Abstract
Colour vision is highly variable in New World monkeys (NWMs). Evidence for the adaptive basis of colour vision in this group has largely centred on environmental features such as foraging benefits for differently coloured foods or predator detection, whereas selection on colour vision for sociosexual communication is an alternative hypothesis that has received little attention. The colour vision of uakaris (Cacajao) is of particular interest because these monkeys have the most dramatic red facial skin of any primate, as well as a unique fission/fusion social system and a specialist diet of seeds. Here, we investigate colour vision in a wild population of the bald uakari, C. calvus, by genotyping the X-linked opsin locus. We document the presence of a polymorphic colour vision system with an unprecedented number of functional alleles (six), including a novel allele with a predicted maximum spectral sensitivity of 555 nm. This supports the presence of strong balancing selection on different alleles at this locus. We consider different hypotheses to explain this selection. One possibility is that trichromacy functions in sexual selection, enabling females to choose high-quality males on the basis of red facial coloration. In support of this, there is some evidence that health affects facial coloration in uakaris, as well as a high prevalence of blood-borne parasitism in wild uakari populations. Alternatively, the low proportion of heterozygous female trichromats in the population may indicate selection on different dichromatic phenotypes, which might be related to cryptic food coloration. We have uncovered unexpected diversity in the last major lineage of NWMs to be assayed for colour vision, which will provide an interesting system to dissect adaptation of polymorphic trichromacy.
Keywords: cacajao, colour vision, trichromacy, sexual selection
1. Introduction
Colour vision is a key trait that has been fertile ground for demonstrating adaptations in animal sensory systems. Most work has focused on adaptations of colour vision to environmental conditions, such as ambient light or food. Several recent studies in invertebrates and vertebrates have shown a link between colour vision and intraspecific signalling (e.g. [1–4]), but there has been relatively little work on mammals. In New World monkeys (NWMs), colour vision is highly variable, and a substantial body of research has investigated the ecological and evolutionary basis of this variation [5], which has implications for the acquisition of trichromacy in humans, apes and Old World monkeys (Catarrhini) [6]. The most frequent colour vision system in NWMs is polymorphic trichromacy, in which individuals of the same species may be either dichromats or trichromats. In these species, there are multiple alleles at the single X-linked opsin locus encoding middle- to long-wave-sensitive (MWS/LWS) visual pigments. This leads to heterozygous females being trichromats, whereas homozygous females and hemizygous males are dichromats [5,7]. Almost all NWM genera studied to date have a polymorphic system, including callithrichids such as marmosets (Callithrix), cebids such as capuchin monkeys (Cebus, Sapajus), atelids such as spider (Ateles) and woolly (Lagothrix) monkeys, and pitheciids such as titi (Callicebus), saki (Pithecia) and bearded saki (Chiropotes) monkeys [8,9]. The two exceptions are the nocturnal owl monkeys (Aotus), which are monochromatic, and the howler monkeys (Alouatta), in which all males and females are trichomatic (routine trichromacy) [10–12].
The selective advantages of trichromacy for primates and the evolutionary forces maintaining colour vision polymorphism have been the subject of considerable discussion. Among environmental targets for selection, there is substantial support for a foraging advantage for trichromats for yellow/red fruit and/or young leaves compared with dichromats [13–17]. The high degree of folivory of howler monkeys has been suggested to account for the evolution of routine trichromacy in this lineage [18]. Predation by snakes and carnivores has also been implicated in the evolution of trichromacy [19,20]. An alternative hypothesis is that trichromacy functions in intraspecific signalling, enabling individuals to discriminate levels of oxygen saturation in red, blood-engorged areas of bare skin [21]. To date, this hypothesis has primarily been applied to catarrhines, with examples including the facial skin in mandrills and macaques, and sexual swellings of chimpanzees and macaques [21]. By contrast, the possibility that trichromacy functions in intraspecific signalling in NWMs has rarely been considered.
A consistent advantage for trichromatic females (such as foraging) would be sufficient to maintain colour vision polymorphism in polymorphic species via overdominance. However, there is evidence for visual tasks in which dichromats outperform trichromats in both captivity and the wild, such as detection of camouflaged food [22–24] and improved foraging at low (mesopic) light levels [25]. In addition, an absence of fitness differences between female dichromats and trichromats in a long-term field study of capuchin monkeys [26] suggests that trichromat advantage alone is not operating. Together, these suggest that frequency-dependent selection may be a more likely explanation for the persistence of colour vision polymorphism in so many NWM species.
The uakaris, Cacajao, comprise a genus of pitheciid NWMs endemic to Amazonia that is unusual in several respects. Uakaris have a specialized diet with a high proportion of seeds from fruits with relatively cryptic coloration [27,28]. They have a unique social system for NWMs, forming very large groups of up to 200 individuals, with a high degree of fission–fusion behaviour [29,30]. Most notably, the bald uakari, C. calvus, has bright red facial skin, in common with several catarrhine primates [31] and two other NWMs (the red-faced black spider monkey, Ateles paniscus, and the red-nosed bearded saki, Chiropotes albinasus [32,33]). The bald uakari provides the most extreme example of red facial skin of all primates. In this species, the red coloration extends over the entire head, which is sparsely haired, unmelanized and an intense scarlet, due to the underlying blood supply [34,35]. The red coloration is subjectively more intense in adult males in comparison with females [36], and the enlarged temporal muscles of the adult males further exaggerate the trait in this sex [37,38]. In addition, the overall pelage colour is reddish orange or reddish golden [32]. Together, these unique features mean that the colour vision of uakaris is of great interest. Foraging on cryptic food might diminish the importance of trichromatic colour vision, whereas sexual selection on male red coloration might enhance selection on trichromacy. However, there is currently no information on colour vision in uakaris, and indeed they are now the only major lineage of NWMs in which colour vision remains unexplored.
There is an excellent understanding of the structure–function relationship of primate MW/LW opsin photopigments [9,39–41], showing that the majority of variation in the maximal spectral sensitivity (λmax) of opsins can be attributed to three amino acid sites encoded in exons 3 and 5. In this study, we investigate colour vision in a wild population of the bald uakari in Peru by genotyping the X-linked opsin locus at these key sites affecting spectral sensitivity. We find that a polymorphic system of colour vision is present in this species, and uncover an unprecedented number of functional alleles for a single NWM population.
2. Material and methods
(a). Sampling
We followed a single group of Peruvian red uakaris (Cacajao calvus ucayalii) in November and December 2010 within an area of 600 ha in the Lago Preto Conservation Concession, on the Yavari River, Peru (4°28′ S, 71°46′ W). Group size was unconfirmed, owing to wide group spread and a high degree of fission–fusion behaviour, but a minimum of 80 animals used the study area during the period. Sixty faecal samples were collected from leaf litter as soon as possible after defaecation, but individual animals were not identified. To ensure rapid desiccation, we preserved one small piece of each sample (0.2–0.5 g) on filter paper in 30 ml container 80% full of dry silica gel. A further two samples were available from the Yanayacu-Pucate River population (4°56′ S, 74°08′ W), which is separated from the Yavari population by a wide river barrier [42].
(b). DNA extraction, sex determination and mtDNA genotyping
For DNA extraction from faecal samples, we used the QIAGEN QIAamp Stool Mini Kit (Qiagen) following the standard protocol as recommended by the supplier with only minor changes [43]. Afterwards, DNA concentration was measured on a NanoDrop ND-1000 spectrophotometer and samples were stored at −20°C until further processing. To determine the sex of individuals, we used a PCR-based gonosomal sexing system (C. Roos 2010, unpublished data). The complete mitochondrial cytochrome b gene (1140 bp) was sequenced in all individuals, resulting in seven haplotypes that were used here to aid identification of individuals from faecal samples. Full details of mitochondrial DNA (mtDNA) sequencing will be published elsewhere (C. Roos et al. 2011, unpublished data). Sequences are available from the authors upon request.
(c). Genotyping of the X-linked opsin locus
We designed oligonucleotide primers to amplify exons 3 and 5 of the LWS/MWS opsin gene from an alignment of existing NWM data from Genbank: Exon 3, Primer3CCF2 5′-CTCTGGTCCCTGGCCATCATT-3′ and Primer3CCR2 5′-CCCCTTACCTGCTCCAACCAA-3′; Exon 5, Primer5CCF2 5′-AGAGTCCGAATCCACCCAGA-3′ and Primer5CCR3 5′-TGCCGGTTCATGAAGACAT-3′. PCR reactions contained approximately 50 ng DNA template, 2.5 mM each dNTP, 1× PCR buffer, 1.5 mM MgCl2, 0.1U Taq DNA polymerase (Bioline), 0.25 µM each primer and ddH2O to a final volume of 20 µl. After an initial denaturation at 94°C for 5 min, the thermocycling profile consisted of 43 cycles at 94°C for 30 s, 57°C for 20 s and 72°C for 30 s, and a final extension at 72°C for 5 min. Each PCR was performed with a negative control consisting of ddH2O instead of template DNA. Each exon was amplified and sequenced one to five times on sense and antisense strands per individual (electronic supplementary material, table S1) to avoid genotyping errors (i.e. to ensure correct scoring of heterozygous sites). Since no repeat genotypes gave conflicting results, allelic dropout in heterozygotes could be excluded.
PCR products were purified by adding 0.05U Exonuclease I, 0.25U Shrimp Alkaline Phosphatase (Affymetrix) and 0.7 µl of ddH2O add to each 6 µl PCR product. They were then heated at 37°C for 30 min followed by 15 min at 80°C. Sequencing on both strands was performed at the DNA Sequencing Facility (Department of Biochemistry, University of Cambridge, UK) with an Applied Biosystems 3730 DNA Analyser. Sequences were edited using SeqMan in Lasergene 10 (DNAstar, Madison, WI, USA) and aligned/translated in MEGA 5 [44].
(d). Data analysis
Colour vision phenotypes for each individual were inferred by combining data from exons 3 and 5 of the LWS/MWS opsin gene, with predicted maximum spectral sensitivity (λmax) from critical positions at exon 3 (position 180) and exon 5 (277 and 285) [45]; we also checked site 294 [46]. Chi-square tests were used to test for heterogeneity in allele frequencies. Simulations of the colour vision of selected uakari opsin genotypes were carried out with the method of [44] with the λmax for the shortwave opsin set to 420 nm.
3. Results
We determined the sex, mtDNA haplotype and X-linked opsin genotypes for 34 faecal samples (electronic supplementary material, table S1). Three pairs of faecal samples (from two males and one female) yielded identical mtDNA and opsin sequences, and we conservatively consider that these are samples from the same individuals in the following analyses. All other samples yielded unique genotypes. We therefore analysed genotypes from 31 total individuals, comprising 11 males and 20 females.
We identified multiple functional alleles at the X-linked opsin locus defined by variation at the three key amino sites determining spectral sensitivity (180, 277, 285; table 1). Two further variable sites occurred (276, 309), which have not been implicated in functional effects. All alleles were verified by the two exons being independently amplified and sequenced in multiple samples (five alleles), or, where only present in a single individual, from amplification and sequencing both exons independently at least twice (one allele; electronic supplementary material, table S1). In addition, all exons with heterozygous sites were sequenced at least twice. No individual presented more than one heterozygous site across the three sites in the two exons, so haplotype inference was straightforward.
Table 1.
X-linked opsin amino acid variants at key functional sites, inferred functional alleles and inferred colour vision in wild uakaris.
| exon 3 | exon 5 |
||||
|---|---|---|---|---|---|
| sample size | 180 | 277 | 285 | inferred alleles (λmax nm) | inferred vision |
| 2 M, 3 F | A | F | A | 535 | dichromatic |
| 1 F | A | Y | A | 543 | dichromatic |
| 4 M, 5 F | A | F | T | 550 | dichromatic |
| 2 M, 1 F | A | Y | T | 556 | dichromatic |
| 3 M, 3 F | S | Y | T | 562 | dichromatic |
| 3 F | A | F | T/A | 535/550 | trichromatic |
| 1 F | A | F/Y | T | 550/556 | trichromatic |
| 2 F | A/S | F | T | 550/555 | trichromatic |
| 1 F | A/S | Y | T | 556/562 | trichromatic |
The results demonstrate a polymorphic system of colour vision in the bald uakari. First, some females were heterozygous for one or more key amino acid sites (35%, 7/20), whereas no heterozygotes were found among males (0/11), following the pattern expected for an X-linked polymorphic system. Second, in total we found six alleles defined by variation across the three key sites, an unprecedented number in a single primate species. Five of these alleles have been described previously in other species. The sixth allele, with the three-site pattern serine–phenylalanine–threonine (SFT), is novel and was found in two heterozygous females. Following the additive rule for the phenotypic effects of substitutions at these sites [40], the alanine (A) to serine (S) transition at site 180 should add approximately 5 nm to the λmax of the alanine–phenylalanine–threonine (AFT) allele (550 nm), giving a predicted λmax of the novel allele of approximately 555 nm; we hereafter refer to this as the 555 nm allele. This is slightly less than the λmax of the previously described allele alanine–tyrosine–threonine (AYT) (556 nm), and functional studies will be needed to determine whether these two opsins have sufficient λmax separation to provide useful red–green colour vision in a trichromat. Across all alleles, there was no variation at site 294 (all alleles with Asparagine (N)), a site which can affect tuning in other primates [46].
The frequencies of the six alleles among the 51 X chromosomes sampled are shown in figure 1. Allele frequencies varied from 0.039 to 0.392, and showed significant heterogeneity (χ² = 27.23, P < 0.05). The three most common alleles had λmax of 535, 550 and 562 nm (figure 1), whereas the 543 nm allele was the rarest allele, sampled in a single individual. Four types of heterozygote were found: 535/550 nm, 550/556 nm, 550/555 nm and 556/562 nm (table 1). Simulated colour vision for representative genotypes is shown in figure 2.
Figure 1.
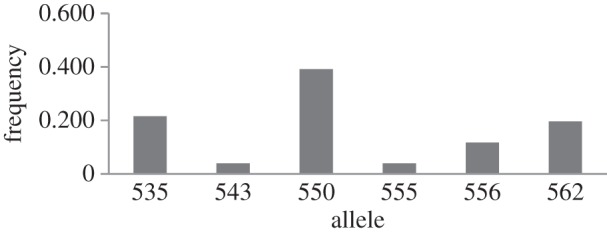
Allele frequencies of inferred LWS/MWS visual pigments in the bald uakari monkey (Cacajao calvus). n = 51 X chromosomes.
Figure 2.
Simulated appearance of a male bald uakari for a trichromatic human observer compared among predicted colour vision phenotypes of bald uakari [47].
4. Discussion
The system of X-linked colour vision polymorphism we have uncovered in the uakari is similar to that of a large majority of other NWMs, occurring in all families and at least 14 genera. With all major lineages now sampled, there are just two NWM genera with other types of colour vision—routine trichromacy in Alouatta and monochromacy in Aotus. However, the presence of six functional allelic types in a wild primate population is unprecedented. Most species of NWM, including all callitrichids, cebids and polymorphic atelids, have two to three, or occasionally four, alleles. Among wild populations of the three other pitheciid genera (a saki, Pithecia irrorata [9,48]; a bearded saki, Chiropotes utahicki [8]; a titi monkey, Callicebus brunneus [45]), the three alleles found (535, 550 and 562 nm) were the same as the three most common alleles in the uakari. Another species of titi monkey (C. moloch) is the only other species of NWM in which more than four alleles have been reported [49]. However, the five alleles (530, 535, 542, 550 and 562 nm) were found in a captive population, and it is unknown whether all of these alleles co-occur in the same wild population. In addition to the novel 555 nm allele found in the uakari, the 556 nm allele has not been previously described in pitheciids.
We now consider the selective forces that are responsible for such a high degree of colour vision polymorphism in uakaris. High allelic diversity may reflect trichromat advantage, since the greater the number of alleles, the higher the potential proportion of female heterozygotes. Discussions of the adaptive advantage of trichromats in NWMs have largely centred on foraging ability. Studies modelling the chromatic signal of food items eaten in the wild and experimental studies in captivity have confirmed that trichromacy is well suited to many foraging tasks involving yellow/red food against a green foliage background [13–16,50]. However, trichromacy may be a disadvantage for cryptic food [22–24], which probably includes the sclerocarpic fruits whose unripe and mature seeds represent a major proportion of the uakaris' diet [28,51,52], although we emphasize that further evidence (such as colour vision modelling of food items of uakaris) is required. Trichromatic NWMs may also be better at detecting predators such as carnivores and snakes [19,20]. So far only raptor predation on uakaris has been documented, where colour vision is probably less relevant [53]. However, the fact that snakes and jaguars may prey on monkeys larger than uakaris within trees suggests that the threat represented by them should not be discounted [54,55].
Another possibility is that the large number of photopigment alleles in the bald uakari could be related to intraspecific signalling involving red coloration, and in particular the uniformly red facial skin of males. The red facial skin of uakaris bears the hallmarks of a sexually selected trait, being more prominent in males than females (with males also having more prominent heads because of enlarged temporal muscles) [56,57]. Although the mating system of uakaris is poorly understood, there is strong circumstantial evidence for a polygynous system related to male coloration. Uakaris have large group sizes and fission–fusion behaviour [30], which are associated with greater male ornamentation in primates [38]; sexual dimorphism in visual traits is stronger in polygynous primates [58], and there is some evidence that subgroups of uakaris are composed of affiliative male units that are outnumbered by females [59]. In addition, large group sizes in NWMs are associated with simple facial patterns, as found in uakaris [60].
In routinely trichromatic Old World monkeys, the intensity of red coloration of male facial skin has been shown to be a sexually selected signal, such as in mandrills and rhesus macaques [61,62]. These results accord with the finding that primate trichromacy is well suited for discriminating the degree of oxygen saturation in the blood in bare skin [21]. There has been good progress in recent years in identifying the proximate factors that lead to variation in sexually selected skin coloration in non-human primates [63,64]. In uakaris, this could be related to testosterone or to specific genotypes, as it is in mandrills [65,66], and/or to parasitism and immune status, following some of the main theoretical explanations for the evolution of sexually selected ornaments [53,67–69]. There is anecdotal evidence that the red face of the bald uakari varies with health, becoming pale in sick uakaris [33,34,70], and the structure of the uakari facial skin allows a direct external assessment of haematological status [34], suggesting that the colour of the face would be an honest indicator of health. Intriguingly, uakaris appear particularly susceptible to infection by two blood-borne parasites: they showed the highest rates of infection by the malaria parasite Plasmodium brasiliensis of any NWM [71] and had an infection rate of 100% for trypanosomatids (Trypanosoma cruzi and T. rangeli) compared with 64% for other primates on the Yavari River in Peru [72]. Hence, the physiological effects of plasmodial and trypanosomal infections might be two aspects of male quality that are signalled by facial coloration in uakaris.
These considerations support the possibility that high levels of colour vision polymorphism in the bald uakari might be an adaptation for sexual selection on male (facial) coloration. Colour vision simulations provide evidence for the conspicuousness of the red male head to female uakari trichromats (figure 2). Trichromacy in female uakaris could serve an important function in discriminating male quality via facial coloration, and give trichromatic females an advantage over dichromats.
If trichromatic advantage alone were operating, we would expect to find a high proportion of heterozygous females, but only 35% of females in our study are heterozygous. This might be simply attributable to inbreeding in this single sampled population. An alternative explanation is that selection is more complex, and involves frequency-dependent selection in which there are some situations in which dichromats outperform trichromats or other dichromats. For example, dichromats may have a foraging advantage for cryptic food, or dichromats with longer wavelength alleles may have a foraging advantage over other dichromats [24,25,50]. However, it is difficult to envisage a scenario in which frequency-dependent selection alone could lead to the low heterozygosity, and a contribution of inbreeding remains likely.
In conclusion, uakaris have surprisingly diverse colour vision, and it is plausible that sexual selection is a contributory factor, although other adaptive advantages may well be involved. Further clarification of the selective forces acting on uakari colour vision will require behavioural studies. It will be of great interest to elucidate these mechanisms further, since uakaris may be a model for the evolution of colour vision in catarrhines, in which ancestrally evolved trichromatic colour vision for foraging then became co-opted for use in sociosexual signalling [73] (but see [74]).
Supplementary Material
Acknowledgements
We thank the Natural History Museum of the National University of San Marcos, Lima and WCS Peru, for assistance in logistics, and Amanda Melin for performing colour vision simulations. Maribel Recharte and Lisseth Lavajos assisted in the field, Christiane Schwarz assisted in the laboratory. We thank Pedro Mayor and four anonymous reviewers for comments on the manuscript.
Ethics
Data were collected with a permit from INRENA (Instituto Nacional de Recursos Naturales) of the Peruvian government, and the methodology complied with their regulations.
Data accessibility
Nucleotide sequences have been deposited in Genbank (accession nos. KP867064–KP867097).
Authors' contributions
M.B. and N.I.M. conceived the study; M.B. collected field samples; J.C., C.R. and M.B. generated the data; J.C. and N.I.M. analysed the data; J.C., M.B., C.R., E.W.H. and N.I.M. wrote the manuscript. All authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
We thank the Los Angeles Zoo for funding the sample collections, and Murray Edwards College, Cambridge for funding opsin genotyping. Part of the genetic analyses was made possible by a DAAD grant to M.B. for a research stay at the Deutsches Primatenzentrum.
References
- 1.Seehausen O, et al. 2008. Speciation through sensory drive in cichlid fish. Nature 455, 620–626. ( 10.1038/nature07285) [DOI] [PubMed] [Google Scholar]
- 2.Briscoe AD, Bybee SM, Bernard GD, Yuan F, Sison-Mangus MP, Reed RD, Warren AD, Llorente-Bousquets J, Chiao CC. 2010. Positive selection of a duplicated UV-sensitive visual pigment coincides with wing pigment evolution in Heliconius butterflies. Proc. Natl Acad. Sci. USA 107, 3628–3633. ( 10.1073/pnas.0910085107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ödeen A, Pruett-Jones S, Driskell AC, Armenta JK, Håstad O. 2012. Multiple shifts between violet and ultraviolet vision in a family of passerine birds with associated changes in plumage coloration. Proc. R. Soc. B 279, 1269–1276. ( 10.1098/rspb.2011.1777) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bloch NI. 2014. Evolution of opsin expression in birds driven by sexual selection and habitat. Proc. R. Soc. B 282, 20142321 ( 10.1098/rspb.2014.2321) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Surridge AK, Osorio D, Mundy NI. 2003. Evolution and selection of trichromatic vision in primates. Trends Ecol. Evol. 18, 198–205. ( 10.1016/S0169-5347(03)00012-0) [DOI] [Google Scholar]
- 6.Jacobs GH. 2008. Primate color vision: a comparative perspective. Visual Neurosci. 25, 619–633. ( 10.1017/S0952523808080760) [DOI] [PubMed] [Google Scholar]
- 7.Mollon JD, Bowmaker JK, Jacobs GH. 1984. Variations of colour vision in a New World primate can be explained by polymorphism of retinal photopigments. Proc. R. Soc. Lond. B 222, 373–399. ( 10.1098/rspb.1984.0071) [DOI] [PubMed] [Google Scholar]
- 8.Lima EM, Pessoa DMA, Sena L, de Melo AGC, de Castro PHG, Oliveira-Mendes AC, Schneider MPC, Pessoa VF. 2015. Polymorphic color vision in captive Uta Hick's cuxiús, or bearded sakis (Chiropotes utahickae). Am. J. Primatol. 77, 66–75. ( 10.1002/ajp.22311) [DOI] [PubMed] [Google Scholar]
- 9.Bonci DMO, Neitz M, Neitz J, Silveira LCL, Ventura DF. 2013. The genetics of New World monkey visual pigments. Psychol. Neurosci. 6, 133–144. ( 10.3922/j.psns.2013.2.02) [DOI] [Google Scholar]
- 10.Levenson DH, Fernandez-Duque E, Evans S, Jacobs GH. 2007. Mutational changes in S-cone opsin genes common to both nocturnal and cathemeral Aotus monkeys. Am. J. Primatol. 69, 757–765. ( 10.1002/ajp.20402) [DOI] [PubMed] [Google Scholar]
- 11.Hiramatsu C, Radlwimmer FB, Yokoyama S, Kawamura S. 2004. Mutagenesis and reconstitution of middle-to-longwave-sensitive visual pigments of New World monkeys for testing the tuning effect of residues at sites 229 and 233. Vis. Res. 44, 2225–2231. ( 10.1016/j.visres.2004.04.008) [DOI] [PubMed] [Google Scholar]
- 12.Jacobs GH, Neitz M, Deegan JF, Neitz J. 1996. Trichromatic colour vision in New World monkeys. Nature 382, 156–158. ( 10.1038/382156a0) [DOI] [PubMed] [Google Scholar]
- 13.Smith AC, Buchanan-Smith HM, Surridge AK, Osorio D, Mundy NI. 2003. The effect of colour vision status on the detection and selection of fruits by tamarins (Saguinus spp.). J. Exp. Biol. 206, 3159–3165. ( 10.1242/jeb.00536) [DOI] [PubMed] [Google Scholar]
- 14.Dominy NJ, Lucas PW. 2001. Ecological importance of trichromatic vision to primates. Nature 410, 363–366. ( 10.1038/35066567) [DOI] [PubMed] [Google Scholar]
- 15.Caine NG, Mundy NI. 2000. Demonstration of a foraging advantage for trichromatic marmosets (Callithrix geoffroyi) dependent on food colour. Proc. R. Soc. Lond. B 267, 439–444. ( 10.1098/rspb.2000.1019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sumner P, Mollon JD. 2000. Catarrhine photopigments are optimized for detecting targets against a foliage background. J. Exp. Biol. 203, 1963–1986. [DOI] [PubMed] [Google Scholar]
- 17.Melin AD, Hiramatsu C, Parr NA, Matsushita Y, Kawamura S, Fedigan LM. 2014. The behavioral ecology of color vision: considering fruit conspicuity, detection distance and dietary importance. Int. J. Primatol. 35, 258–287. ( 10.1007/s10764-013-9730-8) [DOI] [Google Scholar]
- 18.Jacobs GH. 2007. New world monkeys and color. Int. J. Primatol. 28, 729–759. ( 10.1007/s10764-007-9168-y) [DOI] [Google Scholar]
- 19.Isbell LA. 1994. Predation on primates: ecological patterns and evolutionary consequences. Evol. Anthropol. 3, 61–71. ( 10.1002/evan.1360030207) [DOI] [Google Scholar]
- 20.Pessoa DMA, Maia R, de Albuquerque Ajuz RC, de Moraes PZPMR, Spyrides MHC, Pessoa VF. 2014. The adaptive value of primate color vision for predator detection. Am. J. Primatol. 76, 721–729. ( 10.1002/ajp.22264) [DOI] [PubMed] [Google Scholar]
- 21.Changizi MA, Zhang Q, Shimojo S. 2006. Bare skin, blood and the evolution of primate colour vision. Biol. Lett. 2, 217–221. ( 10.1098/rsbl.2006.0440) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caine NG, Surridge AK, Mundy NI. 2003. Dichromatic and trichromatic Callithrix geoffroyi differ in relative foraging ability for red-green color-camouflaged and non-camouflaged food. Int. J. Primatol. 24, 1163–1175. ( 10.1023/B:IJOP.0000005985.18112.25) [DOI] [Google Scholar]
- 23.Melin AD, Fedigan LM, Hiramatsu C, Sendall C, Kawamura S. 2007. Effects of colour vision phenotype on insect capture by a free-ranging population of white faced capuchins (Cebus capucinus). Anim. Behav. 73, 205–214. ( 10.1016/j.anbehav.2006.07.003) [DOI] [Google Scholar]
- 24.Saito A, et al. 2005. Advantage of dichromats over trichromats in discrimination of color camouflaged stimuli in nonhuman primates. Am. J. Primatol. 67, 425–436. ( 10.1002/ajp.20197) [DOI] [PubMed] [Google Scholar]
- 25.Caine NG, Osorio D, Mundy NI. 2010. A foraging advantage for dichromatic marmosets (Callithrix geoffroyi) at low light intensity. Biol. Lett. 6, 36–38. ( 10.1098/rsbl.2009.0591) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fedigan LM, Melin AD, Addicott JF, Kawamura S. 2014. The heterozygote superiority hypothesis for polymorphic color vision is not supported by long-term fitness data from wild Neotropical monkeys. PLoS ONE 9, e84872 ( 10.1371/journal.pone.0084872) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barnett AA, Bowler M, Bezerra BM, Defler TR. 2013. Ecology and behavior of uacaris (genus Cacajao). In Evolutionary biology and conservation of titis, sakis and uacaris (eds Veiga LM, Barnett AA, Ferrari SF, Norconk MA), pp. 151–172. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 28.Bowler M, Bodmer RE. 2011. Diet and food choice in Peruvian red uakaris (Cacajao calvus ucayalii): selective or opportunistic seed predation? Int. J. Primatol. 32, 1109–1122. ( 10.1007/s10764-011-9527-6) [DOI] [Google Scholar]
- 29.Bowler M, Knogge C, Heymann EW, Zinner D. 2012. Multilevel societies in New World primates? Flexibility may characterize the organization of Peruvian red uakaris (Cacajao calvus ucayalii). Int. J. Primatol. 33, 1110–1124. ( 10.1007/s10764-012-9603-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bowler M, Bodmer R. 2009. Social behavior in fission–fusion groups of red uakari monkeys (Cacajao calvus ucayalii). Am. J. Primatol. 71, 976–987. ( 10.1002/ajp.20740) [DOI] [PubMed] [Google Scholar]
- 31.Setchell JM, Wickings EJ, Knapp LA. 2006. Signal content of red facial coloration in female mandrills (Mandrillus sphinx). Proc. R. Soc. B 273, 2395–2400. ( 10.1098/rspb.2006.3573) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hershkovitz P. 1987. Uacaries, new world monkeys of the genus Cacajao (Cebidae, Platyrrhini): a preliminary taxonomic review with the description of a new subspecies. Am. J. Primatol. 12, 1–53. ( 10.1002/ajp.1350120102) [DOI] [PubMed] [Google Scholar]
- 33.Kellogg R, Goldman EA. 1944. Review of the spider monkeys. Proc. US Natl Mus. 96, 1–45. ( 10.5479/si.00963801.96-3186.1) [DOI] [Google Scholar]
- 34.Hill CA. 1965. Maintenance of facial coloration in the red Uakari Cacajao rubicundus. Int. Zoo. Yearb. 5, 140–141. ( 10.1111/j.1748-1090.1965.tb01611.x) [DOI] [Google Scholar]
- 35.Mayor P, Mamani J, Montes D, González-Crespo C, Sebastián MA, Bowler M. 2015. Proximate causes of the red face of the bald uakari monkey (Cacajao calvus). R. Soc. open sci. 2, 150145 ( 10.1098/rsos.150145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hill OW. 1960. Primates: comparative anatomy and taxonomy. Volume 4, part A. Edinburgh, UK: Edinburgh University Press. [Google Scholar]
- 37.Bowler M. 2007. The ecology and conservation of the red uakari monkey on the Yavari River, Peru. Doctoral dissertation, University of Kent at Canterbury, UK.
- 38.Grueter CC, Isler K, Dixson BJ. 2015. Are badges of status adaptive in large complex primate groups? Evol. Hum. Behav. 36, 398–406. ( 10.1016/j.evolhumbehav.2015.03.003) [DOI] [Google Scholar]
- 39.Neitz M, Neitz J. 1991. Spectral tuning of pigments underlying red-green color vision. Science 252, 971–974. ( 10.1126/science.1903559) [DOI] [PubMed] [Google Scholar]
- 40.Shyue SK, et al. 1998. Molecular genetics of spectral tuning in New World monkey color vision. J. Mol. Evol. 46, 697–702. ( 10.1007/PL00006350) [DOI] [PubMed] [Google Scholar]
- 41.Hunt DM, Dulai KS, Cowing JA, Julliot C, Mollon JD, Bowmaker JK, Li W-H, Hewett-Emmett D. 1998. Molecular evolution of trichromacy in primates. Vision Res. 38, 3299–3306. ( 10.1016/S0042-6989(97)00443-4) [DOI] [PubMed] [Google Scholar]
- 42.Bowler M, Noriega Murrieta J, Recharte M, Puertas P, Bodmer R. 2009. Peruvian red uakari monkeys (Cacajao calvus ucayalii) in the Pacaya-Samiria national reserve—a range extension across a major river barrier. Neotrop. Primates 16, 34–37. ( 10.1896/044.016.0108) [DOI] [Google Scholar]
- 43.Haus T, Akom E, Agwanda B, Hofreiter M, Roos C, Zinner D. 2013. Mitochondrial diversity and distribution of African green monkeys (Chlorocebus Gray, 1870). Am. J. Primatol. 75, 350–360. ( 10.1002/ajp.22113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28, 2731–2739. ( 10.1093/molbev/msr121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bunce JA, Isbell LA, Neitz M, Bonci D, Surridge AK, Jacobs GH, Smith DG. 2011. Characterization of opsin gene alleles affecting color vision in a wild population of titi monkeys (Callicebus brunneus). Am. J. Primatol. 73, 189–196. ( 10.1002/ajp.20890) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matsumoto Y, et al. 2014. Evolutionary renovation of L/M opsin polymorphism confers a fruit discrimination advantage to ateline New World monkeys. Mol. Ecol. 23, 1799–1812. ( 10.1111/mec.12703) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Melin AD, Kline DW, Hickey CM, Fedigan LM. 2013. Food search through the eyes of a monkey: a functional substitution approach for assessing the ecology of primate color vision. Vis. Res. 86, 87–96. ( 10.1016/j.visres.2013.04.013) [DOI] [PubMed] [Google Scholar]
- 48.Boissinot S, Tan Y, Shyue SK, Schneider H, Sampaio I, Neiswanger K, Hewett-Emmett D, Li WH. 1998. Origins and antiquity of X-linked triallelic color vision systems in New World monkeys. Proc. Natl Acad. Sci. USA 95, 13 749–13 754. ( 10.1073/pnas.95.23.13749) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jacobs GH, Deegan JF II. 2005. Polymorphic New World monkeys with more than three M/L cone types. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 22, 2072–2080. ( 10.1364/JOSAA.22.002072) [DOI] [PubMed] [Google Scholar]
- 50.Osorio D, Smith AC, Vorobyev M, Buchanan-Smith HM. 2004. Detection of fruit and the selection of primate visual pigments for color vision. Am. Nat. 164, 696–708. ( 10.1086/425332) [DOI] [PubMed] [Google Scholar]
- 51.Smith AC, Surridge AK, Prescott MJ, Osorio D, Mundy NI, Buchanan-Smith HM. 2012. Effect of colour vision status on insect prey capture efficiency of captive and wild tamarins (Saguinus spp.). Anim. Behav. 83, 479–486. ( 10.1016/j.anbehav.2011.11.023) [DOI] [Google Scholar]
- 52.Aquino R, Encarnación F. 1999. Observaciones preliminares sobre la dieta de Cacajao calvus ucayalii en el nor-oriente peruano. Neotrop. Primates 7, 1–5. [Google Scholar]
- 53.Barnett AA, Schiel V, Deveny A, Valsko J, Spironello WR, Ross C. 2011. Predation on Cacajao ouakary and Cebus albifrons (Primates: Platyrrhini) by harpy eagles. Mammalia 75, 169–172. ( 10.1515/mamm.2011.004) [DOI] [Google Scholar]
- 54.Quintino EP, Bicca-Marques JC. 2013. Predation of Alouatta puruensis by Boa constrictor. Primates 54, 325–330. ( 10.1007/s10329-013-0377-z) [DOI] [PubMed] [Google Scholar]
- 55.Peetz A, Norconk MA, Kinzey WG. 1992. Predation by jaguar on howler monkeys (Alouatta seniculus) in Venezuela. Am. J. Primatol. 28, 223–228. ( 10.1002/ajp.1350280307) [DOI] [PubMed] [Google Scholar]
- 56.Dixson AF. 2012. Primate sexuality: comparative studies of the prosimians, monkeys, apes and humans, 2nd edn New York, NY: Oxford University Press. [Google Scholar]
- 57.Ayres JM. 1986. The white uakaris and the Amazonian flooded forests. PhD thesis, Cambridge University, Cambridge UK.
- 58.Dixson AF, Dixson BJ, Anderson M. 2005. Sexual selection and the evolution of visually conspicuous sexually dimorphic traits in male monkeys, apes, and human beings. Annu. Rev. Sex Res. 16, 1–19. [PubMed] [Google Scholar]
- 59.Gregory T, Bowler M. In press. Male–male affiliation and cooperation characterize the social behavior of the large-bodied pitheciids, Chiropotes and Cacajao: a review. Am. J. Primatol. ( 10.1002/ajp.22404) [DOI] [PubMed] [Google Scholar]
- 60.Santana SE, Alfaro JL, Alfaro ME. 2014. Adaptive evolution of facial colour patterns in Neotropical primates. Proc. R. Soc. B 297, 2204–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dubuc C, Allen W, Maestripieri D, Higham JP. 2014. Is male rhesus macaque red color ornamentation attractive to females? Behav. Ecol. Sociobiol. 68, 1215–1224. ( 10.1007/s00265-014-1732-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Setchell JM. 2005. Do female mandrills prefer brightly colored males? Int. J. Primatol. 26, 715–735. ( 10.1007/s10764-005-5305-7) [DOI] [Google Scholar]
- 63.Bergman TJ, Ho L, Beehner JC. 2009. Chest color and social status in male geladas (Theropithecus gelada). Int. J. Primatol. 30, 791–806. ( 10.1007/s10764-009-9374-x) [DOI] [Google Scholar]
- 64.Setchell JM, Wickings JE. 2005. Dominance, status signals and coloration in male mandrills (Mandrillus sphinx). Ethology 111, 25–50. ( 10.1111/j.1439-0310.2004.01054.x) [DOI] [Google Scholar]
- 65.Setchell JM, Dixson AF. 2001. Changes in the secondary sexual adornments of male mandrills (Mandrillus sphinx) are associated with gain and loss of alpha status. Horm. Behav. 39, 177–184. ( 10.1006/hbeh.2000.1628) [DOI] [PubMed] [Google Scholar]
- 66.Setchell JM, Smith T, Wickings EJ, Knapp LA. 2008. Social correlates of testosterone and ornamentation in male mandrills. Horm. Behav. 54, 365–372. ( 10.1016/j.yhbeh.2008.05.004) [DOI] [PubMed] [Google Scholar]
- 67.Setchell JM, Charpentier M, Abbott KA, Wickings EJ, Knapp LA. 2009. Is brightest best? Testing the Hamilton-Zuk hypothesis in mandrills. Int. J. Primatol. 30, 825–844. ( 10.1007/s10764-009-9371-0) [DOI] [Google Scholar]
- 68.Hamilton WD, Zuk M. 1982. Heritable true fitness and bright birds: a role for parasites. Science 218, 384–387. ( 10.1126/science.7123238) [DOI] [PubMed] [Google Scholar]
- 69.Bradley BJ, Mundy NI. 2008. The primate palette: the evolution of primate coloration. Evol. Anthropol. 17, 97–111. ( 10.1002/evan.20164) [DOI] [Google Scholar]
- 70.Lasry JE, Sheridan BW. 1965. Chagas’ myocarditis and heart failure in the red uakari Cacajao rubicundus. Int. Zoo Yearbook. 5, 182–184. ( 10.1111/j.1748-1090.1965.tb01633.x) [DOI] [Google Scholar]
- 71.Davies CR, Ayres JM, Dye C, Deane JM. 1991. Malaria infection rate of Amazonian primates increases with body weight and group size. Funct. Ecol. 5, 655–662. ( 10.2307/2389485) [DOI] [Google Scholar]
- 72.Aysanoa E, et al. 2014. Prevalence of trypanosomatids and Trypanosoma cruzi in wild and captive non-human primates from Perú. Abstract 1738, American Society of Tropical Medicine and Hygiene, 63rd Annual Meeting, New Orleans, LA, USA, 2–6 November 2014. Am. J. Trop. Med. Hygiene, 91(Suppl. 5), 530.
- 73.Fernandez AA, Morris MR. 2007. Sexual selection and trichromatic color vision in primates: statistical support for the preexisting-bias hypothesis. Am. Nat. 170, 10–20. ( 10.1086/518566) [DOI] [PubMed] [Google Scholar]
- 74.Kamilar JM, Heesy CP, Bradley BJ. 2012. Did trichromatic color vision and red hair color coevolve in primates? Am. J. Primatol. 75, 740–751. ( 10.1002/ajp.22099) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Nucleotide sequences have been deposited in Genbank (accession nos. KP867064–KP867097).



