Abstract
The grand challenge facing the chemical and allied industries in the twenty-first century is the transition to greener, more sustainable manufacturing processes that efficiently use raw materials, eliminate waste and avoid the use of toxic and hazardous materials. It requires a paradigm shift from traditional concepts of process efficiency, focusing on chemical yield, to one that assigns economic value to replacing fossil resources with renewable raw materials, eliminating waste and avoiding the use of toxic and/or hazardous substances. The need for a greening of chemicals manufacture is readily apparent from a consideration of the amounts of waste generated per kilogram of product (the E factors) in various segments of the chemical industry. A primary source of this waste is the use of antiquated ‘stoichiometric’ technologies and a major challenge is to develop green, catalytic alternatives. Another grand challenge for the twenty-first century, driven by the pressing need for climate change mitigation, is the transition from an unsustainable economy based on fossil resources—oil, coal and natural gas—to a sustainable one based on renewable biomass. In this context, the valorization of waste biomass, which is currently incinerated or goes to landfill, is particularly attractive. The bio-based economy involves cross-disciplinary research at the interface of biotechnology and chemical engineering, focusing on the development of green, chemo- and biocatalytic technologies for waste biomass conversion to biofuels, chemicals and bio-based materials. Biocatalysis has many benefits to offer in this respect. The catalyst is derived from renewable biomass and is biodegradable. Processes are performed under mild conditions and generally produce less waste and are more energy efficient than conventional ones. Thanks to modern advances in biotechnology ‘tailor-made’ enzymes can be economically produced on a large scale. However, for economic viability it is generally necessary to recover and re-use the enzyme and this can be achieved by immobilization, e.g. as solid cross-linked enzyme aggregates (CLEAs), enabling separation by filtration or centrifugation. A recent advance is the use of ‘smart’, magnetic CLEAs, which can be separated magnetically from reaction mixtures containing suspensions of solids; truly an example of cross-disciplinary research at the interface of physical and life sciences, which is particularly relevant to biomass conversion processes.
Keywords: green chemistry, catalysis, biocatalysis, sustainability, biomass conversion, immobilized enzymes
1. Introduction: efficiency in organic synthesis
The well-being of our society is unimaginable without the myriad products of the chemical and allied industries. Our quality of life depends very much on the products of the pharmaceutical industry for combating disease and relieving pain. Similarly, in our everyday lives we are very much dependent on the synthetic polymers—inter alia, plastics, fibres and synthetic rubber—produced by the chemical industry. However, the flip side of this coin is that many of these processes were developed in an era when waste generation was not a particularly important issue and the negative effects of many chemicals on human health and our natural environment were either not known or not fully understood. This presents a problem but the solution to this problem is not less chemistry but alternative, cleaner technologies that minimize or eliminate waste and avoid the use of toxic and hazardous substances. Enter green chemistry.
Green chemistry can be succinctly defined as: Green chemistry efficiently utilizes (preferably renewable) resources, eliminates waste and avoids the use of toxic and/or hazardous reagents and solvents in the manufacture and application of chemical products [1]. The guiding principle is benign by design, that is, the design of environmentally benign chemical products and processes. The term was first introduced by Anastas and colleagues at the US Environmental Protection Agency in the early 1990s and was probably induced by the introduction of the US Pollution Prevention Act in November 1990. The subject gained formal recognition with the publication of the 12 principles of green chemistry by Anastas & Warner [2] in 1998.
Green chemistry is primary pollution prevention rather than waste remediation based on end-of-pipe solutions. As we have noted elsewhere [3], our attention was drawn to the problem of waste in the (fine) chemical industry, in the early 1980s, by the closure of a plant at Océ Andeno in the Netherlands, which produced approximately 100 tons per annum of phloroglucinol, a pharmaceutical intermediate. The plant was closed when the cost of disposing of the waste approached the selling price of the product. The starting material was 2,4,6-trinitrotoluene (TNT), which was converted, in a three-step process (figure 1), to phloroglucinol in ca 90% yield. So far, so good. Any organic chemist would almost certainly have said that this is an efficient, selective process. Unfortunately, phloroglucinol was not the only product formed. The first step of the process involves an oxidation with potassium dichromate in fuming sulfuric acid and, along with every kilogram of phloroglucinol, 40 kg of solid inorganic waste comprising Cr2(SO4)3, NH4Cl, FeCl2 and KHSO4 are formed. When this is taken into consideration, the process is not looking so efficient or environmentally benign. Apart from forming 40 kg of waste per kilogram of product it uses an explosive material (TNT) as the starting material together with stoichiometric quantities of hexavalent chromium, which is recognized as a human carcinogen, in fuming sulfuric acid.
Figure 1.
Manufacture of phloroglucinol anno 1980.
An important conclusion that could be drawn from our experience with the phloroglucinol process was that a new paradigm was needed for efficiency in organic synthesis to replace the traditional one of chemical yield of product with one that assigns value to waste elimination and avoiding the use of toxic and hazardous materials [4]. We concluded that an environmental factor was missing.
2. E factors and atom economy
Our experience with the phloroglucinol process led us to conduct an inventory of the amount of waste formed in a variety of processes for the manufacture of fine chemicals, pharmaceutical intermediates and even some bulk chemicals. This quickly revealed that the formation of 40 kg of waste per kilogram of desired product, as in the phloroglucinol process, was by no means an exception in the fine chemicals industry. This led us to propose the term E(nvironmental) factor, defined as the amount of waste formed per kg of product, as a convenient metric for quickly assessing the environmental footprint of a manufacturing process. The magnitude of this waste problem in various segments in the chemical industry is illustrated in table 1 which we first published in 1992 [5].
Table 1.
E factors in the chemical industry.
| industry segment | typical plant capacity (tpa) | E factor (kg waste/kg product) |
|---|---|---|
| oil refining | 106–108 | <0.1 |
| bulk chemicals | 104–106 | <1–5 |
| fine chemicals | 102–104 | 5–50 |
| pharmaceuticals | 10–103 | 25–>100 |
The E factor comprises the actual amount of waste formed in the process, including solvents that are not recovered and any other auxiliary materials used in the process and work-up of the product. Knowledge of the stoichiometric equation allows one to predict, without performing any experiments, the minimum amount of waste that can be expected and we began to use what we called the atom utilization, defined as the molecular weight of the product divided by the total molecular weight of all products formed in the stoichiometric equation, expressed as a percentage, to quickly assess the environmental acceptability of alternative processes to a particular product. In an ideal process, all the atoms end up in the product with an atom utilization of 100% and a theoretical E factor of zero. In practice, of course, the E factor will not be zero because auxiliary materials are used, the product yield is not 100%, and excess quantities of reagents are often used. We had previously used the concept of atom utilization, in bulk chemicals manufacture, for assessing commercial viability on the assumption that the more atoms of the starting materials that ended up in the desired product the better the economics would be. In 1991, Trost [6] published his elegant paper on atom economy which became the widely accepted terminology, although sometimes atom efficiency is used. The above described phloroglucinol process, for example, has an atom economy of ca 5% which would predict a (theoretical) E factor of ca 20. That the E factor is actually 40 can be attributed to the fact that the yield is not 100% and excess quantities of reagents are used (fuming sulfuric acid acts as a solvent for the reaction).
3. Waste minimization and catalysis
Where does all this waste originate? It is abundantly clear from the phloroglucinol, and other examples, that the major source of waste is the application of antiquated technologies involving the use of stoichiometric quantities of mainly inorganic reagents in the form of oxidants, reductants, and acids and bases. The solution is evident: substitution of these antiquated stoichiometric methodologies with greener catalytic alternatives. A catalyst, by definition, accelerates the rate of a chemical reaction without being consumed and, consequently, it does not generate any waste in theory. In practice, this is not a perfect world and catalysts are deactivated and can end up as (small amounts of) waste.
The recommended textbook method for the conversion of a secondary alcohol to a ketone (figure 2), for example, involves oxidation with the Jones reagent, which consists of stoichiometric quantities of chromium trioxide in sulfuric acid and affords chromium sulfate as the coproduct. The atom economy of this reaction, using α-methylbenzyl alcohol as the substrate, is 44% which would translate to an E factor of ca 1.3. In practice, the E factor is more than 3 owing to the use of an excess of reagents and the less than 100% yield. This should be compared with the catalytic aerobic oxidation (figure 2), which has an atom economy of 91% and water as the coproduct. The theoretical E factor is ca 0.1. We have described [7] such a process which uses air as the oxidant and a water-soluble palladium complex as the catalyst in an organic solvent free, aqueous biphasic system, affording the corresponding ketone in high yield. It was further shown that the catalyst could be recycled several times, without any loss of activity, via simple phase separation.
Figure 2.
Oxidation of a secondary alcohol to a ketone.
4. Sustainable development and the bio-based circular economy
Sustainable development is defined [8] as development that meets the needs of the present generation without compromising the needs of future generations to meet their own needs. Sustainability consists of three components: societal, ecological and economic, otherwise referred to as the three Ps, people, planet and profit. As Graedel has pointed out [9], in order to be sustainable a technology must fulfil two conditions: (i) natural resources should be used at rates that do not unacceptably deplete supplies over the long term and (ii) residues should be generated at rates no higher than can be assimilated readily by the natural environment. With regard to (i), it is abundantly clear, for example, that non-renewable fossil resources—oil, coal and natural gas—are being used at a much higher rate than they are replaced by natural geological processes and their use is not sustainable in the long term. Furthermore, the use of fossil resources is generating carbon dioxide at rates that cannot be assimilated by the natural environment.
This is illustrated in figure 3 which depicts the petrochemical carbon cycle in which carbon dioxide is converted via the biological process of photosynthesis into plant biomass which is laid down in geological reservoirs where, over a period of millions of years, it is converted to coal, natural gas and oil. The latter is subsequently converted in petrochemical refineries, for example, into liquid fuels which, via combustion, regenerate carbon dioxide which is returned to the atmosphere, but in a much shorter time span. Hence, in the first instance the cycle appears to be complete but closer inspection reveals that the rate of formation of the oil is about five orders of magnitude lower than the rate of its consumption, leading to depletion of these geological reserves and, more importantly, in the shorter term increased carbon dioxide levels in the atmosphere, which are generally believed to be a direct cause of climate change.
Figure 3.
(a) The petrochemical carbon cycle and (b) the bio-based circular economy. (Online version in colour.)
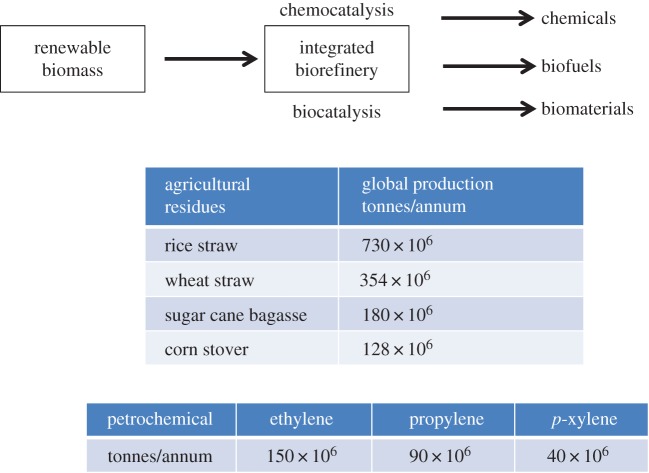
In order to be sustainable the carbon cycle needs to be brought into balance and this can be achieved by replacing the slow formation of oil from plant biomass laid down in geological reservoirs with direct conversion of plant biomass to biofuels and chemical products. This forms the basis for a carbon neutral, bio-based economy comprising the conversion of renewable biomass to biofuels, chemicals and biomaterials in integrated biorefineries using green, resource-efficient chemo- and biocatalytic processes (figure 4). Hence, the ‘bio-based’ refers to the use of renewable biomass as the raw material rather than the technology used for its conversion, which can involve chemical or biotechnological processing.
Figure 4.
The bio-based economy. (Online version in colour.)
Hence, although attention continues to be focused on waste minimization and avoiding the use of toxic and/or hazardous materials in chemicals manufacture, there is currently a growing emphasis on the third element of green chemistry, namely the substitution of non-renewable fossil resources by renewable biomass as a sustainable feedstock for the manufacture of commodity chemicals and liquid fuels [10,11]. A switch to renewable biomass as a feedstock will afford an environmentally beneficial reduction in the carbon footprint of chemicals and liquid fuels. A further benefit could be the substitution of existing products by inherently safer alternatives with reduced environmental footprints, such as biocompatible and biodegradable plastics [12].
However, it is widely accepted that the use of first-generation biomass feedstocks, such as corn and edible oil seeds is not a sustainable option in the longer term because it competes, directly or indirectly, with food production. In the European Union, therefore, emphasis is firmly on the use of second-generation biomass comprising waste lignocellulose and oils and fats as feedstocks. The ideal scenario, involves the valorization of waste biomass generated as agricultural residues by applying resource-efficient chemo- and biocatalytic processes [13]. Global production of sugar cane bagasse, corn stover, wheat straw and rice straw, for example, are in the hundreds of millions of tonnes per annum (figure 4) [14], amounts that far exceed the annual production of the top petrochemicals, ethylene (150 million tonnes), propylene (90 million tonnes) and para-xylene (40 million tonnes) Furthermore, driven by the need to avoid waste, attention has recently focused on a new and promising feedstock for biorefineries: food supply chain waste (FSCW) [15]. Enormous amounts of organic waste, much of which goes to landfill, are generated in the harvesting, processing and use of agricultural products in food and beverages.
Two decades ago, the emphasis was firmly on waste prevention at source but it is now recognized that in cases such as agricultural production where waste cannot be avoided, there is a growing need for creating value by valorization of unavoidable waste. This fits very well with current thinking regarding the so-called the circular economy (http://www.ellenmacarthurfoundation.org/publications). Increased interest in green and sustainable growth, coupled with increasing concern for climate change, has focused attention on resource efficiency. This is stimulating the much needed move from a traditional linear flow of materials in a ‘take–make–consume–dispose economy, to a circular one that seeks to eliminate waste through design of products, processes and business models, and is restorative or regenerative by design. This is embodied in the European Commission's ‘Roadmap to a resource-efficient Europe’ [16].
5. Biocatalysis is green and sustainable
Biocatalysis plays a pivotal role in the production of both first- and second-generation bioethanol (figure 5). Indeed, biocatalysis has numerous benefits to offer in the context of green chemistry and sustainable development. The catalyst (an enzyme) is derived from renewable resources and is biocompatible (sometimes even edible), biodegradable and essentially non-hazardous, i.e. it fulfils the criteria of sustainability remarkably well. Biocatalysis avoids the use of scarce precious metals, the long-term commercial viability of which is questionable. Moreover, the costs of removing traces of noble metals, to an acceptable level, from end products can be substantial. Enzymatic reactions are performed under mild conditions (physiological pH and ambient temperature and pressure) in high selectivities, affording products in higher purity and processes that are more efficient in energy and raw materials consumption and generate less waste than conventional routes.
Figure 5.
First- and second-generation bioethanol. (Online version in colour.)
As shown in figure 5, first-generation bioethanol production involves the enzymatic hydrolysis of starch, derived from, for example, corn or wheat, catalysed by glucoamylase followed by fermentation of glucose to ethanol. The feedstock for second-generation bioethanol, on the other hand, is lignocellulose, the fibrous material that constitutes the cell walls of plants, which is much more difficult to process. Some form of pretreatment, such as a steam explosion, ammonia fibre expansion (AFEX) or lime treatment, is generally necessary to open up the recalcitrant lignocellulose structure and render the targeted glycoside (ether) and ester bonds accessible to the enzyme cocktails. The lignocellulosic biomass is initially hydrolysed into a mixture of cellulose, hemicellulose and lignin. Further hydrolysis of the hemicellulose and cellulose, in a process known as saccharification [17] affords their constituent C5 and C6 monosaccharides which are subsequently converted to bioethanol by fermentation.
Irrespective of which method is used, both the pretreatment costs and the cost of the enzyme cocktail contribute significantly to the overall cost of second-generation bioethanol. The enzyme costs have decreased significantly over the last decade, and are still decreasing, as a result of optimization of the production and properties of the cellulolytic enzyme cocktail [18]. Furthermore, the enzymes are currently applied on a single use, throw-away basis and further significant cost reductions can be achieved by immobilization [19] of the enzyme(s) as insoluble free-flowing powders that are readily separated by filtration or centrifugation and can be recycled multiple times. For example, we developed the technique of immobilizing enzymes as cross-linked enzyme aggregates (CLEAs) [20]. This involves simply precipitating the enzyme from aqueous buffer, as physical aggregates of enzyme molecules, by adding a salt such as ammonium sulfate or a water miscible organic solvent or polymer, followed by cross-linking of the aggregates by reaction with a suitable bifunctional cross-linker such as glutaraldehyde (figure 6). CLEAs have several attractive features in the context of industrial applications. There is no need for highly pure enzyme as they can be prepared from very crude enzyme preparations, even directly from crude cell lysates obtained from fermentation broth. Since they are carrier-free they avoid the costs associated with the use of (often expensive) carriers and they exhibit high catalyst productivities (kilograms product per kilogram biocatalyst) and facile recovery and recycle. Furthermore, they generally have improved storage and operational stability with regard to denaturation by heat, organic solvents and autolysis and are stable towards leaching in aqueous media. In a further elaboration of the CLEA technology, the enzyme aggregates can be cross-linked in the presence of functionalized magnetic particles to afford magnetic CLEAs which can be separated magnetically [21].
Figure 6.
Cross-linked enzyme aggregates (CLEAs). (Online version in colour.)
The application of immobilized enzymes, such as CLEAs, in the hydrolysis of polysaccharides, such as starch and cellulose, presents an extra challenge: the CLEA may have to be separated from other suspended solids present in the reaction mixture. We have demonstrated (R. A. Sheldon, S. van Pelt 2015, unpublished data) that this can be readily achieved, in a cost-effective manner on a large scale, in starch hydrolysis using magnetic CLEAs of glucoamylase and magnetic separation equipment commonly used in the mining industry, such as magnetic wet drum separation. Similarly, the immobilization of cellulolytic enzymes as magnetic CLEAs has been reported [22]. The resulting glucose and pentose sugars are subsequently converted to bioethanol by fermentation. The enzymatic hydrolysis and fermentation can be carried out as separate hydrolysis and fermentation (SHF) or in a one-pot, simultaneous saccharification and fermentation (SSF) [23]. In an SHF process the fermenting organism, usually Saccharomyces cerevisiae (brewer's yeast), and the enzymes can be used at their respective optimum temperature and pH but hydrolysis products can inhibit the glucoamylase or cellulase, thus reducing their efficiency. An advantage of SSF is that the glucose is immediately consumed by the fermenting organism, thus avoiding product inhibition. In an SSF process, in order to recover and recycle the immobilized enzyme, it has to be separated from the yeast in the fermentation broth. This can be readily achieved by applying a ‘smart’ magnetic CLEA in combination with magnetic separation (figure 7), a striking example of cross-disciplinary research at the interface of physical and life sciences.
Figure 7.
Bioethanol production with magnetic separation of the magnetic CLEA of the polysaccharide hydrolysing enzyme. (Online version in colour.)
6. Conclusion and outlook
Over the last two decades the concepts of green chemistry and sustainable technologies, and the underpinning metrics, atom economy and the E factor, have been widely embraced by industry and academia worldwide. It has been a gradual process, which is by no means complete, but much progress has been made from the antiquated technologies illustrated by the TNT to phloroglucinol process. The pharmaceutical industry in particular has made great strides in reducing their E factors and avoiding the use of toxic or hazardous solvents and reagents. Catalysis, both chemical and biological, has played a pivotal role in this ‘greening’ of the pharmaceutical industry.
More recently, much attention is being diverted to a third aspect of green chemistry and sustainability: the use of renewable raw materials as an alternative to the unsustainable use of fossil resources. This shift of emphasis is largely driven by the pressing need to mitigate climate changes resulting from increasing concentrations of fossil resources-derived carbon dioxide in the atmosphere. In this context, the valorization of unavoidable waste lignocellulose and triglycerides is particularly attractive and fits well with the current trend towards a circular economy. In short, we expect that the current developments, at the interface of chemistry and biology, or from an industrial viewpoint, chemical and biotechnology, will continue unabated in the future, on the road to engineering a more sustainable world through catalysis and green chemistry. In the words of William Ford Jr, CEO of the Ford Motor Company: ‘A good company delivers excellent products and services. A great company does all of this and strives to make the world a better place.’
Competing interests
I declare I have no competing interests.
Funding
I received no funding for this study.
References
- 1.Sheldon RA, Arends IWCE, Hanefeld U. 2007. Green chemistry and catalysis. Weinheim, Germany: Wiley-VCH. [Google Scholar]
- 2.Anastas PT, Warner JC. 1998. Green chemistry: theory and practice. Oxford, UK: Oxford University Press. [Google Scholar]
- 3.Sheldon RA. 2007. The E factor: fifteen years on. Green Chem. 9, 1273–1283. ( 10.1039/b713736m) [DOI] [Google Scholar]
- 4.Sheldon RA. 2012. Fundamentals of green chemistry: efficiency in reaction design. Chem. Soc. Rev. 41, 1437–1451. ( 10.1039/C1CS15219J) [DOI] [PubMed] [Google Scholar]
- 5.Sheldon RA. 1992. Organic synthesis: past, present and future. Chem. Ind. (Lond.) 23, 903–906. [Google Scholar]
- 6.Trost BM. 1991. The atom economy: a search for synthetic efficiency. Science 254, 1471–1477. ( 10.1126/science.1962206) [DOI] [PubMed] [Google Scholar]
- 7.Ten Brink GJ, Arends IWCE, Sheldon RA. 2000. Green catalytic oxidation of alcohols. Science 287, 1636–1639. ( 10.1126/science.287.5458.1636) [DOI] [PubMed] [Google Scholar]
- 8.Brundtland CG. 1987. Our common future. Oxford, UK: The World Commission on Environmental Development, Oxford University Press. [Google Scholar]
- 9.Graedel TE. 2002. Green chemistry and sustainable development. In Handbook of green chemistry and technology (eds Clark J, Macquarrie DJ), pp. 56–61. New York, NY: Wiley. [Google Scholar]
- 10.Imhof P, van der Waal JC (eds). 2013. Catalytic process development for renewable materials. Weinheim, Germany: Wiley-VCH. [Google Scholar]
- 11.Sheldon RA. 2014. Green and sustainable manufacture of chemicals from biomass: state of the art. Green Chem. 16, 950–963. ( 10.1039/C3GC41935E) [DOI] [Google Scholar]
- 12.De Jong E, Higson A, Walsh P, Wellisch M. 2012. Product developments in the bio-based chemicals arena. Biofuels Bioprod. Bioref. 6, 606–624. ( 10.1002/bbb.1360) [DOI] [Google Scholar]
- 13.Tuck CO, Perez E, Horvath IT, Sheldon RA, Poliakoff M. 2012. Valorization of biomass: deriving more value from waste. Science 337, 695–699. ( 10.1126/science.1218930) [DOI] [PubMed] [Google Scholar]
- 14.Saini JK, Saini R, Tewari L. 2015. Lignocellulosic agriculture wastes as biomass feedstocks for second-generation bioethanol production: concepts and recent developments. 3 Biotech 5, 337–353. ( 10.1007/s13205-014-0246-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfaltzgraff LA, De Bruyn M, Cooper EC, Budarin V, Clark JH. 2013. Food waste biomass: a resource for high-value chemicals. Green Chem. 15, 307–314. ( 10.1039/c2gc36978h) [DOI] [Google Scholar]
- 16.Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions. 2011 Roadmap to a Resource Efficient Europe, COM/2011/0571 final.
- 17.Bornscheuer U, Buchholz K, Seibel J. 2014. Enzymatic degradation of (ligno)cellulose. Angew. Chem. Int. Ed. 53, 10 876–10 893. ( 10.1002/anie.201309953) [DOI] [PubMed] [Google Scholar]
- 18.Ayrinhac C, Margeot A, Lopes Ferreira N, Chaabane FB, Monot F, Ravot G, Sonet JM, Fourage L. 2011. Saccharification of wheat straw for biofuel production using an engineered secretome of Trichoderma reesei. Org. Proc. Res. Dev. 15, 275–278. ( 10.1021/op100218a) [DOI] [Google Scholar]
- 19.Sheldon RA, van Pelt S. 2013. Immobilisation of biocatalysts: why, what and how? Chem. Soc. Rev. 42, 6223–6235. ( 10.1039/C3CS60075K) [DOI] [PubMed] [Google Scholar]
- 20.Sheldon RA. 2011. Characteristic features and biotechnological applications of cross-linked enzyme aggregates (CLEAs). Appl. Microbiol. Biotechnol. 92, 467–477. ( 10.1007/s00253-011-3554-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheldon RA, Sorgedrager MJ, Kondor B. 2012. Non-leachable magnetic cross-linked enzyme aggregate, PCT Int. Appl. WO 2012/023847 A2 to CLEA Technologies B.V.
- 22.Bhattacharya A, Pletschke BI. 2014. Magnetic cross-linked enzyme aggregates (CLEAs): a novel concept towards carrier free immobilization of lignocellulolytic enzymes. Enz. Microb. Technol. 61–62, 17–27. ( 10.1016/j.enzmictec.2014.04.009) [DOI] [PubMed] [Google Scholar]
- 23.Liu ZH, Qin L, Zhu JQ, Li BZ, Yuan YJ. 2014. Simultaneous saccharification and fermentation of steam-exploded corn stover at high glucan loading and high temperature. Biotechnol. Biofuels 7, 167 ( 10.1186/s13068-014-0167-x) [DOI] [PMC free article] [PubMed] [Google Scholar]