Abstract
Learning and memory are crucial functions which enable insect pollinators to efficiently locate and extract floral rewards. Exposure to pesticides or infection by parasites may cause subtle but ecologically important changes in cognitive functions of pollinators. The potential interactive effects of these stressors on learning and memory have not yet been explored. Furthermore, sensitivity to stressors may differ between species, but few studies have compared responses in different species. Here, we show that chronic exposure to field-realistic levels of the neonicotinoid clothianidin impaired olfactory learning acquisition in honeybees, leading to potential impacts on colony fitness, but not in bumblebees. Infection by the microsporidian parasite Nosema ceranae slightly impaired learning in honeybees, but no interactive effects were observed. Nosema did not infect bumblebees (3% infection success). Nevertheless, Nosema-treated bumblebees had a slightly lower rate of learning than controls, but faster learning in combination with neonicotinoid exposure. This highlights the potential for complex interactive effects of stressors on learning. Our results underline that one cannot readily extrapolate findings from one bee species to others. This has important implications for regulatory risk assessments which generally use honeybees as a model for all bees.
Keywords: Apis mellifera, Bombus terrestris, clothianidin, Nosema ceranae, pollination, proboscis extension response
1. Introduction
Efficient learning and memory formation are essential for insect pollinators by enabling them to relocate floral resources, efficiently collect nectar and pollen, and navigate accurately when foraging in the field [1,2]. For social species such as honeybees and bumblebees, where colonies are dependent on food provided by forager bees, efficient learning, which thus enables efficient foraging, is an important prerequisite for colony growth and success [2,3]. Therefore, anthropogenic or environmental stress factors that may cause subtle changes in behaviour and cognitive abilities have the potential to incur significant negative impacts on pollinators (e.g. [4]).
Both neonicotinoid insecticides and parasites alter associative learning and cognitive functions of bees [5–9]. Acting as nicotinic acetylcholine receptor (nAChR) agonists [10], exposure to very low levels of neonicotinoids can disrupt normal function of neurons in the bee brain [11,12] and lead to disruptions in learning and memory [8,13]. Diseases and parasites disrupt bee behaviour and learning potentially via altering the immune system which is linked to the nervous system [14–16]. There is increasing evidence indicating that interactive effects particularly between insecticides and parasites/diseases may have detrimental impacts on bees (e.g. [17,18]). This is of major concern given that, due to anthropogenic influence, both wild and managed bees are increasingly exposed to sub-lethal concentrations of neonicotinoids [19], and emerging pathogens and parasites [20–22]. However, the potential interactive effects of neonicotinoids and parasites, such as the emergent microsporidian parasite Nosema ceranae [23], on associative learning in bees has received little attention.
Furthermore, the majority of studies of anthropogenic or environmental stress factors on learning and memory has so far focused on honeybees (e.g. [8,9,24,25]), whereas potential impacts on other pollinators such as bumblebees are as yet poorly known [13,26]. In addition to honeybees, bumblebees play a major role in providing pollinating services to wild and crop plants [27]. It has been suggested that bumblebees may be more sensitive than honeybees to the effects of stressors such as neonicotinoids though very few studies have assessed both species simultaneously [28,29]. Thus, there are major gaps in our knowledge as to what extent different pollinator species differ in their sensitivity to stressors.
Here, we test whether chronic exposure to field-realistic sub-lethal levels of the neonicotinoid clothianidin and/or inoculation with the parasite N. ceranae alter associative olfactory learning and memory in both honeybees and bumblebees using a fully factorial design. The microsporidian N. ceranae, transmitted via ingestion of spores that are spread in faeces, recently switched to the European honeybee from its putatively original host, the Asian honeybee [23]. This parasite has been associated with colony losses in some areas in Europe [30,31] and has also recently been found in wild bumblebees, where it may cause significant mortality [21], though not always [32]. We use the proboscis extension response (PER) assay, a widely used method for studying learning and memory in honeybees [33,34] and more recently also in bumblebees [13,15,35,36], to assess potential effects of the aforementioned stress factors on olfactory associative learning and memory.
2. Material and methods
(a). Bees and treatments
Newly emerged honeybee Apis mellifera workers originating from two colonies were divided into 24 groups, regardless of the colony origin, each consisting of 14 (first replicate) or 12 (second replicate) bees. Bumblebee Bombus terrestris workers from six queenright colonies obtained from Biobest (Belgium) via Agralan Ltd (Swindon, UK) were divided into 24 groups (four groups per queenright colony) each consisting of 10 workers. These groups were randomly assigned to one of the four treatments: control (C), neonicotinoid clothianidin (Neo), parasite (N. ceranae) (Para), and exposure to both neonicotinoid and parasite (NP). As for bumblebees, one group from each queenright colony was allocated to each of the four treatments. The experiment was replicated twice, thus, there were 48 groups in total per species. Honeybees resided in aluminium mesh cages (diameter 5 cm, length 20 cm), maintained in the dark at 32°C and 60% relative humidity (RH). A feeder modified from two 1 ml pipette tips (volume approx. 1.8 ml) was attached at the side of the cage. Bumblebees resided in plastic containers (diameter 11 cm, height 9 cm) with an aluminium mesh cover to allow air ventilation, maintained in the dark at 25°C and 50% RH. The tip of a 50 ml bird feeder was pierced through the wall of the container. Bees were provided with an ad libitum supply of 50% sugar (sucrose) water solution for 2–4 days after which Neo and NP groups were provided with an ad libitum supply of 50% sugar water solution contaminated with 4 ppb clothianidin (Sigma-Aldrich, Gillingham, UK) for 11–12 days. Stock solutions were dissolved in acetone and dietary concentrations were made on the day of provisioning. The concentration used was chosen to reflect field-realistic values. Reported values of the maximum amounts of clothianidin residues found in the nectar of treated crops vary from 1 to 14 ppb with the average values ranging from 0.3 to 5.4 ppb [29,37–39]. Fresh sugar water solutions (contaminated with clothianidin or not) were renewed every 2–4 days, and the amount of sugar water collected was recorded by weighting the feeders. All groups were provided ad libitum with untreated pollen (Biobest via Agralan Ltd, sterilized by gamma irradiation with a cobalt-60 source at dose rates between 25 and 45 kGy).
Three days after the start of pesticide treatment, bees were first starved for 2 h, cold anaesthetized for 2–5 min on ice, and then given either 3.6 µl of 30% sugar water (C and Neo groups) or 30% sugar water containing a controlled dose of approx. 180 000 N. ceranae spores (Para and NP groups) (viability 90%, viability test using 0.4% Trypan blue). In total, only 10 honeybees or eight bumblebees within each group (for three groups of six and seven bumblebees, and nine honeybees) were inoculated and any remaining bees were discarded. The rationale for this was that a small percentage of workers were expected to die prior to inoculation, so each group contained surplus bees to ensure that an equal number per group would be available to be treated. A solution of freshly isolated Nosema spores was obtained by homogenizing abdomens of experimentally infected adult honeybees, and purifying the homogenate by centrifugation in 95% Percoll (Sigma-Aldrich). Preliminary tests demonstrated successful infection of bumblebees by N. ceranae (infection success 58.3%, n = 36). After parasite inoculation, bees were monitored daily for worker mortality for 8–9 days until harnessing them for learning assays (see below).
(b). Proboscis extension response assays and memory retention
The PER assays are a standard method of testing learning in bees, which measure the ability of bees to form an association between an odour (conditioned stimulus, CS) and sugar reward (unconditioned stimulus, US) [33,35]. Bees were cold anaesthetized for 2–5 min on ice, and then harnessed in plastic tubes modified from 1 ml pipette tips. The head of the honeybees was restrained with tape, whereas for bumblebees we used a ‘yoke’ made from a paper clip [36]. Harnessed bees were fed to satiation with 40% sugar solution and left in darkness at RT, 60% RH, for approximately 15 h. The following day, responsiveness was tested by touching antennae with a tooth pick dipped in 40% sugar water; only those bees that showed a response were included in the assays. CS was delivered by a continuous air flow (approx. 2 l min−1, aquarium pump, Hidom), by switching the air flow to pass through a 20 ml syringe containing a 2 × 20 mm filter paper with 5 µl of 2 M odorant linalool (Sigma) in mineral oil. Each bee was positioned at 5 cm from the source of the odour, and an extractor fan located behind the bee removed any residual odour. One PER assay was composed of 10 CS–US trials (10 min inter-trial interval (ITI)) with each trial conducted as follows: 14 s air flow, 6 s CS, 4 s US (touching antennae with a toothpick covered with 60% sugar solution and allowing the bee to feed) overlapping with the CS for 3 s, 6 s air flow. Extension of the proboscis was recorded during each CS and US presentation. After 10 CS–US trials, an unrewarded test was conducted to assess the final level of learning by presenting only the CS. Finally, a memory test was performed 2.5 h after PER conditioning, again with the CS only. After the memory test, responsiveness to the sugar stimulus was tested as described above; only those bees who showed a response were included in the analysis. Bees that showed more than 3 sequential negative responses to the US during PER assays were considered unmotivated and were also excluded from analyses.
(c). Parasite screenings
After the experiment, a subset of alive and dead bees was screened by PCR for N. ceranae infection and for the presence of common bumblebee (N. bombi, Crithidia bombi, Apicystis bombi) or honeybee parasites (N. apis). Bees were dissected and abdomens were homogenized in double deionized (dd)H2O. DNA was extracted with 5% Chelex (Bio-Rad, Hemel Hempstead, Hertfordshire, UK). PCR protocols and parasite-specific primers followed Graystock et al. [21] and Martín-Hernández et al. [40] with slight modifications (electronic supplementary material, table S1).
(d). Statistics
We used generalized mixed effect (GLMM) and generalized linear (GLM) models in IBM SPSS v. 21 (IBM SPSS Inc., USA) to analyse the influence of pesticide and parasite treatments on learning. In the analysis of learning acquisition (defined as proportion of positive responses to CS at each 10 CS–US trials), bee identity was added as a subject and trial number (trials 2–10, the first CS–US trial was omitted to increase model fit) as a repeated variable. Binomial error structure with a logit link function was used in models analysing learning acquisition and final level of learning (unrewarded test). The linear mixed effect model (LMM) was used to assess sugar water consumption (honeybees) or collection (bumblebees) where group identity was added as a subject and time point of sugar water measurement (three time points, time point 1 = 5–7 days, time point 2 = 8–10 days, and time point 3 = 11–12 days after the start of the pesticide treatment) as a repeated variable. For bumblebee sugar water collection data, the identity of the original queenright colony was added as a random factor. Assumptions of homogeneity and normality of residuals were checked by inspecting residual plots (residuals against predicted values) and qq-plots. We first fitted the full model after which interaction terms were omitted from the models if they did not increase the model fit based on the Akaike information criterion (AIC). AIC was also used in selecting the repeated covariance type in models with repeated measures structure. Significant interactions were post hoc tested with simple effects tests. To assess the influence of pesticide and parasite treatments on memory, within each treatment group we compared the proportion of positive responses with CS in the unrewarded test (final level of learning) with those in the memory test performed 2.5 h after conditioning using a pairwise repeated samples McNemar test.
3. Results
(a). Nosema infections
PCR screenings of a subset of alive and dead bees (34% of honeybees and 50% of bumblebees, electronic supplementary material, table S2) showed that 100% of Nosema-inoculated honeybees were infected (0% of control honeybees), whereas only 3% of Nosema-inoculated bumblebees were positive. Inspection under the microscope detected no spores in the bumblebee subsamples. Owing to the potential stressful effects ingested parasite spores may exert on bumblebees, even though only 3% became infected (see Discussion), all parasite-treated bumblebees were included in the parasite treatment groups (Para and NP) in the results reported below.
(b). Responsiveness to stimulus
Only a small proportion of honeybees (1.3%, n = 479) and bumblebees (11%, n = 382) died prior to harnessing them for PER assays. Of the tested bees, 73% of honeybees and 74% of bumblebees were responsive to sugar stimulus (GLM: χ2 = 0.03, d.f. = 1, p = 0.85, electronic supplementary material, table S3) and were included in the PER assay. There were no differences in responsiveness among treatment groups (pesticide: χ2 = 2.73, d.f. = 1, p = 0.10; parasite: χ2 = 0.01, d.f. = 1, p = 0.94). Of those bees included in the PER assay, a similar proportion of both species (approx. 18%) failed to complete PER conditioning (GLM: χ2 = 0.06, d.f. = 1, p = 0.81, electronic supplementary material, table S3), either because they died during the PER conditioning, were not sufficiently responsive to US, or showed positive PER to the CS at the 1st trial. There were no differences in completion rate among treatment groups (pesticide: χ2 = 1.59, d.f. = 1, p = 0.69; parasite: χ2 = 0.06, d.f. = 1, p = 0.82). Thus, the final sample size for PER assays was 155 honeybees and 151 bumblebees.
(c). Learning acquisition across 10 conditioned stimulus–unconditioned stimulus trials
There was a significant four-way interaction between species, pesticide exposure, parasite treatment, and trial number on learning acquisition (GLMM, F = 4.18, d.f. = 1, 2738, p = 0.041), thus species were analysed separately. In honeybees, the proportion of positive responses to the odorant (CS), did not significantly increase across the nine CS–US trials (the 1st trial was excluded from the analysis to improve model fit; table 1). This is due to the fact that the level of learning was already high at the second trial (42–68%; figure 1a). Learning acquisition was impaired by pesticide exposure while parasite treatment did not have an effect (table 1 and figure 1a). Initially, pesticide-exposed bees (Neo and NP treatment groups) responded as well as non-exposed ones, but the proportion of positive responses began to decrease during the assay; in the Neo group the proportion of positive responses started to decrease after the 4th trial and was 53% and 72% at the 9th and 10th trial. In the NP group, the proportion of positive responses started to decrease after the 3rd trial reaching 54% by the 10th trial (figure 1a). For non-pesticide-exposed bees (C and Para groups), the level of learning remained fairly high throughout the trials being 81% and 68% by the 10th trial for control and parasite groups, respectively.
Table 1.
Results of repeated generalized linear mixed effect models (GLMMs) on learning acquisition across 10 CS–US trials for honeybee and bumblebee workers exposed to the pesticide clothianidin and inoculated with the parasite N. ceranae spores. Restricted maximum-likelihood procedure was used in the estimation. Honeybees: Akaike information criteria (AIC) = 5 844.03, repeated covariance type: toeplitz. Bumblebees: AIC = 7 259.72, repeated covariance type: scaled identity.
| F | d.f. (numerator (n), denominator (d)) | p-value | |
|---|---|---|---|
| honeybee | |||
| pesticide | 12.04 | 1, 1 391 | 0.001 |
| parasite | 3.27 | 1, 1 391 | 0.071 |
| trial number | 0.09 | 1, 1 391 | 0.77 |
| bumblebee | |||
| pesticide | 3.82 | 1, 1 351 | 0.051 |
| parasite | 0.45 | 1, 1 351 | 0.50 |
| trial number | 165.30 | 1, 1 351 | <0.001 |
| pesticide × parasite | 5.37 | 1, 1 351 | 0.010 |
| pesticide × trial number | 2.44 | 1, 1 351 | 0.12 |
| parasite × trial number | 0.67 | 1, 1 351 | 0.41 |
| pesticide × parasite × trial number | 5.52 | 1, 1 351 | 0.019 |
Figure 1.
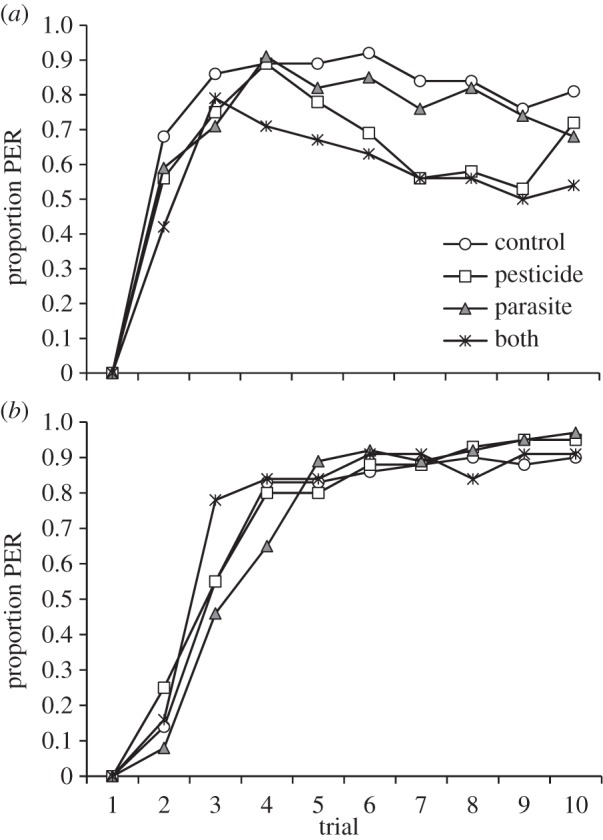
Proportion of proboscis extension responses (PERs) to odour stimulus (CS) for (a) honeybee and (b) bumblebee workers exposed to clothianidin and inoculated with parasite N. ceranae spores across 10 CS–US conditioning trials. Note, only 3% of inoculated bumblebees became infected with Nosema.
In bumblebees, there was a three-way interaction between pesticide exposure, parasite treatment (note, only 3% of parasite-treated bumblebees became infected), and trial number indicating that the rate of learning acquisition differed between treatment groups (table 1). Post hoc analysis revealed that within non-pesticide-exposed bumblebees, parasite-treated bees had a slower rate of learning than control bees (parasite × trial number interaction, p = 0.036, figure 1b), whereas within pesticide-exposed bees, parasite treatment did not affect the rate of learning (pesticide × trial number interaction, p = 0.25). Within non-parasite-treated bumblebees, control and pesticide-exposed bees had similar rates of learning (pesticide × trial number interaction, p = 0.53), whereas within parasite-treated bumblebees, pesticide-exposed bees (NP group) had a faster rate of learning than those not exposed to the pesticide (Para group) (pesticide × trial number interaction, p = 0.014, figure 1b).
The motivation of bees to respond to sugar stimulus (US) during conditioning did not differ between species (GLMM: F = 2.38, d.f. = 1, 3 055, p = 0.12, repeated covariance type toeplitz), nor were there differences among treatment groups (pesticide: F = 0.003, d.f. = 1, 3 055, p = 0.96; parasite: F = 0.68, d.f. = 1, 3 055, p = 0.41, electronic supplementary material, figure S1). The proportion of US responses was slightly lower at the 1st trial (87%) after which it remained close to 100% (94–100%) throughout the rest of the CS–US trials (F = 26.30, d.f. = 1, 3 055, p < 0.001).
(d). Final level of learning and memory
Honeybees showed a lower proportion of positive responses to the CS than bumblebees in the unrewarded test (final level of learning) (GLM: χ2 = 28.72, d.f. = 1, p < 0.001, figure 2a). Pesticide exposure did not affect the final level of learning (χ2 = 0.74, d.f. = 1, p = 0.39) while parasite-treated bees had a lower proportion of positive responses than non-parasitized bees (χ2 = 6.20, d.f. = 1, p = 0.013). From figure 2a, it can be seen that the effect of the parasite treatment on the final level of learning was more strongly manifested in honeybees (C 73% and Neo 75% versus Para 68% and NP groups 44%), whereas in bumblebees (note, only 3% of bumblebees were infected) differences among treatment groups were much smaller (C 93% and Neo 95% versus Para 89% and NP groups 91%).
Figure 2.
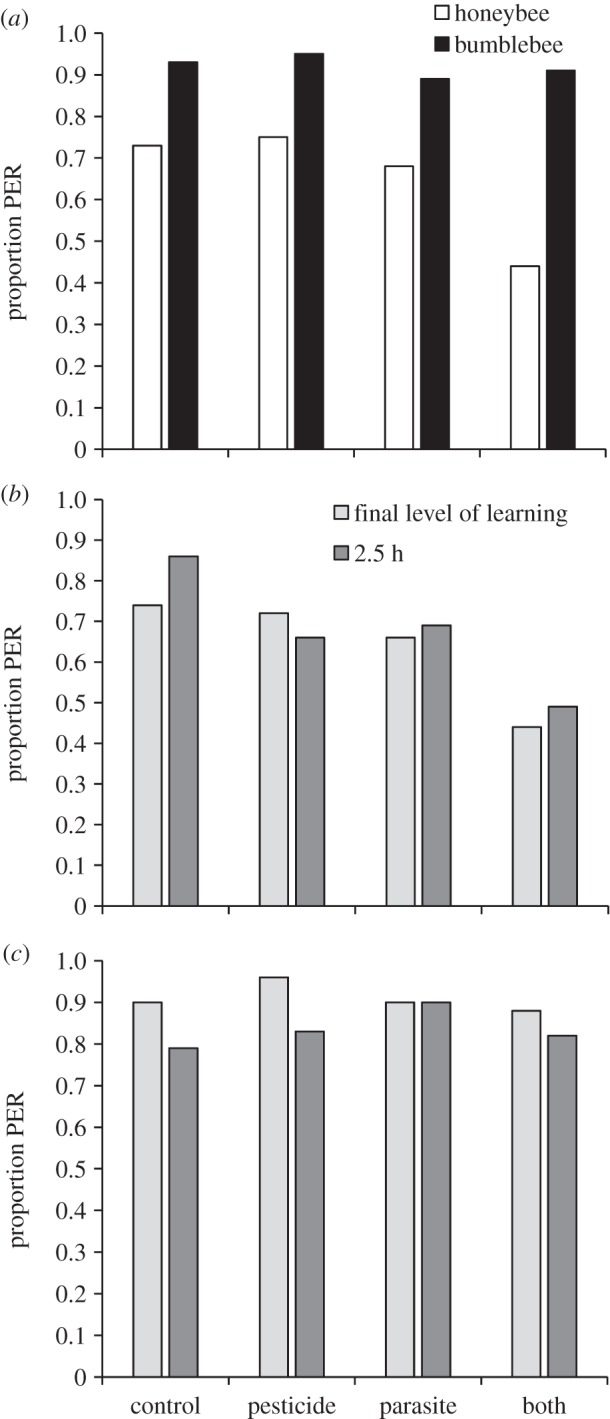
(a) Proportion of proboscis extension responses (PERs) to odour stimulus (CS) for honeybee and bumblebee workers exposed to clothianidin and inoculated with parasite N. ceranae spores in the unrewarded test (final level of learning). Proportion of PERs to CS 2.5 h after PER training (memory test) compared with their responses in the unrewarded test for (b) honeybees and (c) bumblebees. Note, only 3% of inoculated bumblebees became infected with Nosema.
Honeybees and bumblebees in all treatment groups (C, Neo, Para, and NP) remembered the learned association equally well 2.5 h later in the memory test when compared with the final level of learning (pairwise repeated samples McNemar test, honeybees: p = 0.22–1.00; bumblebees: p = 0.38–1.00, figure 2b,c).
(e). Sugar water consumption/collection
In honeybees, neither pesticide exposure nor parasite treatment affected sugar water consumption, which increased with time (LMM, pesticide: F = 0.12, d.f. = 1, 136.9, p = 0.73; parasite: F = 1.63, d.f. = 1, 136.9, p = 0.20; time point: F = 138.18, d.f. = 1, 76, p < 0.001, repeated covariance type diagonal, figure 3a). In bumblebees, pesticide-exposed bees had significantly lower sugar water collection than non-exposed bees while parasite treatment had no effect (LMM, pesticide: F = 31.85, d.f. = 1, 39.9, p < 0.001; parasite: F = 0.56, d.f. = 1, 39.9, p = 0.46, repeated covariance type unstructured, figure 3b). Sugar water collection increased with time (time point: F = 29.89, d.f. = 1, 47.1, p < 0.001).
Figure 3.
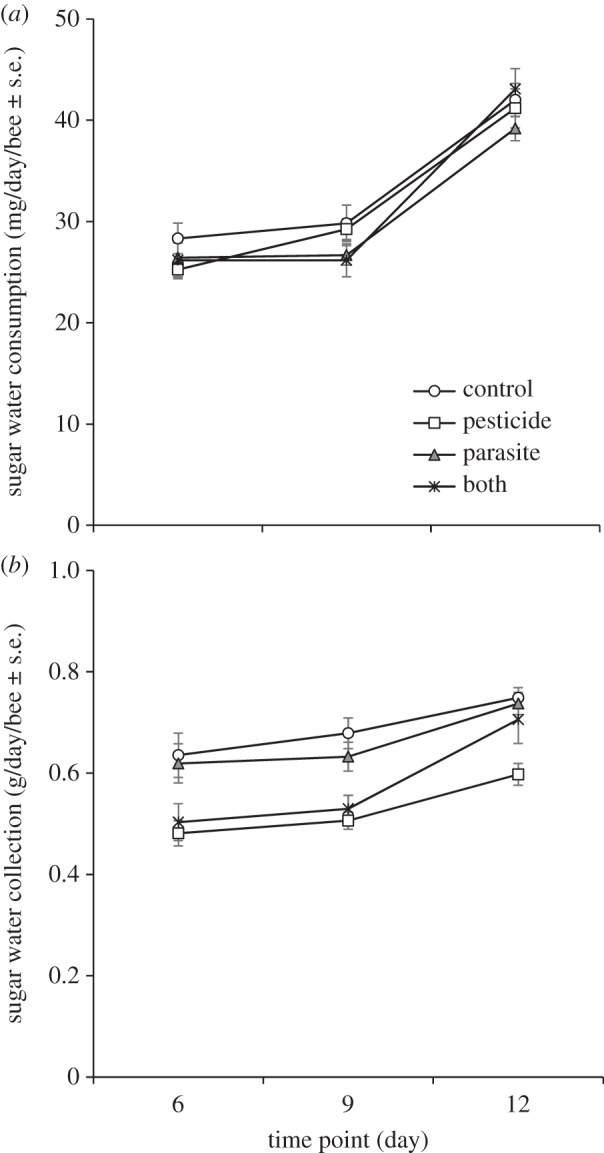
(a) Sugar water consumption (mg/bee/day ± standard error (s.e.)) of honeybees and (b) sugar water collection (g/bee/day ± s.e.) of bumblebees in the control group (control), exposed to clothianidin (pesticide) in the sugar water, inoculated with parasite N. ceranae spores (parasite) and exposed to both clothianidin and the parasite (both). Note, only 3% of inoculated bumblebees became infected with Nosema.
4. Discussion
Associative learning and the recall of memories play a crucial role in enabling bees to forage efficiently [2]. Subtle changes in learning and memory could thus have serious implications for colony fitness. This is the first study to compare the impact of chronic exposure with field-realistic levels of neonicotinoid pesticides on learning and memory between honeybees and bumblebees. We show that, whereas chronic exposure to field-realistic levels of the neonicotinoid clothianidin impaired the acquisition of an olfactory association in honeybees, surprisingly no adverse effects on learning were observed in bumblebees (but see, Stanley et al. [13]). As bumblebees have an annual life cycle and smaller colony size than honeybees, one might expect they would be more vulnerable to the adverse effects of stressors than honeybees, which have a buffer in the form of a large foraging and nest-based workforce. A recent field experiment reported that exposure to clothianidin in seed-coated oilseed rape was associated with lower densities of wild bumblebees and negatively affected colony growth and reproduction in commercially reared bumblebee nests, while no effects on colony strength were observed for honeybees [29]. It has also been shown that neonicotinoid (imidacloprid)-exposed bumblebees have higher levels of bodily residues than honeybees at the same dosage levels, suggesting that they are less efficient at metabolizing these particular pesticides and, therefore, likely to be exposed to higher levels [41], which could make them more vulnerable to their effects. Intriguingly, our results on learning indicate the opposite. One could speculate that the bumblebee neurons may be less sensitive to the effects of clothianidin than those of honeybees. It is known that different nAChR sub-types have different pharmacological properties [42]. Thus, species could differ in their sensitivity to specific neonicotinoid compounds by having differential composition and populations of nAChR sub-types [43,44], or by differential responses in gene expression of nAChR subunits to pesticide exposure [45,46].
An alternative explanation for the differential sensitivity of the two bee species might be that bumblebees may have experienced lower exposure levels, as clothianidin-exposed bumblebees in our study collected less sugar water than those that were not exposed, while no such effect of the pesticide was observed for honeybees, as has been found by others [28,47]. On the other hand, it is noted that the reduced collection rate suggests that clothianidin exposure caused some toxic effects on bumblebees, making them either collect or feed less on pesticide-spiked sugar water. Neither honeybees nor bumblebees are thought to be able to detect neonicotinoids, including clothianidin, in food [47], thus, this effect on sugar collection rate is probably due to toxic effects rather than repellency. It is also noted that the age of bumblebees was not controlled, which could potentially explain some of the differences in sensitivity between the species [47].
Interestingly, the effect of clothianidin exposure on learning in honeybees was manifested as a decline in the proportion of positive responses to CS during conditioning. The potential mechanism underpinning why bees first learned the association but then stopped responding to the odour stimulus while still readily responding to the sugar stimulus probably involves disruption of cholinergic signalling in the olfactory system [11] but not in the neuronal system related to gustation. A similar decline in learning was also observed for honeybees chronically exposed to coumaphos, an organophosphate [8]. However, the decline was observed only when a 30 s ITI was used, but not when bees were trained under an ITI of 10 min, as used here. As the mode of action of neonicotinoids, which are agonists of nAChRs, is different from organophosphates, which are inhibitors of acetylcholinesterase neurotransmitters, the underlying molecular mechanisms of the observed decreases in learning are likely to be somewhat different. Overall, given the importance of olfactory learning in flower recognition and foraging in bees [48], the impaired learning of honeybees exposed to field-realistic levels of clothianidin may have important ecological implications at the colony level, and offers a potential mechanism underlying observed reductions in the homing ability and impaired foraging behaviour of neonicotinoid stressed honeybees [49,50].
Our results show that infection by the parasite N. ceranae impairs learning in honeybees, manifested in the unrewarded test (final level of learning). Such a small reduction in learning acquisition may not have significant relevance in field conditions. At the same time, it is noted that the impact of parasites on learning may become more pronounced in field conditions as indicated by a semi-field study using free-flying bumblebees [51]. The mechanisms underlying pathogen-driven reductions in learning or memory are still relatively poorly understood, but potentially involve interactions between the immune system and the nervous system [14–16]. Previous studies have shown that parasite challenge can induce various alterations of the immune system of bees (e.g. [52,53]), and this, even without actual successful infection and production of spores, can impair the learning and memory of bees as shown by stimulating the immune system of honeybees and bumblebees with non-pathogenic elicitors, lipopolysaccharides [14,15,51].
Although N. ceranae infection slightly impaired learning in honeybees (final level of learning), it did not result in more adverse effects in combination with exposure to the pesticide. In addition to parasites, exposure to pesticides can also induce various alterations of the immune system [54] resulting, for instance, in downregulation of several immune-related genes [55]. Thus, we would have expected that exposure to both these stressors, neonicotinoids and parasite N. ceranae, would be more harmful than one factor alone as previously reported as reduced survival or longevity in honeybees (e.g. [17]). However, we did not observe any interactive effects of these factors when it comes to learning and memory. It could be that the underlying mechanisms by which neonicotinoids and parasites influence the functioning of nervous system are different and do not lead to interactive effects.
Interestingly, even though bumblebees did not become infected with N. ceranae (only 3% of inoculated bumblebees were infected), in contrast with previous studies on N. ceranae infectivity in bumblebees [21,32], there was still a significant negative effect on learning rate. An altered immune system as discussed above [15,53] could potentially explain the lower learning rate in Nosema-inoculated bumblebees, suggesting that ingestion of spores solely may be stressful for bumblebees. The low infectivity of bumblebees is intriguing given our preliminary tests showed that 60% of the inoculated bees became infected. Both the condition and genetic background of the host and the parasite play an essential role in determining the outcome of an infection [56,57]. It could be that the commercial bumblebee strains used in the experiment were resistant to this gut parasite or slight differences in the condition or age of bumblebees may explain the discrepancy between the pilot and the current experiment. Interestingly, Nosema-inoculated bumblebees had a slightly faster rate of learning acquisition when chronically exposed to the pesticide, though pesticide exposure alone had no effect on learning rate. The mechanism underlying enhanced learning rate in our study is not clear. One could speculate that bumblebees exposed to pesticide and inoculated with parasite spores were hungrier and, therefore, more motivated to respond, though further research would be needed to confirm this. Taken together, our results on the effects of N. ceranae on bumblebees are very intriguing and indicate that these stressors may interact in very complex ways. Our results underline the urgent demand for further investigation on the potential factors that affect the infectivity and virulence of newly emerging diseases in bumblebee hosts in order to gain a better understanding of whether N. ceranae presents a threat to bumblebee learning and health.
Studying the impacts of subtle interactions between stressors on bee health under field-realistic conditions poses a major challenge. Our findings emphasize that sensitivity to stressors differs between pollinator species and the effects depend on which endpoint trait is being investigated. Our results also underline the fact that one cannot readily extrapolate findings from one bee species to others, and this has important implications for regulatory risk assessments, which generally use honeybees as a model for all bees.
Supplementary Material
Acknowledgements
We thank Cristina Botías for assisting in the experiment and providing valuable comments on the manuscript, Beth Nicholls and two anonymous reviewers for providing valuable comments on the manuscript, Julia Jones, William O.H. Hughes, and Hasan Mohammed Al Toufailia for kindly providing honeybees for the experiment.
Data accessibility
Raw data can be found in the Dryad repository, http://dx.doi.org/10.5061/dryad.15264.
Authors' contributions
S.P. conceived, designed, collected data, performed statistical analyses, and wrote the manuscript. D.G. conceived, designed, and wrote the manuscript.
Competing interests
We have no competing interests.
Funding
This study was funded by Jenny and Antti Wihuri Foundation and the Foundation's Post Doc Pool.
References
- 1.Menzel R, De Marco RJ, Greggers U. 2006. Spatial memory, navigation and dance behaviour in Apis mellifera. J. Comp. Physiol. A 192, 889–903. ( 10.1007/s00359-006-0136-3) [DOI] [PubMed] [Google Scholar]
- 2.Dukas R. 2008. Evolutionary biology of insect learning. Annu. Rev. Entomol. 53, 145–160. ( 10.1146/annurev.ento.53.103106.093343) [DOI] [PubMed] [Google Scholar]
- 3.Raine NE, Chittka L. 2008. The correlation of learning speed and natural foraging success in bumble-bees. Proc. R. Soc. B 275, 803–808. ( 10.1098/rspb.2007.1652) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitehorn PR, O'Connor S, Wackers FL, Goulson D. 2012. Neonicotinoid pesticide reduces bumble bee colony growth and queen production. Science 336, 351–352. ( 10.1126/science.1215025) [DOI] [PubMed] [Google Scholar]
- 5.Gegear RJ, Otterstatter MC, Thomson JD. 2006. Bumble-bee foragers infected by a gut parasite have an impaired ability to utilize floral information. Proc. R. Soc. B 273, 1073–1078. ( 10.1098/rspb.2005.3423) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iqbal J, Mueller U. 2007. Virus infection causes specific learning deficits in honeybee foragers. Proc. R. Soc. B 274, 1517–1521. ( 10.1098/rspb.2007.0022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goblirsch M, Huang ZY, Spivak M. 2013. Physiological and behavioral changes in honey bees (Apis mellifera) induced by Nosema ceranae infection. PLoS ONE 8, e58165 ( 10.1371/journal.pone.0058165) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williamson SM, Wright GA. 2013. Exposure to multiple cholinergic pesticides impairs olfactory learning and memory in honeybees. J. Exp. Biol. 216, 1799–1807. ( 10.1242/jeb.083931) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wright GA, Softley S, Earnshaw H. 2015. Low doses of neonicotinoid pesticides in food rewards impair short-term olfactory memory in foraging-age honeybees. Sci. Rep. 5, 15322 ( 10.1038/srep15322) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomizawa M, Casida JE. 2005. Neonicotinoid insecticide toxicology: mechanisms of selective action. Annu. Rev. Pharmacol. Toxicol. 45, 247–268. ( 10.1146/annurev.pharmtox.45.120403.095930) [DOI] [PubMed] [Google Scholar]
- 11.Palmer MJ, Moffat C, Saranzewa N, Harvey J, Wright GA, Connolly CN. 2013. Cholinergic pesticides cause mushroom body neuronal inactivation in honeybees. Nat. Commun. 4, 1634 ( 10.1038/ncomms2648) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moffat C, Pacheco JG, Sharp S, Samson AJ, Bollan KA, Huang J, Buckland ST, Connolly CN. 2015. Chronic exposure to neonicotinoids increases neuronal vulnerability to mitochondrial dysfunction in the bumblebee (Bombus terrestris). FASEB J. 29, 2112–2119. ( 10.1096/fj.14-267179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stanley DA, Smith KE, Raine NE. 2015. Bumblebee learning and memory is impaired by chronic exposure to a neonicotinoid pesticide. Sci. Rep. 5, 16508 ( 10.1038/srep16508) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mallon EB, Brockmann A, Schmid-Hempel P. 2003. Immune response inhibits associative learning in insects. Proc. R. Soc. Lond. B 270, 2471–2473. ( 10.1098/rspb.2003.2456) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riddell CE, Mallon EB. 2006. Insect psychoneuroimmunology: immune response reduces learning in protein starved bumblebees (Bombus terrestris). Brain Behav. Immun. 20, 135–138. ( 10.1016/j.bbi.2005.06.008) [DOI] [PubMed] [Google Scholar]
- 16.McDonnell CM, Alaux C, Parrinello H, Desvignes J-P, Crauser D, Durbesson E, Beslay D, Le Conte Y. 2013. Ecto- and endoparasite induce similar chemical and brain neurogenomic responses in the honey bee (Apis mellifera). BMC Ecol. 13, 25 ( 10.1186/1472-6785-13-25) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aufauvre J, et al. 2012. Parasite-insecticide interactions: a case study of Nosema ceranae and fipronil synergy on honeybee. Sci. Rep. 2, 326 ( 10.1038/srep00326) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blanken LJ, van Langevelde F, van Dooremalen C. 2015. Interaction between Varroa destructor and imidacloprid reduces flight capacity of honeybees. Proc. R. Soc. B 282, 20151738 ( 10.1098/rspb.2015.1738) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goulson D. 2013. An overview of the environmental risks posed by neonicotinoid insecticides. J. Appl. Ecol. 50, 977–987. ( 10.1111/1365-2664.12111) [DOI] [Google Scholar]
- 20.Meeus I, Brown MJF, De Graaf DC, Smagghe G. 2011. Effects of invasive parasites on bumble bee declines. Conserv. Biol. 25, 662–671. ( 10.1111/j.1523-1739.2011.01707.x) [DOI] [PubMed] [Google Scholar]
- 21.Graystock P, Yates K, Darvill B, Goulson D, Hughes WOH. 2013. Emerging dangers: deadly effects of an emergent parasite in a new pollinator host. J. Invertebr. Pathol. 114, 114–119. ( 10.1016/j.jip.2013.06.005) [DOI] [PubMed] [Google Scholar]
- 22.Goulson D, Hughes WOH. 2015. Mitigating the anthropogenic spread of bee parasites to protect wild pollinators. Biol. Conserv. 191, 10–19. ( 10.1016/j.biocon.2015.06.023) [DOI] [Google Scholar]
- 23.Gómez-Moracho T, Bartolomé C, Bello X, Martín-Hernández R, Higes M, Maside X. 2015. Recent worldwide expansion of Nosema ceranae (Microsporidia) in Apis mellifera populations inferred from multilocus patterns of genetic variation. Infect. Genet. Evol. 31, 87–94. ( 10.1016/j.meegid.2015.01.002) [DOI] [PubMed] [Google Scholar]
- 24.Decourtye A, Armengaud C, Renou M, Devillers J, Cluzeau S, Gauthier M, Pham-Delègue MH. 2004. Imidacloprid impairs memory and brain metabolism in the honeybee (Apis mellifera L.). Pest. Biochem. Physiol. 78, 83–92. ( 10.1016/j.pestbp.2003.10.001) [DOI] [Google Scholar]
- 25.Williamson SM, Baker DD, Wright GA. 2013. Acute exposure to a sublethal dose of imidacloprid and coumaphos enhances olfactory learning and memory in the honeybee Apis mellifera. Invertebr. Neurosci. 13, 63–70. ( 10.1007/s10158-012-0144-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piiroinen S, Botías C, Nicholls E, Goulson D. 2016. No effect of low-level chronic neonicotinoid exposure on bumblebee learning and fecundity. PeerJ. 4, e1808 ( 10.7717/peerj.1808) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goulson D. 2010. Bumblebees; their behaviour, ecology and conservation. Oxford, UK: Oxford University Press. [Google Scholar]
- 28.Cresswell JE, et al. 2012. Differential sensitivity of honey bees and bumble bees to a dietary insecticide (imidacloprid). Zoology 115, 365–371. ( 10.1016/j.zool.2012.05.003) [DOI] [PubMed] [Google Scholar]
- 29.Rundlöf M, et al. 2015. Seed coating with a neonicotinoid insecticide negatively affects wild bees. Nature 521, 77–80. ( 10.1038/nature14420) [DOI] [PubMed] [Google Scholar]
- 30.Higes M, Martín-Hernández R, Garrido-Bailón E, González-Porto AV, García-Palencia P, Meana A, del Nozal MJ, Mayo R, Bernal JL. 2009. Honeybee colony collapse due to Nosema ceranae in professional apiaries. Environ. Microbiol. Rep. 1, 110–113. ( 10.1111/j.1758-2229.2009.00014.x) [DOI] [PubMed] [Google Scholar]
- 31.Borneck R, Viry A, Martín-Hernández R, Higes M. 2010. Honey bee colony losses in the Jura Region, France and related pathogens. J. Apic. Res. 49, 334–336. ( 10.3896/IBRA.1.49.4.06) [DOI] [Google Scholar]
- 32.Fürst MA, McMahon DP, Osborne JL, Paxton RJ, Brown MJF. 2014. Disease associations between honeybees and bumblebees as a threat to wild pollinators. Nature 506, 364–366. ( 10.1038/nature12977) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bitterman ME, Menzel R, Fietz A, Schäfer S. 1983. Classical conditioning of proboscis extension in honeybees (Apis mellifera). J. Comp. Psychol. 97, 107–119. ( 10.1037/0735-7036.97.2.107) [DOI] [PubMed] [Google Scholar]
- 34.Giurfa M, Sandoz J-C. 2012. Invertebrate learning and memory: fifty years of olfactory conditioning of the proboscis extension response in honeybees. Learn. Mem. 19, 54–66. ( 10.1101/lm.024711.111) [DOI] [PubMed] [Google Scholar]
- 35.Laloi D, Sandoz JC, Picard-Nizou AL, Marchesi A, Pouvreau A, Taséi JN, Poppy G, Pham-Delègue MH. 1999. Olfactory conditioning of the proboscis extension in bumble bees. Entomol. Exp. Appl. 90, 123–129. ( 10.1046/j.1570-7458.1999.00430.x) [DOI] [Google Scholar]
- 36.Riveros AJ, Gronenberg W. 2009. Olfactory learning and memory in the bumblebee Bombus occidentalis. Naturwissenschaften 96, 851–856. ( 10.1007/s00114-009-0532-y) [DOI] [PubMed] [Google Scholar]
- 37.Sanchez-Bayo F, Goka K. 2014. Pesticide residues and bees—a risk assessment. PLoS ONE 9, e94482 ( 10.1371/journal.pone.0094482) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonmatin J-M, et al. 2015. Environmental fate and exposure; neonicotinoids and fipronil. Environ. Sci. Pollut. Res. 22, 35–67. ( 10.1007/s11356-014-3332-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Botías C, David A, Horwood J, Abdul-Sada A, Nicholls E, Hill EM, Goulson D. 2015. Neonicotinoid residues in wildflowers, a potential route of chronic exposure for bees. Environ. Sci. Technol. 49, 12 731–12 740. ( 10.1021/acs.est.5b03459) [DOI] [PubMed] [Google Scholar]
- 40.Martín-Hernández R, Meana A, Prieto L, Salvador AM, Garrido-Bailón E, Higes M. 2007. Outcome of colonization of Apis mellifera by Nosema ceranae. Appl. Environ. Microbiol. 73, 6331–6338. ( 10.1128/AEM.00270-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cresswell JE, Robert F-XL, Florance H, Smirnoff N. 2014. Clearance of ingested neonicotinoid pesticide (imidacloprid) in honey bees (Apis mellifera) and bumblebees (Bombus terrestris). Pest. Manag. Sci. 70, 332–337. ( 10.1002/ps.3569) [DOI] [PubMed] [Google Scholar]
- 42.Lansdell SJ, Millar NS. 2000. The influence of nicotinic receptor subunit composition upon agonist, α-bungarotoxin and insecticide (imidacloprid) binding affinity. Neuropharmacology 39, 671–679. ( 10.1016/S0028-3908(99)00170-7) [DOI] [PubMed] [Google Scholar]
- 43.Dacher M, Lagarrigue A, Gauthier M. 2005. Antennal tactile learning in the honeybee: effect of nicotinic antagonists on memory dynamics. Neuroscience 130, 37–50. ( 10.1016/j.neuroscience.2004.09.006) [DOI] [PubMed] [Google Scholar]
- 44.Jones AK, Sattelle DB. 2010. Diversity of insect nicotinic acetylcholine receptor subunits. Adv. Exp. Med. Biol. 683, 25–43. ( 10.1007/978-1-4419-6445-8_3) [DOI] [PubMed] [Google Scholar]
- 45.Yu X, Wang M, Kang M, Liu L, Guo X, Xu B. 2011. Molecular cloning and characterization of two nicotinic acetylcholine receptor β subunit genes from Apis cerana cerana. Arch. Insect Biochem. Physiol. 77, 163–178. ( 10.1002/arch.20432) [DOI] [PubMed] [Google Scholar]
- 46.Wang X, Sun H, Zhang Y, Liu C, Liu Z. 2015. Transcriptional changes in nAChRs, interactive proteins and P450s in Locusta migratoria manilensis (Orthoptera: Acrididae) CNS in response to high and low oral doses of imidacloprid. J. Insect Sci. 15, 102 ( 10.1093/jisesa/iev080) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kessler SC, Tiedeken EJ, Simcock KL, Derveau S, Mitchell J, Softley S, Stout JC, Wright GA. 2015. Bees prefer foods containing neonicotinoid pesticides. Nature 521, U74–U145. ( 10.1038/nature14414) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wright GA, Choudhary AF, Bentley MA. 2009. Reward quality influences the development of learned olfactory biases in honeybees. Proc. R. Soc. B 276, 2597–2604. ( 10.1098/rspb.2009.0040) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Henry M, Béguin M, Requier F, Rollin O, Odoux J-F, Aupinel P, Aptel J, Tchamitchian S, Decourtye A. 2012. A common pesticide decreases foraging success and survival in honey bees. Science 336, 348–350. ( 10.1126/science.1215039) [DOI] [PubMed] [Google Scholar]
- 50.Schneider CW, Tautz J, Grünewald B, Fuchs S. 2012. RFID tracking of sublethal effects of two neonicotinoid insecticides on the foraging behavior of Apis mellifera. PLoS ONE 7, e30023 ( 10.1371/journal.pone.0030023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alghamdi A, Dalton L, Phillis A, Rosato E, Mallon EB. 2008. Immune response impairs learning in free-flying bumble-bees. Biol. Lett. 4, 479–481. ( 10.1098/rsbl.2008.0331) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Antúnez K, Martín-Hernández R, Prieto L, Meana A, Zunino P, Higes M. 2009. Immune suppression in the honey bee (Apis mellifera) following infection by Nosema ceranae (Microsporidia). Environ. Microbiol. 11, 2284–2290. ( 10.1111/j.1462-2920.2009.01953.x) [DOI] [PubMed] [Google Scholar]
- 53.Brunner FS, Schmid-Hempel P, Barribeau SM. 2013. Immune gene expression in Bombus terrestris: signatures of infection despite strong variation among populations, colonies, and sister workers. PLoS ONE 8, e68181 ( 10.1371/journal.pone.0068181) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.James RR, Xu J. 2012. Mechanisms by which pesticides affect insect immunity. J. Invertebr. Pathol. 109, 175–182. ( 10.1016/j.jip.2011.12.005) [DOI] [PubMed] [Google Scholar]
- 55.Aufauvre J, Misme-Aucouturier B, Viguès B, Texier C, Delbac F, Blot N. 2014. Transcriptome analyses of the honeybee response to Nosema ceranae and insecticides. PLoS ONE 9, e91686 ( 10.1371/journal.pone.0091686) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frank SA, Schmid-Hempel P. 2008. Mechanisms of pathogenesis and the evolution of parasite virulence. J. Evol. Biol. 21, 396–404. ( 10.1111/j.1420-9101.2007.01480.x) [DOI] [PubMed] [Google Scholar]
- 57.Di Pasquale G, Salignon M, Le Conte Y, Belzunces LP, Decourtye A, Kretzschmar A, Suchail S, Brunet J-L, Alaux C. 2013. Influence of pollen nutrition on honey bee health: do pollen quality and diversity matter? PLoS ONE 8, e72016 ( 10.1371/journal.pone.0072016) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data can be found in the Dryad repository, http://dx.doi.org/10.5061/dryad.15264.


