Abstract
The importance of intraspecific trait variability for community dynamics and ecosystem functioning has been underappreciated. There are theoretical reasons for predicting that species that differ in intraspecific trait variability will also differ in their effects on ecosystem functioning, particularly in variable environments. We discuss whether species with greater trait variability are likely to exhibit greater temporal stability in their population dynamics, and under which conditions this might lead to stability in ecosystem functioning. Resolving this requires us to consider several questions. First, are species with high levels of variation for one trait equally variable in others? In particular, is variability in response and effects traits typically correlated? Second, what is the relative contribution of local adaptation and phenotypic plasticity to trait variability? If local adaptation dominates, then stability in function requires one of two conditions: (i) individuals of appropriate phenotypes present in the environment at high enough frequencies to allow for populations to respond rapidly to the changing environment, and (ii) high levels of dispersal and gene flow. While we currently lack sufficient information on the causes and distribution of variability in functional traits, filling in these key data gaps should increase our ability to predict how changing biodiversity will alter ecosystem functioning.
Keywords: biodiversity, intraspecific variation, ecosystem function, functional traits, phenotypic plasticity
1. Introduction
One of the central issues facing ecology is predicting how ecosystem functioning will change as species composition shifts in response to anthropogenic drivers [1]. Early research linked species richness to biodiversity and ecosystem functioning (BEF) [2–4]. Collectively, these studies demonstrated relatively consistent patterns of declining diversity leading to decreased ecosystem functioning [5]. However, this type of research did not provide much insight into how changes in abundance, rather than in diversity, affect ecosystem function. To address this problem, a trait-based approach, predicated on the assumption that species' effects on ecosystem functioning depend on key morphological, chemical, physiological or behavioural traits, has since been developed [6–8]. This approach is powerful in that it can be generalized across species [9,10]. Much progress has been made using trait-based approaches, but typically researchers have ignored intraspecific variability in traits [11,12]. However, in ecosystems experiencing changing environmental conditions, this source of variability could be the key to understanding how the effects of different species on ecosystem functioning might shift as community composition changes in response to fluctuating conditions. In particular, incorporating intraspecific variability may provide valuable insights into the stability of ecosystem function.
One key insight stemming from the use of trait-based approaches to understand BEF was recognizing that changes in ecosystem functioning owing to shifts in composition following a change in environmental conditions is a two-stage process [7]. First, traits determine how the species present in an assemblage will respond to a given driver. Second, traits present in the assemblage determine ecosystem functioning. Critically, the traits predicting responses to a particular driver (response traits) may or may not be the same traits as those traits predicting the effects of species on a particular ecosystem function (effects traits) [13]. There has been much progress in documenting broad-scale correlations between different traits that may reflect fundamental physiological or evolutionary constraints (such as the leaf economic spectrum in plants) [14]. However, it certainly need not be the case that values of response and effects traits are correlated either across or within species, a fact with important implications for considering the effects of trait diversity on the stability of ecosystem function.
While there has been substantial research on the degree of correlation between the values of different traits, there is considerably greater uncertainty about whether variability in different traits is correlated [15]. High levels of variability in response traits should provide a species with a high probability of maintaining stable populations in a variable environment, assuming that species are shifting their trait values primarily in order to produce optimal trait values given environmental conditions (as opposed to, for example, avoiding competition, which theoretically would lead to trait displacement, or owing to genetic constraints). In contrast, high variability in effects traits could destabilize ecosystem functioning, at least under certain conditions (discussed below). Thus, determining whether there are general patterns of variability across species is a key research need.
Variation in trait values within a species can arise from multiple processes, most commonly phenotypic plasticity or local adaptation at the population level [9,16]. While both these mechanisms result in the same general pattern at the local scale, the sources of variation can have important consequences for the temporal and spatial scales of how species respond to environmental variation and how these changes feedback to ecosystem functioning. Considering which processes are more strongly shaping trait variation can allow us to make better predictions about how species will respond to fluctuating environmental conditions and how they will subsequently impact ecosystem function.
Here, we introduce a conceptual model to describe the potential links between intraspecific trait variability and the stability of ecosystem functioning. We have developed this conceptual model based on plants, as they have been the focus of the majority of trait-based research. However, the model should hold for other taxa in principle. Using a dataset from the vegetation of the Sandhills region of North Carolina, we provide an analysis of the degree to which trait variability is correlated across species as a model for future analyses. We close by discussing the implications of different mechanisms for generating trait variability for the stability of ecosystem functioning in a variable environment.
2. Conceptual model
We start by assuming that species' contributions to ecosystem functioning is driven primarily by their abundance, following the mass ratio hypothesis (MRH) [17]. While there are probably other factors that also contribute to the relative importance of a species, such as trait novelty, the MRH is commonly assumed in many studies and has substantial support [18,19]. It is under this assumption that people have adopted the widely used metric of community-weighted means (CWM) of traits as an important predictor of ecosystem functioning. The CWM of trait X (presumed here to be an effect trait) is calculated as follows CWMX = ΣSpSXs, where pS is the relative abundance of species S. From this, it follows that for individual species' contributions to ecosystem functioning to be stable, they must either minimize variance in both abundance and effect trait X, or have variation in abundance and effects traits that offset each other. We now consider how trait variation will affect these two components.
(a). Variation in abundance
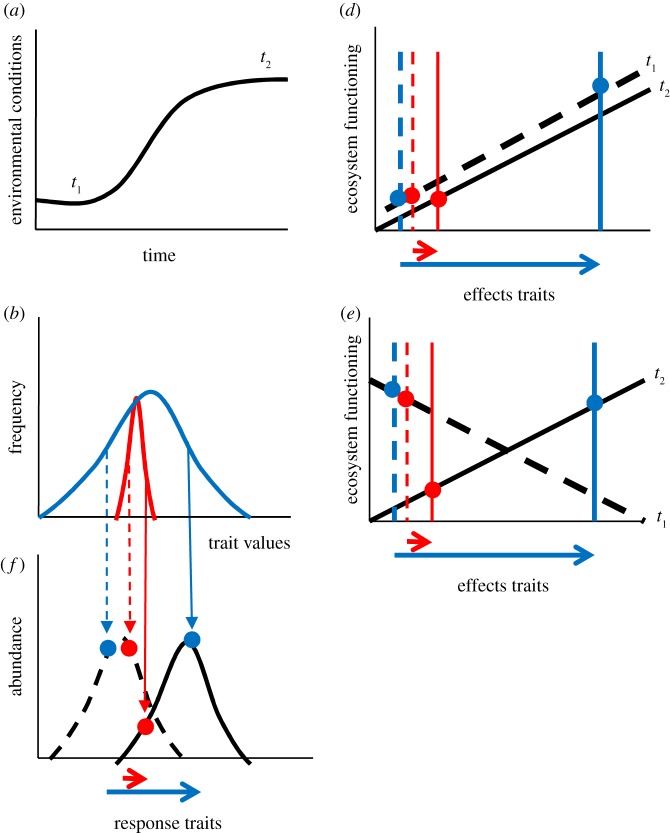
If species are present in an environment where conditions are variable (figure 1a), then we would predict that maintaining maximum population size at different points in time would require different values of response traits. For example, a positive relationship between water availability and specific leaf area (SLA) has frequently been observed [14,20,21], suggesting that under dryer conditions, lower SLA values are optimal, whereas the opposite is true under wetter conditions. It thus follows that species with greater variability in a response trait (figure 1b) should have a greater ability to produce optimal trait values under a wider range of environmental conditions (figure 1c). We note here that we are assuming that species tend to exhibit variation that is adaptive, i.e. moving towards optimal trait values. While this is a common assumption, it has also been proposed that species vary their traits so as to avoid competition with other species that have similar trait values (e.g. equalizing versus stabilizing mechanisms [22]). However, if this assumption holds, we should predict that species with greater variance in response traits should tend to have more stable population sizes.
Figure 1.
(a) Depiction of changes in an environmental condition or resource that affects the abundance of the species of interest across time from period t1 to period t2. (b) Depiction of the range of variability in traits present in two species. The red species exhibits relatively small amounts of trait variation relative to the blue species. (c) The responses of two species (red and blue) to changes in conditions depicted in panel (a). The dotted histogram represents the relationship between a response trait and abundance under conditions at t1, whereas the solid histogram represents this relationship at t2. The red species has limited capacity to shift its trait values and thus exhibits large changes in abundance when compared with the blue species, which has higher levels of trait variability, and thus more stable populations. (d) If we assume that the red species also has limited variability in its effects traits relative to the blue species, in situations where the relationship between effects traits and ecosystem functioning is not contingent on environmental conditions, we predict that the red species should cause greater stability in ecosystem functioning than the blue species. (e) However, if the relationship between effects traits and ecosystem functioning changes in different environments (t1 versus t2), it is the blue species with higher variability in effects traits that is predicted to stabilize ecosystem functioning.
(b). Variation in effects traits
Determining how variation in effects traits will affect the stability of ecosystem functioning is a slightly more complex prospect. This is because, for some ecosystem functions, the relationship between effects traits and ecosystem functioning may be relatively constant under different environmental conditions (as was assumed for the relationship between response traits and abundance above). For example, Cornwell et al. [23] found that SLA predicts decomposition rates of leaf litter across a wide variety of ecosystems, suggesting that in general higher SLA leads to higher decomposition, regardless of the environmental conditions. When the relationship between effects traits and ecosystem functioning is not contingent on environmental conditions, we would predict that species exhibiting high degrees of variability in effects traits should destabilize ecosystem functioning (figure 1d).
However, it is also possible that the relationship between effects traits and ecosystem functioning will be contingent on environmental conditions. For example, when the ecosystem function of interest is primary productivity, response traits and effects traits will often either be identical, or at least tightly linked, as species that reach high abundance do so because they have high productivity. In this case in a wet environment, having a higher SLA might lead to greater productivity, and lower productivity in a dry environment. Under such a situation, it is now the species with higher variability in effects traits that will have a more stable contribution to ecosystem functioning (figure 1e).
Taking all of these together, we can make the following predictions for the interaction between trait variability and ecosystem functioning in a fluctuating environment: (i) for species with low levels of variability in both response and effects traits, we predict low stability in ecosystem functioning owing to large changes in abundance. (ii) For species with low levels of variability in response traits, but high levels of variability in effects traits, we generally predict low stability in ecosystem functioning. However, if the relationship between effects traits and ecosystem functioning is contingent on the environment, and a species changes its effects traits to a large enough degree to offset its changes in abundance, then its contribution to the CWM of the effect trait, and hence its impact on ecosystem functioning, could be constant. (iii) For species with high variability in response traits but low variability in effects traits, we should see stable ecosystem functioning if the relationship between effects traits and ecosystem functioning is not contingent on the environment. (iv) For species with high levels of variability in both response and effects traits, we should see stability in ecosystem functioning only when the relationship between effects traits and ecosystem functioning is contingent on the environment.
3. Is variability in traits correlated across species?
Given the predictions outlined in §2b, we first wanted to investigate what types of correlations appear between trait variabilities across species in natural populations. High variability in functional traits should allow species to maintain high fitness under a broader range of environmental conditions by allowing them to track environmental changes. In fact, high variability in a number of traits has been proposed as a possible explanation for the fact that some invasive species are able to maintain high fitness across a broad range of environmental conditions [15]. Mitchell et al. [24] tested this hypothesis using four common plant traits measured on 49 species and found little evidence for species with high variability in multiple traits, and poor correlation between trait variability and the range of environments occupied.
We conducted a trait analysis of 11 plant traits measured on 107 species (63 685 measurements total) from a longleaf pine forest understory in North Carolina, USA (see [24] for trait collection methods and site description). We included a variety of common plant traits, such as SLA (cm3 g−1), leaf dry matter content (LDMC, gg−1), plant height (cm), and C : N ratio, that are known to be important in a range of systems [10,14,25–27]. These traits are also considered relevant to both plant response to the environment and plant effects on ecosystem functioning [7]. Because our study sites occur along a strong soil moisture gradient from xeric uplands to wet riparian areas, we included water use efficiency (WUE; Δ 13C), a trait known to influence the fitness of individuals along soil moisture gradients in water-limited systems [28–32]. Lastly, because our study area is in a pyrogenic system experiencing frequent prescribed burning, we also included a variety of traits related to leaf flammability that are important to plant fitness [33–35]. These include time to ignition (s), maximum flame height (cm), maximum burn temperature (°C), burn time (s), per cent of matter consumed, and smoulder time (s). Classification of these traits as ‘response’ or ‘effect’ traits depends on the question of interest. For example, SLA can be considered a response trait related to changes in nutrient availability, but can also be considered an effect trait with respect to primary productivity as it is positively associated with photosynthetic rates [7].
We looked at correlation in variability in plant traits (quantified by the coefficient of variation) using pairwise Spearman rank correlations across species. We found variability between traits to be significantly correlated in only about 25% of cases, and in those cases, the strength of correlation was moderate or weak, and often negative (table 1). There was only a single trait pair, SLA and LDMC, whose coefficients of variation had a significant, positive correlation that was of moderate size (ρ = 0.58, p < 0.001). Overall, these findings support Mitchell et al.'s [24] conclusion that there is little evidence for species that are highly variable in multiple traits, a result which has serious implications for maintaining ecosystem functioning in changing environments.
Table 1.
Spearman rank correlations between coefficient of variation for each trait across 107 understory plant species found in the Sandhills of North Carolina, USA.
| coefficient of variation in: | leaf dry-matter content | plant height | water use efficiency | C : N | time to ignition | maximum flame height | maximum burn temperature | burn time | per cent consumed | smoulder time |
|---|---|---|---|---|---|---|---|---|---|---|
| specific leaf area | 0.58a | 0.18 | −0.19 | 0.18 | 0.33a | 0.32a | 0.06 | 0.07 | 0.08 | 0.17 |
| leaf dry-matter content | −0.02 | −0.32a | 0.22 | 0.09 | 0.29a | −0.14 | 0.25a | −0.02 | 0.26a | |
| plant height | 0.05 | 0.02 | 0.18 | 0.03 | −0.05 | −0.23 | 0.00 | −0.07 | ||
| water use efficiency | −0.37a | −0.11 | −0.27a | −0.04 | −0.06 | −0.31a | −0.12 | |||
| C : N | 0.07 | 0.17 | 0.24 | −0.06 | 0.06 | 0.04 | ||||
| time to ignition | 0.36a | 0.06 | −0.08 | 0.21 | 0.04 | |||||
| maximum flame height | 0.23 | 0.20 | 0.39a | 0.24a | ||||||
| maximum burn temperature | −0.05 | 0.23 | −0.15 | |||||||
| burn time | −0.04 | 0.45a | ||||||||
| per cent consumed | −0.03 |
aDenotes significance at α = 0.05.
In our analysis, the majority of species fit predictions 2 and 3, showing variability in multiple traits, but very little correlation between them. Weak correlation in variability between traits, and especially negative correlations (prediction 3), indicates that even if species have enough variability in response traits to maintain their fitness and abundance as the environment shifts, they are unlikely to demonstrate enough variability in critical effects traits to maintain stability in ecosystem functioning. For example, WUE (Δ 13C) is a response trait influencing fitness along soil moisture gradients [28–32], and leaf C : N is an effect trait known to control decomposition rates [36,37]. We found that leaf C : N and WUE variability were moderately negatively correlated across species (ρ = −0.37, p = 0.002). This implies that even if a species has high variability in WUE and is able to track changes in soil moisture and maintain its abundance, the species may not necessarily have enough variation in C : N to maintain the same decomposition rates under the new environmental regime.
4. Implications of phenotypic plasticity versus genetic variability
It is clear from our analysis that, while variation in response and effects traits is not necessarily correlated, there is high variability in several traits. Observed variation in both response and effects traits can be driven by two primary mechanisms: phenotypic plasticity [38] and local adaptation to prevailing conditions [39]. Which of these mechanisms is most prevalent, and under what circumstances, is a matter of some debate [40–42]. However, the underlying source of trait variability will have impacts on how species cope with a changing environment, the linkage between variability in response and effects traits, and whether ecosystem functioning remains stable under fluctuating conditions.
Plants are hypothesized to express functional traits that are at or near the optimum given prevailing environmental conditions and biotic interactions [43]. Given sufficient genetic variability, plants may be able to adapt towards this optimum (local adaptation), or may instead adapt a ‘general-purpose genotype’, or a genotype that displays high phenotypic plasticity in response to encountered conditions. Although plasticity, in particular, has been found to be very prevalent among plants [44], not all traits display appreciable plasticity. Indeed, recent genetic analysis of plant traits has indicated that phenological traits, in particular, exhibited low plasticity, whereas those associated with nutrient capture tended to be the most plastic [45]. Highly canalized traits (e.g. photosynthetic pathway, reliance on specialized pollinators) incorporate no environmental information when constructing an individual's phenotype, and are typically highly invariant, thus any ecosystem functioning reliant on such traits is most directly influenced by species abundance and hence likely to be unstable (figure 1d). For those traits that are not highly canalized, plastic responses can shift trait means and create wide intraspecific variability in traits [46,47], although this variability is not always beneficial at the individual level. A recent meta-analysis [48] examining the prevalence and adaptive value of plastic responses found that whereas half of plastic traits responded with ‘perfect plasticity’ in response to the changing environment (traits shifted towards a common optimum across populations of the same species), nearly one-third of plastic traits responded with non-adaptive plasticity (plastic responses moved individuals further from trait optimums). The maintenance of plasticity within an individual also has costs. Monitoring and responding to environmental stimuli can be energetically expensive, and poor correlation between environmental cues and trait expression can lead to trait–environment mismatches, and a maladaptive phenotype [49].
In contrast, local adaptation is expected to consistently lead to trait expression that is near optimum. A rich literature exists demonstrating that in reciprocal transplant experiments, local populations often show a fitness advantage over transplanted populations (reviewed in [41], but see [40]). In order for local adaptation to arise, a number of conditions must be met. Strong environmental heterogeneity that favours wide differences in phenotype helps drive local adaptation. However, in order to respond to this heterogeneity, species must also possess sufficient genetic variation. Lack of genetic variation can lead to lack of adaptation, regardless of population size. Like phenotypic plasticity, local adaptation is not without cost. Phenotypes that are well adapted to one set of environmental conditions may be maladapted to another, reducing fitness when conditions are altered [50]. Furthermore, offspring derived from highly locally adapted populations may suffer maladaptation, even at relatively small spatial scales, if environmental conditions are significantly different from those of the parent population [51].
Plasticity and local adaptation are expected to arise under specific ecological conditions, which may allow us to predict when to expect either plasticity or local adaptation as a driver of trait variation, and to better understand how these sources of variation impact upon ecosystem functioning. Phenotypic plasticity is expected to arise in systems where abiotic conditions are unpredictable on short timescales, but environmental signals are reliable [52]. Thus, we would predict that for widespread species with high gene flow, high variability owing to phenotypic plasticity is likely to arise in response to strong environmental signals (e.g. resource acquisition traits). Local adaptation is expected to arise when environments are highly divergent but relatively stable over time, and where gene flow is low (e.g. phenotypic traits). Under both mechanisms, under circumstances where variance in response and effects traits is tightly linked, we might expect ecosystem functioning to fluctuate predictably in response to environmental signals, and to maintain higher stability where plasticity in dominant species is prevalent. However, when variance in response and effects traits are driven by different mechanisms or in response to different environmental signals, predicting impacts of environmental signals on ecosystem functioning becomes much more difficult, as variability in response and effects traits are decoupled. In these circumstances, identifying the mechanism, underlying driver and magnitude of variance in effects traits will become critical.
5. Discussion
The ability to predict ecosystem functioning under fluctuating environmental conditions is one of the key goals of ecology. However, research examining how trait variability impacts upon the stability of ecosystem functioning is sorely lacking. Here, we have presented a conceptual model that will allow researchers to develop testable predictions that incorporate both inter- and intraspecific sources of trait variation to better understand the stability of ecosystem functioning.
In our analysis of 11 plant traits across 107 species, we found little evidence that there were tight positive or negative correlations between trait variation. Rather, we found that variation in response traits and effects traits was poorly correlated, and when significantly correlated, this relationship was often negative (high variability in response traits and low variability in effects traits). These results indicate that we may predict stability for ecosystem functioning in this system only for traits that are decoupled from the environment (e.g. SLA and decomposition). Further analysis of the correlation between variation in response and effects across a larger number of species and biomes will indicate whether this trend is indeed general or only specific to this system.
Considering the potential source of this variability is also an important area of future research. For example, in communities that exhibit high gene flow and frequently fluctuating conditions, we may expect to see higher variability in both response and effects traits owing to phenotypic plasticity; and a greater prevalence of prediction 4, high stability in ecosystem functioning as species respond plastically to fluctuating environmental conditions. In contrast, in isolated communities with low gene flow and less-frequent fluctuation, we would predict large changes in ecosystem functioning as environmental conditions fluctuate (prediction 1). Such communities may be of greater conservation value, if, for example, they provide critical ecosystem functions that will be disrupted owing to environmental change.
6. Conclusion
It is clear that intraspecific variability in traits is an important, but often overlooked, link to understanding BEF under fluctuating environmental conditions. Future work in this area should quantify variability in response and effects traits, and identify the role of phenotypic plasticity and local adaptation, in environments experiencing a range of environmental fluctuations to strengthen our understanding of linkages between variability in response and effects traits across ecosystems. We can then build on these trends to make predictions about the stability of ecosystem functioning in the face of fluctuations both endemic to the system, and under possible future conditions.
Acknowledgements
Thanks to U. Brose for the invitation and stimulating discussion with J. Joshi and her laboratory group. The authors thank Janet Gray from the Fort Bragg Endangered Species Branch and Matthew Hohmann from ERDC-CERL for logistical support; and S. Anderson and E. Ungberg for their hard work on this project.
Authors' contributions
All authors contributed equally to the concept and drafting of this paper. All authors gave final approval for publication
Competing interests
We have no competing interests.
Funding
J.W. and G.A. were supported by the US Army Engineer Research Development Center, Construction Engineering Research Laboratory (ERDC-CERL) (cooperative agreement no. W9132T-11-2-0008). R.M. was supported by NSF DBI 1401800.
References
- 1.Brose U, Hillebrand H. 2016. Biodiversity and ecosystem functioning in dynamic landscapes. Phil. Trans. R. Soc. B 371, 20150267 ( 10.1098/rstb.2015.0267) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naeem S, Thompson LJ, Lawler SP, Lawton JH, Woodfin RM. 1994. Declining biodiversity can alter the performance of ecosystems. Nature 368, 734–737. ( 10.1038/368734a0) [DOI] [Google Scholar]
- 3.Tilman D, Knops J, Wedin D, Reich P, Ritchie M, Siemann E. 1997. The influence of functional diversity and composition on ecosystem processes. Science 277, 1300–1302. ( 10.1126/science.277.5330.1300) [DOI] [Google Scholar]
- 4.Hector A, et al. 1999. Plant diversity and productivity experiments in European grasslands. Science 286, 1123–1127. ( 10.1126/science.286.5442.1123) [DOI] [PubMed] [Google Scholar]
- 5.Cardinale BJ, Srivastava DS, Emmett Duffy J, Wright JP, Downing AL, Sankaran M, Jouseau C. 2006. Effects of biodiversity on the functioning of trophic groups and ecosystems. Nature 443, 989–992. ( 10.1038/nature05202) [DOI] [PubMed] [Google Scholar]
- 6.Naeem S, Wright JP. 2003. Disentangling biodiversity effects on ecosystem functioning: deriving solutions to a seemingly insurmountable problem. Ecol. Lett. 6, 567–579. ( 10.1046/j.1461-0248.2003.00471.x) [DOI] [Google Scholar]
- 7.Lavorel S, Garnier E. 2002. Predicting changes in community composition and ecosystem functioning from plant traits: revisting the Holy Grail. Funct. Ecol. 16, 545–556. ( 10.1046/J.1365-2435.2002.00664.X) [DOI] [Google Scholar]
- 8.Díaz S, Cabido M. 2001. Vive la différence: plant functional diversity matters to ecosystem processes. Trends Ecol. Evol. 16, 646–655. ( 10.1016/S0169-5347(01)02283-2) [DOI] [Google Scholar]
- 9.McGill B, Enquist B, Weiher E, Westoby M. 2006. Rebuilding community ecology from functional traits. Trends Ecol. Evol. 21, 178–185. ( 10.1016/j.tree.2006.02.002) [DOI] [PubMed] [Google Scholar]
- 10.Westoby M, Wright IJ. 2006. Land-plant ecology on the basis of functional traits. Trends Ecol. Evol. 21, 261–268. ( 10.1016/j.tree.2006.02.004) [DOI] [PubMed] [Google Scholar]
- 11.Albert C, Thuiller W, Yoccoz N, Soudant A, Boucher F, Saccone P, Lavorel S. 2010. Intraspecific functional variability: extent, structure and sources of variation. J. Ecol. 98, 604–613. ( 10.1111/j.1365-2745.2010.01651.x) [DOI] [Google Scholar]
- 12.Bolnick DI, et al. 2011. Why intraspecific trait variation matters in community ecology. Trends Ecol. Evol. 26, 183–192. ( 10.1016/j.tree.2011.01.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suding KN, et al. 2008. Scaling environmental change through the community-level: a trait-based response-and-effect framework for plants. Glob. Change Biol. 14, 1125–1140. ( 10.1111/j.1365-2486.2008.01557.x) [DOI] [Google Scholar]
- 14.Wright IJ, et al. 2004. The worldwide leaf economics spectrum. Nature 428, 821–827. ( 10.1038/nature02403) [DOI] [PubMed] [Google Scholar]
- 15.Richards CL, Bossdorf O, Muth NZ, Gurevitch J, Pigliucci M. 2006. Jack of all trades, master of some? On the role of phenotypic plasticity in plant invasions. Ecol. Lett. 9, 981–993. ( 10.1111/j.1461-0248.2006.00950.x) [DOI] [PubMed] [Google Scholar]
- 16.Matesanz S, Horgan-Kobelski T, Sultan SE. 2012. Phenotypic plasticity and population differentiation in an ongoing species invasion. PLoS ONE 7, e44955 ( 10.1371/journal.pone.0044955) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grime JP. 1998. Benefits of plant diversity to ecosystems: immediate, filter and founder effects. J. Ecol. 86, 902–910. ( 10.1046/j.1365-2745.1998.00306.x) [DOI] [Google Scholar]
- 18.Garnier E, et al. 2004. Plant functional markers capture ecosystem properties during secondary succession. Ecology 85, 2630–2637. ( 10.1890/03-0799) [DOI] [Google Scholar]
- 19.Violle C, Navas M-L, Vile D, Kazakou E, Fortunel C, Hummel I, Garnier E. 2007. Let the concept of trait be functional! Oikos 116, 882–892. ( 10.1111/j.0030-1299.2007.15559.x) [DOI] [Google Scholar]
- 20.Sandel B, Goldstein LJ, Kraft NJB, Okie JG, Shuldman MI, Ackerly DD, Cleland EE, Suding KN. 2010. Contrasting trait responses in plant communities to experimental and geographic variation in precipitation. New Phytol. 188, 565–575. ( 10.1111/j.1469-8137.2010.03382.x) [DOI] [PubMed] [Google Scholar]
- 21.Wright JP, Sutton-Grier A. 2012. Does the leaf economic spectrum hold within local species pools across varying environmental conditions? Funct. Ecol. 26, 1390–1398. ( 10.1111/1365-2435.12001) [DOI] [Google Scholar]
- 22.Chesson P. 2000. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 31, 343–366. ( 10.1146/annurev.ecolsys.31.1.343) [DOI] [Google Scholar]
- 23.Cornwell WK, et al. 2008. Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol. Lett. 11, 1065–1071. ( 10.1111/j.1461-0248.2008.01219.x) [DOI] [PubMed] [Google Scholar]
- 24.Ames G, Anderson S, Wright J. 2015. Multiple environmental drivers structure plant traits at the community level in a pyrogenic ecosystem. Funct. Ecol. ( 10.1111/1365-2435.12536) [DOI] [Google Scholar]
- 25.Wright IJ, et al. 2005. Modulation of leaf economic traits and trait relationships by climate. Glob. Ecol. Biogeogr. 14, 411–421. ( 10.1111/j.1466-822x.2005.00172.x) [DOI] [Google Scholar]
- 26.Westoby M. 1998. A leaf-height-seed (LHS) plant ecology strategy scheme. Plant Soil 199, 213–227. ( 10.1023/A:1004327224729) [DOI] [Google Scholar]
- 27.Pierce S, Brusa G, Vagge I, Cerabolini BEL. 2013. Allocating CSR plant functional types: the use of leaf economics and size traits to classify woody and herbaceous vascular plants. Funct. Ecol. 27, 1002–1010. ( 10.1111/1365-2435.12095) [DOI] [Google Scholar]
- 28.Smedley MP, Dawson TE, Comstock JP, Donovan LA, Sherill DE, Cook CS, Ehleringer JR. 1991. Seasonal carbon isotope discrimination in a grassland community. Oecologia 85, 314–320. ( 10.1007/bf00320605) [DOI] [PubMed] [Google Scholar]
- 29.Donovan LA, Dudley SA, Rosenthal DM, Ludwig F. 2015. Phenotypic selection on leaf water use efficiency and related ecophysiological traits for natural populations of desert sunflowers. Oecologia 152, 13–25. ( 10.1007/s00442-006-0627-5) [DOI] [PubMed] [Google Scholar]
- 30.Dawson TE, Mambelli S, Plamboeck AH, Templer PH, Tu KP. 2002. Stable isotopes in plant ecology. Annu. Rev. Ecol. Syst. 33, 507–559. ( 10.1146/annurev.ecolsys.33.020602.095451) [DOI] [Google Scholar]
- 31.Monclus R, et al. 2005. Impact of drought on productivity and water use efficiency in 29 genotypes of Populus deltoides × Populus nigra. New Phytol. 169, 765–777. ( 10.1111/j.1469-8137.2005.01630.x) [DOI] [PubMed] [Google Scholar]
- 32.Stewart G, Turnbull M, Schmidt S, Erskine P. 1995. C natural abundance in plant communities along a rainfall gradient: a biological integrator of water availability. Aust. J. Plant Physiol. 22, 51 ( 10.1071/PP9950051) [DOI] [Google Scholar]
- 33.Pérez-Harguindeguy N, et al. 2013. New handbook for standardised measurement of plant functional traits worldwide. Aust. J. Bot. 61, 167–234. ( 10.1071/BT12225) [DOI] [Google Scholar]
- 34.Kane JM, Varner JM, Hiers JK. 2008. The burning characteristics of southeastern oaks: discriminating fire facilitators from fire impeders. For. Ecol. Manage. 256, 2039–2045. ( 10.1016/j.foreco.2008.07.039) [DOI] [Google Scholar]
- 35.De Magalhães RMQ, Schwilk DW. 2012. Leaf traits and litter flammability: evidence for non-additive mixture effects in a temperate forest. J. Ecol. 100, 1153–1163. ( 10.1111/j.1365-2745.2012.01987.x) [DOI] [Google Scholar]
- 36.Enríquez S, Duarte CM, Sand-Jensen K. 1993. Patterns in decomposition rates among photosynthetic organisms: the importance of detritus C:N:P content. Oecologia 94, 457–471. ( 10.1007/BF00566960) [DOI] [PubMed] [Google Scholar]
- 37.Pérez-Harguindeguy N, Díaz S, Cornelissen JHC, Vendramini F, Cabido M, Castellanos A. 2000. Chemistry and toughness predict leaf litter decomposition rates over a wide spectrum of functional types and taxa in central Argentina. Plant Soil 218, 21–30. ( 10.1023/A:1014981715532) [DOI] [Google Scholar]
- 38.Schlichting C. 1986. The evolution of phenotypic plasticity in plants. Annu. Rev. Ecol. Syst. 17, 667–693. ( 10.1146/annurev.es.17.110186.003315) [DOI] [Google Scholar]
- 39.Joshi J, et al. 2001. Local adaptation enhances performance of common plant species. Ecol. Lett. 4, 536–544. ( 10.1046/j.1461-0248.2001.00262.x) [DOI] [Google Scholar]
- 40.Leimu R, Fischer M. 2008. A meta-analysis of local adaptation in plants. PLoS ONE 3, 1–8. ( 10.1371/journal.pone.0004010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hereford J. 2009. A quantitative survey of local adaptation and fitness trade-offs. Am. Nat. 173, 579–588. ( 10.1086/597611) [DOI] [PubMed] [Google Scholar]
- 42.Sultan SE. 2000. Phenotypic plasticity for plant development, function and life history. Trends Plant Sci. 5, 537–542. ( 10.1016/S1360-1385(00)01797-0) [DOI] [PubMed] [Google Scholar]
- 43.Anderson JT, Wagner MR, Rushworth CA, Prasad KVSK, Mitchell-Olds T. 2014. The evolution of quantitative traits in complex environments. Heredity (Edinb). 112, 4–12. ( 10.1038/hdy.2013.33) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ghalambor CK, McKay JK, Carroll SP, Reznick DN. 2007. Adaptive versus non-adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct. Ecol. 21, 394–407. ( 10.1111/j.1365-2435.2007.01283.x) [DOI] [Google Scholar]
- 45.Des Marais DL, Hernandez KM, Juenger TE. 2013. Genotype-by-environment interaction and plasticity: exploring genomic responses of plants to the abiotic environment. Annu. Rev. Ecol. Evol. Syst. 44, 5–29. ( 10.1146/annurev-ecolsys-110512-135806) [DOI] [Google Scholar]
- 46.Mitchell RM, Bakker JD. 2014. Intraspecific trait variation driven by plasticity and ontogeny in Hypochaeris radicata. PLoS ONE 9, e109870 ( 10.1371/journal.pone.0109870) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Jong G. 2005. Evolution of plasticity and the emergence patterns of ecotypes. New Phytol. 166, 101–118. ( 10.1111/j.1469-8137.2005.01322.x) [DOI] [PubMed] [Google Scholar]
- 48.Palacio-López K, Beckage B, Scheiner S, Molofsky J. 2015. The ubiquity of phenotypic plasticity in plants: a synthesis. Ecol. Evol. 5, 3389–3400. ( 10.1002/ece3.1603) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dewitt TJ, Sih A, Wilson DS. 1998. Costs and limits of phenotypic plasticity. Trends Ecol. Evol. 13, 77–81. ( 10.1016/S0169-5347(97)01274-3) [DOI] [PubMed] [Google Scholar]
- 50.Kawecki TJ, Ebert D. 2004. Conceptual issues in local adaptation. Ecol. Lett. 7, 1225–1241. ( 10.1111/j.1461-0248.2004.00684.x) [DOI] [Google Scholar]
- 51.Schmitt J, Gamble SE. 1990. The effect of distance from the parental site on offspring performance and inbreeding depression in Impatiens capensis: a test of the local adaptation hypothesis. Evolution (N.Y). 44, 2022–2030. ( 10.2307/2409612) [DOI] [PubMed] [Google Scholar]
- 52.Van Kleunen M, Fischer M. 2005. Constraints on the evolution of adaptive phenotypic plasticity in plants. New Phytol. 166, 49–60. ( 10.1111/j.1469-8137.2004.01296.x) [DOI] [PubMed] [Google Scholar]