Abstract
Animal behaviour and the ecology and evolution of parasites are inextricably linked. For this reason, animal behaviourists and disease ecologists have been interested in the intersection of their respective fields for decades. Despite this interest, most research at the behaviour–disease interface focuses either on how host behaviour affects parasites or how parasites affect behaviour, with little overlap between the two. Yet, the majority of interactions between hosts and parasites are probably reciprocal, such that host behaviour feeds back on parasites and vice versa. Explicitly considering these feedbacks is essential for understanding the complex connections between animal behaviour and parasite ecology and evolution. To illustrate this point, we discuss how host behaviour–parasite feedbacks might operate and explore the consequences of feedback for studies of animal behaviour and parasites. For example, ignoring the feedback of host social structure on parasite dynamics can limit the accuracy of predictions about parasite spread. Likewise, considering feedback in studies of parasites and animal personalities may provide unique insight about the maintenance of variation in personality types. Finally, applying the feedback concept to links between host behaviour and beneficial, rather than pathogenic, microbes may shed new light on transitions between mutualism and parasitism. More generally, accounting for host behaviour–parasite feedbacks can help identify critical gaps in our understanding of how key host behaviours and parasite traits evolve and are maintained.
Keywords: animal behaviour, parasite, disease ecology, feedback
1. Introduction
Almost every aspect of an animal's behaviour is associated with exposure to some type of parasite. Mating behaviour is critical in the transmission of sexually transmitted bacteria, protozoa, and viruses; foraging is a major route of infection for environmental and trophically transmitted bacteria and helminths; and social behaviour contributes to the dissemination of various contact-transmitted infectious agents [1–3]. Behaviour also plays a central role in how hosts defend themselves against parasites. In fact, behaviour has been referred to as the first line of defence against infection [4,5]. Reciprocally, many behaviours are altered when hosts are infected with parasites. Behavioural change due to infection can occur for a variety of reasons. Parasites may manipulate host behaviour to enhance their own fitness, or changes in host behaviour may result from immunological or pathological consequences of parasite infection [6–8]. On longer timescales, parasites impose selective pressures on their hosts that can drive evolutionary changes in behaviour [9,10]; and in turn, these changes in host behaviour can shape parasite population dynamics and life history including traits such as virulence and transmission mode [11]. Ultimately, behaviour and parasitism are so tightly intertwined that we often cannot understand one without considering the other.
Research that combines information on host behaviour and parasites has been in the mainstream since the 1980s. However, approaches to studying the links between host behaviour and parasites have differed depending on the perspective of the discipline. On the one hand, researchers who are interested in parasite ecology and evolution typically focus on how host behaviour affects parasites. One example includes studies of how host social organization, quantified in different ways, affects parasite transmission dynamics [12,13]. On the other hand, animal behaviourists typically focus on how and why parasites affect behaviour. Examples include comparative studies of the influence of parasites on the evolution of host social behaviour [14,15], and mechanistic studies of how parasites manipulate host behaviour [16,17]. While both research directions have advanced our understanding of the ways in which host behaviour and parasitism interact, merging these two approaches is rare. However, host behaviour–parasite interactions are probably more bidirectional than unidirectional, and explicitly acknowledging this bidirectionality or ‘feedback’ provides at least two key benefits. First, a feedback perspective can uncover important sources of variation in host behaviour and parasite traits that arise due to dynamic interactions between the two. Second, this approach can help identify important gaps in our understanding of the diverse ways in which hosts and parasites interact.
In this paper, we explore host behaviour–parasite feedback as a theme for connecting research at the intersection of animal behaviour and parasite ecology and evolution—an exercise that should be useful to disease ecologists, animal behaviourists, ecological immunologists, and others. To develop this idea, we first describe common feedback processes in ecology and evolution and then relate these ideas to host behaviour–parasite problems. Next, we use a series of case studies focused on current research themes in animal behaviour and disease ecology to highlight insights that can be derived from applying a feedback perspective. Finally, we discuss approaches for evaluating the presence of feedback in behaviour–parasite interactions and highlight outstanding questions.
2. Feedback: a unifying concept for understanding host behaviour–parasite interactions
Across a range of ecological and evolutionary phenomena, reciprocal feedback is an important agent of stability and change [18,19]. For example, negative feedback is a key process involved in density-dependent population regulation [20]. When population sizes exceed some threshold, growth rates are depressed via increasing mortality or decreasing fecundity, effectively regulating individual numbers over short ‘ecological’ timescales. Positive feedback, on the other hand, can produce remarkable change. This type of feedback is widely thought to play a role in the evolution of exaggerated male sexual traits, where an ornamental trait in males and a preference for this trait in females interact in an ongoing causal loop across generations, leading to the exaggeration of male traits (e.g. the peacock's tail) and female preferences (e.g. sensory bias; reviewed in [18]). Antagonistic coevolution is a feedback process of particular interest to disease ecologists (e.g. Red Queen hypothesis; reviewed in [21]). In this case, parasites might select for resistance traits in hosts which then drives the evolution of counter-adaptations in the parasites, such that hosts and parasites are engaged in an ongoing arms race [22]. Arms races create positive feedback loops that manifest in an enhanced capacity of hosts to resist their parasites and parasites to infect their hosts [21,23]. Antagonistic coevolution can also select for rare host and parasite genotypes, resulting in no change in mean levels of host and parasite defences and counter-defences [21,24].
The idea that host behaviour and traits of parasites can be linked in feedback loops is entirely consistent with what we know of other ecological and evolutionary interactions. As with common feedback processes in ecology and evolution, feedbacks between host behaviour and parasites can operate within or across generations of hosts or parasites. As such, these processes can result in plastic shifts in host behaviour or population size changes in parasites on ecological timescales, or in changes in the frequency of host or parasite genotypes on evolutionary timescales. Ecological and evolutionary processes can also affect one another, resulting in eco-evolutionary feedback [25]. Such eco-evolutionary feedback can occur, for example, when ecological changes in one species (e.g. predator abundance) drive evolutionary changes in another species (e.g. prey defence traits) and these evolutionary changes, in turn, affect predator ecology [26]. Although there are few examples of eco-evolutionary feedback in natural systems [25], host–parasite interactions are a promising arena for exploring this phenomenon [26–28].
More generally, all these types of feedback, though often unacknowledged, are probably common in host behaviour–parasite interactions. Consequently, they may play an integral role in shaping variation in host and parasite traits. For example, many instances of parasite manipulation of host behaviour are considered to be adaptations of the parasite to enhance its own fitness. A specific parasite genotype causes a change in host behaviour that has positive repercussions for the parasite, increasing the abundance of the relevant parasite genotype, which then increases the expression of the altered host behaviour, and so on. This is a classic case of positive feedback that can occur on both ecological and evolutionary timescales. Consider interactions between Barley yellow dwarf virus (BYDV), a vector-transmitted pathogen of agricultural grasses, and its insect vector the aphid Rhopalosiphum padi. BYDV alters host plant selection such that non-infected aphids prefer infected host plants, whereas infected aphids prefer non-infected plants [29]. This conditional vector preference increases the likelihood of non-infected vectors acquiring the virus and of infected vectors transmitting the virus to non-infected plants [29], an effect that should promote virus transmission, driving positive ecological feedback between virus abundance and aphid host plant selection. Similarly, malaria parasites (Plasmodium spp.) are known to cause changes in mosquito host-seeking and -feeding behaviour (reviewed in [30]), and mathematical models suggest that these parasite-induced behavioural changes can in turn drastically enhance malaria transmission [31].
The ecological feedbacks represented by the BYDV–aphid and malaria–mosquito systems can lead to evolutionary feedbacks (i.e. antagonistic coevolution) if there is heritable genetic variation in the ability of parasites to manipulate hosts and the ability of hosts to resist such manipulation [32]. Evidence of genetic variation in host and parasite traits associated with behavioural manipulation is rare (but see [33]), however, there are several examples of reciprocal coevolution between parasites and hosts involving other aspects of parasite (e.g. infectivity, virulence) and host (e.g. susceptibility, mortality, competitive ability) life history [23,34–36]. Thus, it is reasonable to expect that behavioural traits may be frequent subjects of host–parasite coevolution. Indeed, parasite-induced selection for behavioural resistance was documented in an artificial selection experiment using Drosophila nigrospiracula and the ectoparasitic mite, Macrocheles subbadius [37]. Perhaps, some of the host life-history traits reported to change in response to selection by parasites (e.g. competitive ability [35]) also involve a behavioural component. Importantly, the outcome of coevolutionary feedback between host behaviour and parasite traits may be profound. A recent theoretical exploration of coevolutionary dynamics between host mate choice and a sexually transmitted parasite showed that in some situations, feedback-induced sustained cycling in host choosiness and parasite virulence. In other situations, the same initial conditions resulted in the evolution of exclusively choosy or non-choosy host mating behaviour [11]. Thus, behaviour–parasite feedback can potentially generate variation in host behaviour and parasite life history at both within- and among-population scales.
One major advantage of the feedback concept is that it highlights that regardless of the initial direction in which a host behaviour–parasite interaction is viewed (behaviour → parasite or parasite → behaviour), changes are potentially occurring in both the parasite and the host. Importantly, asymmetry in generation time between hosts and some parasites means that simultaneous ecological and/or evolutionary changes in parasites, in conjunction with changes in host behaviour, are probably common. For this reason, understanding a host behavioural change caused by a parasite, for example, cannot be divorced from downstream consequences for the parasite. Thus, the interests of animal behaviourists in host outcomes and disease ecologists in parasite outcomes are not easily decoupled. One notable example of this is the loss of sexual signalling in field crickets (Teleogyryllus oceanicus) in response to a deadly parasitoid fly. Male field crickets use song to attract mates, but in Hawaii where these crickets have been introduced, male song also attracts a parasitoid fly, Ormia ochracea [38]. In response to this parasitism, cricket density and male song declined rapidly over an approximately 10-year period on the island of Kauai [39]. A few years later, cricket density rebounded, but almost all the males had female-like wings that made them incapable of producing song. Now, silent males compensate for their inability to produce mating calls by showing greater ‘satellite’ behaviour; they aggregate around the few remaining singing males, which brings them into close proximity of potential mates [39,40]. Recent work also points to parasite-induced selection on other aspects of male mating behaviour, including locomotory behaviours that enhance the encounter rate of silent males with females [41].
Clearly, parasitism drove the rapid evolution of cricket behaviour, but reciprocal effects on the parasite are also extremely likely. Because sound is the only way in which O. ochracea locates its host, and crickets are the only known host for this parasite in Hawaii, the loss of male song has potentially drastic consequences for the parasite. First, O. ochracea could face rapid population declines due to its reduced ability to locate hosts [39,42]. Second, declines in success of locating hosts might in turn drive the evolution of host searching behaviour in the parasitoid. For instance, selection might favour flies that are (i) able to locate silent males using alternative host cues or (ii) oviposit on alternative hosts. Of course, declines in the parasite population should have further consequences for host behaviour. If parasite numbers drop drastically, singing crickets might again be favoured [39]. Indeed, the process could endure, setting the stage for fascinating dynamical changes in both host behaviour and parasite traits.
3. Applying a feedback perspective to research in animal behaviour and disease ecology
The feedback between parasite and host behaviour envisioned in the field cricket system is likely representative of how host behaviour and parasites induce reciprocal ecological and evolutionary change in one another across a range of systems. To explore the types of insight that can be gleaned from applying a feedback perspective to the study of host behaviour and parasites, we discuss three topics that reflect current research themes at the interface of animal behaviour and disease ecology. Focusing on social networks, animal personality, and the microbiome, we examine how a feedback lens can alter predicted outcomes, help explain ecological patterns, and generate novel questions. We expect that the feedback concept applies to a much broader set of topical areas and questions.
(a). Social networks and parasitism
The role of social networks in parasite transmission is an area of rapidly increasing interest to both disease ecologists and animal behaviourists [43–45]. Notably, social networks capture heterogeneous social interactions occurring in a population that can lead to a disproportionate number of transmission events being caused by a few individuals. These ‘super-spreading’ individuals are well documented in animal social network studies [43,44,46]. Thus, most studies on social networks and parasites, to date, have focused almost exclusively on the question of how host position in a social network, or overall network structure, affects the ecological dynamics of parasites. However, the potential for parasites to alter host social networks, in turn, and the downstream consequences for parasite ecology and evolution, are largely unexplored [46,47]. This is somewhat surprising because parasite-induced changes in host behaviour can affect key network metrics such as the rate at which infectious individuals contact susceptible conspecifics [48]. Moreover, susceptible individuals can alter their behaviour in response to the threat of parasitism in ways that affect network topology, for example, by avoiding infectious areas or individuals [49]. As such, parasite-induced behavioural changes that alter social network structure may result in strong negative or positive feedbacks on both parasite ecology (e.g. basic reproductive number: Ro) and evolution (e.g. virulence [50]). For example, there can be significant reductions in parasite transmission when susceptible individuals avoid infected conspecifics. Crucially, simulation models indicate that this type of negative feedback process can significantly alter disease dynamics and parasite prevalence (box 1). By contrast, when infected individuals become more central in a social network, as might be expected from some forms of parasite manipulation, there may be positive feedback on parasite transmission. Notably, the feedback between social networks and behaviour is dynamic and multi-layered because the way in which infection alters social network topology (parasites → behaviour) influences the nature of parasite spread in the population (behaviour → parasites).
Box 1. Exploring the impact of negative feedback on parasite transmission in a social network context. 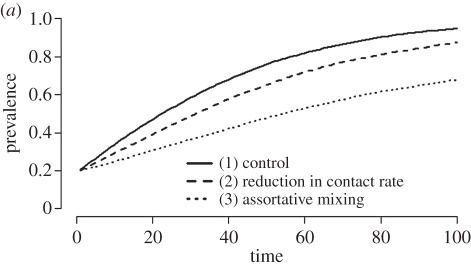
A plot of the mean epidemic curves for simulations that ignore (solid line; scenario #1) or account for infection-induced behavioural changes (dashed and dotted lines; scenarios #2 and #3). We ran each model for 100 time steps and performed 100 simulations per experiment. Each simulation began with a prevalence of 0.20 or 20 infected individuals. The mean degree of both the experimental and control network is approximately 1.1 and the infection probability is 0.1. Simulations were performed using the EpiModel package in R (see the electronic supplementary material for details).
Researchers typically do not consider how parasite-induced behavioural changes influence network structure and the impact these changes have on parasite dynamics during epidemics. However, it is possible to explore these feedbacks using network simulations. Here, we use dynamic networks, which allow for contacts to form and dissolve through time, in order to demonstrate how parasite-induced behavioural changes might affect epidemic outcomes. We simulated a susceptible-infected (SI) model of a directly transmitted parasite through a population of 100 individuals. We tested three cases: (1) a control where contact rate does not change as a result of infection status, (2) an experimental scenario where infected individuals reduce their contact rates upon infection, and (3) an experimental scenario where individuals exhibit assortative mixing—that is, infected individuals were less likely to interact with susceptible individuals than would be expected by chance. We show here (a) a comparison of the resulting epidemic curves and (b) a snapshot comparison of parasite spread on the dynamic networks. Biologically, the two experimental scenarios could result from sickness behaviours (e.g. lethargy, fever; scenario #2) or from susceptibles actively avoiding infected individuals (scenario #3). Accounting for such parasite-induced behavioural changes affects both the rate at which the parasite spreads through the population, as well as the final prevalence in the population. 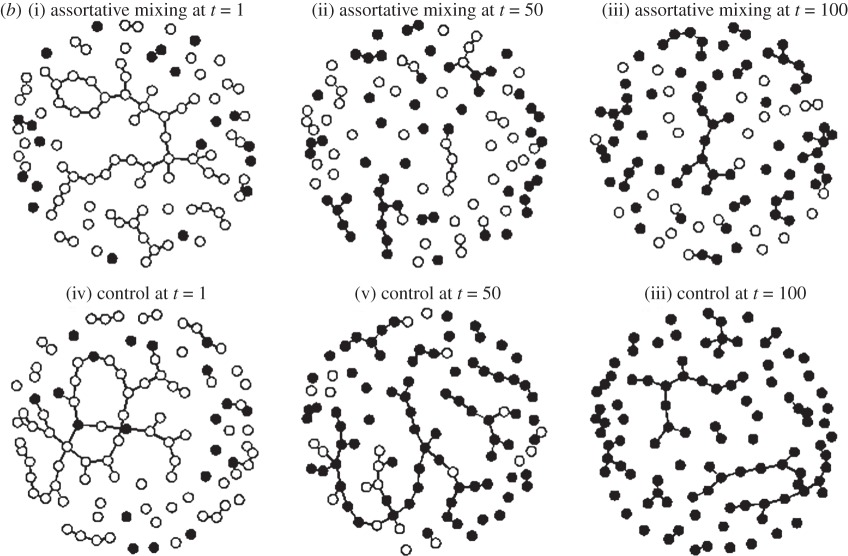
A time-lapse view of the parasite spreading through a dynamic network for both an assortative mixing simulation (top row (i–iii)) and a control simulation (bottom row (iv–vi)) at t = 1, 50, and 100 time steps. Infected nodes (individuals) appear in black and susceptible nodes appear in white. By the end of the simulation, parasite prevalence in the control simulation is 100%, while the assortative mixing simulation only reaches a prevalence of 73%.
The feedback perspective underscores at least two important issues for social network–parasite studies. First, the use of contact networks quantified in solely healthy hosts, something most network studies usually do, can be misleading [46,51]. For example, in humans, a reduction in social contacts due to sickness behaviour reduced the reproductive number of influenza to approximately one-quarter of the value it would have been if contacts made by healthy individuals were used to estimate epidemiological outcomes [48]. Second, the feedback approach addresses a major concern about causality in these studies. A recurring question is whether an individual's social network position determines its infection status, or whether its infection status determines its social network position [43]. The feedback approach explicitly acknowledges that both of these processes probably occur simultaneously. This perspective advocates for studies that monitor both the contact structure and health status of hosts before and during an epidemic, which allows for quantification of related changes in network structure and disease dynamics as an epidemic progresses. Accomplishing this goal in free-living populations is becoming easier due to the availability of new biotelemetry and biomonitoring tools. As one example, temperature-sensing passive integrated transponder (PIT) tags now allow for the simultaneous tracking of behavioural interactions and measures of health-related traits such as fever [52].
(b). Animal personality and infection
Animal personalities, or consistent individual differences in behaviour across contexts, may evolve and persist, in part, as a result of feedback between particular behaviours and intrinsic and extrinsic state variables such as body size, energy reserves, or anthropogenic contaminants [53]. Parasites have recently been discussed as potential state variables that can feedback on and maintain variation in animal personality [53,54]; and animal personalities are especially promising candidates for generating feedback with parasites because they can readily create between-individual heterogeneities in the acquisition and spread of parasites [55]. In terms of parasite acquisition, bolder individuals may be more likely to encounter conspecifics or environmental sources of infection, increasing their risk of acquiring a broad suite of parasite types. For instance, consistent differences in activity and exploratory behaviour in Siberian chipmunks (Tamias sibiricus) predict tick infection risk because the activity patterns and space use of bold individuals make them more prone to encountering ticks in the environment [56]. In other systems, bolder individuals are more likely to interact with conspecifics (great tits, Parus major [57]), and the presence of bold individuals in a group can even attract new individuals to join (sticklebacks, Gasterosteus aculeatus [58]). Thus, across a suite of parasite transmission modes, bold animals may function as ‘super-receivers’, or individuals that are disproportionately more likely to acquire parasites than the general population.
Once infected, bold individuals may also facilitate the spread of parasites by acting as ‘super-spreaders’, if their bold behavioural tendencies persist. For example, Dizney & Dearing [59] found that deer mice (Peromyscus maniculatus) infected with Sin Nombre virus (SNV) were more likely than uninfected mice to engage in bold behaviours that could increase their probability of encountering other mice. Infected mice travelled twice as far and engaged in five times more aggressive interactions than uninfected mice, both behaviours that could increase the likelihood of transmitting the pathogen. Overall, mice that showed a consistently bold suite of behaviours were three times more probably to be infected with SNV than those with shy tendencies [59], but the authors could not determine whether bold behaviour caused greater SNV risk, whether infection caused bold behaviour, or both. Indeed, most studies of personality and parasite risk have been performed on individuals that are naturally infected prior to behavioural assay [56,59–61], making interpretation of causality challenging. However, understanding if boldness changes during infection is critical to identifying the potential for ecological feedback between parasites and personality. If parasite infection exacerbates bold behaviour—as has been previously proposed for cases where hosts have an increased need for resources during infection, thereby reinforcing bold behaviours to acquire those resources—this could result in a positive feedback loop where parasites reinforce bold personality types, and vice versa [53,54]. Studies of personality in both healthy and infected hosts are needed to determine whether boldness predicts parasite risk for healthy individuals, and whether boldness changes during infection in ways that can drive ecological feedbacks between personality and parasites.
Parasites may also shape the relative frequency of personality types on a population scale via eco-evolutionary feedbacks. Specifically, greater exposure risks and higher levels of parasitism in bold individuals may select for traits that ameliorate the costs of infection [62,63], possibly facilitating the maintenance of bold personality types. In support, bolder individuals have recently been reported to show immunological responses that are distinct from those of shy individuals, which may reflect stronger infection resistance mechanisms. In humans, for instance, extraversion is associated with increased expression of pro-inflammatory genes in leucocytes [64]; while, in house finches (Haemorhous mexicanus), individuals with high-risk personalities appear to compensate for heightened parasite exposure risks by investing more in innate immunity [65]. These emerging patterns suggest that parasite-mediated selection on bold individuals may shape physiological responses to infection, allowing bold phenotypes to persist despite the higher parasite risks associated with bold behaviours. One mechanistic hypothesis for how such patterns may arise is that they are a consequence of pleiotropic links between genes coding for behaviour and immune function [66]. More generally, personality–parasite interactions could lead to interesting eco-evolutionary feedbacks, in which personality type affects individual parasite infection risk, and heightened parasite exposure selects for compensatory immunological or genetic resistance mechanisms. At the population level, these compensatory mechanisms may help maintain polymorphism in personality types.
(c). Behaviour and the microbiome
The feedback concept is not only relevant to relationships between host behaviour and parasites; it also applies to microorganisms that have commensal or beneficial relationships with their hosts, including members of the microbiome. Hosts are exposed to these bacteria through a wide range of behaviours, and behaviour is among the most important forces affecting microbiome composition [67,68]. In turn, the microbiome can affect host behaviour, either directly, through microbes that affect host nervous systems [69,70], or indirectly through microbial involvement in olfactory communication between hosts [71,72].
To date, these two phenomena—behavioural effects on the microbiome and microbiome effects on behaviour—have largely been explored in isolation, mirroring the trajectory of host behaviour and parasite studies. However, feedback may be crucial to understanding patterns of microbial abundance and diversity and host behavioural variation [73]. If particular microbes benefit host fitness, these benefits should select for hosts to behave in ways that promote the acquisition of beneficial microbes [74]. This selection pressure could, in turn, generate positive feedback between host behaviour and microbial transmission, leading to the spread of beneficial microbes in host populations. For instance, many young mammals engage in coprophagia, which helps them acquire gastrointestinal microbes necessary to digest complex carbohydrates, and allows microbes to spread to new hosts [75]. However, disease-causing microbes often use the same transmission routes as beneficial microbes, which might lead to negative feedback that dampens the expression of these same pro-transmission behaviours. In response, many mammals have evolved behavioural strategies to avoid faecal contamination and limit the transmission of faecal–oral parasites [4]. These dueling positive and negative feedback loops simultaneously select for behaviours that help hosts acquire good microbes and select against behaviours that increase the transmission of bad microbes. This behavioural tension might lead to novel host–microbe associations that confer protection against parasites. One potential example comes from the social bumblebee Bombus terrestris, which acquires a protective gut microbiota from nest-mates in the first few days of life [76,77]. This microbiota protects individuals against Crithidia bombi, a virulent gut parasite that is also transmitted through faecal–oral contact. While both the protective microbiota and C. bombi are transmitted through faecal–oral routes, microbiota transmission occurs before most parasite exposure, limiting the negative effects of the parasite [76,77].
Positive feedback is also possible when microbes manipulate host behaviour. Certain microbes in the gut microbiome, for example, may directly cause hosts to crave foods that contain resources beneficial to those microbes [78]. In turn, an influx of resources might lead to positive feedback on microbial populations in the gut, possibly amplifying host cravings and leading to further changes in host foraging behaviour. This scenario raises a range of key questions. First, at what point in the positive feedback loop does manipulation of host behaviour by a beneficial microbe lead to health or fitness costs to the host, shifting a microbe along the axis from symbiotic to pathogenic? When that switch-point occurs, the feedback would probably shift from positive to negative, helping to keep beneficial microbes in check. Second, members of the microbiome live in complex communities containing many microbial species, each with their own competing interests and potentially different feedback relationships with hosts. Does the complexity of this ecosystem prevent any single microbial taxon from exerting strong positive feedbacks on host behaviour? Alternatively, do microbes with similar interests cooperate to generate positive feedback loops that manipulate host behaviour in ways that benefit microbial community transmission?
4. Quantifying behaviour–parasite feedbacks
Feedback frequently occurs between host behaviour and parasites. Determining if these behaviour–parasite feedbacks are important sources of variation in host and parasite traits requires researchers to begin connecting typically unlinked observations, i.e. behaviour affects some aspect of parasite biology and parasites affect host behaviour. As is common practice in both animal behaviour and disease ecology, laboratory or field experiments, and in some cases observational studies, can be used to identify linkages between host behaviour and parasites and vice versa. In many cases though, the connections between host behaviour and parasites may be indirect, mediated by changes in host or parasite genetics, physiology, immunity, etc. These indirect pathways can sometimes make it difficult to detect meaningful relationships between the variables of interest, but statistical tools such as structural equation modelling can be used to test the plausibility of alternative direct and indirect causal connections between variables [79,80]. Once there is evidence for reciprocal effects of behaviour on parasites and parasites on behaviour, determining whether these effects are linked via a feedback loop necessitates longitudinal observation. Since feedback loops operate over time, repeated measurements are crucial for establishing whether changes in host behaviour or parasite traits are temporally correlated. When sufficient data are available, methods such as time series analysis can be used to test for associations between temporally matched data on host behaviour and parasite traits (e.g. cross-correlation analysis [81,82]). In less data-rich situations, empirical data can be used to parametrize simulation models to explore the outcomes of hypothesized reciprocal interactions (e.g. box 1). Experimental evolution approaches also provide a powerful tool for studying evolutionary feedback processes [83] and can be readily applied to behaviour–parasite interactions.
What makes identifying behaviour–parasite feedback loops particularly exciting and challenging is that these processes operate on multiple temporal scales, both within and across generations of hosts and parasites. This means that multiple types of feedback loops are possible in any single system, ranging from feedbacks that occur exclusively within (ecological) or across (evolutionary) host and parasite generations to feedbacks that cut across temporal scales (eco-evolutionary). For example, on an ecological timescale, parasites might induce sickness behaviour in hosts, which in turn affects parasite prevalence; while on an evolutionary timescale, behavioural defences of hosts might select for parasite evasion tactics that exert new selection pressure on host behavioural defences. The evolution of behavioural defences in hosts can also feedback on parasite population dynamics, coupling evolutionary and ecological timescales. Likewise, host behaviour could exert selection pressure on parasites, and evolutionary changes in parasite traits could drive ecological changes in host behaviour. Indeed, the enormous potential for rapid evolution by parasites raises the question of whether evolutionary effects of behaviour on parasites might be much more common than the reverse. At present though, very little is known about how host behaviour shapes the evolution of parasite traits and what the subsequent impacts are on host behaviour. More generally, considering the different temporal scales at which behaviour–parasite feedbacks occur highlights a range of outstanding research questions. These questions can apply to specific study systems, particular research topics, as well as the study of parasites and host behaviour more broadly (table 1).
Table 1.
Timescales over which host behaviour–parasite feedback can operate. ‘Eco’ signifies the within-generational scale; ‘evo’ signifies the cross-generation scale, and ‘eco-evo’ indicates feedback that combines both scales. Considering feedback loops from the perspective of multiple timescales raises outstanding research questions at the level of individual study systems, whole research topics, and the study of host behaviour–parasite interactions more generally.
| outstanding research questions |
|||
|---|---|---|---|
| temporal scale | by study system (e.g. field cricket–parasitoid) | by research topic (e.g. social networks and parasites) | host behaviour–parasite interactions (questions that apply to any system) |
| Eco | — How plastic is male mating behaviour and what are the implications of this plasticity for parasite reproductive success? — How flexible is parasite host-seeking behaviour? What are the repercussions for individual host behaviour? |
— How do parasite-induced changes in behaviour (e.g. sickness behaviour) modify host social network structure and parasite transmission? — Can negative feedback between host social network structure and parasite transmission accelerate epidemic fade out? |
— Can feedback between host behaviour and parasites drive fluctuations in individual behaviour over time? — What factors (e.g. physiology, environment) mediate these shifts in behaviour? |
| Evo | — Does the evolution of silence in male crickets impose strong selection on parasite traits? — Do parasite adaptations to male silence select for additional changes in male behaviour? |
— Do certain host social network structures select for certain parasite traits? — Do parasites locally adapt to geographical variation in host social network structure? |
— Do evolutionary arms races occur between host behavioural defences and parasite counter-defences? — How commonly does antagonistic coevolution between hosts and parasites involve host behavioural rather than immunological defences? |
| Eco–Evo | — How does the evolution of silence in crickets affect parasite population dynamics? — Do changes in parasite abundance impose selection on cricket behaviour? — What is the relative effect of evolutionary versus ecological changes in the parasite on host behaviour? |
— Does variation in parasite transmission across types of host social networks influence the evolution of parasite defence behaviours across social systems? — Can social network structure affect the evolution of parasite virulence by modifying the trade-off between transmission and virulence? What is the role of feedback in this process? |
— Can the variable effects of host behaviour on parasite ecology (e.g. transmission, prevalence, distribution) over space and time select for plasticity in host behaviour? — Do mismatches between host and parasite generation times favour host behavioural plasticity as a response to ongoing feedback? |
5. Concluding remarks
Research at the interface of animal behaviour and parasite ecology and evolution is often guided by the perspective of the end-user who might care more about parasite outcomes or behaviour outcomes, but rarely both. However, reciprocal feedback is probably inherent to most host behaviour–parasite interactions, thus merging these traditional perspectives is essential. Feedback processes are well-known agents of ecological and evolutionary stability and change in natural systems, therefore, identifying instances in which host behaviour–parasite feedbacks occur can improve our general ability to describe complex relationships between animal behaviour and parasites. Importantly, the fact that behaviour–parasite feedbacks can operate on different timescales means that within a single system, multiple feedback loops can occur simultaneously, further contributing to variation in host and parasite traits. This unique way of viewing host behaviour–parasite interactions provides a framework for identifying novel pathways of interaction. From a practical perspective, a feedback approach clearly has important benefits. For disease ecologists, feedback loops between behaviour and parasites have clear repercussions for understanding and managing disease dynamics. When behaviour is a major factor influencing transmission, incorporating feedbacks can go a long way towards improving predictions about disease spread. For animal behaviourists, feedback loops between parasites and behaviour impact both ecological and evolutionary variability in behaviour. When parasites impose costs on host behaviour, incorporating feedbacks can help refine our understanding of the diversity and maintenance of animal behaviour.
Supplementary Material
Acknowledgements
We thank G. Hill for discussions that helped shape this paper.
Competing interests
We declare we have no competing interests.
Funding
We thank the Animal Behavior Society and the United States National Science Foundation (NSF DEB-1434365) for supporting the symposium on ‘Animal Behavior and Disease Ecology’ that catalysed this paper. V.O.E. received supported from NSF IOS-1101836; E.A.A. received support from NSF IOS-1053461; L.B.M. was supported by NSF IOS-1257773, 1209747, and 0920475; M.E.C. was funded by NSF DEB-1413925 and the Cooperative State Research Service, U.S. Department of Agriculture, under Projects MINV-62-044 and MINV-62-051; D.M.H. received supported from NSF IOS-1054675 and L.W. received support from the NSF Graduate Research Fellowship Program under grant no. 00039202.
References
- 1.Moore J. 2002. Parasites and the behavior of animals. Oxford, UK: Oxford University Press. [Google Scholar]
- 2.Thrall PH, Antonovics J, Dobson AP. 2000. Sexually transmitted diseases in polygynous mating systems: prevalence and impact on reproductive success. Proc. R. Soc. Lond. B 267, 1555–1563. ( 10.1098/rspb.2000.1178) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altizer S, et al. 2003. Social organization and parasite risk in mammals: integrating theory and empirical studies. Annu. Rev. Ecol. Evol. Syst. 34, 517–547. ( 10.1146/annurev.ecolsys.34.030102.151725) [DOI] [Google Scholar]
- 4.Hart BL. 1990. Behavioral adaptations to pathogens and parasites: 5 strategies. Neurosci. Biobehav. Rev. 14, 273–294. ( 10.1016/S0149-7634(05)80038-7) [DOI] [PubMed] [Google Scholar]
- 5.Hart BL. 2011. Behavioural defences in animals against pathogens and parasites: parallels with the pillars of medicine in humans. Phil. Trans. R. Soc. B 366, 3406–3417. ( 10.1098/rstb.2011.0092) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poulin R. 2010. Parasite manipulation of host behavior: an update and frequently asked questions. Adv. Study Behav. 41, 151–186. ( 10.1016/S0065-3454(10)41005-0) [DOI] [Google Scholar]
- 7.Moore J. 2013. An overview of parasite-induced behavioral alterations—and some lessons from bats. J. Exp. Biol. 216, 11–17. ( 10.1242/jeb.074088) [DOI] [PubMed] [Google Scholar]
- 8.Klein SL. 2003. Parasite manipulation of the proximate mechanisms that mediate social behavior in vertebrates. Physiol. Behav. 79, 441–449. ( 10.1016/S0031-9384(03)00163-X) [DOI] [PubMed] [Google Scholar]
- 9.Poulin R. 1995. ‘Adaptive’ changes in the behaviour of parasitized animals: a critical review. Int. J. Parasitol. 25, 1371–1383. ( 10.1016/0020-7519(95)00100-X) [DOI] [PubMed] [Google Scholar]
- 10.Moller AP, Dufva R, Allander K.. 1993. Parasites and the evolution of host social-behavior. Adv. Stud. Behav. 22, 65–102. ( 10.1016/S0065-3454(08)60405-2) [DOI] [Google Scholar]
- 11.Ashby B, Boots M.. 2015. Coevolution of parasite virulence and host mating strategies. Proc. Natl Acad. Sci. USA 112, 13 290–13 295. ( 10.1073/pnas.1508397112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naug D, Camazine S.. 2002. The role of colony organization on pathogen transmission in social insects. J. Theor. Biol. 215, 427–439. ( 10.1006/jtbi.2001.2524) [DOI] [PubMed] [Google Scholar]
- 13.Craft ME, Volz E, Packer C, Meyers LA. 2011. Disease transmission in territorial populations: the small-world network of Serengeti lions. J. R. Soc. Interface 8, 776–786. ( 10.1098/rsif.2010.0511) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rifkin JL, Nunn CL, Garamszegi LZ. 2012. Do animals living in larger groups experience greater parasitism? A meta-analysis. Am. Nat. 180, 70–82. ( 10.1086/666081) [DOI] [PubMed] [Google Scholar]
- 15.Patterson JE, Ruckstuhl KE. 2013. Parasite infection and host group size: a meta-analytical review. Parasitology 140, 803–813. ( 10.1017/S0031182012002259) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hari Dass SA, Vyas A. 2014. Toxoplasma gondii infection reduces predator aversion in rats through epigenetic modulation in the host medial amygdala. Mol. Ecol. 23, 6114–6122. ( 10.1111/mec.12888) [DOI] [PubMed] [Google Scholar]
- 17.Shi WP, Guo Y, Xu C, Tan SQ, Miao J, Feng YJ, Zhao H, Leger RJS, Fang WG. 2014. Unveiling the mechanism by which microsporidian parasites prevent locust swarm behavior. Proc. Natl Acad. Sci. USA 111, 1343–1348. ( 10.1073/pnas.1314009111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crespi BJ. 2004. Vicious circles: positive feedback in major evolutionary and ecological transitions. Trends Ecol. Evol. 19, 627–633. ( 10.1016/j.tree.2004.10.001) [DOI] [PubMed] [Google Scholar]
- 19.Lehtonen J, Kokko H.. 2012. Positive feedback and alternative stable states in inbreeding, cooperation, sex roles and other evolutionary processes. Phil. Trans. R. Soc. B 367, 211–221. ( 10.1098/rstb.2011.0177) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turchin P. 1999. Population regulation: a synthetic view. Oikos 84, 153–159. ( 10.2307/3546876) [DOI] [Google Scholar]
- 21.Brockhurst MA, Chapman T, King KC, Mank JE, Paterson S, Hurst GD. 2014. Running with the Red Queen: the role of biotic conflicts in evolution. Proc. R. Soc. B 281, 20141382 ( 10.1098/rspb.2014.1382) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dawkins R, Krebs JR. 1979. Arms races between and within species. Proc. R. Soc. Lond. B 205, 489–511. ( 10.1098/rspb.1979.0081) [DOI] [PubMed] [Google Scholar]
- 23.Buckling A, Rainey PB. 2002. Antagonistic coevolution between a bacterium and a bacteriophage. Proc. R. Soc. Lond. B 269, 931–936. ( 10.1098/rspb.2001.1945) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hall AR, Scanlan PD, Morgan AD, Buckling A.. 2011. Host-parasite coevolutionary arms races give way to fluctuating selection. Ecol. Lett. 14, 635–642. ( 10.1111/j.1461-0248.2011.01624.x) [DOI] [PubMed] [Google Scholar]
- 25.Schoener TW. 2011. The newest synthesis: understanding the interplay of evolutionary and ecological dynamics. Science 331, 426–429. ( 10.1126/science.1193954) [DOI] [PubMed] [Google Scholar]
- 26.Becks L, Ellner SP, Jones LE, Hairston NG Jr. 2012. The functional genomics of an eco-evolutionary feedback loop: linking gene expression, trait evolution, and community dynamics. Ecol. Lett. 15, 492–501. ( 10.1111/j.1461-0248.2012.01763.x) [DOI] [PubMed] [Google Scholar]
- 27.Luo S, Koelle K. 2013. Navigating the devious course of evolution: the importance of mechanistic models for identifying eco-evolutionary dynamics in nature. Am. Nat. 181(Suppl 1), S58–S75. ( 10.1086/669952) [DOI] [PubMed] [Google Scholar]
- 28.Tack AJM, Laine A-L. 2014. Spatial eco-evolutionary feedback in plant-pathogen interactions. Eur. J. Plant Pathol. 138, 667–677. ( 10.1007/s10658-013-0353-x) [DOI] [Google Scholar]
- 29.Ingwell LL, Eigenbrode SD, Bosque-Perez NA. 2012. Plant viruses alter insect behavior to enhance their spread. Sci. Rep. 2, 578 ( 10.1038/srep00578) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cator LJ, Lynch PA, Read AF, Thomas MB. 2012. Do malaria parasites manipulate mosquitoes? Trends Parasitol. 28, 466–470. ( 10.1016/j.pt.2012.08.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cator LJ, Lynch PA, Thomas MB, Read AF. 2014. Alterations in mosquito behaviour by malaria parasites: potential impact on force of infection. Malar. J. 13, 164 ( 10.1186/1475-2875-13-164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daoust SP, King KC, Brodeur J, Roitberg BD, Roche B, Thomas F. 2015. Making the best of a bad situation: host partial resistance and bypass of behavioral manipulation by parasites? Trends Parasitol. 31, 413–418. ( 10.1016/j.pt.2015.05.007) [DOI] [PubMed] [Google Scholar]
- 33.Franceschi N, Cornet S, Bollache L, Dechaume-Moncharmont FX, Bauer A, Motreuil S, Rigaud T.. 2010. Variation between populations and local adaptation in acanthocephalan-induced parasite manipulation. Evolution 64, 2417–2430. ( 10.1111/j.1558-5646.2010.01006.x) [DOI] [PubMed] [Google Scholar]
- 34.Koskella B, Lively CM. 2007. Advice of the rose: experimental coevolution of a trematode parasite and its snail host. Evolution 61, 152–159. ( 10.1111/j.1558-5646.2007.00012.x) [DOI] [PubMed] [Google Scholar]
- 35.Zbinden M, Haag CR, Ebert D.. 2008. Experimental evolution of field populations of Daphnia magna in response to parasite treatment. J. Evol. Biol. 21, 1068–1078. ( 10.1111/j.1420-9101.2008.01541.x) [DOI] [PubMed] [Google Scholar]
- 36.Schulte RD, Makus C, Hasert B, Michiels NK, Schulenburg H.. 2010. Multiple reciprocal adaptations and rapid genetic change upon experimental coevolution of an animal host and its microbial parasite. Proc. Natl Acad. Sci. USA 107, 7359–7364. ( 10.1073/pnas.1003113107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luong LT, Polak M.. 2007. Costs of resistance in the Drosophila macrocheles system: a negative genetic correlation between ectoparasite resistance and reproduction. Evolution 61, 1391–1402. ( 10.1111/j.1558-5646.2007.00116.x) [DOI] [PubMed] [Google Scholar]
- 38.Zuk M, Simmons LW, Cupp L. 1993. Calling characteristics of parasitized and unparasitized populations of the field cricket Teleogryllus oceanicus. Behav. Ecol. Sociobiol. 33, 339–343. [Google Scholar]
- 39.Zuk M, Rotenberry JT, Tinghitella RM. 2006. Silent night: adaptive disappearance of a sexual signal in a parasitized population of field crickets. Biol. Lett. 2, 521–524. ( 10.1098/rsbl.2006.0539) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tinghitella RM. 2008. Rapid evolutionary change in a sexual signal: genetic control of the mutation ‘flatwing’ that renders male field crickets (Teleogryllus oceanicus) mute. Heredity 100, 261–267. ( 10.1038/sj.hdy.6801069) [DOI] [PubMed] [Google Scholar]
- 41.Balenger SL, Zuk M.. 2015. Roaming Romeos: male crickets evolving in silence show increased locomotor behaviours. Anim. Behav. 101, 213–219. ( 10.1016/j.anbehav.2014.12.023) [DOI] [Google Scholar]
- 42.Bretman A, Tregenza T.. 2007. Strong, silent types: the rapid, adaptive disappearance of a sexual signal. Trends Ecol. Evol. 22, 226–228. ( 10.1016/j.tree.2007.01.011) [DOI] [PubMed] [Google Scholar]
- 43.Godfrey SS. 2013. Networks and the ecology of parasite transmission: A framework for wildlife parasitology. Int. J. Parasitol. Parasites Wildl. 2, 235–245. ( 10.1016/j.ijppaw.2013.09.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Craft ME. 2015. Infectious disease transmission and contact networks in wildlife and livestock. Phil. Trans. R. Soc. B 370, 20140107 ( 10.1098/rstb.2014.0107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wey T, Blumstein DT, Shen W, Jordan F.. 2008. Social network analysis of animal behaviour: a promising tool for the study of sociality. Anim. Behav. 75, 333–344. ( 10.1016/j.anbehav.2007.06.020) [DOI] [Google Scholar]
- 46.White LA, Forester JD, Craft ME. 2015. Using contact networks to explore mechanisms of parasite transmission in wildlife. Biol. Rev. Camb. Phil. Soc. ( 10.1111/brv.12236) [DOI] [PubMed] [Google Scholar]
- 47.Ferguson N. 2007. Capturing human behaviour. Nature 446, 733 ( 10.1038/446733a) [DOI] [PubMed] [Google Scholar]
- 48.Van Kerckhove K, Hens N, Edmunds WJ, Eames KT. 2013. The impact of illness on social networks: implications for transmission and control of influenza. Am. J. Epidemiol. 178, 1655–1662. ( 10.1093/aje/kwt196) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Croft DP, Edenbrow M, Darden SK, Ramnarine IW, van Oosterhout C, Cable J.. 2011. Effect of gyrodactylid ectoparasites on host behaviour and social network structure in guppies Poecilia reticulata. Behav. Ecol. Sociobiol. 65, 2219–2227. ( 10.1007/s00265-011-1230-2) [DOI] [Google Scholar]
- 50.Kurvers RH, Krause J, Croft DP, Wilson AD, Wolf M.. 2014. The evolutionary and ecological consequences of animal social networks: emerging issues. Trends Ecol. Evol. 29, 326–335. ( 10.1016/j.tree.2014.04.002) [DOI] [PubMed] [Google Scholar]
- 51.Funk S, Bansal S, Bauch CT, Eames KTD, Edmunds WJ, Galvani AP, Klepac P.. 2015. Nine challenges in incorporating the dynamics of behaviour in infectious diseases models. Epidemics 10, 21–25. ( 10.1016/j.epidem.2014.09.005) [DOI] [PubMed] [Google Scholar]
- 52.Adelman JS, Moyers SC, Hawley DM. 2014. Using remote biomonitoring to understand heterogeneity in immune-responses and disease-dynamics in small, free-living animals. Integr. Comp. Biol. 54, 377–386. ( 10.1093/icb/icu088) [DOI] [PubMed] [Google Scholar]
- 53.Sih A, Mathot KJ, Moiron M, Montiglio PO, Wolf M, Dingemanse NJ. 2015. Animal personality and state-behaviour feedbacks: a review and guide for empiricists. Trends Ecol. Evol. 30, 50–60. ( 10.1016/j.tree.2014.11.004) [DOI] [PubMed] [Google Scholar]
- 54.Barber I, Dingemanse NJ. 2010. Parasitism and the evolutionary ecology of animal personality. Phil. Trans. R. Soc. B 365, 4077–4088. ( 10.1098/rstb.2010.0182) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.VanderWaal KL, Ezenwa VO. 2016. Heterogeneity in pathogen transmission: mechanisms and methodology. Funct. Ecol. ( 10.1111/1365-2435.12645) [DOI] [Google Scholar]
- 56.Boyer N, Reale D, Marmet J, Pisanu B, Chapuis JL. 2010. Personality, space use and tick load in an intrdfoduced population of Siberian chipmunks Tamias sibiricus. J. Anim. Ecol. 79, 538–547. ( 10.1111/j.1365-2656.2010.01659.x) [DOI] [PubMed] [Google Scholar]
- 57.Aplin LM, Farine DR, Morand-Ferron J, Cole EF, Cockburn A, Sheldon BC. 2013. Individual personalities predict social behaviour in wild networks of great tits (Parus major). Ecol. Lett. 16, 1365–1372. ( 10.1111/ele.12181) [DOI] [PubMed] [Google Scholar]
- 58.Harcourt JL, Sweetman G, Johnstone RA, Manica A.. 2009. Personality counts: the effect of boldness on shoal choice in three-spined sticklebacks. Anim. Behav. 77, 1501–1505. ( 10.1016/j.anbehav.2009.03.004) [DOI] [Google Scholar]
- 59.Dizney L, Dearing MD. 2013. The role of behavioural heterogeneity on infection patterns: implications for pathogen transmission. Anim. Behav. 86, 911–916. ( 10.1016/j.anbehav.2013.08.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hammond-Tooke CA, Poulin R, Nakagawa S.. 2012. Parasitism and behavioural syndromes in the fish Gobiomorphus cotidianus. Behaviour 149, 601–622. ( 10.1163/156853912X648903) [DOI] [Google Scholar]
- 61.Webber QMR, Willis Craig KR, McGuire LP, Smith SB. 2015. Host behaviour, age and sex correlate with ectoparasite prevalence and intensity in a colonial mammal, the little brown bat. Behaviour 152, 83–105. ( 10.1163/1568539x-00003233) [DOI] [Google Scholar]
- 62.Cezilly F, Favrat A, Perrot-Minnot MJ. 2013. Multidimensionality in parasite-induced phenotypic alterations: ultimate versus proximate aspects. J. Exp. Biol. 216, 27–35. ( 10.1242/jeb.074005) [DOI] [PubMed] [Google Scholar]
- 63.Kortet R, Hedrick AV, Vainikka A.. 2010. Parasitism, predation and the evolution of animal personalities. Ecol. Lett. 13, 1449–1458. ( 10.1111/j.1461-0248.2010.01536.x) [DOI] [PubMed] [Google Scholar]
- 64.Vedhara K, Gill S, Eldesouky L, Campbell BK, Arevalo JM, Ma J, Cole SW. 2015. Personality and gene expression: do individual differences exist in the leukocyte transcriptome? Psychoneuroendocrinology 52, 72–82. ( 10.1016/j.psyneuen.2014.10.028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zylberberg M, Klasing KC, Hahn TP. 2014. In house finches, Haemorhous mexicanus, risk takers invest more in innate immune function. Anim. Behav. 89, 115–122. ( 10.1016/j.anbehav.2013.12.021) [DOI] [Google Scholar]
- 66.MacMurray J, Comings DE, Napolioni V.. 2014. The gene-immune-behavioral pathway: gamma-interferon (IFN-γ) simultaneously coordinates susceptibility to infectious disease and harm avoidance behaviors. Brain Behav. Immun. 35, 169–175. ( 10.1016/j.bbi.2013.09.012) [DOI] [PubMed] [Google Scholar]
- 67.Tung J, et al. 2015. Social networks predict gut microbiome composition in wild baboons. eLife 4, e05224 ( 10.7554/eLife.05224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moeller AH, Foerster S, Wilson ML, Pusey AE, Hahn BH, Ochman H. 2016. Social behavior shapes the chimpanzee pan-microbiome. Sci. Adv. 2, e1500997 ( 10.1126/sciadv.1500997) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Forsythe P, Kunze WA. 2013. Voices from within: gut microbes and the CNS. Cell. Mol. Life Sci. 70, 55–69. ( 10.1007/s00018-012-1028-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mayer EA, Knight R, Mazmanian SK, Cryan JF, Tillisch K.. 2014. Gut microbes and the brain: paradigm shift in neuroscience. J. Neurosci. 34, 15 490–15 496. ( 10.1523/JNEUROSCI.3299-14.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Archie EA, Theis KR. 2011. Animal behaviour meets microbial ecology. Anim. Behav. 82, 425–436. ( 10.1016/j.anbehav.2011.05.029) [DOI] [Google Scholar]
- 72.Ezenwa VO, Williams AE. 2014. Microbes and animal olfactory communication: where do we go from here? BioEssays 36, 847–854. ( 10.1002/bies.201400016) [DOI] [PubMed] [Google Scholar]
- 73.Ezenwa VO, Gerardo NM, Inouye DW, Medina M, Xavier JB. 2012. Microbiology. Animal behavior and the microbiome. Science 338, 198–199. ( 10.1126/science.1227412) [DOI] [PubMed] [Google Scholar]
- 74.Lombardo MP. 2008. Access to mutualistic endosymbiotic microbes: an underappreciated benefit of group living. Behav. Ecol. Sociobiol. 62, 479–497. ( 10.1007/s00265-007-0428-9) [DOI] [Google Scholar]
- 75.Soave O, Brand CD. 1991. Coprophagy in animals—a review. Cornell. Vet. 81, 357–364. [PubMed] [Google Scholar]
- 76.Koch H, Schmid-Hempel P.. 2011. Socially transmitted gut microbiota protect bumble bees against an intestinal parasite. Proc. Natl Acad. Sci. USA 108, 19 288–19 292. ( 10.1073/pnas.1110474108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Koch H, Schmid-Hempel P.. 2012. Gut microbiota instead of host genotype drive the specificity in the interaction of a natural host-parasite system. Ecol. Lett. 15, 1095–1103. ( 10.1111/j.1461-0248.2012.01831.x) [DOI] [PubMed] [Google Scholar]
- 78.Alcock J, Maley CC, Aktipis CA. 2014. Is eating behavior manipulated by the gastrointestinal microbiota? Evolutionary pressures and potential mechanisms. BioEssays 36, 940–949. ( 10.1002/bies.201400071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Corlatti L, Bethaz S, von Hardenberg A, Bassano B, Palme R, Lovari S.. 2012. Hormones, parasites and male mating tactics in Alpine chamois: identifying the mechanisms of life history trade-offs. Anim. Behav. 84, 1061–1070. ( 10.1016/j.anbehav.2012.08.005) [DOI] [Google Scholar]
- 80.Spiesman BJ, Inouye BD. 2013. Habitat loss alters the architecture of plant-pollinator interaction networks. Ecology 94, 2688–2696. ( 10.1890/13-0977.1) [DOI] [PubMed] [Google Scholar]
- 81.Yuan N, Fu Z, Zhang H, Piao L, Xoplaki E, Luterbacher J. 2015. Detrended partial-cross-correlation analysis: a new method for analyzing correlations in complex system. Sci. Rep. 5, 8143 ( 10.1038/srep08143) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.El-Gohary M, McNames J. 2007. Establishing causality with whitened cross-correlation analysis. IEEE Trans. Bio-Med. Eng. 54, 2214–2222. ( 10.1109/TBME.2007.906519) [DOI] [PubMed] [Google Scholar]
- 83.Brockhurst MA, Koskella B.. 2013. Experimental coevolution of species interactions. Trends Ecol. Evol. 28, 367–375. ( 10.1016/j.tree.2013.02.009) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


