Abstract
The Cretaceous Sanagasta neosauropod nesting site (La Rioja, Argentina) was the first confirmed instance of extinct dinosaurs using geothermal-generated heat to incubate their eggs. The nesting strategy and hydrothermal activities at this site led to the conclusion that the surprisingly 7 mm thick-shelled eggs were adapted to harsh hydrothermal microenvironments. We used micro-CT scans in this study to obtain the first three-dimensional microcharacterization of these eggshells. Micro-CT-based analyses provide a robust assessment of gas conductance in fossil dinosaur eggshells with complex pore canal systems, allowing calculation, for the first time, of the shell conductance through its thickness. This novel approach suggests that the shell conductance could have risen during incubation to seven times more than previously estimated as the eggshell erodes. In addition, micro-CT observations reveal that the constant widening and branching of pore canals form a complex funnel-like pore canal system. Furthermore, the high density of pore canals and the presence of a lateral canal network in the shell reduce the risks of pore obstruction during the extended incubation of these eggs in a relatively highly humid and muddy nesting environment.
Keywords: titanosaurs, hydrothermal incubation, burrow-nesting, Sanagasta, micro-CT
1. Introduction
Titanosaurs were Mesozoic mega-herbivorous dinosaurs that inhabited all the land masses. However, the unequivocal record of their nesting sites is to date limited to South America, Europe and Asia [1]. The study of their eggs and eggshells has recently provided new insights on their intriguing palaeobiology, suggesting that these long-necked behemoths were warm-blooded animals that selected globally distributed but highly particular nesting sites, using mound-building and burrow-nesting strategies to incubate their eggs [1–4].
The Sanagasta nesting site (La Rioja, Argentina) was the first, but not the only [1,3,5], confirmed case of extinct dinosaurs incubating their eggs with geothermal-generated heat [2]. The synchronicity between the oviposition and the hydrothermal activities led Grellet-Tinner & Fiorelli [2] to conclude that the Sanagasta eggs were adapted to harsh hydrothermal nesting environments. However, due to methodological limitations (i.e. eggshells are typically studied using destructive thin sections that greatly hinder the understanding of their three-dimensional morphology), it was impossible to understand the physiological impact of the thinning of their surprisingly thick-shelled eggs due to erosion by the geothermal pore fluids.
Here we report the first micro-CT-based three-dimensional reconstruction and microcharacterization of any sauropod dinosaur eggshell. The new three-dimensional analyses provide a robust assessment of gas conductance in the Sanagasta eggshells, allowing for the first time the calculation, rather than an estimation, of gas conductance of these titanosaur eggshells.
2. Results
Microcharacterizations based on three-dimensional reconstructions of a pristine 5.6 mm thick eggshell (figure 1a,b) from the Sanagasta nesting site indicate that it consists of a single thick structural layer, displaying well-defined units formed by calcite crystals radiating from nucleation centres (figure 1c). On average, more than 20% of the shell volume consists of voids (empty spaces). Such a feature has not previously been noticed in the eggshells of extinct dinosaurs and is interesting because of the potential effect of the voids on the mechanical resistance and gas diffusion of these shells.
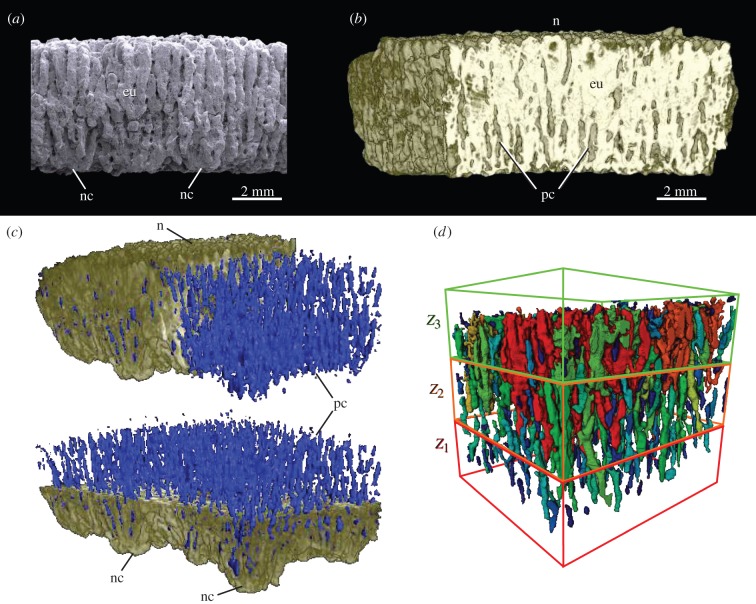
Figure 1.
Sanagasta eggshell in radial view. (a) Scanning electron microscope image, (b) micro-CT-based reconstruction, (c) general view of the eggshell and its pore canal system (highlighted in blue in the online version) and (d) pore canal system showing the shell segmentation in three concentric zones: Z1–Z3. Different grey tones (colours in the online version) were used in an attempt to individualize pore canals. eu, eggshell unit; n, node on the outer shell surface; nc, nucleation centre; pc, pore canal. (Online version in colour.)
The concentration and lateral connection of the pore canals define three distinct eggshell zones (Z1–3; figure 1d). Z1, the innermost third of the shell, is radially crossed by multiple vertical and straight pore canals that are closely aligned and parallel to each other, reaching 10% of the volume in this zone. Their diameter narrows through the first approximately 0.5 mm but then widens towards the shell's external surface, producing a slight but noticeable increase in the proportion of voids across this portion of the shell. Although the pore canal ramifications are visible throughout the shell thickness, they occur more frequently in the outer two-thirds of the eggshell (figure 2a). In Z2, the straight pore canals widen in cross section and branch frequently into lateral interconnections (electronic supplementary material, S1), gradually increasing the proportion of voids outwards, duplicating that of Z1. Lastly, in Z3, the pore canals branch into two or more subcanals of nearly comparable diameters (figure 2b). As branching occurs, pore canals increasingly interconnect laterally, vertically and at random angles (figure 2a,b), forming a lateral canal network that sharply increases the concentration and number of canals. This constitutes the most porous section of the eggshell, containing up to 57% of its total void volume.
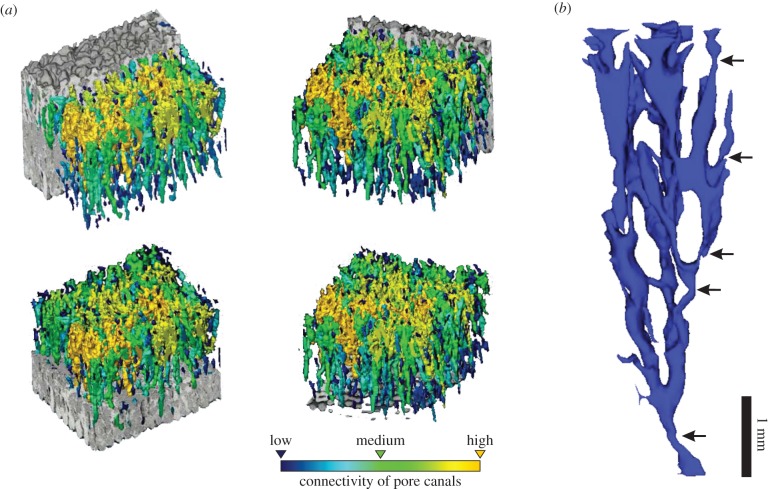
Figure 2.
Pore canal system and pore set. (a) Pore canal system showing the high degree of lateral interconnection. The canals are coloured according to a connectivity gradient. See electronic supplementary material, S1. (b) Restoration of a pore set. Several branches were deliberately cut to enhance viewing. Note that pore canal constrictions appear in different branches at any depth (arrows). (Online version in colour.)
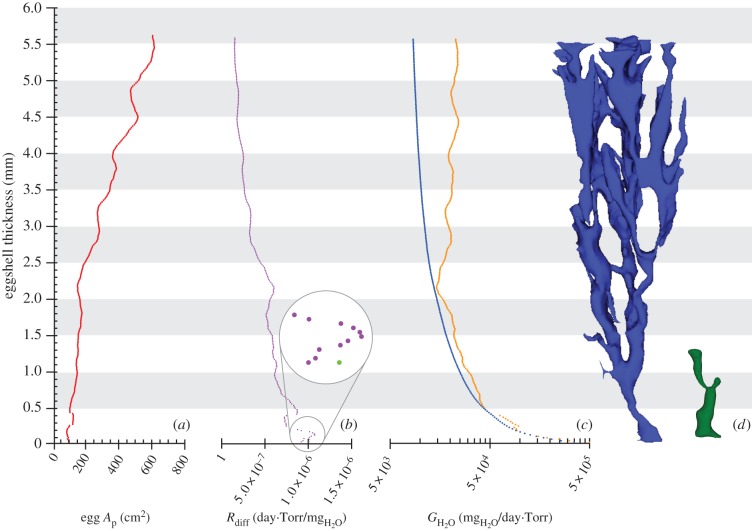
The total egg pore canal area (Ap) calculated at tangential intervals of 20.3 µm indicates a sharp increase outwards (figure 3a), driven by the gradual widening of the pores in addition to their constant branching. Thus, the entire pore canal system could be modelled as a funnel-shaped structure [6,7]. We calculated pore-diffusive resistance and its reciprocal conductance to water vapour [6] of each of the same 20.3 µm thick segments, mirroring the shell thinning process during incubation (electronic supplementary material, S2; Material and methods), as previously reported [2,5]. Considering circular cross sections we obtained the diffusive resistances at each segment (figure 3b) based on Fick's first law, and boundary layer resistance at the inner apertures based on Stefan's law [6–8]. In accordance with Tøien et al. [6,8] (but contra Simkiss [7]) our calculations indicate a very low inner aperture effect, from 0.8% to 2.1% through eggshell thinning. As the diffusive resistances of each segment are cumulative [6], we calculated total egg water vapour conductance (GH2O; figure 3c, coloured blue in the online version). The results (electronic supplementary material, S2) indicate that the shell conductance grows only a little through Z3–2 compared with the exponential increase experienced when shell thickness reduces below approximately 2.2 mm thick (Z1).
Figure 3.
Egg pore area (Ap), diffusive resistance (Rdiff) and water vapour conductance (GH2O) variation through the shell thinning of a 5.6 mm eggshell from Sanagasta. (a) Ap of each shell segment. (b) Rdiff of each shell segment (dark grey; purple in the online version; based on Fick's law) and the inner aperture (light grey; green in the online version; based on Stefan's law). (c) GH2O at each thickness estimated through equation (4.1) (light grey; orange in the online version) and derived from Rdiff (dark grey; blue in the online version); see Material and methods. Data are listed in the electronic supplementary material, S2. (d) Scaled Sanagasta pore set (left) and Auca Mahuevo layer 4 pore canal (right). Restoration of the Auca Mahuevo pore canal based on Grellet-Tinner et al. [5]. (Online version in colour.)
To compare our results with previous studies [5], we calculated egg GH2O by using the equation derived from Fick's first law of gas diffusion (see Material and methods). Here, we considered only the outermost tangential section for each thickness, thus assuming single straight pore canals (figure 3c, coloured orange in the online version; electronic supplementary material, S2). This way, the results present a much more erratic pattern throughout Z2–3, with sudden surges and falls of conductance.
3. Discussion
The Sanagasta eggs (approx. 4850 cm3) are more than 1.5 times larger than those of other titanosaurs [1,5], paralleling the size difference between megapode eggs (e.g. Alectura lathami and Leipoa ocellata) and other galliformes of comparable body size [9]. Such larger eggs allow storage of more nutrients for prolonged incubation and hatching of hyperprecocial chicks [10,11]. Despite the difference in egg size, several morphological features of Z1 are strikingly reminiscent of the Auca Mahuevo titanosaur eggshells [12], including their Y-shaped pore canals (figure 3d). Typically, these eggshells are thinner than 1–1.2 mm at the time embryos hatch because their inner surface thins during embryogenesis [12–19] and the outer surface is eroded as a direct consequence of their incubation in burial conditions with ambient heat and moisture [1,5,12,20] in contrast with the classic avian contact incubation. In addition, calcium absorption from the inner surface of the eggshell is typically 4–8% during embryonic development in birds, but it is much higher (12–21%) in megapodes, which bury their eggs [15–17,19] like the titanosaurs [1]. Although clearly the percentage of shell thinning does not scale isometrically with shell thickness, inner-shell thinning has been observed in the Sanagasta eggshells [5]. Moreover, the geochemical analyses of the Sanagasta nesting sediments coupled with the wide range of eggshell thicknesses [2,21] independently support the hypothesis that the hydrothermal fluids progressively and greatly eroded the outer surface of the 7.9 mm thick eggshell during incubation [2,5,21,22]. This chemical erosion represents the main factor contributing to the shell final thickness of 1.2 mm [2,5,22] that allows an embryo to successfully break through at hatching time.
This study confirms that the pore canal system of the Sanagasta titanosaur eggshells have the most complex pattern known to date (figure 2a). Pore canals can at best be individualized into small sets due to their high density and multiple lateral interconnections (e.g. figure 2b). Moreover, a given pore set and its branches vary greatly and randomly in diameter throughout the eggshell thickness (e.g. arrows in figure 2b), hence hindering the accurate determination of the smallest cross sections that potentially restrict gas diffusion during shell thinning. Therefore, these features represent a notable hurdle to the generalization of Ap, typically used to estimate the conductance of fossil eggshells. Owing to the pore canal system complexity, GH2O calculations of the Sanagasta and probably other titanosaur eggshells using single tangential sections may lead to significantly disparate estimates (figure 3c), questioning the effectiveness of the previously used methods for estimating the humidity in nesting microenvironments of extinct dinosaurs with complex pore canal systems in their eggshells. By contrast, the use of micro-CT scans allows diffusive resistance and GH2O to be accurately calculated in such conditions.
The high degree of lateral interconnection allows the pore canal system to be considered as a unit which can be interpreted as a funnel-shaped structure due to its total Ap increase from the inside out of the shell. Overall, our observations indicate that the Sanagasta shell's narrowest Ap (i.e. the section where the narrowest cross sections are expected) is close to the inner-shell surface (figure 3a), as typically observed in megapode and other sauropod eggshells [6,15,19,23,24]. As such, the GH2O measured through shell thinning mirrors that of modern megapode eggs [15–17], rising exponentially into the last third of the incubation period (figure 3c). Considering that most of the diffusive resistance is concentrated at the innermost Z1, the subtle but constant increase in conductance during removal of Z3–2 must be driven by the strong thickness component of these titanosaur eggshells.
While the Sanagasta eggshells exhibit the most complex lateral canal network so far described, lateral interconnection among vertical pore canals was previously noted in thinner sauropod eggshells [24,25] and, at least, one species of modern megapodes. Two species of mound-building megapodes, the brush-turkey (A. lathami) and the malleefowl (L. ocellata), show differences in their nesting strategies, the brush-turkeys incubating eggs in mound-nests with high relative humidities and malleefowl eggs incubating in dry nests (G Grellet-Tinner 2014, unpublished data). The Y-shaped pores in the eggshells is a shared character of both species, but the single or double horizontal connections between adjacent pores are particular to A. lathami (G Grellet-Tinner 2014, unpublished data). Based on present observations, they would facilitate the lateral diffusion of gases in the event of occlusion of pores on the surface of the egg. Such occlusion is more likely in buried eggs than in eggs incubated in open nests, especially those of brush-turkey, where the nest mound is composed predominantly of moist vegetable matter [16].
Knowing that the Sanagasta egg clutches were deposited in close proximity to hydrothermal structures such as vents, springs and pools [1,2,5,21,22], water and muddy material could have easily obstructed several pores at times [16]. Although the lateral canal network is well developed in Z3–2, it greatly reduces in Z1 (electronic supplementary material, S1), where pore canal obstructions should not have represented a drawback because shell thinning leads to a net increase (double) in the shell's conductance in a few tenths of a millimetre (figure 3c). Therefore, the presence of a highly complex lateral network at the outermost two-thirds of the eggshell reinforces previous hypotheses that suggest that the Sanagasta eggs were able to maintain a sufficient gas and water vapour diffusion through their shell during burial incubation even in the event of partial obstruction [1,2,5].
The extremely thick Sanagasta eggshells were well adapted to minimize the effects of chemical erosion and obstruction of pores by possessing the most complex funnel-like pore canal system so far described. This complex pore system indicates that the gas conductance of these 7.9 mm thick eggshells could have increased up to seven times more than previously estimated [5]. Funnel-shaped pores are extremely important evolutionarily as their low resistance to diffusion allows dissociation of the constraints imposed by respiration from those related to the eggshell size and strength [7]. The presence of a funnel-like pore canal system may have permitted the extremely thick Sanagasta eggshells to act as a mechanism to counteract the external acid erosion in this hydrothermal nesting strategy.
Lastly, the new data demonstrate that gas exchange estimations for sauropod eggs based on the equation derived from Fick's first law using single tangential sections are perhaps an oversimplification for fossil dinosaur eggshells with such complex pore canal systems as observed in the Sanagasta samples.
4. Material and methods
(a). Materials
Fossil eggshells CRILAR-Pv 406, housed at the Centro Regional de Investigaciones Científicas y Transferencia Tecnológica de La Rioja (CRILAR-CONICET), were recovered at sub-site G of the Sanagasta nesting site in La Rioja province, northwest Argentina [2].
(b). Scanning electron microscope imaging
The scanning electron microscope analysis and preparation of eggshell samples were carried out in accordance with Grellet-Tinner's methodology [13]. Observations were carried out with a Philips® Scanning Electron Microscope XL 30 at the Microscopy Laboratory of the Museo Argentino de Ciencias Naturales ‘Bernardino Rivadavia’, Buenos Aires, Argentina.
(c). Micro-CT scanning and analysis
Micro-CT microcharacterizations were performed at the Australian Centre for Microscopy and Microanalysis, University of Sydney, Australia. The eggshell specimens were scanned using an Xradia MicroXCT-400 system operating at 55–60 keV and 127–133 mA. The specimens were mounted in low-density polystyrene to prevent movement during their 360° rotation with projections collected at 0.2° intervals. Geometric and objective magnification was used to scan the Sanagasta samples at a voxel resolution of 20.3 µm. Reconstructed image stacks were rendered using Avizo Fire (VSG|FEI Visualization Sciences Group) with the internal pore networks segmented using built-in thresholding functions following a nonlinear filtering of the dataset to reduce image noise. Pore volume calculations were obtained by running a material calculation on the samples with each voxel being assigned to one of pore, shell or exterior (air) based on the attenuation of the X-ray beam.
(d). Conductance calculations
A section of 44.1 mm2 of a pristine 5.6 mm thick eggshell was used to make volumetric calculations. As a voxel measures 20.3 µm on each side, the sample was divided in 275 tangential segments. It is noteworthy that, given the size of the specimen, the effect of the curvature of the egg is negligible. Pore length (Ls) was considered to be equal to eggshell thickness in all the calculations.
To allow the comparison of our results with previous studies, GH2O of the Sanagasta eggshells was also estimated through the most frequently used formula [5,9,26,27]:
| 4.1 |
where Ap is the total pore area of the egg (mm2), Ls is the pore length (mm) and the constant in the formula is linked to a nesting temperature of 25°C. Although we suspect that the nesting temperature at Sanagasta was above 25°C, this value is widely used in the literature [5–9,16,27–30]. Thus, it allows further comparison with fossil and extant dinosaur eggs and eggshells. Results obtained from the latter (plotted in figure 3c, orange in the online version) were calculated by using only Ap of the outermost tangential segment for each thickness (e.g. 147.8 cm2 egg−1 at 1 mm thick).
Considering the oversimplification of the latter calculations, the volumetric data were also used to evaluate the diffusive resistance of the inner pore apertures (based on Stefan's law) and each of the 275 shell segments (based on Fick's first law). GH2O of each segment was derived from these calculations, according to Tøien et al. [6].
Supplementary Material
Acknowledgements
The authors acknowledge the facilities and the scientific and technical assistance of the Australian Microscopy & Microanalysis Research Facility at the Australian Centre for Microscopy & Microanalysis at the University of Sydney, and the Secretaría de Cultura de La Rioja (Argentina) and S. de la Vega and C. Bustamante (CRILAR-CONICET) for their help and support. E.M.H. thanks L. Leuzinger, S. Rocher and M. Macchioli Grande for discussions and comments on this manuscript and acknowledges the CONICET doctoral fellowship for supporting his studies. G.G.-T. is grateful for the support of the School of Biology and the ACMM of the University of Sydney.
Authors' contributions
E.M.H. and G.G.-T. designed the study, performed comparative work and co-wrote the paper; E.M.H. also carried out the data analysis; M.F. collected data, and with L.E.F. and M.B.T. contributed to the writing and discussion.
Competing interests
We declare we have no competing interests.
Funding
Research funded by CONICET, the Jurassic Foundation (to E.M.H.), the Agencia Nacional de Promoción Científica y Tecnológica (PICT 2012-0421 to L.E.F.) and COFECyT (Asetur 2010 to L.E.F.). Analytic work supported by the University of Sydney International Fellowship (to G.G.-T.).
References
- 1.Hechenleitner EM, Grellet-Tinner G, Fiorelli LE. 2015. What do giant titanosaur dinosaurs and modern Australasian megapodes have in common? PeerJ 3, e1341 ( 10.7717/peerj.1341) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grellet-Tinner G, Fiorelli LE. 2010. A new Argentinean nesting site showing neosauropod dinosaur reproduction in a Cretaceous hydrothermal environment. Nat. Commun. 1, 32 ( 10.1038/ncomms1031) [DOI] [PubMed] [Google Scholar]
- 3.Grellet-Tinner G, Codrea V, Folie A, Higa A, Smith T. 2012. First evidence of reproductive adaptation to ‘island effect’ of a dwarf Cretaceous Romanian titanosaur, with embryonic integument in ovo. PLoS ONE 7, e32051 ( 10.1371/journal.pone.0032051) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eagle RA, et al. 2015. Isotopic ordering in eggshells reflects body temperatures and suggests differing thermophysiology in two Cretaceous dinosaurs. Nat. Commun. 6, 8296 ( 10.1038/ncomms9296) [DOI] [PubMed] [Google Scholar]
- 5.Grellet-Tinner G, Fiorelli LE, Salvador RB. 2012. Water vapor conductance of the Lower Cretaceous dinosaurian eggs from Sanagasta, La Rioja, Argentina: paleobiological and paleoecological implications for South American faveoloolithid and megaloolithid eggs. Palaios 27, 35–47. ( 10.2110/palo.2011.p11-061r) [DOI] [Google Scholar]
- 6.Tøien Ø, Paganelli CV, Rahn H, Johnson RR. 1988. Diffusive resistance of avian eggshell pores. Respir. Physiol. 74, 345–354. ( 10.1016/0034-5687(88)90042-4) [DOI] [PubMed] [Google Scholar]
- 7.Simkiss K. 1986. Eggshell conductance—Fick's or Stefan's law? Respir. Physiol. 65, 213–222. ( 10.1016/0034-5687(86)90051-4) [DOI] [PubMed] [Google Scholar]
- 8.Tøien Ø, Paganelli CV, Rahn H, Johnson RR. 1987. Influence of eggshell pore shape on gas diffusion. J. Exp. Zool. Suppl. 1, 181–186. [PubMed] [Google Scholar]
- 9.Ar A, Rahn H. 1985. Pores in avian eggshells: gas conductance, gas exchange and embryonic growth rate. Respir. Physiol. 61, 1–20. ( 10.1016/0034-5687(85)90024-6) [DOI] [PubMed] [Google Scholar]
- 10.Vleck D, Vleck CM, Seymour RS. 1984. Energetics of embryonic development in the megapode birds, mallee fowl Leipoa ocellata and brush turkey Alectura lathami. Physiol. Zool. 57, 444–456. ( 10.1086/physzool.57.4.30163346) [DOI] [Google Scholar]
- 11.Eiby YA, Booth DT. 2009. The effects of incubation temperature on the morphology and composition of Australian brush-turkey (Alectura lathami) chicks. J. Comp. Physiol. B 179, 875–882. ( 10.1007/s00360-009-0370-4) [DOI] [PubMed] [Google Scholar]
- 12.Grellet-Tinner G, Chiappe LM, Coria RA. 2004. Eggs of titanosaurid sauropods from the Upper Cretaceous of Auca Mahuevo (Argentina). Can. J. Earth Sci. 41, 949–960. ( 10.1139/E04-049) [DOI] [Google Scholar]
- 13.Grellet-Tinner G. 2006. Phylogenetic interpretation of eggs and eggshells: implications for phylogeny of Palaeognathae. Alcheringa 30, 141–182. ( 10.1080/03115510608619350) [DOI] [Google Scholar]
- 14.Board RG, Sparks NH. 1991. Shell structure and formation in avian eggs. In Egg incubation: its effects on embryonic development in birds and reptiles, pp. 71–86. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 15.Booth DT, Seymour RS. 1987. Effect of eggshell thinning on water vapor conductance of malleefowl eggs. Condor 89, 453–459. ( 10.2307/1368635) [DOI] [Google Scholar]
- 16.Seymour RS, Vleck D, Vleck CM, Booth DT. 1987. Water relations of buried eggs of mound building birds. J. Comp. Physiol. B 157, 413–422. ( 10.1007/BF00691824) [DOI] [Google Scholar]
- 17.Booth DT, Thompson MB. 1991. A comparison of reptilian eggs with those of megapode birds. In Egg incubation: its effects on embryonic development in birds and reptiles (eds Deeming DC, Ferguson MWJ), pp. 325–344. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 18.Packard MJ, DeMarco VG. 1991. Eggshell ultrastructure and formation in eggs of oviparous reptiles. In Egg incubation: its effects on embryonic development in birds and reptiles (eds Deeming DC, Ferguson MWJ), pp. 53–69. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 19.Booth D. 1989. Regional changes in shell thickness, shell conductance, and pore structure during incubation in eggs of the mute swan. Physiol. Zool. 62, 607–620. ( 10.1086/physzool.62.2.30156188) [DOI] [Google Scholar]
- 20.Clayburn JK, Smith DL, Hayward JL. 2004. Taphonomic effects of pH and temperature on extant avian dinosaur eggshell. Palaios 19, 170–177. () [DOI] [Google Scholar]
- 21.Fiorelli LE, Grellet-Tinner G, Alasino PH, Argañaraz E. 2012. The geology and palaeoecology of the newly discovered Cretaceous neosauropod hydrothermal nesting site in Sanagasta (Los Llanos Formation), La Rioja, northwest Argentina. Cretac. Res. 35, 94–117. ( 10.1016/j.cretres.2011.12.002) [DOI] [Google Scholar]
- 22.Fiorelli LE, Grellet-Tinner G, Argarañaz E, Salgado L. 2013. Tafonomía del sitio de nidificación de neosaurópodos de Sanagasta (La Rioja, Argentina): ejemplo de preservación excepcional en un paleoambiente hidrotermal del Cretácico. Ameghiniana 50, 389–406. ( 10.5710/AMGH.15.11.2012.523) [DOI] [Google Scholar]
- 23.Deeming DC, Thompson MB. 1991. Gas exchange across reptilian eggshells. In Egg incubation: its effects on embryonic development in birds and reptiles (eds Deeming DC, Ferguson MWJ), pp. 277–284. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 24.Williams DLG, Seymour RS, Kerourio P. 1984. Structure of fossil dinosaur eggshell from the Aix Basin, France. Palaeogeogr. Palaeoclimatol. Palaeoecol. 45, 23–37. ( 10.1016/0031-0182(84)90107-X) [DOI] [Google Scholar]
- 25.Argañaraz E, Grellet-Tinner G, Fiorelli LE, Krause LM, Rauhut OWH. 2013. Huevos de saurópodos del Aptiano–Albiano, Formación Cerro Barcino (Patagonia, Argentina): Un enigma paleoambiental y paleobiológico. Ameghiniana 50, 33–50. ( 10.5710/AMGH.9.11.2012.551) [DOI] [Google Scholar]
- 26.Paganelli CV. 1980. The physics of gas exchange across the avian eggshell. Am. Zool. 20, 329–338. ( 10.1093/icb/20.2.329) [DOI] [Google Scholar]
- 27.Deeming DC. 2006. Ultrastructural and functional morphology of eggshells supports the idea that dinosaur eggs were incubated buried in a substrate. Palaeontology 49, 171–185. ( 10.1111/j.1475-4983.2005.00536.x) [DOI] [Google Scholar]
- 28.Ar A, Paganelli CV, Reeves RB, Greene DG, Rahn H. 1974. The avian egg: water vapor conductance, shell thickness, and functional pore area. Condor 76, 153–158. ( 10.2307/1366725) [DOI] [Google Scholar]
- 29.Birchard GF, Kilgore DLJ. 1980. Conductance of water vapor in eggs of burrowing and nonburrowing birds: implications for embryonic gas exchange. Physiol. Zool. 53, 284–292. ( 10.1086/physzool.53.3.30155791) [DOI] [Google Scholar]
- 30.Tanaka K, Zelenitsky DK, Therrien F. 2015. Eggshell porosity provides insight on evolution of nesting in dinosaurs. PLoS ONE 10, e0142829 ( 10.1371/journal.pone.0142829) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.