Abstract
There is broad consensus that the diversity of functional traits within species assemblages drives several ecological processes. It is also widely recognized that rare species are the first to become extinct following human-induced disturbances. Surprisingly, however, the functional importance of rare species is still poorly understood, particularly in tropical species-rich assemblages where the majority of species are rare, and the rate of species extinction can be high. Here, we investigated the consequences of local and regional extinctions on the functional structure of species assemblages. We used three extensive datasets (stream fish from the Brazilian Amazon, rainforest trees from French Guiana, and birds from the Australian Wet Tropics) and built an integrative measure of species rarity versus commonness, combining local abundance, geographical range, and habitat breadth. Using different scenarios of species loss, we found a disproportionate impact of rare species extinction for the three groups, with significant reductions in levels of functional richness, specialization, and originality of assemblages, which may severely undermine the integrity of ecological processes. The whole breadth of functional abilities within species assemblages, which is disproportionately supported by rare species, is certainly critical in maintaining ecosystems particularly under the ongoing rapid environmental transitions.
Keywords: tropical biodiversity, conservation, extinction, rarity index, functional diversity, null models
1. Introduction
All ecosystems on Earth are facing unprecedented levels of disturbance [1] contributing to the sixth extinction crisis [2], with rare species often being the most vulnerable [3]. Species can be considered rare when they have small population sizes, restricted geographical ranges, or narrow habitat tolerances; these combined characteristics define several forms of rarity [4] and different levels of extinction risk [5]. Therefore, compared with abundant and widespread species, rare species have greater sensitivity to both natural- and human-induced disturbances such as overexploitation, habitat loss, and global environmental changes [6,7]. Rare species have thus received significant attention from conservation biologists; nevertheless, the functional consequences of their decline remain largely overlooked [8].
Beyond the loss of species, there is a growing awareness that the loss of ecological processes that sustain ecosystem functioning can be the most critical impact under accelerating global changes [9]. Given their low representativeness in terms of numerical abundance or total biomass, one may expect a low contribution of rare species to ecosystem functioning as a whole [10]; although less common species have already been reported exerting significant impacts on different processes, such as improving resistance to invasions [11] and acting as keystone [12] or cornerstone species [13]. Nevertheless, most of the research on these relationships has so far examined individual processes even though ecosystem functioning relies on many processes that often require multiple ecological roles to be achieved [14] across many environmental conditions [15]. This multifunctionality of ecosystems and the diversity of ecological processes are increasingly seen as being more closely related to the diversity of functional traits within communities than to the diversity of taxa per se, because species with different traits are more likely to play complementary roles [16,17]. In this context, considering the high vulnerability of rare species to extinction, a critical issue is to assess their contribution to the functional structure of assemblages (FS), i.e. the diversity and distribution of functional traits. If rare species mainly support roles that are also played by common species, then we would expect a low impact following their extinction. On the other hand, if rare species over-contribute to FS then their extinction may lead to a significant loss of ecological processes.
A recent study shows that in three regional species pools (coral reef fish, tropical trees, and alpine plants) the most distinct combinations of traits are mainly supported by rare species [18], which may suggest that they are functionally irreplaceable. At the same time, many rare species support the most common functions and only add functional redundancy to the system [18]. However, Mouillot et al. [18] only focused on individual species through their functional distinctiveness, whereas the contribution of rare species to the FS of assemblages remains uncertain. Very few studies explicitly tackled the functional importance of rare species at the assemblage level [19], and none investigated the consequences of their loss at different spatial scales and on the multiple facets of FS.
Here, we built an integrative measure of species rarity versus commonness (i.e. combining local abundance, geographical range, and habitat breadth), and we considered species traits and their distributions within a functional space (sensu Mouillot et al. [20]) to quantitatively assess the contribution of rare species to three complementary facets of assemblage FS: functional richness [21], functional specialization [20], and functional originality [20]. If rare species tend to support the most extreme and unique combinations of traits, then we expect that their extinction would deeply affect the three functional facets (figure 1). We designed scenarios of species loss, both at the local and regional scales, to test whether rare species over- or under-contribute to the FS of assemblages. Testing these two alternative hypotheses is particularly important in the tropics where a large proportion of species are rare [22,23], and high rates of species loss are expected in the near future [24]. Therefore, we applied this framework to three extensive datasets of species-rich tropical assemblages to enhance the generality of our findings: stream fish from the Brazilian Amazon, rainforest trees from French Guiana, and birds from the Australian Wet Tropics (AWT).
Figure 1.
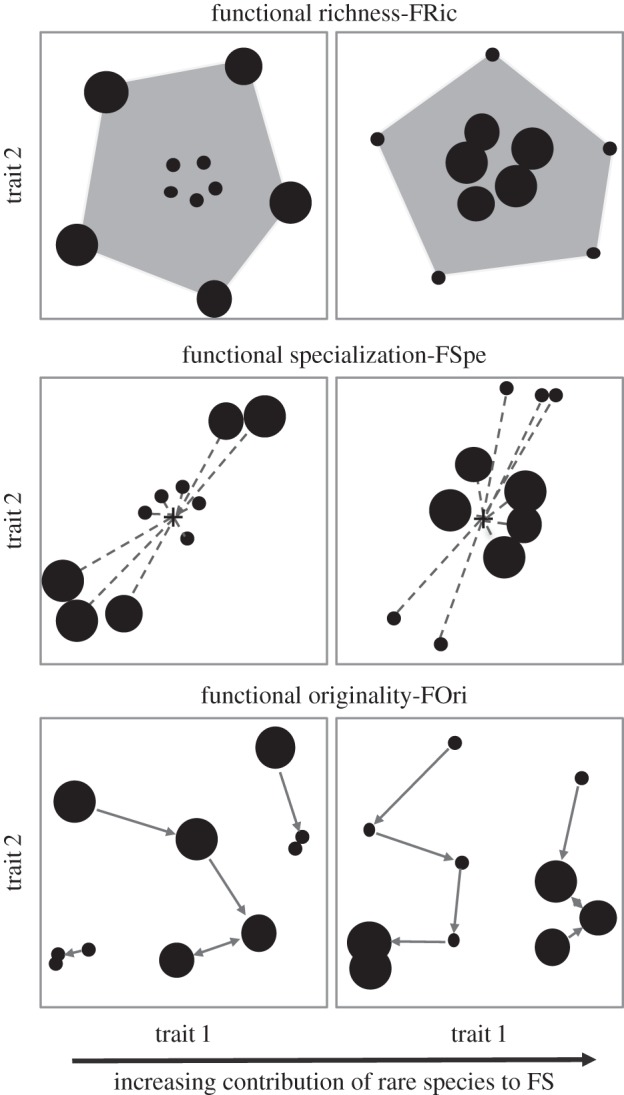
Hypothetical scenarios for the contribution of rare species to the functional structure (FS) of species assemblages. Each plot represents a different study case where species (dots) are distributed across a two-dimensional functional space. The level of species commonness (accounting for abundance, geographical range, and habitat breadth) is illustrated by the size of the dots, rare species being represented by small dots. Three FS indices are illustrated separately: functional richness (convex-hull volume of the functional space filled by all species within the assemblage; grey polygon projected); functional specialization (mean distance between each species and the average position—black cross—of all species; dashed lines); and functional originality (mean distance between a given species and its nearest neighbour; grey arrows). The contribution of rare species to FS increases from the left to the right of the figure in the sense that their loss would significantly reduce the value for each index.
2. Material and methods
(a). Datasets
The datasets were chosen because sampling (i) was carried out in sites covering broad geographical and environmental gradients within well-preserved regions (electronic supplementary material, figure S1), (ii) was standardized for species local abundances and local habitat characterization, and (iii) included a functional characterization of all the species. For each study case, we selected a set of relevant and complementary functional traits to describe the ecological attributes of all the species present.
(i). Fishes
Fishes were sampled in 320 rainforest streams of the Brazilian Amazon between 2004 and 2012, encompassing over 2.3 million km2 along the main tributaries of the cis-Andean Amazon basin. In each stream, we determined a set of 15 environmental parameters describing stream-channel structure, substrate, and water quality (electronic supplementary material, appendix S1). All streams have small dimensions allowing for effective sampling of fish abundances within well-delimited habitat boundaries. The stream extremities (50 m long section) were blocked with fine-mesh nets, and fishes were caught using seine and hand nets. A total of 395 taxa were counted and identified at the species level. Each species was functionally described using a set of 18 ecomorphological traits related to food acquisition, locomotion, and habitat preferences: teeth shape and number; gill-raker shape; mouth protrusion; oral-gape surface, shape and position; eye size and position; body mass, surface, and shape; caudal-peduncle throttling; pectoral-fin shape and position; caudal-fin shape; fin surface ratios (details in the electronic supplementary material, appendix S2).
(ii). Trees
Trees were inventoried in 36 lowland rainforest plots (2 ha) in French Guiana between 2009 and 2010, covering an area of 15 427 km2. In each of the 36 plots, we determined a set of 14 environmental parameters describing climate and soil characteristics (electronic supplementary material, appendix S1), and we counted and identified all trees greater than or equal to 2.5 cm diameter (at 1.3 m height) in 10 2 × 50 m belt transects. Given the operational difficulties to thoroughly measure traits in species-rich tree assemblages, we selected six out of the 36 plots that represented the broader environmental gradient within the region to functionally characterize all species (totalling 262 species). We measured 15 functional traits describing leaf and wood characteristics related to resource capture, nutrient transport, structure, and defence: laminar thickness, toughness and chlorophyll; leaf area, specific area and density; foliar carbon, nitrogen, phosphorus, potassium, C : N ratio and 13C composition; trunk bark thickness; root- and stem-wood specific gravity (details in the electronic supplementary material, appendix S2). Although the functional assessment at the local scale was restricted to the six plots, the estimates of species distribution and abundance included all 36 plots.
(iii). Birds
Birds were sampled between 1992 and 2009 in the AWT, a bioregion that covers 18 000 km2 of mixed tropical forests ranging from sea level to ca 1 600 m. The region is dominated by rainforests with most of the area protected in the AWT World Heritage Area [25]. Birds were recorded within 1 323 standardized dawn surveys across 180 permanent 150 m transects. Each survey was 30 min duration with all individuals counted and identified using calls and visual observations, totalling 86 species. The transects were distributed across 47 subregions delimited by Williams et al. [26] to cover elevational, climatic, and latitudinal gradients across all the AWT. Given that most birds are highly mobile organisms and local-assemblage boundaries are difficult to delimit, we considered each of these 47 subregions as local assemblages in this study. Seven traits describing the key aspects of bird's life history and behaviour were used to functionally characterize the 86 species: body mass; diet; activity period; reproductive seasonality; clutch size; shelter type; vertical strata (details in the electronic supplementary material, appendix S2).
(b). Rarity assessment
Different approaches to define species rarity have been proposed, being most frequently based on three primary characteristics: local abundance, geographical range, and habitat breadth [4]. Because they all determine extinction risk while being complementary to each other [5,25], we embedded these three characteristics within an integrated framework to assess species rarity versus commonness.
For the three datasets, the local abundance of a species i (LAi) was determined as the mean number of individuals counted where that species was present. For fish and tree species, the geographical range (GRi) was estimated by the area (km2) that lies within the outermost limits of the occurrence of each species, based exclusively on their distribution across our sample sites (320 streams, 36 tree plots). For species recorded only in one sample site, GRi was defined as the area of the site in which that species occurred. For species recorded only in two sites, GRi was estimated as the area of a polygon whose sides are the mean extension of the two sites and the distance between them. We chose to restrict geographical range estimates to our own data, because secondary information is mostly lacking for fish and tree species; a lacuna widely recognized for the Amazon forest [23]. For AWT birds, however, species geographical ranges had been previously well established during decades of intensive studies across the region. Therefore, we used the GRi data compiled in Williams et al. [26]. For fish and tree species, habitat breadth (HBi) was estimated by the ‘tolerance’ metric from Outlying Mean Index analyses [27], which is a measure of the species-specific niche breadth relative to the available niche space of the region (i.e. environmental parameters measured across sites). For bird species, HBi was estimated by the proportion of occurrences in different structural vegetation types (‘vegetation specialization’ in Williams et al. [26]).
We built an integrative measure of rarity by combining the three metrics into a single index. Each metric was log-transformed to decrease the magnitude across observed values, and then standardized between 0 and 1 by dividing them by the respective maximum value observed over all species in each dataset. To take into account the degree of dependence between the three metrics, we down-weighted each one by its correlation with the two others [28]. The rarity index for a species i (RIi) is thus calculated as
where wla, wgr, and whb are the weighting parameters that represent the degree of independence of each rarity metric to the others. For instance, the weighting parameter for rarity in terms of local abundance wla is calculated as
where rlagr is the Pearson's correlation coefficient between local abundance and geographical range and rlahb is the Pearson's correlation coefficient between local abundance and habitat breadth (electronic supplementary material, figure S2).
Because each metric scales between 0 and 1, and their weighted values are relativized by the sum of the weighting parameters, RIi also varies between 0 (the potential value reached by the rarest species) and 1 (the potential value reached by the most common species). Therefore, even considering that rarity might be differently expressed for each of the three taxonomic groups, the use of RIi makes them more comparable. However, RIi is a contextual measure, because a species can be rare in a given dataset but common elsewhere and because it highly depends on the species pool (comparatively to the most common species).
(c). Functional structure of species assemblages
For each taxonomic group, we first computed the functional distance between each pair of species. All traits were continuous for trees, so we computed the Euclidean distance on the scaled and centred trait values. Functional traits were not all continuous for fish and birds, so we used the Gower distance, which allows considering different types of traits while standardizing them [21]. We then ran a principal coordinate analysis (PCoA) on each functional distance matrix to build a multidimensional functional space and estimate the different functional facets of assemblage structure [20]. For each taxonomic group, the number of dimensions (i.e. PCoA axes) was chosen based on the quality of the functional space, i.e. the extent to which it accurately represents the initial functional distances between species pairs, quantified by the mean squared-deviation index—mSD [29]. We kept the minimum number of axes that provides a high-quality functional space to minimize the amount of assemblages we had to exclude to attain computation requirements (i.e. higher number of species than PCoA axes [21]). We thus kept the first four, nine, and five PCoA axes for fishes, trees, and birds, respectively (see details in the electronic supplementary material, figure S3 and table S1).
We used three complementary indices to quantitatively describe the FS (figure 1): functional richness (FRic), functional specialization (FSpe), and functional originality (FOri). FRic is the convex-hull volume of the functional space filled by all species within the assemblage, indicating the range of trait combinations [21]. We standardized FRic values by expressing them as a proportion of the volume filled by the pool of species in each dataset. FSpe represents the distinctiveness of species traits in the assemblage [20]. FSpe is expressed as the mean Euclidean distance between each species and the average position of all species (i.e. barycentre) in the functional space. FOri reflects the degree of uniqueness of species traits in the assemblage [20]. FOri is expressed as the mean distance between each species and its nearest neighbour in the functional space. The raw values of FSpe and FOri were standardized between 0 and 1 by dividing them, respectively, by the maximum distance to the barycentre and by the maximum nearest-neighbour distance observed over all species present in each dataset [20]. These functional indices are complementary to each other in the sense that they describe different facets of the assemblage FS. For instance, a species can be placed at the centre of the functional space (i.e. low contribution to FSpe and no contribution to the assemblage FRic) while being isolated from other species (i.e. high contribution to FOri). Because none of the rarity metrics was taken into account in the calculation of functional indices, there were no trivial relationships between rarity and FS assessments.
(d). Scenarios of species loss
To assess the consequences of potential extinctions on the FS of each of the three regional assemblages (395 fishes, 262 trees, and 86 birds), we simulated a set of species-loss scenarios. We first sequentially removed species from the rarest to the most common and we computed the three FS indices at each step. We compared the level of functional erosion obtained under this scenario with the ones obtained from a scenario simulating an unrealistic sequential species loss from the most common to the rarest and from a null scenario simulating a random sequential extinction (i.e. independently of species commonness). To perform this null scenario, we shuffled 1 000 times the order of the species while keeping their trait values constant. Because the 22 rarest tree species have an equal RIi value, we randomized their rank 100 times and used the median value of FS for each deletion step.
While regional or global species extinctions have been scarcely reported, local species extirpations are more and more common worldwide. Therefore, we conducted another set of simulations to assess the potential consequences of local extirpations on the FS of the local assemblages (320 streams, six tree plots, and 47 subregions). We defined nine levels of species loss for each of them, removing from 10% to 90% of the species, and then computed FS indices, following three different scenarios: rarest species lost first; most common species lost first; and random loss of species (1 000 times). We then carried out a Friedman paired test to compare the three scenarios. This allows removing the effects of local specificities (e.g. species richness) on FS while comparing the scenarios. When the remaining number of species after species removal was lower than the number of functional dimensions, we excluded that local assemblage from the analysis (see final sample sizes in the electronic supplementary material, table S2). Because the estimation of the commonness status (RIi) for a given species takes into account both local and regional characteristics, we considered the same RIi value for each species in the two sets of simulations.
All computations were carried out using R software [30]. A list of the main functions used with the respective packages and the scripts to run all simulations are provided in the electronic supplementary material (table S3 and appendices S3–4).
(e). Sensitivity analyses
We conducted a set of sensitivity analyses to assess the robustness of our findings to three methodological aspects: (i) the influence of each particular trait, (ii) the potential effects of the uneven number of traits among the study cases, and (iii) the influence of the number of dimensions (see methodological details and results in the electronic supplementary material, appendix S5).
3. Results
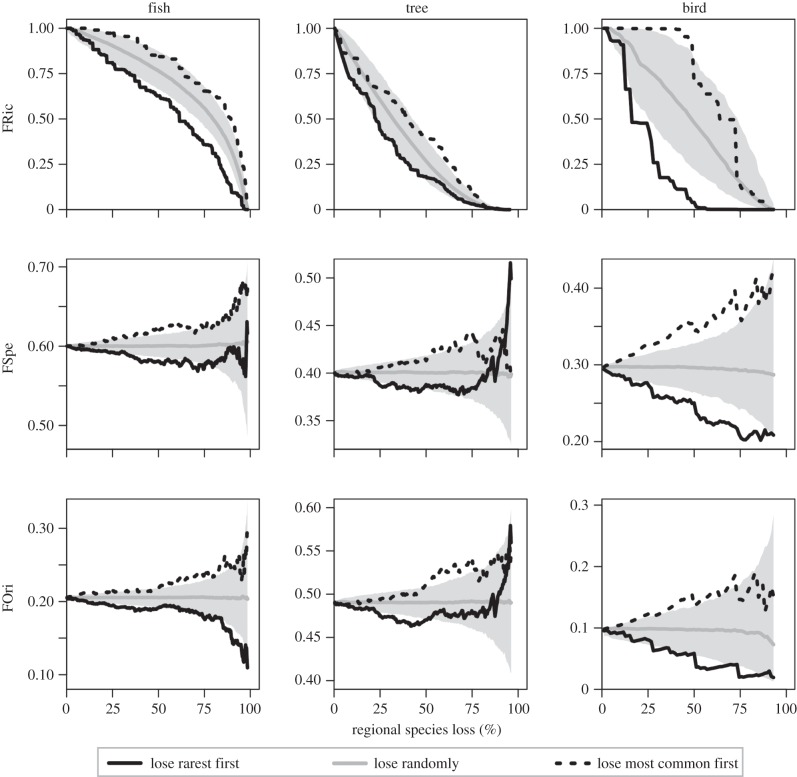
Simulations of species removal from regional pools showed a consistent and significant pattern: a faster decrease of functional richness (FRic) when species were lost from the rarest to the most common compared with a random species loss (figure 2). For example, losing the 20% rarest species of fishes and trees led to a supplemental loss of, respectively, 7.2% and 9% of regional FRic when compared with a random loss. For birds, the impact of rarest species loss was even more critical, with the extinction of the 20% rarest species inducing an extra decrease of 28.3% for FRic compared with a random loss. The extinction of the rarest species also led to a decrease of mean functional specialization (FSpe) and originality (FOri) for the three taxonomic groups. Conversely, when the most common species were removed first in each of the three datasets, a general trend of increasing FSpe and FOri was observed (figure 2).
Figure 2.
Impact of regional species extinction on the functional structure (functional richness, FRic; specialization, FSpe; and originality, FOri) of three tropical assemblages: stream fish from the Brazilian Amazon, rainforest trees from French Guiana, and birds from the Australian Wet Tropics. Rarest species sequential loss (black solid line) is compared with the opposite scenario where most common species are lost first (black dashed line) and with a null scenario simulating a random sequential extinction (grey line indicates the median of this scenario among 1 000 replicates and the 95% confidence interval is represented as the shaded area). Due to computation constraints the maximum species removals from the regional pools of fish, trees, and birds were, respectively, 99%, 96%, and 93%.
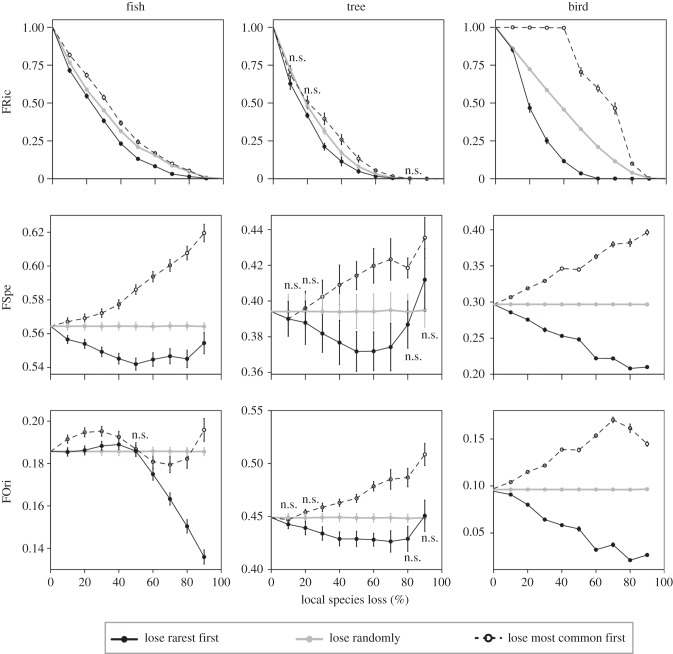
At the local scale, the loss of the rarest species also induced a severe decrease of the functional indices. The erosion of FRic when the rarest species were removed first was significantly higher than in the random loss scenario for all three groups (figure 3). Conversely, FRic generally dropped less than expected under a random loss scenario when common species were firstly removed (figure 3). FSpe of local assemblages decreased more than expected under a random loss scenario when the rarest species were firstly removed for the three study cases (figure 3). Local FOri of tree and bird assemblages decreased more than expected when the rarest species were first removed, whereas it increased when most common species were lost first. For fish, FOri values did not differ from null model expectations when the top 50% rarest species were removed first, but they were significantly higher than expected when the top 50% most common species were removed first (figure 3).
Figure 3.
Impact of local species extinction on the functional structure (functional richness, FRic; specialization, FSpe; and originality, FOri) of local assemblages of stream fish from the Brazilian Amazon, rainforest trees from French Guiana, and birds from the Australian Wet Tropics. For computations of functional indices, 10–90% of the species of each local assemblage were removed according to three different scenarios: lose the rarest species first (black solid line); lose the most common species first (black dashed line); and lose species randomly (grey solid line). Dots and vertical bars represent mean values and standard error at the species-removal level among all local assemblages. ‘n.s.' indicates no significant difference (p > 0.05) when comparing scenarios for a given level of species removal (see detailed results of Friedman paired test and sample sizes in the electronic supplementary material, table S2).
4. Discussion
Our scenarios of species loss demonstrate a disproportional influence of rare species on the functional structure of tropical assemblages, both at local and regional scales. Losing rare species would reduce the functional richness, specialization, and originality of assemblages more than expected under a random loss of species. Therefore, beyond taxonomic or aesthetic loss, extirpating rare species may represent the loss of irreplaceable functions and a reduced diversity of ecological niches within assemblages, potentially disrupting refined interactions among species, eradicating highly specialized forms of resource utilization and undermining the integrity of important ecological processes. Given that the multifunctionality of ecosystems can be strongly predicted by the FS [17,31], the loss of rare species may ultimately result in significant impacts on the long-term provisioning of ecosystem goods and services. The generality of these findings and potential implications is strengthened by the strong convergent patterns observed among three taxonomic groups highly distinct in terms of evolutionary history and ecology.
We assumed that the rarest species are those that combine the three basic characteristics of having small local populations, restricted geographical range, and narrow habitat breadth. Small populations are more vulnerable to demographic, environmental, and genetic stochasticity [32], and investigations of these processes (i.e. small-population paradigm) led to several theoretical and applied insights into conservation biology [33]. Restricted geographical distribution is also widely recognized as a strong predictor of species extinction risk, mainly because even a punctual impact (e.g. a river impoundment for fishes or a fire event for trees) may severely impair the persistence of a species [34]. Finally, less-tolerant species in terms of habitat conditions are obviously under higher vulnerability, because even small environmental changes can be lethal to these organisms. Therefore, our scenarios simulating the loss of rare species first could be considered as the most realistic.
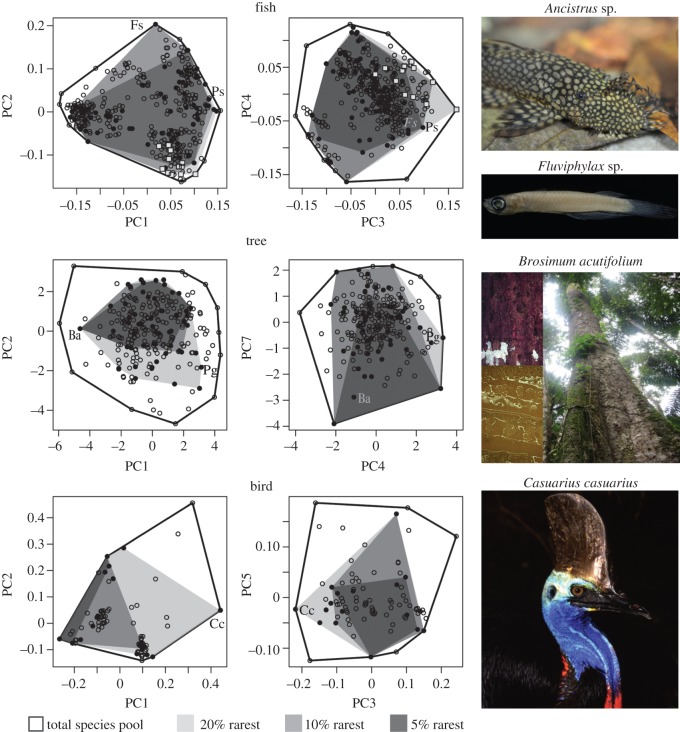
Using a single and individual species-based functional index, Mouillot et al. [18] showed that in some regional species pools the most distinct combinations of traits are supported by rare species but that, at the same time, many rare species support common traits and thus redundant functions. On balance, the impact of losing rare species remains unknown. Scaling up at the assemblage level and using a multifaceted framework, we demonstrate that beyond supporting the most unusual traits, rare species over-contribute to the functional structure of species assemblages in several ways as illustrated by a closer examination of their positions in functional space (see figure 4 and electronic supplementary material, figures S4–S6). For instance, the southern cassowary Casuarius casuarius (Casuariidae), ranked within the top 16% rarest species and listed as vulnerable by the International Union for Conservation of Nature (IUCN) [35], strongly contributes to the functional structure of bird assemblages in the AWT because of its high functional specialization and originality (figure 4). This species is the only remaining large-bodied plant disperser over long distances in Australian tropical rainforests [36]. Losing C. casuarius could therefore affect plant population dynamics across the landscape, particularly for large-seeded species.
Figure 4.
Multidimensional functional spaces built with the species pool of three tropical assemblages: stream fish from the Brazilian Amazon (395 species), rainforest trees from French Guiana (262), and birds from the Australian Wet Tropics (86). Each panel represents two axes (PC) of the functional space where species are plotted according to their respective trait values (see biplots for all PC combinations in the electronic supplementary material, figures S4–S6). Convex hull volumes filled by the 5%, 10%, and 20% rarest species are illustrated as the nested grey areas. Circles filled with black are the 20% rarest species. Grey squares on top panels are periphyton-grazing fishes (illustrated by the loricariid Ancistrus sp.). Ps, Paravandellia sp.; Fs, Fluviphylax simplex; Ba, Brosimum acutifolium; Pg, Protium giganteum; and Cc, Casuarius casuarius. (Online version in colour.)
Rare fish species also tend to be placed relatively isolated and on the edge of the functional space (figure 4), increasing the FS. For instance, the vampire catfish Paravandellia sp. (Trichomycteridae), ranked within the top 2% rarest species, has a very particular oral apparatus to feed on blood from other small-bodied fishes [37], being one of the few haematophagous species recorded in small Amazonian streams. The poeciliid Fluviphylax simplex, ranked within the top 5% rarest species, is a miniature fish with highly specialized morphology (e.g. superior-oriented mouth and extremely large eyes) allowing for feeding on fine particulate detritus and on very small prey at the water–air interface (i.e. neustophagia). Beyond individual species, some rare functional groups have critical roles in aquatic systems. That is the case of periphyton-grazing fishes, which have restricted geographical ranges and are often found in low local abundances in small Amazonian forest streams (13 species among the top 20% rarest; figure 4). These species use particular traits to directly exploit the periphyton, being the only fish group responsible for the early incorporation of autotrophic carbon along the fluvial continuum (M Anjos 2015, personal communication).
The rainforest trees Brosimum acutifolium (Moraceae) and Protium giganteum (Burseraceae), both within the top 20% rarest tree species in French Guiana, are relatively isolated and at opposite extremes in the functional space (figure 4). The former is characterized by dense wood and high specific leaf area with milky latex on the leaves, which is typically associated with defence capacity against herbivores and fungal pathogens [38]. On the other functional extreme, P. giganteum holds high values of laminar and trunk bark thickness, which ensures protection against the increasing frequency and intensity of wildfires that may occur in the region [39]. The asynchrony of species responses to environmental fluctuations is an important mechanism through which biodiversity can stabilize ecosystem properties [40]. In this context, maintaining rare species and the high diversity of traits within assemblages may provide resistance and resilience to a variety of disturbances in a changing world.
We demonstrate that losing rare species negatively over-influenced assemblage functional structure not only at the local scale, but also at the regional scale. Biodiversity can provide insurance for ecosystem functioning across several spatial scales producing alpha, beta, and gamma diversity–stability relationships [41]. As a consequence, losing species may impair ecosystem stability and functioning at large spatial scales by reducing the capacity of connected systems to share or replace potential key functions. This is particularly important for management decisions which are often made at the landscape scale [41]. Given that this spatial biodiversity–stability relationship is primarily driven by differences in the fundamental niches and complementarity of the species [42], keeping the pool of traits and the functional structure of regional assemblages is critical to maintain the functional insurance within and across ecosystems.
In addition to the implications for biodiversity conservation, our findings bring new theoretical insights into community ecology, particularly about the contrasting hypotheses proposed to explain the assembly of species into communities. Neutral models assume ecological equivalence among species, with their abundances mainly driven by dispersal limitation and demographic stochasticity [43]. In contrast, niche differentiation hypotheses postulate that species rarity and commonness will be better explained by differences in functional traits and their interactions with prevailing environmental conditions [44]. Under this latter assumption, rare species should be ecologically distant from common species and from each other, with the mechanism of resource partitioning mainly driving community assembly (see Mi et al. [45] for an example of how these opposing paradigms were tested drawing on rare species contribution to the phylogenetic diversity of communities). Although our study has not been designed to test community assembly, the high functional diversity supported by rare species indicates that niche differentiation mechanisms may be important determinants in tropical assemblages.
Although broad in scale, our study includes some limitations. First, we assume that traits are relevant proxies for species roles while this is sometimes not so straightforward [46]. Traits certainly matter for defining functions, but some functions are still ignored, because corresponding traits cannot be easily measured (e.g. ecophysiological characteristics in animals). This research gap prevents us reaching broader conclusions about the vulnerability of ecosystem functioning (e.g. biogeochemical cycles in streams) and should be considered in the agenda of functional-based approaches. Second, although we avoid spurious relationships, one might expect background associations between some functional traits and the patterns of species distribution (e.g. organisms with higher body mass tend to have broader geographical ranges). However, our sensitivity analyses show that no particular trait drives the patterns alone. Finally, we recognize that, particularly for tree species, using additional data to improve the estimation of their geographical range would improve the strength of our rarity versus commonness estimates. However, we believe this would not change our main conclusions as we found consistency between the rarity ranking for several species from our estimates and from a broader assessment of Amazon tree distribution [47].
Given the operational difficulties involving the study of rare species (e.g. poor ecological knowledge), they have frequently been neglected in community ecology and in experimental tests on the effects of biodiversity on ecosystem functioning [8]. According to the ‘commonness-dominance’ paradigm, the focus on the common species is also justified because they often account for the major overall biomass and energy use in a community (i.e. mass-ratio hypothesis [10]), whereas restricted-range and less abundant species supposedly use marginal resources or habitats (i.e. resource availability hypothesis [48]), playing a negligible role in the short-term structure and functioning of ecosystems [44]. However, our results indicate that this overlooked attention on rare species can be a misjudgement in the long term, because they are irreplaceable components of the functional structure of assemblages. Moreover, the loss of rare species could have deep impacts on community and ecosystem functioning if they exhibit compensatory growth to common species declines or are favoured by environmental changes [19]. An appropriate management of ecosystems should thus not only focus on the provision of functions and services under current conditions, but also consider their maintenance under future changes [49].
Tropical ecosystems are facing unprecedented levels of pressure from multiple sources and at all scales. Our empirical knowledge of tropical biodiversity is still too limited to make robust predictions about its conservation value [50]. However, we can reasonably assume that not just common and dominant species are functionally important. Disproportionately supporting the whole breadth of functional abilities within assemblages, rare species potentially play critical roles in maintaining ecological processes in space and time particularly under the ongoing rapid environmental transitions in the tropics. This justifies the application of the precautionary principle for conservation strategies and undermines arguments that many species are functionally redundant in highly diverse systems.
Supplementary Material
Acknowledgements
D. Bastos, L. Carvalho, F. Cabeceira, R.N. Júnior, and RAS Project shared part of the fish data. F. Guilhaumon, F. Leprieur, and V. Parravicini helped with R-codes. N. Rabelo and S. Cunha helped with ecomorphological analysis. T. Gardner, F. Teresa, T. Oberdorff, B. Forsberg, C. Cornelius, S. Amadio, C. Deus, and D. Kasper gave valuable suggestions on earlier versions of the manuscript. Pictures courtesy of D. Bastos, M. Hopkins, and R. Van Loon. This is contribution #40 of the Projeto Igarapés.
Ethics
All fish sampling procedures were carried out under licence from the Brazilian Institute of Environment and Renewable Natural Resources (IBAMA/592016) and subject to local ethical review.
Data accessibility
The datasets supporting this article are available from the Dryad digital repository: http://dx.doi.org/10.5061/dryad.8s58v.
Authors' contributions
R.P.L., D.M., J.Z., and S.V. designed the study; R.P.L., D.M., and S.V. performed the data analyses; R.P.L., J.Z., and F.P.M. built the fish dataset; S.E.W. built the bird dataset; C.B. and C.F. built the tree dataset. All authors discussed the results and gave final approval for publication.
Competing interests
We have no competing interests.
Funding
Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq 482209/2010-0, 156915/2011-1, 168227/2014-2, 313183/2014-7), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES/PDSE 1914-13-8), Fundação de Amparo à Pesquisa do Estado do Amazonas (FAPEAM 062.00202/2013), National Science Foundation (NSF/DEB 0743103, 0743800), INRA Package and Agence Nationale de la Recherche (CEBA 10-LABX-25-01).
References
- 1.Vitousek PM, Mooney HA, Lubchneco J, Melillo JM. 1997. Human domination of Earth's ecosystems. Science 277, 494–499. ( 10.1126/science.277.5325.494) [DOI] [Google Scholar]
- 2.Barnosky AD, et al. 2011. Has the Earth's sixth mass extinction already arrived? Nature 471, 51–57. ( 10.1038/nature09678) [DOI] [PubMed] [Google Scholar]
- 3.Purvis A, Agapow PM, Gittleman JC, Mace GM. 2000. Non-random extinction and the loss of evolutionary history. Science 288, 328–330. ( 10.1126/science.288.5464.328) [DOI] [PubMed] [Google Scholar]
- 4.Rabinowitz D. 1981. Seven forms of rarity. In Biological aspects of rare plant conservation (ed. Synge H.), pp. 205–217. Chichester, UK: John Wiley. [Google Scholar]
- 5.Harnik PG, Simpson C, Payne L. 2012. Long-term differences in extinction risk among the seven forms of rarity. Proc. R. Soc. B 279, 4969–4976. ( 10.1098/rspb.2012.1902) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies KF, Margules CR, Lawrence JF. 2004. A synergistic effect puts rare, specialized species at greater risk of extinction. Ecology 85, 265–271. ( 10.1890/03-0110) [DOI] [Google Scholar]
- 7.Sekercioglu CH, Schneider SH, Fay JP, Loarie SR. 2008. Climate change, elevational range shifts, and bird extinctions. Conserv. Biol. 22, 140–150. ( 10.1111/j.1523-1739.2007.00852.x) [DOI] [PubMed] [Google Scholar]
- 8.Lyons KG, Brigham CA, Traut BH, Schwartz MW. 2005. Rare species and ecosystem functioning. Conserv. Biol. 19, 1019–1024. ( 10.1111/j.1523-1739.2005.00106.x) [DOI] [Google Scholar]
- 9.Naeem S, Duffy JE, Zavaleta E. 2012. The functions of biological diversity in an age of extinction. Science 336, 1401–1406. ( 10.1126/science.1215855) [DOI] [PubMed] [Google Scholar]
- 10.Grime JP. 1998. Benefits of plant diversity to ecosystems: immediate, filter and founder effects. J. Ecol. 86, 902–910. ( 10.1046/j.1365-2745.1998.00306.x) [DOI] [Google Scholar]
- 11.Lyons KG, Schwartz M. 2001. Rare species loss alters ecosystem function: invasion resistance. Ecol. Lett. 4, 358–365. ( 10.1046/j.14610248.2001.00235.x) [DOI] [Google Scholar]
- 12.Power ME, et al. 1996. Challenges in the quest for keystones. Bioscience 46, 609–620. ( 10.2307/1312990) [DOI] [Google Scholar]
- 13.Bracken MES, Low NHN. 2012. Realistic losses of rare species disproportionately impact higher trophic levels. Ecol. Lett. 15, 461–467. ( 10.1111/j.1461-0248.2012.01758.x) [DOI] [PubMed] [Google Scholar]
- 14.Hector A, Bagchi R. 2007. Biodiversity and ecosystem multifunctionality. Nature 448, 188–190. ( 10.1038/nature05947) [DOI] [PubMed] [Google Scholar]
- 15.Isbell F, et al. 2011. High plant diversity is needed to maintain ecosystem services. Nature 477, 199–202. ( 10.1038/nature10282) [DOI] [PubMed] [Google Scholar]
- 16.Hooper DU, et al. 2005. Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol. Monogr. 75, 3–35. ( 10.1890/04-0922) [DOI] [Google Scholar]
- 17.Mouillot D, Villéger S, Scherer-Lorenzen M, Mason NWH. 2011. Functional structure of biological communities predicts ecosystem multifunctionality. PLoS ONE 6, e17476 ( 10.1371/journal.pone.0017476) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mouillot D, et al. 2013. Rare species support vulnerable functions in high-diversity ecosystems. PLoS Biol. 11, e1001569 ( 10.1371/journal.pbio.1001569) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jain M, et al. 2014. The importance of rare species: a trait-based assessment of rare species contributions to functional diversity and possible ecosystem function in tall-grass prairies. Ecol. Evol. 4, 104–112. ( 10.1002/ece3.915) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mouillot D, Graham NAJ, Villéger S, Mason NWH, Bellwood DR. 2013. A functional approach reveals community responses to disturbances. Trends Ecol. Evol. 28, 167–177. ( 10.1016/j.tree.2012.10.004) [DOI] [PubMed] [Google Scholar]
- 21.Villéger S, Mason NWH, Mouillot D. 2008. New multidimensional functional diversity indices for a multifaceted framework in functional ecology. Ecology 89, 2290–2301. ( 10.1890/07-1206.1) [DOI] [PubMed] [Google Scholar]
- 22.Hercos AP, Sobansky M, Queiroz HL, Magurran AE. 2012. Local and regional rarity in a diverse tropical fish assemblage. Proc. R. Soc. B 280, 20122076 ( 10.1098/rspb.2012.2076) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hubbell SP. 2013. Tropical rain forest conservation and the twin challenges of diversity and rarity. Ecol. Evol. 3, 3263–3274. ( 10.1002/ece3.705) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brook BW, Bradshaw CJA, Koh LP, Sodhi NS. 2006. Momentum drives the crash: mass extinction in the tropics. Biotropica 38, 302–305. ( 10.1111/j.1744-7429.2006.00141.x) [DOI] [Google Scholar]
- 25.Williams SE, Williams YM, VanDerWal J, Isaac J, Shoo LP, Johnson CN. 2009. Ecological specialization and population size in a biodiversity hotspot: How rare species avoid extinction. Proc. Natl Acad. Sci. USA 106, 19 737–19 741. ( 10.1073/pnas.0901640106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams SE, et al. 2010. Distributions, life-history specialization, and phylogeny of the rain forest vertebrates in the Australian Wet Tropics. Ecology 91, 2493 ( 10.1890/09-1069.1) [DOI] [Google Scholar]
- 27.Dolédec S, Chessel D, Gimaret-Carpentier C. 2000. Niche separation in community analysis: a new method. Ecology 81, 2914–2927. ( 10.1890/0012-9658) [DOI] [Google Scholar]
- 28.Kark S, Mukerji T, Safriel UN, Noy-Meir I, Nissani R, Darvasi A. 2002. Peak morphological diversity in an ecotone unveiled in the chukar partridge by a novel estimator in a dependent sample (EDS). J. Anim. Ecol. 71, 1015–1029. ( 10.1046/j.1365-2656.2002.00665.x) [DOI] [Google Scholar]
- 29.Maire E, Grenouillet G, Brosse S, Villéger S. 2015. How many dimensions are needed to accurately assess functional diversity? A pragmatic approach for assessing the quality of functional spaces. Glob. Ecol. Biogeogr. 24, 728–740. ( 10.1111/geb.12299) [DOI] [Google Scholar]
- 30.R CoreTeam. 2013. R: a language and environment for statistical computing. Vienna, Austria. See http://www.R-project.org.
- 31.Duncan C, Thompson JR, Pettorelli N. 2015. The quest for a mechanistic understanding of biodiversity–ecosystem services relationships. Proc. R. Soc. B 282, 20151348 ( 10.1098/rspb.2015.1348) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simberloff DS. 1986. The proximate causes of extinction. In Patterns and processes in the history of life (eds Raup DM, Jablonski D), pp. 259–276. Berlin, Germany: Springer. [Google Scholar]
- 33.Caughley G. 1994. Directions in conservation biology. J. Anim. Ecol. 63, 215–244. ( 10.2307/5542) [DOI] [Google Scholar]
- 34.Nogueira C, Buckup PA, Menezes NA, Oyakawa OT, Kasecker TP, Neto MR, Silva JMC. 2010. Restricted-range fishes and the conservation of Brazilian freshwaters. PLoS ONE 5, e11390 ( 10.1371/journal.pone.0011390) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.IUCN. 2014. IUCN red list of threatened species. See http://www.iucnredlist.org.
- 36.Westcott DA, Bentrupperbäumer J, Bradford MG, McKeown A. 2005. Incorporating patterns of disperser behaviour into models of seed dispersal and its effects on estimated dispersal curves. Oecologia 146, 57–67. ( 10.1007/s00442-005-0178-1) [DOI] [PubMed] [Google Scholar]
- 37.Zuanon J, Sazima I. 2005. Free meals on long-distance cruisers: the vampire fish rides giant catfishes in the Amazon. Biota Neotrop. 5, 109–114. ( 10.1590/S1676-06032005000100012) [DOI] [Google Scholar]
- 38.Chave J, Coomes D, Jansen S, Lewis SL, Swenson NG, Zanne AE. 2009. Towards a worldwide wood economics spectrum. Ecol. Lett. 12, 351–366. ( 10.1111/j.1461-0248.2009.01285.x) [DOI] [PubMed] [Google Scholar]
- 39.Brando PM, Nepstad DC, Balch JK, Bolker B, Christman MC, Coe M, Putz FE. 2012. Fire-induced tree mortality in a neotropical forest: the roles of bark traits, tree size, wood density and fire behavior. Glob. Change Biol. 18, 630–641. ( 10.1111/j.1365-2486.2011.02533.x) [DOI] [Google Scholar]
- 40.Loreau M, de Mazancourt C. 2013. Biodiversity and ecosystem stability: a synthesis of underlying mechanisms. Ecol. Lett. 16, 106–115. ( 10.1111/ele.12073) [DOI] [PubMed] [Google Scholar]
- 41.Wang S, Loreau M. 2014. Ecosystem stability in space: α, β and γ variability. Ecol. Lett. 17, 891–901. ( 10.1111/ele.12292) [DOI] [PubMed] [Google Scholar]
- 42.Loreau M, Hector A. 2001. Partitioning selection and complementarity in biodiversity experiments. Nature 412, 72–76. ( 10.1038/35083573) [DOI] [PubMed] [Google Scholar]
- 43.Hubbell SP. 2001. The unified neutral theory of biodiversity and biogeography. Princeton, NJ: Princeton University Press. [Google Scholar]
- 44.Gaston KJ. 2011. Common ecology. Bioscience 61, 354–362. ( 10.1525/bio.2011.61.5.4) [DOI] [Google Scholar]
- 45.Mi X, et al. 2012. The contribution of rare species to community phylogenetic diversity across a global network of forest plots. Am. Nat. 180, E17–E30. ( 10.1086/665999) [DOI] [PubMed] [Google Scholar]
- 46.Kraft NJB, Godoy O, Levine JM. 2015. Plant functional traits and the multidimensional nature of species coexistence. Proc. Natl Acad. Sci. USA 112, 797–802. ( 10.1073/pnas.1413650112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.ter Steege H, et al. 2013. Hyperdominance in the Amazonian tree flora. Science 342, 1243092 ( 10.1126/science.1243092) [DOI] [PubMed] [Google Scholar]
- 48.Venier LA, Fahrig L. 1996. Habitat availability causes the species abundance-distribution relationship. Oikos 76, 564–570. ( 10.2307/3546349) [DOI] [Google Scholar]
- 49.Oliver TH, et al. 2015. Biodiversity and resilience of ecosystem functions. Trends Ecol. Evol. 30, 673–684. ( 10.1016/j.tree.2015.08.009) [DOI] [PubMed] [Google Scholar]
- 50.Gardner TA, Barlow J, Parry LW, Peres CA. 2007. Predicting the uncertain future of tropical forest species in a data vacuum. Biotropica 39, 25–30. ( 10.1111/j.1744-7429.2006.00228.x) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article are available from the Dryad digital repository: http://dx.doi.org/10.5061/dryad.8s58v.