Abstract
The way in which we move influences our ability to perceive, interpret and predict the actions of others. Thus movements play an important role in social cognition. This review article will appraise the literature concerning movement kinematics and motor control in individuals with autism, and will argue that movement differences between typical and autistic individuals may contribute to bilateral difficulties in reciprocal social cognition.
Keywords: autism, movement, motor control, kinematics, social cognition
1. Introduction
Already in the earliest descriptions of autism a variety of movement atypicalities have been noted including atypical postural control, gait, upper limb movements and fine motor control. However, these neurologically important signs have not been investigated as much as the social impairments in autism. Recent research has significantly advanced our understanding of the contribution of movements to socio-cognitive function. This literature suggests that processes such as action perception, prediction and interpretation are critical to social communication. For instance, these processes may be facilitated between two individuals who move similarly and impeded between individuals who move differently. In this paper, §2 briefly summarizes the literature suggesting that autistic and typical individuals move differently; §3 examines the contribution of one's own movement patterns to the perception, prediction and interpretation of the movements of others and, finally, §4 proposes that movement differences between typical and autistic individuals may contribute to bilateral difficulties in reciprocal social cognition. If so, autistic individuals will have difficulties perceiving, predicting and interpreting the actions of typical individuals and, conversely, typical individuals will have difficulties perceiving, predicting and interpreting the actions of individuals with autism. This interpretation goes some way towards the increasing recognition that the roots of the social difficulties that autistic individuals experience, are deeply embedded in compromised interactions, and are not solely due to processing deficits that are internal to the autistic person.
2. Are movements atypical in autism?
Autism spectrum disorder1 (henceforth autism) is a developmental disorder characterized by impaired communication and social interaction, and restricted and repetitive interests [1]. Movement atypicalities have been linked with autism as far back as the work of Kanner [6] and Asperger [7], who noted motor abnormalities such as ‘sluggish’ reflexes, ‘clumsy’ gait and an absence, from an early age, of anticipatory postures when being picked up.
While most studies have focused primarily on social impairments in autism, there are also a number of reviews that have focused on movement atypicalities and abnormalities in areas of the brain relating to movement such as the cerebellum, striatum and brainstem (e.g. [3,8–10]). Here we give a brief overview of behavioural differences between autistic and typical individuals that have been noted with regard to various different types of movement. As this literature has been reviewed in depth elsewhere [11–16], we briefly summarize the main findings. These illustrate the wide-range of movement atypicalities that have been linked to all forms of autism.
(a). A note on movements and actions
When reviewing the literature concerning movements and actions, there are many possibilities for sub-categorizing the topic. For example, Gowen & Hamilton [12] decompose actions into constituent computational processes including motor planning, feed-forward control and motor execution; in doing so they demonstrate the utility of this approach in starting to isolate particular computational processes that may drive atypical movements in autism. In a review of the action understanding literature, Kilner [17] describes actions at four, non-independent, hierarchically organized levels: (i) the kinematic level: the trajectory and the velocity profile of the action; (ii) the motor level: the processing and pattern of muscle activity required to produce the kinematics; (iii) the goal level: the immediate purpose of the action; and (iv) the intention level: the overall reason for executing the action. This approach is particularly useful in illustrating that, due to the non-independence between different levels of the action hierarchy, an atypicality at one level (e.g. atypical goal identification) can impact upon other levels (e.g. atypical kinematics). Although both approaches are useful to bear in mind throughout this article, this paper will initially adopt a functional perspective in order to demonstrate that atypical movements are not restricted to one functional domain such as handwriting but may impact on many aspects of everyday life for individuals with autism.
(b). Posture and balance
At least 11 studies, to date, have investigated differences between autistic individuals2 and non-autistic individuals in terms of postural control [19–29]. In an early study, Kohen-Raz et al. [25] measured autistic and typical participants' (aged 6–20 years) weight distribution while standing on stable and unstable surfaces with or without the benefit of vision. Autistic participants were generally less stable in their posture and typically exhibited a tendency to put most of their weight on one heel/toe. Similar patterns have been observed in subsequent studies of postural sway; for instance, autistic children demonstrate abnormalities when standing and looking straight ahead [21,22,30], standing while dual-tasking [31], standing with eyes closed [27,32], standing on unstable surfaces [27] and standing on a sway-referenced platform [26]. With a view to investigating the development of postural control in autism, Minshew et al. [26] recruited participants ranging from 5 to 52 years; they concluded that the development of postural control was delayed in autistic participants and differed from typical postural control even in adulthood.
(c). Gait
At least seven separate studies have assessed gait or the ‘style of walking’ in autistic children and adults, and a number of atypicalities have been observed [33–37]. For example, Nobile et al. showed that, compared with typical individuals, autistic children (6–14 years) exhibited trunk postural abnormalities, difficulties in walking in a straight line, a marked loss of smoothness (an increase in the jerkiness of movement) and, in general, a stiffer gait in which the usual fluidity of walking was lost. In a comprehensive review of gait atypicalities in autistic children, Kindregan et al. [13] found that the most commonly reported atypicalities concerned step width, step and stride length, reduced velocity and increased time in the stance phase of gait. On the basis that increased step width provides a wider base of support, and reduced velocity and step and stride lengths help a walker to keep their centre of gravity within this base of support, they argue that together these results suggest a tendency for individuals with autism to augment their stability during walking—and, therefore, that autistic children have a more unstable gait compared with typical children. Extending this research into the adolescent years, Weiss et al. [38] found that 16- to 19-year olds with autism differed from typical controls with respect to various spatio-temporal aspects of gait, including step and stride length, foot positioning, cadence, velocity and step time. Hallett et al. [35] report mild clumsiness of gait and reduced range of motion of the ankle in autistic adults.
(d). Upper limb movements
Paradigms investigating upper limb movements in autism typically measure arm movement preparation and execution times and kinematic parameters, i.e. parameters referring to joint motions and angles at specific points in a movement and typically reported in terms of the velocity, acceleration (change in velocity) and jerk (change in acceleration) of a particular point on the body. Such studies have revealed differences between autistic and typical individuals [39–45]. To illustrate, Glazebrook et al. [41,42] found that adults with autism required more time both during movement initiation and execution for manual aiming movements, while Rinehart and colleagues have reported that autistic children [40] and young adults [46] require more time to prepare point-to-point movements (moving from one point in space to another).
Further work uses the reach-to-grasp task where, upon presentation of a cue, participants move their hand from a start position to grasp a target object. Using such a task, Stoit et al. [45] found that autistic children and adolescents exhibited longer movement times from the start of the movement to the grasp of the object. Yang et al. [47] found that children with autism showed significantly longer movement times for reach-to-grasp actions and executed their movements with more jerky kinematics. In line with this, Cook et al. [48] demonstrated that high-functioning adults with autism make more jerky movements that proceed with greater acceleration and velocity, even when these movements are not goal directed and are thus relatively unconstrained.
(e). Fine motor control
Fine motor control has typically been examined through analysis of handwriting in those with autism. While these studies have generally revealed autistic individuals to have atypical handwriting, the specific details of how handwriting deviates from the norm vary somewhat across studies [49–53]. In a comprehensive review of the literature concerning handwriting produced by children with autism, Kushki et al. [15] note consistent atypicalities in the overall legibility of handwriting and letter formation. For example, autistic children have been found to produce more poorly formed letters, though they do not exhibit difficulties in correctly aligning and spacing letters [51]. Macrographia (atypically large handwriting) has also been noted in both children [53] and adults with autism [50]. These features have been related to atypical movement kinematics [53]. Johnson et al. [53] demonstrated that handwriting-related movements were considerably larger, peak velocity was significantly greater and movement trajectory more variable, in autistic children. An analysis of the velocity of movements suggested that autistic children may require higher energy input to achieve the same smoothness of movement as typical controls.
(f). Summary
Compared with typical individuals, children and adults with autism have, on average, been reported to exhibit increased instability during both standing and walking, atypical kinematics with respect to various movements, poor fine motor control as illustrated by atypical handwriting and, when making goal-directed or point-to-point arm movements, increased preparation and execution times. These findings, which are highly reliable and robust over many studies, suggest that, at a low level of cognitive processing, autistic individuals are likely to make movements which deviate from those made by individuals without autism. Adopting a bottom-up view, it is plausible that these ‘low level’ movement differences might impact on ‘higher level’ processing. This does not rule out that separate difficulties also exist at a higher level. However, it is possible that a bottom-up account would result in a parsimonious explanation of at least some of the symptoms of autism. In §3, we consider how the established movement atypicalities may influence higher level processes such as the perception, prediction and interpretation of others' actions, and how in turn this may disrupt very high level social interaction.
3. Movements influence socio-cognitive processes
Perceptual and motor systems are tightly linked: action influences perception and perception influences action. Research over the past few decades has demonstrated that this reciprocal relationship between action and perception may play a role in wider socio-cognitive functions, including action prediction, estimation of others' mental states, imitation and the development of positive social attitudes.
(a). Action and perception
Watching another person perform a movement evokes activity (often referred to as ‘motor resonance’) in one's own motor system. Evidence for this claim comes from a variety of fields: single cell recording studies have found that neurons in the motor system of the macaque (subsequently labelled ‘mirror neurons’) fire when the monkey passively observes an action [54], and research using a range of neuroimaging methods including functional magnetic resonance imaging (fMRI), transcranial magnetic stimulation, magnetoencephalography (MEG) and electroencephalography (EEG) provides strong evidence for similar responses to action execution and action perception in the human brain. fMRI experiments have identified overlapping activity for action perception and execution in a network of regions (subsequently referred to as the human mirror neuron system (MNS)), including the inferior frontal gyrus (e.g. [55]), inferior parietal lobe [56,57], ventral and dorsal premotor cortex [58,59], anterior intraparietal sulcus [60,61] and the superior temporal sulcus [62]. Furthermore, cross-modal repetition suppression, where a reduced response is seen for observation following execution or vice versa, has been observed in both frontal [63] and parietal MNS areas [64].
Studies using MEG and EEG have also shown that sensorimotor oscillatory activity in both the 8–12 Hz (µ) and 15–30 Hz (β, beta) ranges is attenuated both when observing and executing actions [65–70]. However, electrical activity is not simply suppressed during action execution but is modulated dynamically [71,72]. Correspondingly, studies have demonstrated that sensorimotor oscillatory activity is also modulated dynamically during action observation according to the kinematics of the observed movement [73–76]. For example, Press et al. [76] demonstrated that beta power was dynamically modulated according to the acceleration profile of an observed arm movement, mirroring what would be expected during execution of the same action. Such automatic activation of the motor system during action observation can influence behaviour; that is, observing others' actions can interfere with ongoing action selection and execution such that we automatically imitate actions we observe [77–85].
Just as perception influences action, action influences perception. For example, inducing a motor load through performance of a concurrent task has been shown to modulate perceptual judgements about the weight of an object being lifted by an actor [86] or speed of a walker [87]. Similarly, perceptual judgements can be impaired through application of disruptive transcranial magnetic stimulation to motor regions [88]. Furthermore, in clinical populations, deficits in action production resulting from either cortical lesions and/or apraxia are correlated with deficits in action recognition [89–91]. Thus, there is widespread evidence that the motor and visual systems are intrinsically linked and mutually influence each other.3
(b). The importance of being similar: a worked example
Several theoretical accounts of the relationship between the visual and motor systems predict that the more similar two people are in their action execution the more likely they are to engage in motor resonance when observing each other's actions [93–96]. The following worked example describes such a situation in detail and elucidates how such effects might come about.
This example concerns three people, Fred, Jill and George. When Fred performs a reach-to-grasp movement he typically accelerates his hand towards the object until he has covered 50% of the distance, then begins to gradually decelerate. Jill performs this action with the same kinematics as Fred. George is different; George continues to accelerate his hand forward until he has covered 65% of the distance to the object. Fred has made movements like this for most of his life. He has a wealth of experience of observing his kinematic profile and simultaneously activating the motor codes for executing this reach-to-grasp movement (i.e. experience of simultaneously seeing and doing). This vast amount of experience means that for Fred the visual representation of a reach-to-grasp movement with a 50% acceleration phase has become tightly associated with his motor programmes for executing a reach-to-grasp movement [97]. Consequently, when Fred sees Jill make this movement it automatically activates his motor codes for executing a reach-to-grasp movement. By contrast, Fred has very little experience with seeing reach-to-grasp movements that follow George's (unusual) kinematic profile. Thus for Fred the visual representation of reach-to-grasp movements with George's kinematics is only weakly associated with his motor code for executing a reach-to-grasp movement and, therefore, George's movement only weakly activates Fred's motor system. It can, therefore, be seen that movements that are more similar to one's own movements are more likely to result in motor resonance (e.g. Fred and Jill's movements) than those that are dissimilar (e.g. Fred and George's movements).
The argument that movement similarity boosts motor resonance is not merely theoretical: various laboratories have tested this hypothesis. For example, Cross et al. [98] trained expert dancers to learn complex whole-body dance sequences that were not in their motor repertoire prior to training. They found that motor system activity during passive observation of videoed dance sequences covaried as a function of the observer's ability to execute the dance move; greater activity was seen for movements that the dancer had mastered. Thus, motor resonance increased as participants' own movements became increasingly similar to the videoed movements.
(c). The importance of being similar: repercussions for socio-cognitive processes
As discussed in §3b, movements that are similar to one's own movement patterns are more likely to result in motor resonance. A number of studies suggest that a by-product of this motor resonance is the facilitation of various socio-cognitive functions, including action perception, prediction, interpretation and imitation. This point is illustrated here with various examples from the literature.
(i). Movement similarity and action perception
Casile & Giese [99] used motor training to ascertain the contribution of movement similarity to perception. Participants learned a novel upper-body movement while blindfolded, meaning that they received verbal and haptic, but not visual, feedback. Before and after training point-light stimuli were used to test the visual recognition of the learned movement. Despite the absence of visual stimulation during training, participants demonstrated an enhanced ability to visually recognize the trained movement. Furthermore, visual recognition performance after training correlated strongly with the accuracy of the execution of the learned movement. Thus, the more similar a participant's executed movements were to the observed movement, the better their visual recognition performance.
(ii). Movement similarity and action prediction
Aglioti et al. [100] demonstrated that professional basketball players could predict the success of free shots at a basket earlier and more accurately than individuals with comparable visual experience (coaches or sports journalists) but reduced motor experience. Moreover, Aglioti and colleagues found that only basketball players showed time-specific motor activation during observation of erroneous shots. They suggest that individuals who can move more similarly to the observed stimuli (i.e. basketball players) are more successful in their predictions, and that such results are a function of enhanced motor resonance.
(iii). Movement similarity and the mental state of confidence
Theoretical accounts predict that motor similarity should promote mental state inference [94]. Patel et al. [101] tested this hypothesis with respect to a particular mental state: confidence. In an initial execution condition, participants performed a visual discrimination task wherein they successively viewed two images, one a target and one a foil. Participants indicated whether the first or second image contained the target by picking up a marble and placing it in the appropriately labelled slot, and subsequently rated their confidence in their decision. In this phase of the experiment, increasing confidence was associated with faster movements. In an ensuing observation task, participants watched a series of video clips showing the hands of anonymized actors performing the execution task and judged how confident they considered the actor to be. Patel and colleagues found that participants' judgements depended upon their own movement speed in the execution condition—if a participant watched an actor who moved faster than themselves then they were more likely to rate this actor as being confident, whereas movements performed slower than a participant's own movements were more likely to be rated as low in confidence. Participants were therefore more likely to accurately estimate confidence for movements that were similar in speed to their own movements.
(iv). Movement similarity and behavioural imitation
Kilner et al. [82] demonstrated that behavioural imitation of observed movements is greater for movements that are similar to one's own. Kilner et al. tracked participants' arm movements while they executed vertical sinusoidal arm ‘waving’ movements. Simultaneously, participants watched a video of an actor making incongruent horizontal movements. The video was experimentally manipulated such that the arm moved either with typical human kinematics (in a smooth, fluid manner) or at constant velocity (i.e. like a traditional robot). They found that observing videos of a person moving with human kinematics interfered with participants' on-going actions such that they subtly imitated the observed movement. By contrast, there were no subtle signs of imitation for the constant velocity movements. Thus, imitation was enhanced for movements that were similar to the participants' own movements relative to movements that were dissimilar.
(v). Movement similarity and positive affect
Movement similarity has been associated with positive affect. For example, Kirsch et al. [102] found that participants reported greater enjoyment and interest when observing dance movements from within their own motor repertoire, and an associated body of literature suggests that behavioural correlates of motor resonance such as movement synchronicity and automatic imitation may be intrinsically rewarding. For instance, Hove & Risen [103] demonstrated that participants who tapped synchronously with an experimenter liked the experimenter more than participants who tapped asynchronously. They argued that synchronicity of movements between interactants can promote the development of positive attitudes. Similarly, numerous studies have demonstrated that being imitated increases positive evaluations of interactions [104–107], and after being imitated people are more helpful, increase the amount they donate to charity [108], and feel closer to others [109]. Thus, a number of studies support the notion that movement similarity and behavioural correlates of motor resonance, such as movement synchronicity and automatic imitation, promote positive affect.
(vi). How important is motor resonance?
The literature described in §3c(i–v) shows that, compared with people who move in dissimilar ways, people who move in similar ways will probably experience more fluid action perception and prediction, be better at estimating each others' mental states, be more likely to imitate each other and be more inclined to develop positive affective ties to each other. It is possible that these diverse benefits of motor similarity are all due to enhanced motor resonance.
However, such effects may also be mediated by a visual experience route. To illustrate, imagine you have had a well-spent afternoon mastering the art of balancing a teaspoon on the tip of your finger. In doing so, you have learned that success is associated with a particular pattern of muscle contractions. Now imagine your friend attempts this complex balancing act. After watching only their initial bodily positioning, you successfully predict that the teaspoon will fall. According to the motor resonance account, observing your friend's initial positioning activates the corresponding motor codes within your system, generating a forward model (a prediction of the sensory consequences of the pattern of muscle contractions) from which you can predict the probability of success. However, while mastering the art of teaspoon balancing, in addition to motor experience, you also received visual experience. For example, you may have learned that the sight of your finger being at a particular angle relative to the ground and a certain distance from your body is highly predictive of success. If your motor system were temporarily lesioned you would still be able to use this visual experience to estimate your friend's chances of success.
Thus, both motor and visual experiences are important in our processing of others' actions. For many of the studies discussed above it has been empirically demonstrated that motor resonance adds predictive power over and above that contributed by the visual system alone [100,110]. However, when thinking about the repercussions of movement atypicalities in clinical populations, it is important to remember that if an individual tends to move differently compared with typical individuals they will have both different motor and visual experience of actions.
(d). Summary
Whether due to the natural development of their movements throughout their lifetime, or intense targeted training (e.g. dance classes), people who move similarly to each other will have comparable motor and visual experiences. Conversely, motor and visual experience is less comparable for individuals who move differently. Further, similar motor and visual experiences appear to facilitate socio-cognitive processes, including action perception, prediction, estimation of mental states, imitation and the development of positive affective ties. Thus, these processes are probably enhanced for people that move similarly and (relatively) impaired for those who do not.
4. Atypical movements and socio-cognitive function in autism
In 1996, Leary & Hill [111] published a controversial comment on the autism literature. They suggested that autism research had virtually ignored movement atypicalities, instead focusing on social and communicative problems. They argued that social descriptions of behaviours such as ‘a failure to cuddle’, ‘socially inappropriate gestures’ and ‘an indifference to affection’ could be recast in terms of neurological motor symptoms such as ‘abnormal posture and tone’, ‘dyskinesia’ and ‘marked underactivity’. Critically, they asserted that the application of a social context to motor behaviours diverts attention from the possible neurological explanations and thus hinders appropriate treatment interventions. Although Leary & Hill's [111] focus concerned social interaction—actions and reactions that occur between people—they also commented on social cognition—internal processes relating to the perception, prediction and interpretation of others:
Many individuals who experience movement disturbance report differences in internal mental processes, such as perception, changes in attention, consciousness, motivation, and emotion [112–115].
[111, p. 40]
Sections 2 and 3 summarized the literature demonstrating that autistic individuals move differently from typical individuals, and argued that socio-cognitive tasks such as perceiving, predicting and interpreting others may be made more difficult between people who move differently compared with those who move similarly. This section elaborates on Leary & Hill's comment by making the case that—at least in part due to movement differences—autistic individuals may have difficulties in perceiving, predicting and interpreting the actions of typical individuals, and, conversely, typical individuals may have difficulties perceiving, predicting and interpreting the actions of autistic individuals. I conclude by highlighting in §4d–g outstanding questions to be addressed by research in this area.
(a). Movement similarity and action perception in autism
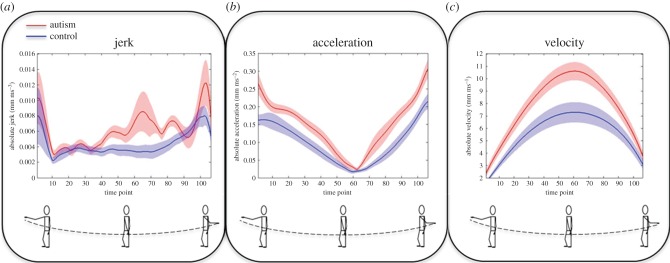
Using motion-tracking technology, Cook et al. [48] examined the relationship between movement kinematics and action perception in autism. Adults with autism and typical individuals matched in terms of age, gender and intelligence performed simple sinusoidal arm ‘waving’ movements while the kinematics (velocity, acceleration and jerk) of their movements were recorded. Autistic individuals produced arm movements that were more jerky, and which proceeded with greater acceleration and velocity (figure 1) , than those produced by typical individuals. The magnitude of these kinematic atypicalities was significantly positively correlated with autism symptom severity as measured by the Autism Diagnostic Observation Schedule semi-structured questionnaire [116]. Such results are consistent with reports from other laboratories of atypically jerky arm [47] and whole-body [36] movements in autism.
Figure 1.
Kinematics of arm movements for autistic and typical individuals. When executing simple sinusoidal arm movements individuals with autism made more jerky movements (a) and travelled with faster absolute acceleration (b) and velocity (c). Mean movement vectors are plotted in red for the autism group and blue for the typical control group. Shaded regions indicate the standard error of the mean. Image adapted from Cook et al. [48] (fig. 3; CC BY).
In a separate perception task, participants watched a series of visual stimuli comprising an image of a human hand that made vertical sinusoidal movements (down and then up) across the computer screen. The velocity profile of the hand was generated by motion-morphing between human-like minimum jerk motion and robot-like constant velocity. Participants also completed a non-biological control condition which featured a falling tennis ball, the velocity profile of which was a motion-morph between gravitational motion and constant velocity. Participants were required to label the movement of the stimulus as ‘natural’ or ‘unnatural’. Results showed that the degree to which kinematic profiles were atypical when executing arm movements was significantly correlated with biased responding when observing motion of a human hand but not a tennis ball. In other words, the more atypical an autistic participant's kinematics (relative to kinematics exhibited by typical individuals), the less likely they were to classify movements that follow typical kinematics as ‘natural’. Such results are consistent with the conclusions of Patel et al. [101] drawn from their studies of typical individuals; in the same way that a typical observer's perception of a confident movement was modelled on their own confident movements, autistic individuals' perception of natural movements is likely to be modelled on their own movements.
(b). Movement similarity and imitation in autism
Cattaneo et al. [117] investigated the link between action execution and automatic imitation of others' actions in children with autism and a matched group of typically developing children. In an action execution condition, participants were required to pick up a piece of paper and place it in a container, or pick up a piece of food and eat it. During both actions, the activity of the mouth-opening mylohyoid (MH) muscle was recorded using electromyography. In a separate ‘observation condition’ participants passively observed a typical child pick up a piece of (i) food and place it in their mouth or (ii) paper and place it in a container while activity from the MH muscle was recorded. Cattaneo et al. found that during the execution condition, MH muscle activity from typical children started to increase several hundreds of milliseconds before their hand grasped the food. It continued to increase during actual grasping, and reached its peak when the child started to open its mouth. MH muscle activity for autistic children was strikingly different: no activity increase was found during the entire reaching and grasping phases; the muscle only became active as the food was brought to the mouth.
These group differences during action execution translated into group differences during action observation: for typical children MH activity was observed when they passively observed another child reach and grasp a piece of food. By contrast, the autistic children did not show MH activation during the observation of either reaching or grasping phases. Thus, atypical action execution in autistic children (i.e. a lack of anticipatory activation of the MH muscles when bringing food to their own mouth) was associated with atypical imitative responses.
(c). Is the mirror neuron system broken in autism?
Much of the past decade's literature concerning autism has debated the integrity of the MNS in this population (e.g. [118–123]). Thus, it is important to be clear about the claims made in this paper. Although the difference between the current stance and the broken mirror stance may appear subtle, it is important. Mirror neurons are active both when a person executes a movement and when they observe a movement. Hence, they can be considered a ‘link’ between the visual and motor system. Indeed, the broken mirror account of autism focuses on the link between action observation and execution: its key tenet is that what is broken is the link between seeing and doing. The current focus is different: here I focus on action execution, that is, not on any link between seeing and doing, but on the doing itself. This assumption is neutral as to whether mirror system activity measured independently, is atypical in autism. Several accounts have rivalled the broken mirror theory of autism [118,119,122]. This does not affect the current claim. Even if one assumes that the link between action observation and action execution is intact in individuals with autism they may still exhibit atypical imitation, and other socio-cognitive functions, due to atypical movement execution and their subsequently atypical visual and motor experience.
(i). Further questions
Section 4b highlights that reduced similarity between autistic and non-autistic movement kinematics and anticipatory muscle activation may impact on socio-cognitive functions (i.e. biological motion categorization and imitation). Clearly, much further work is required to elucidate the link between various movement differences between autistic and typical individuals (e.g. postural control, gait, fine motor control) and such socio-cognitive functions as intentional inferences, reading emotions from actions, estimating mental states, etc. In addition to widening the scope of this literature, there are a number of important questions that also need to be addressed by this growing research field.
(d). What is atypical in the social interactions between autistic and non-autistic people?
The thesis outlined above supposes that impaired perception, interpretation and prediction of a typical person's movements can arise because an autistic individual has had a lifetime of visual and motor experience with their own movements—which differ from those of typical individuals. The same applies to the typical individual who encounters an autistic person. That is, most typical individuals have little visual and no motor experience with autistic movement patterns; thus they will probably have poor representations of autistic movements and thus potential deficits in the perception, prediction and interpretation of autistic behaviour. This is an important insight. It suggests that social interaction difficulties lie not with the autistic individual themself but, rather, with both interaction partners: the autistic person has difficulties perceiving, predicting and interpreting the actions of the non-autistic person and vice versa. This shift in focus away from autistic individuals, towards the interaction between autistic and non-autistic people, is consistent with recent calls to develop a ‘second person neuropsychiatry’ with an increased focus on social interaction [124,125].
The question arises whether social interactions between partners who are both autistic are more fluid and whether such individuals show enhanced motor resonance due to greater movement similarity. A plausible alternative is that each atypical movement pattern is atypical in its own way and therefore dissimilar to every other individual. Preliminary support for the former comes from anecdotal evidence that high-functioning individuals with autism describe social interactions with other autistic individuals to be less effortful and more efficient compared with interactions with non-autistic people [124]. This argument also applies to the comparison of different conditions with neurological movement disorder. Further research is therefore necessary to investigate social interaction and its relationship to movement execution for autistic–autistic dyads and dyads comprising an autistic individual and an individual with a different movement disorder.
(e). Are atypical movements unique to autism?
The answer to this question is assuredly no. There are many conditions in which individuals exhibit movements that are different from those exhibited by typical controls including attention deficit hyperactivity disorder (ADHD), specific language impairment (SLI), Huntingdon's disease, Parkinson's disease and developmental coordination disorder. Indeed, finding a ‘movement signature’ that can differentiate individuals with autism from those with other conditions has become an important aim for the field due to its potential to expedite early detection. Initial studies show promise in differentiating autistic and typical children on the basis of movement patterns [39]. However, an important goal is to be able to differentiate autistic children from those with other developmental conditions, such as ADHD and SLI.
Differentiating autistic movements from those exhibited by children with ADHD is perhaps the most promising avenue in this literature so far [126–133]. MacNeil & Mostofsky [129] have argued that whereas both children with ADHD and autism show impairments in basic motor control, difficulties with the formation of perceptual-motor action models are specific to autism. In line with this, Ament et al. [126] suggest that impairments in motor skills requiring the coupling of visual and temporal feedback to guide and adjust movement can differentiate ADHD, autism and developmental delay. McPhillips et al. [134] have begun to extend this line of research to other developmental conditions by comparing children with autism and SLI. However, much further work is required before a ‘movement signature’ differentiating autism from other conditions can be identified.
(f). Are movement atypicalities in other conditions, such as ADHD, associated with socio-cognitive function?
If moving atypically (i.e. different from typical controls) is associated with atypical perception, prediction and interpretation of controls' movements, and, if atypical movements occur in various conditions—from ADHD to Parkinson's disease—the current theory implies that individuals with these conditions might exhibit socio-cognitive atypicalities.
Socio-cognitive function in conditions including ADHD [135], Parkinson's disease [136] and Huntington's disease [137] is an active area of research, and it may be the case that further work in this field uncovers atypicalities in socio-cognitive function that cut across traditional diagnostic labels. However, it should be noted that for many conditions there may be additional factors, such as attentional control and executive function deficits, which feed into both motor control and social cognition impairments (this also applies to autism, see below). It is therefore important that future research attempts to ascertain the relative contribution of these various factors and/or uses tasks with minimal executive function, attention and memory requirements.
In studies where clinical groups are compared, the onset and duration of atypicalities matter. To give an example, if an individual has a sudden insult resulting in atypical movements (e.g. a torn ligament) this is unlikely to impact on socio-cognitive function; for that individual, their lifetime's visual and motor experience with typical movement patterns will probably outweigh the acute episode of atypical movements. This reasoning should apply to Parkinson's disease and other movement disorders acquired in late adulthood. It is likely that the impact of atypical movements on social cognition is a function of the length of time one has experienced atypical movements. At present, further research is required to ascertain the influence of the duration of movement atypicalities, and whether an individual's developmental stage at the time of onset is important.
(g). Are movement atypicalities the root cause of autistic cognition?
Using a bottom-up explanatory framework, can atypical movements in autism be considered the root cause of autistic cognition? Such an account is likely to be too simplistic. Rather, I argue that, though movement atypicalities may not explain all features of autistic behaviour, the role of movements in autistic socio-cognitive function should not be overlooked.
Contemporary accounts of autism suggest atypical computations that may pervade many cognitive functions from visual perception to decision-making. Recent examples are the notions of atypical priors [138] and aberrant precision of sensory information [139]. The latter, for instance, proposes that the precision of (i.e. reliability or confidence attributed to) incoming sensory information is too high relative to the precision of prior beliefs. This account provides a compelling explanation for visual perceptual atypicalities in autism: for instance, suggesting that autistic individuals' immunity to many visual illusions [140] may be due to abnormally high precision attributed to incoming sensory information relative to prior beliefs [139]. In addition, it has been argued that this account may help to explain difficulties with social interaction due to the heavy reliance of social interactions on prior beliefs [124]. Although the aberrant precision account has also been extended to repetitive and stereotyped behaviours [139], further work would be required to apply this account to the wide-ranging movement atypicalities documented in §2 of this paper. However, it is not impossible to imagine such an account. With respect to the atypically jerky gait characteristic of autism [36], the ability to walk in a smooth fluid manner is learned and refined during early development [141]. This process can be recast within a predictive coding framework whereby prior beliefs about how to optimally move are refined according to incoming sensory information. Atypically jerky gait in autism could therefore conceivably be due to an imbalance in the precision of incoming sensory information relative to prior beliefs.
(h). A final note
This paper has argued that visual and motor experience with own—atypical—movements in autism can result in the development of atypical (visual and/or motor) representations of movements, which is likely to impact on the perception, prediction and interpretation of others' movements. Perhaps the most interesting implication of this claim is that the same argument should be true for typical individuals. That is, due to reduced experience with autistic movements, typical individuals may exhibit deficits in the perception, prediction and interpretation of autistic behaviour. Support for this hypothesis comes from a recent study showing poor recognition of autistic emotional facial expressions by typical control observers [142]. The real-world implications of this proposition should not be overlooked: it may be the case that many typical individuals who provide services for individuals with autism are poor at understanding the actions of their autistic service users. Thus, a final suggestion for further research is a comprehensive test of the hypothesis that typical controls exhibit poor perception, prediction and interpretation of autistic movements and an investigation of suitable training programmes.
Acknowledgement
The author thanks Geoff Bird and Uta Frith for their comments on the manuscript, and Clare Press and Rosy Edey for many insightful discussions relating to the ideas in this paper.
Endnotes
This review focuses on studies of autism spectrum disorder (referred to as autism for brevity) as defined in the DSM V [1]. Studies focusing exclusively on participants with Asperger's disorder have been excluded given the on-going debate concerning differences in motor function between autism spectrum disorder and Asperger's disorder [2–5].
‘Disability-first’ terminology is used throughout in line with the majority preference expressed in a recent survey of the autistic community [18].
Though note that recent accounts argue that motor system activity has widespread effects on perception that are not restricted to the action domain [92].
Competing interests
The author has no competing interests
Funding
The author is supported by the Birmingham Fellows programme
References
- 1.American Psychiatric Association 2013. Diagnostic and statistical manual of mental disorder, 5th edn Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- 2.Macintosh KE, Dissanayake C. 2004. Annotation: the similarities and differences between autistic disorder and Asperger's disorder: a review of the empirical evidence. J Child Psychol. Psychiatry 45, 421–434. ( 10.1111/j.1469-7610.2004.00234.x) [DOI] [PubMed] [Google Scholar]
- 3.Nayate A, Bradshaw JL, Rinehart NJ. 2005. Autism and Asperger's disorder: are they movement disorders involving the cerebellum and/or basal ganglia? Brain Res. Bull. 67, 327–334. ( 10.1016/j.brainresbull.2005.07.011) [DOI] [PubMed] [Google Scholar]
- 4.Nayate A, Tonge BJ, Bradshaw JL, McGinley JL, Iansek R, Rinehart NJ. 2011. Differentiation of high-functioning autism and Asperger's disorder based on neuromotor behaviour. J. Autism Dev. Disord. 42, 707–717. ( 10.1007/s10803-011-1299-5) [DOI] [PubMed] [Google Scholar]
- 5.Rinehart NJ, Tonge BJ, Bradshaw JL, Iansek R, Enticott PG, McGinley J. 2006. Gait function in high-functioning autism and Asperger's disorder. Eur. Child Adolesc. Psychiatry 15, 256–264. ( 10.1007/s00787-006-0530-y) [DOI] [PubMed] [Google Scholar]
- 6.Kanner L. 1943. Autistic disturbances of affective contact. Acta Paedopsychiatr. 35, 100–136. [PubMed] [Google Scholar]
- 7.Asperger H. 1944. Die autistischen psychopathien im kindersalter. Arch. Psychiatr. Nervenkrankheiten 117, 76–136. ( 10.1007/BF01837709) [DOI] [Google Scholar]
- 8.Bauman M. 1992. Motor dysfunction in autism. In Movement disorders in neurology and neuropsychiatry (eds Joseph AB, Young RR), pp. 658–661. Boston, MA: Blackwell Scientific. [Google Scholar]
- 9.Courchesne E. 1997. Brainstem, cerebellar and limbic neuroanatomical abnormalities in autism. Curr. Opin. Neurobiol. 7, 269–278. ( 10.1016/S0959-4388(97)80016-5) [DOI] [PubMed] [Google Scholar]
- 10.Courchesne E, Yeung-Courchesne R, Press G, Hesselink J, Jernigan T. 1988. Hypoplasia of cerebellar vermal lobules VI and VII in autism. N. Engl. J. Med. 318, 1349–1354. ( 10.1056/NEJM198805263182102) [DOI] [PubMed] [Google Scholar]
- 11.Braddick O, Atkinson J. 2013. Visual control of manual actions: brain mechanisms in typical development and developmental disorders. Dev. Med. Child Neurol. 55(Suppl 4), 13–18. ( 10.1111/dmcn.12300) [DOI] [PubMed] [Google Scholar]
- 12.Gowen E, Hamilton A. 2013. Motor abilities in autism: a review using a computational context. J. Autism Dev. Disord. 43, 323–344. ( 10.1007/s10803-012-1574-0) [DOI] [PubMed] [Google Scholar]
- 13.Kindregan D, Gallagher L, Gormley J. 2015. Gait deviations in children with autism spectrum disorders: a review. Autism Res. Treat. 2015, 741480 ( 10.1155/2015/741480) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mostofsky SH, Ewen JB. 2011. Altered connectivity and action model formation in autism is autism. Neuroscientist 17, 437–448. ( 10.1177/1073858410392381) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kushki A, Chau T, Anagnostou E. 2011. Handwriting difficulties in children with autism spectrum disorders: a scoping review. J. Autism Dev. Disord. 41, 1706–1716. ( 10.1007/s10803-011-1206-0) [DOI] [PubMed] [Google Scholar]
- 16.Sacrey L-AR, Germani T, Bryson SE, Zwaigenbaum L. 2014. Reaching and grasping in autism spectrum disorder: a review of recent literature. Front. Neurol. 5, 6 ( 10.3389/fneur.2014.00006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kilner J. 2011. More than one pathway to action understanding. Trends Cogn. Sci. 15, 352–357. ( 10.1016/j.tics.2011.06.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kenny L, Hattersley C, Molins B, Buckley C, Povey C, Pellicano E. In press. Which terms should be used to describe autism? Perspectives from the UK autism community. Autism 1362361315588200 ( 10.1177/1362361315588200) [DOI] [PubMed] [Google Scholar]
- 19.Chen F-C, Tsai C-L. 2015. A light fingertip touch reduces postural sway in children with autism spectrum disorders. Gait Posture 43, 137–140. ( 10.1016/j.gaitpost.2015.09.012) [DOI] [PubMed] [Google Scholar]
- 20.Doumas M, McKenna R, Murphy B. 2015. Postural control deficits in autism spectrum disorder: the role of sensory integration. J. Autism Dev. Disord. 46, 853–861. ( 10.1007/s10803-015-2621-4) [DOI] [PubMed] [Google Scholar]
- 21.Fournier KA, Kimberg CI, Radonovich KJ, Tillman MD, Chow JW, Lewis MH, Bodfish JW, Hass CJ. 2010. Decreased static and dynamic postural control in children with autism spectrum disorders. Gait Posture 32, 6–9. ( 10.1016/j.gaitpost.2010.02.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fournier KA, Amano S, Radonovich KJ, Bleser TM, Hass CJ. 2014. Decreased dynamical complexity during quiet stance in children with autism spectrum disorders. Gait Posture 39, 420–423. ( 10.1016/j.gaitpost.2013.08.016) [DOI] [PubMed] [Google Scholar]
- 23.Graham SA, Abbott AE, Nair A, Lincoln AJ, Müller R-A, Goble DJ. 2014. The influence of task difficulty and participant age on balance control in ASD. J. Autism Dev. Disord. 45, 1419–1427. ( 10.1007/s10803-014-2303-7) [DOI] [PubMed] [Google Scholar]
- 24.Greffou S, Bertone A, Hahler E-M, Hanssens J-M, Mottron L, Faubert J. 2011. Postural hypo-reactivity in autism is contingent on development and visual environment: a fully immersive virtual reality study. J. Autism Dev. Disord. 42, 961–970. ( 10.1007/s10803-011-1326-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kohen-Raz R, Volkmar FR, Cohen DJ. 1992. Postural control in children with autism. J. Autism Dev. Disord. 22, 419–432. ( 10.1007/BF01048244) [DOI] [PubMed] [Google Scholar]
- 26.Minshew NJ, Sung K, Jones BL, Furman JM. 2004. Underdevelopment of the postural control system in autism. Neurology 63, 2056–2061. ( 10.1212/01.WNL.0000145771.98657.62) [DOI] [PubMed] [Google Scholar]
- 27.Molloy CA, Dietrich KN, Bhattacharya A. 2003. Postural stability in children with autism spectrum disorder. J. Autism Dev. Disord. 33, 643–652. ( 10.1023/B:JADD.0000006001.00667.4c) [DOI] [PubMed] [Google Scholar]
- 28.Morris SL, Foster CJ, Parsons R, Falkmer M, Falkmer T, Rosalie SM. 2015. Differences in the use of vision and proprioception for postural control in autism spectrum disorder. Neuroscience 307, 273–280. ( 10.1016/j.neuroscience.2015.08.040) [DOI] [PubMed] [Google Scholar]
- 29.Stins JF, Emck C, de Vries EM, Doop S, Beek PJ. 2015. Attentional and sensory contributions to postural sway in children with autism spectrum disorder. Gait Posture 42, 199–203. ( 10.1016/j.gaitpost.2015.05.010) [DOI] [PubMed] [Google Scholar]
- 30.Memari AH, Ghanouni P, Gharibzadeh S, Eghlidi J, Ziaee V, Moshayedi P. 2013. Postural sway patterns in children with autism spectrum disorder compared with typically developing children. Res. Autism Spectr. Disord. 7, 325–332. ( 10.1016/j.rasd.2012.09.010) [DOI] [Google Scholar]
- 31.Memari AH, Ghanouni P, Shayestehfar M, Ziaee V, Moshayedi P. 2014. Effects of visual search vs. auditory tasks on postural control in children with autism spectrum disorder. Gait Posture 39, 229–234. ( 10.1016/j.gaitpost.2013.07.012) [DOI] [PubMed] [Google Scholar]
- 32.Travers BG, Powell PS, Klinger LG, Klinger MR. 2012. Motor difficulties in autism spectrum disorder: linking symptom severity and postural stability. J. Autism Dev. Disord. 43, 1568–1583. ( 10.1007/s10803-012-1702-x) [DOI] [PubMed] [Google Scholar]
- 33.Esposito G, Venuti P. 2008. Analysis of toddlers’ gait after six months of independent walking to identify autism: a preliminary study. Percept. Mot. Skills 106, 259–269. ( 10.2466/pms.106.1.259-269) [DOI] [PubMed] [Google Scholar]
- 34.Esposito G, Venuti P, Apicella F, Muratori F. 2011. Analysis of unsupported gait in toddlers with autism. Brain Dev. 33, 367–373. ( 10.1016/j.braindev.2010.07.006) [DOI] [PubMed] [Google Scholar]
- 35.Hallett M, Lebiedowska MK, Thomas SL, Stanhope SJ, Denckla MB, Rumsey J. 1993. Locomotion of autistic adults. Arch. Neurol. 50, 1304–1308. ( 10.1001/archneur.1993.00540120019007) [DOI] [PubMed] [Google Scholar]
- 36.Nobile M, Perego P, Piccinini L, Mani E, Rossi A, Bellina M, Molteni M. 2011. Further evidence of complex motor dysfunction in drug naïve children with autism using automatic motion analysis of gait. Autism 15, 263–283. ( 10.1177/1362361309356929) [DOI] [PubMed] [Google Scholar]
- 37.Shetreat-Klein M, Shinnar S, Rapin I. 2014. Abnormalities of joint mobility and gait in children with autism spectrum disorders. Brain Dev. 36, 91–96. ( 10.1016/j.braindev.2012.02.005) [DOI] [PubMed] [Google Scholar]
- 38.Weiss MJ, Moran MF, Parker ME, Foley JT. 2013. Gait analysis of teenagers and young adults diagnosed with autism and severe verbal communication disorders. Front Integr Neurosci 7, 33 ( 10.3389/fnint.2013.00033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crippa A, Salvatore C, Perego P, Forti S, Nobile M, Molteni M, Castiglioni I. 2015. Use of machine learning to identify children with autism and their motor abnormalities. J. Autism Dev. Disord. 45, 2146–2156. ( 10.1007/s10803-015-2379-8) [DOI] [PubMed] [Google Scholar]
- 40.Dowd AM, McGinley JL, Taffe JR, Rinehart NJ. 2011. Do planning and visual integration difficulties underpin motor dysfunction in autism? A kinematic study of young children with autism. J. Autism Dev. Disord. 42, 1539–1548. ( 10.1007/s10803-011-1385-8) [DOI] [PubMed] [Google Scholar]
- 41.Glazebrook C, Gonzalez D, Hansen S, Elliott D. 2009. The role of vision for online control of manual aiming movements in persons with autism spectrum disorders. Autism 13, 411–433. ( 10.1177/1362361309105659) [DOI] [PubMed] [Google Scholar]
- 42.Glazebrook C, Elliott D, Lyons J. 2006. A kinematic analysis of how young adults with and without autism plan and control goal-directed movements. Motor Control 10, 244–264. [DOI] [PubMed] [Google Scholar]
- 43.Hughes C. 1996. Brief report: planning problems in autism at the level of motor control. J. Autism Dev. Disord. 26, 99–107. ( 10.1007/BF02276237) [DOI] [PubMed] [Google Scholar]
- 44.Mari M, Castiello U, Marks D, Marraffa C, Prior M. 2003. The reach-to-grasp movement in children with autism spectrum disorder. Phil. Trans. R. Soc. Lond. B 358, 393–403. ( 10.1098/rstb.2002.1205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stoit AMB, Schie HT. van Slaats-Willemse DIE, Buitelaar JK. 2013. Grasping motor impairments in autism: not action planning but movement execution is deficient. J. Autism Dev. Disord. 43, 2793–2806. ( 10.1007/s10803-013-1825-8) [DOI] [PubMed] [Google Scholar]
- 46.Rinehart NJ, Bellgrove MA, Tonge BJ, Brereton AV, Howells-Rankin D, Bradshaw JL. 2006. An examination of movement kinematics in young people with high-functioning autism and Asperger's disorder: further evidence for a motor planning deficit. J. Autism Dev. Disord. 36, 757–767. ( 10.1007/s10803-006-0118-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang H-C, Lee I-C, Lee I-C. 2014. Visual feedback and target size effects on reach-to-grasp tasks in children with autism. J. Autism Dev. Disord. 44, 3129–3139. ( 10.1007/s10803-014-2165-z) [DOI] [PubMed] [Google Scholar]
- 48.Cook J, Blakemore S, Press C. 2013. Atypical basic movement kinematics in autism spectrum conditions. Brain 136, 2816–2824. ( 10.1093/brain/awt208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alaniz ML, Galit E, Necesito CI, Rosario ER. 2015. Hand strength, handwriting, and functional skills in children with autism. Am. J. Occup. Ther. 69, 6904220030. ( 10.5014/ajot.2015.016022) [DOI] [PubMed] [Google Scholar]
- 50.Beversdorf DQ, Anderson JM, Manning SE, Anderson SL, Nordgren RE, Felopulos GJ, Bauman ML. 2001. Brief report: macrographia in high-functioning adults with autism spectrum disorder. J. Autism Dev. Disord. 31, 97–101. ( 10.1023/A:1005622031943) [DOI] [PubMed] [Google Scholar]
- 51.Fuentes CT, Mostofsky SH, Bastian AJ. 2009. Children with autism show specific handwriting impairments. Neurology 73, 1532–1537. ( 10.1212/WNL.0b013e3181c0d48c) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fuentes CT, Mostofsky SH, Bastian AJ. 2010. Perceptual reasoning predicts handwriting impairments in adolescents with autism. Neurology 75, 1825–1829. ( 10.1212/WNL.0b013e3181fd633d) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnson BP, Phillips JG, Papadopoulos N, Fielding J, Tonge B, Rinehart NJ. 2013. Understanding macrographia in children with autism spectrum disorders. Res. Dev. Disabil. 34, 2917–2926. ( 10.1016/j.ridd.2013.06.003) [DOI] [PubMed] [Google Scholar]
- 54.di Pellegrino G, Fadiga L, Fogassi L, Gallese V, Rizzolatti G. 1992. Understanding motor events: a neurophysiological study. Exp. Brain Res. 91, 176–180. ( 10.1007/BF00230027) [DOI] [PubMed] [Google Scholar]
- 55.Iacoboni M, Woods R, Brass M, Bekkering H, Mazziotta J, Rizzolatti G. 1999. Cortical mechanisms of human imitation. Science 286, 2526–2528. ( 10.1126/science.286.5449.2526) [DOI] [PubMed] [Google Scholar]
- 56.Grèzes J, Armony JL, Rowe J, Passingham RE. 2003. Activations related to ‘mirror’ and ‘canonical’ neurones in the human brain: an fMRI study. Neuroimage 18, 928–937. ( 10.1016/S1053-8119(03)00042-9) [DOI] [PubMed] [Google Scholar]
- 57.Aziz-Zadeh L, Koski L, Zaidel E, Mazziotta J, Iacoboni M. 2006. Lateralization of the human mirror neuron system. J. Neurosci. 26, 2964–2970. ( 10.1523/JNEUROSCI.2921-05.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Buccino G. et al. . 2001. Action observation activates premotor and parietal areas in a somatotopic manner: an fMRI study. Eur. J. Neurosci. 13, 400–404. ( 10.1046/j.1460-9568.2001.01385.x) [DOI] [PubMed] [Google Scholar]
- 59.Gazzola V, Rizzolatti G, Wicker B, Keysers C. 2007. The anthropomorphic brain: the mirror neuron system responds to human and robotic actions. Neuroimage 35, 1674–1684. ( 10.1016/j.neuroimage.2007.02.003) [DOI] [PubMed] [Google Scholar]
- 60.Shmuelof L, Zohary E. 2006. A mirror representation of others’ actions in the human anterior parietal cortex. J. Neurosci. 26, 9736–9742. ( 10.1523/JNEUROSCI.1836-06.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dinstein I, Hasson U, Rubin N, Heeger DJ. 2007. Brain areas selective for both observed and executed movements. J. Neurophysiol. 98, 1415–1427. ( 10.1152/jn.00238.2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gazzola V, Aziz-Zadeh L, Keysers C. 2006. Empathy and the somatotopic auditory mirror system in humans. Curr. Biol. 16, 1824–1829. ( 10.1016/j.cub.2006.07.072) [DOI] [PubMed] [Google Scholar]
- 63.Kilner J, Neal A, Weiskopf N, Friston K, Frith C. 2009. Evidence of mirror neurons in human inferior frontal gyrus. J. Neurosci. 29, 10 153–10 159. ( 10.1523/JNEUROSCI.2668-09.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chong TT-J, Cunnington R, Williams MA, Kanwisher N, Mattingley JB. 2008. fMRI adaptation reveals mirror neurons in human inferior parietal cortex. Curr. Biol. 18, 1576–1580. ( 10.1016/j.cub.2008.08.068) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cochin S, Barthelemy C, Roux S, Martineau J. 1999. Observation and execution of movement: similarities demonstrated by quantified electroencephalography. Eur. J. Neurosci. 11, 1839–1842. ( 10.1046/j.1460-9568.1999.00598.x) [DOI] [PubMed] [Google Scholar]
- 66.Cochin S, Barthelemy C, Lejeune B, Roux S, Martineau J. 1998. Perception of motion and qEEG activity in human adults. Electroencephalogr. Clin. Neurophysiol. 107, 287–295. ( 10.1016/S0013-4694(98)00071-6) [DOI] [PubMed] [Google Scholar]
- 67.Hari R, Forss N, Avikainen S, Kirveskari E, Salenius S, Rizzolatti G. 1998. Activation of human primary motor cortex during action observation: a neuromagnetic study. Proc. Natl Acad. Sci. USA 95, 15 061–15 065. ( 10.1073/pnas.95.25.15061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Babiloni C, Babiloni F, Carducci F, Cincotti F, Cocozza G, Del Percio C, Moretti DV, Rossini PM. 2002. Human cortical electroencephalography (EEG) rhythms during the observation of simple aimless movements: a high-resolution EEG study. NeuroImage 17, 559–572. ( 10.1006/nimg.2002.1192) [DOI] [PubMed] [Google Scholar]
- 69.Caetano G, Jousmäki V, Hari R. 2007. Actor's and observer's primary motor cortices stabilize similarly after seen or heard motor actions. Proc. Natl Acad. Sci. USA 104, 9058–9062. ( 10.1073/pnas.0702453104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kilner J, Marchant JL, Frith CD. 2009. Relationship between activity in human primary motor cortex during action observation and the mirror neuron system. PLoS ONE 4, e4925 ( 10.1371/journal.pone.0004925) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kilner J, Salenius S, Baker SN, Jackson A, Hari R, Lemon RN. 2003. Task-dependent modulations of cortical oscillatory activity in human subjects during a bimanual precision grip task. NeuroImage 18, 67–73. ( 10.1006/nimg.2002.1322) [DOI] [PubMed] [Google Scholar]
- 72.Kilner J, Baker SN, Salenius S, Hari R, Lemon RN. 2000. Human cortical muscle coherence is directly related to specific motor parameters. J. Neurosci. 20, 8838–8845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Avanzini P, Fabbri-Destro M, Dalla Volta R, Daprati E, Rizzolatti G, Cantalupo G. 2012. The dynamics of sensorimotor cortical oscillations during the observation of hand movements: an EEG study. PLoS ONE 7, e37534 ( 10.1371/journal.pone.0037534) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marty B, Bourguignon M, Jousmäki V, Wens V, Op de Beeck M, Van Bogaert P, Goldman S, Hari R, De Tiège X. 2015. Cortical kinematic processing of executed and observed goal-directed hand actions. Neuroimage 119, 221–228. ( 10.1016/j.neuroimage.2015.06.064) [DOI] [PubMed] [Google Scholar]
- 75.Zhou G, Bourguignon M, Parkkonen L, Hari R. 2016. Neural signatures of hand kinematics in leaders vs. followers: a dual-MEG study. Neuroimage 125, 731–738. ( 10.1016/j.neuroimage.2015.11.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Press C, Cook J, Blakemore S, Kilner J. 2011. Dynamic modulation of human motor activity when observing actions. J. Neurosci. 31, 2792–2800. ( 10.1523/JNEUROSCI.1595-10.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bach P, Tipper SP. 2007. Implicit action encoding influences personal-trait judgments. Cognition 102, 151–178. ( 10.1016/j.cognition.2005.11.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brass M, Bekkering H, Prinz W. 2001. Movement observation affects movement execution in a simple response task. Acta Psychol. (Amst.) 106, 3–22. ( 10.1016/S0001-6918(00)00024-X) [DOI] [PubMed] [Google Scholar]
- 79.Cook J, Bird G. 2011. Social attitudes differentially modulate imitation in adolescents and adults. Exp. Brain Res. 211, 601–612. ( 10.1007/s00221-011-2584-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cook J, Bird G. 2012. Atypical social modulation of imitation in autism spectrum conditions. J. Autism Dev. Disord. 42, 1045–1051. ( 10.1007/s10803-011-1341-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gillmeister H, Catmur C, Liepelt R, Brass M, Heyes C. 2008. Experience-based priming of body parts: a study of action imitation. Brain Res. 1217, 157–170. ( 10.1016/j.brainres.2007.12.076) [DOI] [PubMed] [Google Scholar]
- 82.Kilner J, Hamilton A, Blakemore S. 2007. Interference effect of observed human movement on action is due to velocity profile of biological motion. Soc. Neurosci. 2, 158–166. ( 10.1080/17470910701428190) [DOI] [PubMed] [Google Scholar]
- 83.Kilner J, Paulignan Y, Blakemore S. 2003. An interference effect of observed biological movement on action. Curr. Biol. 13, 522–525. ( 10.1016/S0960-9822(03)00165-9) [DOI] [PubMed] [Google Scholar]
- 84.Leighton J, Heyes C. 2010. Hand to mouth: automatic imitation across effector systems. J. Exp. Psychol. Hum. Percept. Perform. 36, 1174–1183. ( 10.1037/a0019953) [DOI] [PubMed] [Google Scholar]
- 85.Press C, Bird G, Flach R, Heyes C. 2005. Robotic movement elicits automatic imitation. Brain Res. Cogn. Brain Res. 25, 632–640. ( 10.1016/j.cogbrainres.2005.08.020) [DOI] [PubMed] [Google Scholar]
- 86.Hamilton A, Wolpert D, Frith U. 2004. Your own action influences how you perceive another person's action. Curr. Biol. 14, 493–498. ( 10.1016/j.cub.2004.03.007) [DOI] [PubMed] [Google Scholar]
- 87.Jacobs A, Shiffrar M. 2005. Walking perception by walking observers. J. Exp. Psychol. Hum. Percept. Perform. 31, 157–169. ( 10.1037/0096-1523.31.1.157) [DOI] [PubMed] [Google Scholar]
- 88.Pobric G, de C. Hamilton AF. 2006. Action understanding requires the left inferior frontal cortex. Curr. Biol. 16, 524–529. ( 10.1016/j.cub.2006.01.033) [DOI] [PubMed] [Google Scholar]
- 89.Buxbaum LJ, Kyle KM, Menon R. 2005. On beyond mirror neurons: internal representations subserving imitation and recognition of skilled object-related actions in humans. Cogn. Brain Res. 25, 226–239. ( 10.1016/j.cogbrainres.2005.05.014) [DOI] [PubMed] [Google Scholar]
- 90.Pazzaglia M, Smania N, Corato E, Aglioti SM. 2008. Neural underpinnings of gesture discrimination in patients with limb apraxia. J. Neurosci. 28, 3030–3041. ( 10.1523/JNEUROSCI.5748-07.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Negri GAL, Rumiati RI, Zadini A, Ukmar M, Mahon BZ, Caramazza A. 2007. What is the role of motor simulation in action and object recognition? Evidence from apraxia. Cogn. Neuropsychol. 24, 795–816. ( 10.1080/02643290701707412) [DOI] [PubMed] [Google Scholar]
- 92.Press C, Cook R. 2015. Beyond action-specific simulation: domain-general motor contributions to perception. Trends Cogn. Sci. 19, 176–178. ( 10.1016/j.tics.2015.01.006) [DOI] [PubMed] [Google Scholar]
- 93.Friston K, Mattout J, Kilner J. 2011. Action understanding and active inference. Biol. Cybern. 104, 137–160. ( 10.1007/s00422-011-0424-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kilner J, Friston KJ, Frith CD. 2007. Predictive coding: an account of the mirror neuron system. Cogn. Process 8, 159–166. ( 10.1007/s10339-007-0170-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rizzolatti G, Craighero L. 2004. The mirror-neuron system. Annu. Rev. Neurosci. 27, 169–192. ( 10.1146/annurev.neuro.27.070203.144230) [DOI] [PubMed] [Google Scholar]
- 96.Wolpert DM, Doya K, Kawato M. 2003. A unifying computational framework for motor control and social interaction. Phil. Trans. R. Soc. Lond. B 358, 593–602. ( 10.1098/rstb.2002.1238) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cook R, Bird G, Catmur C, Press C, Heyes C. 2014. Mirror neurons: from origin to function. Behav. Brain Sci. 37, 177–192. ( 10.1017/S0140525X13000903) [DOI] [PubMed] [Google Scholar]
- 98.Cross ES, de C. Hamilton AF. Grafton ST. 2006. Building a motor simulation de novo: observation of dance by dancers. NeuroImage 31, 1257–1267. ( 10.1016/j.neuroimage.2006.01.033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Casile A, Giese MA. 2006. Nonvisual motor training influences biological motion perception. Curr. Biol. 16, 69–74. ( 10.1016/j.cub.2005.10.071) [DOI] [PubMed] [Google Scholar]
- 100.Aglioti SM, Cesari P, Romani M, Urgesi C. 2008. Action anticipation and motor resonance in elite basketball players. Nat. Neurosci. 11, 1109–1116. ( 10.1038/nn.2182) [DOI] [PubMed] [Google Scholar]
- 101.Patel D, Fleming SM, Kilner JM. 2012. Inferring subjective states through the observation of actions. Proc. R. Soc. B 279, 4853–4860. ( 10.1098/rspb.2012.1847) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kirsch L, Drommelschmidt KA, Cross ES. 2013. The impact of sensorimotor experience on affective evaluation of dance. Front. Hum. Neurosci 7, 521 ( 10.3389/fnhum.2013.00521) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hove MJ, Risen JL. 2009. It's all in the timing: interpersonal synchrony increases affiliation. Soc. Cogn. 27, 949–960. ( 10.1521/soco.2009.27.6.949) [DOI] [Google Scholar]
- 104.Bailenson J, Yee N. 2005. Digital chameleons: automatic assimilation of nonverbal gestures in immersive virtual environments. Psychol. Sci. 16, 814–819. ( 10.1111/j.1467-9280.2005.01619.x) [DOI] [PubMed] [Google Scholar]
- 105.Chartrand T, Bargh J. 1999. The chameleon effect: the perception-behavior link and social interaction. J. Pers. Soc. Psychol. 76, 893–910. ( 10.1037/0022-3514.76.6.893) [DOI] [PubMed] [Google Scholar]
- 106.Kouzakova M, Karremans JC, van Baaren RB, van Knippenberg A. 2010. A stranger's cold shoulder makes the heart grow fonder: why not being mimicked by a stranger enhances longstanding relationship evaluations. Soc. Psychol. Pers. Sci. 1, 87–93. ( 10.1177/1948550609355718) [DOI] [Google Scholar]
- 107.Suzuki N, Takeuchi Y, Ishii K, Okada M. 2003. Effects of echoic mimicry using hummed sounds on human–computer interaction. Speech Commun. 40, 559–573. ( 10.1016/S0167-6393(02)00180-2) [DOI] [Google Scholar]
- 108.van Baaren R, Holland R, Kawakami K, van Knippenberg A. 2004. Mimicry and prosocial behavior. Psychol. Sci. 15, 71–74. ( 10.1111/j.0963-7214.2004.01501012.x) [DOI] [PubMed] [Google Scholar]
- 109.Ashton–James C, van Baaren RB, Chartrand TL, Decety J, Karremans J. 2007. Mimicry and me: the impact of mimicry on self-construal. Soc. Cogn. 25, 518–535. ( 10.1521/soco.2007.25.4.518) [DOI] [Google Scholar]
- 110.Calvo-Merino B, Grèzes J, Glaser DE, Passingham RE, Haggard P. 2006. Seeing or doing? Influence of visual and motor familiarity in action observation. Curr. Biol. 16, 1905–1910. ( 10.1016/j.cub.2006.07.065) [DOI] [PubMed] [Google Scholar]
- 111.Leary MR, Hill DA. 1996. Moving on: autism and movement disturbance. Ment. Retard. 34, 39–53. [PubMed] [Google Scholar]
- 112.Bliss J. 1980. Sensory experiences of Gilles de la Tourette syndrome. Arch. Gen. Psychiatry 37, 1343–1347. ( 10.1001/archpsyc.1980.01780250029002) [DOI] [PubMed] [Google Scholar]
- 113.Kahlbaum K. 1874. Catatonia [Transl. Y. Levij & T. Pridan] Baltimore, MD: John Hopkins University Press. [Google Scholar]
- 114.Rogers D. 1992. Motor disorder in psychiatry: towards a neurological psychiatry. New York, NY: Wiley. [Google Scholar]
- 115.Sacks O. 1990. Awakenings. New York, NY: Harper Perennial. [Google Scholar]
- 116.Lord C, Rutter M, Goode S, Heemsbergen J, Jordan H, Mawhood L, Schopler E. 1989. Autism diagnostic observation schedule: a standardized observation of communicative and social behavior. J. Autism Dev. Disord. 19, 185–212. ( 10.1007/BF02211841) [DOI] [PubMed] [Google Scholar]
- 117.Cattaneo L, Fabbri-Destro M, Boria S, Pieraccini C, Monti A, Cossu G, Rizzolatti G. 2007. Impairment of actions chains in autism and its possible role in intention understanding. Proc. Natl Acad. Sci. USA 104, 17 825–17 830. ( 10.1073/pnas.0706273104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bird G, Leighton J, Press C, Heyes C. 2007. Intact automatic imitation of human and robot actions in autism spectrum disorders. Proc. R. Soc. B 274, 3027–3031. ( 10.1098/rspb.2007.1019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Leighton J, Bird G, Charman T, Heyes C. 2008. Weak imitative performance is not due to a functional ‘mirroring’ deficit in adults with autism spectrum disorders. Neuropsychologia 46, 1041–1049. ( 10.1016/j.neuropsychologia.2007.11.013) [DOI] [PubMed] [Google Scholar]
- 120.Ramachandran V, Oberman L. 2006. Broken mirrors: a theory of autism. Sci. Am. 295, 62–69. ( 10.1038/scientificamerican1106-62) [DOI] [PubMed] [Google Scholar]
- 121.Rogers S, Pennington B. 1991. A theoretical approach to the deficits in infantile autism. Dev. Psychopathol. 3, 137–162. ( 10.1017/S0954579400000043) [DOI] [Google Scholar]
- 122.Southgate V, de C Hamilton AF. 2008. Unbroken mirrors: challenging a theory of autism. Trends Cogn. Sci. 12, 225–229. ( 10.1016/j.tics.2008.03.005) [DOI] [PubMed] [Google Scholar]
- 123.Williams J, Whiten A, Suddendorf T, Perrett DI. 2001. Imitation, mirror neurons and autism. Neurosci. Biobehav. Rev. 25, 287–295. ( 10.1016/S0149-7634(01)00014-8) [DOI] [PubMed] [Google Scholar]
- 124.Schilbach L. 2016. Towards a second-person neuropsychiatry. Phil. Trans. R. Soc. B 371, 20150081 ( 10.1098/rstb.2015.0081) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schilbach L, Timmermans B, Reddy V, Costall A, Bente G, Schlicht T, Vogeley K. 2013. Toward a second-person neuroscience. Behav. Brain Sci. 36, 393–414. ( 10.1017/S0140525X12000660) [DOI] [PubMed] [Google Scholar]
- 126.Ament K, Mejia A, Buhlman R, Erklin S, Caffo B, Mostofsky S, Wodka E. 2015. Evidence for specificity of motor impairments in catching and balance in children with autism. J. Autism Dev. Disord. 45, 742–751. ( 10.1007/s10803-014-2229-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gidley Larson JC, Mostofsky SH. 2008. Evidence that the pattern of visuomotor sequence learning is altered in children with autism. Autism Res. 1, 341–353. ( 10.1002/aur.54) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Izawa J, Pekny SE, Marko MK, Haswell CC, Shadmehr R, Mostofsky SH. 2012. Motor learning relies on integrated sensory inputs in ADHD, but over-selectively on proprioception in autism spectrum conditions. Autism Res. 5, 124–136. ( 10.1002/aur.1222) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.MacNeil LK, Mostofsky SH. 2012. Specificity of dyspraxia in children with autism. Neuropsychology 26, 165–171. ( 10.1037/a0026955) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mahajan R, Dirlikov B, Crocetti D, Mostofsky SH. 2015. Motor circuit anatomy in children with autism spectrum disorder with or without attention deficit hyperactivity disorder. Autism Res. 9, 67–81. ( 10.1002/aur.1497) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mahone EM, Powell SK, Loftis CW, Goldberg MC, Denckla MB, Mostofsky SH. 2006. Motor persistence and inhibition in autism and ADHD. J. Int. Neuropsychol. Soc. 12, 622–631. ( 10.1017/S1355617706060814) [DOI] [PubMed] [Google Scholar]
- 132.Pan C-Y, Tsai C-L, Chu C-H. 2009. Fundamental movement skills in children diagnosed with autism spectrum disorders and attention deficit hyperactivity disorder. J. Autism Dev. Disord. 39, 1694–1705. ( 10.1007/s10803-009-0813-5) [DOI] [PubMed] [Google Scholar]
- 133.Van Waelvelde H, Oostra A, Dewitte G, Van Den Broeck C, Jongmans MJ. 2010. Stability of motor problems in young children with or at risk of autism spectrum disorders, ADHD, and or developmental coordination disorder. Dev. Med. Child Neurol. 52, e174–e178. ( 10.1111/j.1469-8749.2009.03606.x) [DOI] [PubMed] [Google Scholar]
- 134.McPhillips M, Finlay J, Bejerot S, Hanley M. 2014. Motor deficits in children with autism spectrum disorder: a cross-syndrome study. Autism Res. 7, 664–676. ( 10.1002/aur.1408) [DOI] [PubMed] [Google Scholar]
- 135.Reiersen A, Constantino J, Todd R. 2008. Co-occurrence of motor problems and autistic symptoms in attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adoles. Psychiatry 47, 662–672. ( 10.1097/CHI.0b013e31816bff88) [DOI] [PubMed] [Google Scholar]
- 136.Cao R, Ye X, Chen X, Zhang L, Chen X, Tian Y, Hu P, Wang K. 2015. Exploring biological motion processing in Parkinson's disease using temporal dilation. PLoS ONE 10, e0138502 ( 10.1371/journal.pone.0138502) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Eddy CM, Sira Mahalingappa S, Rickards HE. 2014. Putting things into perspective: the nature and impact of theory of mind impairment in Huntington's disease. Eur. Arch. Psychiatry Clin. Neurosci. 264, 697–705. ( 10.1007/s00406-014-0498-4) [DOI] [PubMed] [Google Scholar]
- 138.Pellicano E, Burr D. 2012. When the world becomes ‘too real’: a Bayesian explanation of autistic perception. Trends Cogn. Sci. (Regul. Ed.) 16, 504–510. ( 10.1016/j.tics.2012.08.009) [DOI] [PubMed] [Google Scholar]
- 139.Lawson RP, Rees G, Friston KJ. 2014. An aberrant precision account of autism. Front. Hum. Neurosci. 8, 302 ( 10.3389/fnhum.2014.00302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Happé F, Frith U. 2006. The weak coherence account: detail-focused cognitive style in autism spectrum disorders. J. Autism Dev. Disord. 36, 5–25. ( 10.1007/s10803-005-0039-0) [DOI] [PubMed] [Google Scholar]
- 141.Sutherland DH, Olshen R, Cooper L, Woo SL. 1980. The development of mature gait. J. Bone Joint Surg. Am. 62, 336–353. [PubMed] [Google Scholar]
- 142.Brewer R, Biotti F, Catmur C, Press C, Happé F, Cook R, Bird G. 2015. Can neurotypical individuals read autistic facial expressions? Atypical production of emotional facial expressions in autism spectrum disorders. Autism Res. 9, 67–81. ( 10.1002/aur.1508) [DOI] [PMC free article] [PubMed] [Google Scholar]