubMed] [Google Scholar]
- 33.De Backer D, Scolletta S. Clinical management of the cardiovascular failure in sepsis. Curr Vasc Pharmacol. 2013;11:222–42. [PubMed] [Google Scholar]
- 34.Hornig D. Distribution of ascorbic acid, metabolites and analogues in man and animals. Ann N Y Acad Sci. 1975;258:103–18. doi: 10.1111/j.1749-6632.1975.tb29271.x. [DOI] [PubMed] [Google Scholar]
- 35.May JM, Qu ZC, Nazarewicz R, Dikalov S. Ascorbic acid efficiently enhances neuronal synthesis of norepinephrine from dopamine. Brain Res Bull. 2013;90:35–42. doi: 10.1016/j.brainresbull.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Levine M. Ascorbic acid specifically enhances dopamine beta-monooxygenase activity in resting and stimulated chromaffin cells. J Biol Chem. 1986;261:7347–56. [PubMed] [Google Scholar]
- 37.Deana R, Bharaj BS, Verjee ZH, Galzigna L. Changes relevant to catecholamine metabolism in liver and brain of ascorbic acid deficient guinea-pigs. Int J Vitam Nutr Res. 1975;45:175–82. [PubMed] [Google Scholar]
- 38.Hoehn SK, Kanfer JN. Effects of chronic ascorbic acid deficiency on guinea pig lysosomal hydrolase activities. J Nutr. 1980;110:2085–94. doi: 10.1093/jn/110.10.2085. [DOI] [PubMed] [Google Scholar]
- 39.Bornstein SR, Yoshida-Hiroi M, Sotiriou S, Levine M, Hartwig HG, Nussbaum RL, et al. Impaired adrenal catecholamine system function in mice with deficiency of the ascorbic acid transporter (SVCT2) FASEB J. 2003;17:1928–30. doi: 10.1096/fj.02-1167fje. [DOI] [PubMed] [Google Scholar]
- 40.Duggan M, Browne I, Flynn C. Adrenal failure in the critically ill. Br J Anaesth. 1998;81:468–70. doi: 10.1093/bja/81.3.468. [DOI] [PubMed] [Google Scholar]
- 41.Nieboer P, van der Werf TS, Beentjes JA, Tulleken JE, Zijlstra JG, Ligtenberg JJ. Catecholamine dependency in a polytrauma patient: Relative adrenal insufficiency? Intensive Care Med. 2000;26:125–7. doi: 10.1007/s001340050024. [DOI] [PubMed] [Google Scholar]
- 42.Treschan TA, Peters J. The vasopressin system: Physiology and clinical strategies. Anesthesiology. 2006;105:599–612. doi: 10.1097/00000542-200609000-00026. [DOI] [PubMed] [Google Scholar]
- 43.Russell JA. Bench-to-bedside review: Vasopressin in the management of septic shock. Crit Care Med. 2011;15:226. doi: 10.1186/cc8224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharshar T, Blanchard A, Paillard M, Raphael JC, Gajdos P, Annane D. Circulating vasopressin levels in septic shock. Crit Care Med. 2003;31:1752–8. doi: 10.1097/01.CCM.0000063046.82359.4A. [DOI] [PubMed] [Google Scholar]
- 45.Prigge ST, Mains RE, Eipper BA, Amzel LM. New insights into copper monooxygenases and peptide amidation: Structure, mechanism and function. Cell Mol Life Sci. 2000;57:1236–59. doi: 10.1007/PL00000763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Landry DW, Levin HR, Gallant EM, Ashton Rc, Jr, Seo S, D’Alessandro D, et al. Vasopressin deficiency contributes to the vasodilation of septic shock. Circulation. 1997;95:1122–5. doi: 10.1161/01.cir.95.5.1122. [DOI] [PubMed] [Google Scholar]
- 47.Giusti-Paiva A, Domingues VG. Centrally administered ascorbic acid induces antidiuresis, natriuresis and neurohypophyseal hormone release in rats. Neuro Endocrinol Lett. 2010;31:87–91. [PubMed] [Google Scholar]
- 48.Kahn SA, Beers RJ, Lentz CW. Resuscitation after severe burn injury using high-dose ascorbic acid: A retrospective review. J Burn Care Res. 2011;32:110–7. doi: 10.1097/BCR.0b013e318204b336. [DOI] [PubMed] [Google Scholar]
- 49.Morelli A, Ertmer C, Rehberg S, Lange M, Orecchioni A, Laderchi A, et al. Phenylephrine versus norepinephrine for initial hemodynamic support of patients with septic shock: A randomized, controlled trial. Crit Care Med. 2008;12:R143. doi: 10.1186/cc7121. [DOI] [PMC free article] [PubMed] [Google Scholar]


