Abstract
In groups of socially foraging animals, feeding behaviour may change with group size in response to varying cost–benefit trade-offs. Numerous studies have described group-size effects on group-average feeding behaviour, particularly emphasizing an increase in scrounging incidence for larger groups, where individuals (scroungers) feed from the food sources others (producers) discovered. However, individual variation in feeding behaviour remains unconsidered in the vast majority of these studies even though theoretical models predict individuals to specialize in feeding tactic and anticipate higher scrounger-type frequencies in larger groups. We combined group-level and individual-level analyses of group-size effects on social foraging in the subsocial spider Australomisidia ergandros. Lending novel experimental support to model predictions, we found that individuals specialize in feeding tactic and that higher scrounging and lower producing incidence in larger groups were mediated through shifts in the ratio of feeding types. Further, feeding-type specialization was not explained by innate individual differences in hunting ability as all feeding types were equally efficient in prey capture when foraging alone. Context adaptivity of feeding behaviour might allow this subsocial species to succeed under varying socioecological conditions.
Keywords: behavioural type, feeding tactics, group size, scrounging, social foraging
1. Introduction
The ecological determinants of group living fall into two major categories: anti-predator and foraging trade-offs [1,2]. Individuals in larger groups commonly benefit from a reduced per capita risk of being predated owing to phenomena known as the ‘many-eyes hypothesis' or the ‘dilution effect’ [2]. Conversely, costs of food competition increase with group size and hence present counteracting selective pressures on group living [3,4]. A negative relationship between group size and foraging is, however, not universal [5]. In some species, food acquisition can improve with increasing group size owing to cooperation between individuals, thus promoting sociality. This seems to hold true especially for groups of predators that benefit from a reduced per capita cost of attacking and succeed more frequently in subduing prey when hunting in bigger packs [4,6]. Improved foraging in larger groups has been demonstrated for cooperatively hunting mammals such as African wild dogs [7] and bison-hunting wolves [8], but also for communally feeding spiders [9,10].
Critically viewed, the above-mentioned studies describe only parts of the biological phenomena influencing group formation as they portray selective pressures on sociality at the level of averaged group-size effects [11,12]. However, the payoffs of group living are often not evenly distributed between the members of a social group [13]. Individuals vary in their average level of behaviour, their ‘behavioural type’, and given this variation, the impacts of group size probably operate on individual level [14]. More specifically, a group-size effect, such as improved foraging in larger groups, might result from a shift in the expression or frequency of behavioural types rather than from a uniform behavioural change that all individuals undergo. Therefore, linking group-level and individual-level effects of group size is essential for a comprehensive understanding of the evolutionary processes leading to sociality [12,13,15].
Revisiting the relationship between group size and foraging trade-offs from this individual perspective, much of the literature describes a particular intraspecific pattern of behavioural types: the existence of ‘producers' and ‘scroungers' in groups of socially foraging animals [16–18]. Whenever only a few members of the group are necessary to create a collective good, e.g. a food source, individuals may specialize in either producing, where the individual actively acquires the food source, or scrounging, where the individual joins to consume parts of the food source other members acquired [16]. These producer–scrounger dynamics are presumed to be highly influential on the ecology of group living, as scrounging behaviour can exert a substantial cost on social living [4]. Producer–scrounger models predict that the proportion of producers and scroungers alters as group size increases, with higher frequencies of scroungers in larger groups [17,18]. Although the theory of producer–scrounger dynamics is well developed, surprisingly few studies have experimentally tested the influence of group size on feeding behaviour from an individual level (but see [19]).
Here, we present an experimental approach that combines group-level and individual-level analyses of group-size effects on producer–scrounger dynamics in the subsocial crab spider Australomisidia ergandros. These spiders make an excellent model for studying the ecology of social foraging. They naturally occur in groups of varying sizes and feed communally on large insects that only one to a few individuals captured [20,21], whereby the scene for the emergence of producing and scrounging tendencies is set [17]. We focused our study on the relationship between group size, group-level effects on social foraging and individual specialization in the three possible feeding tactics: producing, scrounging and feeding alone. Specifically, we tested the model prediction that average feeding behaviour alters with increasing group size, showing higher incidence of scrounging in larger groups. By measuring individual behaviour over repeated trials, we furthermore tested the hypothesis that group-size effects on social foraging are mediated through shifts of behavioural types rather than through uniform behavioural changes of all individuals.
In some social spiders, individual differences in behaviour have been shown to result from innate phenotypic differences, thus persisting across context and subsequently being termed ‘animal personalities' [22]. For A. ergandros and other subsocial spiders, where group living is temporary [20,23], a persistence of the non-attacking scrounger type when not surrounded by group members would pose a substantial disadvantage for survival. We therefore extended our study to the persistence of feeding behaviour in a non-social context and examined innate individual differences in hunting ability as a possible factor explaining individual feeding type.
2. Material and methods
(a). Study species
Australomisidia ergandros (former Diaea ergandros, revised by Szymkowiak [24]) is an annual, subsocial crab spider inhabiting leaf nests in Eucalyptus trees along the Great Dividing Range from Victoria to Queensland, Australia. Group size within the nests ranges from five to 45 spiderlings (27 ± 10.81, n = 39 nests; [20]), usually the offspring of one female that provides maternal care by feeding her young [25]. After the mother's death, the spiderlings continue living jointly until maturation over a time period of five to seven months [25,26]. During this post-maternal social period, they display cooperative behaviour by contributing to nest construction and feeding communally on large prey [20,21].
(b). Collection and group establishment
We collected 25 A. ergandros nests containing juvenile spiders from Eucalyptus trees between Yass and Murrumbateman, NSW, Australia in February 2015. During that time of year, A. ergandros spiderlings are very young (four to six weeks after hatching), and the likelihood of having immigrant spiderlings within the nest is low [26]. Hence, we can assume that spiderlings from the same nest are related; an essential condition for studying A. ergandros feeding interactions as foreign spiderlings affect the group's social foraging structure [27]. The nests were removed from their host trees by cutting off supporting branches and bagged separately for transport to the laboratory at the Macquarie University Sydney, NSW, Australia.
In the laboratory, we dissected the nests and counted the number of spiderlings within. In cases where the number of individuals exceeded 15 (n = 16 nests), we visually selected six, 10 or 14 similar-sized spiderlings to compose an experimental group. These spiderlings were colour-marked individually (© Plaka Farbe) and jointly transferred into Petri dishes containing Eucalyptus leaves to offer shelter. We chose to create groups as opposed to using natural nests to achieve a sufficient number of replicates for group sizes. We established 15 experimental groups, equally divided over three group-size treatments: small (S) with six individuals, medium (M) with 10 individuals and large (L) with 14 individuals. Petri dish size was chosen depending on group size, with diameters of 80 mm for small, 100 mm for medium and 120 mm for large groups. Likewise, we varied the number of provided Eucalyptus leaves. The specific group sizes and the corresponding Petri dish sizes were chosen in order to optimally standardize spiderling density.
Groups were given a 14 day habituation phase to encourage silk weaving before we tested them in a series of repeated feeding trials. Throughout this phase, the groups were fed a diet of Drosophila or Musca domestica flies equal to the diet provided during the experiment. In six groups, one spiderling died prior to the start of the trials. Consequently, we redefined group-size treatments as ranges: small with five to six individuals, medium with nine to 10 individuals and large with 13–14 individuals. We excluded groups from the study if they fell out of these ranges over the course of the experiment owing to the death of further spiders. This was the case in one group per treatment.
(c). Communal feeding experiment
To investigate the effect of group size on feeding behaviour and the existence of behavioural types, we assessed each individual's feeding behaviour in 10 consecutive communal feeding trials over 36 days. This duration is equivalent to approximately 10% of A. ergandros's lifespan. Every fourth day, the groups were presented with large alive Drosophila or M. domestica flies, to provide the possibility of communal feeding. Testing every fourth day ensured that the groups, which were not fed between the trials, were sufficiently hungry to attack. To homogenize food availability across treatments, small groups were offered one, medium groups two, and large groups three flies. On average, three to four A. ergandros spiderlings feed on the captured prey [27]. Thus, the feeding possibility fi per individual and trial, defined as the potentially available feeding spots, hovered around fi ≈ 0.8 for all group sizes. Two days after the 10th feeding trial, all individuals were weighted to determine individual end mass measures (table 1).
Table 1.
Parameters describe individual feeding behaviour in the communal feeding experiment for the subsocial crab spider Australomisidia ergandros.
| parametera | definition | data analyses |
|---|---|---|
| time measurements | ||
| feeding time | total no. of 30 min intervals the ind. was observed feeding over the 10 trials, maxfeeding time = 60 | group-size effect, cluster differences |
| produce.time | proportion of feeding time the ind. spent producing (=feeding communally on a self-captured fly) | group-size effect, cluster analysis, cluster differences |
| alone.time | proportion of feeding time the ind. spent feeding alone (=feeding alone on a self-captured fly) | group-size effect, cluster analysis, cluster differences |
| scrounge.time | proportion of feeding time the ind. spent scrounging (=feeding communally on a fly others captured) | group-size effect, cluster analysis, cluster differences |
| frequency measurements | ||
| feeding frequency | total no. of trials the ind. was observed feeding, maxfeeding frequency = 10 | cluster differences |
| produce.freq | proportion of feeding frequency the ind. spent producing | group-size effect, cluster differences |
| alone.freq | proportion of feeding frequency the ind. spent feeding alone | group-size effect, cluster differences |
| scrounge.freq | proportion of feeding frequency the ind. spent scrounging | group-size effect, cluster differences |
| mass measurements | ||
| body massb | ind. body mass 2 days after the 10th trial in mg | group-size effect |
| mass rank | ranked value of an ind. body mass within its group, divided by the number of group members | group-size effect |
aAll parameters (except feeding time, feeding frequency and body mass) take proportional values to allow for comparison of individuals.
bMeasured with the electronic balance Mettler Toledo New Classic MS.
Trials were commenced by placing the adequate number of CO2-anaesthesized flies into the Petri dishes. After a few seconds, the flies started moving, consequently noted and captured. In each trial, we documented: (i) the ID of the individuals that captured a fly and (ii) the ID of all spiderlings feeding at a fly every 30 min for 3 h. Based on this data, we determined a series of feeding parameters describing individual behaviour on its different axes (table 1).
(d). Single feeding experiment
To test for persistence of individual attacking behaviour across context (following [28]) and thereby for innate individual differences in hunting ability, we assessed each spiderling's prey capture performance in a single-feeding experiment after communal feeding trials. We therefore separated all individuals into individual Petri dishes (40 mm diameter) and standardized hunger level by starving the spiderlings for 4 days. In the subsequent test, we placed a big Drosophila fly into each Petri dish and continuously monitored spiderling behaviour for 90 min. We determined each spiderling's attack success (binary: yes/no) and where applicable the attack latency, quantified as the time span from the moment the spiderling oriented itself towards the prey until the successful attack. To approximate food intake rate, calculated as consumed mg per minute, we measured the time each spiderling spent feeding after the attack and weighted the spiderlings before and after the experiment to the fourth decimal with an electronic balance (Mettler Toledo New Classic MS).
(e). Data analyses
Statistical analyses were carried out in R (R Core Team 2014). We excluded individuals from the analyses if their feeding time value (table 1) was below a threshold of 7 (25% quantile of the set of individual feeding time parameters) and therefore, insufficient to reflect feeding type tendency. Owing to this restriction, final sample size comprised n = 90 spiderlings (nsmall = 16, nmedium = 31, nlarge = 43).
We analysed the effect of group size on feeding behaviour using binomial generalized estimating equations (GEEs) and fitted an exchangeable correlation structure to the within-group observations. Similar to mixed modelling, GEEs account for dependence in data, but an important difference is that GEEs do not require distributional assumptions, so that potentially misleading estimates made through distributional misspecification can be avoided [29]. In case of heteroscedasticity, we used generalized least-squares (GLS) and applied the constant variance function varIdent. In separate models, each parameter of interest (table 1) was treated as the response and group size as the explanatory variable. We added body mass and mass rank as covariates to all our models to factor in possible mass correlated behavioural variation. Model selection was done by stepwise elimination of the least significant predictors and refitting the model until all remaining predictors were significant.
We visually chose similar-sized spiderlings for our experiments as their small size did not allow precise mass determination. However, we do not interpret end mass differences between spiderlings, measured after the communal feeding experiment, as an indicator of fitness differences. End mass variation probably reflects variation that already existed at the start of the experiment but was visually not detectable. This argument is supported by an analysis in which we tested for change in mass rank after communal feeding trials and before single feeding trials (14 days). We found no significant change (paired Wilcoxon signed-rank test, V = 419.5, n = 78, p = 0.6466). Thus, incorporating the mass measurements as covariates in our models is justified. We do not discuss body mass effects on feeding behaviour here as these will be investigated in a future study with targeted experimental design, where body mass is determined before measuring feeding behaviour.
To establish the existence of behavioural types, we used cluster analysis—a technique that aims at identifying groups of similar elements (here: similarly behaving individuals) in datasets [30]. We applied a fuzzy clustering algorithm with Euclidean distance measure on a set of feeding parameters (table 1). Group ID was included as a cluster variable to account for group-dependency. Elements clustered via fuzzy algorithms obtain a probability degree of belonging to each cluster rather than getting assigned with a membership level of either 0 or 1 [30]. We selected the fuzzy method over hard clustering, because our data were visually not well separated and thus might be best modelled probabilistically. For cluster validation, we calculated the average silhouette width s, (−1 < s < 1), a goodness-of-fit-measure of how appropriate the data have been clustered with values close to 1 indicating natural groupings [31,32]. We compared the silhouette width between a clustering into two and a clustering into three clusters, appropriate to the three axes of feeding behaviour, to find the clustering that explained individual variation best.
To characterize the discovered groupings, descriptive statistics of feeding parameters per cluster are given as mean ± s.e. To analyse pairwise cluster differences in feeding behaviour, we performed non-parametric Mann–Whitney U-tests as normal distribution of parameters could not be achieved. The effect of group size on behavioural type frequency was analysed based on a contingency table of individual cluster membership per group size using the Freeman–Halton extension of Fisher's exact probability test.
In the analysis of the single feeding experiment, our main interest was the existence of innate individual differences in hunting ability between feeding types. Specifically, we tested whether individuals with scrounging tendencies in the communal feeding experiment showed lower attack tendencies in the single feeding experiment compared with non-scroungers. We used GEEs with scrounger-cluster membership as a predictor, group ID as a grouping variable and attack success or attack latency as the response variable. Individual body mass was included as a covariate. Sample size comprised n = 78 individuals as a few spiderlings died between experiments.
3. Results
(a). Group-level effects
(i). Effects of group size on producing
Group size had a significant effect on the percentage of feeding time individuals spent producing, which generally decreased with increasing group size (figure 1 and table 2). Large groups spent significantly less of their overall feeding time producing than medium groups (produce.time M/L: χ2 = 12.49, p = 0.0004) and tended to spend less than small groups (produce.time S/L: χ2 = 2.61, p = 0.106). Hardly any difference existed between small and medium groups (figure 1). Similarly, individuals in larger groups showed fewer incidences of producing (produce.freq S: 0.35 ± 0.07, M: 0.25 ± 0.03, L: 0.21 ± 0.03), but this effect was not significant (table 2).
Figure 1.
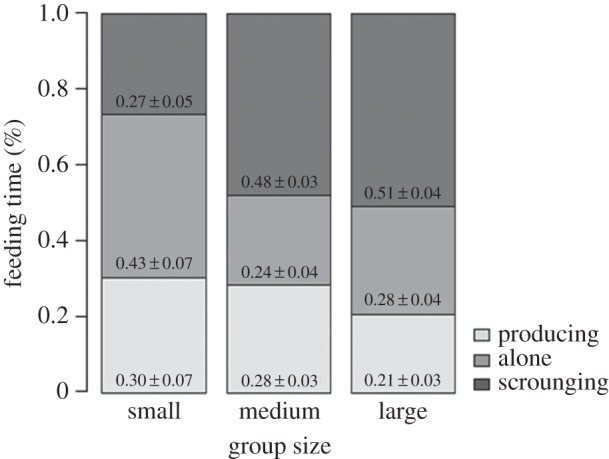
Stacked percentage bars show the relative differences in the proportion of feeding time spent producing, feeding alone and scrounging (given as mean ± s.e.) between the group-size treatments.
Table 2.
Model analyses of group-size effect on social foraging behaviour of the subsocial crab spider Australomisidia ergandros. (We tested for significant differences in different feeding parameters (table 1) between three group-size treatments: small with five to six individuals, medium with 9–10 individuals and large with 13–14 individuals.)
| response | analysisa | test statistic | p-valueb |
|---|---|---|---|
| produce.time | GEE, binomial error structure, exchangeable correlation | Wald, 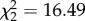
|
p = 0.0003 |
| produce.freq | GEE, binomial error structure, exchangeable correlation | Wald, 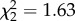
|
p = 0.44 |
| alone.time | GEE, binomial error structure, exchangeable correlation | Wald, 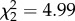
|
p = 0.083 |
| alone.freq | GEE, binomial error structure, exchangeable correlation | Wald, 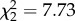
|
p = 0.021 |
| scrounge.time | GEE, binomial error structure, exchangeable correlation | Wald, 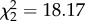
|
p = 0.0001 |
| scrounge.freq | GLS, binomial error structure, exchangeable correlation, varIdent for homogeneity | L-ratio = 21.41 d.f. = 2 | p < 0.0001 |
aThe explanatory variables of all models are group size, body mass and mass rank.
bSignificant p-values for the effect of group size are indicated in bold, trends in italic.
(ii). Effect of group size on feeding alone
Group size also affected the spiderlings’ tendency to feed alone (figure 1). While the GEE analysis showed marginal significance in the overall group-size effect on the percentage of feeding time that was spent alone (table 2), the overall group-size effect on feeding alone frequency was significant (table 2). Here, individuals in small groups fed alone considerably more frequently than individuals in medium and large groups (alone.freq S/M: χ2 = 6.78, p = 0.0092; S/L: χ2 = 5.91, p = 0.015), whereas medium groups did not differ significantly from large groups.
(iii). Effect of group size on scrounging
As predicted, scrounging behaviour significantly increased with group size (figure 1 and table 2). Comparing each possible pair of treatments, scrounging was considerably more pronounced in medium and large groups, both in time (scrounge.time S/M: W = 14.54, p = 0.0001; S/L: χ2 = 21.79, p < 0.0001) and frequency (scrounge.freq S/M: t = 5.56, p < 0.0001; S/L: t = 4.25, p = 0.0001). Individuals in medium and large groups spent on average twice as much of their feeding time scrounging than individuals in small groups (figure 1) and the same applies to the scrounging percentage of feeding frequency (scrounge.freq S: 0.31 ± 0.05, M: 0.57 ± 0.03, L: 0.59 ± 0.04). Following the general pattern, medium and large groups did not differ in these parameters.
(b). Individual-level effects
(i). Existence of behavioural types
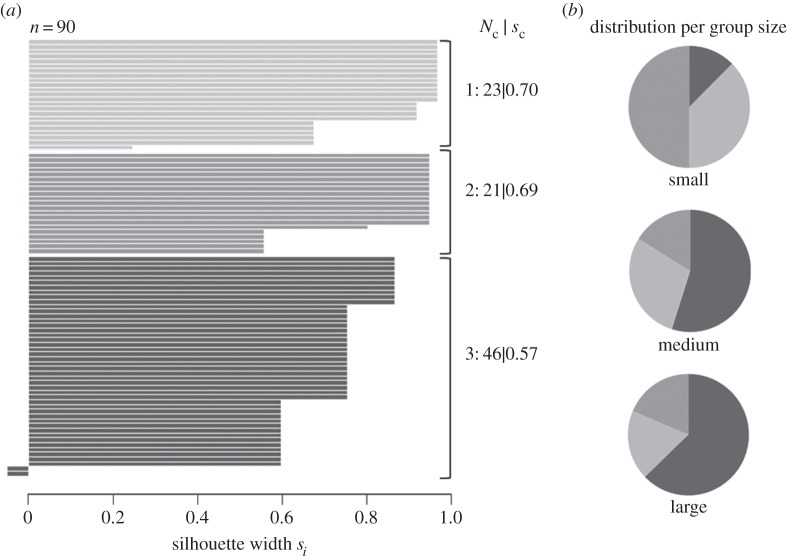
The fuzzy cluster analysis indicated that the variation in individual feeding behaviour was best expressed by three clusters that were clearly separated with cluster-specific silhouette widths close to 1 (figure 2a). Consequently, the average silhouette width of the overall grouping was s = 0.628, indicating high clustering validity. All clusters were well pronounced in terms of their number of members (figure 2a). These findings suggest the existence of three behavioural types, as such a cluster pattern would not emerge if all individuals on average behaved similarly [32]. The characteristics of these behavioural types can be best described with graphical and statistical comparisons of feeding parameter means between the clusters.
Figure 2.
(a) Silhouette plot for cluster validation; it shows how well each individual lies within its assigned cluster. Each grouping of bars represents a cluster, each bar stands for the membership level (si) of an individual to its assigned cluster. Nc denotes the number of individuals within the cluster, sc the average silhouette width of the cluster. (b) The pie charts illustrate the percentage of individuals assigned to cluster 1 (light grey), 2 (grey) and 3 (dark grey) per group-size treatment.
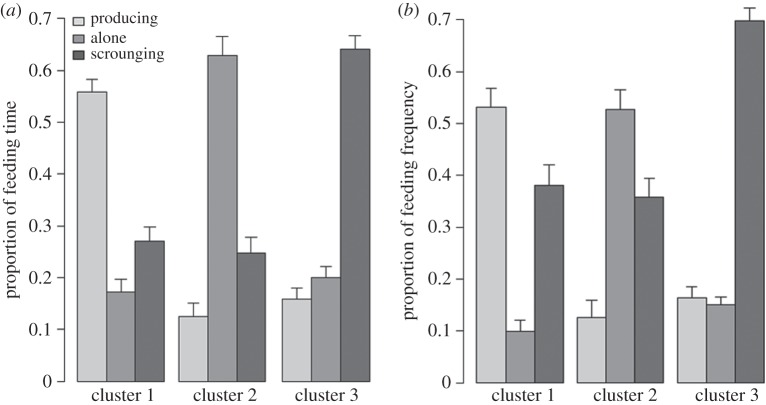
In cluster 1, individuals spent more than 50% of their feeding time and feeding frequency producing (figure 3), thus differing considerably from cluster 2 (produce.time c1/c2: Z = 5.69, p < 0.0001; produce.freq c1/c2: Z = 5.39, p < 0.0001) and cluster 3 individuals (produce.time c1/c3: Z = 6.58, p < 0.0001; produce.freq c1/c3: Z = 6.12, p < 0.0001). By contrast, no significant difference in producing behaviour was found between clusters 2 and 3 (figure 3).
Figure 3.
Barplots describe cluster characteristics. Barplot (a) shows differences between the individuals assigned to clusters 1, 2 or 3 in the proportions of feeding time they spent producing, feeding alone and scrounging (time measurements, table 1). Analogously, barplot (b) shows differences in the producing, feeding alone and scrounging proportion of feeding frequency (frequency measurements, table 1).
Cluster 2 stood out from the other clusters in the average tendency to feed alone. Unlike in clusters 1 and 3, feeding alone made up the largest proportion of feeding time and feeding frequency (figure 3). These differences were significant between clusters 1 and 2 (alone.time c1/c2: Z = −5.68, p < 0.0001; alone.freq c1/c2: Z = −5.65, p < 0.0001) and between clusters 2 and 3 (alone.time c2/c3: Z = 6.42, p < 0.0001; alone.freq c2/c3: Z = 6.36, p < 0.0001).
In cluster 3, scrounging behaviour accounted for over 60% of feeding time and feeding frequency (figure 3). These values proved to be significantly higher than in cluster 1 (scrounge.time c1/c3: Z = −6.57, p < 0.0001; scrounge.freq c1/c3: Z = −5.38, p < 0.0001) and in cluster 2 (scrounge.time c2/c3: Z = −6.40, p < 0.0001; scrounge.freq c2/c3: Z = −5.73, p < 0.0001). Cluster 1 and cluster 2 individuals did not differ in scrounging tendency (figure 3).
Summarizing the results, cluster 1 characterized the expected producer-type and cluster 3 the expected scrounger-type. Cluster 2 individuals represented a third, ‘loner’ behavioural type. The clusters did not differ in absolute feeding time (c1: 18.57 ± 1.44, c2: 17.29 ± 1.53, c3: 18.04 ± 0.8), implying that all behavioural types result in equal success in terms of per capita food availability. In cluster 2, however, absolute feeding frequency (c1: 5.48 ± 0.39, c2: 5 ± 0.37, c3: 6.52 ± 0.25) was notably lower (feeding frequency c2/c3: Z = −3.55, p = 0.0003). This indicates that loners secure their food share through rarer, but longer feeding events, whereas the opposite applies to scroungers.
(ii). Frequency of behavioural types per group size
All three behavioural types were found in any of the group sizes (figure 2b), but the quantitative ratio altered significantly between group size treatments (Fisher–Freeman–Halton, p = 0.0058). In particular, the percentage of producers decreased with increasing group size (S: 0.31 ± 0.14, M: 0.28 ± 0.08, L: 0.19 ± 0.02), whereas the percentage of the scroungers increased (S: 0.13 ± 0.08 M: 0.55 ± 0.12, L: 0.63 ± 0.06). In addition, small groups contained proportionally much more loners than the other treatments (figure 2b).
(iii). Individual differences in hunting ability
The analysis of feeding behaviour of single spiders did not reveal any difference in hunting ability between spiderlings assigned to the scrounger-type and spiderlings assigned to either one of the two other behavioural clusters, the producer-type and the loner-type. There was no effect of cluster membership on attack success, meaning that scroungers did not attack significantly more or less flies than non-scroungers when facing the fly alone (χ2 = 0.17, p = 0.68). Similarly, no difference was found in the attack latency of scroungers and non-scroungers (χ2 = 0.14, p = 0.71). We therefore extended our GEE analysis to testing for behavioural type (scrounger/non-scrounger) differences in the time until the spiderlings’ first attempt to grasp the fly. In the event of a successful attack, we tested for differences in intake rate (consumed mg per minute). Consistent with the above results, scroungers and non-scroungers differed in neither of these two parameters (time until first attempt: χ2 = 0.20, p = 0.66; intake rate: χ2 = 0.23, p = 0.63).
4. Discussion
We found that group size significantly affected social foraging behaviour in the communally feeding spider A. ergandros. On group level, scrounging behaviour increased with group size, while producing and feeding alone behaviour decreased. Small groups were especially distinguished by low scrounging and high feeding alone tendencies. Large groups stood out through considerably low producing as well as high scrounging tendencies, whereas medium groups represented an intermediate stage. It has been argued that a positive relationship between group size and scrounger-tactic use corresponds to lower per capita prey density in larger groups, so that scrounging becomes increasingly necessary to obtain sufficient food [19]. Here, however, scrounging increased with group size, irrespective of this pressure as we experimentally controlled for it. Independence between scrounging and prey density was also found in nutmeg mannikins (Lonchura punctulata; [19]). Therefore, scrounging incidence might be primarily influenced by other group-size-dependent factors.
In isolation, the above findings do not allow for individual-level interpretations as they portrait group-average changes in social foraging behaviour [11]. However, group members could still diverge substantially in feeding tactic preference [13–15]. We investigated individual-level effects and found strong evidence for the stable coexistence of three alternative feeding types: producers, loners and scroungers. Although all feeding types were present in all group sizes, their frequency altered with group size concurring with the group-level effects on feeding behaviour. This implies that the impacts of group size on social foraging operate on an individual-specific level in this subsocial spider, with group size influencing the ratio of behavioural types within the group.
Our study thus provides novel experimental support for predictions of producer–scrounger models by Packer & Ruttan [17] and Vickery et al. [18], which presume individuals to specialize in feeding tactic and anticipate higher scrounger-type frequencies in larger groups. Further, our results support the existence of ‘loners', matching Packer & Ruttan's [17] expectation of a solitary feeding type within foraging societies. In agreement with these models, laboratory research showed that nutmeg mannikins (L. punctulata) specialize in feeding tactics and increase their use of the scrounger tactic with group size [19,33], but group-size dependency in the mix of behavioural types was not directly investigated.
Outside the context of social foraging, the finding that behavioural type ratio alters with group size is not entirely new. In Scottish blackface sheep (Ovis aries), where individuals display variability along the bold–shy continuum, behavioural type positively influences an individual's propensity to move away from conspecifics. Bold sheep leave at smaller group sizes than shy sheep and hence, larger foraging groups feature a higher frequency of the shy behavioural type [34]. Moreover, Pruitt & Riechert [35] reported a positive association between colony size and frequency of an aggressive, ‘asocial’ phenotype in the socially polymorphic spider Anelosimus studiosus. However, their results suggest that the greater proportion of asocial spiders increases capture success [36] and thus, a scrounging tendency of the asocial phenotype seems unlikely.
It has been proposed that individuals within foraging societies choose feeding tactic depending on predation risk, feeding efficiency and/or individual phenotypic differences [16,37,38]. Our experimental groups were not exposed to predation risk but scrounger-type frequency still increased with group size. However, we cannot neglect a possible impact of predation risk on scrounging. Predation risk has been shown to affect scrounger-tactic use in multiple bird species [39] and is assumed to be group-size-dependent in group-living spiders [10,21]. Testing the effect of predator presence on behavioural-type frequency across group size in A. ergandros would be an interesting extension to the research presented here.
Improved feeding efficiency probably explains the increase in scrounger-type frequency found in this study. In this context, Packer & Ruttan [17] propose that the advantage of avoiding the costs of attacking outweighs the improvement in food acquisition through producing behaviour as group size increases. Consequently, in larger groups, proportionally more individuals can benefit from the costly prey capture of a few producers and the group's feeding efficiency is enhanced (also discussed in Rypstra [40]). In line with the theory, we found that both the percentage of feeding time spent producing and the producer-type frequency declined with larger group size. We could not provide a measure of average feeding efficiency (such as mean individual weight gain) for different group sizes. However, a recent study of group size and predation risk in A. ergandros by Unglaub et al. [21] further supports feeding efficiency as the likely mechanism influencing scrounger-type frequency. In that study, larger groups comprising 10 and 25 individuals were found to grow better than smaller groups irrespective of predator presence.
This gives rise to the idea of ‘beneficial scrounging’ as a factor promoting group living. Such dynamics, however, can only be stable as long as prey biomass is sufficient to saturate all feeding individuals [17,40]. The number of communal feeders exceeding this equilibrium is presumably higher than the group sizes investigated in Unglaub et al. [21] and in this study. For even larger group sizes, scrounging might become increasingly costly, so that scrounger-type frequency would again decline [4]. Accordingly, research on African subsocial and social spiders reports negative effects of group size on feeding efficiency [41,42].
Our finding that all behavioural types were present in all group sizes further points to the relevance of a factor besides feeding efficiency, that specifically influences which individuals specialize in producing or feeding alone even when scrounging would be equally (or more) efficient. For social spiders, it has been suggested that individuals tend to be scroungers when they are larger than the group average and thus unlikely to be hindered [43]. Alternatively, we argue that the costs of the producer-tactic are lower for larger individuals because they need to invest proportionally less venom to subdue the prey [44]. Hence, the threshold to attack might be lower in larger individuals, which may drive producing tendencies. The solitary feeding type is unlikely to be an artefact of ‘loners' compulsorily monopolizing a fly, because most other group members already feed on another. The highest loner-frequency was found in small groups, which received only one fly. This indicates a group-size effect on the threshold to feed alone. Response-threshold variation is believed to promote task differentiation in insect societies and can be mediated by morphological size differences as well as group size [13,45].
Possibly consistent with the above assumptions, Ruch et al. [27] reported that body mass relative to group members influenced feeding interactions in A. ergandros groups, with small and large individuals being more involved in communal feeding events than medium-sized spiders. The individuals tested in our study also differed in body mass, at least as measured at the end of the communal feeding trials, but our experimental design did not allow for a specific analysis of body mass effects on social foraging. Relative body mass is therefore a likely predictor of an individual's feeding type in this system and should be investigated in a subsequent study.
We found no evidence for innate individual differences in hunting ability, which thus cannot explain the existence of different individual feeding types. On their own, all individuals were equally efficient and likely to attack prey. Based on this result, we further disfavour considering feeding types as behavioural expressions of personalities in A. ergandros. The personality definition demands behavioural consistency in time and context [22], but although we found individual feeding behaviour to be temporally stable, scrounger attacking behaviour did not persist across context. Our individual-level results from the communal feeding trials underline this assertion, as individuals assigned to a certain feeding type did not exclusively produce, feed alone or scrounge. From an ecological and evolutionary perspective, context-adaptivity of feeding behaviour makes sense in the light of the life history of a subsocial spider, where after adulthood dispersal, individuals depend on procuring food for themselves or in the case of females for their offspring [9].
5. Conclusion
The subsocial crab spider A. ergandros shows behavioural specialization and between-individual polymorphism in feeding tactic use. Group-size effects on social foraging, such as higher scrounging and lower producing incidence in larger groups, are explained on this individual level, being mediated through ratio shifts of feeding types. We thus give first experimental evidence in accordance with established individual-level predictions from producer–scrounger models. We suggest that feeding efficiency is a likely ecological determinant of feeding-type ratio and that an individual's feeding type may further depend on body size differences between group members. In this context, relative body size variation may correlate with attack-threshold variation, driving feeding type polymorphism. The flexibility of feeding behaviour under novel, non-social conditions stands in line with these assumptions and may further be evolutionary adaptive, allowing individuals to succeed under different socioecological conditions.
Acknowledgements
We thank K.J. for help with spider collection. Moreover, we thank Jasmin Ruch for sharing her knowledge on A. ergandros as well as for helpful advice concerning the study design.
Data accessibility
The datasets supporting this article have been uploaded as part of the supplementary material to Dryad: http://dx.doi.org/10.5061/dryad.v3sg2.
Authors' contributions
All authors contributed essentially to conception and experimental design of the study. M.D. conducted the experiments, performed all data analyses and wrote the first draft of the manuscript. J.M.S. and M.E.H. made substantial contributions to revisions.
Competing interests
We have no competing interests.
Funding
M.D. is supported by a PhD scholarship of the Konrad-Adenauer Foundation.
References
- 1.Alexander RD. 1974. The evolution of social behavior. Annu. Rev. Ecol. Syst. 5, 325–383. ( 10.1146/annurev.es.05.110174.001545) [DOI] [Google Scholar]
- 2.Krause J, Ruxton GD. 2002. Living in groups, pp. 6–54. New York, NY: Oxford University Press. [Google Scholar]
- 3.Grand TC, Dill LM. 1999. The effect of group size on the foraging behaviour of juvenile coho salmon: reduction of predation risk or increased competition? Anim. Behav. 58, 443–451. ( 10.1006/anbe.1999.1174) [DOI] [PubMed] [Google Scholar]
- 4.Giraldeau L-A, Caraco T. 2000. Social foraging theory, pp. 51–196. Princeton, NJ: Princeton University Press. [Google Scholar]
- 5.Shen S-F, Akcay E, Rubenstein DR. 2014. Group size and social conflict in complex societies. Am. Nat. 183, 301–310. ( 10.1086/674378) [DOI] [PubMed] [Google Scholar]
- 6.Macdonald DW. 1983. The ecology of carnivore social behaviour. Nature 301, 379–384. ( 10.1038/301379a0) [DOI] [Google Scholar]
- 7.Creel S, Creel NM. 1995. Communal hunting and pack size in African wild dogs, Lycaon pictus. Anim. Behav. 50, 1325–1339. ( 10.1016/0003-3472(95)80048-4) [DOI] [Google Scholar]
- 8.MacNulty DR, Tallian A, Stahler DR, Smith DW. 2014. Influence of group size on the success of wolves hunting bison. PLos ONE 9, e0112884 ( 10.1371/journal.pone.0112884) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitehouse MEA, Lubin Y. 2005. The functions of societies and the evolution of group living: spider societies as a test case. Biol. Rev. 80, 347–361. ( 10.1017/s1464793104006694) [DOI] [PubMed] [Google Scholar]
- 10.Yip EC, Powers KS, Aviles L. 2008. Cooperative capture of large prey solves scaling challenge faced by spider societies. Proc. Natl Acad. Sci. USA 105, 11 818–11 822. ( 10.1073/pnas.0710603105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sih A, Hanser SF, McHugh KA. 2009. Social network theory: new insights and issues for behavioral ecologists. Behav. Ecol. Sociobiol. 63, 975–988. ( 10.1007/s00265-009-0725-6) [DOI] [Google Scholar]
- 12.Morand-Ferron J, Wu G-M, Giraldeau L-A. 2011. Persistent individual differences in tactic use in a producer-scrounger game are group dependent. Anim. Behav. 82, 811–816. ( 10.1016/j.anbehav.2011.07.014) [DOI] [Google Scholar]
- 13.Dall SRX, Bell AM, Bolnick DI, Ratnieks FLW. 2012. An evolutionary ecology of individual differences. Ecol. Lett. 15, 1189–1198. ( 10.1111/j.1461-0248.2012.01846.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sih A, Bell A, Johnson JC. 2004. Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol. Evol. 19, 372–378. ( 10.1016/j.tree.2004.04.009) [DOI] [PubMed] [Google Scholar]
- 15.Sih A, Cote J, Evans M, Fogarty S, Pruitt J. 2012. Ecological implications of behavioural syndromes. Ecol. Lett. 15, 278–289. ( 10.1111/j.1461-0248.2011.01731.x) [DOI] [PubMed] [Google Scholar]
- 16.Barnard CJ, Sibly RM. 1981. Producers and scroungers: a general model and its application to captive flocks of house sparrows. Anim. Behav. 29, 543–550. ( 10.1016/S0003-3472(81)80117-0) [DOI] [Google Scholar]
- 17.Packer C, Ruttan L. 1988. The evolution of cooperative hunting. Am. Nat. 132, 159–198. ( 10.1086/284844) [DOI] [Google Scholar]
- 18.Vickery WL, Giraldeau LA, Templeton JJ, Kramer DL, Chapman CA. 1991. Producers, scroungers, and group foraging. Am. Nat. 137, 847–863. ( 10.1086/285197) [DOI] [Google Scholar]
- 19.Coolen I. 2002. Increasing foraging group size increases scrounger use and reduces searching efficiency in nutmeg mannikins (Lonchura punctulata). Behav. Ecol. Sociobiol. 52, 232–238. ( 10.1007/s00265-002-0500-4) [DOI] [Google Scholar]
- 20.Ruch J, Herberstein ME, Schneider JM. 2014. Families hunt more successfully: effect of group composition on hunting and communal feeding. Anim. Behav. 91, 171–178. ( 10.1016/j.anbehav.2014.03.013) [DOI] [Google Scholar]
- 21.Unglaub B, Ruch J, Herberstein ME, Schneider JM. 2013. Hunted hunters? Effect of group size on predation risk and growth in the Australian subsocial crab spider Diaea ergandros. Behav. Ecol. Sociobiol. 67, 785–794. ( 10.1007/s00265-013-1502-0) [DOI] [Google Scholar]
- 22.Pruitt JN, Riechert SE, Jones TC. 2008. Behavioural syndromes and their fitness consequences in a socially polymorphic spider, Anelosimus studiosus. Anim. Behav. 76, 871–879. ( 10.1016/j.anbehav.2008.05.009) [DOI] [Google Scholar]
- 23.Ruch J, Herberstein ME, Schneider JM. 2014. Offspring dynamics affect food provisioning, growth and mortality in a brood-caring spider. Proc. R. Soc. B 281, 20132180 ( 10.1098/rspb.2013.2180) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szymkowiak P. 2014. Revision of Australian species of the genus Diaea (Araneae: Thomisidae) with redefinition of their taxonomic status. Ann. Zool. 64, 333–477. ( 10.3161/000345414X684795) [DOI] [Google Scholar]
- 25.Evans TA. 1998. Offspring recognition by mother crab spiders with extreme maternal care. Proc. R. Soc. Lond. B 265, 129–134. ( 10.1098/rspb.1998.0273) [DOI] [Google Scholar]
- 26.Evans TA, Goodisman MAD. 2002. Nestmate relatedness and population genetic structure of the Australian social crab spider Diaea ergandros (Araneae: Thomisidae). Mol. Ecol. 11, 2307–2316. ( 10.1046/j.1365-294X.2002.01623.x) [DOI] [PubMed] [Google Scholar]
- 27.Ruch J, Dumke M, Schneider JM. 2015. Social network structure in group-feeding spiders. Behav. Ecol. Sociobiol. 69, 1429–1436. ( 10.1007/s00265-015-1955-4) [DOI] [Google Scholar]
- 28.Kralj-Fiser S, Schneider JM. 2012. Individual behavioural consistency and plasticity in an urban spider. Anim. Behav. 84, 1–8. ( 10.1016/j.anbehav.2012.04.032) [DOI] [Google Scholar]
- 29.Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with R, pp. 295–342. New York, NY: Springer Press. [Google Scholar]
- 30.Halkidi M, Batistakis Y, Vazirgiannis M. 2001. On clustering validation techniques. J. Intell. Inf. Syst. 17, 107–145. ( 10.1023/a:1012801612483) [DOI] [Google Scholar]
- 31.Rousseeuw PJ. 1987. Silhouettes: a graphical aid to the interpretation and validation of cluster-analysis. J. Comput. Appl. Math. 20, 53–65. ( 10.1016/0377-0427(87)90125-7) [DOI] [Google Scholar]
- 32.Kaufman L, Rousseeuw PJ. 1991. Finding groups in data: an introduction to cluster analysis, pp. 64–198. Hoboken, NJ: John Wiley & Sons. [Google Scholar]
- 33.Coolen I, Giraldeau L-A, Lavoie M. 2001. Head position as an indicator of producer and scrounger tactics in a ground-feeding bird. Anim. Behav. 61, 895–903. ( 10.1006/anbe.2000.1678) [DOI] [Google Scholar]
- 34.Michelena P, Sibbald AM, Erhard HW, McLeod JE. 2009. Effects of group size and personality on social foraging: the distribution of sheep across patches. Behav. Ecol. 20, 145–152. ( 10.1093/beheco/arn126) [DOI] [Google Scholar]
- 35.Pruitt JN, Riechert SE. 2009. Frequency-dependent success of cheaters during foraging bouts might limit their spread within colonies of a socially polymorphic spider. Evolution 63, 2966–2973. ( 10.1111/j.1558-5646.2009.00771.x) [DOI] [PubMed] [Google Scholar]
- 36.Pruitt JN, Riechert SE. 2011. How within-group behavioural variation and task efficiency enhance fitness in a social group. Proc. R. Soc. B 278, 1209–1215. ( 10.1098/rspb.2010.1700) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anders NP. 2001. Predator behaviour and prey density: evaluating density-dependent intraspecific interactions on predator functional responses. J. Anim. Ecol. 70, 14–19. ( 10.1111/j.1365-2656.2001.00472.x) [DOI] [Google Scholar]
- 38.Barta Z, Giraldeau L-A. 1998. The effect of dominance hierarchy on the use of alternative foraging tactics: a phenotype-limited producing-scrounging game. Behav. Ecol. Sociobiol. 42, 217–223. ( 10.1007/s002650050433) [DOI] [Google Scholar]
- 39.Barta Z, Liker A, Mónus F. 2004. The effects of predation risk on the use of social foraging tactics. Anim. Behav. 67, 301–308. ( 10.1016/j.anbehav.2003.06.012) [DOI] [Google Scholar]
- 40.Rypstra AL. 1993. Prey size, social competition, and the development of reproductive division of labor in social spider groups. Am. Nat. 142 868–880. ( 10.1086/285577) [DOI] [Google Scholar]
- 41.Ruch J, Heinrich L, Bilde T, Schneider JM. 2009. Relatedness facilitates cooperation in the subsocial spider, Stegodyphus tentoriicola. BMC Evol. Biol. 9, 257 ( 10.1186/1471-2148-9-257) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Whitehouse MEA, Lubin Y. 1999. Competitive foraging in the social spider Stegodyphus dumicola. Anim. Behav. 58, 677–688. ( 10.1006/anbe.1999.1168) [DOI] [PubMed] [Google Scholar]
- 43.Ward PI. 1986. Prey availability increases less quickly than nest size in the social spider Stegodyphus mimosarum. Behaviour 97, 213–225. ( 10.1163/156853986X00603) [DOI] [Google Scholar]
- 44.Foelix R. 2010. Biology of spiders, pp. 88–217. New York, NY: Oxford University Press. [Google Scholar]
- 45.Duarte A, Weissing FJ, Pen I, Keller L. 2011. An evolutionary perspective on self-organized division of labor in social insects. Annu. Rev. Ecol. Evol. Syst. 42, 91–110. ( 10.1146/annurev-ecolsys-102710-145017) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting this article have been uploaded as part of the supplementary material to Dryad: http://dx.doi.org/10.5061/dryad.v3sg2.