Abstract
At present, there is substantive evidence that the nutritional content of agriculturally important food crops will decrease in response to rising levels of atmospheric carbon dioxide, Ca. However, whether Ca-induced declines in nutritional quality are also occurring for pollinator food sources is unknown. Flowering late in the season, goldenrod (Solidago spp.) pollen is a widely available autumnal food source commonly acknowledged by apiarists to be essential to native bee (e.g. Bombus spp.) and honeybee (Apis mellifera) health and winter survival. Using floral collections obtained from the Smithsonian Natural History Museum, we quantified Ca-induced temporal changes in pollen protein concentration of Canada goldenrod (Solidago canadensis), the most widespread Solidago taxon, from hundreds of samples collected throughout the USA and southern Canada over the period 1842–2014 (i.e. a Ca from approx. 280 to 398 ppm). In addition, we conducted a 2 year in situ trial of S. canadensis populations grown along a continuous Ca gradient from approximately 280 to 500 ppm. The historical data indicated a strong significant correlation between recent increases in Ca and reductions in pollen protein concentration (r2 = 0.81). Experimental data confirmed this decrease in pollen protein concentration, and indicated that it would be ongoing as Ca continues to rise in the near term, i.e. to 500 ppm (r2 = 0.88). While additional data are needed to quantify the subsequent effects of reduced protein concentration for Canada goldenrod on bee health and population stability, these results are the first to indicate that increasing Ca can reduce protein content of a floral pollen source widely used by North American bees.
Keywords: bee health, carbon dioxide, Solidago canadensis, planetary health, pollen, protein
1. Introduction
As has been observed in nearly a 100 individual studies and several meta-analyses, as atmospheric carbon dioxide (Ca) increases, nitrogen (protein) concentration declines in a wide range of plant species [1–5]. This decline can be associated with dilution resulting from increased carbohydrate production [4,6]; reduced transpiration and a reduction in mass flow of N and other mobile elements [4,7,8] and/or the need for less Rubisco with a subsequent lowering of plant demand for N, particularly in photosynthetic tissues [9]. The Ca-caused reduction in protein has been observed in a wide range of plant tissues, including leaves, stems, roots, tubers, seeds and grains [1–3,5] and has been correlated with negative effects on human nutrition on a global scale [3,10]. However, whether such reductions also occur for pollen, with subsequent effects on pollinator nutrition, are unknown.
At present, there is considerable concern regarding the widespread decline in native bee and honeybee colony numbers and increasing annual colony losses, particularly in the US and Europe. Drivers that have been associated with these declines include, but are not limited to, socioeconomic concerns (e.g. agricultural intensification), invasive pests and/or pathogens (e.g. Varroa, Nosema), agrochemicals (e.g. neonicotinoids) and a decline in genetic diversity of bee populations and food sources (e.g. over-reliance on one floral source) [11–17]. These factors may act singly or in combination to influence bee health [14,15].
Vulnerability to these reported environmental stressors is, in part, linked to pollinator nutrition [13,18]. For example, nutrition/diet can affect bee immunocompetence and the response to parasites (e.g. Varroa destructor or Nosema ceranae), which, in turn, can feedback to exacerbate nutritional stress [19–21]. Good nutrition can also aid in pesticide detoxification [22–24].
As all bees, wild or domesticated, obtain their energy and nutrition from flowering plants, any stressor that affects floral physiology on a panoptic scale could, potentially, alter long-term bee health. Nectar is the primary source of energy for the colony; however, pollen is the sole source of protein for all bees, wild and domesticated, and fulfils dietary requirements for the lipids, sterols, vitamins and minerals needed for larvae development [25–27]. In contrast to nectar, only a small amount of pollen is stored in the colony at any given time, making bee colonies susceptible to sudden fluctuations in pollen quantity or quality [28–30]. Lower pollen quality has been shown to negatively impact adult longevity in bees in a number of studies [31–34].
In northern latitudes in North America and Europe where little pollen is available during winter, plants that serve as late-season pollen sources are especially important for winter survival [35]. Among such sources, goldenrod (Solidago spp.) is a late-season perennial with a long bloom period from late July through to October. Although national estimates of pollen sources for bee diets do not exist; goldenrod has been recognized as a primary autumn pollen source for many pollinators in North America, including wild and domesticated bees, by apiarists and extension agents throughout Canada [36,37], New York [38], Ohio [39], Wisconsin [40], Michigan [41] and Minnesota [42], inter alia. The USDA Handbook of Agriculture lists goldenrod as an important nectar and pollen plant for all regions of the USA except the West [43].
Although there are numerous Solidago species, Solidago canadensis (Canada goldenrod) is the widest spread taxon, and includes several taxa which are frequently granted species status including Solidago altissima L., lepida DC (sensu Fernald), Solidago gilvocanescens (Rydb.) Smyth, Solidago scabra Muhl., Solidago elongate Nutt., Solidago salebrosa (Piper) Rydb. and Solidago pruinosa Greene [36]. Solidago canadensis is found in almost every state in the USA and throughout Canada (http://www.fs.fed.us/database/feis/plants/forb/solcan/all.html).
Whether recent or projected increases in Ca can induce changes in pollen protein concentration that could, potentially, also impact pollinator health, has not been established. Yet, such information may be particularly relevant for bees and other pollinators, given their role in global food production; hence, we wished to determine whether Ca has, or could, affect pollen protein levels using S. canadensis as a test case.
2. Material and methods
(a). Anatomical considerations
To determine variation between floral parts with respect to nitrogen and carbon, pollen was collected in situ for tall or Canada goldenrod at three locations within Indiana and two locations in Maryland in 2012. Pure pollen was compared to pollen/anther composition with respect to carbon and nitrogen concentration using carbon, hydrogen, nitrogen (CHN) analysis (see CHN analysis section). Pollen and anther C and N were similar in concentration and highly correlated (r2 > 0.96). This allowed a relative comparison between pollen per se and the anther/pollen samples taken from the historical and experimental studies.
(b). Historical
Canada goldenrod (S. canadensis) is the largest goldenrod taxon and includes a number of taxa that have species status such as S. altissima (http://plants.usda.gov/core/profile?symbol=soal6). Taxonomically identified specimens of S. canadensis were obtained from the Smithsonian Institution's Museum of Natural History's collection. Pressed plants contained both vegetative and floral tissue, as well as date and location of the collected specimen. All samples contained fully developed flowers. Because these flowers were dry and subject to protein degradation, elemental analysis (C, H and N) was used to estimate protein concentration.
The collected plants from the USA were from Arizona, California, Colorado, Indiana, Maryland, Texas and the District of Columbia. Canadian samples, all from Ontario province, were also examined. Floral branches, usually 4–6 per plant and 4–10 cm in length were excised from each sample. Each branch was placed in a scintillation vial and labelled. A hand lens with razor blade or tweezers was used at the laboratory to remove florets from involucres. Anthers and pollen were placed into tin capsules (8 × 5 mm) and weighed on a Perkin-Elmer autobalance. Overall, four to six floral branches were collected from each of 350 individual plant specimens in the USA and Canada that spanned a time period from 1842 through to 1998. Florets from each branch were analysed separately then averaged for a given herbarium sample. Additional field samples were processed from Maryland (2008, 2012, 2014) and Texas (2012, 2014).
(c). Experimental: floral demography and insect visitation
To provide a more updated assessment of autumn floral demographics and to quantify Solidago populations, we measured the frequency of flowering stems and mapped their distribution using a 24 × 24 m area gridded into 1 m2 quadrats in Williamstown, Berkshire County, Massachusetts. We identified all stems to the species level and for quadrats with less than 15 stems, we recorded the x- and y-coordinates; for quadrats with more than 15 stems, we assigned coordinates so that stems were evenly distributed in the quadrat. To determine the type and frequency of flower visitors to autumn-blooming Asteraceae, we marked out ten 1 m2 quadrats and directly scored visitors to flowers in five 3 min observations periods (15 min) for 10 quadrats for a total of 750 min. We recorded the identity of each insect that visited a flower in the quadrat. Both sets of observations were from 2010; September and early October, up until the first frost.
(d). Experimental: field trials
Solidago canadensis was grown in assemblages of prairie plants along a pre-industrial (subambient) to projected Ca gradient (500 ppm) in the Lysimeter CO2 Gradient (LYCOG) facility located in central Texas USA (31°05′ N, 97°20′ W). The LYCOG consists of two transparent and tunnel-shaped chambers, aligned in parallel along a north–south axis. At present, this is the only field-based facility capable of exposing plant assemblages to a continuous gradient of Ca spanning pre-industrial to elevated concentrations [44,45].
Each chamber in LYCOG is divided into 10 consecutive compartments each 5 m long and 1.2 m (LYCOG) wide and tall. Chambered vegetation was enclosed in a transparent polyethylene film. Photosynthesis by enclosed vegetation progressively depleted the CO2 concentration in air as it was moved by blowers towards the air outlet of each chamber to create daytime CO2 gradients of 500–395 ppm (elevated chamber) and 395–250 ppm (subambient chamber). Night-time CO2 concentrations were regulated at 130–150 ppm above daytime values along each chamber. Air temperature and vapour pressure deficit were regulated near ambient values by cooling and dehumidifying air at 5 m intervals along chambers. CO2 treatments are maintained each growing season from April through to mid-November.
The LYCOG facility was constructed atop 1.2 m wide × 1.6 m deep steel containers that were buried to 1.2 m depth and into which were placed intact soil monoliths (each 1 × 1 × 1.5 m deep) of three soil types: silty clay, clay and sandy loam [44]. Four perennial C4 grass species and three perennial C3 forb species, all characteristic of tallgrass prairie in central Texas, were transplanted into each monolith in June 2003, 3 years prior to CO2 treatment [44,45]. Eventual dominants included the C4 grasses Bouteloua curtipendula (Michx.) Torr. and Sorghastrum nutans (L.) Nash and the forb S. canadensis (Canada goldenrod). CO2 treatments were initiated in 2006. Each monolith in the LYCOG was irrigated twice weekly during each growing season. Irrigation was applied to simulate the seasonal distribution and average of growing season precipitation in central Texas (560 mm). Solidago canadensis was sufficiently abundant to assess relationships between pollen N and Ca only for monoliths from the clay soil.
In early October of 2012 and again in 2014, we collected 10–15 floral branches (3–6 cm in length) with pollen-bearing anthers from monoliths of the clay soil along the Ca gradient (i.e. from 280 to 500 ppm). (Samples were also taken in 2013, but were not analysed owing to the government shutdown.) Floral collections were accessed through zippered-openings in the polyethylene film enclosing vegetation. Samples were collected from each inflorescence present in each monolith (more than three flowering stems/monolith), combined for a given monolith, and stored in labelled vials. All vials were sent to Beltsville, MD, USA where they were processed as with the historical samples. The Ca to which plants in each monolith was exposed was calculated from the linear relationship between Ca and the physical position of each monolith along the gradient [44,45]. The effect of Ca on floral quality did not differ as a function of year, so both years were combined for analysis; however, S. canadensis biomass production was not correlated with Ca for either year (p = 0.26 and 0.87 in 2012 and 2014, respectively). The forb contributed an average of 12.5% of above-ground production of prairie assemblages along the Ca gradient.
(e). Carbon, hydrogen, nitrogen analysis
Elemental concentrations of carbon and nitrogen were determined using a Perkin-Elmer 2400 CHN/O analyser (Perkin-Elmer, Waltham, MA, USA). Samples from all floral branches for a given specimen/sample were collected and pooled, and three subsamples were run to establish an average value. Nitrogen and carbon content were determined as a percentage of the dry weight of the sample. Because it has been used previously for assessing nitrogen to protein conversion for pollen in bee diets, a conversion factor (N to protein) of 6.25 was used [46]. The protein concentration reported for S. canadensis in this study for ambient Ca is consistent with that of other studies [47].
(f). Statistical considerations
For analysis of both historical and experimental data, we used regression analysis to test for significance between Ca and the response variable using Sigmaplot (v. 12, 2014). We tested different functions for data fitness and selected the model with the highest adjusted r2. If no differences were evident, we report results for the linear ‘best-fit’ model.
3. Results
Although there are a number of older quantitative estimates of demography and predominance of S. canadensis and related taxa (e.g. S. altissima) [38]; we wished to update these data by documenting the floral dominance and pollinator visits for Solidago during the autumn, including domesticated and wild bees. We observed that of 11 718 flowering stems, 10 993 (87%) are Solidago with S. altissima representing 5964 (50%) and Apis, or honeybees, being the dominant pollinator (figure 1a,b). The relative abundance of Solidago observed was consistent with previous observations (e.g. [38]).
Figure 1.
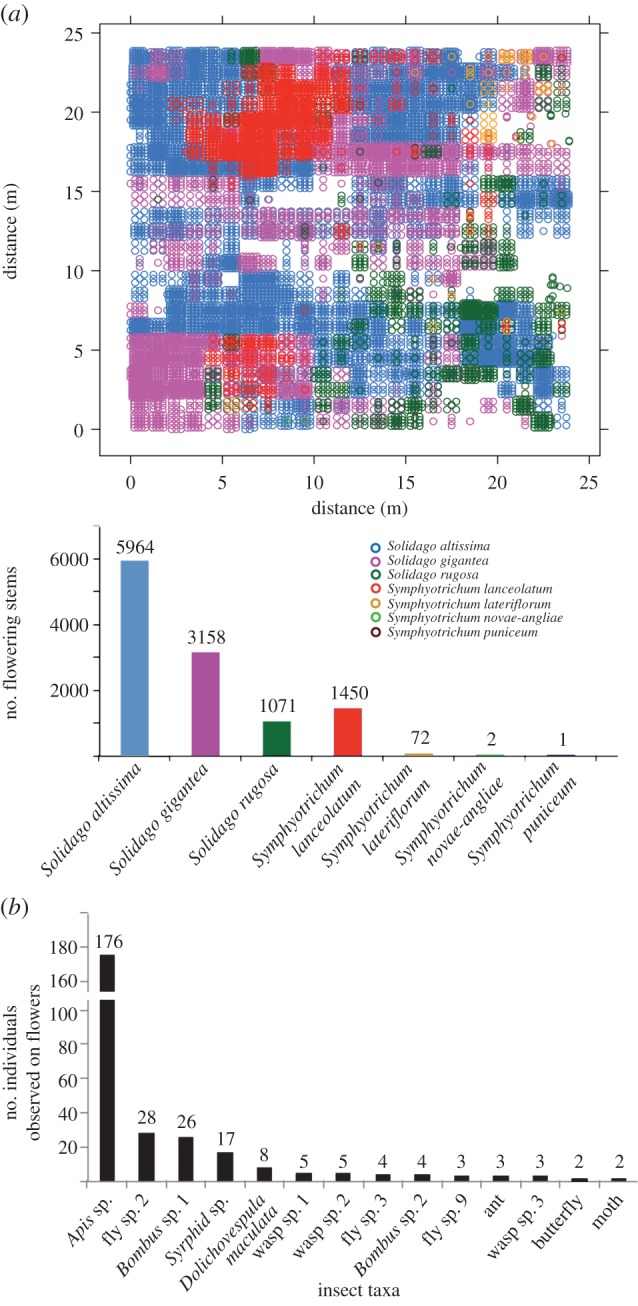
Widely distributed over North America, goldenrod (Solidago spp.) is a key floral pollen source for pollinator populations just prior to winter. Canada goldenrod (S. canadensis) is the largest goldenrod taxon and includes a number of taxa that have species status such as S. altissima [36] (http://plants.usda.gov/core/profile?symbol=soal6). (a) Map of all flowering stems (Sep–Oct) in a 24 × 24 m area plotted by species. (b) Frequency distribution of insect visitors to 1 m2 quadrats within map based on 750 min of observations (Sep–Oct). Among pollinator populations, Apis, or honeybees are the most frequent visitors. See Material and methods for additional details.
Having confirmed the dominant role of Solidago in pollen availability during the autumn; we then determined whether Ca influenced the nutritional value of S. canadensis pollen. We employed two independent methodological approaches. Both approaches; one historical, one experimental, make use of a continuous Ca gradient. Results from continuous Ca gradients can be highly informative, as plant properties, including tissue chemistry, do not always respond in a linear manner with ambient Ca versus 2× ambient Ca comparisons [48].
Historical data were obtained through the Smithsonian Institution's National Museum of Natural History archives. These archives contain floral S. canadensis plant samples collected between 1842 and 1998 across a wide range of biogeographic locations throughout the USA and southern Canada. In addition, we supplemented these historical data with S. canadensis samples obtained in situ from Maryland (2008, 2012, 2014) and Texas (2012, 2014). The increase in Ca, from the onset of the industrial revolution to the beginning of the twenty-first century, was highly correlated with the observed decline in pollen protein (r2 = 0.81, p < 0.001) with overall pollen protein declining by approximately one-third (from approx. 18 to 12%; figure 2a). Although the entire Ca record is over a 170 year period, the bulk of the Ca increase has, in fact, occurred since the latter half of the twentieth and early twenty-first century (i.e. Ca has risen from approx. 315 ppm in 1960 to 398 ppm in 2014); consequently, the largest decrease in pollen protein for S. canadensis has occurred during that time. The observed decrease in protein is concomitant to a parallel increase in the ratio of carbon to nitrogen (figure 2b). Such an increase is consistent with previous studies and is likely to indicate more substantial increases in carbohydrate to protein ratio as increasing Ca tends to increase the concentration of starch and sugars while reducing the concentration of protein (nitrogen) in plant tissues (e.g. [5,9]).
Figure 2.
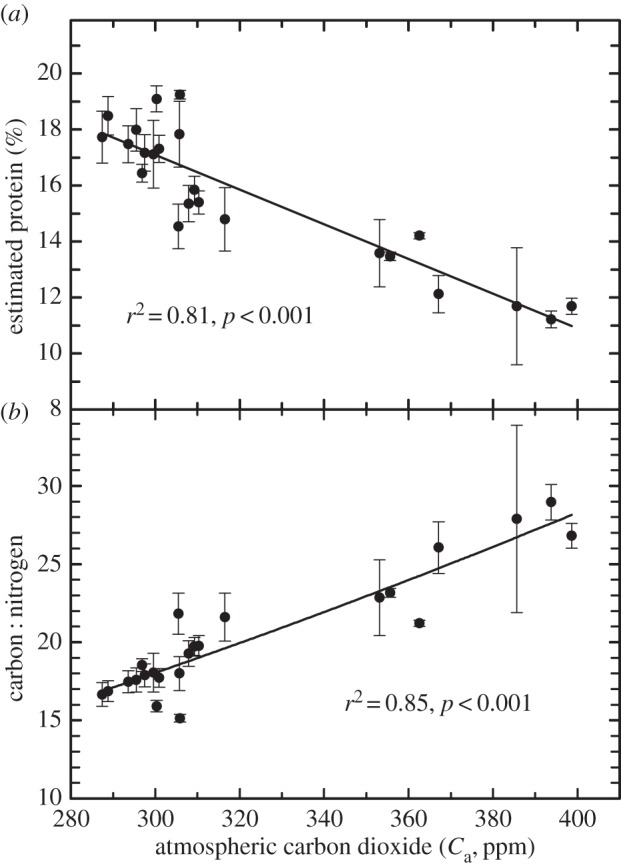
Average and variation (±s.e.) with time (1842–1998) in estimated protein concentration (a) and carbon to nitrogen ratio (b) for historical samples from floral (anthers and pollen) tissue for S. canadensis from the Smithsonian Natural History Museum. Atmospheric CO2 (Ca) for a given set of sample dates were obtained prior to 1960 from [49]; after 1960 using http://www.esrl.noaa.gov/gmd/ccgg/trends/. Each point is the average of approximately 6–40 samples by year from different biogeographic regions within North America. See Material and methods for additional details.
The experimental study was conducted in situ, using parallel, elongated chambers to maintain a continuous Ca gradient spanning pre-industrial to projected mid-twenty-first century (500 ppm) concentrations [44,45]. Carbon and nitrogen were quantified and pollen protein estimated for S. canadensis flowers grown along this Ca continuum during 2012 and 2014. Although the absolute numbers differed, the pollen-Ca pattern observed was consistent with that derived from historical data; i.e. at higher Ca levels along the experimental tunnels, pollen protein concentration declined and carbon : nitrogen ratios increased (figure 3a,b). In addition, both approaches showed a similar decline in protein content and concurrent increase in carbon : nitrogen ratio for S. canadensis pollen in response to increased Ca.
Figure 3.
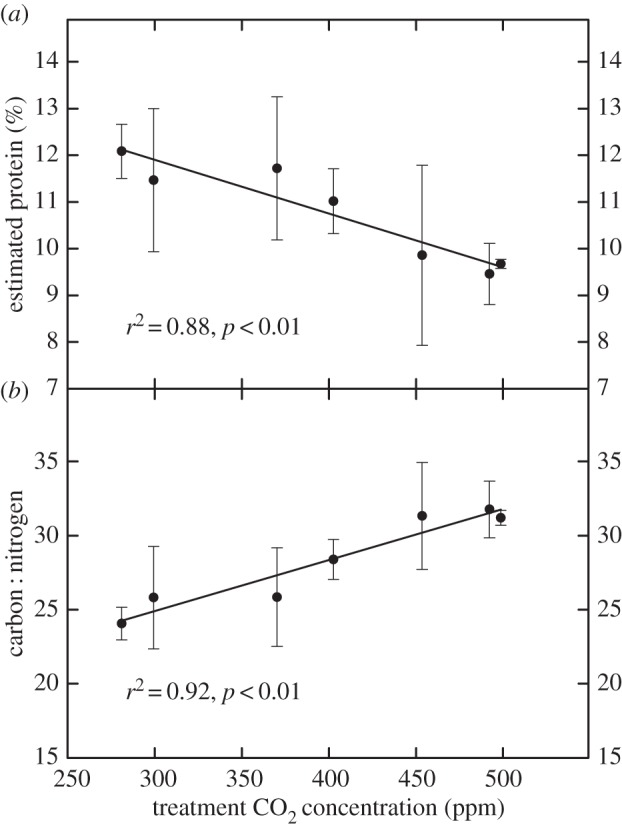
Average and variation (±s.e.) in estimated protein concentration (a) and carbon to nitrogen ratio (b) for experimental samples from floral (anthers and pollen) tissue for S. canadensis growing along a continuous gradient of Ca in clay soil for a mixed prairie community. Ca treatments were initiated in 2006. Data were averaged for 2012 and 2014 for each Ca treatment sampled within the LYCOG facility, n = 6–14 per Ca.
4. Discussion
The decline in pollen protein concentration with Ca for S. canadensis observed in both the historical and experimental analyses is consistent with many studies and meta-analyses which have shown that increased Ca systemically reduces nitrogen and protein concentration in non-leguminous plant tissues [1–6,8,9]. Decreases in nitrogen concentration have also been reported for herbaria plant specimens in response to the increases in Ca during the twentieth century [50]. Overall, the data from the current study provide strong evidence that rising Ca since the start of the industrial age has, and will continue in the near term, to reduce the pollen protein concentration of Solidago, an important autumn pollen source for bees and other pollinators.
Because pollen provides all of the essential amino acids needed for bee development and can, in turn, affect hypopharyngeal gland and ovary development, pathogen susceptibility, immunocompetence and overall bee longevity [21,25,51–53], reductions in pollen protein concentration of Solidago have the potential to negatively affect bee health and survival.
These results for Solidago may be particularly relevant to potential health impacts for bees as it is the source of some of the last seasonal pollen acquired prior to winter, and thus constitute the nutrient load available for overwintering. Bees that overwinter require substantial pollen stores because late winter brood rearing occurs prior to the availability of spring pollen in temperate zones. For example, Farrar [54] found that the spring bee population as a percentage of the autumn population was positively correlated with the amount of stored pollen. In general, for temperate climates, early spring is recognized as a time of pollen protein shortages and colony starvation [22,27,35].
But can bees distinguish protein concentration among diverse pollen sources; and by doing so, compensate for any Ca-induced decrease? It has long been known that honeybees can differentiate sugar content among nectar sources and convey such information to the colony (e.g. the ‘waggle dance’). However, while amino acid composition has been suggested as a learned aspect of honeybee protein foraging [55]; the overall consensus is that bees do not collect high-quality pollen preferentially [56,57].
There is widespread agreement then that: (i) pollen protein is an essential aspect of bee diet, and (ii) bees do not appear able to compensate by choosing other floral sources with higher protein concentration. Given the temporal importance of autumn pollen in pollinator life cycles in North America, the increase in Ca associated with climatic change and the resultant decline in pollen protein concentration of S. canadensis, could adversely affect bee health and overwintering capacity on a continental scale.
However, there are a number of caveats that need to be considered. First, it cannot be assumed that projected increases in Ca above those considered here will be proportionate to protein loss; i.e. there is the potential for saturating effects on protein concentration with rising Ca. Second, the subsequent influence of reduced protein concentration from S. canadensis on bee feeding, health or demographics has not been explicitly determined. Finally, whether Ca is also resulting in similar reductions in pollen protein in other floral species needs to be quantified (e.g. [58]). Overall the specific consequences of declining pollen concentration are likely to be dependent on these factors, as well as other environmental characteristics (e.g. Varroa, neonicotinoids), percentage of Solidago among flowering species in the autumn, etc. These environmental parameters and their potential interactions will require further elucidation in the context of Ca-induced nutritional changes in order to fully quantify impacts to bee health and population stability.
Although additional information is clearly needed, the current data do indicate a clear and unequivocal link, both historically and experimentally, between rising Ca and a qualitative decline in pollen protein for S. canadensis; among the most widely recognized and widely available food source for bees in North America (e.g. [43]). Given the economic and environmental importance of bees, and because the rise in Ca is global in nature, these data provide an urgent and compelling case for establishing the Ca sensitivity of pollen protein for other floral species and, in turn, quantifying the potential consequences for pollinator physiology around the globe.
Acknowledgements
The authors thank the staff and taxonomists at the Smithsonian Museum of Natural History; and Dr Dennis vanEngelsdorp of the University of Maryland and Dr Scott Cornman of the US Geological Survey for their comments and suggestions to the manuscript. We thank the 2010 Ecology class at Williams College for assistance in mapping the Asteraceae in figure 1. The suggestions and comments from Dr Marla Spivak of the University of Minnesota and Ms Allison Crimmons, Environmental Scientist at the EPA are also gratefully acknowledged.
Data accessibility
Data are available at http://dx.doi.org/10.5061/dryad.8bb66.
Authors' contributions
L.H.Z. conceived the experiments. A.C. provided the herbarium samples; J.E and J.E.H. provided the data on plant demographics of autumn Solidago and insect visitations; H.W.P. provided the LYCOG samples; M.B.T. did the CHN analysis; L.H.Z. wrote the paper; J.S.P, M.B.T., J.S.D., I.L. and H.W.P. analysed the data and/or edited the manuscript.
Competing interest
Authors acknowledge no competing interests.
Funding
We received no funding for this study.
References
- 1.Cotrufo MF, Ineson P, Scott A. 1998. Elevated CO2 reduces the nitrogen concentration of plant tissues. Glob. Change Biol. 4, 43–54. ( 10.1046/j.1365-2486.1998.00101.x) [DOI] [Google Scholar]
- 2.Jablonski LM, Wang X, Curtis PS. 2002. Plant reproduction under elevated CO2 conditions: a meta-analysis of reports on 79 crop and wild species. New Phytol. 156, 9–26. ( 10.1046/j.1469-8137.2002.00494.x) [DOI] [Google Scholar]
- 3.Taub DR, Miller B, Allen H. 2008. Effects of elevated CO2 on the protein concentration of food crops: a meta-analysis. Glob. Change Biol. 14, 565–575. ( 10.1111/j.1365-2486.2007.01511.x) [DOI] [Google Scholar]
- 4.Loladze I. 2002. Rising atmospheric CO2 and human nutrition: toward globally imbalanced plant stoichiometry? Trends Ecol. Evol 17, 457–461. ( 10.1016/S0169-5347(02)02587-9) [DOI] [Google Scholar]
- 5.Loladze I. 2014. Hidden shift of the ionome of plants exposed to elevated CO2 depletes minerals at the base of human nutrition. eLife 3, e02245 ( 10.7554/eLife.02245) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conroy J, Hocking P. 1993. Nitrogen nutrition of C3 plants at elevated atmospheric CO2 concentrations. Physiol. Plant 89, 570–576. ( 10.1111/j.1399-3054.1993.tb05215.x) [DOI] [Google Scholar]
- 7.McDonald EP, Erickson JE, Kruger EL. 2002. Research note: can decreased transpiration limit plant nitrogen acquisition in elevated CO2? Funct. Plant Biol. 29, 1115–1120. ( 10.1071/FP02007) [DOI] [PubMed] [Google Scholar]
- 8.McGrath JM, Lobell DB. 2013. Reduction of transpiration and altered nutrient allocation contribute to nutrient decline of crops grown in elevated CO2 concentrations. Plant Cell Environ. 36, 697–705. ( 10.1111/pce.12007) [DOI] [PubMed] [Google Scholar]
- 9.Taub DR, Wang X. 2008. Why are nitrogen concentrations in plant tissues lower under elevated CO2? A critical examination of the hypotheses. J. Integ. Plant Biol. 50, 1365–1374. ( 10.1111/j.1744-7909.2008.00754.x) [DOI] [PubMed] [Google Scholar]
- 10.Myers SS, et al. 2014. Increasing CO2 threatens human nutrition. Nature 510, 139–142. ( 10.1038/nature13179) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, Kunin WE. 2010. Global pollinator declines: trends, impacts and drivers. Trends Ecol. Evol. 25, 344–353. ( 10.1016/j.tree.2010.01.007) [DOI] [PubMed] [Google Scholar]
- 12.vanEngelsdorp D, Hayes J, Underwood RM, Pettis J. 2008. A survey of honey bee colony losses in the U.S., fall 2007 to spring 2008. PLoS ONE 3, e4071 ( 10.1371/journal.pone.0004071) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winfree R, Aguilar R, Vázquez DP, LeBuhn G, Aizen MA. 2009. A meta-analysis of bees’ responses to anthropogenic disturbance. Ecology 90, 2068–2076. ( 10.1890/08-1245.1) [DOI] [PubMed] [Google Scholar]
- 14.Cameron SA, Lozier JD, Strange JP, Koch JB, Cordes N, Solter LF, Griswold TL. 2011. Patterns of widespread decline in North American bumble bees. Proc. Natl Acad. Sci. USA 108, 662–667. ( 10.1073/pnas.1014743108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith KM, Loh EH, Rostal MK, Zambrana-Torrelio CM, Mendiola L, Daszak P. 2013. Pathogens, pests, and economics: drivers of honeybee colony declines and losses. EcoHealth 10, 434–445. ( 10.1007/s10393-013-0870-2) [DOI] [PubMed] [Google Scholar]
- 16.Wilfert L, Long G, Leggett HC, Schmid-Hempel P, Butlin R, Martin SJM, Boots M. 2016. Deformed wing virus is a recent global epidemic in honeybees driven by Varroa mites. Science 351, 594–597. ( 10.1126/science.aac9976) [DOI] [PubMed] [Google Scholar]
- 17.Mullin CA, Frazier M, Frazier JL, Ashcraft S, Simonds R, vanEngelsdorp D, Pettis JS. 2010. High levels of miticides and agrochemicals in North American apiaries: implications for honey bee health. PLoS ONE 5, e9754 ( 10.1371/journal.pone.0009754) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tuell JK, Fiedler AK, Landis D, Isaacs R. 2008. Visitation by wild and managed bees (Hymenoptera: Apoidea) to eastern U.S. native plants for use in conservation programs. Comm. Ecosys. Ecol. 37, 707–718. ( 10.1603/0046-225x(2008)37%5B707:vbwamb%5D2.0.co;2) [DOI] [PubMed] [Google Scholar]
- 19.Mayack C, Naug D. 2009. Energetic stress in the honeybee Apis mellifera from Nosema ceranae infection. J. Invertebr. Pathol. 100, 185–188. ( 10.1016/j.jip.2008.12.001) [DOI] [PubMed] [Google Scholar]
- 20.Naug D, Gibbs A. 2009. Behavioral changes mediated by hunger in honeybees infected with Nosemaceranae. Apidologie 40, 595–599. ( 10.1051/apido/2009039) [DOI] [Google Scholar]
- 21.Alaux C, Ducloz F, Crauser D, Le Conte Y. 2010. Diet effects on honeybee immunocompetence. Biol. Lett. 6, 562–565. ( 10.1098/rsbl.2009.0986) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.vanEngelsdorp D, et al. 2010. Weighing risk factors associated with bee colony collapse disorder by classification and regression tree analysis. J. Econ. Entomol. 103, 1517–1523. ( 10.1603/EC09429) [DOI] [PubMed] [Google Scholar]
- 23.Wahl O, Ulm K. 1983. Influence of pollen feeding and physiological condition on pesticide sensitivity of the honey bee Apis mellifera carnica. Oecologia 59, 106–128. ( 10.1007/BF00388082) [DOI] [PubMed] [Google Scholar]
- 24.Mao W, Shuler MA, Berenbaum MR. 2013. Honey constituents up-regulate detoxification and immunity genes in the western honey bee, Apis mellifera. Proc. Natl Acad. Sci. USA 110, 8763–8764. ( 10.1073/pnas.1306617110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeGroot AP. 1953. Protein and amino acid requirements of the honeybee (Apid mellifica L.). Physiol. Comp. Oecol. 3, 197–285. [Google Scholar]
- 26.Haydak MH. 1970. Honey bee nutrition. Annu. Rev. Entomol. 15, 143–156. ( 10.1146/annurev.en.15.010170.001043) [DOI] [Google Scholar]
- 27.Brodschneider R, Crailsheim K. 2010. Nutrition and health in honey bees. Apidologie 41, 278–294. ( 10.1051/apido/2010012) [DOI] [Google Scholar]
- 28.Schmickl T, Crailsheim K. 2001. Cannibalism and early capping: strategies of honeybee colonies in times of experimental pollen shortages. J. Comp. Physiol. 187, 541–547. ( 10.1007/s003590100226) [DOI] [PubMed] [Google Scholar]
- 29.Schmickl T, Crailsheim K. 2002. How honeybees (Apis mellifera L.) change their broodcare behavior in response to non-foraging conditions and poor pollen conditions. Behav. Ecol. Sociobiol. 51, 415–425. ( 10.1007/s00265-002-0457-3) [DOI] [Google Scholar]
- 30.Schmid-Hempel P, Winston ML, Ydenbreg RC. 1993. Foraging of individual workers in relation to colony state in the social Hymenoptera. Can. Entomol. 125, 129–160. ( 10.4039/Ent125129-1) [DOI] [Google Scholar]
- 31.Crailsheim K. 1990. The protein balance of the honey bee worker. Apidologie 21, 417–429. ( 10.1051/apido:19900504) [DOI] [Google Scholar]
- 32.Eischen FA, Rothenbuhler WC, Kulincevic JM. 1982. Length of life and dry weight of worker honeybees reared in colonies with different worker-larva rations. J. Apicult. Res. 21, 19–25. [Google Scholar]
- 33.Herbert EW, Shimanuki H, Caron DM. 1977. Optimum protein levels required by honeybees (Hymenoptera, Apidae) to initiate and maintain brood rearing. Apidologie 8, 141–146. ( 10.1051/apido:19770204) [DOI] [Google Scholar]
- 34.Li C, Xu B, Wang Y, Yang Z, Yang W. 2014. Protein content in larval diet affects adult longevity and antioxidant gene expression in honey bee workers. Ento Exp. Appicata 151, 19–26. ( 10.1111/eea.12167) [DOI] [Google Scholar]
- 35.Mattila HR, Otis GW. 2006. Influence of pollen diet in spring on development of honey bee (Hymenoptera: Apidae) colonies. J. Econ. Entomol. 99, 604–613. ( 10.1093/jee/99.3.604) [DOI] [PubMed] [Google Scholar]
- 36.Werner PA, Bradbury IK, Gross RS. 1980. The biology of Canadian weeds. 45. Solidago canadensis L. Can. J. Plant Sci. 60, 193–1409. ( 10.4141/cjps80-194) [DOI] [Google Scholar]
- 37.Stimec J, Scott-Dupree CD, McAndrews JH. 1997. Honey bee, Apis mellifera, pollen foraging in southern Ontario. Can. Field-Nat. 111, 454–456. [Google Scholar]
- 38.Ginsberg HS. 1983. Foraging ecology of bees in an old field. Ecology 64, 165–165. ( 10.2307/1937338) [DOI] [Google Scholar]
- 39.Sponsler DB, Johnson RM. 2015. Honey bee success predicated by landscape composition in Ohio, USA. Peer J. 3, e838 ( 10.7717/peerj.838) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fye RE, Medler JT. 1954. Spring emergence and floral hosts of Wisconsin bumblebees Wise. Acad. Sci. Arts Lett. 43, 75–82. [Google Scholar]
- 41.Evans FC. 1986. Bee–flower interactions on an old field in southeastern Michigan. In Proc. of the 9th North American Prairie Conf., Moorhead, Minnesota, 1984. (eds Clambey GK, Pemble RH), pp. 103–109. Fargo, North Dakota: Tricollege University Centre for Environmental Studies. [Google Scholar]
- 42.Haydak MH. 1962. Beekeeping in Minnesota Haydak MH. Revised, 21 pages. St Paul, MN: University of Minnesota, Agricultural Extension Service.
- 43.Oertel E. 1967. Nectar and pollen plants. US Dep. Agr. Handbook. 335, 16–23. [Google Scholar]
- 44.Fay PA, Kelley AM, Procter AC, Hui D, Jin VL, Jackson RB, Johnson HB, Polley HW. 2009. Primary productivity and water balance of grassland vegetation on three soils in a continuous CO2 gradient: initial results from the Lysimeter CO2 Gradient Experiment. Ecosys 12, 699–714. ( 10.1007/s10021-009-9247-3) [DOI] [Google Scholar]
- 45.Polley HW, Johnson HB, Fay PA, Sanabria J. 2008. Initial response of evapotranspiration from tallgrass prairie vegetation to CO2 at subambient to elevated concentrations. Funct. Ecol. 22, 163–171. ( 10.1111/j.1365-2435.2007.01351.x) [DOI] [Google Scholar]
- 46.Keller I, Fluri P, Imdorf A. 2005. Pollen nutrition and colony development in honey bees. Part I. Bee World 86, 3–10. ( 10.1080/0005772X.2005.11099641) [DOI] [Google Scholar]
- 47.Pernel SF, Currie RW. 2001. The influence of pollen quality on foraging behavior in honeybees (Apis mellifera L.). Behav. Ecol. Sociobiol. 51, 53–68. ( 10.1007/s002650100412) [DOI] [Google Scholar]
- 48.Gill RA, Polley H, Johnson HB, Anderson LJ, Maherali H, Jackson RB. 2002. Nonlinear grassland responses to past and future atmospheric CO2. Nature 417, 279–282. ( 10.1038/417279a) [DOI] [PubMed] [Google Scholar]
- 49.Friedli H, Lötscher H, Oeschger H, Siegenthaler U, Stauffer B. 1986. Ice core record of 13C/12C ratio of atmospheric CO2 in the past two centuries. Nature 324, 237–238. ( 10.1038/324237a0) [DOI] [Google Scholar]
- 50.Penuelas J, Estiarte M. 1997. Trends in plant carbon concentration and plant demand for N throughout this century. Oecology 109, 69–73. ( 10.1007/s004420050059) [DOI] [PubMed] [Google Scholar]
- 51.Rinderer TE, Rothenbuhler WC, Gochnauer TA. 1974. The influence of pollen on the susceptibility of honey-bee larvae to Bacillus larvae. J. Invertebr. Pathol. 23, 347–350. ( 10.1016/0022-2011(74)90100-1) [DOI] [PubMed] [Google Scholar]
- 52.Rinderer TE, Elliott KD. 1977. Worker honey bee response to infection with Nosema apis: influence of diet. J. Econ. Entomol. 70, 431–433. ( 10.1093/jee/70.4.431) [DOI] [Google Scholar]
- 53.Schmidt JO, Thoenes SC, Levin MD. 1987. Survival of honey bees, Apis mellifera (Hymenoptera: Apidae), fed various pollen sources. Annu. Entomol. Soc. Am. 80, 176–183. ( 10.1093/aesa/80.2.176) [DOI] [Google Scholar]
- 54.Farrar CL. 1936. Influence of pollen reserves on the surviving populations of over-wintered colonies. Am. Bee J. 76, 452–454. [Google Scholar]
- 55.Cook SM, Awmack CS, Murray DA, Williams IH. 2003. Are honey bees’ foraging preferences affected by pollen amino acid composition? Ecol. Entomol. 28, 622–627. ( 10.1046/j.1365-2311.2003.00548.x) [DOI] [Google Scholar]
- 56.Roulston TAH, Cane JH, Buchmann SL. 2000. What governs protein content of pollen: pollinator preferences, pollen-pistil interactions, or phylogeny? Ecol. Monogr. 70, 617–643. ( 10.1890/0012-9615(2000)070%5B0617:WGPCOP%5D2.0.CO;2) [DOI] [Google Scholar]
- 57.Roulston TH, Cane JH. 2002. The effect of pollen protein concentration on body size in the sweat bee Lasioglossum zephyrum (Hymenoptera: Apiformes). Evol. Ecol. 16, 49–65. ( 10.1023/A:1016048526475) [DOI] [Google Scholar]
- 58.Singer BD, Ziska LH, Frenz DA, Gebhard DE, Straka JG. 2005. Research note: increasing Amb a 1 content in common ragweed (Ambrosia artemisiifolia) pollen as a function of rising atmospheric CO2 concentration. Funct. Plant Biol. 7, 667–670. ( 10.1071/FP05039) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available at http://dx.doi.org/10.5061/dryad.8bb66.


