Abstract
Some of the most promising vaccines in the pipeline for tuberculosis (TB) target adolescents and adults. Unlike for childhood vaccines, high-coverage population-wide vaccination is significantly more challenging for adult vaccines. Here, we aimed to estimate the impact of vaccine delivery strategies that were targeted to high-incidence geographical ‘hotspots’ compared with randomly allocated vaccination. We developed a spatially explicit mathematical model of TB transmission that distinguished these hotspots from the general population. We evaluated the impact of targeted and untargeted vaccine delivery strategies in India—a country that bears more than 25% of global TB burden, and may be a potential early adopter of the vaccine. We collected TB notification data and conducted a demonstration study in the state of Gujarat to validate our estimates of heterogeneity in TB incidence. We then projected the impact of randomly vaccinating 8% of adults in a single mass campaign to a spatially targeted vaccination preferentially delivered to 80% of adults in the hotspots, with both strategies augmented by continuous adolescent vaccination. In consultation with vaccine developers, we considered a vaccine efficacy of 60%, and evaluated the population-level impact after 10 years of vaccination. Spatial heterogeneity in TB notification (per 100 000/year) was modest in Gujarat: 190 in the hotspots versus 125 in the remaining population. At this level of heterogeneity, the spatially targeted vaccination was projected to reduce TB incidence by 28% after 10 years, compared with a 24% reduction projected to achieve via untargeted vaccination—a 1.17-fold augmentation in the impact of vaccination by spatially targeting. The degree of the augmentation was robust to reasonable variation in natural history assumptions, but depended strongly on the extent of spatial heterogeneity and mixing between the hotspot and general population. Identifying high-incidence hotspots and quantifying spatial mixing patterns are critical to accurate estimation of the value of targeted intervention strategies.
Keywords: tuberculosis, spatially targeted tuberculosis vaccine, mathematical model, tuberculosis in India
1. Introduction
Despite the availability of affordable and effective treatment, tuberculosis (TB) remains one of the largest sources of infectious disease burden. There are estimated 9 million new TB cases and 1.5 million deaths every year, most of which occur in resource-limited settings [1]. The Stop TB Partnership's post 2015 End TB strategy aims to reduce TB globally by 50% from 2015 to 2025, and through 90% by 2035. But given modest levels of current decline in TB incidence (1.5% per year), these goals can only be achieved by rapidly increasing the rates of declines in TB incidence. Two critical factors have been identified by the World Health Organization that can accelerate this decline: (i) optimal use of current and new tools and (ii) introduction of new innovations, such as new drugs and new vaccines, which have the potential to substantially reduce TB prevalence [2].
In this context, one potential avenue for optimization of current and future resources may be to take advantage of geographical heterogeneity in TB prevalence, a phenomenon also observed across many other disease systems [3,4]. In these other systems, many successful control strategies have targeted such heterogeneity [5–7]. Factors associated with risk of TB, including socio-economic status [8–13], living conditions [14], migration status [15] and prevalence of HIV [16,17], tend to be geographically heterogenous themselves—thereby also leading to heterogeneity in TB incidence [18]. Targeting these high-incidence geographical ‘hotspots’ of TB can have a twofold effect. First, targeting the high-incidence setting would serve to directly protect the population that is at relatively higher risk of TB. Second, ‘hotspots’ can serve as drivers in fueling the TB epidemic in the general community, and targeting them may indirectly serve to also protect the general population [19]. Hence, it is important to examine the effectiveness of intervention strategies that target TB hotspots, relative to strategies that do not discriminate between geographical locations on the basis of TB incidence.
Vaccines are among the interventions in TB control that hold the greatest promise for approaching or achieving the aggressive targets discussed above [20]. The last decade has seen rapid progress in the development of TB vaccines, generating hope that such a vaccine may become available in the near future [21,22]. Unlike many infectious diseases, some of the most promising vaccines for TB target adolescents and adults rather than children. Since high-coverage mass vaccination may be difficult to achieve among adolescents and adults, it is important to design effective and efficient vaccine implementation strategies that maximize the impact for the resources and effort spent. Hence, this provides a unique opportunity to assess the impact of an intervention that is both a technological innovation and dependent on appropriate use of existing data for optimal implementation.
In this study, we aimed to estimate the relative benefit of targeting an intervention, in the form of a hypothetical adult TB vaccine, to geographical hotspots and to identify key factors that affect their value. We developed a dynamic model of TB transmission in a population that consisted of high-incidence TB hotspots. We modelled vaccination campaigns in which vaccines were delivered either in a targeted manner to these hotspots or in an untargeted fashion to the rest of the population but used the same number of vaccine doses. We compared the impact of the two strategies and estimated the relative benefit of the hotspot-targeted vaccine strategies. We calibrated our model to represent a setting of the state of Gujarat in India. India is home to 25% of all new TB cases worldwide, more than twice the total burden in any other country [1], making it the likely centerpiece of any global TB control strategy. The state of Gujarat is representative of the country, in terms of both TB incidence and population density [23]. The Revised National Tuberculosis Control Program (RNTCP) in Gujarat maintains an active programme of TB surveillance and data reporting, and Gujarat has higher quality infrastructure than many other states, thereby making Gujarat a potential early implementer of a new TB vaccine.
We collected TB notification data to quantify the level of spatial heterogeneity, and identified areas of high TB incidence in Gujarat. We conducted demonstration study to validate the observed heterogeneity in the notification data. Subsequently, we incorporated heterogeneity in the transmission model, and using this model, we evaluated the impact of vaccine strategies that targeted geographical hotspots compared with ones that were untargeted but used the same number of vaccine doses.
2. Material and methods
2.1. Data
2.1.1. Tuberculosis incidence
Data on TB incidence used in this study came from the state of Gujarat in India as notified by the RNTCP. Located in the midwestern part of the country, Gujarat has a population of over 60 million, and is both representative of the country and home to a well-functioning state-wide TB control office. We obtained data from the Indian RNTCP at the level of the subdistrict/TB Unit (TU)—an administrative unit for TB control that covers a source population of approximately 500 000 people each. These data were obtained for all TUs throughout the state of Gujarat, on a quarterly basis, from the first quarter of 2009 to the second quarter of 2012, for a total of 14 quarters. The TU was the smallest administrative level at which vaccination strategies are likely to be implemented through the public sector. The average incidence of total TB cases in Gujarat during this period was 132 per 100 000 per year. Data aggregated at the level of TUs are shown in figure 1, arranged in descending order of TB incidence from left to right.
Figure 1.
Spatial heterogeneity in TB incidence in Gujarat, and identification of ‘hotspots’. Incidence of TB at the level of TB units (n = 151) across the state of Gujarat, arranged in descending order of notified incidence from left to right. Notifications were based on the RNTCP's quarterly reports of TB cases from Gujarat, averaged between the first quarter of 2009 to the second quarter of 2012, inclusive. The vertical lines represent the interquartile range in the reported quarterly incidences. The incidence of TB on average across Gujarat was approximately 132 per 100 000 (indicated by the grey dashed horizontal line). The TB incidence among the highest 10% of TB units, which were collectively defined as ‘hotspots’ for this analysis, was 190 per 100 000, indicated by the dashed red line; these 17 TB units are indicated by blue dots in the map of Gujarat in the upper right. The TB incidence in the other 90% of the population was 125 per 100 000, indicated by the dashed blue line.
2.2. Demonstration study
We verified the reporting practices at the TU level by conducting a demonstration study in 15 district microscopy centres (DMCs), which are TB facilities that aim to serve an underlying population of 100 000 people. We selected nine DMCs with high incidence that were located within our geographically defined ‘hotspot’, and six DMCs with low incidence that were located outside of the hotspot. These are shown in electronic supplementary material, figure S-3. In each DMC, we verified the monthly reports of TB incidence by checking local records against those reported to the TU as well as through direct observation of TB diagnosis, treatment, and reporting practices in each DMC. We also used the heterogeneity in TB incidence observed at the DMC level to inform sensitivity analyses around our main model (which assumed that vaccine would be targeted at the TU level).
2.3. Model structure
The model was structured to take into account four important factors: (i) spatial heterogeneity of TB, (ii) transmission dynamics of TB, (iii) population age structure, and (iv) vaccine-derived protection.
2.3.1. Spatial heterogeneity
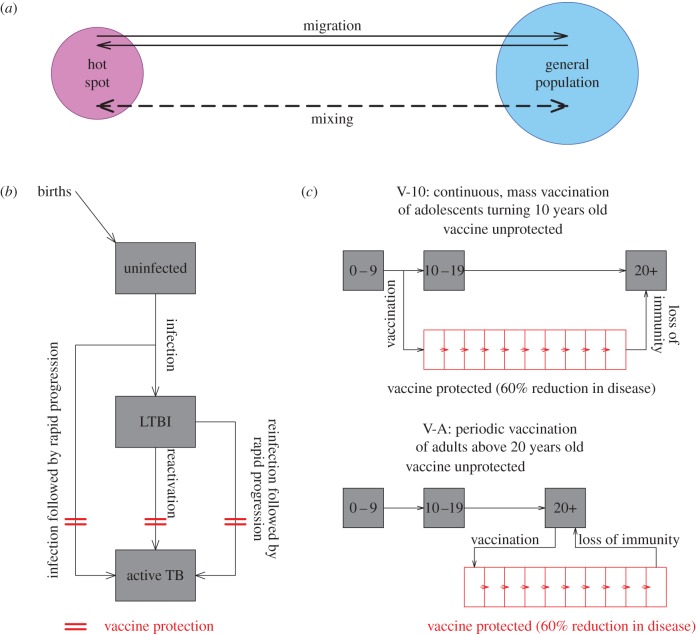
The spatial heterogeneity of TB risk was modelled by subdividing the population into two sub-populations: (i) hotspot—the sub-population with the highest incidence of TB and (ii) the remaining general population. In the base case, we modelled the hotspot to consist of those 10% of all TUs that reported the highest incidence of TB, using the mean notified value over the 14 quarters evaluated (2009–2012). The dynamics of TB transmission were considered separately in the two sub-populations. Individuals were assumed to mix homogeneously within the sub-populations, which were connected via two mechanisms: (i) migration and (ii) mixing. Migration was thought of as a permanent relocation from one sub-population to another, and modelled as explicit movement of individuals between the two sub-populations. For simplicity, the migration in and out of sub-populations (hotspot and general population) was balanced such that the size of the sub-populations remained constant. In contrast with migration, mixing was conceptualized as short-term movement of individuals between the two sub-populations. This may consist, for example, of populations that commute between sub-populations for work, business or schooling, or short-term visits for business or vacation. This was modelled as the fraction of the per capita hazard of TB infection that is generated in the home sub-population but that results in TB transmission to members of the other sub-population (σ). Hence, σ = 0 would imply two isolated sub-populations with no transmissions from an individual in one sub-population to an individual in the other sub-population; σ = 1 would imply that two sub-populations mix homogeneously; and a mixing of 3% (σ = 0.03), for example, would imply that an individual with TB in one sub-population is 0.03 times as likely to transmit to a randomly selected individual in the other sub-population as she is to transmit to a randomly selected individual in the home sub-population.
2.3.2. Transmission dynamics of tuberculosis
Following the lead of other models of TB [24,25], we generated a simple and parsimonious description of the natural history of TB for the model of transmission dynamics. In this model, the population was divided into three epidemiological compartments of TB status: individuals that are uninfected; individuals that are latently infected with TB; and individuals that are infectious with active TB disease. Upon exposure to TB, uninfected individuals may either develop latent infection or progress immediately to develop active TB. Latently infected individuals can develop active TB disease, either by endogenous reactivation or by exogenous reinfection. Only active TB was considered infectious, and transmission was modelled to be frequency-dependent. The transmission dynamics of TB were otherwise assumed to be identical in both sub-populations, except for the transmission rate, which was calibrated on the basis of the incidence levels in each sub-population. Successful treatment of TB was modelled as return to the latent class. The model is represented in figure 2, the model parameters are presented in table 1, and a set of ordinary differential equations that fully describe the model are presented in ‘Model details’ section of the electronic supplementary material.
Figure 2.
Simplified schematic of the model. (a) In this model, the population was divided geographically into two sub-populations; the hotspot and the general population. The dynamics of TB were considered separately in the two sub-populations, but allowed individuals to permanently migrate from one sub-population to another, and to mix between sub-populations (without permanently migrating). Within each sub-population, we modelled the progression of TB, vaccine dynamics (mechanism and implementation) and ageing. (b) Uninfected individuals, upon exposure to TB, could either develop latent infection (LTBI) or progress rapidly to develop active TB. Latently infected individuals could either reactivate or be exogenously reinfected to develop active TB. Successful treatment of TB was modelled as a return to the latent class. Vaccine was modelled to impart protection against TB disease and not infection (shown by red obstruction); this included rapid progression of new and re-infections, and endogenous reactivation. (c) Individuals were divided into three age categories: 0–9 years, 10–19 years and 20+ years, with progression along the age categories signifying ageing. Vaccine campaign was implemented in two parts: (i) V-10: individuals turning 10 were continuously vaccinated with a coverage of 80% and (ii) V-A: adults 20 years and above were vaccinated periodically once every 10 years with a coverage of 8%. Vaccine-derived protection was modelled to last on average for 10 years and individuals returned to the unprotected class after loss of vaccine-derived immunity.
Table 1.
Model parameters and inputs. The table lists model parameters and inputs used in the model, along with the values and ranges considered during the analyses.
| model parameters/inputs | value (range in parentheses) | references |
|---|---|---|
| per capita mortality rate for individuals actively infected with TB | 0.182 per year | [26] |
| (0.15–0.25) | ||
| fraction of infections that progress rapidly to active TB | 0.14 | [27] |
| (0.1–0.2) | ||
| per capita reactivation rate | 0.001 per year | [28] |
| (0.0005–0.002) | ||
| average duration of active TB until diagnosis and initiation of treatment | 12 months | [1] |
| (8–24) | ||
| percentage of TB cases with successful treatment | 95% | [1] |
| (85–97.5%) | ||
| relative hazard of reinfection in a host with LTBI | 0.33 | [29–32] |
| (0.25–0.5) | ||
| per capita transmission rates in hotspot general population | variable (per infectious person-year) | fit to data |
| hotspot: 6.31–10.37 | ||
| general population: 3.29–6.98 | ||
| percentage of the population in hotspot and general population | 10%, 90% | assumed |
| total TB incidence in hotspot and general population | 190, 125 per 100 000 per year | data |
| migration; percentage of population that migrated within the last year | 1% | [33] |
| (0–3%) | ||
| mixing; percentage of shared contacts between hotspot and general population | 3% | assumed |
| (1–5%) | ||
| vaccine efficacy; percentage protection against active TB | 60% | assumed |
| (40–80%) | ||
| mean duration of vaccine-derived immunity | 10 years | assumed |
2.3.3. Ageing and demography
Individuals were divided into three age categories: 0–9 years, 10–19 years and 20+ years. The choice of these specific age categories reflected vaccine campaigns that might target specific age groups. Ageing of the population was modelled as the flow of individuals between two consecutive age categories. Demographic turnovers (births and mortalities) were constrained to maintain a constant population size, with births occurring only into the TB uninfected class.
2.3.4. Vaccine-derived protection
We simulated the properties of a hypothetical TB vaccine in consultation with the Aeras Foundation (Rockville, MD, USA). Vaccine protection was modelled by subdividing the population into 12 categories, 1 representing individuals that are vaccine naive, 10 representing individuals with vaccine protection and 1 representing individuals who were vaccinated but whose vaccine protection has waned. The 10 categories of vaccine protection correspond to 1 year each, thus representing a mean of 10 years following vaccination as vaccinated populations progress from one stratum to the next. Upon moving out of the tenth year-long vaccine-protected compartment, populations move to the vaccine-unprotected state. Each vaccine-protected category is assumed to provide equal protection in the base case. The vaccine was modelled to prevent disease (whether through initial infection or reinfection with rapid progression, or through endogenous reactivation) with a given efficacy (60% in the base case) but to have no effect on infection (figure 2b). In the reference case, we assumed that the vaccine had 60% protective efficacy agains the development of TB disease.
2.4. Sensitivity and uncertainty analyses
To account for uncertainties in the parameter estimates and data, as well as to assess the sensitivity of the results to variations in these parameters and data, we carried out two kinds of sensitivity and uncertainty analyses. For natural history parameters, we carried out a multivariate uncertainty analysis. Using the reference scenario as the baseline, we conducted 5000 simulations in which all TB natural history parameters were varied uniformly across biologically plausible ranges (as provided in electronic supplementary material, figure S-7) using Latin hypercube sampling. Based on these simulations, we calculated partial ranked correlation coefficients for each of the parameters to assess the sensitivity of the results to individual parameters (see electronic supplementary material, figure S-12), and calculated corresponding uncertainty ranges in the estimates.
Additionally, to address the sensitivity of our assumptions regarding (i) vaccine efficacy, (ii) migration rates, (iii) size of the targeted hotspot, (iv) vaccine delivery, and (v) the level of vaccine coverage, we conducted several one-way and multi-way sensitivity analyses in which we varied each parameter to meaningful high and low values and reported the augmented impact of spatial vaccine targeting on TB incidence. These analyses are described in the electronic supplementary material, Sensitivity analyses section.
3. Results
3.1. Heterogeneity in tuberculosis incidence in Gujarat
The overall TB incidence in Gujarat averaged over 14 quarters (2009–2012) of RNTCP notification data was 132 per 100 000 per year. TB incidence showed only modest heterogeneity at the TU level, varying from 75 to 225 per 100 000 per year (figure 1). The incidence rates in the top decile of TUs (hotspot) and the remaining population (general population) were 190 and 125 per 100 000 pear year, respectively, yielding an incidence ratio in the hotspot relative to the general population of 1.5 : 1.
We validated the above data on a small scale by verifying the reporting practices in 15 designated microscopy centres (DMCs) in five randomly selected TUs across Gujarat. We counted the number of new smear-positive cases entered in the laboratory register for third quarter of 2013, verified (on one day per DMC) that the laboratory register contained all patients who submitted sputum for evaluation at the DMC and cross-checked the case counts with both the monthly laboratory abstracts and the quarterly Peripheral Health Insititution (PHI) report, which is a document that is generated on a quarterly basis and sent to the overseeing TU. Case registration and notification were generally consistent (table 2), and all patients whom we observed to submit sputum were registered by the laboratory. In one of 15 DMCs (Halol), the PHI report differed grossly from the other two reports, a discrepancy that was explained as reflecting patients who were diagnosed at the selected DMC but who resided elsewhere. TB incidence was much more heterogeneous at the DMC level (100 000 population) than the TU level (500 000 population), with a range of 2–170 cases notified per quarter among DMCs serving underlying populations of similar size (table 2).
Table 2.
Validation of registration and notification of new smear-positive TB cases. Fifteen designated microscopy centres (DMC)s from five TB units (TUs) were covered as part of the demonstration study. (Each TU includes five DMCs; three were selected from each of the TUs studied.) Presented are counts of new smear-positive TB cases during the third quarter of 2013 (i) as they were registered in the laboratory register, (ii) after they were totalled and reported in the monthly laboratory abstract and (ii) notified via quarterly Peripheral Health Institution (PHI) report sent to the respective TUs.
| TB units | DMC | laboratory register | laboratory abstract | PHI report |
|---|---|---|---|---|
| Ahmadabad East, Ahmadabad | Rakhial | 30 | 29 | 30 |
| Gomtipur | 33 | 34 | 34 | |
| Odhav | 20 | 20 | 20 | |
| Halol, Pachmahal | Halol | 54 | 53 | 28 |
| Sansoli | 12 | 12 | 12 | |
| Vejalpur | 21 | 22 | 22 | |
| Hirabaug, Surat | Fulpada | 16 | 16 | 16 |
| Smimer | 170 | 170 | 170 | |
| Hirabaug | 44 | 44 | 44 | |
| Jetpur, Rajkot | Gondal | 39 | 39 | 39 |
| Jetpur | 51 | 51 | 51 | |
| Virpur | 2 | 2 | 2 | |
| Keshod, Junagadh | Keshod | 33 | 33 | 33 |
| Balagam | 4 | 4 | 4 | |
| TB Hospital | 11 | 11 | 11 |
3.2. Reduction in tuberculosis incidence achieved through spatially targeted vaccination
We simulated a mass campaign that vaccinated 8% of adults older than 20 years (V-A), augmented by a continuous vaccine campaign that achieved 80% coverage in individuals turning 10 years of age. We assumed that continuous vaccination of 10-year-olds would achieve high coverage throughout the population, and thus primarily explored the role of spatially targeting the periodic adult campaigns. After 10 years following the launch of vaccination, the percentage of the total population that was vaccine-protected reached 14%, and varied between 14 and 18% after 10 years depending on the timing of the periodic adult vaccine campaigns (figure 3, black line). When the vaccine campaign was implemented in an untargeted fashion (UTV), TB incidence fell by 24% after 10 years (figure 3, grey line). In the spatially targeted vaccine (STV) strategy, the adult vaccine (V-A) coverage was 80% in the hotspot and 0% in the general population. When the hotspot and the general populations were isolated with neither migration nor mixing, the reductions in TB incidence were similar for both STV and UTV (figure 3, tan versus grey lines). Even though individuals in the hotspot are at greater risk of TB, the number of individuals that could be potentially infected by these individuals could be smaller when mixing is limited to a smaller population in which targeted vaccination may reduce TB incidence over time (see electronic supplementary material, figure S-8).
Figure 3.

Reduction in TB incidence achieved by untargeted (UTV) and spatially targeted (STV) vaccine campaigns. Plotted in black is the percentage of the population estimated to be vaccine-protected in the first 20 years after the deployment of the vaccine. The vaccine campaigns consist of two parts: (i) continuous vaccination of adolescents that turn 10 years old (V-10) at 80% coverage; and (ii) periodic vaccination of adults older than 20 years old (V-A) at 8% coverage (indicated by the hatched area). Plotted in colour are the corresponding percentage reductions in TB incidence through the first 20 years after vaccine introduction in five different scenarios: (i) untargeted vaccination (UTV, in grey) and spatially targeted vaccination (STV) with: (ii) no migration and mixing (‘no mix’, in solid tan), (iii) annual migration at 1% and mixing at 1% (1% mix, in dashed pink), (iv) annual migration at 1% and mixing at 3% (3% mix, in dashed red), and (v) annual migration at 1% and mixing at 5% (5% mix, in dashed brown).
However, with increased rates of short-term mixing, the STV strategy began to show greater impact. The degree to which STV improved the impact of the vaccine depended on the level of mixing: for mixing levels of 1%, 3% and 5%, the reductions in TB incidence after 10 years of vaccination with STV were 25%, 28% and 31%, respectively (figure 3, dashed coloured lines), as compared to a uniform 24% reduction with UTV. Spatial targeting augmented the impact of TB vaccination by a factor of 1.17 in the reference scenario. Variation of natural history parameters across the pre-specified ranges in table 1 resulted in a 95% uncertainty range of this augmentation factor of 1.11–1.33 (see electronic supplementary material, figure S-11). This augmentation factor was fairly robust to variation in the natural history parameters (see the electronic supplementary material for sensitivity analyses), and increased from 1.06 to 1.25 when the mixing rate was increased from 1 to 5%.
3.3. Key determinants that drive the effectiveness of spatially targeted vaccines
In subsequent analysis, we explored the roles of two key factors that emerged as primary determinants of the relative impact of STV. Specifically, we varied the level of spatial heterogeneity—defined as the ratio of incidence in the top decile of the population with the highest TB incidence (i.e. hotspot) to the incidence in the remaining 90% general population—and the degree of short-term mixing between the hotspot and the general population. We set the TB incidence in the population to 132 per 100 000 per year, and the annual migration rate to 1%, as seen in Gujarat data. We then estimated the reduction in TB incidence in the first 10 years after the launch of the vaccine for the two vaccine strategies, UTV and STV. We compared the reduction in incidence achieved by the two strategies and reported this as the ratio of reduction achieved by STV compared with that achieved by UTV (figure 4). The relative impact of STV versus UTV increased with greater spatial heterogeneity and intensity of mixing. When TB was perfectly homogeneous in a population with no mixing, STV led to slightly worse outcomes than UTV (bottom left corner of figure 4). But as both spatial heterogeneity and mixing increased, the advantage of STV gradually increased (figure 4). For example, when the ratio of incidence in hotspot versus general population was 3 : 1 and the mixing between the hotspot and the general population was 5% (i.e. red circle on figure 4), STV could achieve more than 1.6 times the reduction in incidence compared with UTV. This result was robust to variation in annual migration rate and vaccine efficacy (see electronic supplementary material, figures S-5 and S-6).
Figure 4.

The relative benefit of spatially targeted versus untargeted TB vaccine, as a function of spatial heterogeneity and short-term mixing. The figure depicts the roles of spatial heterogeneity and mixing in the relative benefit of spatially targeted TB vaccination (STV) over untargeted vaccination (UTV). Plotted on the vertical axis are different levels of spatial heterogeneity, defined as the ratio of TB incidence in the decile of the population with the highest TB incidence (i.e. hotspot) to the incidence in the rest of the population, holding the average incidence in the total population constant at 132 per 100 000 per year. Plotted on the horizontal axis are different levels of mixing (as a percentage of infections that originate from residents of the hotspot but occur in the residents of the general population). The contours show the benefit of spatial targeting, expressed as the ratio of the reduction in TB incidence under an STV versus UTV strategy, 1 : 1 denoting equivalent impact of the two strategies (and thus no benefit or harm from spatial targeting). Points marked A, B and C correspond to spatially targeted vaccinations deployed in Gujarat (with the observed heterogeneity in the TB unit level data of 1.5 : 1) at 1%, 3% and 5% annual mixing, respectively, as shown by dashed lines in figure 3. The point marked by the red circle considers a scenario where a sub-population could be identified that had three times the TB incidence of the general population, and in which 5% of all respiratory contacts were shared with the general population.
4. Discussion
In this study, we aimed to assess the benefits of spatially targeted TB vaccine strategies in a high-incidence, South Asian setting. This was achieved in two parts. First, we estimated the level of spatial heterogeneity in TB incidence. At the resolution of TUs (approx. 500 000 population), heterogeneity in TB incidence was low, with ‘hotspot’ TUs having only 50% more notified cases than the mean. Second, we developed a model of TB transmission that represented TB incidence as well as the heterogeneity observed in these data, and used it to simulate STV delivery. Specifically, we compared the reduction in incidence achieved by the two strategies, spatially targeted (STV) and untargeted (UTV). We found that targeting a 10% hotspot sub-population resulted in only modest gains, equal to only 1–7% absolute additional reduction in incidence (relative reduction of 1.05–1.26) relative to UTV. The gains were highly dependent on the degree of mixing between the hotspot and the general population. As such, this impact could be markedly augmented (to a relative reduction of 1.6 or more) in settings characterized by high heterogeneity and mixing between hotspots and the general population.
We observed a low degree of spatial heterogeneity in TB incidence at the TU level in this study, particularly in comparison to what has been observed elsewhere [18], with the result that the estimated impact of spatial targeting was small in our base case. At least three factors may explain this observation. First, spatial resolution at the level of the TU (approx. 500 000 people each) may not be sufficiently fine to detect substantive heterogeneity that occurs at smaller scales. Indeed, the level of heterogeneity observed at the level of DMCs (100 000) was much higher, up to 10-fold within the same TU and nearing 100-fold across different TUs. Thus, to make a STV strategy effective, one may need to focus on targeting sub-populations with finer geographical resolution—a strategy that may be more difficult logistically. Second, the designation of TUs by the RNTCP was designed to optimize provision of TB services by allocating resources in a more equitable manner. This may reduce the potential impact of a STV planned for delivery within this same system. Finally, during the period over which we evaluated TB notifications, incentives existed within India to reach World Health Organization targets for 70% case detection of smear-positive TB, at the TU level. Although these incentives have now been replaced with process-based measures, trends from 2009 to 2012 may have led to reporting of TB incidence that was more uniform than was actually the case.
Geographical heterogeneity is a pattern that is not limited to TB, and has been observed across many infectious diseases [3], including vector-borne diseases such as malaria [34] and dengue [35], and sexually transmitted diseases [36]. Interventions that target this heterogeneity have also been recommended [6,7]. However, both the drivers of such heterogeneity and the impact of such heterogeneity on disease transmission likely differ across diseases. Unlike other major infectious diseases, TB is an airborne pathogen that can be transmitted across distances of hundreds of metres and times of hours (even days). TB is also a chronic infection, with an infectious period that can last from months to years. Thus, TB is likely to differ from most other infectous diseases [37,38] in terms of the degree of observed heterogeneity, the importance of different mixing patterns and the relative benefit of spatially targeted disease control strategies.
Our study has several limitations. The study data are based on official notifications, which may not fully represent underlying TB dynamics in the population. While our demonstration study suggested that errors in notifications are uncommon, notified data at the TU level may not accurately reflect the spatial heterogeneity in TB incidence when individuals receive diagnosis and treatment in TUs that are different from their residence (for example, the TUs covering their workplaces). More importantly, official notifications do not account for individuals with TB who receive diagnosis and treatment through the private sector, which in India constitutes a sizable portion of TB care [39,40]. The rate of mixing (the proportion of TB transmission events originating from cases in the hotspot that occur to members of the general community) was found to be an important determinant of the added value of spatially targeted vaccination, but mixing is particularly difficult to quantify for an airborne disease. We have explored a range of mixing rates between 1 and 5%, which is comparable with the proportions of longer range contacts seen in mixing studies [41,42], albeit contacts investigated in these studies were in the context of influenza transmission and not TB. Given its importance, there is a need to better understand the movement of highest-risk populations on a short-term (e.g. daily commuting) basis, particularly with respect to airborne transmission events.
As with any modelling study, this analysis was based on several simplifying assumptions. The model does not consider several forms of heterogeneity such as in population growth rates and mixing within each sub-population, TB risks among people that migrate and people that receive vaccine. Research aimed at gathering data on these forms of heterogeneity is important to inform future modelling efforts. The model was deterministic and did not account for demographic or other kinds of stochasticity. Population-level dynamics of TB tend to be quite stable, especially in the context of a large population such as that of Gujarat (approx. 50 million), and less likely to be affected by demographic stochasticity compared with the dynamics of other acute infectious diseases such as measles or cholera. This can also be seen in the trend of TB incidence in Gujarat in each of the quarters from 2009 through 2012 (electronic supplementary material, figure S-2, left).
From a logistic standpoint, it may be infeasible to implement a STV campaign at a spatial scale that is small enough to merit targeting, and those areas with the greatest infrastructure (and therefore probably the lowest TB incidence) may be the easiest sites for vaccine campaigns to target. Many vaccine characteristics (e.g. mechanism and duration of effect, heterogeneity of immunological ‘take’) are not known, and are difficult to assess prior to implementation, even with a large-scale clinical trial. Finally, this work evaluated only one pre-specified form of geographical targeting, at the TU level based on incidence. Other targeting strategies (e.g. occupational targeting, targeting of congregate living settings) may have greater impact, and future research to evaluate optimal targeting strategies across different epidemiological settings would be valuable.
In summary, our results suggest that the degree of spatial heterogeneity and frequency of mixing are key determinants of the value of spatially targeted TB vaccine strategies. In our studied setting with relatively little reported spatial heterogeneity, spatial targeting was estimated to provide only a modest benefit over an untargeted vaccination strategy. In other settings, accurately delineating high-incidence and high-mixing sub-populations (including the development of systems capable of capturing such data) will be essential to fully harness the potential advantage of spatial targeting of TB vaccines—and by extension, also other TB interventions. These benefits of spatial targeting must be weighed against the feasibility of delivering a vaccine at the spatial scale on which such heterogeneity is observed. Identifying the appropriate spatial scale for quantifying heterogeneity [43], including understanding the movement and mixing behaviour of populations in TB hotspots, is therefore an important area for future research. Where heterogeneity in TB incidence is small and mixing between populations relatively unimportant (as may be the case in Gujarat at the TU level), spatially targeted vaccines may have little additional benefit. However, in well-connected hotspots with high TB incidence, STV delivery can augment the impact of TB vaccines on incidence by 1.6-fold or greater. Identification of such hotspots and targeted delivery to these areas may therefore be critical if TB vaccines are to achieve their maximum impact at the population level.
Supplementary Material
Supplementary Material
Acknowledgements
We thank the Revised National Tuberculosis Control Program (RNTCP) for their help and support during this study: Dr K. S. Sachdeva and Dr Amar Niranjan Shah in Delhi office for their help with coordination, the approval process and connecting us to the Gujarat RNTCP; and Dr P. V. Dave and Dr Sandeep Bharaswadkar in Gujarat for their help and support during the demonstration study. We are grateful to Preety Rajbangshi for her help in carrying out the demonstration study, and to Hae-Young Kim for her help in the literature review.
Authors' contributions
All authors contributed in the overall design of the project and the writing of the manuscript. S.S. and D.W.D. conceived the model and analysed the results. S.S. coded the model and carried out model simulations. S.C. and K.D.R. contributed in the demonstration study.
Competing interests
We declare we have no competing interests.
Funding
This research was funded by a grant from the Aeras Foundation.
References
- 1.WHO. 2014. Global tuberculosis report 2014. See http://www.who.int/tb/publications/global_report/.
- 2.WHO. 2015. Global strategy and targets for tuberculosis prevention, care and control after 2015. http://www.who.int/tb/post2015_strategy/en/.
- 3.Woolhouse M, et al. 1997. Heterogeneities in the transmission of infectious agents: implications for the design of control programs. Proc. Natl Acad. Sci. USA 94, 338–342. ( 10.1073/pnas.94.1.338) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stoddard ST, et al. 2013. House-to-house human movement drives dengue virus transmission. Proc. Natl Acad. Sci. USA 110, 994–999. ( 10.1073/pnas.1213349110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keeling MJ, Woolhouse MEJ, May RM, Davies G, Grenfell BT. 2003. Modelling vaccination strategies against foot-and-mouth disease. Nature 421, 136–142. ( 10.1038/nature01343) [DOI] [PubMed] [Google Scholar]
- 6.Carter R, Mendis KN, Roberts D. 2000. Spatial targeting of interventions against malaria. Bull. World Health Organ. 78, 1401–1411. [PMC free article] [PubMed] [Google Scholar]
- 7.Azman AS, Luquero FJ, Rodrigues A, Palma PP, Grais RF, Banga CN, Grenfell BT, Lessler J. 2012. Urban cholera transmission hotspots and their implications for reactive vaccination: evidence from Bissau city, Guinea Bissau. PLoS Negl. Trop. Dis. 6, e1901 ( 10.1371/journal.pntd.0001901) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shetty N, Shemko M, Vaz M, D'Souza G. 2006. An epidemiological evaluation of risk factors for tuberculosis in South India: a matched case control study. Int. J. Tuberc. Lung Dis. 10, 80–86. [PubMed] [Google Scholar]
- 9.Spence DPS, Hotchkiss J, Williams CSD, Davies PDO. 1993. Tuberculosis and poverty. Br. Med. J. 307, 759–761. ( 10.1136/bmj.307.6907.759) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krieger N, Waterman PD, Chen JT, Soobader M-J, Subramanian S. 2003. Monitoring socioeconomic inequalities in sexually transmitted infections, tuberculosis, and violence: geocoding and choice of area-based socioeconomic measures—the public health disparities geocoding project (US). Public Health Rep. 118, 240–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Souza WV, Ximenes R, Albuquerque MFM, Lapa TM, Portugal JL, Lima MLC, Martelli CMT. 2000. The use of socioeconomic factors in mapping tuberculosis risk areas in a city of northeastern Brazil. Rev. Panam. Salud Publica 8, 403–410. ( 10.1590/S1020-49892000001100005) [DOI] [PubMed] [Google Scholar]
- 12.Harling G, Ehrlich R, Myer L. 2008. The social epidemiology of tuberculosis in South Africa: a multilevel analysis. Soc. Sci. Med. 66, 492–505. ( 10.1016/j.socscimed.2007.08.026) [DOI] [PubMed] [Google Scholar]
- 13.Oxlade O, Murray M. 2012. Tuberculosis and poverty: why are the poor at greater risk in India? PLoS ONE 7, e47533 ( 10.1371/journal.pone.0047533) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Munch Z, Van Lill SWP, Booysen CN, Zietsman HL, Enarson DA, Beyers N. 2003. Tuberculosis transmission patterns in a high-incidence area: a spatial analysis. Int. J. Tuberc. Lung Dis. 7, 271–277. [PubMed] [Google Scholar]
- 15.Haase I, et al. 2007. Use of geographic and genotyping tools to characterise tuberculosis transmission in Montreal. Int. J. Tuberc. Lung Dis. 11, 632–638. [PubMed] [Google Scholar]
- 16.Chaisson RE, Martinson NA. 2008. Tuberculosis in Africa—combating an HIV-driven crisis. New Engl. J. Med. 358, 1089–1092. ( 10.1056/NEJMp0800809) [DOI] [PubMed] [Google Scholar]
- 17.Corbett E, Watt C, Walker N, Maher D, Williams B, Raviglione MC, Dye C. 2003. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch. Intern. Med. 163, 1009–1021. ( 10.1001/archinte.163.9.1009) [DOI] [PubMed] [Google Scholar]
- 18.Dowdy DW, Golub JE, Chaisson RE, Saraceni V. 2012. Heterogeneity in tuberculosis transmission and the role of geographic hotspots in propagating epidemics. Proc. Natl Acad. Sci. USA 109, 9557–9562. ( 10.1073/pnas.1203517109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dowdy DW, Azman AS, Kendall EA, Mathema B. 2014. Transforming the fight against tuberculosis: targeting catalysts of transmission. Clin. Infect. Dis. 59, 1123–1129. ( 10.1093/cid/ciu506) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abu-Raddad LJ, Sabatelli L, Achterberg JT, Sugimoto JD, Longini IM, Dye C, Halloran ME. 2009. Epidemiological benefits of more-effective tuberculosis vaccines, drugs, and diagnostics. Proc. Natl Acad. Sci. USA 106, 13 980–13 985. ( 10.1073/pnas.0901720106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaufmann SH, Hussey G, Lambert P-H. 2010. New vaccines for tuberculosis. Lancet 375, 2110–2119. ( 10.1016/S0140-6736(10)60393-5) [DOI] [PubMed] [Google Scholar]
- 22.Marinova D, Gonzalo-Asensio J, Aguilo N, Martin C. 2013. Recent developments in tuberculosis vaccines. Expert Rev. Vaccines 12, 1431–1448. ( 10.1586/14760584.2013.856765) [DOI] [PubMed] [Google Scholar]
- 23.TBFacts.org. 2015. TB statistics for India. See http://www.tbfactors.org/tb-statistics-india.html.
- 24.Blower SM, Mclean AR, Porco TC, Small PM, Hopewell PC, Sanchez MA, Moss AR. 1995. The intrinsic transmission dynamics of tuberculosis epidemics. Nat. Med. 1, 815–821. ( 10.1038/nm0895-815) [DOI] [PubMed] [Google Scholar]
- 25.White PJ, Garnett GP. 2010. Mathematical modelling of the epidemiology of tuberculosis. In Modelling parasite transmission and control (eds Michael E, Spear RC). Advances in Experimental Medicine and Biology, vol. 673, pp. 127–140. New York, NY: Springer. [Google Scholar]
- 26.Dye C, Garnett GP, Sleeman K, Williams BG. 1998. Prospects for worldwide tuberculosis control under the WHO DOTS strategy. Lancet 352, 1886–1891. ( 10.1016/S0140-6736(98)03199-7) [DOI] [PubMed] [Google Scholar]
- 27.Vynnycky E, Fine P. 1997. The natural history of tuberculosis: the implications of age-dependent risks of disease and the role of reinfection. Epidemiol. Infect. 119, 183–201. ( 10.1017/S0950268897007917) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horsburgh CR Jr, O'Donnell M, Chamblee S, Moreland JL, Johnson J, Marsh BJ, Narita M, Johnson LS, von Reyn CF. 2010. Revisiting rates of reactivation tuberculosis: a population-based approach. Am. J. Respir. Crit. Care Med. 182, 420 ( 10.1164/rccm.200909-1355OC) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sutherland I, Švandová E, Radhakrishna S. 1982. The development of clinical tuberculosis following infection with tubercle bacilli: 1. A theoretical model for the development of clinical tuberculosis following infection, linking from data on the risk of tuberculous infection and the incidence of clinical tuberculosis in the Netherlands. Tubercle 63, 255–268. ( 10.1016/S0041-3879(82)80013-5) [DOI] [PubMed] [Google Scholar]
- 30.Vynnycky E, Fine P. 1997. The annual risk of infection with Mycobacterium tuberculosis in England and Wales since 1901. Int. J. Tuberc. Lung Dis. 1, 389–396. [PubMed] [Google Scholar]
- 31.Basu S, Orenstein E, Galvani AP. 2008. The theoretical influence of immunity between strain groups on the progression of drug-resistant tuberculosis epidemics. J. Infect. Dis. 198, 1502–1513. ( 10.1086/592508) [DOI] [PubMed] [Google Scholar]
- 32.Andrews JR, Noubary F, Walensky RP, Cerda R, Losina E, Horsburgh CR. 2012. Risk of progression to active tuberculosis following reinfection with Mycobacterium tuberculosis. Clin. Infect. Dis. 54, 784–791. ( 10.1093/cid/cir951) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Office of the Registrar General and Census Commissioner of India. 2001. D-Series: migration tables. http://www.censusindia.gov.in/Tables_Published/D-Series/Tables_on_Migration_Census_of_India_2001.aspx.
- 34.Bousema T, et al. 2010. Identification of hot spots of malaria transmission for targeted malaria control. J. Infect. Dis. 201, 1764–1774. ( 10.1086/652456) [DOI] [PubMed] [Google Scholar]
- 35.Mammen MP, Jr, et al. 2008. Spatial and temporal clustering of dengue virus transmission in Thai villages. PLoS Med. 5, e205 ( 10.1371/journal.pmed.0050205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomas JC, Tucker MJ. 1996. The development and use of the concept of a sexually transmitted disease core. J. Infect. Dis. 174, S134–S143. ( 10.1093/infdis/174.Supplement_2.S134) [DOI] [PubMed] [Google Scholar]
- 37.Viboud C, Bjørnstad ON, Smith DL, Simonsen L, Miller MA, Grenfell BT. 2006. Synchrony, waves, and spatial hierarchies in the spread of influenza. Science 312, 447–451. ( 10.1126/science.1125237) [DOI] [PubMed] [Google Scholar]
- 38.Mossong J, et al. 2008. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med. 5, e74 ( 10.1371/journal.pmed.0050074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Satyanarayana S, et al. 2011. From where are tuberculosis patients accessing treatment in India? Results from a cross-sectional community based survey of 30 districts. PLoS ONE 6, e24160 ( 10.1371/journal.pone.0024160) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hazarika I. 2011. Role of private sector in providing tuberculosis care: evidence from a population-based survey in India. J. Glob. Infect. Dis. 3, 19 ( 10.4103/0974-777X.77291) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Read JM, Lessler J, Riley S, Wang S, Tan LJ, Kwok KO, Guan Y, Jiang CQ, Cummings DAT. 2014. Social mixing patterns in rural and urban areas of southern China. Proc. R. Soc. B 281, 20140268 ( 10.1098/rspb.2014.0268) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garske T, Yu H, Peng Z, Ye M, Zhou H, Cheng X, Wu J, Ferguson N. 2011. Travel patterns in China. PLoS ONE 6, e16364 ( 10.1371/journal.pone.0016364) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Riley S, Eames K, Isham V, Mollison D, Trapman P. 2014. Five challenges for spatial epidemic models. Epidemics 10, 68–71. ( 10.1016/j.epidem.2014.07.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.