Abstract
The ability to move safely between obstacles is critical for animals that fly rapidly through cluttered environments but surprisingly little is known about how they achieve this. Do they reactively avoid obstacles or do they instead fly towards the gaps between them? If they aim towards gaps, what information do they use to detect and fly through them? Here, we aim to answer these questions by presenting orchid bees with different apertures. When negotiating gaps, orchid bees locate and fly close to the point that gives them greatest clearance from the edges. The cue that they use to pinpoint this spot is the brightness gradient formed across the aperture. Furthermore, we find that orchid bees also rely on brightness cues to locate gaps that are sufficiently large to negotiate safely. The advantage of using brightness for locating and negotiating gaps in a cluttered environment is that it provides information about the safest path through obstacles, at least in a forest environment. This brightness-based guidance strategy for gap detection and negotiation represents a fast, computationally simple and efficient mechanism to identify the clearest path through a forest and is, therefore, likely to represent a more general mechanism used by other animals.
Keywords: flight, insect, vision, guidance, brightness, orchid bee
1. Introduction
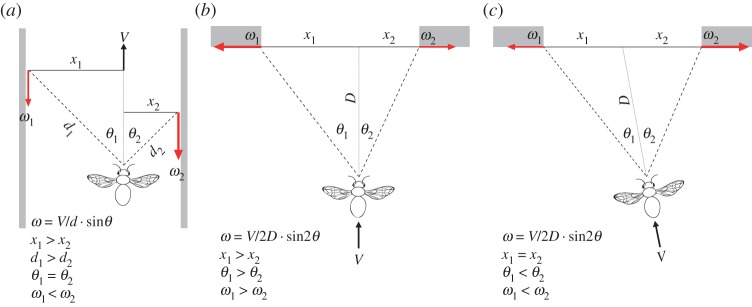
The tropical rainforest is arguably one of the most spatially complex environments on the Earth. Nonetheless, birds, bats and insects routinely fly at high speeds through the dense tropical undergrowth, a feat that requires them to quickly and efficiently locate the safest path through clutter. Despite decades of research investigating how flying animals control flight, we know surprisingly little about how they safely guide their path in complex environments. When flying through an artificial forest, pigeons tend to aim towards the largest visual gaps [1] although it is not clear exactly what cues they use to do this. Honeybees, bumblebees and budgerigars negotiate narrow corridors by extracting information from the wide-field image motion (known as optic flow) that is generated on their eyes as they move [2–6]. However, the nature of the image motion in a narrow corridor is quite specific: the angular velocity of the optic flow generated by the nearby obstacles—that is to say, the walls—on each eye varies with the following relationship: V/d · sin θ, where V is the velocity, d is the distance to the wall and θ is the viewing angle at which the optic flow is being measured. If the animal is not moving along the midline of the corridor, the angular velocity of optic flow experienced in the eye viewing the nearer wall will be higher (figure 1a). Honeybees, bumblebees and budgerigars respond to such imbalances by increasing their distance from the near wall, apparently adjusting their position until the angular velocities in the left and right eye are equalized, or balanced [2,3,5,6]. This simple strategy, known as the optic flow balancing hypothesis, ensures that the risk of collision with the walls is minimized by maximizing the distance to surrounding obstacles without the need for absolute distance information.
Figure 1.
The pattern of motion generated on the eye of a bee flying along a corridor or through apertures. The relative difference in the magnitude of optic flow (ω, red arrows), generated by points on the walls on each side of a corridor on the eyes of a bee flying with a different distance to each wall (d) but viewing them at the same angle (Θ, a). The relationship between the direction and magnitude of optic flow (red arrows) generated by the edges of an aperture on the eye of a bee flying on a perpendicular but off-centre trajectory (b) or diagonally towards the centre of an aperture (c). D, the perpendicular distance to the aperture; X, lateral distance to the point generating the optic flow.
The natural forest habitat, however, is very different from a corridor. Unlike the walls in a corridor, obstacles in a cluttered environment (such as trees in a forest) create a non-uniform series of shallow, essentially two-dimensional, apertures. The most efficient strategy for safely negotiating a narrow aperture is to locate the point that provides the greatest clearance from the edges and to adjust the trajectory accordingly before the gap is reached. A crucial difference between corridor walls and aperture edges is that they generate very different patterns of image motion. Instead of forming a pattern of front-to-back optic flow (figure 1a), the edges of an aperture would generate a pattern of expansion in the frontal visual field as it is approached (figure 1b,c). The magnitude of this expansion pattern is defined by: V/2D · sin 2θ, where V is the velocity, D is the perpendicular distance to the aperture and θ is the viewing angle at which the optic flow of the edge is measured (see [7] for a detailed explanation and derivation). An important consequence of this relationship is that the relative difference in the angular velocities generated by the aperture's edges changes with different approach trajectories. For trajectories that are perpendicular to the aperture but off-centre, the edge that is further away will generate a higher magnitude of angular velocity than the one that is nearer (figure 1b). This is effectively the opposite of the relative motion differences that would be generated by an off-centre trajectory in a corridor (figure 1a). An attempt to rectify the imbalance by moving away from the edge that generates the higher angular velocity would cause the animal to move increasingly further from the centre of the aperture, elevating the chance of colliding with an edge. Similarly, a safe but oblique trajectory towards the centre of the aperture will result in the near edge generating a higher angular velocity than the far one (figure 1c) and any attempt to equalize the angular velocities of the edges would cause the animal to move away from the centre of the aperture, enhancing the risk of collision by decreasing the distance to one of the edges. If animals use an optic flow balancing strategy to negotiate an aperture as has been previously proposed [5], they will therefore only be able to locate and fly through the safest point if the approach is perpendicular to the plane of the aperture and is directed towards its centre. This raises several important questions: do animals try to fly through the safest point when negotiating narrow apertures, and if so, do they do this from the rate of expansion of optic flow of the edges or do they use a different strategy?
In this study, we aim to answer these questions using orchid bees Euglossa imperialis that have inhabited tropical rainforests for the past 20 Myr [8] and that are therefore likely to have developed an efficient method for guiding flight through their cluttered habitat. In their pursuit of scent from spatially rare orchid flowers, male orchid bees routinely fly over large distances [9,10] at high speed through the dense rainforest undergrowth, adeptly avoiding collisions with trees, branches and leaves. This feat is particularly impressive considering the limitations imposed upon them by their miniature brains and sensory systems, which cannot make use of stereo vision or other sophisticated mechanisms for determining the absolute distance to nearby obstacles. Understanding how they overcome the challenges imposed on them by their habitat and lifestyle is important for understanding the sensory, neural and behavioural mechanisms that animals have evolved in order to cope with the demands of moving through cluttered and unpredictable environments. In addition, this information can provide important insights for the development of small lightweight guidance systems that could allow autonomous robots to perform similar feats in spatially complex environments.
2. Results
(a). Orchid bees locate and fly close to the point of greatest clearance in an aperture
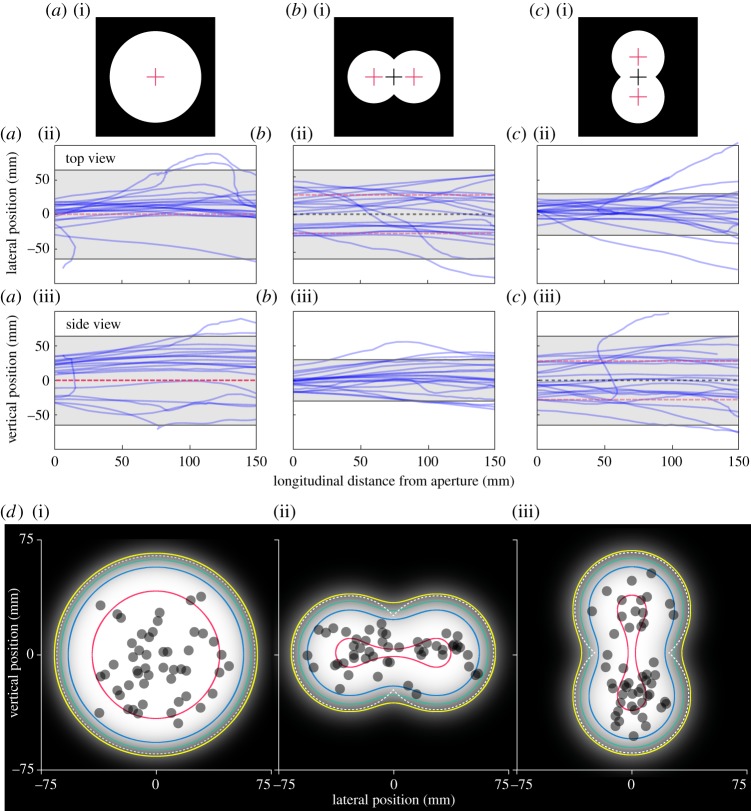
The trajectories of free flying orchid bees were recorded as they flew out of two different apertures, a 130 mm diameter circle (figure 2a(i)) and a ‘double circle’ made of two 75 mm diameter overlapping circular apertures that was 130 mm along its long axis (figure 2b(i),c(i)). Top and side views of 20 bees flying out of each aperture are shown in figure 2. The approach paths are not necessarily straight or perpendicular to the plane of the aperture; nonetheless, in the circular aperture the bees positioned themselves very close to the centre (lateral position: 12(14) mm, vertical position: 21(17) mm, median (25%–75% interquartile range), n = 40). In a circular aperture, the centre overlaps with the point of greatest clearance (i.e. the safest point to fly through). A double circle rather has two points of greatest clearance; one at the centre of each overlapping circle (28.5 mm from the centre, red pluses, figure 2b(i),c(i)). If the bees use an optic flow balancing strategy to guide their flight through apertures they should fly through the centre of the double circle (grey pluses, figure 2b(i),c(i)), if they are using a strategy that allows them to locate the point of greatest clearance they should instead fly close to the centre of one of the overlapping circles, that is, either 28.5 mm laterally or vertically from the centre (depending on whether the long axis of the double circle is oriented horizontally or vertically, respectively). When the long axis of the double circle was oriented horizontally, the lateral position of bees was further from the centre (21(16) mm, n = 40) and closer to a point of greatest clearance (7.5(16) mm, if the origin is placed at a point of greatest clearance) than in the circle aperture (p = 0.0019, Z = 3). Similarly, the vertical position of bees in the vertically oriented double circle (24(17) mm, n = 40) was also closer to a point of greatest clearance (4.5(17) mm) than to the centre although this was not significantly different from the already large vertical distance from the centre recorded in the circular aperture (p = 0.7, Z = 0.4). It is important to note that there appears to be a slight bias in the flight paths towards the right-hand side of the tunnel, this is most probably due to an unevenness of the illumination outside of the tunnel. Nonetheless, the flight paths indicate that orchid bees do not necessarily make centred, or perpendicular approaches when flying out of apertures and that they fly closer to the point of greatest clearance, rather than the geometric centre if these are not concurrent. It, therefore, seems unlikely that they rely primarily on an optic flow balancing strategy to guide their flight through apertures.
Figure 2.
Orchid bees fly towards the point of greatest clearance of an aperture. Bees were presented with a 130 mm circular aperture (a)(i) or double circle aperture oriented horizontally (b)(i) or vertically (c)(i). Red crosses mark the point of greatest clearance in the aperture (which coincides with the geometric centre in the circle), grey crosses mark the geometric centre. The flight paths (blue lines) of the bees flying out of these apertures are shown in top view ((a)(ii)–(c)(ii)) and side view ((a)(iii)–(c)(iii)). Grey areas indicate the width of the aperture, red dashed lines indicate the position of the point of greatest clearance and black dashed lines indicate the geometric centre of the double circle aperture for each orientation. ((d)(i–iii)) The apertures shown in ((a)(i)–(c)(i), respectively) processed with a Gaussian blur to represent how they might be perceived by orchid bees at a distance of 400 mm. Contours enclose levels of contrast between the bright pixels and the dark edge beginning with 40% (yellow), 60% (green), 80% (blue) and 100% (red). White dashed lines mark the physical edge of the aperture. Overlaid on the plots are the positions of orchid bees (grey dots) when presented with the 130 mm diameter circular aperture ((d)(i), see also (a)(i)) or a double circular aperture oriented horizontally ((d)(ii), see also (b)(i)) or vertically ((d)(iii), see also (c)(i)).
A possible mechanism for position control when negotiating narrow gaps is to extract information about the distance to the aperture's edges from their relative motion against the background. To investigate whether such motion parallax cues play a role in orchid bee gap negotiation behaviour, we presented them with the same apertures as above but this time blocked the view of the background by placing a diffuser behind them. Interestingly, the bees did not appear to detect the presence of the diffuser but instead reliably crashed into it as they tried to exit the chamber. In the absence of motion parallax information, the position of the bees just prior to contacting the diffuser was once again centred in the circle (lateral position = 16(18) mm, vertical position = 15(21) mm, figure 2d(i)). In the double circle, the bees flew very close to a point of greatest clearance irrespective of whether the long axis was oriented horizontally (lateral position = 26(24) mm, circle versus horizontal double circle: Plateral = 0.004, Zlateral = −2.9, figure 2d(ii)) or vertically (vertical position = 27(17) mm, circle versus vertical double circle: Pvertical = 0.004, Zvertical = −2.9, figure 2d(iii)). These findings suggest that orchid bees do not require motion parallax cues, or optic flow cues, to negotiate narrow apertures.
What other cues might orchid bees use for locating the point of greatest clearance when flying through an aperture? One possible cue that would be available in both the open and closed apertures used above is the brightness gradient that forms across an aperture and its edges. Although not initially obvious when considering a high-resolution, high-contrast image of an aperture (as in figure 2a(i)–c(i)), the concept of using a brightness gradient to control position becomes apparent if we consider how the bees might perceive the aperture through their low-resolution optics. Figure 2d provides an approximation of how the different apertures tested in the experiments above might appear through the eyes of an orchid bee at a distance of 400 mm (images processed with a Gaussian blur assuming a maximum resolution of 1.8° as measured for similarly sized bumblebees in [11]), with contour lines encircling regions with different levels of contrast (used in the images here used as a proxy for brightness in a real aperture) with respect to the black background. This analysis demonstrates that the region of highest contrast is focused on the point of greatest clearance from the edges, showing that the brightness gradient across an aperture can be a useful cue for avoiding collisions when negotiating gaps. The grey dots in figure 2d indicate the position of bees when attempting to exit the apertures that were covered with a diffuser. Interestingly, the distribution of the points tends to follow the shape of the contrast contour lines, providing further evidence that they use the brightness gradient to guide their flight through apertures.
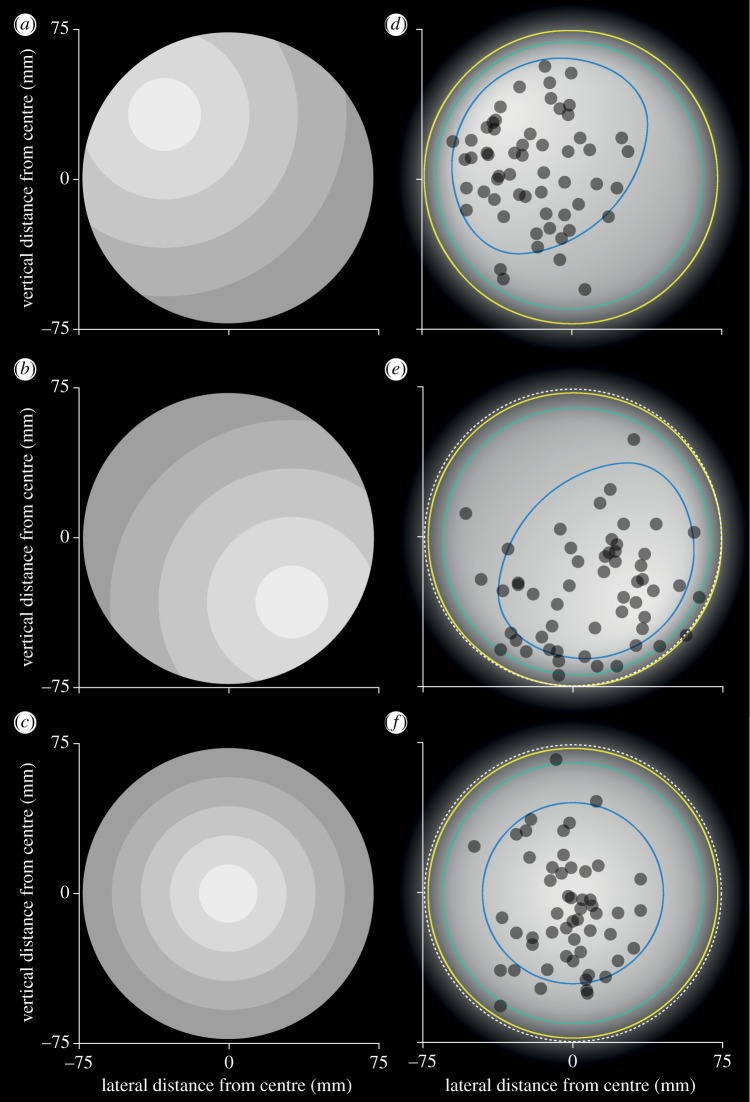
To test the hypothesis that orchid bees use brightness cues to negotiate gaps safely, we presented them with an aperture in which the brightness gradient was artificially shifted 32.7 mm laterally and vertically away from the point of greatest clearance (i.e. the centre of the circle)—either upwards and left (‘Up45’, figure 3a) or downwards and right (‘Down45’, figure 3b) along a 45° axis—using a series of overlapping neutral density (ND) filters. These results were compared with the position of bees when attempting to fly through a closed aperture in which the artificial brightness gradient was centred (‘Centre’, figure 3c). If the bees use brightness cues to control their position when negotiating apertures, we would expect them to position themselves close to the shifted centre of brightness, which is indeed what happened. In the Up45 condition, the bees flew to the left of the vertical midline of the aperture (lateral position = −25(32) mm) and above the horizontal midline of the aperture (vertical position 10(28) mm), In the Down45 condition, the bees instead flew to the right of the aperture's vertical midline (lateral position = 13(39) mm) and below its horizontal midline (vertical position = −25(39) mm). By contrast, the bees flew very close to the centre of the aperture in the Centre condition (lateral position = 0(19) mm, vertical position = −10(43) mm). The change in position caused by shifting the brightest point in Up45 and Down45 were significantly different from the Centre stimulus in both the lateral (centre versus Up45: 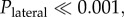 Zlateral = 5; centre versus Down45: Plateral = 0.047) and vertical (Pvertical = 0.03, Zvertical = 3; Pvertical = 0.007, Zvertical = −3) dimensions.
Zlateral = 5; centre versus Down45: Plateral = 0.047) and vertical (Pvertical = 0.03, Zvertical = 3; Pvertical = 0.007, Zvertical = −3) dimensions.
Figure 3.
Orchid bees fly towards the brightest point in an aperture. (a–c) Bees were presented with 150 mm circular apertures in which the position of the centre of brightness had been manipulated using a series of overlapping neutral density filters. The centre of brightness was moved 45° up and left (a), down and right (b) or centred (c). (d–f) The apertures shown in (a–c) processed to represent how it might be perceived by orchid bees (other details as in figure 2d).
A summary of the lateral and vertical position data across all experimental conditions described above (figure 4) illustrates that when the brightness cues are aligned with the geometric centre of the stimulus (figure 4, solid lines), the bees fly towards this point. The distributions are clearly shifted away from the centre towards the brighter region in all the conditions where the brightest point does not coincide with its geometric centre (figure 4, dotted and dashed lines). Taken together, these results provide compelling evidence that orchid bees use brightness cues to control their position when negotiating apertures.
Figure 4.
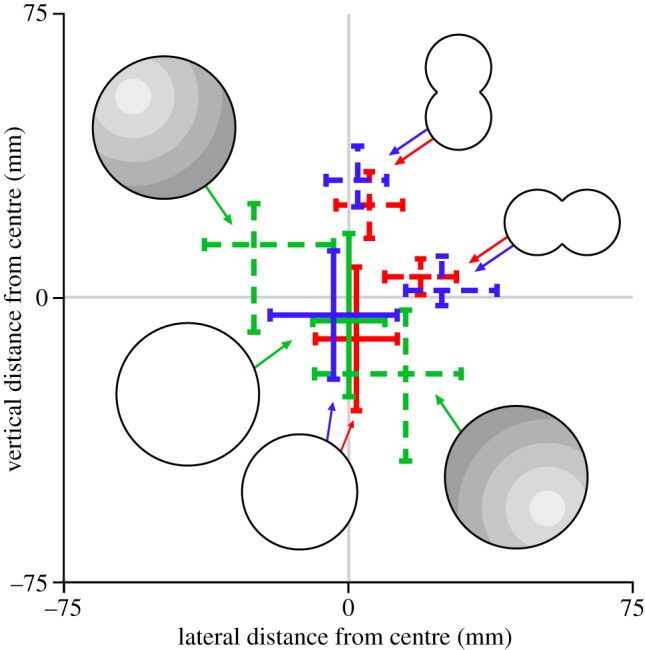
A summary of the positions of orchid bees attempting to exit the apertures from all treatments. Each cross indicates the median and the extent of the IQR for the lateral and vertical distance from the geometric centre of the apertures presented in this study. Solid lines indicate the extent of data from the open (blue) or covered with a diffuser 130 mm diameter circular aperture (red). Dashed lines represent the data from treatments when the centre of brightness was shifted away from the geometric centre of the aperture either by using a horizontal or vertically oriented double circle (open: blue; covered with a diffuser: red; note: the calculation for the double circle apertures was performed using absolute values for direct comparison with the 130 mm diameter circular aperture) or by creating an artificial brightness gradient shifted 45° up and left, or 45° down and right (green).
(b). Orchid bees use brightness cues to locate apertures that are sufficiently large to negotiate safely
In addition to providing information about the safest place to pass through an aperture, brightness could also potentially be used to locate apertures that are large enough to pass through safely. This is because the brightness of an aperture will increase with its area so that small apertures are darker than those that are larger. To investigate if orchid bees also use brightness information to determine if an aperture is large enough to fly through, we presented them with a series of circular apertures, 25 mm, 50 mm and 75 mm in diameter. Of the 15 bees tested, only one (7%) flew out of the smallest aperture (which afforded them a maximum clearance of approximately 2.5 mm considering their approximately 20 mm wingspan, as measured from the recorded images), while eight (53%) and 15 (100%) flew out of the 50 mm and 75 mm diameter apertures, respectively. This result suggests that the bees are somehow capable of determining the size of apertures and whether they are sufficiently large enough to fly through safely.
To test if brightness was being used as a cue to determine aperture size, we presented the bees with a choice of two apertures of similar area (and, therefore, similar relative brightness) but different clearances; a 60 mm diameter circle and a triangle, 60 mm wide and 90 mm high. The maximum clearance (considering the bees' approx. 20 mm wing span) for the circular aperture was approximately 10 mm (note that this is greater than the approx. 5 mm clearance for the 50 mm diameter aperture that 53% of the bees flew through in the previous experiment), while the maximum clearance for the triangular aperture was 1.7 mm (note that this is smaller than the maximum clearance of the 25 mm wide circular aperture that only one bee flew through in the previous experiment). Interestingly, the bees were equally likely to fly out of the triangle aperture (11 bees, 44%) as the circular one (14 bees, 56% p = 0.56, G-test using the expected proportion of 0.5 for each aperture, n = 25; figure 5a). To investigate if this result was due to the equal brightness of the apertures, we also presented the bees with two circular apertures, 60 mm and 43.4 mm diameter. These apertures had the same clearance as the circle and the triangle aperture but had areas, and therefore brightness, that differed by a factor of approximately 2 (area of large circle = 28 cm2, area of small circle = 15 cm2). When faced with this choice, the majority of bees flew out of the larger aperture (22 bees, 88%), while only three bees chose to fly out of the small aperture (12%, p < 0.0001, G-test using the expected proportion of 0.5 for each aperture; figure 5a). These results suggest that orchid bees use brightness cues to locate apertures that provide sufficient clearance to fly through safely. To rigorously test this hypothesis, we manipulated the relative brightness of the two circular apertures (60 mm and 43.4 mm) using lights placed behind them. When the lights behind each aperture had the same intensity (1018 photons cm−2 s−1), only four of 30 bees (13%) flew out of the smaller aperture. However, when the intensity of the light behind the larger aperture was reduced by three orders of magnitude (to 1018 photons cm−2 s−1), the proportion of bees flying out of the small aperture increased significantly from 13% to 40% (12 of 30, p = 0.0003, G-test using the expected proportion of 0.13 for the small aperture; figure 5b). In combination, these results strongly indicate that orchid bees use brightness cues to locate suitable apertures to fly through.
Figure 5.
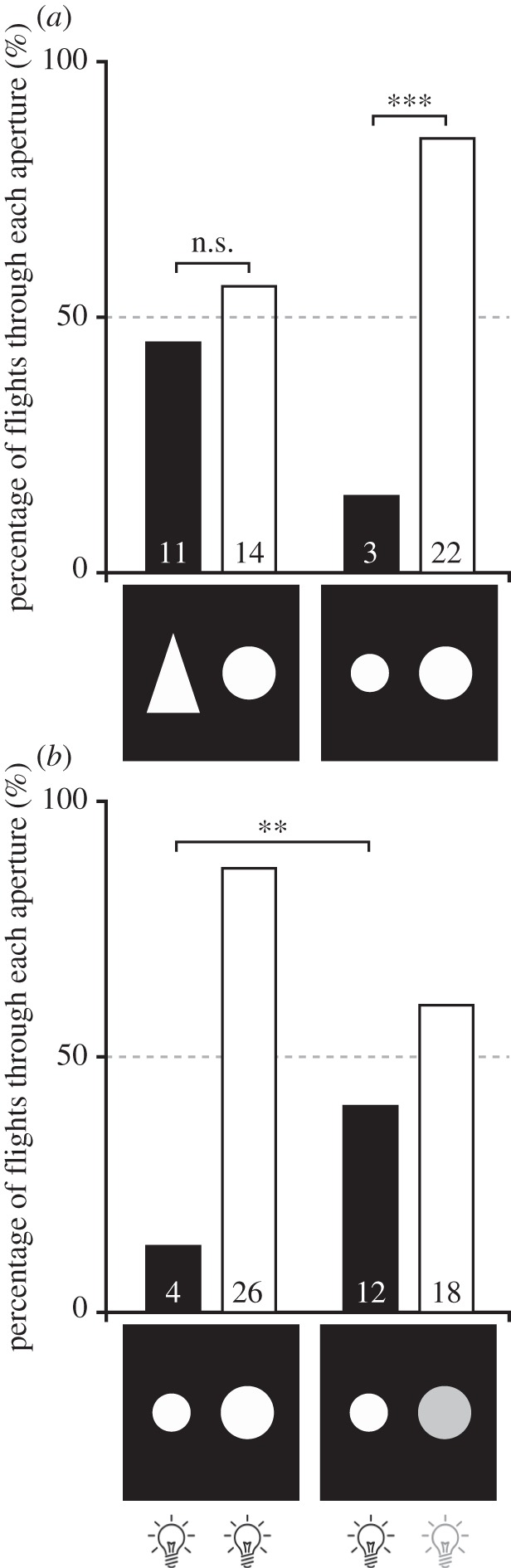
Orchid bees use brightness cues to determine the size of an aperture. (a) The percentage choice of aperture when presented with a triangle (60 mm wide, 90 mm high) and a 60 mm diameter circle or with two circles, 43.4 mm and 60 mm in diameter. Black bars indicate the percentage of bees that flew through the aperture with the smallest greatest clearance to the edges; white bars represent the percentage of bees that flew through the 60 mm diameter aperture. (b) The aperture choice when bees were presented with the 43.4 mm and 60 mm diameter apertures that were either illuminated with lights of the same intensity (left bars) or when the intensity of the light behind the larger aperture was dimmed (right bars). Numbers indicate the number of individuals that flew through each aperture.
3. Discussion
Safe flight through a cluttered environment, such as a rainforest, requires an ability to quickly and efficiently find and negotiate the gaps between obstacles. Here, we analysed the trajectories and positions of orchid bees flying out of apertures with different shapes, sizes and visual properties. We find that, when negotiating apertures, the bees position themselves close to the ‘safest’ point, that is, the point that provides them with the greatest clearance from all edges. Interestingly, brightness cues appear to be the most important for guiding this behaviour; when the centre of brightness is shifted away from the point of greatest clearance, the bees fly closer to the brightest point of the aperture even when this causes them to fly close to an edge that they would otherwise avoid. In addition, our results also suggest that orchid bees use brightness as a cue for locating apertures that are large enough to fly through safely. Overall, our results suggest that orchid bees use a hitherto undescribed brightness-based strategy to locate gaps and to fly safely through them.
(a). Brightness provides information about the clearest path through a forest
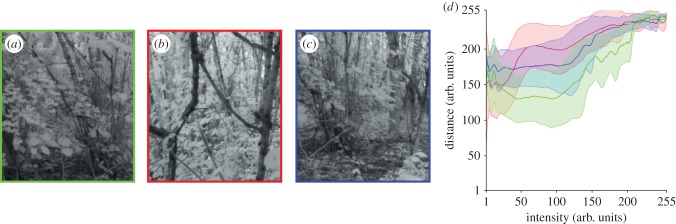
For brightness information to be useful for guiding flight through apertures in the orchid bees' natural forest habitat, these cues must be a reliable indicator of clear space between obstacles. To investigate if this is the case, we used a three-dimensional camera to take a series of images (figure 6a–c) from within a forest. Our analysis shows that there is an overall positive relationship between relative intensity and distance to obstacles (figure 6d), suggesting that the brighter parts of the visual scene do indeed indicate open apertures as well as the most ‘obstacle-free’ paths. This relationship arises because the high density of vegetation blocks out sunlight coming from low angles about the horizon so that the visual scene is illuminated only by downwelling light, causing obstacles to appear dark and obstacle-free spaces to appear bright. One exception to this is when the downwelling light is reflected off shiny leaves. However, brightness caused by leaf reflectance would not remain stable in the visual scene as the animal moves in relation to them and might therefore not have a strong influence, at least not over a significant period of time, on the flight trajectory. In other environments, such as at the forest edge where obstacles may be illuminated from the side, open spaces would appear darker than the obstacles. In this situation, brightness cues could still be used to find the clearest path if the animal moved towards the darker areas of the scene rather than the brighter ones. In either case, additional information from motion cues could provide information about whether bright or dark areas represent obstacles because nearby obstacles would move faster over the visual field than those that are further away. It is therefore important to note that using brightness cues alone would not necessarily be sufficient for avoiding collisions with obstacles in a forest. Instead, we hypothesize that orchid bees and other animals that might use this strategy would most probably use brightness cues in combination with motion information to find the safest path through a cluttered forest environment. Interestingly, pigeons and budgerigars appear to be able to determine the size of narrow gaps [1,12], but it remains unclear how they do this. One possible explanation is that these birds are using a brightness-based guidance strategy, as orchid bees do, although further detailed analysis would be necessary to test this. The main advantage of using brightness cues to guide flight through a forest is that they provide information about the point of greatest clearance in an aperture, the relative size of an aperture as well as the clearest path through a forest. Unlike motion cues, the relative brightness across the visual scene could, at least theoretically, be assessed in one sample of the image using very little processing, making it a rapid and relatively simple guidance method.
Figure 6.
The relationship between brightness and distance in a forest. (a–c) Images taken in a forest using a three-dimensional camera (Raytrix, Germany). (d) An analysis of the images in (a) (green data), (b) (blue data) and (c) (red data) showing the mean and standard deviation of relative distance as a function of relative intensity (both in arbitrary units).
(b). A brightness-based guidance strategy could exploit a combination of phototaxis and other direction information
What mechanism might be underlying the orchid bees' use of brightness cues for guidance? It is likely that the bees are simply exploiting their innate phototactic behaviour. Phototaxis is an innate light response that guides animals to bright or dark areas of the environment and is arguably the simplest and most general form of visual guidance in animals. If the bees were using phototaxis alone to guide their flight, however, they would invariably fly upwards towards the bright sky and never be able to find the orchid flowers and nectar under the canopy. Nonetheless, the brightness-based guidance strategy that we propose here could be achieved if phototactic behaviour were combined with the cues that are leading them to their goal, which may be, for example, a scent source, a food source, a mate or shelter. The preferred direction that is set by this goal could then define the region within which brightness cues have a higher attraction, or weighting within the neuronal circuits that process these cues. With such a task-controlled preference weighting across the visual field, a bee cruising through the forest would be attracted to the brighter areas that are closer to their goal direction but would still be able to escape from a ‘dead end’ or a predator by using cues behind or above them if there are no sufficiently bright areas in the desired direction. Another advantage of using phototactic information for guidance is that, due to its relative simplicity, it is potentially much faster than motion vision to process. Indeed, evidence from flies suggests that, in the visual pathways of insects, phototactic information is processed separately from motion [12]. Thus, we propose that, if combined with other directional cues, the strategy of using brightness cues to find the safest, clearest path has several advantages for any animal or agent that must quickly and efficiently navigate through a cluttered environment. The brightest regions of a scene can, in principle, be located near-instantaneously from one image using minimal processing power as all it requires is a relatively simple brightness comparison across the visual field or region of interest (this region could be set by other directional cues).
(c). Conclusion
In this study, we describe a brightness-based guidance strategy that would enable fast and efficient flight through a forest. Because of its low-computational requirements, it is likely that this strategy for visual guidance is used by animals other than orchid bees for avoiding collisions when moving through the world. Moreover, the simplicity of this approach makes it ideal for development as a lightweight, low-power collision avoidance system for autonomous robots. Thus, rather than being specific to orchid bees, using brightness cues to navigate cluttered and complex natural environments probably represents a universal strategy that any agent (be it animal or machine) could use to detect apertures and to position themselves safely when moving through them.
4. Material and methods
All experiments were performed in shade under a roof on Barro Colorado Island, Panama. The experimental set-up consisted of a Perspex chamber, 300 mm high, 300 mm wide and 750 mm long. The outer surface of the walls and roof of the chamber were covered in a randomized black and white (in equal proportions) 10 × 10 mm check pattern printed on semi-diffuse paper, which provided strong visual cues for flight control and allowed diffuse light to enter the experimental chamber. The floor of the chamber was left uncovered for observation. The back wall was made of 5 mm thick matte black foam board and was covered on the inside by the check pattern. The front wall of the chamber was constructed from matte black foam board (5 mm thick) into which the different apertures were cut. A clear 250 mm Perspex section was fitted onto the end of the chamber and the open end covered with white netting such that bees that flew through the aperture could be trapped enabling recapture. The geometric centres of single apertures were aligned with the centre of the chamber, while in the choice experiments the apertures were laterally positioned 100 mm from the wall. The chamber was oriented so that it faced the open sunlit forest, with the nearest trees being a minimum of 5 m away.
Male E. imperialis were captured at scent baits, marked using coloured paint and transferred to clear plastic vials. It was not possible to mark the bees individually but by marking them with different colours for each treatment we could ensure that the same individual was not used twice in any single treatment. Bees were introduced into the chamber by inserting the open end of the vial into a small hole in the back wall that was centred 150 mm from each wall with a height of 200 mm from the floor of the tunnel.
Once in the chamber, most bees would fly directly towards the aperture, either flying out (‘open’ aperture and choice experiments) or crashing into the filter covering the aperture. Bees that did not attempt to fly out from the aperture within 150 s were released and not included in the dataset.
Flight trajectories through the circular and double circle apertures (described below) were recorded at 100 Hz using a pair of synchronized cameras placed orthogonally; one positioned under the tunnel looking upward, the other recording flights from the side though a small hole cut into the pattern. The flights of 20 bees were digitized and reconstructed in three dimensions [13].
Position data in all other experiments were recorded using cameras (as described above) but with one camera mounted in the back wall of the tunnel. The camera mounted beneath the tunnel was used to determine the moment when the bee came into contact with the diffuser. The frame that preceded this was saved for analysis. The horizontal and vertical position of the bee with respect to the geometric centre of the aperture was determined in the image by converting pixel distances to millimetre distances using the known size of the aperture. Median and 25–75% interquartile range are reported for all treatments, Wilcoxon rank-sum analyses were used to compare the distribution of distances from the aperture's centre; G-tests were used to compare proportion data.
To investigate how bees guided their flight through apertures, we presented them with a 130 mm diameter circular aperture and a ‘double circle’ aperture composed of two 75 mm diameter circles overlapping by 20 mm. The long axis of the double circle (130 mm) was oriented either horizontally (figure 2b(i)) or vertically (figure 2c(i)). Because the distribution of positions along the short axis of this aperture was highly constrained in comparison to the circular aperture, comparisons with the circular aperture were only made using absolute lateral or vertical distances from the centre for the horizontal or vertical orientations, respectively. In the second experiment, the apertures were covered with a diffuser (LF252, LEE Filters, UK) and illuminated evenly (1018 photons cm−2 s−1) using a lamp (Manfrotto Maxima, Manfrotto, Italy) placed at a distance of 400 mm behind the aperture. In the first experiment, different individuals were used for each condition, in the second experiment the same individuals were presented with the three treatments in a randomized order.
To investigate the importance of brightness cues, an artificial brightness gradient was created across a 150 mm diameter circular aperture by layering 0.15 ND filters (LEE Filters, UK) in concentric circles. In the brightness-shifted aperture, the central opening (37.5 mm diameter) of the ND filters was centred 32.7 mm to the left and above or to the right and below the centre of the aperture (‘Up45’, figure 4a or ‘Down45’, figure 4b, respectively). Each ND filter step increased in diameter by 50 mm. In the brightness-centred aperture (figure 4c), the inner (brightest) circle was 30 mm in diameter with each diameter step increasing by 30 mm. This configuration ensured that the lowest contrast between the edge and the brightness gradient was the same in both the brightness-centred and the brightness-shifted apertures. In both cases, a diffuser (as above) was placed over the brightness gradients to minimize the visibility of the contrast edges created between the ND filter layers. The pattern was fixed to the outer side of the aperture. A lamp (as above) was used to provide even illumination across the patterns. All three treatments were presented to individual bees in a randomized order.
To investigate whether orchid bees use brightness cues to locate gaps that are safe to fly through, 15 bees were presented with three circular apertures of 25 mm, 50 mm and 75 mm diameter in a randomized order. A bee was considered to have made a successful exit if it flew out of the aperture within 150 s of being released. This time limit was set because preliminary experiments showed that bees that did not exit within this time were unlikely to exit after periods exceeding 5 min. We next presented 25 bees with two pairs of apertures placed beside each other and centred 100 mm from the wall of the tunnel. The first set of apertures consisted of a 60 mm diameter circle (28 cm2 area) and a triangle, 60 mm wide and 90 mm high that had approximately the same area (27 cm2). The second set of apertures consisted of two circles 60 mm and 43.4 mm in diameter. The two different aperture sets, as well as the relative position (left or right) of the 60 mm diameter circle, were alternated between trials.
In the final experiment, we placed two lamps (as above) at a distance of 250 mm behind two circular apertures (60 mm and 43.4 mm in diameter) to manipulate the relative brightness of each aperture when viewed from within the chamber. To ensure that each aperture was only illuminated by the lamp placed behind it and that the light across each aperture was evenly distributed, the section of the chamber after the aperture was lined and divided longitudinally with matte black foam and covered at the back with a diffuser (as above). Two different conditions were presented to each of 30 bees: (i) both apertures illuminated with the lamps on a ‘bright’ (1018 photons cm−2 s−1) setting, (ii) the smaller aperture illuminated on this bright setting, the larger aperture on the ‘dim’ (1015 photons cm−2 s−1) setting. The relative position and brightness of the apertures was alternated between trials.
Acknowledgements
We are grateful to Klaus Lunau for inspiring our work on orchid bees, the staff at STRI for their support and Dr Gavin Taylor for analysing the three-dimensional forest images and assisting with the experiments.
Data accessibility
All raw data are available from Dryad (doi:10.5061/dryad.34dr2).
Authors' contributions
E.B. and M.D. designed and performed the experiments; E.B. analysed and interpreted the data and wrote the manuscript. M.D. edited the manuscript prior to the submission.
Competing interests
We have no competing interests.
Funding
This work was supported by grants from the Air Force Office of Scientific Research (FA8655-12-1-2136, E.B.), the Swedish Research Council (2014-4762, E.B.), the Royal Physiographic Society of Lund (E.B.) and the Swedish Foundation for Strategic Research (FFL09-056, M.D.).
References
- 1.Lin H-T, Ros IG, Biewener AA. 2014. Through the eyes of a bird: modelling visually guided obstacle flight. J. R. Soc. Interface 11, 20140239 (doi:10.1098/rsif.2014.0239) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhagavatula Partha S, Claudianos C, Ibbotson Michael R, Srinivasan Mandyam V. 2011. Optic flow cues guide flight in birds. Curr. Biol. 21, 1794–1799. (doi:10.1016/j.cub.2011.09.009) [DOI] [PubMed] [Google Scholar]
- 3.Dyhr JP, Higgins CM. 2010. The spatial frequency tuning of optic-flow-dependent behaviours in the bumblebee Bombus impatiens. J. Exp. Biol. 213, 1643–1650. (doi:10.1242/jeb.041426) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Portelli G, Serres J, Ruffier F, Franceschini N. 2010. Modelling honeybee visual guidance in a 3-D environment. J. Physiol. Paris 104, 27–39. (doi:10.1016/j.jphysparis.2009.11.011) [DOI] [PubMed] [Google Scholar]
- 5.Srinivasan MV, Lehrer M, Kirchner WH, Zhang SW. 1991. Range perception through apparent image speed in freely flying honeybees. Vis. Neurosci. 6, 519–535. (doi:10.1017/S095252380000136X) [DOI] [PubMed] [Google Scholar]
- 6.Linander N, Dacke M, Baird E. 2015. Bumblebees measure optic flow for position and speed control flexibly within the frontal visual field. J. Exp. Biol. 218, 1051–1059. (doi:10.1242/jeb.107409) [DOI] [PubMed] [Google Scholar]
- 7.Baird E, Boeddeker N, Ibbotson MR, Srinivasan MV. 2013. A universal strategy for visually guided landing. Proc. Natl Acad. Sci. USA 110, 18 686–18 691. (doi:10.1073/pnas.1314311110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roubik D, Hanson P. 2004. Orchid bees of tropical America. Santo Domingo de Heredia, Costa Rica: Editorial INBio. [Google Scholar]
- 9.Wikelski M, Moxley J, Eaton-Mordas A, López-Uribe MM, Holland R, Moskowitz D, Roubik DW, Kays R. 2010. Large-range movements of neotropical orchid bees observed via radio telemetry. PLoS ONE 5, e10738 (doi:10.1371/journal.pone.0010738) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janzen DH. 1971. Euglossine bees as long-distance pollinators of tropical plants. Science 171, 203–205. (doi:171/3967/20310.1126/science.171.3967.203) [DOI] [PubMed] [Google Scholar]
- 11.Spaethe J, Chittka L. 2003. Interindividual variation of eye optics and single object resolution in bumblebees. J. Exp. Biol. 206, 3447–3453. (doi:10.1242/jeb.00570) [DOI] [PubMed] [Google Scholar]
- 12.Schiffner I, Vo HD, Bhagavatula P, Srinivasan MV. 2014. Minding the gap: in-flight body awareness in birds. Front. Zool. 11, 27–39. (doi:10.1186/s12983-014-0064-y)24666790 [Google Scholar]
- 13.Bouguet J-Y. 1999. Visual methods for three-dimensional modelling. Pasadena, CA: California institute of Technology. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All raw data are available from Dryad (doi:10.5061/dryad.34dr2).