Abstract
Climate change is expected to increase the frequency and magnitude of extreme weather events. It is therefore of major importance to identify the community attributes that confer stability in ecological communities during such events. In June 2013, a flood event affected a plant diversity experiment in Central Europe (Jena, Germany). We assessed the effects of plant species richness, functional diversity, flooding intensity and community means of functional traits on different measures of stability (resistance, resilience and raw biomass changes from pre-flood conditions). Surprisingly, plant species richness reduced community resistance in response to the flood. This was mostly because more diverse communities grew more immediately following the flood. Raw biomass increased over the previous year; this resulted in decreased absolute value measures of resistance. There was no clear response pattern for resilience. We found that functional traits drove these changes in raw biomass: communities with a high proportion of late-season, short-statured plants with dense, shallow roots and small leaves grew more following the flood. Late-growing species probably avoided the flood, whereas greater root length density might have allowed species to better access soil resources brought from the flood, thus growing more in the aftermath. We conclude that resource inputs following mild floods may favour the importance of traits related to resource acquisition and be less associated with flooding tolerance.
Keywords: biodiversity, resistance, resilience, grasslands, biomass
1. Introduction
Climate change is one of the greatest human-induced ecological concerns facing the world today [1]. As one of the consequences, an increase in the frequency and intensity of extreme weather events is expected, including an increased occurrence of floods [2]. Assessing the stability of ecological communities in the face of such environmental change is a major goal of ecologists in the 21st century. Past work has defined post-disturbance stability as at least two temporally separated measurements: resistance is the capacity of a community to maintain baseline ecosystem functions (e.g. biomass production) throughout a disturbance, compared with a pre-disturbance level [3,4]; and resilience is the ability to recover ecosystem functions following the disturbance [5] (figure 1).
Figure 1.
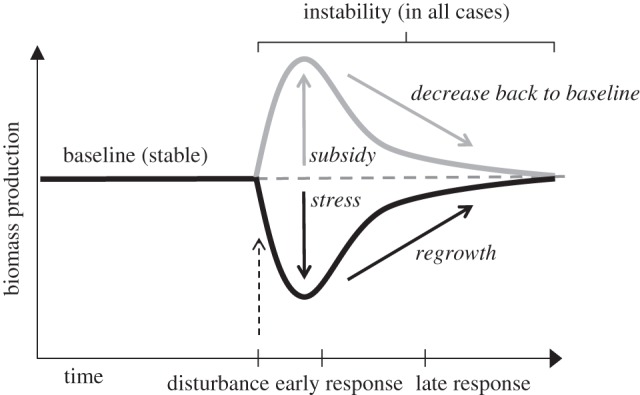
Theoretical scheme of how biomass production may increase (owing to subsidy) or decrease (owing to stress) following a disturbance. Both instances lead to decreased stability compared with pre-disturbance conditions. We show a disturbance that increases biomass production (grey line) and a disturbance that decreases biomass production (black line). In the early-response period (sometimes ‘resistance’) a subsidy-based disturbance will increase biomass production and a stress-based disturbance will decrease biomass production. Both cases will lead to decreased stability. In the late-response period (sometimes ‘resilience’), the reverse will occur: a subsidy-based disturbance will decrease biomass production back to a baseline (unless a new stable state is attained); a stress-based disturbance will increase biomass production back to a baseline.
Depending on the type, magnitude, duration and frequency of the disturbance, the effects on the community may vary [6,7]. Drought and high temperature events may cause mostly physiological stress for plants (e.g. water stress, photoinhibition and reduced photosynthetic rates), causing a decrease in community biomass [4,5]. Fires and floods may increase resource availability, and the effect of the disturbance, in this case, can result in a resource subsidy and an increase in biomass production [5,8] (figure 1). Importantly, as disturbance severity increases, physiological stress may also increase [9]. So, the outcome of any disturbance is probably the result of the combined effects of the physiological stress experienced by the constituent species and changes in resource availability.
Community characteristics, such as species richness [10], species evenness [11] and functional trait diversity [12], may improve community stability during a disturbance. Higher diversity plant communities can maintain ecosystem functions during stress-based perturbations owing to the increased probability that a more diverse community contains tolerant species that persist during, or recover quickly after, the event [4,12–14]. In this case, the presence and low performance of sensitive species may be buffered by the presence of other species that are more tolerant during the disturbance, or have the ability to regrow after the disturbance [15]. Higher diversity communities may also increase ecosystem functions during subsidy-based perturbations owing to the increased probability that a more diverse community contains fast-growing species that capitalize on resource influxes [8]. Community responses to disturbance may therefore be related to particular functional traits (e.g. disturbance sensitivity) and/or trait diversity.
A trait-based approach to studying biodiversity–stability relationships may allow us to develop a better mechanistic understanding of the stabilizing mechanisms of ecological communities. Different functional traits may determine stability at different points in time and with different consequences for resistance and resilience. Furthermore, the greater diversity of species with different traits within the community (functional diversity) may increase community stability in response to different kinds of disturbances.
In the case of flooding disturbances, negative effects of flooding on plants are most strongly related to lack of access to oxygen, reduced cellular respiration and an ATP crisis for the cell [16,17]. Traits that maintain oxygen exchange (e.g. leaf area, aerenchyma production and plant height) can slow this process and may thus increase the resistance of these species and their resident communities [12,13]. Conversely, investment in below-ground structures that increase resource acquisition and rapid regrowth following a flood (e.g. underground storage organs and increased root density) may be more tightly associated with subsidy-based disturbances. With increased resource availability below ground, such as during a minor to moderate flood [8], dense roots may increase species and community growth following a flood event. Finally, traits associated with plant phenology may also be important for plant community responses to flooding. A disturbance may not coincide with the active growing season of some species and may therefore have little effect on those resident species or the community overall [16,17]. For early summer flooding, such as the case in this study, species that grow later in the season may be less affected. Consequently, plant communities with a predominance of late-season species (not diversity per se) may be more resistant to disturbances. Conversely, resilience may be unaffected, owing to little deviation from the baseline, and therefore little regrowth possible.
Here, we used a unique trait-based experiment (TBE) [18] to assess the effects of flooding index, trait diversity and species richness on community stability (resistance and resilience) and changes in biomass production (both positive and negative) after a mild flooding disturbance that occurred in Central Europe in June 2013. In the framework of the TBE of the Jena Experiment [18], we separately manipulated diversity in terms of spatial resource acquisition traits (rooting depth, root length density, plant height and leaf area) and temporal resource phenology traits (growth starting date and flowering starting date).
Specifically, we assessed two hypotheses concerning biodiversity–stability relationships:
(1) Increasing species richness and trait diversity should result in increased biomass production following the flood (in comparison with the previous year). This will be paired with decreased community stability (resistance and resilience).
(2) With stronger flood intensity, increasingly negative effects (stress) should overwhelm the benefits of any resource inputs (subsidies) resulting from the flood. Increased biomass production in higher-diversity communities during mild floods should become weaker with increasing flooding intensity, as fewer species are physiologically capable of persisting during severe stress [8].
We also assessed two hypotheses related to the role of plant traits and their effects on community biomass changes following the flood:
(3) During a stress-based disturbance, plants with greater plant height and leaf area, related to oxygen exchange, should be important for flooding tolerance, whereas phenological traits associated with late post-flood growth may be important for flood avoidance. Both should maintain biomass production of the community immediately following the flood (early response). For longer-term responses (late response), communities dominated by late-season growers may be the most capable of regrowth.
(4) During a subsidy-based disturbance (figure 1), traits associated with rapid acquisition of below-ground resources (e.g. dense roots) and phenological traits associated with early growth should increase biomass production of the community immediately following the flood (early response). For longer-term responses (late response), these same traits should be correlated with greater declines in growth as the community returns back to a baseline.
2. Methods
The TBE was established in 2010 in the floodplain of the river Saale, near the city of Jena, Germany (50°55′ N, 11°35′ E, 130 m.a.s.l.). The area has a mean annual air temperature of 9.4°C and mean annual precipitation of 587 mm [19]. The soil is a Eutric Fluvisol developed from up to 2 m thick fluvial sediments that are almost free of stones [20].
The experiment is based on plant communities that were created by sowing different combinations of species into 3.5 × 3.5 m experimental plots [18]. For defining the trait-based species mixtures, native species from the area were described according to functional traits indicative of spatial and temporal resource acquisition strategies. These trait data were analysed by principal components analysis (PCA), and species were then selected for the mixtures according to the species scores on the two main ordination axes. The first principal component was positively related to leaf area, plant height and root depth, and negatively related to root length density. This axis therefore represented a trade-off in allocation patterns: between short plants with dense roots on one end of the PCA axis, and tall plants with large leaves and sparse roots on the opposite end of the PCA axis. The second principal component was related to phenological traits (‘temporal resources’): species with late growth and flowering start had positive PCA scores, whereas species with early growth and flowering had negative PCA scores (electronic supplementary material, figure S1).
We established communities with variation in either spatial resource acquisition strategies or temporal resource acquisition strategies. To do this, we held temporal traits constant (at intermediate phenological score values) and selected species from the full range of spatial resource acquisition strategies. Conversely, we held spatial resource acquisition traits constant (at intermediate values) and selected species from the full range of temporally (phenology-) based trait values. Finally, a third species pool was formed using species with extreme scores on both ordination axes, thus named ‘mixed’ species pool (see [18] and electronic supplementary material, figure S1 and table S1).
For each resource acquisition trait pool (spatial and temporal), the sown communities covered a species richness gradient (SR; 1, 2, 3, 4 and 8 species). The total of 138 plots were arranged in three blocks to account for any underlying differences in soil type and elevation at the field site. All plots were weeded three times per year, intending to maintain the treatment only with the sown species. The whole experiment was mown two times per year, and mown biomass was removed in order to mimic the usual management of extensively used hay meadows in the region. Plots did not receive any fertilization.
In June 2013, an extreme flood event with an estimated 200 years average return time occurred across much of Central and Eastern Europe [8,21]. This resulted in moderate flooding in the TBE at the Jena experiment. The flood duration (maximum 12 days) and depth of water (maximum of 40 cm) was variable among plots. The selected species of the experiment were not necessarily flood-adapted, but past work has indicated that even non-flood-adapted species can survive floods up to 7 days [17]. Thus, it was unclear whether the selected species for the TBE would experience severe stress or tolerance under a flood of this duration and type. To define the flood intensity that each plot experienced during the flood, we calculated an index based on the daily proportion of the plot that was flooded and the number of days that each plot was flooded [8]:
 |
Plant above-ground biomass was harvested in late May and late August 2012 just before mowing the experimental plots (pre-disturbance conditions), and July and September 2013 (early and late post-disturbance, respectively) by clipping the plants 3 cm above the ground in two randomly placed rectangles of 0.2 × 0.5 m per plot. Samples were separated into target (sown) species and weeds, dried at 70°C for 48 h, and weighed. The two replicates per plot within the same sampling campaign were averaged. We used total target species biomass to calculate stability indices.
For the analysis of biodiversity–stability relationships, we calculated resistance:
and resilience:
after Isbell et al. [5], where 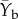 is the average biomass in May and August 2012, here taken as pre-flood condition; Ye is early post-disturbance biomass (July 2013); and Yl is late post-disturbance biomass (September 2013). As such, both numbers are always positive. Short-term biomass losses or gains of 100% result in a resistance value of 1, and losses or gains of 50% result in a resistance value of 2. If biomass increase surpasses 100%, resistance will be lower than 1 and approach 0. Similarly, if these biomass losses or gains return towards pre-flood values by another 50% when late post-disturbance biomass is measured, this results in a resilience value equal to 2. Resilience, as defined here, measures the rate at which the system is approaching pre-flood conditions.
is the average biomass in May and August 2012, here taken as pre-flood condition; Ye is early post-disturbance biomass (July 2013); and Yl is late post-disturbance biomass (September 2013). As such, both numbers are always positive. Short-term biomass losses or gains of 100% result in a resistance value of 1, and losses or gains of 50% result in a resistance value of 2. If biomass increase surpasses 100%, resistance will be lower than 1 and approach 0. Similarly, if these biomass losses or gains return towards pre-flood values by another 50% when late post-disturbance biomass is measured, this results in a resilience value equal to 2. Resilience, as defined here, measures the rate at which the system is approaching pre-flood conditions.
We also explored raw changes in biomass (as opposed to absolute value measures of stability discussed above) in order to explore complementary information on the exact community response (increase or decrease in biomass production in relation to previous conditions). These types of responses should more accurately explore the mechanisms for the types of responses we observed (e.g. figure 1). We computed indices of biomass change relative to the previous year (early biomass change index):
This comparison was conducted during July 2013 (Ye), and the average biomass from May 2012 and August 2012 (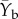 ) in order to normalize the seasonal differences that may have occurred by taking measurements in different months during the two different years. We also calculated a late biomass change index:
) in order to normalize the seasonal differences that may have occurred by taking measurements in different months during the two different years. We also calculated a late biomass change index:
For this index, a fair seasonal comparison could be made directly between September 2013 and late August 2012.
To test biodiversity–stability relationships, we fitted linear mixed-effects models using flood index, plant species richness (as log-linear term), trait pool (as factor with three levels: spatial, temporal or mixed) and all higher-order interactions as fixed effects. We included block as a random effect. We used log-transformed resistance, resilience and early and late biomass change indices as response variables.
To investigate the role of particular trait strategies and functional diversity, we used the original species scores on the PCA ordination (the same one we used to define the three species pools to establish the experiment, electronic supplementary material, figure S1) to compute community mean scores (CMS) for the first two ordination axes (PCA1 and PCA2). Plots with higher values of community mean scores for PCA axis 1 contained a higher proportion of tall-statured species with large leaves and deep sparse roots, whereas negative values of community mean scores for PCA1 represented a community with a high proportion of small-statured plants with small leaves and dense shallow roots (grasses mostly). Communities with low values of community mean scores for PCA axis 2 contained a high proportion of early-growing and -flowering species (see experimental design, electronic supplementary material, figure S1).
We also computed Rao quadratic entropy [22] to calculate functional diversity using the species scores on the ordination axes as ‘traits’. That is, instead of considering each one of the original trait values for computing community mean scores and functional diversity, we used computed traits based on the species scores for the two first axes of the ordination. We fitted linear mixed-effects models using functional diversity and community mean scores for PCA1 and PCA2 and all higher-order interactions as fixed effects. We included block and flooding index as random effects. We used log-transformed early and late biomass change indices as our response variables.
To fit and testing linear mixed-effects models, we used the function lme available in the nlme R package [23], followed by the R function ANOVA (using option for marginal sums of squares) to obtain additional p values for the terms involving nominal predictors. To compute community mean scores and Rao entropy, we used the SYNCSA R package [24]. All input data of the analysis are available in electronic supplementary material, table S3.
3. Results
(a). Biodiversity–stability hypotheses
Community resistance to disturbance decreased with increasing richness of sown species, but only when the flooding index was very low (significant plant species richness × flooding index interaction, table 1 and electronic supplementary material, figure S2). There was no difference in how communities with different types of traits (spatial versus temporal resource acquisition traits) affected resistance (table 1). None of the investigated plant community characteristics or flood index had a significant effect on resilience (table 1).
Table 1.
Linear mixed-effects model results using plant species richness (LogPSR), pool, flood index and all higher-order interactions as fixed effects. We analysed responses in resistance, resilience, and early and late biomass change indices. Significant effects (p < 0.05) are given in italics.
| resistance |
resilience |
early biomass change |
late biomass change |
|||||
|---|---|---|---|---|---|---|---|---|
| F-value | p-value | F-value | p-value | F-value | p-value | F-value | p-value | |
| pool | 1.90 | 0.154 | 0.94 | 0.394 | 0.97 | 0.381 | 1.35 | 0.263 |
| flood | 5.55 | 0.020 | 2.19 | 0.141 | 4.15 | 0.044 | 1.84 | 0.177 |
| LogPSR | 7.40 | 0.008 | 3.90 | 0.051 | 3.97 | 0.049 | 1.24 | 0.268 |
| pool : flood | 2.19 | 0.116 | 1.00 | 0.372 | 1.24 | 0.294 | 1.84 | 0.164 |
| pool : LogPSR | 2.35 | 0.099 | 1.56 | 0.213 | 0.59 | 0.558 | 0.50 | 0.610 |
| flood : LogPSR | 7.48 | 0.007 | 3.60 | 0.060 | 3.91 | 0.050 | 1.07 | 0.304 |
| pool : flood : LogPSR | 2.56 | 0.081 | 1.52 | 0.222 | 0.71 | 0.494 | 0.76 | 0.472 |
Species richness increased biomass production in the first month after the flood (early biomass change index, table 1), and this trend was reversed in the plots with the highest flooding index (figure 2). None of the measured variables had a significant effect on the later biomass change index (table 1).
Figure 2.
The effects of plant species richness (log axis) on early biomass change index (unitless, log-transformed) after the flooding event depended on the degree of flooding. The plots shown here in the low flood index category experienced 8–9.25 days of whole-plot flooding. The intermediate flood index plots experienced 9.5–9.75 days of whole-plot flooding. The high flood index plots experienced 10–12 days of whole-plot flooding. The division of flooding index into three bins is done for display purposes only; all analyses are based on continuous variation. Shaded areas represent 95% CIs.
(b). Trait and functional diversity hypotheses
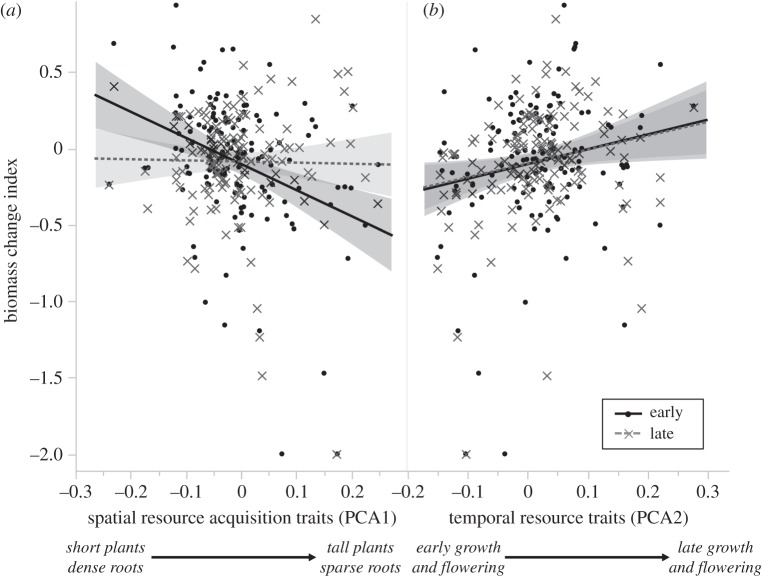
Community mean scores on PCA axis 1 (spatial resource traits) and 2 (temporal resource traits) affected plant biomass production right after the flood and two months later (early and late biomass change indices; table 2 and figure 3). Communities with lower values of community mean scores on PCA1, that is plant communities with a higher proportion of small-statured species with small leaves and shallow, dense roots, grew up to 50% more immediately following the flood in comparison with the previous year (early biomass change index, figure 3a). Communities with a higher proportion of tall plants with large leaves and deep sparse roots grew nearly 50% less than in the previous year. Phenological traits, represented by PCA2, also affected biomass change indices for both early and late biomass change indices: communities with an increasing proportion of late-season (growing and flowering) species grew more than communities dominated by early-growing and early-flowering species. There was an interaction between PCA1 and PCA2 for both early and late biomass change indices: plots dominated by tall plants with sparse roots responded negatively to the flood, and this was exacerbated in plots with mostly early-growing/early-flowering species (electronic supplementary material, figure S3). The late biomass change index increased significantly with increasing functional diversity, though this was only true in the plots dominated by tall plants with sparse roots (electronic supplementary material, figure S4).
Table 2.
Linear mixed-effects model results using community mean scores in the first two axes of the ordination (CMS PCA1, CMS PCA2) and Rao Quadratic entropy and all higher-order interactions as our fixed effects. We analysed responses in resistance, resilience, and early and late biomass change indices. Significant effects (p < 0.05) are given in italics.
| resistance |
resilience |
early biomass change |
late biomass change |
|||||
|---|---|---|---|---|---|---|---|---|
| F-value | p-value | F-value | p-value | F-value | p-value | F-value | p-value | |
| CMSPCA1 | 0.83 | 0.366 | 0.82 | 0.368 | 15.81 | <0.001 | 12.23 | 0.001 |
| CMSPCA2 | 0.1 | 0.751 | 0.27 | 0.607 | 7.63 | 0.007 | 6.03 | 0.016 |
| RaoQ | 0.99 | 0.323 | 2.11 | 0.149 | 0.81 | 0.371 | 6.77 | 0.011 |
| CMSPCA1 : CMSPCA2 | 0.24 | 0.624 | 0.00 | 0.971 | 9.01 | 0.003 | 6.75 | 0.011 |
| CMSPCA1 : RaoQ | 1.53 | 0.219 | 0.57 | 0.451 | 0.41 | 0.524 | 12.76 | 0.001 |
| CMSPCA2 : RaoQ | 0.11 | 0.741 | 0.00 | 1.000 | 2.77 | 0.099 | 2.14 | 0.147 |
| CMSPCA1 : CMSPCA2 : RaoQ | 0.35 | 0.555 | 0.11 | 0.738 | 0.61 | 0.437 | 2.30 | 0.132 |
Figure 3.
The effects of community mean scores in PCA axis 1 and 2 for early and late biomass change indices (unitless, log-transformed). Communities with lower values in PCA1 have a higher proportion of short species with shallow dense roots; communities with higher values in PCA1 have a higher proportion of tall plants with large leaves and deep sparse roots. PCA2 represents temporal resource (phenology) traits. The solid line represents early changes and the dotted grey line represents late changes. Shaded areas represent 95% CIs.
4. Discussion
Here we show that positive biodiversity–stability relationships may not be applicable to disturbances of all types and intensities. Although this study focused on the short-term responses of plant biomass production, the trend observed following a flooding event in a temperate European grassland was the opposite of that expected by biodiversity–stability theory [4]: species diversity did not increase but reduced post-disturbance resistance (electronic supplementary material, figure S2). While recent work demonstrated similar negative trends for short-term biodiversity–stability relationships [8], the novelty of the combined findings indicates that we should be assessing biodiversity–stability relationships during different types of disturbances along a subsidy–stress gradient.
After droughts or other stress-based disturbances that cause biomass losses, highly diverse communities are more stable because they can persist and maintain biomass production over time [4,25]. This maintenance is often related to increased functional diversity: more species with more functional responses to the event can insure the community against biomass losses [26]. During disturbances that increase resource availability (subsidy-based), but do not strongly increase physiological stress and mortality (such as a mild flood), higher-diversity communities may be less stable because they are more likely to include highly productive species that may take advantage of a resource pulse. This results in increased biomass production and decreased stability in higher-diversity communities (figures 1 and 2). Thus, the use of stability indices based on absolute values [5] can be counterintuitive. Raw measurements of increases or decreases in biomass (biomass change indices) can complement these approaches and give more insight into mechanisms.
Our results show that species-rich plant communities (in terms of both species diversity and functional diversity) can grow more than species-poor communities following a mild disturbance (figure 2 and electronic supplementary material, figure S4). In areas where the water stayed longer (10–12 days), this trend reversed in terms of species diversity. Higher-diversity communities grew less than lower-diversity communities, probably due to a sampling effect. Specifically, the Poa pratensis monoculture (grass species) experienced the longest flooding period. This species invests heavily in dense roots (low value on PCA axis 1) and may have been a fast grower following the flood when resource availability increased. This may have been true even when growing in lower-diversity mixtures. In this case, the presence of a single species with important flood response traits may have been more important than diversity per se.
(a). Spatial resource acquisition traits
The spatial resource acquisition traits, represented by the first PCA ordination axis, were rooting depth, root length density, maximum plant height and leaf area. Our experimental design allowed us to explore an energetic trade-off between tall plants with large leaves and sparse roots at one end of the PCA axis and short plants with dense roots at the other end of the PCA axis. Had the flooding event been a stress-based disturbance, we would have expected tall plants with large leaves to be more flood tolerant [27], and therefore, more important in terms of early flood responses. We expected small-statured plants to be more sensitive to flooding stress, as their ability to maintain contact with oxygen above the flood waters is reduced [27]. However, because shorter plants were also those plants capable of investing in greater root length density, they may be more efficient in resource uptake below ground [16,28]. Thus, during a subsidy-based disturbance (such as a mild flood), communities dominated by short-stature plants with greater root length density may grow most in the weeks following the disturbance.
In terms of community mean scores, we found that early biomass change indices were highest for communities dominated by short plants with high root length density (figure 3a). We speculate that these communities may have experienced no extreme oxygen limitation and no severe physiological stress. Furthermore, as seen in previous work, flooding may increase water and nitrogen availability below ground [8]. Consequently, stress tolerance traits were less important for plant performance. Instead, rapid community growth following a resource subsidy drove the observed patterns. Community responses to some disturbances may therefore be most tightly linked to those ‘opportunistic’ species that have the capacity to access rapid influxes of nitrogen and water below ground.
(b). Phenology traits and timing strategies
Flood disturbance timing is an important determinant of species distributions within river floodplains in environments with a well-defined growing season [29]. A severe flood during the winter does not represent a disturbance when organisms are less active [30,31]. In our study, communities with a higher proportion of late-growing and late-flowering species (positively related to PCA axis 2, figure 3b) grew more than those with early-season (growing and flowering) species. Late-growing species were probably growing less and still had not begun investing in flowering structures at the time of the flood. This may have meant they were less affected by the stress of submersion. Further, the increase in resources brought from the flood might also have affected this trend. Late-season species may have been capable of taking up the influx of nutrients (associated with the flood) in the initial phases of their development, and, in comparison with the previous year (pre-flood conditions), these species may have grown more.
(c). Conclusion
Our study is one of the first reporting a negative biodiversity–stability relationship in terms of short-term plant community responses to a flooding disturbance. This response was mostly driven by an increase in biomass production in higher-diversity communities following a mild flood. Specifically, communities with a higher proportion of species with dense roots and an increased capacity to absorb below-ground resources grew more immediately following the flood. Both trends are related to the mild nature of the event: the disturbance acted as a subsidy, and probably not as a stress. Understanding the mechanisms behind these responses necessitated an exploration of both stability indices [5] and raw changes in biomass. In the face of ongoing climate change, it is essential that we have a comprehensive understanding of the drivers of ecosystem functioning following disturbances. Only then can we start to tease out a mechanistic framework for maintaining ecosystem functions and services going forward.
Supplementary Material
Acknowledgements
We thank the technical staff for their work in maintaining the field site and also many student helpers for weeding the experimental plots. F.M.F. initiated the work during an internship at the Chair of Restoration Ecology, Technische Universität München, hosted by Prof. Johannes Kollmann through the German-Brazilian network TUMBRA.
Authors' contributions
F.M.F. and A.J.W. carried out the analyses and wrote the first draft of the manuscript. N.E., A.E., C.R., H.d.K., C.W., A.W. and W.W.W. conceived of the study, designed the study, and carried out the data collection for the study. V.D.P. helped conceive of the study, revised the analyses and the final version of the manuscript. All authors gave final approval for publication.
Competing interests
The authors state that they have no competing interests for this research.
Funding
The Jena Experiment is funded by the Deutsche Forschungsgemeinschaft (FOR 1451). Further support came from the German Centre for Integrative Biodiversity Research (iDiv) Halle-Jena-Leipzig, funded by the German Science Foundation (FZT 118). F.M.F. is granted with a PhD scholarship by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Ministry of Education, Federal Government of Brazil. The German-Brazilian network TUMBRA was funded by the German Academic Exchange Service (DAAD, grant 54 417 975). V.D.P. has been supported by CNPq (grant no. 307689/2014-0).
References
- 1.Stocker TF, et al. 2013. Climate change 2013 the physical science basis working group I contribution to the fifth assessment report of the intergovernmental panel on climate change. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 2.IPCC. 2012. Managing the risks of extreme events and disasters to advance climate change adaptation. A special report of working groups I and II of the Intergovernmental Panel on Climate Change. 1st edn. Cambridge, UK, and New York, NY, USA: Cambridge University Press. [Google Scholar]
- 3.Van Ruijven J, Berendse F.. 2010. Diversity enhances community recovery, but not resistance, after drought. J. Ecol. 98, 81–86. ( 10.1111/j.1365-2745.2009.01603.x) [DOI] [Google Scholar]
- 4.Tilman D, Downing JA. 1994. Biodiversity and stability in grasslands. Nature 367, 363–365. ( 10.1038/367363a0) [DOI] [Google Scholar]
- 5.Isbell F, et al. 2015. Biodiversity increases the resistance of ecosystem productivity to climate extremes. Nature 526, 574–577. ( 10.1038/nature15374) [DOI] [PubMed] [Google Scholar]
- 6.Vervuren PJA, Blom CWPM, de Kroon H.. 2003. Extreme flooding events on the Rhine and the survival and distribution of riparian plant species. J. Ecol. 91, 135–146. ( 10.1046/j.1365-2745.2003.00749.x) [DOI] [Google Scholar]
- 7.Caplat P, Anand M.. 2009. Effects of disturbance frequency, species traits and resprouting on directional succession in an individual-based model of forest dynamics. J. Ecol. 97, 1028–1036. ( 10.1111/j.1365-2745.2009.01541.x) [DOI] [Google Scholar]
- 8.Wright AJ, et al. 2015. Flooding disturbances increase resource availability and productivity but reduce stability in diverse plant communities. Nat. Commun. 6, 6092 ( 10.1038/ncomms7092) [DOI] [PubMed] [Google Scholar]
- 9.Odum EP, Finn JT, Franz EH. 1979. Perturbation theory and the subsity–stress gradient. Bioscience 29, 349–352. ( 10.2307/1307690) [DOI] [Google Scholar]
- 10.Chapin FS III, Walker BH, Hobbs RJ, Hooper DU, Lawton JH, Sala OE, Tilman D.. 1997. Biotic control over the functioning of ecosystems. Science (80-.). 277, 500–504. ( 10.1126/science.277.5325.500) [DOI] [Google Scholar]
- 11.Wall DH. 1999. Biodiversity and ecosystem functioning: A special issue devoted to belowground biodiversity in soils and freshwater and marine sediments. Bioscience 49, 107–108. ( 10.2307/1313535) [DOI] [Google Scholar]
- 12.Díaz S, Cabido M. 2001. Vive la différence: plant functional diversity matters to ecosystem processes. Trends Ecol. Evol. 16, 646–655. ( 10.1016/S0169-5347(01)02283-2) [DOI] [Google Scholar]
- 13.Isbell F, et al. 2011. High plant diversity is needed to maintain ecosystem services. Nature 477, 199–202. ( 10.1038/nature10282) [DOI] [PubMed] [Google Scholar]
- 14.Elmqvist T, Folke C, Nyström M, Peterson G, Bengtsson J, Walker B, Norberg J.. 2003. Response diversity, ecosystem change, and resilience. Front. Ecol. Environ. 1, 488–494. ( 10.1890/1540-9295(2003)001%5B0488:RDECAR%5D2.0.CO;2) [DOI] [Google Scholar]
- 15.Yachi S, Loreau M. 1999. Biodiversity and ecosystem productivity in a fluctuating environment: the insurance hypothesis. Proc. Natl Acad. Sci. USA 96, 1463–1468. ( 10.1073/pnas.96.4.1463) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mommer L, Lenssen JPM, Huber H, Visser EJW, De Kroon H.. 2006. Ecophysiological determinants of plant performance under flooding: a comparative study of seven plant families. J. Ecol. 94, 1117–1129. ( 10.1111/j.1365-2745.2006.01175.x) [DOI] [Google Scholar]
- 17.Voesenek LACJ, Bailey-Serres J.. 2013. Flooding tolerance: O2 sensing and survival strategies. Curr. Opin. Plant Biol. 16, 647–653. ( 10.1016/j.pbi.2013.06.008) [DOI] [PubMed] [Google Scholar]
- 18.Ebeling A, et al. 2014. A trait-based experimental approach to understand the mechanisms underlying biodiversity–ecosystem functioning relationships. Basic Appl. Ecol. 15, 229–240. ( 10.1016/j.baae.2014.02.003) [DOI] [Google Scholar]
- 19.Kluge G, Müller-Westermeier G. 2000. Das Klima ausgewählter Orte der Bundesrepublik Deutschland: Jena. In Berichte des Deutschen Wetterdienstes, p. 213. [Google Scholar]
- 20.Roscher C, Schumacher J, Baade J, Wilckec W, Gleixnerd G, Weissera WW, Schmide B, Schulzed E-D. 2004. The role of biodiversity for element cycling and trophic interactions: an experimental approach in a grassland community. Basic Appl. Ecol. 121, 107–121. ( 10.1078/1439-1791-00216) [DOI] [Google Scholar]
- 21.Blöschl G, Nester T, Komma J, Parajka J, Perdigão RAP. 2013. The June 2013 flood in the Upper Danube basin, and comparisons with the 2002, 1954 and 1899 floods. Hydrol. Earth Syst. Sci. Discuss. 10, 9533–9573. ( 10.5194/hessd-10-9533-2013) [DOI] [Google Scholar]
- 22.Rao CR. 1982. Diversity and dissimilarity coefficients: a unified approach. Theor. Popul. Biol. 21, 24–43. ( 10.1016/0040-5809(82)90004-1) [DOI] [Google Scholar]
- 23.Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team 2016. nlme: linear and nonlinear mixed effects models. R package version 3.1-126, http://CRAN.R-project.org/package=nlme.
- 24.Debastiani VJ, Pillar VD. 2012. SYNCSA-R tool for analysis of metacommunities based on functional traits and phylogeny of the community components. Bioinformatics 28, 2067–2068. ( 10.1093/bioinformatics/bts325) [DOI] [PubMed] [Google Scholar]
- 25.Proulx R, et al. 2010. Diversity promotes temporal stability across levels of ecosystem organization in experimental grasslands. PLoS ONE 5, e13382 ( 10.1371/journal.pone.0013382) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chapin FS, et al. 2000. Consequences of changing biodiversity. Nature 405, 234–242. ( 10.1038/35012241) [DOI] [PubMed] [Google Scholar]
- 27.Striker GG. 2012. Flooding stress on plants: anatomical, morphological and physiological responses. In Botany (ed. Mworia J.), p. 226. Rijeka, Croatia and Shanghai, China: InTech. [Google Scholar]
- 28.Casper BB, Jackson RB. 1997. Plant competition underground. Annu. Rev. Ecol. Syst. 28, 545–570. ( 10.1146/annurev.ecolsys.28.1.545) [DOI] [Google Scholar]
- 29.van Eck WHJM, Steeg HM, van de Steeg HM, Blom CWPM, de Kroon H. 2004. Is tolerance to summer flooding correlated with distribution patterns in river floodplains? A comparative study of 20 terrestrial grassland species. Oikos 2, 393–405. ( 10.1111/j.0030-1299.2004.13083.x) [DOI] [Google Scholar]
- 30.Klimešová J. 1994. The effects of timing and duration of floods on growth of young plants of Phalaris arundinacea L. and Urtica dioica L.: an experimental study. Aquat. Bot. 48, 21–29. ( 10.1016/0304-3770(94)90071-X) [DOI] [Google Scholar]
- 31.van Eck WHJM, Lenssen JPM, van de Steeg HM, Blom CWPM, de Kroon H.. 2006. Seasonal dependent effects of flooding on plant species survival and zonation: a comparative study of 10 terrestrial grassland species. Hydrobiologia 565, 59–69. ( 10.1007/s10750-005-1905-7) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.