Abstract
The amygdala is a complex structure that plays its role in perception and threat-related behaviour by activity of its specific nuclei and their separate networks. In the present functional magnetic resonance imaging study, we investigated the role of the basolateral amygdala in face and context processing. Five individuals with focal basolateral amygdala damage and 12 matched controls viewed fearful or neutral faces in a threatening or neutral context. We tested the hypothesis that basolateral amygdala damage modifies the relation between face and threatening context, triggering threat-related activation in the dorsal stream. The findings supported this hypothesis. First, activation was increased in the right precentral gyrus for threatening versus neutral scenes in the basolateral amygdala damage group compared with the control group. Second, activity in the bilateral middle frontal gyrus, and left anterior inferior parietal lobule was enhanced for neutral faces presented in a threatening versus neutral scene in the group with basolateral amygdala damage compared with controls. These findings provide the first evidence for the neural consequences of basolateral amygdala damage during the processing of complex emotional situations.
Keywords: amygdala, threat, emotion, face perception, basolateral amygdala, Urbach–Wiethe disease
1. Introduction
Facial expressions represent a substantial part of the threat signals humans have to deal with on a daily basis. We are scared because someone shows anger and we feel threatened when other people show signals of fear. The importance these threat signals acquire for our behaviour may often be a function of the whole context in which we encounter those signals. In real life, we rarely see a facial expression completely isolated from any other source of information. Instead, we recognize it, taking into account the information provided by the person's identity, the voice, the scene and other aspects that constitute the everyday context. Even seeing a neutral face feels different in broad daylight compared with when it is observed in a dark and empty alley. These intuitions indicate that to understand face perception and emotion recognition one must reckon with the role of the context.
Recent evidence indicates that the natural context plays an important role in determining how facial and also bodily expressions of emotion are processed and reacted to [1–13]. A facial expression is recognized faster when presented in a congruent emotional background (for example, a fearful face in a threatening context) [9]. On the other hand, the context can also bias the processing of the face towards the emotional valence of the scene in which it is seen. Naturalistic face processing is still relatively unexplored but several recent studies have provided the first insight into the neural basis of face context perception. For instance, scene context influence occurs early in time. The N170, an event-related potential that is linked to the first stages of face processing, shows an increase or decrease in amplitude depending on the context [3,8]. The few studies that have used functional magnetic resonance imaging (fMRI) to investigate the effect of affective context information on one or another stimulus category (faces, bodies, scenes) reported that the presence of affective information influences activity in the relevant category representation in the ventral stream, that is in the fusiform face area (FFA), the extrastriate body area (EBA), and the parahippocampal place area (PPA) [2,6,7]. The standard view is that the amygdala (AMG) is the central structure in orchestrating such up or down modulation [10,13,14]. For example, when a neutral face is seen in a threatening context, increased activation is observed in the FFA [6]. The presumed mechanism is based on feedback connections between the FFA and the AMG, similar to what was argued previously for the finding that a fearful face compared with a neutral face leads to increased activation in the FFA [15]. This picture reflects the standard function attributed to the AMG in human fMRI studies, whereby the AMG detects the valence or relevance of a stimulus [16,17].
So far, most previous studies on the human AMG did not specify the contributions of the different AMG subnuclei to the processing of the face and the context in which a facial expression is perceived. In order to push forward our understanding of the mechanisms whereby the AMG represents the affective, relevance and motivation dimension of information [18–20], one must take into account that the AMG is a heterogeneous structure. It consists of a large number of subnuclei with different connectivity patterns to major cortical areas involved in emotion perception and in adaptive reaction [21]. It would then seem that it is at the level of these nuclei, their interconnections and their connection profile to cortical structures that the effects of the AMG must be understood. In view of the complexity of the AMG, it may be that understanding its role is not only simply a matter of the range of stimuli in themselves that the AMG is sensitive to, but also of the context in which they appear [22]. Indeed, the AMG integrates the stimulus valence with task goals and context in a flexible way [23–25]. Viewed from this perspective, investigations of context rich stimuli provide a means of unravelling the complex machinery of the AMG.
The central-medial amygdala (CMA) and the basolateral amygdala (BLA) are presumably the most important subnuclei for understanding the role of the AMG in threat signal perception and the subsequent behavioural response. These two major subnuclei have very different connectivity profiles [26,27], but cooperate closely in regulating perception and action in the face of threat. The BLA and BLA-driven connections play a crucial role in the integration of emotional cues and computation of the affective value of the stimulus [28,29]. On the other hand, a CMA-network mediates reflexive reactions to threat [30,31], and this process is modulated by the BLA. Such modulation would seem to be needed when a neutral face is shown in a fear-triggering context, or, in the opposite direction, when a fearful face is seen against a bucolic scene background. It is known from animal studies that the BLA has strong connections with different brain areas not only in the ventral, but also dorsal stream [32–36], and influences activity in these networks [37]. These direct connections between the BLA and areas known to contribute to emotion expression perception in the ventral stream area, such as the fusiform gyrus, superior temporal sulcus and in action-related dorsal stream areas such as precentral gyrus, have also been observed in humans [38]. Both feed-forward and feedback connections between the amygdala, possibly the BLA [39] and ventral regions, such as the FFA, mediate contextual emotional face perception [5,40,41].
Studies of individuals with BLA damage offer a unique opportunity to clarify the role of the BLA and thereby that of the CMA in the overall picture of context-sensitive threat processing of the AMG. We tested a group of individuals with selective bilateral BLA damage and twelve matched controls. Several findings already suggest that damage to this structure would impact the processing in the dorsal stream more than the ventral stream during contextual emotional face perception. No consistent effect of damage to the AMG, including the BLA, on activity in ventral regions has been reported [15,42]. Against this background, we expected that damage to BLA would modify activity in the dorsal stream. We used the validated faces-in-context task [6,7], in which fearful or neutral faces are presented in a threatening or neutral background scene. In this task, attention is directed to the central target face such that the information from the scene is not relevant to the task and is processed implicitly. This is also in keeping with ecological situations, as the gaze of the observer tends to be primarily attracted by the face. Under these conditions, the context is preferentially engaging the dorsal stream areas [39,43–45]. We expected this implicit processing of unattended emotional contextual information in the dorsal stream to be enhanced in participants with BLA damage. In line with this suggestion, our previous behavioural results showed that BLA damage does not lead to impairments in emotion recognition per se, but to a deficit in ignoring task-irrelevant threat signals [46,47], suggesting no impairment in ventral stream (recognition), but possibly an enhancement of dorsal stream (detection) processing. In addition, previous studies highlight the notion of reflexive reactions when confronted with threat [48–50], something that is possibly increased after BLA damage [46]. BLA normally inhibits reflexive responsiveness to threat when a neutral face is presented in a threatening context or vice versa. Therefore, our prediction is that after BLA-damage, increased activation in dorsal action and specifically motor perception and preparation regions, such as the inferior parietal lobule and (pre)motor area, will be observed for faces presented in a threatening scene, even if the face is neutral in emotion. Furthermore, for participants with BLA damage, seeing a fearful face with a neutral background may not dampen activity in dorsal, action-related areas.
2. Material and method
(a). Participants
Five female participants with Urbach–Wiethe disease (UWD) partly from the South African UWD cohort [51] and 12 matched controls took part in the experiment. UWD, also known as lipoid proteinosis [52], is a rare genetic disorder that leads to a variety of symptoms such as thickening of the skin and beaded eyelid papules, and also results in calcification of brain tissue. Mutations in the extracellular matrix protein (ECM1) gene on chromosome 1 (1q21) are responsible for UWD symptoms [53]. The five UWD participants in the present study have a Q276X mutation in exon 7 of the ECM1 gene [54]. Both UWD and control participants live in the mountain desert areas of Namaqualand in the Northern Cape region in South Africa, are of mixed Western European and Indigenous Nama/Khoisan descent, and were matched on demographics and neurophysiological characteristics (table 1, see also [55]). None of the participants had a history of secondary psychopathology or epileptic insults. Participants were unaware of the aim of the study.
Table 1.
Demographic data. VIQ, verbal IQ; PIQ, performance IQ; FSIQ, full-scale IQ. Means and standard deviations are reported. No significant differences between groups, p's ≥ 0.78.
| UWDs (n = 5) |
controls (n = 12) |
||||||
|---|---|---|---|---|---|---|---|
| UWD 1 | UWD 2 | UWD 3 | UWD 4 | UWD 6 | mean | mean | |
| age | 27 | 34 | 38 | 52 | 39 | 38 ± 9.14 | 37.17 ± 5.20 |
| VIQ | 97 | 84 | 93 | 82 | 83 | 87.80 ± 6.76 | 86.67 ± 4.68 |
| PIQ | 99 | 87 | 85 | 84 | 87 | 88.40 ± 6.07 | 88.17 ± 5.39 |
| FSIQ | 98 | 84 | 87 | 81 | 83 | 86.60 ± 6.73 | 85.83 ± 4.43 |
(b). Basolateral amygdala lesion
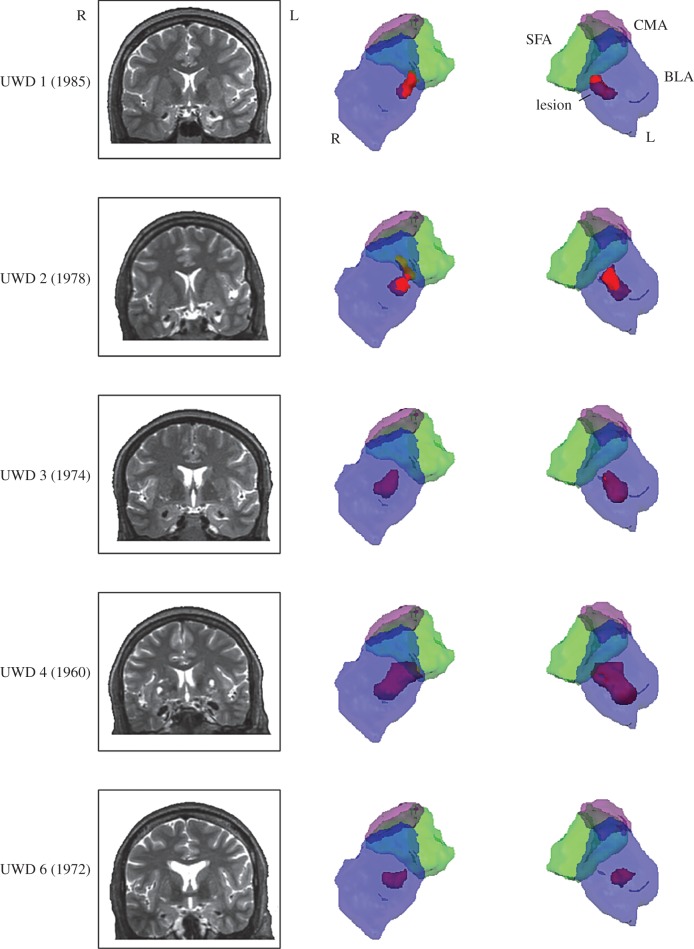
Previous work that assessed the extent of the lesion by means of structural and functional MRI showed that the calcification is restricted to the BLA, with other AMG regions unaffected [46,55,56]. The cytoarchitectonic probability that lesion voxels were located in the BLA was greater than 80% across the UWD group with minimal overlap with the CMA and superficial amygdala. Moreover, functional analysis revealed increased activity in the CMA during an emotion versus shape-matching task. In figure 1, the location and size as well as a three-dimensional reconstruction of the lesion are presented. The three-dimensional reconstruction of the AMG subnuclei was based on cytoarchitectonic probability maps from Amunts et al. [57] in Eickhoff et al. [58].
Figure 1.
Location and size of the BLA damage. Coronal view of T2-weighted magnetic resonance images (left) and three-dimensional reconstruction (right) of the lesion for the five UWD participants with birth year indicated.
(c). Stimuli and task
An adaptation of the faces-in-context task [6,7] was used in which fearful or neutral faces or a control shape were paired with a threatening, neutral or scrambled background. Figure 2 presents examples of the stimuli. Male and female faces were taken from the MacBrain Face Stimulus Set. We used different ethnicities to match the diversity of the population and thereby increase ecological validity. Background scenes were taken from Sinke et al. [7] and Van den Stock et al. [6]. Scenes (buildings, cars, and landscapes) with either a neutral or threatening value were used (for example, a parked car versus a car accident). No humans or animals were present in the scenes. Scrambled versions of the scenes were created by randomly rearranging the picture in 10 000 squares. Faces or triangles were presented in the middle of the background scene. To avoid floating faces, a black body-like shape accompanied the faces.
Figure 2.
Examples of the stimuli. Neutral (first column) or fearful faces (second column) or control shapes (third column) were presented on a neutral (first row), threatening (second row) or scrambled (third row) background.
A passive oddball task [59] was used. Participants were instructed to fixate on the cross overlaid on the nose of the face and pay attention to the transition of this cross into a red circle. To counteract any possible contamination of the blood-oxygenation-level dependent signal (BOLD) response by motor responses, participants did not have to make an overt response. The task and procedure were explained to the participant outside the scanner by a nurse familiar to the participants. The experiment commenced when participants indicated that they completely understood the instructions.
A block design was used and participants completed two runs of 6.5 min. Each run consisted of 18 stimulation blocks (nine different conditions repeated twice) and three oddball blocks presented in a random order, and two rest blocks presented at a fixed time point (block 11 and 22). The inter block interval was 6 s. During a stimulation block, 12 stimuli belonging to the same category (for example, fearful face in threatening background) were presented in a random order for 800 ms each, with an inter stimulus interval of 200 ms (total duration 12 s). In total, 48 trials per condition were presented (four blocks balanced over two runs). Rest blocks were included for a dynamic presentation, and no stimuli were shown during these blocks. Stimuli were presented using E-Prime 2.0 software (Psychology Software Tools, Pittsburgh, PA, USA), projected onto a screen located at the end of the scanner bore. Each new event was synchronous with a new scan volume.
(d). Image acquisition
Functional and anatomical data were acquired with a Siemens Magnetom Allegra 3 Tesla head-only scanner (Siemens Medical Systems GmBH, Erlangen, Germany) at the Cape Universities Brain Imaging Centre in Cape Town, South Africa. Earplugs attenuated the scanner noise and padding was used to reduce head movements. A two-dimensional echo-planar images sequence was used for functional whole-brain coverage. Each volume contained 36 slices acquired in ascending order with a 3.5 mm isotropic resolution (interslice gap = 0.525, repetition time (TR) = 2000 ms, echo time (TE) = 27 ms, flip angle (FA) = 70°, field of view (FOV) = 225 × 225 mm2, matrix size = 64 × 64). In total, 201 functional volumes were collected. A high-resolution T1-weighted anatomical scan was obtained with 1 mm isotropic resolution (no gap, TR = 2300 ms and TE = 39 ms, FA = 9°, FOV = 240 × 256 mm2, matrix size = 256 × 256).
(e). Functional magnetic resonance imaging preprocessing and analyses
Data preprocessing and analyses were carried out using BrainVoyager QX v. 2.8.4 (Brain Innovation, The Netherlands, www.brainvoyager.com). The first four volumes of each run were discarded from the analyses to avoid T1 saturation effects. Preprocessing of the functional data consisted of three steps. First, slice time correction (using sinc interpolation), a rigid-body algorithm to correct for small movements between scan (trilinear/sinc estimation and interpolation) and temporal high-pass filtering (GLM-Fourier with two cycles sine/cosine per run including linear trend removal) were applied. Next, functional data were co-registered to the anatomical data and normalized into Talairach space. Third, cortex-based alignment [60,61] was used to reduce individual macro-anatomical differences between participants and the two groups, thereby increasing statistical power. Using the individual curvature information derived from the gyri and sulci folding pattern, this high-resolution cortical mapping procedure results in non-rigid and superior alignment of different brains. Electronic supplementary material figure S1 presents the pre- and post-CBA alignment between the participants. No further spatial smoothing was used as the CBA procedure already applies smoothing to the data.
Data analyses consisted of two steps. First, at the single-subject level fixed-effects whole-brain general linear models (GLMs) were applied using a regression model with each condition and oddball block defined as predictors. Several predictors of no interest were added to the model. Besides the z-transformed motion predictors, outlier predictors were included [62,63]. This was done to further reduce error variance. For each run of each participant an outlier map was created. In this map, clusters with a time course value of greater than 6 s.d. above the mean were identified and manually inspected. The z-transformed time course of a cluster was only included in the design matrix if not related to incidental spike or movement. To prevent overfitting, we checked each design matrix for shared variance. If a predictor of no interest was explained by the combination of other predictors of no interest (R2 > 0.80), it was removed from the design matrix. Thus, besides the task predictors (9 + 1 oddball), motion predictors and possible outlier predictors were included in the design matrix. The number of predictors of no interest ranged between 5 and 7 and did not differ between groups, p's > 0.99.
Second, at the group level, a random-effects GLM was performed. We first assessed general categorical task effects to validate and compare the current dataset with the two previous studies by Van den Stock et al. [2,6] that used the same task design in healthy students. We used the two main categorical contrasts to map functional activation for the two groups combined by contrasting faces versus control shapes, and scenes versus scrambled scenes. Next, a between-subjects dummy-coded GLM was used to test our a priori hypotheses. First, we tested the overall effect of a threatening versus neutral scene regardless of the face condition (analysis I), as well as overall effects of fearful versus neutral faces regardless of the context condition (analysis II). Next, we mapped the specific effect of a neutral scene on the processing of fearful faces (analysis III) by contrasting fearful faces in a neutral scene versus fearful faces in a threatening scene. Lastly, we tested the specific effect of a threatening scene on processing of neutral faces (analysis IV) by contrasting neutral faces in a threatening scene with neutral faces in a neutral scene. All between-subjects maps were tested for UWDs > controls and controls > UWDs. The latter contrast revealed no significant clusters. Besides the between-subjects maps, we also reported the within-subjects maps for the UWD and control group separately and combined. For the between-subjects maps a whole-brain correction was applied using cluster-size correction that corresponds to a cluster-level false-positive rate (α) of 5% [64]. Recently, it was shown that a liberal initial threshold can increase false positives and result in low spatial specificity with large clusters [65]. However, given the unique sample, the use of cortex-based alignment and a random-effects GLM, the fine trade-off between type I and type II errors [66], as well as the observation of small clusters, we used a liberal initial single-voxel threshold of p = 0.01 with 1000 Monte-Carlo simulation iterations [61]. In line with guidelines for data visualization [67], we report if clusters survived a more stringent initial single-voxel threshold. The within-subjects maps for UWDs and controls were tested against zero using a one-sample t-test with a threshold set at p < 0.01 and with an extended cluster size of 25. All statistical maps are shown on the average group-aligned surface reconstruction, and Talairach coordinates and t- and p-values of peak vertices are reported.
Besides whole-brain analyses, we performed exploratory region of interest (ROI) analyses based on the main results of the previous studies using the same paradigm in neurotypical populations [6,7]. The following ROIs (5 × 5 × 5 mm sphere; x, y, z coordinates of centre of the ROI reported) were created: right occipital face area (OFA: 39, −72, −11), right FFA (39, −41, −20), bilateral PPA (RH: 19, −50, 0/LH: −22, −51, −4), right EBA (38, −69, 4), bilateral superior temporal sulcus (RH: 45, −50, 13/LH: −61, −41, 3) and the right cuneus (15, −94, −2). The majority of these regions were also activated in the study that used bodies instead of faces [2]. After extracting the β values from the ROIs, we tested the four main contrasts as well as a GLM for repeated measurements with faces (3) and scenes (3) as within subject factors, and group (2) as between subject factor.
3. Results
In line with previous results [2,6], faces versus control shapes elicited stronger activation in face selection regions in the fusiform gyrus, while scenes versus scrambled scenes resulted in increased activation in the transverse occipital sulcus, as well as the lingual and fusiform gyrus (electronic supplementary material, figure S2, tables S1 and S2). No differences were observed between UWD and controls for faces versus control shapes. However, UWD compared with control showed increased activation to scenes versus scrambled scenes in the left superior frontal gyrus, providing preliminary evidence for potential differences in the processing of contextual information.
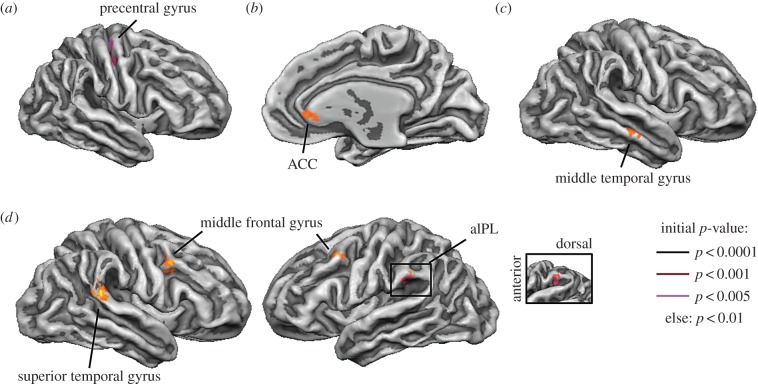
Compared with controls, UWDs showed more activation to threatening versus neutral scenes regardless of the type of face in the right precentral gyrus (figure 3a). Controls showed only increased activation in the cuneus for this contrast. Dorsal structures, such as the precentral gyrus and the inferior parietal lobule were less activated for threatening compared with neutral scenes (electronic supplementary material, table S2). Secondly, UWDs compared with controls showed enhanced activation for fearful faces versus neutral faces independent of context in the right anterior cingulate gyrus (ACC) (figure 3b). Thirdly, more activation was observed in the UWDs compared with the controls in the right middle temporal gyrus for fearful faces in a neutral scene compared with fearful faces in a threatening scene (figure 3c). Testing the specific effect of a threatening versus neutral scene on neutral face processing indicated that UWDs displayed increased activation in the right and left middle frontal gyrus, right superior temporal gyrus and left anterior inferior parietal lobule (aIPL) compared with controls (figure 3d). We ran the last two between-group analyses again, only with scrambled scenes as a control condition. When contrasting fearful faces in neutral scenes with fearful faces in scrambled scenes no significant between-group clusters emerged, even when an initial single voxel threshold of p = 0.05 was used. Using the same threshold revealed a similar between-group map when contrasting neutral faces in a threatening scene versus neutral faces in a scrambled scene. UWDs compared with controls showed more activation in the left aIPL and bilateral middle frontal gyrus during this contrast (electronic supplementary material, figure S3). The clusters in the middle frontal gyrus were slightly more anterior to the premotor cortex. Together, these results suggest that damage to BLA enhances activity in the dorsal stream when processing faces in a threatening context. Table 2 and electronic supplementary material tables S2–S5 report the results in detail.
Figure 3.
Basolateral amygdala damage and neural activation during contextual emotional face processing. UWDs compared with controls showed increased activation in the right precentral gyrus for threatening versus neutral scenes (a). UWDs in contrast with controls showed increased activation in the right ACC for fearful versus neutral faces (b), the middle temporal gyrus for fearful faces presented in a neutral compared with a threatening scene (c), and the right superior temporal gyrus, left aIPL and the bilateral middle frontal gyrus for neutral faces presented in a threatening compared with a neutral scene (d). Inset shows the location of the IPL cluster. All activations are cluster-size corrected and lines denote if clusters survive a more stringent initial single voxel threshold.
Table 2.
Outcome of the between-group functional activation analyses. For all clusters, UWDs > controls and p < 0.01, cluster-size corrected.
| Talairach coordinates |
||||||||
|---|---|---|---|---|---|---|---|---|
| hemisphere | x | y | z | Brodmann | t | p-values | no. vertices | |
| threatening versus neutral context | ||||||||
| precentral gyrus | RH | 37 | −18 | 56 | 4 | 5.777 | 0.000037 | 98 |
| fearful faces versus neutral faces | ||||||||
| anterior cingulate | RH | 7 | 36 | 7 | 24 | 4.108 | 0.000932 | 38 |
| fearful faces in a neutral scene compared with fearful faces in a threatening scene | ||||||||
| middle temporal gyrus | RH | 60 | −16 | −7 | 21 | 3.779 | 0.001820 | 36 |
| neutral faces in a threatening scene compared with neutral faces in a neutral scene | ||||||||
| middle frontal gyrus | RH | 44 | 8 | 37 | 9 | 5.060 | 0.000141 | 51 |
| superior temporal gyrus | RH | 59 | −36 | 17 | 40 | 9.166 | <0.000001 | 134 |
| anterior inferior parietal lobule | LH | −56 | −35 | 27 | 40 | 4.662 | 0.000307 | 102 |
| middle frontal gyrus | LH | −40 | 15 | 42 | 8 | 5.701 | 0.000042 | 29 |
(a). Exploratory region of interest analyses
What are the effects on activity in the ventral stream? We tested the effect of BLA damage on activity in ventral regions that were previously implicated in contextual face processing [2,6,7]. In line with the previous results[6], increased activation in face-selective areas (OFA and FFA) was observed for (fearful) faces compared with control shapes, while scenes compared with scrambled scenes increased activation in the right PPA for UWDs and controls combined. Only two between-group effects were found. First, a general between-group effect was found for the right Cuneus, with UWDs exhibiting more activation compared with controls for all conditions. Second, more activation was observed in the UWDs compared with the controls for fearful faces in a neutral scene, compared with fearful faces in a threatening scene in the right STS. While the cuneus lies at the beginning of the ventral and dorsal stream division, the STS receives input from both the ventral and dorsal streams [68,69]. Overall, the results suggest that after BLA damage no impairment is visible in the ventral stream during contextual face perception. See the electronic supplementary material table S6 for the ROI results.
4. Discussion
In this study, we investigated the role of the BLA on the neural circuitry of context-sensitive threat processing. The central hypothesis was that after BLA damage, faces presented in a threatening scene would lead to enhanced activation in action and motor regions of the dorsal stream. Results confirmed these expectations. BLA-damaged participants in contrast with control participants showed more activity in the right precentral gyrus for threatening versus neutral scenes. Furthermore, activity was also increased in the bilateral middle frontal gyrus and left aIPL for neutral faces presented in a threatening versus a neutral scene. When comparing fearful faces with neutral faces, regardless of the context, increased activation was found for BLA-damaged participants in the right ACC compared with controls. We also did not find decreased activation in action-related areas for fearful faces presented in a neutral scene in the BLA-damaged compared with the control group. Only activity in the right middle temporal gyrus was increased in this contrast. In addition, we did not observe a decrease in activity in ventral regions. Together, these findings suggest that BLA damage selectively influences dorsal stream activity.
(a). Motor and action regions
The present results suggest that after BLA damage, processing of facial expressions is more controlled by a dorsal-based route, especially when these are encountered in a threatening scene. Indeed, we observed increased activation for BLA-damaged participants compared with controls in the precentral gyrus during the perception of threatening versus neutral scenes, but also enhanced activation in the anterior part of the IPL and the bilateral middle frontal gyrus during the perception of neutral faces in a threatening scene. Activation of these regions may point to enhanced action-related mechanisms. First, the IPL, including the anterior part has been linked not only to action processes [70] but also to fear processing [48,71–73]. The aIPL is connected to premotor regions [74], and the areas found in the middle frontal gyrus correspond to the premotor cortex. Direct connections between the BLA and the primary and premotor cortex have been observed in primates [75] and humans [38]. This pathway would sustain a direct influence of the BLA on the mechanisms implementing reflexive reactions to threat. In the presence of BLA damage reflexive reactions may be seen irrespective of the contextual relevance of the threatening signal. Thus, when perceiving a neutral face in a threatening context, processing of the face stimulus is biased by the presence of irrelevant threat. In this study, we did not have behavioural measures but previous findings support this possibility [46,47]. Individuals with BLA damage have a lower threshold for recognition of fearful faces, a longer gaze duration to the eye region of fearful faces, and more interference from non-consciously perceived fearful faces [46]. And in line with this, they are more distracted by task-irrelevant threatening aspects of a stimulus like a happy face in combination with an angry body [47]. It is likely that an impaired ability to distinguish between relevant and irrelevant threat leads to increased preparation for reflexive reactions to threat.
(b). Prefrontal cortex
The observed increase in activity in the subgenual part of the ACC for fearful in contrast with neutral faces for the BLA-damaged compared with control participants fits this reasoning. The BLA has bidirectional connections with regions in the medial prefrontal cortex including the anterior cingulate [32,33], and these connections are proposed to mediate safety signalling and emotion regulation [28]. For example, a study in humans found that coupling between the subgenual part of the ACC and the AMG was related to emotion regulation [76]. Interestingly, the previous neurotypical study reported increased activity in this region for faces presented in threatening versus neutral scenes, and this was influenced by the personality of the observer [6]. Related to this, social anxiety is correlated with activity in the subgenual ACC [77]. While the importance of the subgenual ACC in concert with the AMG in adaptive emotional reactions is well appreciated, an outstanding question remains of how BLA damage influences these processes.
(c). Visual regions
Previous studies investigating context effects on emotional face, body or scene perception in neurotypical populations found activation predominantly in category-selective regions in the ventral stream, such as the EBA and PPA [2,6,7]. For example, two previous studies by Van den Stock et al. [2,6] increased activation in the EBA for threatening compared with neutral scenes, but found no activation of dorsal stream regions. The increased activation in the EBA has been interpreted as enhanced ambiguity reduction due to a cross-categorical bias between emotional expression and scenes [2]. Interestingly, we did not observe that activity in the EBA or in the PPA was changed in the BLA damage group compared with the control group for threatening versus neutral scenes. This suggests a relative independence of encoding of stimulus valence between ventral and dorsal structures. BLA neurons presumably encode the valence of the stimulus, and connections between the BLA and temporal regions have been implicated in emotion categorization [29]. As we did not observe any substantial differences in the ventral stream between the BLA-damaged and control group in the present study, it remains to be investigated whether BLA damage leads to reduced activation in the ventral stream, to selective increase in activity in the dorsal stream, or both. No clear indications are available from the literature. An early study that tested face processing in patients with hippocampus and amygdala damage, revealed decreased activity in the fusiform cortex for fearful compared with neutral faces, but normal activity for faces versus houses [15]. This suggests that the fusiform gyrus remains functional, but that AMG-mediated contextual regulation is disrupted. In line with our results, a recent study reported no differences in ventral stream activity between participants with unilateral amygdala damage and healthy controls during the processing of emotional images [42].
(d). Ventral and dorsal stream
The present results are consistent with a broader framework for understanding the complex way in which the brain deals with affective signals. Our proposal of a dual route of affective perception [39], and related perspectives [50,78–80], suggest that relatively independent detection and recognition routes underlie affective signal processing. These routes correspond to a dorsal and ventral division, respectively [69,81,82]. The first route comprises subcortical regions, such as the pulvinar and AMG as well as the dorsal stream and orbitofrontal cortex, and sustains early emotion processing, automatic action preparation and reflexive reactions. The second and parallel route sustains more elaborate stimulus recognition, decision-making and reflective action. Recent studies report on the reorganization of ventral and dorsal stream processing in a variety of populations [83,84]. For example, a recent study investigating visual processing in Williams syndrome found substantial reorganization of the dorsal stream, while the ventral stream was relatively unchanged [83]. As Williams syndrome has been associated with changes in AMG functioning [85–87], it provides important clues for the role of the AMG. One study reported that long-term damage to the entire AMG did lead to morphometric changes in the ventral stream, but not the dorsal stream [88]. On this account, BLA damage reveals the relative independence of these two routes. Moreover, our results suggest that the BLA is a crucial organizational hub that influences ventral and dorsal stream processing.
5. Conclusion
In this fMRI study, we investigated the role of the BLA in perception of faces in natural contexts focusing on the neural consequences of bilateral BLA calcification during contextual emotional face processing. Taken together, our results indicate that the BLA plays an important role in contextualizing threat perception as shown by the finding that bilateral BLA calcification leads to a ventral-to-dorsal processing shift. In other words, the BLA-driven changes in the network could result in a dominant role of a dorsal-based route sustaining rapid detection and reflexive defensive behaviour.
Supplementary Material
Acknowledgements
We are grateful to the volunteers for their participation in this study, and to Armin Heinecke and Minye Zhan for assistance in functional magnetic resonance imaging analyses. Development of the MacBrain Face Stimulus Set was overseen by Nim Tottenham and supported by the John D. and Catherine T. MacArthur Foundation Research Network on Early Experience and Brain Development. Contact Nim Tottenham at tott0006@tc.umn.edu for more information concerning the stimulus set.
Ethics
The study was approved by the Health Sciences Faculty Human Research Ethics Committee of the University of Cape Town and carried out in accordance with the standards set by the Declaration of Helsinki. Participants provided written informed consent.
Authors' contributions
R.H. designed the study, analysed and interpreted the data, and wrote the paper. D.T. designed the study, acquired and interpreted the data, and wrote the paper. B.M. acquired and interpreted the data, and wrote the paper. D.J.S. conceived the study, interpreted the data and wrote the paper. J.v.H. conceived and designed the study, interpreted the data and wrote the paper. B.d.G. conceived and designed the study, interpreted the data and wrote the paper. All authors provided final approval.
Competing interests
We have no competing interests.
Funding
B.d.G. and R.H. were partly funded by the project TANGO. The project TANGO acknowledges the financial support of the Future and Emerging Technologies (FET) programme within the Seventh Framework Programme for Research of the European Commission, under FET-Open grant no. 249858. B.d.G. has also received funding from the European Research Council under the European Union's Seventh Framework Programme (FP7/2007-2013)/ERC grant agreement no. 295673. D.T. was supported by grants from the Netherlands Organization for Scientific Research (NWO): VENI 451-13-004. D.J.S. was supported by the Medical Research Council of South Africa. J.v.H. was supported by grants from Utrecht University, the Netherlands Society of Scientific Research (Brain and Cognition: 056-24-010), the South African MRC/DST Professional Development Program and the University of Cape Town (Brain Behavior Initiative).
References
- 1.de Gelder B, Meeren HKM, Righart R, Van den Stock J, van de Riet WAC, Tamietto M. 2006. Beyond the face: exploring rapid influences of context on face processing. Prog. Brain Res. 155, 37–48. ( 10.1016/S0079-6123(06)55003-4) [DOI] [PubMed] [Google Scholar]
- 2.Van den Stock J, Vandenbulcke M, Sinke CBA, de Gelder B. 2014. Affective scenes influence fear perception of individual body expressions. Hum. Brain Mapp. 35, 492–502. ( 10.1002/hbm.22195) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Righart R, de Gelder B. 2008. Rapid influence of emotional scenes on encoding of facial expressions: an ERP study. Soc. Cogn. Affect. Neurosci. 3, 270–278. ( 10.1093/scan/nsn021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kret ME, de Gelder B. 2010. Social context influences recognition of bodily expressions. Exp. Brain Res. 203, 169–180. ( 10.1007/s00221-010-2220-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wieser MJ, Brosch T. 2012. Faces in context: a review and systematization of contextual influences on affective face processing. Front. Psychol. 3, 471 ( 10.3389/fpsyg.2012.00471) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van den Stock J, Vandenbulcke M, Sinke CBA, Goebel R, de Gelder B. 2014. How affective information from faces and scenes interacts in the brain. Soc. Cogn. Affect. Neurosci. 9, 1481–1488. ( 10.1093/scan/nst138) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sinke CBA, Van den Stock J, Goebel R, de Gelder B. 2012. The constructive nature of affective vision: seeing fearful scenes activates extrastriate body area. PLoS ONE 7, e38118 ( 10.1371/journal.pone.0038118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Righart R, de Gelder B. 2006. Context influences early perceptual analysis of faces---an electrophysiological study. Cereb. Cortex 16, 1249–1257. ( 10.1093/cercor/bhj066) [DOI] [PubMed] [Google Scholar]
- 9.Righart R, de Gelder B. 2008. Recognition of facial expressions is influenced by emotional scene gist. Cogn. Affect. Behav. Neurosci. 8, 264–272. ( 10.3758/CABN.8.3.264) [DOI] [PubMed] [Google Scholar]
- 10.Kim H, Somerville LH, Johnstone T, Polis S, Alexander AL, Shin LM, Whalen PJ. 2004. Contextual modulation of amygdala responsivity to surprised faces. J. Cogn. Neurosci. 16, 1730–1745. ( 10.1162/0898929042947865) [DOI] [PubMed] [Google Scholar]
- 11.Wieser MJ, Gerdes ABM, Büngel I, Schwarz KA, Mühlberger A, Pauli P. 2014. Not so harmless anymore: how context impacts the perception and electrocortical processing of neutral faces. NeuroImage 92, 74–82. ( 10.1016/j.neuroimage.2014.01.022) [DOI] [PubMed] [Google Scholar]
- 12.Van den Stock J, de Gelder B. 2012. Emotional information in body and background hampers recognition memory for faces. Neurobiol. Learn. Mem. 97, 321–325. ( 10.1016/j.nlm.2012.01.007) [DOI] [PubMed] [Google Scholar]
- 13.Mobbs D, Weiskopf N, Lau HC, Featherstone E, Dolan RJ, Frith CD. 2006. The Kuleshov effect: the influence of contextual framing on emotional attributions. Soc. Cogn. Affect. Neurosci. 1, 95–106. ( 10.1093/scan/nsl014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim H, Somerville LH, Johnstone T, Alexander AL, Whalen PJ. 2003. Inverse amygdala and medial prefrontal cortex responses to surprised faces. Neuroreport 14, 2317–2322. [DOI] [PubMed] [Google Scholar]
- 15.Vuilleumier P, Richardson MP, Armony JL, Driver J, Dolan RJ. 2004. Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nat. Neurosci. 7, 1271–1278. ( 10.1038/nn1341) [DOI] [PubMed] [Google Scholar]
- 16.Pessoa L. 2010. Emotion and cognition and the amygdala: from ‘what is it?’ to ‘what's to be done?’ Neuropsychologia 48, 3416–3429. ( 10.1016/j.neuropsychologia.2010.06.038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sander D, Grafman J, Zalla T. 2003. The human amygdala: an evolved system for relevance detection. Rev. Neurosci. 14, 303–316. ( 10.1515/REVNEURO.2003.14.4.303) [DOI] [PubMed] [Google Scholar]
- 18.Murray RJ, Brosch T, Sander D. 2014. The functional profile of the human amygdala in affective processing: insights from intracranial recordings. Cortex 60, 10–33. ( 10.1016/j.cortex.2014.06.010) [DOI] [PubMed] [Google Scholar]
- 19.Janak PH, Tye KM. 2015. From circuits to behaviour in the amygdala. Nature 517, 284–292. ( 10.1038/nature14188) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sabatinelli D, Fortune EE, Li Q, Siddiqui A, Krafft C, Oliver WT, Beck S, Jeffries J. 2011. Emotional perception: meta-analyses of face and natural scene processing. NeuroImage 54, 2524–2533. ( 10.1016/j.neuroimage.2010.10.011) [DOI] [PubMed] [Google Scholar]
- 21.McDonald AJ. 1998. Cortical pathways to the mammalian amygdala. Prog. Neurobiol. 55, 257–332. ( 10.1016/S0301-0082(98)00003-3) [DOI] [PubMed] [Google Scholar]
- 22.Ishai A, Pessoa L, Bikle PC, Ungerleider LG. 2004. Repetition suppression of faces is modulated by emotion. Proc. Natl Acad. Sci. USA 101, 9827–9832. ( 10.1073/pnas.0403559101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cunningham WA, Van Bavel JJ, Johnsen IR. 2008. Affective flexibility: evaluative processing goals shape amygdala activity. Psychol. Sci. 19, 152–160. ( 10.1111/j.1467-9280.2008.02061.x) [DOI] [PubMed] [Google Scholar]
- 24.Stillman PE, Van Bavel JJ, Cunningham WA. 2015. Valence asymmetries in the human amygdala: task relevance modulates amygdala responses to positive more than negative affective cues. J. Cogn. Neurosci. 27, 842–851. ( 10.1162/jocn_a_00756) [DOI] [PubMed] [Google Scholar]
- 25.Hindi Attar C, Müller MM, Andersen SK, Büchel C, Rose M. 2010. Emotional processing in a salient motion context: integration of motion and emotion in both V5/hMT+ and the amygdala. J. Neurosci. 30, 5204–5210. ( 10.1523/JNEUROSCI.5029-09.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bzdok D, Laird AR, Zilles K, Fox PT, Eickhoff SB. 2013. An investigation of the structural, connectional, and functional subspecialization in the human amygdala. Hum. Brain Mapp. 34, 3247–3266. ( 10.1002/hbm.22138) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swanson LW, Petrovich GD. 1998. What is the amygdala? Trends Neurosci. 21, 323–331. ( 10.1016/S0166-2236(98)01265-X) [DOI] [PubMed] [Google Scholar]
- 28.Likhtik E, Paz R. 2015. Amygdala–prefrontal interactions in (mal)adaptive learning. Trends Neurosci. 38, 158–166. ( 10.1016/j.tins.2014.12.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benarroch EE. 2015. The amygdala: functional organization and involvement in neurologic disorders. Neurology 84, 313–324. ( 10.1212/WNL.0000000000001171) [DOI] [PubMed] [Google Scholar]
- 30.Fox AS, Oler JA, Tromp DPM, Fudge JL, Kalin NH. 2015. Extending the amygdala in theories of threat processing. Trends Neurosci. 38, 319–329. ( 10.1016/j.tins.2015.03.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mosher CP, Zimmerman PE, Gothard KM. 2010. Response characteristics of basolateral and centromedial neurons in the primate amygdala. J. Neurosci. 30, 16 197–16 207. ( 10.1523/JNEUROSCI.3225-10.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heimer L, Harlan RE, Alheid GF, Garcia MM, de Olmos J. 1997. Substantia innominata: a notion which impedes clinical–anatomical correlations in neuropsychiatric disorders. Neuroscience 76, 957–1006. ( 10.1016/S0306-4522(96)00405-8) [DOI] [PubMed] [Google Scholar]
- 33.Ghashghaei HT, Barbas H. 2002. Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience 115, 1261–1279. ( 10.1016/S0306-4522(02)00446-3) [DOI] [PubMed] [Google Scholar]
- 34.Mogenson GJ, Jones DL, Yim CY. 1980. From motivation to action: functional interface between the limbic system and the motor system. Prog. Neurobiol. 14, 69–97. ( 10.1016/0301-0082(80)90018-0) [DOI] [PubMed] [Google Scholar]
- 35.Amaral DG. 2003. The amygdala, social behavior, and danger detection. Ann. NY Acad. Sci. 1000, 337–347. ( 10.1196/annals.1280.015) [DOI] [PubMed] [Google Scholar]
- 36.Freese JL, Amaral DG. 2005. The organization of projections from the amygdala to visual cortical areas TE and V1 in the macaque monkey. J. Comp. Neurol. 486, 295–317. ( 10.1002/cne.20520) [DOI] [PubMed] [Google Scholar]
- 37.Hart G, Leung BK, Balleine BW. 2014. Dorsal and ventral streams: the distinct role of striatal subregions in the acquisition and performance of goal-directed actions. Neurobiol. Learn Mem. 108, 104–118. ( 10.1016/j.nlm.2013.11.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grèzes J, Valabrègue R, Gholipour B, Chevallier C. 2014. A direct amygdala-motor pathway for emotional displays to influence action: a diffusion tensor imaging study. Hum. Brain Mapp. 35, 5974–5983. ( 10.1002/hbm.22598) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Gelder B, Hortensius R, Tamietto M. 2012. Attention and awareness each influence amygdala activity for dynamic bodily expressions-a short review. Front. Integr. Neurosci. 6, 54 ( 10.3389/fnint.2012.00054) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vuilleumier P, Driver J. 2007. Modulation of visual processing by attention and emotion: windows on causal interactions between human brain regions. Phil. Trans. R. Soc. B 362, 837–855. ( 10.1098/rstb.2007.2092) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rudrauf D, David O, Lachaux J-P, Kovach CK, Martinerie J, Renault B, Damasio AR. 2008. Rapid interactions between the ventral visual stream and emotion-related structures rely on a two-pathway architecture. J. Neurosci. 28, 2793–2803. ( 10.1523/JNEUROSCI.3476-07.2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Edmiston EK, McHugo M, Dukic MS, Smith SD, Abou-Khalil B, Eggers E, Zald DH. 2013. Enhanced visual cortical activation for emotional stimuli is preserved in patients with unilateral amygdala resection. J. Neurosci. 33, 11 023–11 031. ( 10.1523/JNEUROSCI.0401-13.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tamietto M, de Gelder B. 2010. Neural bases of the non-conscious perception of emotional signals. Nat. Rev. Neurosci. 11, 697–709. ( 10.1038/nrn2889) [DOI] [PubMed] [Google Scholar]
- 44.Zhan M, Hortensius R, de Gelder B. 2015. The body as a tool for anger awareness—differential effects of angry facial and bodily expressions on suppression from awareness. PLoS ONE 10, e0139768 ( 10.1371/journal.pone.0139768) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Corbetta M, Kincade JM, Ollinger JM, McAvoy MP, Shulman GL. 2000. Voluntary orienting is dissociated from target detection in human posterior parietal cortex. Nat. Neurosci. 3, 292–297. ( 10.1038/73009) [DOI] [PubMed] [Google Scholar]
- 46.Terburg D, Morgan BE, Montoya ER, Hooge IT, Thornton HB, Hariri AR, Panksepp J, Stein DJ, van Honk J. 2012. Hypervigilance for fear after basolateral amygdala damage in humans. Transl. Psychiatry 2, e115 ( 10.1038/tp.2012.46) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Gelder B, Terburg D, Morgan B, Hortensius R, Stein DJ, van Honk J. 2014. The role of human basolateral amygdala in ambiguous social threat perception. Cortex 52, 28–34. ( 10.1016/j.cortex.2013.12.010) [DOI] [PubMed] [Google Scholar]
- 48.de Gelder B, Snyder J, Greve D, Gerard G, Hadjikhani N. 2004. Fear fosters flight: a mechanism for fear contagion when perceiving emotion expressed by a whole body. Proc. Natl Acad. Sci. USA 101, 16 701–16 706. ( 10.1073/pnas.0407042101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schutter DJLG, Hofman D, van Honk J. 2008. Fearful faces selectively increase corticospinal motor tract excitability: a transcranial magnetic stimulation study. Psychophysiology 45, 345–348. ( 10.1111/j.1469-8986.2007.00635.x) [DOI] [PubMed] [Google Scholar]
- 50.Grèzes J, Dezecache G. 2014. How do shared-representations and emotional processes cooperate in response to social threat signals? Neuropsychologia 55, 105–114. ( 10.1016/j.neuropsychologia.2013.09.019) [DOI] [PubMed] [Google Scholar]
- 51.Thornton HB, Nel D, Thornton D, van Honk J, Baker GA, Stein DJ. 2008. The neuropsychiatry and neuropsychology of lipoid proteinosis. J. Neuropsychiatry Clin. Neurosci. 20, 86–92. ( 10.1176/appi.neuropsych.20.1.86) [DOI] [PubMed] [Google Scholar]
- 52.Quirici MB, da Rocha AJ. 2013. Teaching neuroimages: lipoid proteinosis (Urbach–Wiethe disease): typical findings in this rare genodermatosis. Neurology 80, e93 ( 10.1212/WNL.0b013e3182840741) [DOI] [PubMed] [Google Scholar]
- 53.Hamada T, et al. 2002. Lipoid proteinosis maps to 1q21 and is caused by mutations in the extracellular matrix protein 1 gene (ECM1). Hum. Mol. Genet. 11, 833–840. ( 10.1093/hmg/11.7.833) [DOI] [PubMed] [Google Scholar]
- 54.van Honk J, Terburg D, Thornton HB, Stein DJ, Morgan B. In press Consequences of selective bilateral lesions to the basolateral amygdala in humans. In Living without an amygdala (eds Amaral DG, Adolphs R). New York, NY: Guilford Press. [Google Scholar]
- 55.Morgan B, Terburg D, Thornton HB, Stein DJ, van Honk J. 2012. Paradoxical facilitation of working memory after basolateral amygdala damage. PLoS ONE 7, e38116 ( 10.1371/journal.pone.0038116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klumpers F, Morgan B, Terburg D, Stein DJ, van Honk J. 2015. Impaired acquisition of classically conditioned fear-potentiated startle reflexes in humans with focal bilateral basolateral amygdala damage. Soc. Cogn. Affect. Neurosci. 10, 1161–1168. ( 10.1093/scan/nsu164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, Habel U, Schneider F, Zilles K. 2005. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat. Embryol. 210, 343–352. ( 10.1007/s00429-005-0025-5) [DOI] [PubMed] [Google Scholar]
- 58.Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. 2005. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage 25, 1325–1335. ( 10.1016/j.neuroimage.2004.12.034) [DOI] [PubMed] [Google Scholar]
- 59.Carretié L, Hinojosa JA, Martín-Loeches M, Mercado F, Tapia M. 2004. Automatic attention to emotional stimuli: neural correlates. Hum. Brain Mapp. 22, 290–299. ( 10.1002/hbm.20037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Frost MA, Goebel R. 2012. Measuring structural–functional correspondence: spatial variability of specialised brain regions after macro-anatomical alignment. NeuroImage 59, 1369–1381. ( 10.1016/j.neuroimage.2011.08.035) [DOI] [PubMed] [Google Scholar]
- 61.Goebel R, Esposito F, Formisano E. 2006. Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: from single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Hum. Brain Mapp. 27, 392–401. ( 10.1002/hbm.20249) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luo W-L, Nichols TE. 2003. Diagnosis and exploration of massively univariate neuroimaging models. NeuroImage 19, 1014–1032. ( 10.1016/S1053-8119(03)00149-6) [DOI] [PubMed] [Google Scholar]
- 63.Carter CS, Heckers S, Nichols T, Pine DS, Strother S. 2008. Optimizing the design and analysis of clinical functional magnetic resonance imaging research studies. Biol. Psychiatry 64, 842–849. ( 10.1016/j.biopsych.2008.06.014) [DOI] [PubMed] [Google Scholar]
- 64.Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. 1995. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn. Reson. Med. 33, 636–647. ( 10.1002/mrm.1910330508) [DOI] [PubMed] [Google Scholar]
- 65.Woo C-W, Krishnan A, Wager TD. 2014. Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. NeuroImage 91, 412–419. ( 10.1016/j.neuroimage.2013.12.058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lieberman MD, Cunningham WA. 2009. Type I and type II error concerns in fMRI research: re-balancing the scale. Soc. Cogn. Affect. Neurosci. 4, 423–428. ( 10.1093/scan/nsp052) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Allen EA, Erhardt EB, Calhoun VD. 2012. Data visualization in the neurosciences: overcoming the curse of dimensionality. Neuron 74, 603–608. ( 10.1016/j.neuron.2012.05.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ungerleider LG, Haxby JV. 1994. 'What' and ‘where’ in the human brain. Curr. Opin. Neurobiol. 4, 157–165. ( 10.1016/0959-4388(94)90066-3) [DOI] [PubMed] [Google Scholar]
- 69.Goodale MA, Milner AD. 1992. Separate visual pathways for perception and action. Trends Neurosci. 15, 20–25. ( 10.1016/0166-2236(92)90344-8) [DOI] [PubMed] [Google Scholar]
- 70.Rizzolatti G, Matelli M. 2003. Two different streams form the dorsal visual system: anatomy and functions. Exp. Brain Res 153, 146–157. ( 10.1007/s00221-003-1588-0) [DOI] [PubMed] [Google Scholar]
- 71.Sinke CBA, Sorger B, Goebel R, de Gelder B. 2010. Tease or threat? Judging social interactions from bodily expressions. NeuroImage 49, 1717–1727. ( 10.1016/j.neuroimage.2009.09.065) [DOI] [PubMed] [Google Scholar]
- 72.Becker B, et al. 2012. Fear processing and social networking in the absence of a functional amygdala. Biol. Psychiatry 72, 70–77. ( 10.1016/j.biopsych.2011.11.024) [DOI] [PubMed] [Google Scholar]
- 73.Engelen T, de Graaf TA, Sack AT, de Gelder B. 2015. A causal role for inferior parietal lobule in emotion body perception. Cortex 73, 195–202. ( 10.1016/j.cortex.2015.08.013) [DOI] [PubMed] [Google Scholar]
- 74.Mars RB, et al. 2011. Diffusion-weighted imaging tractography-based parcellation of the human parietal cortex and comparison with human and macaque resting-state functional connectivity. J. Neurosci. 31, 4087–4100. ( 10.1523/JNEUROSCI.5102-10.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Avendaño C, Price JL, Amaral DG. 1983. Evidence for an amygdaloid projection to premotor cortex but not to motor cortex in the monkey. Brain Res. 264, 111–117. ( 10.1016/0006-8993(83)91126-5) [DOI] [PubMed] [Google Scholar]
- 76.Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. 2007. Amygdala–frontal connectivity during emotion regulation. Soc. Cogn. Affect. Neurosci. 2, 303–312. ( 10.1093/scan/nsm029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ball TM, Sullivan S, Flagan T, Hitchcock CA, Simmons A, Paulus MP, Stein MB. 2012. Selective effects of social anxiety, anxiety sensitivity, and negative affectivity on the neural bases of emotional face processing. Neuroimage 59, 1879–1887. ( 10.1016/j.neuroimage.2011.08.074) [DOI] [PubMed] [Google Scholar]
- 78.de Gelder B, Rouw R. 2001. Beyond localisation: a dynamical dual route account of face recognition. Acta Psychol. (Amst.) 107, 183–207. ( 10.1016/S0001-6918(01)00024-5) [DOI] [PubMed] [Google Scholar]
- 79.Vuilleumier P. 2005. How brains beware: neural mechanisms of emotional attention. Trends Cogn. Sci. 9, 585–594. ( 10.1016/j.tics.2005.10.011) [DOI] [PubMed] [Google Scholar]
- 80.Grèzes J, Adenis M-S, Pouga L, Armony JL. 2013. Self-relevance modulates brain responses to angry body expressions. Cortex 49, 2210–2220. ( 10.1016/j.cortex.2012.08.025) [DOI] [PubMed] [Google Scholar]
- 81.Binkofski F, Buxbaum LJ. 2013. Two action systems in the human brain. Brain Lang 127, 222–229. ( 10.1016/j.bandl.2012.07.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ungerleider LG, Mishkin M. 1982. Two cortical visual systems. In Analysis of visual behavior (eds Goodale MA, Ingle DJ, Mansfield RJW), pp. 549–586. Cambridge, MA: MIT Press. [Google Scholar]
- 83.Bernardino I, Rebola J, Farivar R, Silva E, Castelo-Branco M. 2014. Functional reorganization of the visual dorsal stream as probed by 3-D visual coherence in Williams syndrome. J. Cogn. Neurosci. 26, 2624–2636. ( 10.1162/jocn_a_00662) [DOI] [PubMed] [Google Scholar]
- 84.Crockford DN, Goodyear B, Edwards J, Quickfall J, el-Guebaly N. 2005. Cue-induced brain activity in pathological gamblers. Biol. Psychiatry 58, 787–795. ( 10.1016/j.biopsych.2005.04.037) [DOI] [PubMed] [Google Scholar]
- 85.Meyer-Lindenberg A, Mervis CB, Faith Berman K. 2006. Neural mechanisms in Williams syndrome: a unique window to genetic influences on cognition and behaviour. Nat. Rev. Neurosci. 7, 380–393. ( 10.1038/nrn1906) [DOI] [PubMed] [Google Scholar]
- 86.Meyer-Lindenberg A, Hariri AR, Munoz KE, Mervis CB, Mattay VS, Morris CA, Berman KF. 2005. Neural correlates of genetically abnormal social cognition in Williams syndrome. Nat. Neurosci. 8, 991–993. ( 10.1038/nn1494) [DOI] [PubMed] [Google Scholar]
- 87.Bellugi U, Adolphs R, Cassady C, Chiles M. 1999. Towards the neural basis for hypersociability in a genetic syndrome. Neuroreport 10, 1653–1657. ( 10.1097/00001756-199906030-00006) [DOI] [PubMed] [Google Scholar]
- 88.Boes AD, Mehta S, Rudrauf D, Van Der Plas E, Grabowski T, Adolphs R, Nopoulos P. 2012. Changes in cortical morphology resulting from long-term amygdala damage. Soc. Cogn. Affect. Neurosci. 7, 588–595. ( 10.1093/scan/nsr047) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.