Abstract
Elevated carbon dioxide levels and the resultant ocean acidification (OA) are changing the abiotic conditions of the oceans at a greater rate than ever before and placing pressure on marine species. Understanding the response of marine fauna to this change is critical for understanding the effects of OA. Population-level variation in OA tolerance is highly relevant and important in the determination of ecosystem resilience and persistence, but has received little focus to date. In this study, whether OA has the same biological consequences in high-salinity-acclimated population versus a low-salinity-acclimated population of the same species was investigated in the marine isopod Idotea balthica. The populations were found to have physiologically different responses to OA. While survival rate was similar between the two study populations at a future CO2 level of 1000 ppm, and both populations showed increased oxidative stress, the metabolic rate and osmoregulatory activity differed significantly between the two populations. The results of this study demonstrate that the physiological response to OA of populations from different salinities can vary. Population-level variation and the environment provenance of individuals used in OA experiments should be taken into account for the evaluation and prediction of climate change effects.
Keywords: climate change, CO2, Idotea balthica, macrophysiology, pH, physiology
1. Introduction
The Earth is currently undergoing a period of rapid environmental change. In the marine environment, this change is most notable as increasing temperature and of ocean acidification (OA) resulting from anthropogenic carbon dioxide (CO2) emissions [1]. With atmospheric CO2 predicted to increase significantly by the end of this century, the increased CO2 is absorbed into seawater, which then creates a shift in carbonate chemistry, and a resultant reduction in pH. A now conservative estimate places atmospheric CO2 at 1000 ppm by the year 2100, and this will cause a reduction in seawater pH to an average of 7.7, which corresponds to a 150% increase in acidity compared with today's oceans. The biological effects of OA have been demonstrated to be broad [2] and varied [3,4]. To complicate matters further, marine organisms are facing an environment that is changing on many fronts, not just acidity. In addition to increasing temperature, there will also be localized effects of climate change including changes in salinity and turbidity, particularly in coastal regions more affected by land run off and thus rain patterns [5].
The marine isopod Idotea balthica is an abundant grazer in the shallow sublittoral macroalgal belts; as the primary grazer of Fucus vesiculosus, this isopod has a controlling influence on the macroalgae community and is ecosystem engineer within this habitat. Idotea balthica occurs on both sides of the Atlantic ocean, throughout the waters of Northern Europe and also within the Baltic sea down to salinities as low as 4 practical salinity units (PSU) around Öregrund on the Swedish coast [6]. Idotea balthica is one of very few marine invertebrates to be found at such permanently low salinities in the Baltic sea. The Baltic sea is a marine system with a pronounced spatial gradient in salinity. The salinity gradient of the Baltic sea characterizes the fauna that is found there; osmotic stress is a limiting factor in colonization for many marine species, with some entire groups (e.g. echinoderms) missing from the Baltic ecosystem as a result.
The low salinity of the Baltic sea results in lower alkalinity, and lower pH compared with fully saline seawater at the same pCO2 levels [7]. Further, lowered salinity also reduces total inorganic carbon [7]. As such, the chemical changes in seawater as a result of reduced salinity overlap with the changes from OA (increased pCO2). There is potential for both lowered pH and salinity to impact upon an organism's ion regulation, acid–base regulation and metabolism [8–10], and have interactive effects [11]. Both lowered pH (OA) and salinity require greater internal regulation, be it ionic or osmotic; these processes are energetically expensive and as such both of these stressors can result in secondary effects of reduced energy availability. Increased energy for osmoregulation under suboptimal salinity conditions is well documented [12,13]; indeed, increased enzymatic activity was seen in the pleopods of I. balthica in diluted seawater [14]. Indirect evidence of the principle of knock-on effects to the energetic budget under OA was seen as muscle wastage in the brittlestar Amphiura filiformis [15], whereas the combination of low salinity and pH resulted in lower lipid and glycogen stores in juvenile Crassostrea virginica [11]. There is significant overlap in physiological processes affected by lowered pH and salinity, and thus the potential of synergism in effects, particularly as changing weather patterns predict greater rainfall and thus salinity changes of near shore waters [16]. Given the likelihood of occurrence and effect, further research into these combined stressors is still required.
The I. balthica populations at low salinities may have undergone local adaptation within the Baltic and be more capable of coping with the similar physiological challenges (ion regulation, high energetic requirement for homeostasis) presented by OA. Alternatively, the environmental stress of the low-salinity environment may mean further stress (and energetic demands) of physiological regulation under OA, which may cause the combined effect of low salinity and pH to be unsustainable.
This study aims to address the question of whether high-salinity-acclimated populations and low-salinity-acclimated populations of the same species respond in the same way to OA. We do this by comparing the responses of organisms from the Baltic sea (brackish environment) and the North sea (fully marine environment) exposed to CO2 levels expected by the end of the century.
2. Material and methods
(a). Field collection
The Baltic population of I. balthica was collected from Öregrund (salinity (S): 5, temperature (T): 12.8°C). This site was selected to represent I. balthica from the Baltic sea based on the results of a previous study [17]. Isopods were collected by hand from a depth of 0.5–1 m by gathering F. vesiculosus plants at the site. Each algal frond was searched, and I. balthica were placed into a separate container of fresh F. vesiculosus free from other fauna; the macroalgae was used as both refuge and to keep the isopods moist. Wet algae also helped in maintaining stable temperature during transport. Containers with animals and macroalgae were transported back to the laboratory in temperature-regulated containers within 7 h of collection. The marine (fully saline) population were taken from the Biological Institute Helgoland (BAH; S: 32.3, T: 13°C), collected from the shores of Helgoland Island, Germany in the North Sea. The animals were kept at Helgoland in a large flow-through system with fresh seawater and live F. vesiculosus, and regularly supplemented with newly caught individuals. The marine I. balthica (together with fresh F. vesiculosus free from other fauna) were placed in a ventilated cool box with an electric cooling unit, and transported to the laboratory within 24 h. Neither population had observed mortalities during transport. Both populations originate from similar habitats of shallow sublittoral waters dominated by F. vesiculosus. Following arrival at The Sven Lovén Centre for Marine Science-Kristineberg (58°14′58.5″ N 11°26′40.4″ E), both populations were maintained in the laboratory where they were divided between two pre-prepared 75 l containers (per population) of aerated water of field site salinity (based on seasonal average data from the Swedish Meteorological and Hydrological Institute for Öregrund, (S: 5), and BAH records for Helgoland, (S: 32). The water temperature was controlled to 13°C, and growing F. vesiculosus was added as food and shelter. Animals were kept for a period of 21 days to acclimate prior to being placed in the experimental system.
(b). Experimental set-up
Pre-existing genetic information indicating the two chosen populations were genetically distinct [18], and previously published physiological data demonstrated that the Baltic population chosen for this study represented the physiology of populations along the span of the Baltic distribution [17]. As such, each population was considered to reliably represent either Baltic or Marine I. balthica. Isopods from each population (Baltic and Marine) measuring between 11 and 16 mm were weighed and measured before being randomly allocated to a CO2 treatment of either 400 (control) or 1000 ppm. Twenty replicate individuals were used in each treatment. In this size range, the sex ratio was approximately 50 : 50, and no brooding females were used. Each isopod was kept individually in a 250 ml container with two 5 × 4 cm panels (2 mm mesh size) to allow water exchange and then placed in 5 l aquaria (five pots per aquaria and four aquaria per treatment).
Fucus vesiculosus was used as the primary food source, and owing to the potential for the F. vesiculosus to react differently to the salinity treatments; the algae (apical parts) were processed into an artificial food matrix with agar (see [7] for full methodology), which created comparable wet-to-dry weight ratio (ww : dw) to the live macroalgae. Approximately 1 g (ww) of artificial Fucus food was placed in each pot with the isopod for ad libitum feeding. Food was changed twice weekly, and water was changed weekly for the course of the 63 day (nine week) experimental exposure.
Both populations were kept in water of the same salinity as their origin: 32 PSU for the marine population and 5 PSU for the Baltic population. Five PSU was achieved by mixing deep seawater (approx. 32 PSU) with tap water and bubbling with air as a precaution to remove any traces of chlorine before it was used for the animal containers, a methodology shown to be effective in salinity manipulation [17].
All isopods were within the experimental set-up for a period of one week prior to the start of the experimental exposure. Aquaria were maintained at 13°C in a constant temperature room with continuous aeration. Acidification was achieved by manipulating air CO2 levels, owing to the effect that salinity has on seawater pH. Thus, by choosing atmospheric CO2 levels predicted for 2100, both treatments received seawater conditions as will be experienced, even though this results in different pH. The control group (CO2 : 400 ppm) were aerated with unaltered air. In the high CO2 group, the CO2 level was gradually increased over one week to achieve the CO2 level required (1000 ppm) prior to the start of the experiment. CO2 adjustment was achieved by adding CO2 to air according to the methodology described in Findlay et al. [19], and monitored using a LICOR gas analyser. CO2 levels in the air supplying the control treatment were also periodically measured using the LICOR gas analyser. Following acclimation, the experiment ran for nine weeks, at which time a steady physiological state response was expected. Water parameters were monitored weekly in randomly chosen aquaria within each treatment: temperature and pH (Mettler Toledo LE413), salinity (WTW LF197) and CO2 (LICOR LI-820).
(c). Survival and growth
Growth in I. balthica is stochastic and related to moulting events. Length is thus not considered a reliable measurement of growth and as such animal weight was used to record growth. Each individual was measured at the start and end of the experiment. Blotted wet weight was measured on a Mettler Toledo AT200 balance accurate to 0.1 mg. Any mortalities were recorded daily and such animals removed.
(d). Food consumption
Food consumption was measured for each individual in week three of the exposure. Food sticks were weighed before and after feeding, and the weight difference was corrected for water uptake (measured using controls in each salinity treatment). Consumption was calculated as percentage of each animal's bodyweight.
(e). Metabolic rate
Metabolic rate (MR) was measured by oxygen consumption as a proxy, using closed bottle respirometry. After the 63 day exposure, individuals were placed in a 120 ml glass titration bottle with oxygen-saturated filtered water of the same salinity as the experimental exposure. Bottles were sealed with a glass stopper and placed in a waterbath (13°C) in darkness for 60 min. The bottles were gently turned every 15 min to mix the water within the bottle and avoid an oxygen gradient build up. Start and end oxygen values for each bottle were measured with a Unisense OXY-meter with a Clark-type oxygen probe (Unisense OX 100 microsensor). Blank chambers were run with each set of incubations to measure background respiration. Oxygen consumption was calculated as the difference between the start and end oxygen values, corrected for background respiration salinity and atmospheric pressure (using standard correction factors), and standardized per gram of animal (wet weight). Many crustaceans are known to depress their metabolism at lower oxygen availability (nearing 50%: [20,21]). As the limits for oxygen conformation are not available for I. balthica, assay design ensured oxygen did not drop below 90% saturation in any chamber to ensure measurement of actual MR. All individuals were starved for 24 h prior to respiration measurements being taken.
(f). Oxidative stress
Oxidative stress, measured as amount of protein carbonyls, was evaluated in abdomen (to exclude stomach content and head) tissue samples of each I. balthica. Samples were quick-frozen in dry ice and stored at –80°C at the end of the experimental treatment prior to processing in the laboratory. Oxidative stress (nmol protein carbonyls mg protein−1) was measured via a reaction with 2,4-dinitrophenylhydrazine (DNPH), followed by trichloroacetic acid (TCA) precipitation, as previously described [22–24]. The samples were homogenized in ice-cold 50 mM phosphate buffer (pH 7.4), containing 0.1% (w/v) digitonin and protease inhibitor cocktail (Sigma-Aldrich P8340). The homogenates were centrifuged at 11 000g for 20 min at 4°C. Supernatant samples containing 1 mg protein, measured using the BCA Protein Assay Reagent kit (Pierce, Thermo-Scientific), were incubated with 10 mM DNPH in 2 M HCl for 2 h and then precipitated with TCA on ice before being centrifuged at 11 000g for 10 min at 4°C. Pellets were washed three times with ethanol–ethyl acetate (1 : 1) followed by centrifugation. After re-solubilizing the pellets in guanidine hydrochloride solution at pH 2.3 by incubation at 37°C, absorbance was read at 360 nm using a plate reader. Negative controls not subjected to DNPH were run in parallel, and these values were subtracted from the DNPH-treated samples. Data were expressed as nmol protein carbonyls mg protein−1 using the molar absorption coefficient of 22 000 M−1 cm−1 for DNPH derivatives. Reagents not further specified were from Sigma-Aldrich (St Louis, MO).
(g). Osmoregulatory activity
Osmoregulatory activity was measured on pleopod tissue, the primary site of osmoregulation in I. balthica previously shown to exhibit salinity-dependent changes in Na+–K+–ATPase activity [24]. In this tissue, Na+–K+–ATPase (NKA) activity was measured using a kinetic microassay validated and optimized for I. balthica from McCormick [25]. At the end of the experimental period, all pleopods were dissected out using microscissors, rinsed in SEI buffer (150 mM sucrose, 10 mM Na2EDTA, 50 mM imidazole), frozen immediately on dry-ice and stored at −80°C until analysis. The pleopod samples were thawed approximately 5 min prior to assay. The buffer was removed by centrifugation and replaced by 200 µl ice-cold SEI-buffer with 0.1% Na–deoxycholate, and the samples were homogenized in a glass–glass homogenizer. The homogenates were centrifuged at 3000g for 30 s, the supernatants removed and assayed for NKA activity [25]. The protein content of the supernatants was determined by the BCA Protein Assay Kit (Pierce, Thermo-Scientific).
(h). Statistical analysis
All data were analysed using the statistical programme Primer 6 with the PERMANOVA+ add-on. PERMANOVA analysis provides an ANOVA analysis without the limitations of a normally distributed dataset. This provides the advantage of having no transformation requirements if data do not fit a normal distribution, and allows the same statistical methodology to be applied to all data. Data were analysed with a 95% significance threshold, and significant interactions were investigated by conducting pairwise tests within Primer. Where relevant, measures were standardized per gram of animals to correct from differences in body size; where this is the case, it is stated in the measurement unit of that graph and statistical data. For ease of reference all statistical analyses refer to ‘CO2’ as a treatment factor that represents the altered CO2 treatment.
3. Results
(a). Experimental conditions
Salinity, CO2 and temperature remained stable for the course of the experiment (table 1).
Table 1.
Water parameters over course of experiment. (Values shown are mean ± 95% CIs. Temperature, salinity, pH (NBS scale), and CO2 and were measured and the remaining parameters (total alkalinity (TA), CO2 (CO2), bicarbonate (HCO3) and carbonate (CO3)) calculated using CO2sys [26] using constants from Mehrbach et al. [27] refitted by Dickson & Millero [28] and KHSO4 values from Dickson [29].)
| treatment S and CO2 | T (°C) | salinity | pH | CO2 (ppm) | TA (μmol per kgSW) | tCO2 (μmol per kgSW) | HCO3 (μmol per kgS W) | CO3 (μmol per kgSW) | |
|---|---|---|---|---|---|---|---|---|---|
| 32 | present | 13.0 ± 0.2 | 32.7 ± 0.2 | 8.13 ± 0.01 | 390 ± 0 | 2036 ± 44 | 1870 ± 38 | 1737 ± 34 | 117 ± 5 |
| 5 | present | 12.8 ± 0.1 | 5.3 ± 0.1 | 8.01 ± 0.01 | 390 ± 0 | 955 ± 7 | 950 ± 7 | 913 ± 6 | 18 ± 0.4 |
| 32 | future | 12.9 ± 0.1 | 32.7 ± 0.2 | 7.78 ± 0.01 | 1034 ± 24 | 2233 ± 27 | 2180 ± 27 | 2076 ± 25 | 63 ± 2 |
| 5 | future | 12.7 ± 0.1 | 5.1 ± 0.1 | 7.68 ± 0.02 | 1034 ± 24 | 1246 ± 23 | 1246 ± 23 | 1186 ± 23 | 11 ± 1 |
(b). Survival
Survival was not significantly different between populations (70%), whereas there was a significant (p = 0.0015, F = 11.19) reduction in survival as a result of high CO2. Over the nine weeks experimental exposure survival was reduced by 50% in the high CO2 treatment compared with the control in both populations.
(c). Metabolic rate
There was a significant interaction between CO2 treatment and population on the MR (table 2). Both populations had comparable MR at control CO2 (400 ppm). At 1000 ppm, the Marine population exhibited metabolic depression, whereas the Baltic population demonstrated metabolic upregulation (figure 1).
Table 2.
PERMANOVA analysis of metabolic rate (MR), oxidative stress (ox. stress) and NaKATPase activity for the factors of pH (CO2) and population (pop).
| test | source | d.f. | SS | MS | pseudo-F | p(perm) | perms |
|---|---|---|---|---|---|---|---|
| MR | pop. | 1 | 2507 | 2507 | 40 | 0.0001 | 9939 |
| CO2 | 1 | 286 | 286 | 5 | 0.0311 | 9944 | |
| pop. × CO2 | 1 | 3756 | 3756 | 59 | 0.0001 | 9947 | |
| res. | 32 | 2023 | 63 | ||||
| total | 35 | 7070 | |||||
| ox. stress | pop. | 1 | 31 | 31 | 0 | 0.835 | 999 |
| CO2 | 1 | 809 | 809 | 4 | 0.029 | 998 | |
| pop. × CO2 | 1 | 380 | 380 | 2 | 0.175 | 998 | |
| res. | 37 | 7232 | 195 | ||||
| total | 40 | 8413 | |||||
| NaKATPase | pop. | 1 | 2073 | 2073 | 11.1 | 0.0013 | 9954 |
| CO2 | 1 | 518 | 518 | 2.8 | 0.0841 | 9940 | |
| pop × CO2 | 1 | 686 | 686 | 3.7 | 0.0452 | 9956 | |
| res. | 28 | 5236 | 187 | ||||
| total | 31 | 9054 |
Figure 1.
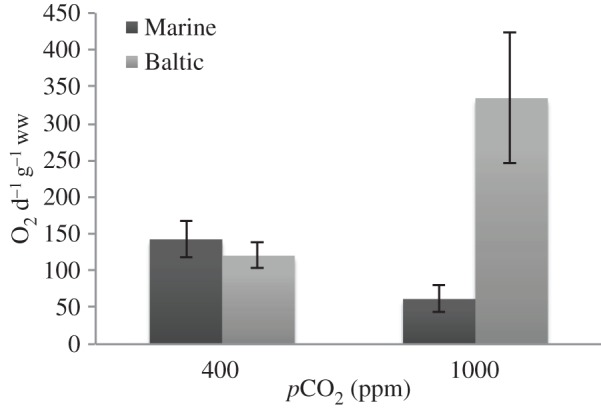
Mean metabolic rate, measured as oxygen consumption per day, per gram of Idotea balthica. Data shown for two populations of I. balthica at two CO2 levels: control (400 ppm) and elevated (1000 ppm). Error bars show 95% CIs.
(d). Food consumption
Food consumption was significantly different between populations (p = 0 0.027) with the Baltic population consuming more food than the Marine population; however, the combined analysis showed no effect of the increased CO2 treatment on this relationship.
(e). Growth
All individuals gained weight over the course of the experiment. There was no significant effect of either population or CO2 treatment. There was a large amount of within treatment variation that made analysis of these results difficult. An effect of population or CO2 treatment cannot be ruled out based on this data.
(f). Oxidative stress
Oxidative stress was significantly higher in the 1000 ppm CO2 treatment than the control (table 2 and figure 2) in both Marine and Baltic populations, which were not significantly different from each other.
Figure 2.
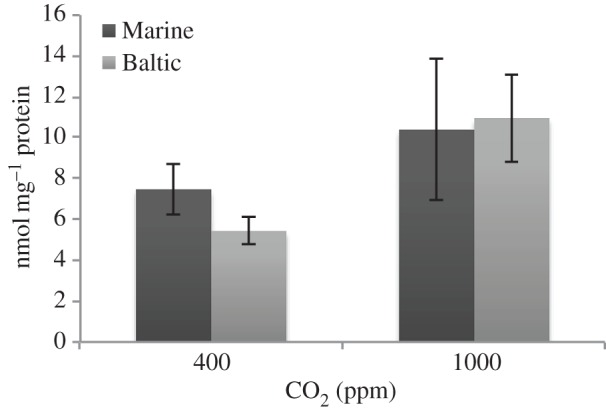
Oxidative stress, measured as protein carbonyls, of two populations of I. balthica at two CO2 levels: control (400 ppm) and elevated (1000 ppm). Error bars show 95% CIs.
(g). Osmoregulatory potential
NKA activity showed a significant interaction between population and CO2 treatment (table 2). As expected owing to the greater osmotic pressure of water of 5 PSU, the Baltic population showed a significantly greater NKA activity at control CO2 (400 ppm) compared with the Marine population from 32 PSU (post hoc pairwise tests, p = 0.0001). However, the osmoregulatory activity of the Baltic population decreases in the high CO2 (1000 ppm) treatment (figure 3), whereas the Marine population remains unaffected by the high CO2 treatment.
Figure 3.
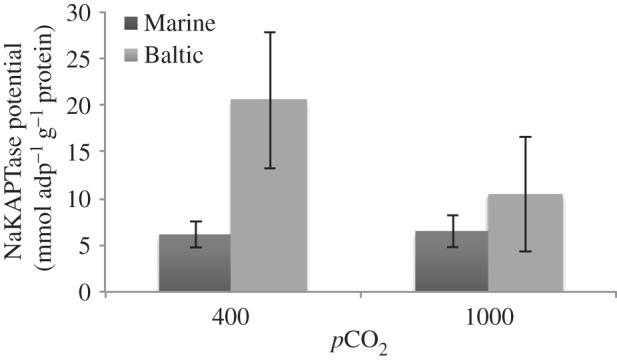
Osmoregulatory potential of two populations of Idotea balthica under control (400 ppm) and elevated (1000 ppm) levels of carbon dioxide. Error bars show 95% CIs.
4. Discussion
This study demonstrates that different populations within the same species may respond very differently to OA. While survival rate was not different between the two populations, the physiological changes that occurred were different between the populations, specifically regarding ion regulation and MR.
The question of how different populations of the same species respond to OA is a pertinent one. The marine environment is influenced by many abiotic factors, and species are often spread over a large geographical and abiotic range. Thus, populations within a species will experience OA under differing base abiotic conditions; therefore, how these different populations and base conditions affect a species response to OA is essential to our overall understanding. This concept is encapsulated within the field of macrophysiology, which is the study of variability in physiological tolerance over geographical and temporal ranges [30]. Macrophysiology in the marine realm is an effective and important approach in understanding large-scale issues including species' responses to climate change [30–32]. This has been effectively demonstrated in a calanoid copepod where populations most vulnerable to global climate change were identified through analysis of population-level physiology [32].
(a). Macrophysiological approach to ocean acidification studies
The application of a macrophysiology approach of population-level comparisons with OA studies has to date been limited. Despite the recognized potential and importance of inter-population variation in understanding how a species will respond to this climate change stressor [31,32], there are few published invertebrate studies on this topic at the time of this publication (but see [33–37] as good examples). Parker et al. [33] demonstrated differences in shell growth reduction between populations of the Sydney rock oyster Saccostrea glomerata. Wood et al. [37] found reduced different immunocompetence levels in I. balthica, but a cross population effect by OA of reduced immunocompetence and increase oxidative stress. Lardies et al. [34] found a significant pCO2 × population interaction for heat shock protein expression and MR in an intertidal snail. The findings in this study of significantly different physiological responses to OA are consistent with these few studies published to date. Further, Lardies et al. [34] showed increased MR, attributed to the higher cost of homeostasis, which is consistent with the results in this study for the low-salinity Baltic population.
(b). Population differences in metabolism and ion exchange under ocean acidification
Changes to MR are commonly seen in OA studies [34,38,39]. The increased energy required for ion regulation to achieve homeostasis in lowered pH OA water has been suggested as a cause of such metabolic upregulation, and this theory is supported by evidence of an energy deficit under OA conditions [14,39]. In this study, it has been demonstrated that despite a similar survival rate between two geographically and abiotically distant populations of the marine isopod I. balthica, the metabolic response of the two populations was quite different, with upregulation in the low-salinity population (‘Baltic’) in response to near future OA (1000 ppm atmospheric CO2) conditions, and in contrast, metabolic depression in the other (Marine) population. Metabolic depression has previously been described as a response to OA by Langenbuch & Pörtner [40] in a sipunculid worm. Such depression is typically considered a short-term response to OA, although it has also been reported in longer-term studies on other species, and as such the long-term viability of the marine population, demonstrating metabolic depression, remains unclear. It is interesting that this same population also showed no change in NKA activity, despite a large decrease in this same activity in the other population. What this means in terms of the whole organism response is as yet unclear. By contrast, a reduction in NKA activity is seen in the Baltic population (lower salinity). The mechanism at the pleopod levels is to absorb Na+ and Cl–, and for Na+ this absorption is probably driven by Na+-channels in electrochemical connection to H+-ATPases, as such an increase in H+-pumping owing to increased H+-load could therefore lead to the observed decrease in the NKA activity. These channels have been shown to be used in osmoregulation [41], thus our results provide evidence to put forward the hypothesis that this mechanism has a role in responding to the increased H+ associated with OA. The metabolic upregulation in the low-salinity population indicates a response to greater energetic demand under OA; potentially, a demonstration of the physiological plasticity that also allows survival in low salinity.
(c). Implications of intraspecies variation in ocean acidification response
The Baltic population of the isopod I. balthica in this study has demonstrated phenotypic plasticity in physiological activity under OA compared with little effect of OA on the physiology of marine counterparts. While plasticity is not in itself an evolutionary process, selective pressure may be on the trait of plasticity itself [42,43]. How a species responds to stress determines not only its immediate tolerance level, but also its potential to evolve increased tolerance over the long term. Given the rate of climate change, evolutionary adaptations may rely on standing (but potentially hidden or cryptic) genetic variation within species [44]. The variation in OA response between these two populations of I. balthica may be an example of such genetic variation that will be fundamental to the long-term survival of the species under OA. This same physiological plasticity may also be responsible for the success of one population in the low-salinity Baltic sea.
Making predictions of species prevalence with respect to climate change is a complicated issue, not least because: (i) environmental stressors such as OA as yet have little unified principles, thus relying on empirical data species by species, and (ii) environmental stressors will not occur in isolation, and interactions, synergies and antagonisms need to be considered. This study highlights the importance of considering intraspecies variability in the study of anthropogenic impacts. In addition, this study demonstrates that populations which appear to be under existing environmental stress—such as osmoregulatory stress—can be at least as tolerant of further environmental stress.
(d). Different home salinities: comparing apples and pears?
Salinity affects osmoregulatory ion regulation in I. balthica, which means that there are underlying differences in the energy budget of the two populations. The higher NKA activity shown in the Baltic population suggests that it has a higher cost for osmotic regulation than the Marine population and therefore, there is a basic difference in energy allocation in the organisms of the two populations. It is likely this influenced the physiological response to OA. Further, there is potential for the osmoregulatory ion exchange to be further affected by pH regulatory ion exchange in the OA treatment. However, maintaining the representative salinity condition from the location of each population in the treatments was essential in order to investigate how these populations respond to OA relative to their natural environment.
5. Conclusion
Species occur over large geographical and abiotic ranges, and this study clearly demonstrates that the physiological response of a species to OA cannot be understood or predicted without the incorporation of population-level variation and consideration of the environmental provenance of the test population(s).
Acknowledgements
This work was supported through The Centre for Marine Evolutionary Biology (CeMEB), a Linnaeus Centre of Excellence, with additional project funding from GRiP. Special thanks to L. Svanberg for assistance in the laboratory and Dr Bale for chemistry advice. Thanks also to the two anonymous reviewers whose suggestions and insights greatly improved a previous version of this manuscript. Data from this paper is available in Dryad.
Authors' contributions
S.E. participated in the design of the study and data analysis, and contributed to the drafting of the manuscript. K.S. designed the NKA protocol optimization, carried out NKLA laboratory work and contributed to data analysis and manuscript drafting. H.S. designed and contributed to laboratory work for oxidative stress, and helped with manuscript drafting. B.C.A. ran the oxidative stress laboratory work, contributed to data analysis and drafting of manuscript. H.L.W. conceived of the study, designed the study, drafted the manuscript, coordinated the study, ran data analysis and carried out the water, food consumption, growth, NKA, MR and survival laboratory work. All authors gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
We received no funding for this study.
References
- 1.Caldeira K, Wickett ME. 2003. Anthropogenic carbon and ocean pH. Nature 425, 365 ( 10.1038/425365a) [DOI] [PubMed] [Google Scholar]
- 2.McCauley DJ, Pinsky ML, Palumbi SR, Estes JA, Joyce FH, Warner RR. 2015. Marine defaunation: animal loss in the global ocean. Science 347, 1255641 ( 10.1126/science.1255641) [DOI] [PubMed] [Google Scholar]
- 3.Kroeker KJ, Kordas RL, Crim RN, Singh GG. 2010. Meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms. Ecol. Lett. 13, 1419–1434. ( 10.1111/j.1461-0248.2010.01518.x) [DOI] [PubMed] [Google Scholar]
- 4.Widdicombe S, Spicer JI. 2008. Predicting the impact of ocean acidification on benthic biodiversity: what can animal physiology tell us? J. Exp. Mar. Biol. Ecol. 366, 187–197. ( 10.1016/j.jembe.2008.07.024) [DOI] [Google Scholar]
- 5.IPCC. 2012. Managing the risks of extreme events and disasters to advance climate change adaptation. A special report of Working Groups I and II of the Intergovernmental Panel on Climate Change, pp. 231–290. New York, NY: Cambridge University Press. [Google Scholar]
- 6.Leidenberger S, Harding K, Jonsson P. 2012. Ecology and distribution of the isopod genus Idotea in the Baltic sea: key species in a changing environment. J. Crust. Biol. 32, 359–381. ( 10.1163/193724012X626485) [DOI] [Google Scholar]
- 7.Hoffmann AF, Middleburg JJ, Soetaert K, Meysman FJR. 2009. pH modelling in aquatic systems with time-variable acid-base dissociation constants applied to the turbid, tidal Scheldt estuary. Biogeosciences 6, 1539–1561. ( 10.5194/bg-6-1539-2009) [DOI] [Google Scholar]
- 8.Kinne O. 1971. Salinity: animals: invertebrates. In Marine ecology (ed. Kinne O.), vol. 1 pp. 821–996. New York, NY: Wiley. [Google Scholar]
- 9.Truchot JP. 1988. Problems of acid-base balance in rapidly changing intertidal environments. Am. Zool. 28, 55–64. ( 10.1093/icb/28.1.55) [DOI] [Google Scholar]
- 10.Lannig G, Eilers S, Pörtner HO, Sokolova IM, Bock C. 2010. Impact of ocean acidification on energy metabolism of oyster Crassostrea gigas: changes in metabolic pathways and thermal response. Mar. Drugs 8, 2318–2339. ( 10.3390/md8082318) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dickinson GH, Ivanina AV, Matoo OB, Pörtner HO, Lannig G, Bock C, Beniash E, Sokolova IM. 2012. Interactive effects of salinity and elevated CO2 levles on juvenile eastern oysters Crassostrea virginica. J. Exp. Biol. 215, 29–43. ( 10.1242/jeb.061481) [DOI] [PubMed] [Google Scholar]
- 12.Kinne O. 1964. Slinity and temperature combinations. Oceanogr. Mar. Biol. Annu. Rev. 2, 281–339. [Google Scholar]
- 13.Gyllenberg G, Lundqvist G. 1979. The effects of temperature and salinity on the oxygen consumption of Eurytemora hirundoides (Crustacea, Copepoda). Ann. Zool. Fenn. 16, 205–208. [Google Scholar]
- 14.Holliday CW. 1988. Branchial NA+/K + ATPase and osmoregulation in the isopod, Idotea wosnesenskii. J. Exp. Biol. 136, 259–272. [Google Scholar]
- 15.Wood HL, Spicer JI, Widdicombe S. 2008. Ocean acidification may increase calcification rates, but at a cost. Proc. R. Soc. B 275, 1767–1773. ( 10.1098/rspb.2008.0343) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.IPCC. 2012. Managing the risks of extreme events and disasters to advance climate change adaptation. In A special report of working groups I and II of the IPCC (eds Field GB et al.), p. 582. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 17.Wood HL, Nylund G, Eriksson SP. 2014. Physiological plasticity is key to the presence of the isopod Idotea baltica (Pallas) in the Baltic sea. J. Sea Res. 85, 255–262. ( 10.1016/j.seares.2013.05.009) [DOI] [Google Scholar]
- 18.Leidenberger S. 2013. Adaptation to the Baltic Sea: the case of isopod genus Idotea. Doctoral thesis, University of Gothenburg, Faculty of Science, Department of Biological and Environmental Sciences, Gothenburg, Sweden.
- 19.Findlay HS, Kendall MA, Spicer JI, Turley CM, Widdicombe S. 2008. Novel microcosm system for investigating the effects of elevated carbon dioxide and temperature on intertidal organisms. Aquat. Biol. 3, 51–62. ( 10.3354/ab00061) [DOI] [Google Scholar]
- 20.Wolvekamp HP, Waterman TH. 1960. Respiration. In Physiology of Crustacea. 1. Metabolism and growth (ed. Waterman TH.), pp. 35–100. New York, NY: Academic Press. [Google Scholar]
- 21.DeJours P, Beekenkamp H. 1977. Crayfish respiration as a function of water oxygenation. Respir. Physiol. 30, 241–251. ( 10.1016/0034-5687(77)90033-0) [DOI] [PubMed] [Google Scholar]
- 22.Reznick AZ, Packer L. 1994. Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Mol. Enzymol. 233, 357–363. ( 10.1016/S0076-6879(94)33041-7) [DOI] [PubMed] [Google Scholar]
- 23.Yan B, Dai Q, Liu X, Huang S, Wang Z. 1996. Flooding induced membrane damage, lipid oxidation and activated oxygen generation in corn leaves. Plant Soil 179, 261–268. ( 10.1007/BF00009336) [DOI] [Google Scholar]
- 24.Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman ER. 1990. Determination of carbonyl content in oxidatively modified proteins. Mol. Enzymol. 186, 464–478. ( 10.1016/0076-6879(90)86141-H) [DOI] [PubMed] [Google Scholar]
- 25.McCormick SD. 1993. Methods for nonlethal gill biopsy and measurement of Na+,K+-ATPase activity. Can. J. Fish Aquat. Sci. 50, 656–658. ( 10.1139/f93-075) [DOI] [Google Scholar]
- 26.Pierrot D, Lewis E, Wallace DWR. 2006. Co2sys Dos program developed for CO2 SYSTEMCALCULATIONS. ORNL/CDIAC-105. Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory US. Department of Energy, Oak Ridge, TN.
- 27.Mehrbach C, Culberson CH, Hawley JE, Pytkowicz RM. 1973. Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol. Oceanogr. 18, 897–907. ( 10.4319/lo.1973.18.6.0897) [DOI] [Google Scholar]
- 28.Dickson AG, Millero FJ. 1987. Comparison of the equilibrium constants for the dissociation of carbonic-acid in seawater media. Deep Sea Res. 34, 1733–1743. ( 10.1016/0198-0149(87)90021-5) [DOI] [Google Scholar]
- 29.Dickson AG. 1990. Standard potential of the reaction: AgCl(S) 1/2H– 2(g) = AG(S) + HCL(aq) and the standard acidity constant of the ion HSO4- in synthetic sea-water from 27 315-K to 31 815-K. J. Chem. Thermodyn. 22, 113–127. ( 10.1016/0021-9614(90)90074-Z) [DOI] [Google Scholar]
- 30.Gaston KJ, et al. 2011. Macrophysiology: a conceptual reunification. Am. Nat. 174, 595–612. ( 10.1086/605982) [DOI] [PubMed] [Google Scholar]
- 31.Chown SL, Gaston KJ. 2008. Macrophysiology for a changing world. Proc. R. Soc. B 275, 1469–1478. ( 10.1098/rspb.2008.0137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Osovitz CJ, Hofmann GE. 2007. Marine macrophysiology: studying physiological variation across large spatial scales in marine systems. Comp. Biochem. Physiol. A, Mol. Integr. Physiol. 147, 821–827. ( 10.1016/j.cbpa.2007.02.012) [DOI] [PubMed] [Google Scholar]
- 33.Parker LM, Ross PM, O'Connor WA. 2011. Populations of the Sydney rock oyster, Saccostrea glomerata, vary in response to ocean acidification. Mar. Biol. 158, 689–697. ( 10.1007/s00227-010-1592-4) [DOI] [Google Scholar]
- 34.Lardies MA, et al. 2014. Differential response to ocean acidification in physiological traits of Concholepas concholepas populations. J. Sea Res. 90, 127–134. ( 10.1016/j.seares.2014.03.010) [DOI] [Google Scholar]
- 35.Vargas CA, Aguilera VM, San Martin V, Manriquez PH, Navarro JM, Duarte C, Torres R, Lardies MA, Lagos NA. 2014. CO2-driven ocean acidification disrupts the filter feeding behaviour in Chilean gastropod and bivalve species from different geographic localities. Estuar. Coasts 37, 1–15. [Google Scholar]
- 36.Thor P, Oliva EO. 2015. Ocean acidification elicits different energetic responses in an arctic and a boreal population of the copepod Pseudocalanus acuspes. Mar. Biol. 16, 799–807. ( 10.1007/s00227-015-2625-9) [DOI] [Google Scholar]
- 37.Wood HL, Sköld HN, Eriksson SP. 2014. Health and population-dependent effects of ocean acidification on the marine isopod Idotea balthica. Mar. Biol. 161, 2423–2431. ( 10.1007/s00227-014-2518-3) [DOI] [Google Scholar]
- 38.Wood HL, Spicer JI, Lowe DM, Widdicombe S. 2010. Interaction of ocean acidification and temperature; the high cost of survival in the brittlestar Ophiura ophiura. Mar. Biol. 157, 2001–2013. ( 10.1007/s00227-010-1469-6) [DOI] [Google Scholar]
- 39.Harms L, Frickenhaus S, Schiffer M, Mark F, Storch D, Held C, Pörtner H-O, Lucassen M. 2014. Gene expression profiling in gills of the great spider crab Hyas araneus in response to ocean acidification and warming. BMC Genomics 15, 789 ( 10.1186/1471-2164-15-789) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Langenbuch M, Pörtner HO. 2004. High sensitivity to chronically elevated CO2 levels in a eurybathic marine sipunculid. Aquat. Toxicol. 70, 55–61. ( 10.1016/j.aquatox.2004.07.006) [DOI] [PubMed] [Google Scholar]
- 41.Hu M, Lee J-R, Lin L-Y, Shih T-H, Stumpp M, Lee M-F, Hwang P-P, Tseng Y-C. 2013. Development in a naturally acidified environment: Na+/H+-exchanger 3-based proton secretion leads to CO2 tolerance in cephalopod embryos. Front. Zool. 10, UNSP51. ( 10.1186/1742-9994-10-51) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baythavong BS, Stanton ML. 2010. Characterizing selection on phenotypic plasticity in response to natural environmental heterogeneity. Evolution 64, 2904–2920. ( 10.1111/j.1558-5646.2010.01057.x) [DOI] [PubMed] [Google Scholar]
- 43.de Jong G. 2004. Evolution of phenotypic plasticity: patterns of plasticity and the emergence of ecotypes. New Phycol. 166, 101–118. ( 10.1111/j.1469-8137.2005.01322.x) [DOI] [PubMed] [Google Scholar]
- 44.Runcie DE, Garfield DA, Babbitt CC, Wygoda JA, Mukherjee S, Wray GA. 2012. Genetics of gene expression responses to temperature stress in a sea urchin gene network. Mol. Ecol. 21, 4547–4562. ( 10.1111/j.1365-294X.2012.05717.x) [DOI] [PMC free article] [PubMed] [Google Scholar]


