Abstract
Establishing homologies between cortical areas in animal models and humans lies at the heart of translational neuroscience, as it demonstrates how knowledge obtained from these models can be applied to the human brain. Here, we review progress in using parallel functional imaging to ascertain homologies between parietal areas of human and non-human primates, species sharing similar behavioural repertoires. The human homologues of several areas along monkey IPS involved in action planning and observation, such as AIP, LIP and CIP, as well as those of opercular areas (SII complex), have been defined. In addition, uniquely human areas, such as the tool-use area in left anterior supramarginal gyrus, have also been identified.
Keywords: fMRI, homology, action planning, action observation
1. Introduction
Following the work of Mountcastle [1], it became generally accepted that the posterior parietal cortex (PPC) is involved in sensorimotor transformations underlying the planning of human actions [2]. The PPC has also been implicated in more cognitive functions, such as attention [3], working and long-term memory [4,5], numerical processing [6] and tool use [7]. One important initial step in characterizing PPC functions is the definition of parietal areas, those building blocks providing the foundation of the functional studies.
Although in exceptional circumstances neuronal activity in human PPC can be accessed directly [8], functional studies of PPC generally rely on imaging (which is limited in spatio-temporal resolution and maps neuronal selectivity only indirectly), using repetition suppression [9], or multivoxel pattern analysis [10]. The limitations of these methods have become apparent [11,12], underscoring the necessity of animal models. Animal models should be appropriate (i.e. share the brain functions under investigation). Non-human primates (NHPs)—who, like humans, use dexterous hands and mobile eyes to explore and interact with the environment—are the most valuable models for PPC. To be useful, however, any knowledge derived from the monkey brain must translate to human brain function. Hence, using the NHP to define human PPC regions requires that homologies between PPC regions be established and uniquely human areas identified.
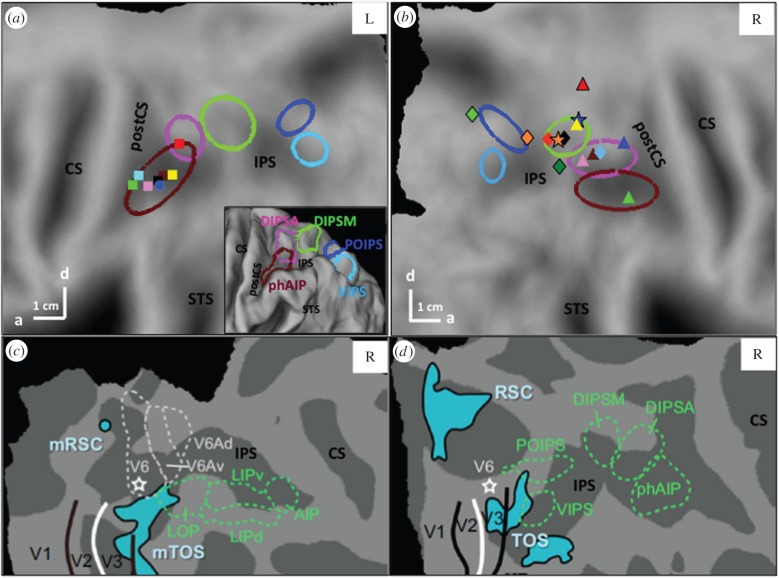
Monkey single-cell studies have established that LIP, AIP and the parietal reach region encompassing MIP and V6A are involved in planning saccades, grasping and reaching, respectively [13]. However, applying single-cell NHP results directly to human fMRI involves changing both experimental technique and species [14], and often incorrectly assumes that an area is unique in having neurons endowed with a given property. For example, activation by saccades is often considered the signature of LIP [15,16], although single-cell [17] and imaging [18] studies have shown more widespread activation of monkey PPC. Indeed, saccades activate a substantial part of human PPC (figure 1). Such difficulties can be avoided using fMRI in alert monkeys as an intermediate step between human fMRI and NHP single-cell studies. This review builds on such parallel imaging studies.
Figure 1.
Human PPC areas hypothetically homologous to areas in the lateral bank of monkey IPS. (a,b) Confidence ellipses of five areas (brown, phAIP; pink, DIPSA; green, DIPSM; dark blue, POIPS; light blue, VIPS) in posterior parts of (a) left and (b) right flattened hemispheres (folded view in inset). (c,d) Parietal areas in (c) monkey and (d) human relative to V1–3, V6, retrosplenial cortex (RSC), transverse occipital sulcus (TOS) area and possible monkey counterparts (mRSC, mTOS); modified from Vanduffel et al. [18]. LOP is synonymous with CIP; IPS, intraparietal sulcus; CS, central sulcus. Symbols in (a)—local maxima (LM) for grasping: circle: [19]; squares: [20] (yellow), [21] (red), [22] (black), [23] (green), [24] (pink); visuo-tactile: [25] (brown); and 3D hand orientation: [26] (blue). In (b)—stars: LM of human LIP using spatial attention (orange, [27]) or saccades (blue, [28]); diamonds: other saccade LM: [15] (light and dark green), [29] (orange and red), [16] (black) and [28] (light blue); triangles: proposed human VIP: [30] (yellow), [31] (red), [32], (pink), [33] (green), [34], (blue), [16] (brown). Last two LM: visuo-tactile, others optic flow. Putative VIP LM stretch over 40 mm in medio-lateral direction, putative LIP LM over 40 mm in rostro-caudal direction, preventing the computation of useful confidence ellipses.
Establishing homologies between cortical areas in humans and macaque monkeys considers only a very small subset of primates, while ideally one would examine multiple species within this order. The alternative is to consider as many different properties of the areas under investigation as possible in both species [35]. In NHP, visual cortical areas are defined by four criteria: (i) cyto- and myeloarchitectonics, (ii) anatomical connections with other areas, (iii) retinotopic organization, and (iv) functional properties. We suggest using those criteria to establish cortical homologies, adding area topology—localization with respect to neighbouring areas—as a valuable fifth criterion [35], following the tradition of comparative anatomy. While progress has been made in mapping cyto- and myeloarchitectonic architecture in the human brain, methodologies differ considerably from those classically used in monkeys, thus far preventing systematic comparison. Although diffusion tensor imaging (DTI) [36] was potentially seen as a measure of connectivity between areas, recent comparative studies in the monkey question the value of DTI as a proxy for tract tracing in animals [37]. Thus, the chief criteria for establishing homologies remain assessing retinotopic organization and as many functional properties as possible, both of which can be adequately tested by parallel imaging of macaque and humans. These can be supplemented by topological arguments, and, where possible, by cyto- or myeloarchitectonic data.
Human cerebral hemispheres have 9.2 times the surface area of macaque hemispheres [38,39]. The number of human cortical areas is estimated at 150–200, a 1.3-fold increase over monkeys (130–140 areas per hemisphere), suggesting that new areas have appeared in humans to support typically human behaviour, such as tool use or language. In this review, we concentrate on two sets of parietal areas as candidates for homologous areas: those along the intraparietal sulcus (IPS) and the parietal opercular areas. Conversely, species differences are documented in the inferior parietal lobule (IPL).
2. Similarities along the intraparietal sulcus
We have described five regions along human IPS whose homology is becoming clear (figure 1). Because activation studies mainly yield local maxima (LM), these areas were defined [40] as confidence ellipses surrounding these maxima (figure 1a,b). The four caudal ellipses representing motion-responsive regions [41] follow the dorsal/posterior bank of the IPS: the dorsal IPS anterior (DIPSA), dorsal IPS medial (DIPSM), parieto-occipital IPS (POIPS) and ventral IPS (VIPS), rostrally to caudally. Rostral to DIPSA, we described the putative homologue of AIP (phAIP) from maxima of motor activation during grasping and multimodal activations (see legend, figure 1). These latter LM cluster very well, allowing computation of a confidence ellipse (figure 1a), unlike activations by saccades identifying LIP, or activations by visuo-tactile convergence or optic flow, identifying VIP (figure 1b). We propose (table 1) that phAIP and DIPSA correspond to anterior (motor), and posterior (visual) parts of monkey AIP, respectively [18,42], while DIPSM corresponds to anterior LIP and VIPS to monkey CIP (figure 1). These areas are discussed together, stressing topological relationships. Note that AIP–CIP are located along the lateral/ventral bank of monkey IPS, DIPSA-VIPS along the dorsal/medial bank of human IPS, in agreement with Grefkes & Fink [43]. In both species, these regions lie rostral to the V6 and V3A complexes. The homology of POIPS is less clear: it probably corresponds to an area on the medial bank of monkey IPS rostral to V6/V6A.
Table 1.
Proposed homologies of IPS regions.
| monkey area | human area(s) | criteria |
|---|---|---|
| AIP | phAIP+vDIPSA | 6/8 functional tests of table 2 |
| anterior LIP | DIPSM | 8/9 functional tests of table 2 |
| VIP | dDIPSA | visuo-tactile sensitivity optic flow sensitivity intrusion-into-peripersonal-space sensitivity numerosity selectivity size selectivity topological relationships |
| CIP | VIPS (V7/V7A) | retinotopy 3D shape-from-disparity sensitivity 2D shape sensitivity topological relationships |
As stated, establishing a homology necessitates examining as many functional characteristics as possible. Indeed, the property initially considered, sensitivity to three-dimensional (3D) structure from motion (SFM) using random line stimuli, revealed marked differences between human and monkey PPC [44]. In many other respects, however, the lateral bank of monkey IPS and dorsal bank of human IPS are functionally similar [45,46]. Rostrally to caudally, three regions emerge along the banks of IPS in both species: a rostral 3D shape-from-disparity (SFD)-sensitive region (red in figure 2a,c), a mid-region with sensitivity to disparity but not 3D SFD (yellow), and a caudal region with mixed sensitivities for 3D SFD and simple disparity (orange/red). The rostral region corresponded to a single, large two-dimensional (2D) shape-sensitive region encompassing posterior AIP and anterior LIP, and to two 2D shape-sensitive regions, DIPSA and DIPSM in humans. The caudal region, also 2D shape-sensitive, corresponded to CIP in monkeys and VIPS in humans (figure 2b,d). In both species, saccade sensitivities differentiated two components in the rostral region: anterior LIP/posterior AIP in the monkey and DIPSA/DIPSM in humans (figure 2b,e). These and similar studies [42] indicate that DIPSM and anterior LIP share 8 of 9 characteristics investigated, 3D SFM being the exception (table 2). Similarly, posterior AIP and DIPSA share 6 of 8 characteristics. Significantly, combined single-cell and fMRI experiments in monkey [47] suggest that 3D SFD activations in DIPSA and DIPSM actually correspond to two neuronal clusters selective for 3D SFD in the rostral lateral bank of monkey IPS. This underscores how parallel imaging in humans and NHP disclose neuronal operations in the human brain.
Figure 2.
Parallel imaging of 2D and 3D shape sensitivity: (a,b) lateral bank of left monkey IPS (from [45]) (a) 3D shape-from-disparity, (b) 2D shape sensitivity; (c–e) left human IPS (from [46])—(c) 3D shape-from-disparity; (d) 2D shape sensitivity; (e) saccade sensitivity (dark hatching); colours: see text. In (a,b) white dotted lines indicate the AIP/LIP borders derived from the saccade-related activation.
Table 2.
Criteria for homology of DIPSM and anterior LIP.
| criterion | anterior LIP | DIPSM |
|---|---|---|
| central representationa | + | + |
| motion sensitivityb | + | + |
| 2D shape sensitivity | + | + |
| 3D shape from disparity (random lines) sensitivity | + | + |
| 3D shape from disparity (surfaces) sensitivity | + | + |
| 3D shape from motion sensitivity | − | + |
| saccade sensitivityc | + | + |
| observed-action sensitivity | + | + |
| topological sequence for 2D shape and disparity | + | + |
aNot tested in AIP.
bFails in AIP.
cAbsent in both AIP and DIPSA.
Initially, only phAIP was considered homologous to monkey AIP, but this was based upon motor response during grasping and somato-sensory convergence, characteristics of rostral AIP [48,49]. This supports phAIP plus DIPSA being the human counterpart of monkey AIP [46]. The devotion of such a large region to planning hand actions is consistent with their importance in the human motor repertoire. The homology of phAIP/DIPSA with monkey AIP is further supported by action-observation studies in both species. Nelissen et al. [50] showed AIP activation in monkeys observing grasping actions, in agreement with Pani et al. [51] and Maeda et al. [52]. Similarly, three human studies [40,53,54] documented phAIP sensitivity to observed manipulative actions (figure 3a).
Figure 3.
Anterior IPS regions on left flatmap: (a) voxels specific for observation of manipulative (red, phAIP) and interpersonal actions (green, dDIPSA); (b,d) dDIPSA (black outline) relative to local maxima of studies as indicated (b), and to myelin density peak near middle IPS (d). (a,b,d) Modified from Ferri et al. [54]. Dots in (a)—IPS3 (purple), 4 (light blue) and 5 (yellow) from Konen et al. [55]. Symbols in (b)—stars: centre of numerosity (yellow) and size (red) maps averaged over four subjects from [56,57]; other symbols: LM of [34] (red), [32] (yellow), [58] (black), [59] (green). (c) Parietal part of human-specific resting-state network (red to yellow voxels) compared with hIPS (black outline): from [60], and outline of left anterior SMG region specific for human tool use: from [61] (pink), [62] (yellow) compared with phAIP (brown). Black dot indicates LM of interaction execution/tool [63].
The phAIP, DIPSA and DIPSM regions are mere confidence ellipses whose sizes depend on the variability of the LM locations defining them. While subsequent work suggests that phAIP is indeed functionally homogeneous, this may not be true of DIPSA. A recent study [54] suggested that dorsal DIPSA (dDIPSA) is functionally distinct from the ventral part located in the extension of phAIP (figure 1). Because the activation by observed interpersonal actions (figure 3a,b) was located between other activations reflecting typical VIP characteristics such as somato-visual convergence [34,59], intrusion into peripersonal space [58] and optic flow [32], we have proposed that dDIPSA may correspond to VIP, while only ventral DIPSA (vDIPSA) corresponds to posterior AIP. The activation by observing interpersonal actions was thus interpreted to reflect the intrusion or movement of the target person in the peripersonal space of the actor. Indeed, visuo-tactile VIP neurons also react to visual stimuli in the peripersonal space of the experimenter [64]. This identification is also supported by the overlap with size and numerosity maps [56,57], as VIP neurons are selective for numerosity and size [65,66]. It is also consistent with the proximity of dDIPSA to the myelin density peak in dorsal IPS (figure 3c) [67], which may correspond to ventral LIP [68]. Our most recent work suggests that DIPSM may also require splitting into a ventral part and a dorsal part extending the homologue of VIP more caudally. Such subdivisions are unsurprising, because areas in lateral bank and fundus of monkey IPS occupy narrow, parallel strips [68].
Progress establishing homology between monkey CIP and human VIPS has been slower. Support, beyond that reviewed above, comes chiefly from retinotopic studies. A region in posterior PPC, dorsal to human V3A/B, overlapping posterior VIPS (figure 4c), has long been designated V7 [70] or IPS0 [55]. A recent study [71] using the stereoscopic stimuli very effective in caudal human IPS [72] has revealed a central (C) cluster (two areas sharing a central representation) in the occipital part of IPS, rostral to V6 and separated from rostral clusters by a broad representation of far eccentricities (figure 4d,e). We propose that this V7/V7A cluster is the retinotopic counterpart of functionally defined VIPS. Its organizational features strongly resemble those of monkey CIP, which also corresponds to a C cluster of two areas (figure 4a,b) [69] and is separated from anterior LIP by a representation of the far periphery in posterior LIP. Thus, there is some support for identifying VIPS with monkey CIP, both of which comprise two areas.
Figure 4.
Retinotopic organizations: human V7/V7A cluster homologue of monkey CIP. (a,b) Eccentricity and azimuth CIP maps in the monkey (from [69])—stars: central representation, dotted lines: horizontal meridians, dashed lines: vertical meridians. (c) Overlap between functional VIPS and retinotopic V7: the rostral part of VIPS probably corresponds to V7A; (d,e) eccentricity and azimuth maps of posterior right hemisphere of subject 1 (same as in fig. 1 of [67]) centred on the V7/V7A cluster. Purple lines: far eccentricity borders of clusters; black stars: central representation, full and stippled black lines: lower and upper vertical meridians, white stippled lines: horizontal meridians. Arrows indicate rostral C clusters (1, 2), eccentricity ridge rostral to the V7/V7A cluster (3), centre of V6 (4) and a retinotopic map potentially corresponding to monkey DP (5).
3. Similarities in the parietal operculum
Although strictly speaking the parietal operculum is not part of PPC, which includes only areas 5 and 7 in the monkey and 5, 7, 40 and 39 in humans, we discuss opercular homology here because it also contains higher-order sensorimotor areas. Eickhoff et al. [73] described four cytoarchitectonic regions in the operculum, labelled OP1-4, located anterior to the various PF regions [74] constituting the rostral IPL (figure 5a). Using somatotopic mapping with fMRI, Eickhoff et al. [75] provided evidence that OP1 and OP4 correspond to monkey S2 and PV (figure 5b,c). They suggested that OP3 corresponds to the third somatotopic map in the monkey area VS and speculated that remaining OP2 might be vestibular in nature. Recently, we have begun using stereo EEG, intracerebral recordings of local field potentials in epileptic patients, to complement our fMRI studies. Recording from many patients, reconstructing lead locations and warping hemispheres to a template has allowed us to reconstruct four-dimensional maps of human cortex, combining millimetre spatial localization with millisecond time resolution, in response to median nerve stimulation [76]. This study has shown that OP2 processes somato-sensory information as much as OP1 or OP4. Hence further work is needed to understand the homology of area VS, as some monkeys have two VS areas [77], but the evidence relating OP1 and OP4 to S2 and PV is rather convincing.
Figure 5.
Homologies of parietal opercular areas. (a) Somatotopy of monkey areas (from [75]); (b) flatmap of left operculum indicating the four opercular areas (white outlines) and the five PF areas (coloured outlines); (c) somatopic organization of OP1, OP3 and OP4 [75]; (d) response to electrical stimulation median nerve in OP areas (from [76]); (e,f) activation sites for observation of skin-displacing action (e) and their activity profiles (f) from Ferri et al. [54].
Recently, we [54] have shown that OP1, and neighbouring PFop, are activated by the observation of skin-displacing actions, such as rubbing or scratching (figure 5d). Control tests indicated that this activation reflects the dynamic nature of the actions observed, not the viewing of tactile contact. The co-activation of OP1 and PFop (figure 5e) was reminiscent of the robust projections between monkey S2 and PF [78], whereby PF provides output to OP1 (human S2), complementing OP4 (human PV) in this role. In fact, we have suggested [54] that OP1 and PFop contributions to the observed-action activation may correspond to the sensory and motor parts, respectively, of the transformation underlying planning of skin-displacing action, resembling the respective roles of posterior and anterior AIP.
4. Species differences in the inferior parietal lobule
Warping monkey cortex to its human counterpart, then performing cluster analysis of the resting-state networks of the two species, Mantini et al. [60] found three human networks with no functional or topological monkey counterparts. Two of these evolutionarily novel networks were lateralized, but both included a common IPL region (figure 3d). This novel IPL region overlapped with hIPS, implicated in numerical processing [6] and with anatomical regions undergoing intense evolutionary expansion in humans [79]. Although the hIPS region has been associated with monkey VIP [80], recent data indicate that numerosity and size maps [56,57], consisting of voxels tuned to small numerosities or size, like VIP neurons [65], are located dorsal to hIPS, overlapping the proposed homologue of VIP (figure 3b). Hence, I suggest that human PPC hosts two numerosity processing regions separated by phAIP/vDIPSA: one common with the monkey in dDIPSA, supporting subitizing, and another specifically human, in hIPS, supporting counting.
Another functionally defined region exemplifying cortical expansion in human IPL is the left anterior supramarginal (aSMG) tool-use region (figure 3d) [61,62]. This region responds specifically to observation of tool actions, but not hand actions with similar goals, unlike phAIP, which responds to either. Videos used to define aSMG yielded no such specific IPL activation in monkeys, even after extensive training using pliers or rake, the tools featuring in those videos [61]. This aSMG region corresponds precisely to a region active when humans use tools [63]. We have suggested that this region, corresponding to cytoarchitectonic PFt [74], is a typically human area, underlying the development of tool use in humans [81]. Most likely, the use and creation of tools, technology, is based on the interaction of this area with several others in PPC and temporal lobe [82].
It is unlikely, despite its expansion, that all human IPL is evolutionary novel. For example, it has been recently shown that a region in monkey PG connected to the hippocampus is activated by the retrieval of the first of several previously seen items [5], very much like the human angular gyrus [83].
5. Discussion and conclusion
The studies reviewed here have begun to illuminate challenging questions concerning homologies of macaque and human parietal regions, and many objectives defined a decade ago [35] have now been met. Critical elements were parallel imaging in these two species and employing multiple functional criteria, revealing a substantial number of homologous PPC areas. This approach resolves the translational question of how knowledge accumulated through invasive experiments in macaques can be applied to humans, where investigations are more limited for ethical reasons. Monkey single-cell studies can thus provide particularly valuable information about neuronal mechanisms underlying human behavioural competences. For example, the homology between phAIP/vDIPSA and monkey AIP implies that the canonical and mirror neurons observed in single-cell studies [49,52] also exist in this human area.
The studies also suggest two avenues for further progress. One is to leverage the topological relationships between areas, which are generally retained across species. A set of homologous regions, once identified, can provide a seed for extending functional correspondences, and ultimately homology, to neighbouring regions. For example, regions dorsal to DIPSM are involved in the execution and observation of reaching [84], suggesting homology with macaque MIP and V6A in the medial bank of IPS, befitting topological relationships in both species. Second, some studies reviewed here suggest action observation can serve as proxy for action planning and execution. This may circumvent the limitations on the range of sensorimotor transformations observable in a monkey sitting in a chair with its head fixed, or in human subjects lying supine in a scanner (largely grasping, reaching and saccades). Moreover, videos are easily shown to both monkeys and humans, facilitating attribution of sensorimotor transformations to discrete PPC regions and establishing homologies.
Finally, taking a broader perspective, the few PPC regions present in rodents [85] are probably involved in locomotion and coarse use of the forepaws. These areas probably correspond to the medial wall of primate PPC, though they surely have undergone substantial modification to accommodate the navigational needs [86] of primates, especially bipedal humans. NHP Brodmann areas 5 and 7 have been added to those ancestral PPC regions in a medial-to-lateral direction for the sophisticated control of mobile eyes and dexterous hands. This medio-lateral trend was further amplified in humans with the expansion of IPL, generating areas 39 and 40, to control vocal and other communication as well as the use of artefacts, extending the potential of biological effectors.
Acknowledgements
The author is indebted to W. Vanduffel, G. Rizzolatti, G. Luppino, K. Nelissen, J. B. Durand, P. Avanzini, R. Abdollahi, R. Peeters, J. Jastorff and S. Ferri for the collaboration in the studies reviewed here. He is also thankful to S. Raiguel for comments on an earlier version of the manuscript and to S. Ferri for help with the figures.
Competing interests
I declare I have no competing interests.
Funding
This study was supported by ERC Parietalaction.
References
- 1.Mountcastle VB, Lynch JC, Georgopoulos A, Sakata H, Acuna C. 1975. Posterior parietal association cortex of the monkey: command functions for operations within extrapersonal space. J. Neurophysiol. 38, 871–908. [DOI] [PubMed] [Google Scholar]
- 2.Andersen RA, Buneo CA. 2002. Intentional maps in posterior parietal cortex. Annu. Rev. Neurosci. 25, 189–220. ( 10.1146/annurev.neuro.25.112701.142922) [DOI] [PubMed] [Google Scholar]
- 3.Wojciulik E, Kanwisher N. 1999. The generality of parietal involvement in visual attention. Neuron 23, 747–764. ( 10.1016/S0896-6273(01)80033-7) [DOI] [PubMed] [Google Scholar]
- 4.Gnadt JW, Andersen RA. 1988. Memory related motor planning activity in posterior parietal cortex of macaque. Exp. Brain Res. 70, 216–220. [DOI] [PubMed] [Google Scholar]
- 5.Miyamoto K, Osada T, Adachi Y, Matsui T, Kimura HM, Miyashita Y. 2013. Functional differentiation of memory retrieval network in macaque posterior parietal cortex. Neuron 77, 787–799. ( 10.1016/j.neuron.2012.12.019) [DOI] [PubMed] [Google Scholar]
- 6.Dehaene S, Piazza M, Pinel P, Cohen L. 2003. Three parietal circuits for number processing. Cogn. Neuropsychol. 20, 487–506. ( 10.1080/02643290244000239) [DOI] [PubMed] [Google Scholar]
- 7.Goldenberg G, Spatt J. 2009. The neural basis of tool use. Brain 132, 1645–1655. ( 10.1093/brain/awp080) [DOI] [PubMed] [Google Scholar]
- 8.Aflalo T, et al. 2015. Decoding motor imagery from the posterior parietal cortex of a tetraplegic human. Science 348, 906–910. ( 10.1126/science.aaa5417) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grill-Spector K, Malach R. 2001. fMR-adaptation: a tool for studying the functional properties of human cortical neurons. Acta Psychol. (Amst.) 107, 293–321. ( 10.1016/S0001-6918(01)00019-1) [DOI] [PubMed] [Google Scholar]
- 10.Kamitani Y, Tong F. 2005. Decoding the visual and subjective contents of the human brain. Nat. Neurosci. 8, 679–685. ( 10.1038/nn1444) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sawamura H, Orban GA, Vogels R. 2006. Selectivity of neuronal adaptation does not match response selectivity: a single-cell study of the FMRI adaptation paradigm. Neuron 49, 307–318. ( 10.1016/j.neuron.2005.11.028) [DOI] [PubMed] [Google Scholar]
- 12.Bulthé J, De Smedt B, Op de Beeck HP. 2015. Visual number beats abstract numerical magnitude: format-dependent representation of Arabic digits and dot patterns in human parietal cortex. J Cogn. Neurosci. 27, 1376–1387. ( 10.1162/jocn_a_00787) [DOI] [PubMed] [Google Scholar]
- 13.Andersen RA, Buneo CA. 2002. Intentional maps in posterior parietal cortex. Annu. Rev. Neurosci. 25, 189–220. ( 10.1146/annurev.neuro.25.112701.142922) [DOI] [PubMed] [Google Scholar]
- 14.Orban GA. 2002. Functional MRI in the awake monkey: the missing link. J. Cogn. Neurosci. 14, 965–969. ( 10.1162/089892902760191171) [DOI] [PubMed] [Google Scholar]
- 15.Simon O, Mangin JF, Cohen L, Le Bihan D, Dehaene S. 2002. Topographical layout of hand, eye, calculation, and language-related areas in the human parietal lobe. Neuron 33, 475–487. ( 10.1016/S0896-6273(02)00575-5) [DOI] [PubMed] [Google Scholar]
- 16.Eger E, Pinel P, Dehaene S, Kleinschmidt A. 2015. Spatially invariant coding of numerical information in functionally defined subregions of human parietal cortex. Cereb. Cortex 25, 1319–1329. ( 10.1093/cercor/bht323) [DOI] [PubMed] [Google Scholar]
- 17.de Lafuente V, Jazayeri M, Shadlen MN. 2015. Representation of accumulating evidence for a decision in two parietal areas. J. Neurosci. 35, 4306–4318. ( 10.1523/JNEUROSCI.2451-14.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vanduffel W, Zhu Q, Orban GA. 2014. Monkey cortex through fMRI glasses. Neuron 83, 533–550. ( 10.1016/j.neuron.2014.07.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Binkofski F, Buccino G, Posse S, Seitz RJ, Rizzolatti G, Freund H. 1999. A fronto-parietal circuit for object manipulation in man: evidence from an fMRI-study. Eur. J. Neurosci. 11, 3276–3286. ( 10.1046/j.1460-9568.1999.00753.x) [DOI] [PubMed] [Google Scholar]
- 20.Begliomini C, Wall MB, Smith AT, Castiello U. 2007. Differential cortical activity for precision and whole-hand visually guided grasping in humans. Eur. J. Neurosci. 25, 1245–1252. ( 10.1111/j.1460-9568.2007.05365.x) [DOI] [PubMed] [Google Scholar]
- 21.Culham JC, Danckert SL, DeSouza JFX, Gati JS, Menon RS, Goodale MA. 2003. Visually guided grasping produces fMRI activation in dorsal but not ventral stream brain areas. Exp. Brain Res. 153, 180–189. ( 10.1007/s00221-003-1591-5) [DOI] [PubMed] [Google Scholar]
- 22.Cavina-Pratesi C, Goodale MA, Culham JC. 2007. fMRI reveals a dissociation between grasping and perceiving the size of real 3D objects. PLoS ONE 2, e424 ( 10.1371/journal.pone.0000424) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frey SH, Vinton D, Norlund R, Grafton ST. 2005. Cortical topography of human anterior intra-parietal cortex active during visually guided grasping. Brain Res. Cogn. Brain Res. 23, 397–405. ( 10.1016/j.cogbrainres.2004.11.010) [DOI] [PubMed] [Google Scholar]
- 24.Kroliczak G, Cavina-Pratesi C, Goodman DA, Culham JC. 2007. What does the brain do when you fake it? An FMRI study of pantomimed and real grasping. J. Neurophysiol. 97, 2410–2422. ( 10.1152/jn.00778.2006) [DOI] [PubMed] [Google Scholar]
- 25.Jäncke L, Kleinschmidt A, Mirzazade S, Shah NJ, Freund HJ. 2001. The role of the inferior parietal cortex in linking the tactile perception and manual construction of object shapes. Cereb. Cortex 11, 114–121. ( 10.1093/cercor/11.2.114) [DOI] [PubMed] [Google Scholar]
- 26.Shikata E, Hamzei F, Glauche V, Koch M, Weiller C, Binkofski F, Buchel C. 2003. Functional properties and interaction of the anterior and posterior intraparietal areas in humans. Eur. J. Neurosci. 17, 1105–1110. ( 10.1046/j.1460-9568.2003.02540.x) [DOI] [PubMed] [Google Scholar]
- 27.Sereno MI, Pitzalis S, Martinez A. 2001. Mapping of contralateral space in retinotopic coordinates by a parietal cortical area in humans. Science 294, 1350–1354. ( 10.1126/science.1063695) [DOI] [PubMed] [Google Scholar]
- 28.Koyama M, Hasegawa I, Osada T, Adachi Y, Nakahara K, Miyashita Y. 2004. Functional magnetic resonance imaging of macaque monkeys performing visually guided saccade tasks: comparison of cortical eye fields with humans. Neuron 41, 795–807. ( 10.1016/S0896-6273(04)00047-9) [DOI] [PubMed] [Google Scholar]
- 29.Hagler DJ Jr, Riecke L, Sereno MI. 2007. Parietal and superior frontal visuospatial maps activated by pointing and saccades. NeuroImage 35, 1562–1577. ( 10.1016/j.neuroimage.2007.01.033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peuskens H, Sunaert S, Dupont P, Van Hecke P, Orban GA. 2001. Human brain regions involved in heading estimation. J. Neurosci. 21, 2451–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bartels A, Zeki S, Logothetis NK. 2008. Natural vision reveals regional specialization to local motion and to contrast-invariant, global flow in the human brain. Cereb. Cortex 18, 705–717. ( 10.1093/cercor/bhm107) [DOI] [PubMed] [Google Scholar]
- 32.Cardin V, Smith AT. 2010. Sensitivity of human visual and vestibular cortical regions to egomotion-compatible visual stimulation. Cereb. Cortex 20, 1964–1973. ( 10.1093/cercor/bhp268) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bremmer F, Schlack A, Shah NJ, Zafiris O, Kubischik M, Hoffmann K, Zilles K, Fink GR. 2001. Polymodal motion processing in posterior parietal and premotor cortex: a human fMRI study strongly implies equivalencies between humans and monkeys. Neuron 29, 287–296. ( 10.1016/S0896-6273(01)00198-2) [DOI] [PubMed] [Google Scholar]
- 34.Sereno MI, Huang R-S. 2006. A human parietal face area contains aligned head-centered visual and tactile maps. Nat. Neurosci. 9, 1337–1343. ( 10.1038/nn1777) [DOI] [PubMed] [Google Scholar]
- 35.Orban GA, Van Essen D, Vanduffel W. 2004. Comparative mapping of higher visual areas in monkeys and humans. Trends Cogn. Sci. 8, 315–324. ( 10.1016/j.tics.2004.05.009) [DOI] [PubMed] [Google Scholar]
- 36.Dougherty RF, Ben-Shachar M, Deutsch G, Potanina P, Bammer R, Wandell BA. 2005. Occipital-callosal pathways in children: validation and atlas development. Ann. NY Acad. Sci. 1064, 98–112. ( 10.1196/annals.1340.017) [DOI] [PubMed] [Google Scholar]
- 37.Reveley C, Seth AK, Pierpaoli C, Silva AC, Yu D, Saunders RC, Leopold DA, Ye FQ. 2015. Superficial white matter fiber systems impede detection of long-range cortical connections in diffusion MR tractography. Proc. Natl Acad. Sci. USA 112, E2820–E2828. ( 10.1073/pnas.1418198112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Essen DC, Glasser MF, Dierker DL, Harwell J, Coalson T. 2012. Parcellations and hemispheric asymmetries of human cerebral cortex analyzed on surface-based atlases. Cereb. Cortex 22, 2241–2262. ( 10.1093/cercor/bhr291) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Essen DC, Glasser MF, Dierker DL, Harwell J. 2012. Cortical parcellations of the macaque monkey analyzed on surface-based atlases. Cereb. Cortex 22, 2227–2240. ( 10.1093/cercor/bhr290) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jastorff J, Begliomini C, Fabbri-Destro M, Rizzolatti G, Orban GA. 2010. Coding observed motor acts: different organizational principles in the parietal and premotor cortex of humans. J. Neurophysiol. 104, 128–140. ( 10.1152/jn.00254.2010) [DOI] [PubMed] [Google Scholar]
- 41.Sunaert S, Van Hecke P, Marchal G, Orban GA. 1999. Motion-responsive regions of the human brain. Exp. Brain Res. 127, 355–370. ( 10.1007/s002210050804) [DOI] [PubMed] [Google Scholar]
- 42.Orban GA, Claeys K, Nelissen K, Smans R, Sunaert S, Todd JT, Wardak C, Durand J-B, Vanduffel W. 2006. Mapping the parietal cortex of human and non-human primates. Neuropsychologia 44, 2647–2667. ( 10.1016/j.neuropsychologia.2005.11.001) [DOI] [PubMed] [Google Scholar]
- 43.Grefkes C, Fink GR. 2005. The functional organization of the intraparietal sulcus in humans and monkeys. J. Anat. 207, 3–17. ( 10.1111/j.1469-7580.2005.00426.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vanduffel W, Fize D, Peuskens H, Denys K, Sunaert S, Todd JT, Orban GA. 2002. Extracting 3D from motion: differences in human and monkey intraparietal cortex. Science 298, 413–415. ( 10.1126/science.1073574) [DOI] [PubMed] [Google Scholar]
- 45.Durand J-B, Nelissen K, Joly O, Wardak C, Todd JT, Norman JF, Janssen P, Vanduffel W, Orban GA. 2007. Anterior regions of monkey parietal cortex process visual 3D shape. Neuron 55, 493–505. ( 10.1016/j.neuron.2007.06.040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Durand J-B, Peeters R, Norman JF, Todd JT, Orban GA. 2009. Parietal regions processing visual 3D shape extracted from disparity. NeuroImage 46, 1114–1126. ( 10.1016/j.neuroimage.2009.03.023) [DOI] [PubMed] [Google Scholar]
- 47.Van Dromme ICL, Vanduffel W, Janssen P. 2015. The relation between functional magnetic resonance imaging activations and single-cell selectivity in the macaque intraparietal sulcus. NeuroImage 113, 86–100. ( 10.1016/j.neuroimage.2015.03.023) [DOI] [PubMed] [Google Scholar]
- 48.Borra E, Belmalih A, Calzavara R, Gerbella M, Murata A, Rozzi S, Luppino G. 2008. Cortical connections of the macaque anterior intraparietal (AIP) area. Cereb. Cortex 18, 1094–1111. ( 10.1093/cercor/bhm146) [DOI] [PubMed] [Google Scholar]
- 49.Murata A, Gallese V, Luppino G, Kaseda M, Sakata H. 2000. Selectivity for the shape, size, and orientation of objects for grasping in neurons of monkey parietal area AIP. J. Neurophysiol. 83, 2580–2601. [DOI] [PubMed] [Google Scholar]
- 50.Nelissen K, Borra E, Gerbella M, Rozzi S, Luppino G, Vanduffel W, Rizzolatti G, Orban GA. 2011. Action observation circuits in the macaque monkey cortex. J. Neurosci. 31, 3743–3756. ( 10.1523/JNEUROSCI.4803-10.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pani P, Theys T, Romero MC, Janssen P. 2014. Grasping execution and grasping observation activity of single neurons in the macaque anterior intraparietal area. J. Cogn. Neurosci. 26, 2342–2355. ( 10.1162/jocn_a_00647) [DOI] [PubMed] [Google Scholar]
- 52.Maeda K, Ishida H, Nakajima K, Inase M, Murata A. 2015. Functional properties of parietal hand manipulation-related neurons and mirror neurons responding to vision of own hand action. J. Cogn. Neurosci. 27, 560–572. ( 10.1162/jocn_a_00742) [DOI] [PubMed] [Google Scholar]
- 53.Abdollahi RO, Jastorff J, Orban GA. 2013. Common and segregated processing of observed actions in human SPL. Cereb. Cortex 23, 2734–2753. ( 10.1093/cercor/bhs264) [DOI] [PubMed] [Google Scholar]
- 54.Ferri S, Rizzolatti G, Orban GA. 2015. The organization of the posterior parietal cortex devoted to upper limb actions: an fMRI study. Hum. Brain Mapp. 36, 3845–3866. ( 10.1002/hbm.22882) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Konen CS, Mruczek REB, Montoya JL, Kastner S. 2013. Functional organization of human posterior parietal cortex: grasping- and reaching-related activations relative to topographically organized cortex. J. Neurophysiol. 109, 2897–2908. ( 10.1152/jn.00657.2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harvey BM, Klein BP, Petridou N, Dumoulin SO. 2013. Topographic representation of numerosity in the human parietal cortex. Science 341, 1123–1126. ( 10.1126/science.1239052) [DOI] [PubMed] [Google Scholar]
- 57.Harvey BM, Fracasso A, Petridou N, Dumoulin SO. 2015. Topographic representations of object size and relationships with numerosity reveal generalized quantity processing in human parietal cortex. Proc. Natl Acad. Sci. USA 112, 13 525–13 530. ( 10.1073/pnas.1515414112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holt DJ, Cassidy BS, Yue X, Rauch SL, Boeke EA, Nasr S, Tootell RBH, Coombs G. 2014. Neural correlates of personal space intrusion. J. Neurosci. 34, 4123–4134. ( 10.1523/JNEUROSCI.0686-13.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gentile G, Petkova VI, Ehrsson HH. 2011. Integration of visual and tactile signals from the hand in the human brain: an FMRI study. J. Neurophysiol. 105, 910–922. ( 10.1152/jn.00840.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mantini D, Corbetta M, Romani GL, Orban GA, Vanduffel W. 2013. Evolutionarily novel functional networks in the human brain? J. Neurosci. 33, 3259–3275. ( 10.1523/JNEUROSCI.4392-12.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peeters R, Simone L, Nelissen K, Fabbri-Destro M, Vanduffel W, Rizzolatti G, Orban GA. 2009. The representation of tool use in humans and monkeys: common and uniquely human features. J. Neurosci. 29, 11 523–11 539. ( 10.1523/JNEUROSCI.2040-09.2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peeters RR, Rizzolatti G, Orban GA. 2013. Functional properties of the left parietal tool use region. NeuroImage 78, 83–93. ( 10.1016/j.neuroimage.2013.04.023) [DOI] [PubMed] [Google Scholar]
- 63.Brandi M-L, Wohlschläger A, Sorg C, Hermsdörfer J. 2014. The neural correlates of planning and executing actual tool use. J. Neurosci. 34, 13 183–13 194. ( 10.1523/JNEUROSCI.0597-14.2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ishida H, Nakajima K, Inase M, Murata A. 2010. Shared mapping of own and others’ bodies in visuotactile bimodal area of monkey parietal cortex. J. Cogn. Neurosci. 22, 83–96. ( 10.1162/jocn.2009.21185) [DOI] [PubMed] [Google Scholar]
- 65.Nieder A, Miller EK. 2004. A parieto-frontal network for visual numerical information in the monkey. Proc. Natl Acad. Sci. USA 101, 7457–7462. ( 10.1073/pnas.0402239101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tudusciuc O, Nieder A. 2007. Neuronal population coding of continuous and discrete quantity in the primate posterior parietal cortex. Proc. Natl Acad. Sci. USA 104, 14 513–15 518. ( 10.1073/pnas.0705495104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Abdollahi RO, Kolster H, Glasser MF, Robinson EC, Coalson TS, Dierker D, Jenkinson M, Van Essen DC, Orban GA. 2014. Correspondences between retinotopic areas and myelin maps in human visual cortex. NeuroImage 99, 509–524. ( 10.1016/j.neuroimage.2014.06.042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lewis JW, Van Essen DC. 2000. Mapping of architectonic subdivisions in the macaque monkey, with emphasis on parieto-occipital cortex. J. Comp. Neurol. 428, 79–111. () [DOI] [PubMed] [Google Scholar]
- 69.Arcaro MJ, Pinsk MA, Li X, Kastner S. 2011. Visuotopic organization of macaque posterior parietal cortex: a functional magnetic resonance imaging study. J. Neurosci. 31, 2064–2078. ( 10.1523/JNEUROSCI.3334-10.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Press WA, Brewer AA, Dougherty RF, Wade AR, Wandell BA. 2001. Visual areas and spatial summation in human visual cortex. Vis. Res. 41, 1321–1332. ( 10.1016/S0042-6989(01)00074-8) [DOI] [PubMed] [Google Scholar]
- 71.Kolster H, Peeters R, Orban GA. 2011. Ten retinotopically organized areas in human parietal cortex. In Program 851.10 Neuroscience meeting planner. Society for Neuroscience: Washington, DC.
- 72.Tsao DY, et al. 2003. Stereopsis activates V3A and caudal intraparietal areas in macaques and humans. Neuron 39, 555–568. ( 10.1016/S0896-6273(03)00459-8) [DOI] [PubMed] [Google Scholar]
- 73.Eickhoff SB, Schleicher A, Zilles K, Amunts K. 2006. The human parietal operculum. I. Cytoarchitectonic mapping of subdivisions. Cereb. Cortex 16, 254–267. ( 10.1093/cercor/bhi105) [DOI] [PubMed] [Google Scholar]
- 74.Caspers S, Geyer S, Schleicher A, Mohlberg H, Amunts K, Zilles K. 2006. The human inferior parietal cortex: cytoarchitectonic parcellation and interindividual variability. NeuroImage 33, 430–448. ( 10.1016/j.neuroimage.2006.06.054) [DOI] [PubMed] [Google Scholar]
- 75.Eickhoff SB, Grefkes C, Zilles K, Fink GR. 2007. The somatotopic organization of cytoarchitectonic areas on the human parietal operculum. Cereb. Cortex 17, 1800–1811. ( 10.1093/cercor/bhl090) [DOI] [PubMed] [Google Scholar]
- 76.Avanzini P, et al. In press. Four dimensional maps of the human somatosensory system. Proc. Natl Acad. Sci. USA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Coq JO, Qi H, Collins CE, Kaas JH. 2004. Anatomical and functional organization of somatosensory areas of the lateral fissure of the New World titi monkey (Callicebus moloch). J. Comp. Neurol. 476, 363–387. ( 10.1002/cne.20237) [DOI] [PubMed] [Google Scholar]
- 78.Disbrow E, Litinas E, Recanzone GH, Padberg J, Krubitzer L. 2003. Cortical connections of the second somatosensory area and the parietal ventral area in macaque monkeys. J. Comp. Neurol. 462, 382–399. ( 10.1002/cne.10731) [DOI] [PubMed] [Google Scholar]
- 79.Van Essen DC, Dierker DL. 2007. Surface-based and probabilistic atlases of primate cerebral cortex. Neuron 56, 209–225. ( 10.1016/j.neuron.2007.10.015) [DOI] [PubMed] [Google Scholar]
- 80.Nieder A, Dehaene S. 2009. Representation of number in the brain. Annu. Rev. Neurosci. 32, 185–208. ( 10.1146/annurev.neuro.051508.135550) [DOI] [PubMed] [Google Scholar]
- 81.Osiurak F, Jarry C, Le Gall D. 2010. Grasping the affordances, understanding the reasoning: toward a dialectical theory of human tool use. Psychol. Rev. 117, 517–540. ( 10.1037/a0019004) [DOI] [PubMed] [Google Scholar]
- 82.Orban GA, Caruana F. 2014. The neural basis of human tool use. Front. Psychol. 5, 310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yonelinas AP, Otten LJ, Shaw KN, Rugg MD. 2005. Separating the brain regions involved in recollection and familiarity in recognition memory. J. Neurosci. 25, 3002–3008. ( 10.1523/JNEUROSCI.5295-04.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Filimon F, Nelson JD, Hagler DJ, Sereno MI. 2007. Human cortical representations for reaching: mirror neurons for execution, observation, and imagery. NeuroImage 37, 1315–1328. ( 10.1016/j.neuroimage.2007.06.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Reep RL, Chandler HC, King V, Corwin JV. 1994. Rat posterior parietal cortex: topography of corticocortical and thalamic connections. Exp. Brain Res. 100, 67–84. ( 10.1016/S0079-6123(08)60770-0) [DOI] [PubMed] [Google Scholar]
- 86.Kravitz DJ, Saleem KS, Baker CI, Mishkin M. 2011. A new neural framework for visuospatial processing. Nat. Rev. Neurosci. 12, 217–230. ( 10.1038/nrn3008) [DOI] [PMC free article] [PubMed] [Google Scholar]