Abstract
There is a growing interest in using trait-based approaches to characterize the functional structure of animal communities. Quantitative methods have been derived mostly for plant ecology, but it is now common to characterize the functional composition of various systems such as soils, coral reefs, pelagic food webs or terrestrial vertebrate communities. With the ever-increasing availability of distribution and trait data, a quantitative method to represent the different roles of animals in a community promise to find generalities that will facilitate cross-system comparisons. There is, however, currently no theory relating the functional composition of food webs to their dynamics and properties. The intuitive interpretation that more functional diversity leads to higher resource exploitation and better ecosystem functioning was brought from plant ecology and does not apply readily to food webs. Here we appraise whether there are interpretable metrics to describe the functional composition of food webs that could foster a better understanding of their structure and functioning. We first distinguish the various roles that traits have on food web topology, resource extraction (bottom-up effects), trophic regulation (top-down effects), and the ability to keep energy and materials within the community. We then discuss positive effects of functional trait diversity on food webs, such as niche construction and bottom-up effects. We follow with a discussion on the negative effects of functional diversity, such as enhanced competition (both exploitation and apparent) and top-down control. Our review reveals that most of our current understanding of the impact of functional trait diversity on food web properties and functioning comes from an over-simplistic representation of network structure with well-defined levels. We, therefore, conclude with propositions for new research avenues for both theoreticians and empiricists.
Keywords: ecological networks, trait matching, ecosystem functioning, biodiversity
1. Introduction
The investigation of functional traits has been proposed as a means to find generalities in the structure and dynamics of ecological communities, beyond the mere study of pairwise interactions [1]. It is prohibitive to study and document all potential pairwise interactions, as their number increases in proportion to the square of species richness. Instead, traits are common currencies, allowing comparisons across communities and regional pools [2,3]. Trait-based approaches are commonly used to explore and understand the diversity of forms and functions within an ecosystem, and they have been used to approximate some aspects of ecosystem functioning [4]. While they were originally developed for plants (e.g. [5,6]), there are now more and more studies investigating the functional composition of animal communities [7], including birds, mammals, arthropods, fishes, marine invertebrates, reptiles and amphibians (e.g. [8–12]). A quantitative approach to represent the different roles of animals in a community promises to find generalities and will definitely facilitate cross-system comparisons. It is not so clear, however, how the current trait-based approaches apply to food webs, and more generally to interaction networks.
The difficulty arising with animals is their involvement in complex interaction networks, making it hard to measure and understand the role of functional structure and diversity. The theory developed for plants is applicable for a single trophic group driven by competition [13], ignoring the complexity arising with animals involved in a more diverse array of interactions such as herbivory, predation, parasitism, seed dispersal and pollination. The study of plant community structure from functional traits is commonly based on the quantification of the average trait value of a community weighted by relative abundance (community-weighted mean, CWM). CWM is meant to represent the most dominant traits of the community [14], while its associated variance (CWV) represents how variable is the trait in the community [15]. The functional structure of a community is often described to understand the role of key traits and how they relate to environmental gradients [16], and to identify causal links on ecosystem properties and functions [17,18]. Functional trait diversity is also often used to assess complementarity and redundancy among species, which ultimately should give insights on the assembly mechanisms in play [19]. Both CWM and CWV, therefore, nicely relate to the mechanisms driving a positive relationship between the diversity and productivity of plant communities [20]. The sampling effect is based on the hypothesis that some dominant species, because of their traits (such as leaf traits [21]), drive ecosystem functioning [22] and that they are more likely to be found in a diverse community. The complementarity effect, on the other hand, proposes the increase of resource use efficiency with the diversity of ecological strategies (e.g. diversity of rooting depth). The CWM and the CWV, therefore, represent the sampling and the complementarity effects, respectively [23]. While the biodiversity and ecosystem functioning (BEF) theory is clearly established for plants [24] and well supported by experiments [25], it was found not so easy to generalize for food webs because of the complex interactions that could either reverse expectations or simply blur any relationship [26]. Trophic regulation and the complexity of food web topologies including omnivory, intra-guild predation, cannibalism and loops prevent such generalization.
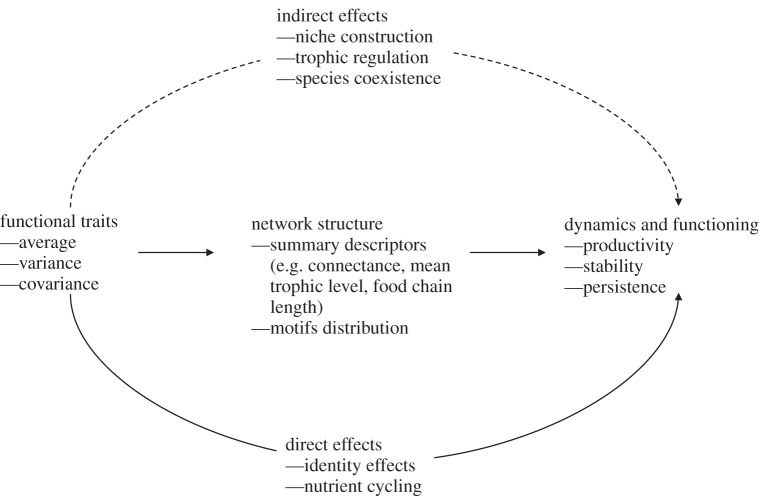
It is not straightforward to appreciate the role of food web functional trait structure and diversity for ecosystem functioning. Traits are involved both directly and indirectly in the processes of shaping ecological communities and driving their dynamics (figure 1). First, traits have a direct effect on ecosystem functioning through the traditional mechanisms of resource use complementarity and identity effects. The presence of top predators, for instance, has been recognized for a long time as being essential for ecosystem functioning [27]. Their extinctions due to human actions have often resulted in dramatic shifts of ecosystem properties. As an example, in the marine ecosystem, sharks play a key role through top-down control on lower trophic levels. The loss of this trophic regulation could have drastic consequences on ecosystem functioning such as declines in fish production, sudden increase of some populations, decrease in stability, and nutrient losses [28]. Secondly, traits have an indirect effect via their impact on the structure of the food web, and thereby on trophic cascades. Further, predators can change the composition of the lower trophic level and thereby influence nutrient cycling processes [29]. There are some hints as to how the topology of ecological networks might impact ecosystem functioning (for instance via the mechanism of trophic complementarity [30]), but we are yet to fully understand this relationship.
Figure 1.
Conceptual relationship between functional composition, food web structure, and dynamics. The functional composition of the community has a direct effect on food webs via identity effects and an indirect effect via its impact on network structure.
The objective of this paper is to provide a conceptual framework to guide the interpretation of functional trait structure and diversity in food webs. We first propose a terminology for the investigation of the functional structure and diversity of food webs. We briefly review the types of traits that are typically used in studies of animal communities. We then review how functional structure and diversity of animal communities have been studied, and contrast these studies to the investigation of plant communities. Next, we describe how traits have been used to understand pairwise interactions between animals, and assess the implication of functional diversity on food web structure. Finally, we explore the implications of trait diversity for ecosystem functioning. Our framework highlights major gaps in knowledge, and we consequently end with a discussion of the most promising and exciting research avenues opened by the investigation of the functional structure and diversity of food webs.
2. Classifying traits in food webs
We focus on three categories of traits that are essential to food web structure and dynamics. These categories are not exclusive, as some traits—such as body size or movement ability—can be found in multiple categories. We provide definitions, and support this classification with the interpretation of a classical food web model in box 1 and examples of traits that are commonly studied in table 1.
Box 1. Interpretation of functional traits in a food web model.
We illustrate how the different categories of traits influence food web dynamics directly and indirectly. A tri-trophic diamond food web model (figure B1) is commonly used to study coexistence [31] and stability [32]. The model represents with ordinary differential equations the dynamics of a producer compartment P, herbivores H1 and H2, and a top carnivore C. For simplicity, the dynamics of the producer follows a logistic equation with intrinsic growth rate r and carrying capacity K. Consumers harm the resource population at a given rate, and only a fraction of the resource ingested is converted into consumer biomass. The dynamics could be described by the following set of differential equations:
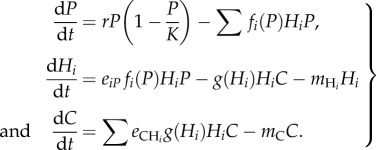 |
2.1 |
The definition of the different fluxes illustrates how the classification of traits we propose in the main text impacts directly on the dynamics of this system. The herbivores consume the plant at rate fi(P)HiP and only a fraction eiP of that flux is converted into biomass. Similarly, the carnivore consumes the herbivores at rate g(Hi). The functions fi(P) and gi(Hi) vary across systems, types of interactions and pairs of species (see Jeschke et al. [33] and Jeschke [34] for an extensive discussion on the topic). In a type II functional response, for instance, such as aij/(1 + aijbijRj), the maximal attack rate aij is a single parameter embedding foraging characteristics of the consumer (e.g. its mobility) and the vulnerability of the resource (e.g. defensive traits). Similarly, the handling time bij is a consumption trait that depends on the characteristics of both the consumer (its capacity to catch and process resources) and the resource (mainly its digestibility). For the same reasons, the assimilation efficiency depends on both consumer and resource traits. The natural mortality rate mi is a life history trait that depends only on the characteristics of the consumer, not its prey. Resource selection impacts on network structure and will be found in the variation of the attack rates. For instance, a preference for one of the herbivores by the carnivore would be reflected by a higher 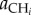 or a better conversion efficiency
or a better conversion efficiency 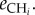
Solving the system at equilibrium also illustrates how the different traits affect the abundance of the different species indirectly. The analytical solution to the equilibrium densities of the four species web is tedious to write down and not so useful for this example, but the tri-trophic chain P−H1−C is nonetheless instructive. At equilibrium we get:
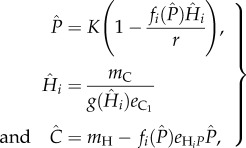 |
2.2 |
which shows that the equilibrium abundances of the different species depends on all types of traits. The per capita effect of the carnivore on the herbivore is simply the functional response gHi, and inversely the per capita effect of the herbivore on the carnivore is 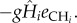 Similarly, the per population effects are, respectively,
Similarly, the per population effects are, respectively, 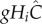 and
and 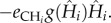 This simple example shows that while per capita effects only depend on the set of traits of the species pair, per population effects also depend on the entire distribution of traits of the community because indirect interactions propagate through the food chain and affect all species. As an example, the traits of the plant will affect the per population effect of the carnivore on the herbivore because the more productive is the plant community (high r and K), the larger will be the carnivore population, and consequently, the total amount of herbivore consumed at equilibrium. Accordingly, we expect that the entire distribution of traits (mean, variance, covariance and higher moments) will impact net interactions between all pairs of species in a food web.
This simple example shows that while per capita effects only depend on the set of traits of the species pair, per population effects also depend on the entire distribution of traits of the community because indirect interactions propagate through the food chain and affect all species. As an example, the traits of the plant will affect the per population effect of the carnivore on the herbivore because the more productive is the plant community (high r and K), the larger will be the carnivore population, and consequently, the total amount of herbivore consumed at equilibrium. Accordingly, we expect that the entire distribution of traits (mean, variance, covariance and higher moments) will impact net interactions between all pairs of species in a food web.
 .
.
Figure B1. Schematic representation of the modelled food web (note that only the outgoing flux is represented for clarity, the assimilation efficiency is not included).
Table 1.
Classification of common traits used in studies of animal functional diversity. References for terrestrial communities are [7,10,35] and references for marine communities include [8,36–38] and references therein.
| type of trait | fishes | marine invertebrates | birds | mammals | amphibians | reptiles | invertebrates |
|---|---|---|---|---|---|---|---|
| topological | body length | maximum size | body mass | body mass | body length | body length | body mass |
| body mass | maximum growth rate | body shape | body shape | body shape | body shape | body shape | |
| gut length | body design | plumage colour | skin colour | skin colour | aposematic colour | ||
| median paired fin length | living habit | diet type | diet type | diet type | diet type | substrate mimicry | |
| pectoral fins surface to caudal fin surface | living location/environmental position | foraging behaviour | foraging behaviour | foraging behaviour | foraging behaviour | diet type | |
| pectoral fin shape | exposure potential | nesting behaviour | nesting behaviour | poisonous skin | nesting behaviour | foraging behaviour | |
| peduncle throttling | degree of attachment to substrate | feeding behaviour | nesting behaviour | nesting behaviour | feeding behaviour | sociality | |
| caudal fin shape | degree of flexibility | activity time | feeding habitat | feeding habitat | activity time | specific weapons | |
| body shape | strength of attachment to substrate | wing morphology | activity time | activity time | swimming traits | toxicity/resistance | |
| eye position | resource capture method | social behaviour | social behaviour | swimming traits | mouthparts size | ||
| skin coloration | movement method | mouthparts strength | |||||
| swimming traits | mobility | escape strategy | |||||
| body size | defence strategy | adhesion strength | |||||
| physical protection—spines, bony carapaces | water column migration | palatability | |||||
| chemical defense | cognitive ability | ||||||
| consumption | fish body mass | body design | metabolic rate | metabolic rate | metabolic rate | metabolic rate | metabolic rate |
| visual acuity: maximum eye diameter relative to head size | degree of flexibility | body mass | body mass | body mass | body mass | body mass | |
| barbel length | resource capture method | diet type | diet type | diet type | diet type | diet type | |
| oral gape surface/shape | food type | bill morphology | gape width | gape width | gape width | mouthparts size | |
| vertical position of oral gape | movement method | wing morphology | mouthparts strength | ||||
| shape of teeth | mobility | feet morphology | feet morphology | feet morphology | feet morphology | defence strategy | |
| density and maximal length of gill rakers | defence strategy | tarsus/leg length | tarsus/leg length | tarsus/leg length | tarsus/leg length | escape strategy | |
| maximal length of gill rakers | water column migration | social behaviour | social behaviour | toxicity/resistance | |||
| competitive ability | competitive ability | competitive ability | competitive ability | sociality | |||
| local movement | local movement | local movement | local movement | competitive ability | |||
| life history | egg size | maximum growth rate | reproductive effort | reproductive effort | reproductive effort | reproductive effort | egg size |
| number of eggs | reproductive method | reproductive behaviour | reproductive behaviour | reproductive behaviour | reproductive behaviour | egg number | |
| larval life history | time to maturity | metabolism | metabolism | metabolism | metabolism | length of larval stage | |
| type of reproduction sites | fecundity | fecundity | fecundity | fecundity | fecundity | number of larval stages | |
| parental care | propagule dispersal | digestive physiology | digestive physiology | digestive physiology | digestive physiology | dispersal | |
| relative brain size | relative brain size | relative brain size | relative brain size | temperature requirements | |||
| social behaviour | social behaviour | larval diet type | |||||
| migratory status | migratory status | competitive ability | |||||
| home range size | home range size | home range size | home range size | survival rate | |||
| dispersal strategy | dispersal strategy | dispersal strategy | dispersal strategy | metabolism | |||
| competitive ability | competitive ability | competitive ability | competitive ability | resistance to starvation | |||
| survival rate | survival rate | survival rate | survival rate | resistance to dessication | |||
| reproduction system | |||||||
| sociality |
‘Topological traits’ determine whether a given predator species can feed on the given prey species. Topological traits could be further divided into two subcategories [39,40]: ‘foraging traits’ determine the type of preys a predator will look for. For instance, the shape of mouthparts of several arthropods such as ground beetles will tell us much about their optimal prey type [41]. Such traits are often regrouped into broad categories such as herbivores, filter feeders or carnivores. By contrast, ‘vulnerability traits’ determine the type of predators for a given prey species. They include traits providing resistance to predation, such as particular morphological attributes (e.g. spines) or chemical defense. A trophic interaction is the result of a match between foraging and vulnerability traits (see the section below on trait-matching models). Topological traits could include physiological characteristics of a species, morphological, behavioural (e.g. hiding strategies) and microhabitat preferences (e.g. foraging location in canopy trees). They should be precise and measurable attributes of the species, rather than the common representation into feeding guilds (e.g. [42]). Topological traits also encompass any characteristic of species related to phenology and use of space. The occurrence of an interaction requires a match between the time of the activity, its location, and the occurrence of both predators and preys.
‘Consumption traits’ determine the rate at which trophic interactions harm the prey population and benefit the predator. They determine the functional and numerical responses of the prey and the predator, respectively (see extensive discussions of the related traits in [33,34]). These traits are less documented and more difficult to measure (table 1). There are several steps in the process of a trophic interaction and all of them correspond to specific traits (these are more formally interpreted mathematically in box 1). First, the attack rate (which is the result of foraging speed) is influenced by both predator and prey characteristics, such as movement, the capacity to hide, temporal match of activities, spatial distribution of the prey, etc. Further, prey density and the capacity of the predator to handle individuals also influence the rate of interactions. Handling time is impacted by prey characteristics, such as digestibility—for instance, mussels and gastropods are harder to digest than crustaceans because of their shell. The handling time is also impacted by the characteristics of the predator, for instance, the recovery time between attacks, specialized morphological characteristics to process preys, or the time needed to digest them. Secondly, resource assimilation efficiency determines the fraction of the prey biomass that is ingested by the predator and converted into biomass. Again, both predator and prey characteristics will determine this quantity, as some preys have a larger proportion of edible biomass (e.g. relative weight of bones, lignified tissues in plants), and predators cannot process all of the prey (e.g. parasitoid larvae do not consume the entire biomass of their host). The resource assimilation efficiency is an important characteristic for indirect trophic interactions between scavengers and their resources. Finally, it is also a characteristic of the predator determining the fraction of the consumed prey biomass that is converted into its own biomass and into offspring. Several traits are related to it, such as fecundity and the stoichiometry of the predator [43].
‘Life history traits’ are characteristics of preys and predators affecting their demography, and consequently, equilibrium abundances. Life history traits are not involved directly in the trophic interaction, they do not influence the per capita rate at which the interaction happens. However, they affect population dynamics and could thus indirectly determine population size and the per population effect of one species on another. The R* principle [44], for instance, states that the equilibrium abundance of a resource is determined by the ratio of mortality and attack rates of the consumer. Examples include the rate of ontogenic development, age at sexual maturity, duration of the larval stage, fecundity, or natural mortality rate (examples with references are provided in table 1).
In summary, topological traits determine the pairwise interactions in a food web, the consumption traits impact the per capita interaction strength, in both directions, and finally, the life history traits will be involved in the per population interaction strength. All these traits will combine to set trophic regulation [45], species composition and population size of both prey and predator at equilibrium (box 1), community stability in face of a disturbance [46–48], and the direct and indirect effects of perturbations [49]. Note that we have thus far kept aside traits that are involved in species-specific responses to environmental variation (e.g. optimal temperature, habitat requirements), as they are not directly involved in the trophic interactions and food web dynamics. They should nonetheless be kept in mind, as they influence the co-distribution of predators and preys over environmental gradients and consequently the occurrence of an interaction [50], they could condition interaction rates [51], as well as temporal dynamics. We also ignored trait-mediated interactions, which are well known to impact community dynamics and ecosystem functioning [52]. Some interactions impact the expression of species traits, and indirectly affect other interactions [51]. Trait-mediated interactions are susceptible to affect all three categories of traits we described here.
The trait-based conceptual framework for plant ecology is based on a different classification of traits. Lavorel & Garnier [13] distinguished ‘effect traits’, determining the effect species have on ecosystem dynamics and properties, from ‘response traits’, which determine the response of species to the environment. The framework has been successfully used to analyse how environmental gradients and disturbances shape the functional structure of plant communities (e.g. [16,53]), and how effect traits can be used to link biodiversity, ecosystem functioning and even ecosystem services [54,55]. It has also been applied directly to animal communities (e.g. [56]). Plant ecologists have recently recognized that other compartments of the interaction network might influence the link between biodiversity and ecosystem functioning. Plant–pollination or plant–herbivory are two examples of interactions that can influence indirectly the relationship between response traits and the environment, as well as effect traits and ecosystem functioning. To address this issue, the framework was extended to measure the functional linkages that cascade through the primary producers to the consumers [7]. The general idea is to link response traits at a given trophic level to effect traits at another. As an example, the corolla length and flower colour of a grassland community would be considered as effect traits because they influence the pollinator community. In turn, body size and proboscis length of the pollinators would be considered response traits. The problem with this classification is that it remains strongly influenced by a plant-centred perspective, where the primary producers are structuring the community, and causal relationships are from the bottom to the top of the food web. But a predator harms a prey, just as the prey sustains the needs of the predator, and consequently, it is simply impossible to determine effect and response traits in such an interaction. In addition, consumption and life history traits can be viewed as effect traits, as they influence the per capita and the per population effects one species has on another, respectively. Our classification thereby appears more appropriate to food webs than the more traditional effect/response dichotomy. The classification of some response traits, however, stays relevant when used to describe attributes related to abiotic or habitat preferences.
3. Quantifying the functional structure and diversity of food webs
The functional composition of a community is the multivariate distribution of traits across co-occurring organisms. It is characterized by the different moments of the distribution of each trait (mean, variance, skewness, etc.), and the covariance among them. The functional structure is characterized by the average for each trait, which could be weighted by abundance (CWM). The ordination of CWMs for all traits allows a visual representation of the central aspects of the multivariate trait structure. The functional diversity represents the dispersion of the traits around their mean. There are also several techniques to quantify the dispersion of traits around the central moments of the distribution [57], the more direct being the CWV. The relevant question is, therefore, should we measure functional diversity of food webs in the same way we do for plants?
There are several tools to quantify the functional composition of communities, some redundant and others emphasizing particular aspects of the multivariate distribution. First analyses of functional diversity were based on expert classification, with functional diversity quantified from the number of functional groups [58], but many functional diversity indices have been proposed since [59]. A group of indices is based on the computation of pairwise distances between species (e.g. [17,60]). A major breakthrough was the proposition of a decomposition of the functional diversity in three components: the richness, the divergence, and the evenness [61]. The computation of functional volume is also a powerful tool to explore variations in the functional composition of assemblages through space and time due to biotic (e.g. species invasions) or abiotic (e.g. climate change) perturbations [57]. Computation of functional diversity and dispersion has been widely applied to different types of organisms such as fishes [11], birds [62], plants [63] or insects [64]. This class of methods, however, considers all organisms the same way, and the application to food webs, or more generally to interaction networks, has not been explored in detail. Further, there is currently no method to relate the functional composition (or occupation of the functional space) to network structure.
An important aspect of functional composition that is relevant to food webs but overlooked in these indices is the covariance among traits. Such covariance could arise, for instance, from coevolution of the enemy and the victim in an evolutionary arms race [65]. They could also occur when traits are correlated in the same way to an underlying environmental gradient [16]. Relationships among traits are susceptible to have important implications for the structure of the interaction network and thus indirectly on ecosystem functioning (see box 2 for a formal mathematical argument and figure 2 for an illustration). It could make interactions more or less likely to occur relative to the situation where they are independently distributed. The effect of such correlation depends on the function relating foraging and vulnerability traits to the occurrence of an interaction. We currently have a minimal empirical documentation of the existence of such covariance structure in the distribution of traits. Further, numerical methods to analyse it are currently limited and thus new techniques to characterize this particular aspect of functional composition are required.
Box 2. Correlation among traits influences the occurrence of interactions.
We are interested in understanding if a predator species i will feed on a prey species j. It is convenient to treat the problem as a stochastic event resulting from the random draw of two species from the multivariate trait distribution. Species i, therefore, has the trait value Ti with probability P(Ti), and similarly for the species j. The observation of an interaction between these species, Lij, should thus be expressed as a joint probability event:
| 2.3 |
A trait-matching function describes the probability of observing this interaction, given the traits of the two species [66]:
| 2.4 |
Now, a correlation between traits indicates that trait Ti is more likely to be observed if Tj is observed. Such correlation can arise because of non-random co-occurrence of species [67,68]). Because the traits are not independent, the joint probability of observing both traits is:
| 2.5 |
Finally, the probability of observing the interaction could be decomposed as:
| 2.6 |
This last equation shows that the occurrence of the interaction will increase with the conditional probability of observing trait i given the observation of trait j. In other words, the interaction will be more likely to happen if the two traits are more susceptible to be found together than by chance alone.
Figure 2.
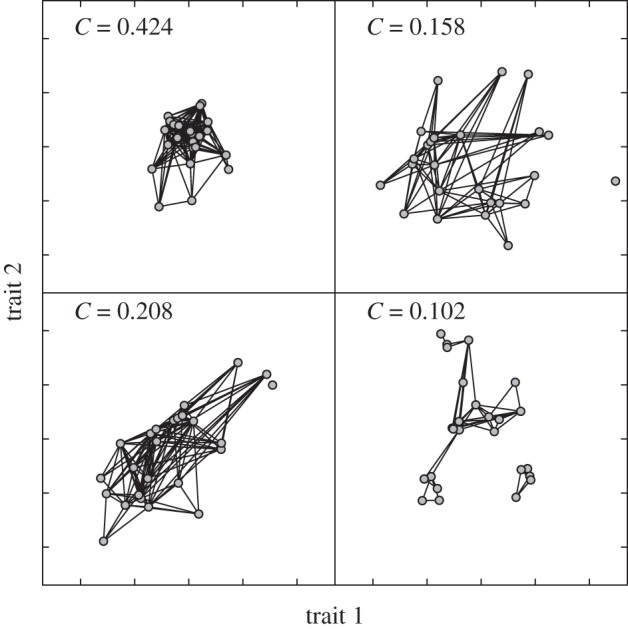
The effect of functional trait structure and diversity on food web topology. The figure illustrates the potential impact of different aspects of the multivariate trait distribution while keeping the trait-matching rules constant: trait variability (top left compared with top right), trait correlation (top left compared with bottom-left) and non-uniform trait distribution (clustering, top left compared with bottom right). Formal proof of the effect of trait correlation is provided in box 2. The simulations are run with a two-dimensional adaptation of the niche model [69]. We generalized the model to several niche axes and flexible distributions of the different traits, but keeping the trait-matching function based on the ordering of niche positions and the existence of a diet optimum. Each point represents the position of a species in a two-dimensional trait space (foraging and vulnerability traits). Each line represents a trophic interaction. The predator has the largest trait value (for both traits) and the prey the smallest. The connectance of the network, C, is indicated on each panel. It emerges from the underlying rules, instead of being predetermined, as in the original model. Niche position for each species is drawn from a bivariate normal distribution with mean trait value of 0. Correlation between niche axes is null for all panels, except for the bottom left panel that has a correlation of 0.8. The standard deviation for each trait is set to 10, except for the top left panel that has standard deviation of 5. A non-uniform distribution of traits is simulated by five niche positions ni from the bivariate normal with mean 0 and standard deviation of 10, and then after drawing four additional species with niche positions of mean ni and standard deviation of 3.16. The range r for each axis is obtained by drawing a random number from a uniform distribution between 0 and 30. The centroid o for each axis is obtained by drawing a random number from a uniform distribution between the minimal niche position and r/2. Preys are of a given predator i are the species whose niche falls within the interval oi − ri/2 and oi + ri/2.
Representing the spatial variation of functional structure and diversity of food webs is of great interest as it can reveal the effects of environmental filtering between regions or along gradients. However, despite the large availability of trait data, this is not a common practice in ecology. Although several studies have analysed how environment and biotic interactions may influence functional structure and diversity, this is usually carried out at small spatial scales and for some specific groups [70]. Few attempts have been made to analyse the covariation of diversity across groups and plants. Macro-ecologists have mapped the spatial variation of some traits (e.g. maximal body size) for various groups [71,72], but the covariation among groups is currently overlooked. The observation of systematic variation in functional structure and diversity of different groups is susceptible to be instructive for understanding network assembly. For instance, it is known that high floral diversity usually stimulates a diversity of functional groups of pollinators [73]. Most published studies, however, have either analysed covariation between different biodiversity facets within a given group (e.g. birds, [74]) or covariation of a given facet (e.g. phylogenetic diversity) across different groups [75,76]. However, as far as we are aware, there is no systematic analysis of large-scale functional diversity between different groups of animals or/and with plants. We argue that it should give some insights on the link between functional structure and diversity and food webs.
A promising avenue in the study of functional structure and diversity is the measure of the contribution of a given species to the functional diversity of a community. There are various methods to measure the functional distinctiveness of a given species in respect to the others [77]. Unlike phylogenetic distinctiveness, which emphasizes the evolutionary heritage of a species, the functional distinctiveness rather emphasizes how a given species uses a different set of resources, behaves differently or uniquely affects ecosystem functioning. It has important implications when it comes to food webs as functionally distinct species are likely to be particularly important for the stability of the entire food web and act as unique hubs gluing the network together [78]. A recent meta-analysis has also shown that functionally distinct species are usually geographically rare or of low abundance, for both plants and animals [79]. Functional distinctiveness could reveal unique species that have specific diet or strong identity effects.
4. Relating trait structure to network topology
How do we move from a given trait distribution to a network? There is currently no clear answer to this question, but the many theoretical models of food web structure do provide some preliminary intuition. The first one proposed, the cascade model [80], ranks species along an abstract axis, and any predator interacts with a given probability with all species with an inferior position along this axis. The niche model of food web structure [69] is similarly based on a single niche axis, and also considers that predators feed on preys whose niche falls within a range around an optimum. The minimum potential niche model [81] extends this approach to multiple niche axes. The nested hierarchy model is based on similar rules, with the addition of phylogenetic constraints [82]. All of these models have in common that species could be ordered according to their position along a niche axis, which for the purpose of generality, is never associated with a particular trait. It was recently shown, however, that most ecological networks could be summarized with only a few of such axes, which could easily match to some functional traits [50].
All of these theoretical models of network structure have been fitted to empirical data and compared with each other [81,83], opening the way for the development of trait-matching models of interactions [84]. Indirect methods of inference of ecological interactions based from traits were developed first. For instance, Rohr et al. [40] represents the structure of interaction networks using latent traits (basically the niche position and optimum), which could be later related to functional traits and to phylogenies. Williams et al. [83] did similarly by fitting a probabilistic version of the niche model to empirical data, evaluating the niche, the optimum and the range for each species, and then by regressing these parameters to body size. Direct methods of trait-matching were proposed after. The niche model was fitted directly with the assumption that body size is the main niche axis and that the predator–prey body size relationship represents the optimal niche of a species [85]. This method was successfully extended to other types of interactions, such as plant–herbivores where traits such as leaf dry matter content and incisive strength are good predictors of interactions [66]. Crea et al. [86] considered multinomial regression to model interaction probability based on plant and pollinator traits and abundance. A totally different approach, derived from multivariate statistics and ordination techniques [87], also confirmed that physiological and morphological traits are driving the diet of marine mammals. At the end, despite some important differences in the statistical approach used to evaluate trait-matching functions, we find that a common denominator of all of these methods is the classification of traits involved in trophic interactions as foraging and vulnerability traits (see above and the detailed table in [84]).
However, while trait-matching constraints are fundamental to establish potential interactions between pairs of species, their realization also requires that species encounter each other. Abundance and prevalence consequently have a fundamental importance to food web structure [51]. Neutral models of trophic structure are exclusively based on the law of mass action; they propose that the frequency of an interaction happening and the strength of that interaction should be proportional to the product of abundances between pairs of species [88]. In other words, they hypothesize that consumers do not select their resources; they only take them in the proportion they find them. Not surprisingly, rare species interact with much fewer species than abundant ones, and predominantly with the most abundant ones. Neutral models can fit well empirical data, even for interactions that are hypothesized to be strongly driven by trait-matching constraints [89,90]. An important point to remember for the purpose of our framework is that traits, in particular life history ones, involved in the determination of abundance could also indirectly impact both the topology of food webs and the intensity of the interactions.
The key question, now that it is established that traits impact the occurrence of pairwise interactions, is how do we move from the entire trait distribution to the network structure? The mean of the different traits, their variance and their covariance are all moments of the multivariate distribution that will all affect the network structure. We illustrate in figure 2 with an adaptation of the niche model [69] that increasing functional diversity in a food web whose interactions are determined by trait-matching constraints could significantly impact on its emerging properties. The niche model is based on the match between the niche position of the prey (a vulnerability trait) and the centroid of the diet of the predator (a foraging trait). The distribution of the foraging and vulnerability traits in the niche model were originally fixed by hypothesis, but the figure 2 illustrates that more flexible distributions with a variation of the average, the variance and the covariance could alter the properties of the resulting network. Increasing the variance (figure 2, top left to top right) of both traits could decrease the connectance and impact the way the links are distributed. Similarly, a correlation between traits (figure 2, bottom left) or their clustering (figure 2, bottom right) can also significantly impact the network structure. These properties are, in turn, expected to impact the dynamics, stability, persistence and functioning of food webs. This figure is only a specific example, and further investigations will be required to clarify the roles that the different moments of the multivariate trait distribution have on network structure, both in theory and in empirical systems.
5. Impacts of trait diversity on ecosystem functioning
Completing the loop from the distribution of traits to network structure and ecosystem functioning is far from trivial. Fortunately, layered food webs can give some preliminary insights. First studies on the impact of trophic interactions on the relationship between biodiversity and ecosystem functioning were mostly restricted to the investigation of extreme cases where networks were composed of either generalists or specialists, but nothing in between [91]. It was proposed recently that ‘trophic complementarity’ is a mechanism driving the shape of the BEF relationship [30] in networks of intermediate complexity. The principle is simple; it mirrors the one of resource-use complementarity. Species in a given layer of a food web interact by both exploitative and apparent competition through shared resources and predators. The contribution of a given species to ecosystem functioning is expected to increase with its trophic complementarity. It is defined as the overlap of interactions between pairs of species (across resources and predators). According to the models described above relating traits to interactions, species sharing similar traits are also expected to share similar interactions. We thus intuit that there should be a positive relationship between functional distinctiveness and trophic complementarity. A key question at this stage is how traits responsible for trophic interactions are independent of the ones determining resource use. If they were uncorrelated, it would imply that quantifying trophic complementarity would be as important as functional complementarity in predicting ecosystem functioning and even coexistence. The BEF relationship in food webs has been discussed [26] and some experiments have provided evidence of a positive relationship ([92], but see [93]), but only species richness has been investigated thus far, not functional diversity.
We note at least two hypotheses by which functional diversity of the food web could promote ecosystem functioning. First, increasing functional diversity at one layer could provide niches for above layers, such as plant diversity sustaining a diverse herbivore community (e.g. [94]). In other words, diversity could beget diversity, simply because the higher trophic layers do require a diversity of preys at the lower level to establish and persist [67]. Such ‘niche construction’ is based on the assumption of a sequential build-up of the food web, where bottom-up processes dominate. It predicts a positive correlation between functional diversity at the different trophic levels. High plant functional diversity should promote the diversity of herbivores, which in turn would promote diversity of predators [95]. It is important, however, to understand the role of trophic regulation in this relationship to adequately distinguish the cause and the effect. A strong top-down control, as imposed by functional diversity of the top layer, could either promote diversity through keystone predation [96] or via reduced apparent competition [31]. Functional diversity could also have a positive effect on nutrient retention and cycling within the food web [29]. Primary productivity will be promoted by a tight nutrient cycling loop and thus the diversity of feeding modes, including scavengers and detritivores, will ensure that primary and secondary production remains local and is not exported out of the system [97]. Further, a diversity of stoichiometry in the various organisms, as long as exudates, could promote the efficiency of detritivores [98].
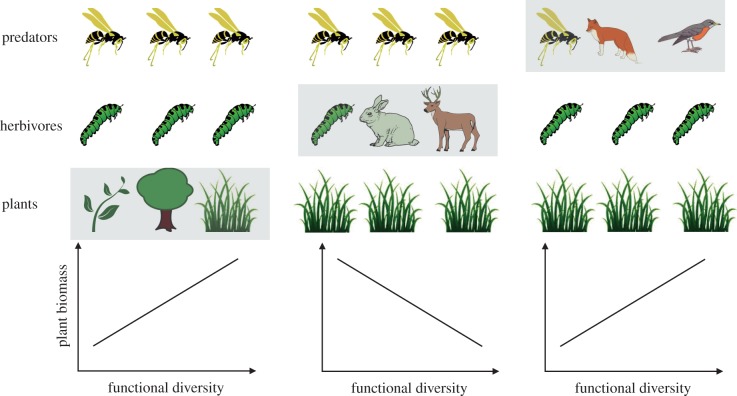
Functional diversity could, on the other hand, increase the strength of top-down regulation and consequently have various effects on primary and secondary productivity, depending of the trophic level considered. Increased functional diversity of a given trophic level will increase the regulation of the lower level by enhanced trophic complementarity. Consequently, the diversity of foraging traits of herbivores will promote their ability to exploit the plant level, and the diversity of vulnerability traits will help to reduce the regulation by carnivores and reduce apparent competition (figure 3, left panel). As a consequence, we would expect a negative relationship between functional diversity of herbivores and primary productivity (figure 3, central panel, see experimental support in [99]). Conversely, the diversity of foraging traits of carnivores will help them to control the herbivore layer, thereby releasing the plants from grazing (figure 3, right panel). The key question, still unresolved, is what will happen in between if we add all of the complexity that is typical of unipartite food webs, such as intra-guild predation, omnivory, cannibalism and loops.
Figure 3.
Cartoon representation of the effect of trait diversity in layered and top-down control food webs. The figure represents hypothesized relationships between functional diversity and plant biomass in BEF experiments where the functional diversity is varied at different trophic levels (indicated by shading). (Online version in colour.)
What remains to be understood is the importance of functional identity effects in food webs [100]. Functional traits should be used to better quantify their underlying drivers. The interpretation of strong identity effects is often intuited from knowledge of species traits. The trait-based approach formalizes this interpretation with quantitative analyses, such as the use of functional distinctiveness and CWM. The food web literature has been instrumental in the establishment of the ‘keystone species’ concept [96]. It is well documented that some predators, because they are highly efficient at controlling some populations, could promote coexistence and have a significant impact on ecosystem structure [27]. Despite the fact that they are often located at the top of the food chain, we have a poor understanding of the traits determining the keystoneness of a species in a given food web. Further, the keystone role of a predator has to be the result of a match between its traits and the ones of the prey species in the food web. The problem of keystoneness was addressed with the investigation of the determinants of direct and indirect interactions in modelled food webs [101]. It was found that the best predictors of single species removal effects were essentially the biomass of the removed species (which is a dominance effect) and the network path length between the removed species and the focal one (the shorter the path, the stronger the effect). Predator–prey body mass ratio surprisingly was only a poor predictor of the interaction strength. As all the interactions were, however, determined by a single trait, body size, we do not know to what extent this result is generalizable to more complex functional structures.
Answering the classic question of whether an ecosystem is bottom-up or top-down regulated remains of primary importance to understand the role of functional diversity in food webs. For bottom-up systems, we could intuitively predict that in most situations, increasing functional diversity, whatever is the complexity of the food web, will always be positive because it will promote niche construction and close the nutrient cycle. The challenge is thus found in trophically regulated systems. Identity effects could also occur with functionally distinct species, which could either be beneficial or harmful for food web dynamics. The main problem is that functional diversity of preys could promote their escape from trophic regulation, while the diversity of predators could enhance it, making underdetermined the final relationship between functional diversity of the entire food web and ecosystem functioning.
6. What next?
Understanding the relationship between functional diversity and the structure and dynamics of food webs is a key problem to solve in order to predict ecosystem functioning. The investigation of the functional structure and diversity of communities has been mostly restricted to plants, but recent investigations of animal communities are promising. Traits could both directly and indirectly impact food web dynamics. The food web perspective promises a more integrated perspective of the relationship between biodiversity and ecosystem functioning [102]. A trait-based approach suggests that it could be possible to find generalities and circumvent the idiosyncracies commonly arising in food webs. It extends a fundamental principle linking food web ecology and ecosystem functioning [103]: ‘interactions occur between functional units according to a topology that is driven by trait-matching constraints’. Further, it helps representing energy and material fluxes and the multiple types of species interactions [103].
We have identified three broad categories of traits that are relevant to the characterization of food webs: topological traits, consumption traits and life history traits. These traits combine to determine the per capita and per population interaction strengths. We have also pointed out that in food webs the characterization of the functional structure should include not only the mean and the variance of the different traits, but also their covariance. Finally, we have emphasized that determining the strength of trophic regulation is of primary importance to understand the role of the functional structure of food web dynamics. The review also highlighted some key gaps in knowledge and pointed to some of the most critical problems to solve. In conclusion, we see the following main steps for a research agenda on functional diversity of food webs:
(1) Gathering and integrating interaction data with functional traits to improve our understanding of trait-matching constraints. While there has been some work on this question already, only the low-hanging fruits have been picked; more specific data will be required to infer the identity and the strength of trophic interactions. New platforms for interaction data, such as ‘mangal’ [104] need to be coupled with platforms for trait data. A key problem to solve is finding and integrating relevant traits across different groups of organisms such as vertebrates, arthropods and plants.
(2) Developing theory relating functional composition, network structure and ecosystem functioning. As described above and illustrated in figure 1, we only have a partial understanding of the steps going from traits to ecosystem functioning. Investigations of this problem will require data-driven simulation models and to integrate the entire chain of causal relationships.
(3) Large-scale analysis of the covariation between functional composition of trophic guilds and network characteristics. The above review highlighted that a key aspect of functional trait structure and diversity to quantify is the multivariate nature of the problem. The covariation of traits among predators and preys is key to understand the emergent properties at the network level. There is a critical gap in knowledge to solve, as we do not know how functional structures covary among trophic levels; neither do we understand the covariation between functional structure and emerging network properties. There are also some aspects of the trait distribution that are not qualified by current metrics describing functional composition.
(4) Understanding the role of a species in a network from its traits. This question is key to several fields, from conservation, where it could help to identify keystone species, to invasion ecology, where it could help to predict not only the invasibility, but most of all, the direct and indirect impacts of invaders. We now have tools to document species role in complex networks [105]; the next step will be to predict it from traits. Answering this question will require theoretical investigations of pairwise interactions in trait-based food web models, as well as extensive mining of the literature on predator removal experiments.
(5) The relationship between identity effects and extinction risks in food webs. Traits not only determine the role of a species in a food web and its impact on other species, but also its vulnerability to global changes. Understanding their relationship is essential to make the field of network ecology predictive and relevant for biodiversity managers.
Acknowledgement
The authors thank Sébastien Ibanez for his help on filling table 1 for terrestrial invertebrates.
Competing interests
We declare we have no competing interests.
Funding
W.T. received funding from the European Research Council under the European Community's Seven Framework Programme FP7/2007-2013 grant agreement no. 281422 (TEEMBIO). W.T. belongs to the Laboratoire d’Écologie Alpine, which is part of Labex OSUG2020 (ANR10 LABX56). D.G. thanks financial support from the Canada Research Chair Program and for a NSERC Collaborative Research and Development grant.
References
- 1.McGill B, Enquist B, Weiher E, Westoby M. 2006. Rebuilding community ecology from functional traits. Trends Ecol. Evol. 21, 178–185. ( 10.1016/j.tree.2006.02.002) [DOI] [PubMed] [Google Scholar]
- 2.Violle C, Navas M-L, Vile D, Kazakou E, Fortunel C, Hummel I, Garnier E. 2007. Let the concept of trait be functional! Oikos 116, 882–892. ( 10.1111/j.0030-1299.2007.15559.x) [DOI] [Google Scholar]
- 3.Violle C, Reich PB, Pacala SW, Enquist BJ, Kattge J. 2014. The emergence and promise of functional biogeography. Proc. Natl Acad. Sci. USA 111, 13 690–13 696. ( 10.1073/pnas.1415442111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naeem S, Duffy J, Zavaleta E. 2012. The functions of biological diversity in an age of extinction. Science 336, 1401–1406. ( 10.1126/science.1215855) [DOI] [PubMed] [Google Scholar]
- 5.Cornelissen JHC, Cerabolini B, Castro-Díez P, Villar-Salvador P, Montserrat-Martí G, Puyravaud JP, Maestro M, Werger MJA, Aerts R. 2003. Functional traits of woody plants: correspondence of species rankings between field adults and laboratory-grown seedlings? J. Veg. Sci. 14, 311–322. ( 10.1111/j.1654-1103.2003.tb02157.x) [DOI] [Google Scholar]
- 6.Garnier E, Navas M-L. 2012. A trait-based approach to comparative functional plant ecology: concepts, methods and applications for agroecology. A review. Agron. Sustain. Dev. 32, 365–399. ( 10.1007/s13593-011-0036-y) [DOI] [Google Scholar]
- 7.Lavorel S, et al. 2013. A novel framework for linking functional diversity of plants with other trophic levels for the quantification of ecosystem services. J. Veg. Sci. 24, 942–948. ( 10.1111/jvs.12083) [DOI] [Google Scholar]
- 8.Albouy C, Guilhaumon F, Villéger S, Mouchet M, Mercier L, Culioli J, Tomasini J, Le Loc'h F, Mouillot D. 2011. Predicting trophic guild and diet overlap from functional traits: statistics, opportunities and limitations for marine ecology. Mar. Ecol. Prog. Ser. 436, 17–28. ( 10.3354/meps09240) [DOI] [Google Scholar]
- 9.Sekercioglu CH. 2011. Functional extinctions of bird pollinators cause plant declines. Science 331, 1019–1020. ( 10.1126/science.1202389) [DOI] [PubMed] [Google Scholar]
- 10.Luck G, Lavorel S, McIntyre S, Lumb K. 2012. Improving the application of vertebrate trait-based frameworks to the study of ecosystem services. J. Anim. Ecol. 81, 1065–1076. ( 10.1111/j.1365-2656.2012.01974.x) [DOI] [PubMed] [Google Scholar]
- 11.Mouillot D, et al. 2014. Functional over-redundancy and high functional vulnerability in global fish faunas on tropical reefs. Proc. Natl Acad. Sci. USA 111, 13 757–13 762. ( 10.1073/pnas.1317625111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thuiller W, Maiorano L, Mazel F, Guilhaumon F, Ficetola GF, Lavergne S, Renaud J, Roquet C, Mouillot D. 2015. Conserving the functional and phylogenetic trees of life of European tetrapods. Phil. Trans. R. Soc. B 370, 20140005 ( 10.1098/rstb.2014.0005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lavorel S, Garnier E. 2002. Predicting changes in community composition and ecosystem functioning from plant traits: revisiting the Holy Grail. Funct. Ecol. 16, 545–556. ( 10.1046/j.1365-2435.2002.00664.x) [DOI] [Google Scholar]
- 14.Pakeman RJ, et al. 2008. Impact of abundance weighting on the response of seed traits to climate and land use. J. Ecol. 96, 355–366. ( 10.1111/j.1365-2745.2007.01336.x) [DOI] [Google Scholar]
- 15.Sonnier G, Shipley B, Navas M-L. 2010. Quantifying relationships between traits and explicitly measured gradients of stress and disturbance in early successional plant communities. J. Veg. Sci. 21, 1014–1024. ( 10.1111/j.1654-1103.2010.01210.x) [DOI] [Google Scholar]
- 16.Shipley B, Vile D, Garnier E. 2006. From plant traits to plant communities: a statistical mechanistic approach to biodiversity. Science 314, 812–814. ( 10.1126/science.1131344) [DOI] [PubMed] [Google Scholar]
- 17.Petchey O, Gaston K. 2006. Functional diversity: back to basics and looking forward. Ecol. Lett. 9, 741–758. ( 10.1111/j.1461-0248.2006.00924.x) [DOI] [PubMed] [Google Scholar]
- 18.Cadotte M, Carscadden K, Mirotchnick N. 2011. Beyond species: functional diversity and the maintenance of ecological processes and services. J. Appl. Ecol. 48, 1079–1087. ( 10.1111/j.1365-2664.2011.02048.x) [DOI] [Google Scholar]
- 19.Chalmandrier L, Münkemüller T, Gallien L, de Bello F, Mazel F, Lavergne S, Thuiller W. 2013. A family of null models to distinguish between habitat filtering and biotic interactions in functional diversity patterns. J. Veg. Sci. 24, 853–864. ( 10.1111/jvs.12031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loreau M. 1998. Biodiversity and ecosystem functioning: a mechanistic model. Proc. Natl Acad. Sci. USA 95, 5632–5636. ( 10.1073/pnas.95.10.5632) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wright IJ, et al. 2004. The worldwide leaf economics spectrum. Nature 428, 821–827. ( 10.1038/nature02403) [DOI] [PubMed] [Google Scholar]
- 22.Garnier E, et al. 2004. Plant functional markers capture ecosystem properties during secondary succession. Ecology 85, 2630–2637. ( 10.1890/03-0799) [DOI] [Google Scholar]
- 23.Tobner C, Paquette A, Gravel D, Reich P, Williams L, Messier C. In press Functional identity is themain driver of diversity effects in young tree communities. Ecol. Lett. [DOI] [PubMed] [Google Scholar]
- 24.Loreau M, et al. 2001. Biodiversity and ecosystem functioning: current knowledge and future challenges. Science 294, 804–808. ( 10.1126/science.1064088) [DOI] [PubMed] [Google Scholar]
- 25.Cardinale BJ, et al. 2012. Biodiversity loss and its impact on humanity. Nature 486, 59–67. ( 10.1038/nature11148) [DOI] [PubMed] [Google Scholar]
- 26.Duffy JE, Cardinale BJ, France KE, McIntyre PB, Thébault E, Loreau M. 2007. The functional role of biodiversity in ecosystems: incorporating trophic complexity. Ecol. Lett. 10, 522–38. ( 10.1111/j.1461-0248.2007.01037.x) [DOI] [PubMed] [Google Scholar]
- 27.Estes JA, et al. 2011. Trophic downgrading of planet Earth. Science 333, 301–306. ( 10.1126/science.1205106) [DOI] [PubMed] [Google Scholar]
- 28.Ferretti F, Worm B, Britten GL, Heithaus MR, Lotze HK. 2010. Patterns and ecosystem consequences of shark declines in the ocean. Ecol. Lett. 13, 1055–1071. ( 10.1111/j.1461-0248.2010.01489.x) [DOI] [PubMed] [Google Scholar]
- 29.Schmitz OJ. 2008. Herbivory from Individuals to Ecosystems. Annu. Rev. Ecol. Evol. Syst. 39, 133–152. ( 10.1146/annurev.ecolsys.39.110707.173418) [DOI] [Google Scholar]
- 30.Poisot T, Mouquet N, Gravel D. 2013. Trophic complementarity drives the biodiversity–ecosystem functioning relationship in food webs. Ecol. Lett. 16, 653–661. ( 10.1111/ele.12118) [DOI] [PubMed] [Google Scholar]
- 31.Chesson P, Kuang JJ. 2008. The interaction between predation and competition. Nature 456, 235–238. ( 10.1038/nature07248) [DOI] [PubMed] [Google Scholar]
- 32.McCann K, Hastings A, Huxel GR. 1998. Weak trophic interactions and the balance of nature. Nature 395, 794–798. ( 10.1038/27427) [DOI] [Google Scholar]
- 33.Jeschke JM, Kopp M, Tollrian R. 2002. Predator functional responses: discriminating between handling and digesting prey. Ecol. Monogr. 72, 95–112. ( 10.1890/0012-9615(2002)072%5B0095:PFRDBH%5D2.0.CO;2) [DOI] [Google Scholar]
- 34.Jeschke JM. 2006. Density-dependent effects of prey defenses and predator offenses. J. Theor. Biol. 242, 900–907. ( 10.1016/j.jtbi.2006.05.017) [DOI] [PubMed] [Google Scholar]
- 35.Ibanez S. 2012. Optimizing size thresholds in a plant–pollinator interaction web: towards a mechanistic understanding of ecological networks. Oecologia 170, 233–242. ( 10.1007/s00442-012-2290-3) [DOI] [PubMed] [Google Scholar]
- 36.Boyle KS, Horn MH. 2006. Comparison of feeding guild structure and ecomorphology of intertidal fish assemblages from central California and central Chile. Mar. Ecol. Prog. Ser. 319, 65–84. ( 10.3354/meps319065) [DOI] [Google Scholar]
- 37.Bremner J. 2008. Species’ traits and ecological functioning in marine conservation and management. J. Exp. Mar. Biol. Ecol. 366, 37–47. ( 10.1016/j.jembe.2008.07.007) [DOI] [Google Scholar]
- 38.Villéger S, Miranda JR, Hernández DF, Mouillot D. 2010. Contrasting changes in taxonomic vs. functional diversity of tropical fish communities after habitat degradation. Ecol. Appl. 20, 1512–1522. ( 10.1890/09-1310.1) [DOI] [PubMed] [Google Scholar]
- 39.Rossberg A, Matsuda H, Amemiya T, Itoh K. 2006. Food webs: experts consuming families of experts. J. Theor. Biol. 241, 552–563. ( 10.1016/j.jtbi.2005.12.021) [DOI] [PubMed] [Google Scholar]
- 40.Rohr RP, Scherer H, Kehrli P, Mazza C, Bersier LF. 2010. Modeling food webs: exploring unexplained structure using latent traits. Am. Nat. 176, 170–177. ( 10.1086/653667) [DOI] [PubMed] [Google Scholar]
- 41.Evans M, Forsythe T. 1985. Feeding mechanisms, and their variation in form, of some adult ground-beetles (coleoptera: Caraboidea). J. Zool. 206, 113–143. ( 10.1111/j.1469-7998.1985.tb05640.x) [DOI] [Google Scholar]
- 42.Wilman H, Belmaker J, Simpson J, de la Rosa C, Rivadeneira MM, Jetz W. 2014. Eltontraits 1.0: species-level foraging attributes of the world's birds and mammals. Ecology 95, 2027–2027 ( 10.1890/13-1917.1) [DOI] [Google Scholar]
- 43.Sterner R, Elser JJ. 2002. Ecological stoichiometry: the biology of elements from molecules to the biosphere. Princeton, NJ: Princeton University Press. [Google Scholar]
- 44.Tilman D. 1982. Resource competition and community structure. Princeton, NJ: Princeton University Press. [PubMed] [Google Scholar]
- 45.Berlow E, et al. 2004. Interaction strengths in food webs: issues and opportunities. J. Anim. Ecol. 73, 585–598. ( 10.1111/j.0021-8790.2004.00833.x) [DOI] [Google Scholar]
- 46.Stouffer DB, Bascompte J. 2011. Compartmentalization increases food-web persistence. Proc. Natl Acad. Sci. USA 108, 3648–3652. ( 10.1073/pnas.1014353108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Allesina S, Tang S. 2012. Stability criteria for complex ecosystems. Nature 483, 205–208. ( 10.1038/nature10832) [DOI] [PubMed] [Google Scholar]
- 48.Zhang L, Thygesen UH, Knudsen K, Andersen KH. 2012. Trait diversity promotes stability of community dynamics. Theor. Ecol. 6, 57–69. ( 10.1007/s12080-012-0160-6) [DOI] [Google Scholar]
- 49.Montoya JM, Woodward G, Emmerson MC, Solé RV. 2009. Press perturbations and indirect effects in real food webs. Ecology 90, 2426–2433. ( 10.1890/08-0657.1) [DOI] [PubMed] [Google Scholar]
- 50.Eklöf A, et al. 2013. The dimensionality of ecological networks. Ecol. Lett. 16, 577–583. ( 10.1111/ele.12081) [DOI] [PubMed] [Google Scholar]
- 51.Poisot T, Stouffer DB, Gravel D. 2015. Beyond species: why ecological interactions vary through space and time. Oikos 124, 243–251. ( 10.1111/oik.01719) [DOI] [Google Scholar]
- 52.Schmitz OJ, Buchkowski RW, Burghardt KT, Donihue CM. 2015. Chapter ten—functional traits and trait-mediated interactions: connecting community-level interactions with ecosystem functioning. Adv. Ecol. Res. 52, 319–343. ( 10.1016/bs.aecr.2015.01.003) [DOI] [Google Scholar]
- 53.de Bello F, Lavorel S, Lavergne S, Albert CH, Boulangeat I, Mazel F, Thuiller W. 2013. Hierarchical effects of environmental filters on the functional structure of plant communities: a case study in the French Alps. Ecography 36, 393–402. ( 10.1111/j.1600-0587.2012.07438.x) [DOI] [Google Scholar]
- 54.Suding K, Lavorel S, Chapin F, Cornelissen JHC, Diaz S, Garnier E, Goldberg D, Hooper DY, Jackson TS, Navas M-L. 2008. Scaling environmental change through the community-level: a trait-based response-and-effect framework for plants. Glob. Change Biol. 14, 1125–1140. ( 10.1111/j.1365-2486.2008.01557.x) [DOI] [Google Scholar]
- 55.Lavorel S, Grigulis K. 2012. How fundamental plant functional trait relationships scale-up to trade-offs and synergies in ecosystem services. J. Ecol. 100, 128–140. ( 10.1111/j.1365-2745.2011.01914.x) [DOI] [Google Scholar]
- 56.Moretti M, Legg C. 2009. Combining plant and animal traits to assess community functional responses to disturbance. Ecography 32, 299–309. ( 10.1111/j.1600-0587.2008.05524.x) [DOI] [Google Scholar]
- 57.Mouillot D, Graham NAJ, Villéger S, Mason NWH, Bellwood DR. 2013. A functional approach reveals community responses to disturbances. Trends Ecol. Evol. 28, 167–177. ( 10.1016/j.tree.2012.10.004) [DOI] [PubMed] [Google Scholar]
- 58.Hooper D, Vitousek P. 1997. The effects of plant composition and diversity on ecosystem processes. Science 277, 1302–1305. ( 10.1126/science.277.5330.1302) [DOI] [Google Scholar]
- 59.Chiu C-H, Chao A. 2014. Distance-based functional diversity measures and their decomposition: a framework based on hill numbers. PLoS ONE 9, e100014 ( 10.1371/journal.pone.0100014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Walker B, Kinzig A, Langridge J. 1999. Plant attribute diversity, resilience, and ecosystem function: the nature and significance of dominant and minor species. Ecosystems 2, 95–113. ( 10.1007/s100219900062) [DOI] [Google Scholar]
- 61.Mason N, Mouillot D, Lee WG, Wilson JB. 2005. Functional richness, functional evenness and functional divergence: the primary components of functional diversity. Oikos 111, 112–118. ( 10.1111/j.0030-1299.2005.13886.x) [DOI] [Google Scholar]
- 62.Boyer A, Jetz W. 2014. Extinctions and the loss of ecological function in island bird communities. Glob. Ecol. Biogeogr. 23, 679–688. ( 10.1111/geb.12147) [DOI] [Google Scholar]
- 63.Ordonez A, Wright IJ, Olff H. 2010. Functional differences between native and alien species: a global-scale comparison. Funct. Ecol. 24, 1353–1361. ( 10.1111/j.1365-2435.2010.01739.x) [DOI] [Google Scholar]
- 64.Poff N, Older JD, Vieira NKM, Finn DS, Simmons MP, Kondratieff BC. 2006. Functional trait niches of North American lotic insects: traits-based ecological applications in light of phylogenetic relationships. J. N. Am. Benthol. Soc. 25, 730–755. ( 10.1899/0887-3593(2006)025%5B0730:FTNONA%5D2.0.CO;2) [DOI] [Google Scholar]
- 65.Thompson J. 2005. The geographic mosaic of coevolution. Chicago, IL: University of Chicago Press. [Google Scholar]
- 66.Bartomeus I, Gravel D, Tylianakis J, Aizen M, Dickie I, Bernard-Verdier M. In press. A common framework for identifying linkage rules across different types of interaction networks. Funct. Ecol. [Google Scholar]
- 67.Gravel D, Massol F, Canard E, Mouillot D, Mouquet N. 2011. Trophic theory of island biogeography. Ecol. Lett. 14, 1010–1016. ( 10.1111/j.1461-0248.2011.01667.x) [DOI] [PubMed] [Google Scholar]
- 68.Cazelles K, Araújo MB, Mouquet N, Gravel D. 2015. A theory for species cooccurrence in interaction networks. Theor. Ecol., 1–10. ( 10.1007/s12080-015-0281-9) [DOI] [Google Scholar]
- 69.Williams R, Martinez N. 2000. Simple rules yield complex food webs. Nature 404, 180–183. ( 10.1038/35004572) [DOI] [PubMed] [Google Scholar]
- 70.Chalmandrier L, Münkemüller T, Lavergne S, Thuiller W. 2015. Effects of species’ similarity and dominance on the functional and phylogenetic structure of a plant meta-community. Ecology 96, 143–153. ( 10.1890/13-2153.1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ruggiero A, Werenkraut V. 2007. One-dimensional analyses of Rapoport's rule reviewed through meta-analysis. Glob. Ecol. Biogeogr. 16, 401–414. ( 10.1111/j.1466-8238.2006.00303.x) [DOI] [Google Scholar]
- 72.Fortes R, Absalao R. 2010. The latitudinal and bathymetric ranges of marine fishes: a global analysis to test the application of Rapoport's Rule. Mar. Ecol. Evol. Perspect. 31, 483–493. ( 10.1111/j.1439-0485.2010.00357.x) [DOI] [Google Scholar]
- 73.Potts S, Vulliamy B, Dafni A, Ne'eman G, Willmer P. 2003. Linking bees and flowers: how do floral communities structure pollinator communities? Ecology 84, 2628–2642. ( 10.1890/02-0136) [DOI] [Google Scholar]
- 74.Devictor V, Mouillot D, Meynard C, Jiguet F, Thuiller W, Mouquet N. 2010. Spatial mismatch and congruence between taxonomic, phylogenetic and functional diversity: the need for integrative conservation strategies in a changing world. Ecol. Lett. 13, 1030–1040. ( 10.1111/j.1461-0248.2010.01493.x) [DOI] [PubMed] [Google Scholar]
- 75.Zupan L, Cabeza M, Maiorano L, Roquet C, Devictor V, Lavergne S, Mouillot D, Mouquet N, Renaud J, Thuiller W. 2014. Spatial mismatch of phylogenetic diversity across three vertebrate groups and protected areas in Europe. Divers. Distrib. 20, 674–685. ( 10.1111/ddi.12186) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Albouy C, Leprieur F, Le Loc'h F, Mouquet N, Meynard CN, Douzery EJP, Mouillot D. 2015. Projected impacts of climate warming on the functional and phylogenetic components of coastal mediterranean fish biodiversity. Ecography 38, 681–689. ( 10.1111/ecog.01254) [DOI] [Google Scholar]
- 77.Pavoine S, Ollier S, Dufour A-B. 2005. Is the originality of a species measurable? Ecol. Lett. 8, 579–586. ( 10.1111/j.1461-0248.2005.00752.x) [DOI] [Google Scholar]
- 78.Dunne JA, Williams RJ, Martinez ND. 2002. Food-web structure and network theory: the role of connectance and size. Proc. Natl Acad. Sci. USA 99, 12 917–12 922. ( 10.1073/pnas.192407699) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mouillot D, et al. 2013. Rare species support vulnerable functions in high-diversity ecosystems. PLoS Biol. 11, e1001569 ( 10.1371/journal.pbio.1001569) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cohen JE, Briand F, Newman C. 1990. Community food webs: data and theory. Berlin, Germany: Springer. [Google Scholar]
- 81.Allesina S, Alonso D, Pascual M. 2008. A general model for food web structure. Science 320, 658–661. ( 10.1126/science.1156269) [DOI] [PubMed] [Google Scholar]
- 82.Cattin MF, Bersier LF, Banasek-Richter C, Baltensperger R, Gabriel JP. 2004. Phylogenetic constraints and adaptation explain food-web structure. Nature 427, 835–839. ( 10.1038/nature02327) [DOI] [PubMed] [Google Scholar]
- 83.Williams RJ, Anandanadesan A, Purves D. 2010. The probabilistic niche model reveals the niche structure and role of body size in a complex food web. PLoS One 5, e12092 ( 10.1371/journal.pone.0012092) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Morales-Castilla I, Matias MG, Gravel D, Araújo MB. 2015. Inferring biotic interactions from proxies. Trends Ecol. Evol. 30, 347–356. ( 10.1016/j.tree.2015.03.014) [DOI] [PubMed] [Google Scholar]
- 85.Gravel D, Poisot T, Albouy C, Velez L, Mouillot D. 2013. Inferring food web structure from predator–prey body size relationships. Methods Ecol. Evol. 4, 1083–1090. ( 10.1111/2041-210X.12103) [DOI] [Google Scholar]
- 86.Crea C, Ali RA, Rader R. 2015. A new model for ecological networks using species-level traits. Methods Ecol. Evol. 7, 232–241. ( 10.1111/2041-210X.12471) [DOI] [Google Scholar]
- 87.Spitz J, Ridoux V, Brind'Amour A. 2014. Let's go beyond taxonomy in diet description: testing a trait-based approach to prey–predator relationships. J. Anim. Ecol. 83, 1137–1148. ( 10.1111/1365-2656.12218) [DOI] [PubMed] [Google Scholar]
- 88.Canard E, Mouquet N, Marescot L, Gaston K, Gravel D, Mouillot D. 2012. Emergence of structural patterns in neutral trophic networks. PLoS ONE 7, e38295 ( 10.1371/journal.pone.0038295) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vázquez D, Chacoff N, Cagnolo L. 2009. Evaluating multiple determinants of the structure of plant-animal mutualistic networks. Ecology 90, 2039–2046. ( 10.1890/08-1837.1) [DOI] [PubMed] [Google Scholar]
- 90.Canard E, Mouquet N, Mouillot D, Stanko M, Miklisova D, Gravel D. 2014. Empirical evaluation of neutral interactions in host-parasite networks. Am. Nat. 183, 468–479. ( 10.1086/675363) [DOI] [PubMed] [Google Scholar]
- 91.Thébault E, Loreau M. 2003. Food-web constraints on biodiversity–ecosystem functioning relationships. Proc. Natl Acad. Sci. USA 100, 14 949–14 954. ( 10.1073/pnas.2434847100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cardinale BJ, Srivastava DS, Duffy JE, Wright JP, Downing AL, Sankaran M, Jouseau C. 2006. Effects of biodiversity on the functioning of trophic groups and ecosystems. Nature 443, 989–992. ( 10.1038/nature05202) [DOI] [PubMed] [Google Scholar]
- 93.Harvey E, Séguin A, Nozais C, Archambault P, Gravel D. 2013. Identity effects dominate the impacts of multiple species extinctions on the functioning of complex food webs. Ecology 94, 169–179. ( 10.1890/12-0414.1) [DOI] [PubMed] [Google Scholar]
- 94.Haddad NM, Crutsinger GM, Gross K, Haarstad J, Knops JM, Tilman D. 2009. Plant species loss decreases arthropod diversity and shifts trophic structure. Ecol. Lett. 12, 1029–1039. ( 10.1111/j.1461-0248.2009.01356.x) [DOI] [PubMed] [Google Scholar]
- 95.Douglass JG, Duffy JE, Bruno JF. 2008. Herbivore and predator diversity interactively affect ecosystem properties in an experimental marine community. Ecol. Lett. 11, 598–608. ( 10.1111/j.1461-0248.2008.01175.x) [DOI] [PubMed] [Google Scholar]
- 96.Paine R. 1966. Food web complexity and species diversity. Am. Nat. 100, 65–75. ( 10.1086/282400) [DOI] [Google Scholar]
- 97.Loreau M. 2010. From populations to ecosystems: theoretical foundations for a new ecological synthesis. Princeton, NJ: Princeton University Press. [Google Scholar]
- 98.Daufresne T, Hedin LO. 2005. Plant coexistence depends on ecosystem nutrient cycles: extension of the resource-ratio theory. Proc. Natl Acad. Sci. USA 102, 9212–9217. ( 10.1073/pnas.0406427102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Deraison H, Badenhausser I, Loeuille N, Scherber C, Gross N. 2015. Functional trait diversity across trophic levels determines herbivore impact on plant community biomass. Ecol. Lett. 18, 1346–1355. ( 10.1111/ele.12529) [DOI] [PubMed] [Google Scholar]
- 100.Séguin A, Harvey É, Archambault P, Nozais C, Gravel D. 2014. Body size as a predictor of species loss effect on ecosystem functioning. Sci. Rep. 4, 4616 ( 10.1038/srep04616) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Berlow EL, Dunne JA, Martinez ND, Stark PB, Williams RJ, Brose U. 2009. Simple prediction of interaction strengths in complex food webs. Proc. Natl Acad. Sci. USA 106, 187–191. ( 10.1073/pnas.0806823106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Thompson RM, et al. 2012. Food webs: reconciling the structure and function of biodiversity. Trends Ecol. Evol. 27, 689–697. ( 10.1016/j.tree.2012.08.005) [DOI] [PubMed] [Google Scholar]
- 103.Hines J, et al. 2015. Chapter four—towards an integration of biodiversity–ecosystem functioning and food web theory to evaluate relationships between multiple ecosystem services. Adv. Ecol. Res. 53, 161–199. ( 10.1016/bs.aecr.2015.09.001) [DOI] [Google Scholar]
- 104.Poisot T, et al. 2015. mangal—making ecological network analysis simple. Ecography 37 ( 10.1111/ecog.00976) [DOI] [Google Scholar]
- 105.Stouffer DB, Sales-Pardo M, Sirer MI, Bascompte J. 2012. Evolutionary conservation of species’ roles in food webs. Science 335, 1489–1492. ( 10.1126/science.1216556) [DOI] [PubMed] [Google Scholar]