Abstract
Global change drivers are rapidly altering resource availability and biodiversity. While there is consensus that greater biodiversity increases the functioning of ecosystems, the extent to which biodiversity buffers ecosystem productivity in response to changes in resource availability remains unclear. We use data from 16 grassland experiments across North America and Europe that manipulated plant species richness and one of two essential resources—soil nutrients or water—to assess the direction and strength of the interaction between plant diversity and resource alteration on above-ground productivity and net biodiversity, complementarity, and selection effects. Despite strong increases in productivity with nutrient addition and decreases in productivity with drought, we found that resource alterations did not alter biodiversity–ecosystem functioning relationships. Our results suggest that these relationships are largely determined by increases in complementarity effects along plant species richness gradients. Although nutrient addition reduced complementarity effects at high diversity, this appears to be due to high biomass in monocultures under nutrient enrichment. Our results indicate that diversity and the complementarity of species are important regulators of grassland ecosystem productivity, regardless of changes in other drivers of ecosystem function.
Keywords: plant diversity, global change drivers, resource amendment, resource reduction, soil nutrients, drought
1. Introduction
Anthropogenically driven environmental change presently affects a considerable proportion of Earth's ecosystems [1] and is rapidly altering their capacity to provide the many ecosystem functions and services needed by human societies [2,3]. Global change drivers significantly impact on ecosystem functioning and biodiversity [4], which also plays a vital role in controlling ecosystem functioning [3,5]. While it is well established that increased biodiversity [6] and resource availability [7] enhance productivity, particularly in grasslands, the effect of the interaction between these factors is less well understood [4,8]. Furthermore, there is evidence to suggest that interactions between resource availability and biodiversity could be positive [9], negative [10] or non-significant [4,11,12].
High biodiversity could enhance productivity responses to increases in resource availability, e.g. increased precipitation or nutrient enrichment, through a number of mechanisms [13]. These include the greater likelihood of responsive species, e.g. nitrophilous grasses, being present and dominating diverse mixtures (selection effects) and increased resource-use efficiency for nitrogen with diversity, even in the absence of legumes [14]. This latter mechanism could lead to a more efficient exploitation of additional resources [9]. However, resource amendment may also diminish the positive effects of biodiversity. For example, where nitrogen (N) is added, legumes may decline in abundance and reduce their rates of N fixation, thus reducing their effects on resource supply [15,16]. Complementarity effects operating via nutrient-based niche differentiation, e.g. differing nutrient foraging strategies [17], could also be lost if resource limitation shifts to another resource for which complementarity effects are weaker, e.g. other soil nutrients [18], light [19], CO2 [20] or water [21]. Furthermore, under ambient conditions the availability of growth-limiting resources changes along plant diversity gradients. In three long-term grassland diversity experiments, N mineralization rates increased with plant species diversity [22–25], potentially lowering nutrient limitation and leading to a weaker response to nutrient addition in high-diversity communities.
Where there is a reduction in resource availability, biodiversity can buffer productivity declines via a number of mechanisms. These include the greater likelihood of tolerant species being present (insurance effects of biodiversity; e.g. [26]), which maintain productivity in periods of drought or nutrient limitation [27]. Furthermore, high-diversity communities may be more likely to contain species that are able to access scarce resources during periods of stress, e.g. by accessing water from deeper soil layers [28] or nutrients from different depths in the rhizosphere [29]. While such mechanisms might buffer decreases of productivity in high-diversity communities on a proportional scale, as predicted by biodiversity–stability theory [30] and confirmed in experimental studies [11,31], more diverse communities might suffer greater losses in productivity on an absolute scale, because they have more biomass to lose [10].
Given this wide range of possible interactions between biodiversity and resource availability on productivity, we examined the net balance of these mechanisms by measuring the strength and direction of the interaction between plant species richness and resource availability. This was achieved by performing a meta-level analysis, which is distinct from meta-analysis in that we used raw data and did not calculate effect sizes [32,33]. This was done using data from 16 experimental grassland studies, where plant species richness and resource availability were orthogonally manipulated. In all cases, resource reduction took the form of water reduction, i.e. drought, while resource amendment was nutrient addition. Secondly, we quantified net biodiversity, complementarity and selection effects [34] within treatments to test the extent to which diversity effects were altered by plant species richness, resource availability and their interaction.
2. Material and methods
(a). Data collection and preparation
We created our database by consulting recently published meta-analyses on biodiversity–ecosystem function relationships [4,35,36]. We identified three additional studies (A. Hector, D. Deutschman, S. Levin & S. Pacala, unpublished; C.K.M. Palmborg, unpublished; C. Rosher & A. Siebenkäs, unpublished) that met our selection criteria. These were grassland experiments across Europe and North America that crossed a sown (or planted) plant species richness gradient with global-change drivers that increased or reduced resource availability. Given the low number of experiments that fully crossed sown plant diversity with other global-change drivers, such as CO2 enrichment or temperature, we further narrowed our selection criteria to only include studies that increased resource availability by adding nutrients or decreased resource availability by reducing water availability, thus ensuring comparability of studies. We obtained datasets from 16 experiments (6 drought, 10 nutrient addition; electronic supplementary material, appendix 1 and table S1). Hector et al. unpublished was treated as two independent studies because sown plant species richness was independently crossed with nutrient addition and drought treatments. In the selected experiments, nutrients were added once per year as NPK fertilizer or ammonium nitrate (NH4NO3) and water availability was generally reduced using rain-out shelters (electronic supplementary material, table S1).
In total, the nutrient addition dataset comprises observations from 1199 plots (n = 4032), and the drought dataset consists of 788 plots (n = 2150). All observations are of above-ground plant productivity (g m−2) for each plot in each year of each experiment (electronic supplementary material, table S1 and figures S1 and S2). For experiments that increased nutrient availability, we used peak biomass (a proxy for ANPP), while for experiments that reduced water availability, we used post-drought biomass harvested immediately (usually within one week) following the termination of experimental drought treatments.
We calculated net biodiversity, complementarity and selection effects for the studies that recorded species level biomass (six nutrient addition studies and three drought studies; electronic supplementary material, table S1), following Loreau & Hector [34]. This subset of studies exhibited qualitatively similar above-ground productivity responses to both plant species richness and drought or nutrient addition using the complete dataset (electronic supplementary material, table S2 and figure S3). As the experimental treatments were also expected to drive variation in above-ground productivity, we standardized net diversity, complementarity and selection effects by the mean monoculture biomass of the corresponding treatment to compare the strengths of these effects across treatments. The standardized net diversity effect is the difference between the observed and expected above-ground productivity of a mixture, i.e. 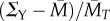 , where ΣY is the observed yield,
, where ΣY is the observed yield, 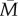 is the average above-ground productivity in a monoculture of each species in a plot, and
is the average above-ground productivity in a monoculture of each species in a plot, and 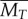 is the mean monoculture biomass of the treatment. The standardized complementarity effect reflects the extent to which species produce more (or less) biomass in a mixture than in a monoculture:
is the mean monoculture biomass of the treatment. The standardized complementarity effect reflects the extent to which species produce more (or less) biomass in a mixture than in a monoculture: 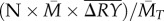 , where N is the number of species and
, where N is the number of species and 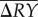 is
is 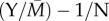 . The standardized selection effect is
. The standardized selection effect is 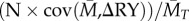 and describes whether species with high yields in monocultures also dominate in mixtures. Species with a mean monoculture biomass less than 2.5 g m−2 in a given year were excluded because relative yield can approach infinity when monoculture biomass values are close to zero [37].
and describes whether species with high yields in monocultures also dominate in mixtures. Species with a mean monoculture biomass less than 2.5 g m−2 in a given year were excluded because relative yield can approach infinity when monoculture biomass values are close to zero [37].
(b). Data analysis
For nutrient addition and drought studies, we fitted separate linear mixed-effects models that test for the effects of plant species richness, treatment and the interaction of the two on above-ground productivity. Plant species richness was the natural log of sown plant species richness and treatment was a binary variable (0, control; 1, treatment). Random effects were included for a study factor, interactions of study with plant species richness, treatment, time (year of experiment) and their interactions, and a plot (within study) term. We accounted for repeated measurements within plots by using a first-order autoregressive covariance structure, which fitted the data better than a compound symmetry covariance structure based on the Akaike information criterion. To test for the effects of plant species richness, treatment and the interaction of the two on net biodiversity, complementarity and selection effects, we fitted separate linear mixed-effects models for nutrient addition and drought studies. For nutrient addition studies, we used the same model structure described above; for drought studies, we simplified the aforementioned model due to model convergence issues. The simplified model contained the same fixed- and random-effects structure as the previous models, but did not include a temporal correlation covariance structure. Above-ground productivity was square-root transformed to meet model assumptions, which were checked by visually inspecting residual plots for homogeneity and quantile–quantile plots for normality. Models were fitted using the asreml function in the asreml package in R, and Wald tests and variance components were extracted using the test.asreml function in the pascal package (electronic supplementary material, appendix S2).
We performed sensitivity analyses to test whether results differed when excluding either low- (sown plant species richness = 1) or high-diversity communities (sown plant species richness greater than 20). To do so, we fitted the linear mixed-effects models to both subsets for each type of resource alteration as described above. For nutrient addition studies, we also tested the sensitivity of our results to the type of fertilizer used (NPK or NH4NO3) by fitting a linear mixed effects model where treatment changed to a variable with three levels (0, control; 1, NPK; 2, NH4NO3). Additionally, we assessed the robustness of our results to covariates: for nutrient addition experiments, the amount of nutrients added (N g m−2; electronic supplementary material, table S1), and for water reduction experiments, drought duration. The latter was used as a proxy for drought severity because of its significantly negative correlation with plant productivity across biomes [38]. Drought duration was strongly right-skewed and nonlinear because most studies excluded precipitation for fewer than 60 days, with the exception of two studies that did so for more than 130 days (electronic supplementary material, table S1). Thus, drought duration was treated as a categorical variable, where short droughts were those lasting less than 60 days and long droughts lasted longer than 60 days. We tested the influence of the above-mentioned covariates by fitting separate linear mixed effects models where a fixed effect for nutrient addition or drought duration and the corresponding interactions with plant species richness and treatment were added to the original models. All analyses were performed in R v. 3.2 [39].
3. Results
(a). Above-ground productivity
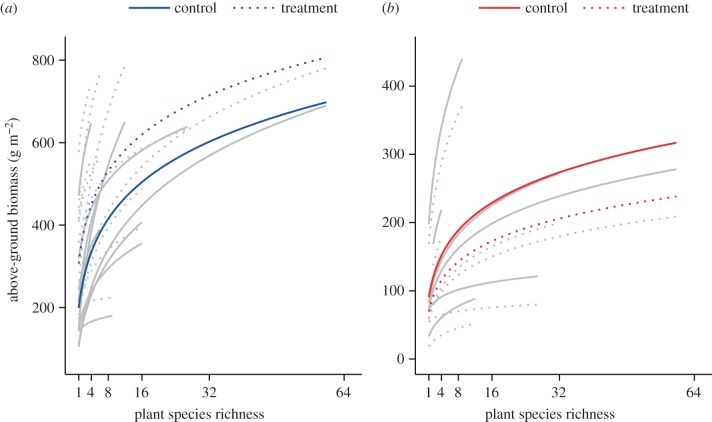
For both types of resource alteration, above-ground productivity varied significantly by plant species richness and treatment but there was no significant interaction between the two (table 1). Productivity increased significantly with plant species richness across both nutrient addition and drought studies (figure 1), yet exhibited contrasting responses to each treatment: productivity increased with nutrient addition and decreased with drought (on log-square root scale; table 2). While the effects of plant species richness and treatment were consistent among studies (see variance components, table 1), the interaction between plant species richness and resource alteration was highly variable among studies for both types of resource alteration (table 1). Among studies, productivity also varied strongly across experimental years for both types of resource alteration (table 1).
Table 1.
Fixed effects and variance component estimates (standard error) for linear mixed-effects models of above-ground productivity response to plant species richness and nutrient addition or drought.
| nutrient addition | drought | |
|---|---|---|
| fixed effects | ||
| intercept | F1,8.5 = 141.1*** | F1,5.1 = 46.93*** |
| species richness | F1,8.1 = 29.29*** | F1,4.2 = 17.13* |
| treatment | F1,7.9 = 18.13** | F1,3.8 = 19.08* |
| species richness × treatment | F1,801.9 = 1.29 | F1,309 = 1.02 |
| variance components | ||
| study | 14.69 (9.07) | 9.66 (7.10) |
| study × species richness | 2.11 (1.25) | 0.94 (0.77) |
| study × treatment | 1.97 (1.27) | 0.08 (0.22) |
| study × species richness × treatment | 0.000002 (0.00000006)a | 0.000002 (0.00000008)a |
| study × time | 4.20 (1.35)a | 2.92 (1.21)a |
| Plot | 13.86 (1.08)a | 11.50 (0.99)a |
| temporal autocorrelation | ||
| ρAR(1) | 0.10 (0.03)a | 0.05 (0.05) |
*p < 0.05; **p < 0.01; ***p < 0.001.
aThe z ratio of the variance component is greater than 1.96. Above-ground productivity (square-root transformed for analysis) is the response variable for both models. Species richness is the number of sown plant species (natural-log transformed), Treatment is a factor where 0 is Control and 1 is Treatment (either nutrient addition or drought) and Time is the experimental year. Fixed effects were tested sequentially. Kenward–Roger approximations are given for denominator degrees of freedom.
Figure 1.
Plant species richness effects on productivity in response to (a) nutrient addition or (b) drought. Lines are mixed-effects model fits for each treatment within each study (light grey lines) or for each treatment across all studies (black lines). Solid lines refer to Control and dashed lines correspond to Treatment, where nutrient or water availability was experimentally manipulated. (Online version in colour.)
Table 2.
Fixed effects estimates and 95% CIs (on log-square root scale) for linear mixed effects models of above-ground productivity response to plant species richness and nutrient addition or drought.
| nutrient addition | drought | |
|---|---|---|
| fixed effects | ||
| intercept | 14.12 (11.32, 16.91) | 9.52 (6.77, 12.27) |
| species richness | 3.01 (1.94, 4.08) | 2.03 (1.10, 2.96) |
| treatment | 3.39 (1.87, 4.91) | −1.18 (−2.13, −0.23) |
| species richness × treatment | −0.34 (−0.94, 0.25) | −0.29 (−0.86, 0.28) |
Results from the sensitivity analyses, in which low- or high-diversity communities were excluded, were in agreement with the results derived from full datasets for both types of resource alteration (electronic supplementary material, tables S3 and S4). In all cases, productivity increased significantly with increasing plant species richness and varied significantly by treatment; but the interaction of plant species richness and treatment did not have significant effects on productivity. When accounting for the type of fertilizer (NPK or NH4NO3), we found similar results to the main analysis (i.e. figure 1a and table 1); above-ground productivity varied significantly with plant species richness (F1, 8 = 30.05, p = 0.0006) and treatment (F2, 7.2 = 14.74, p = 0.003) but there was no significant interaction between the two fixed effects (p > 0.10). Lastly, including the amount of nutrients added or drought duration as covariates did not explain significant amounts of variation in above-ground productivity (p > 0.10), nor did they alter the significant effects of either plant species richness or treatment on above-ground productivity.
(b). Net diversity, complementarity and selection effects
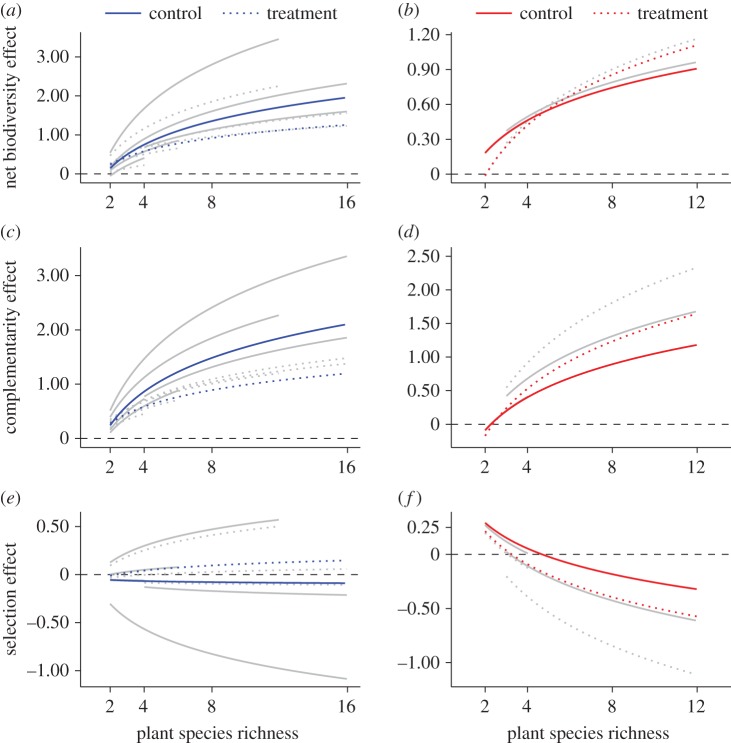
With increasing plant species richness, net biodiversity and complementarity effects increased significantly in both nutrient addition and drought studies (figure 2; electronic supplementary material, table S5a and S5b). For nutrient addition studies, but not drought studies, the effect of plant species richness on net biodiversity and complementarity effects was significantly greater under control conditions than under nutrient enrichment (see interaction of plant species richness × treatment; electronic supplementary material, table S5a). Selection effects were not significantly reduced by plant species richness, treatment or their interaction in either nutrient addition or drought studies. The effects of plant species richness and the interaction between plant species richness and treatment on net biodiversity, complementarity and selection effects were consistent among nutrient addition studies (see variance components; electronic supplementary material, table S5a), as were those of plant species richness on net biodiversity, complementarity and selection effects among drought studies (electronic supplementary material, table S5b). However, the effects of resource alteration on net biodiversity, complementarity and selection effects among both nutrient addition and drought studies were highly variable.
Figure 2.
Plant species richness effects on net biodiversity, complementarity and selection effects in response to (a,c,e) nutrient addition and (b,d,f) drought. Lines are mixed-effects model fits for each treatment within each study (light grey lines) or for each treatment across all studies (black lines). Solid lines refer to Control and dashed lines correspond to Treatment, where nutrient or water availability was experimentally manipulated. Net biodiversity, complementarity and selection effects are dimensionless because they were standardized by the mean monoculture biomass of their corresponding treatment. (Online version in colour.)
4. Discussion
(a). Effects of diversity on productivity
In our meta-level study, we found that plant diversity increased ecosystem productivity irrespective of nutrient or water availability. Our results suggest that nutrient enrichment and drought did not alter the strength or the direction of biodiversity–ecosystem function relationships in grassland plant communities despite significantly increasing and reducing productivity, respectively. This finding is consistent with previous studies that have found that positive biodiversity–ecosystem function relationships are robust to intensive management activities of grasslands, such as fertilization and mowing frequency [40,41], although function could still be impaired by biodiversity loss caused by fertilization and land use intensification [42–44]. These relationships appear to be driven primarily by increases in complementarity effects along plant species richness gradients, which were reduced by nutrient addition yet not affected by drought. Our results highlight the essential role of plant diversity in maintaining grassland productivity, which could be compromised by anthropogenic activities associated with biodiversity loss [45].
(b). Effects of diversity and nutrient addition
Contrary to expectations that high-diversity communities may exploit additional resources to a greater extent than low-diversity communities [9], we found that changes in productivity caused by nutrient addition were constant along plant diversity gradients. Our results suggest that high-diversity communities failed to take advantage of nutrient enrichment due to its impact on complementarity, which was lower than in high-diversity communities under ambient conditions. The reduction of complementarity effects by nutrient enrichment probably reflects that above-ground productivity in monocultures was high under nutrient enrichment. This, as well as the non-significant reductions in selection effects under ambient conditions, contributed to the significant interaction of plant species richness and nutrient enrichment for net biodiversity effects, which was not observed for above-ground productivity. Nutrient enrichment probably reduces N inputs from legumes by decreasing N fixation and legume abundance [15,16,46], thus dampening the overall response of productivity to nutrient addition. Furthermore, our results suggest that diverse nutrient-uptake strategies that facilitate nutrient acquisition during different periods of the growing season [47] and from alternate sources and soil depths [28,29,48] probably become redundant when grassland communities are subjected to chronic nutrient enrichment. Thus, our results indicate that nutrient enrichment alters a crucial mechanism that maintains biodiversity. In the long term and in naturally assembled communities, the loss of this mechanism may result in the loss of species richness. Because biodiversity–productivity relationships are consistently positive, this in turn could offset some of the productivity gains made by increasing N availability [43,49,50].
(c). Effects of diversity and drought
Irrespective of water availability, above-ground productivity increased significantly with plant diversity. This result is unexpected because drought is usually expected to strongly reduce above-ground productivity of high-diversity communities due to their greater overall biomass [27,51], as greater evapotranspiration increases vulnerability to drought [52], a weakening of complementarity effects, or the presence of species or plant functional groups that are sensitive to drought, such as legumes [10]. However, the non-significant effect of the interaction of plant species richness and drought on above-ground productivity suggests that such mechanisms were not operating. That drought did not affect net biodiversity or complementarity effects along plant species richness gradients supports the idea that diversity effects on above-ground productivity are robust to reductions in water availability. Repeated experimental droughts may select for specific, ‘conservative’ plant traits [53] that enhance drought tolerance [54,55] yet are more strongly correlated with survival than productivity [56]. In other words, while shifts in functional composition may occur in response to drought, they may not translate directly into changes in productivity [57]. Although grassland communities lose biomass during experimental droughts, they may conserve vital ecosystem functions by becoming progressively more tolerant to future droughts due to shifts in functional composition.
(d). Variability across studies and underlying mechanisms
In this study, we found that the effect of the interaction between diversity and both types of resource alterations—nutrient enrichment and drought—on above-ground productivity was not significant. Non-significant interaction effects do not indicate that the hypothesized mechanisms were not operating (as indicated by our analysis of net biodiversity, complementarity and selection effects), but rather that they produced a response that was neutral across studies. The strong variation of site-level responses, as evidenced by the large variation in the effect of the interaction between plant diversity and treatment on above-ground productivity among studies, suggests that the relative balance of these mechanisms varied between studies, possibly due to differences in experimental design, site-level variation in resource limitations, and the adaptation of the local species pool to these limitations.
Lastly, environmental conditions, such as soil nutrient pools and precipitation, also varied over time and could have altered the realized magnitude of experimental treatments. Future research should therefore investigate the cause and importance of between-site variation in determining the interaction between resource availability and diversity, as it is possible that interactions could be stronger in certain ecosystems. This question may be best addressed using standardized methodology, e.g. by using global networks of standardized experiments that enable sources of across-site variation in environmental conditions to be quantified precisely [50,58,59].
5. Conclusion
The present results show that the positive effects of biodiversity on above-ground productivity are robust to resource alterations. This finding is consistent with other recent meta-analyses, which have found that the interaction between diversity and other drivers of ecosystem function is surprisingly weak in determining a range of ecosystem functions and properties, such as decomposition and soil microbial biomass carbon [4,12]. Importantly, our results revealed that one of the key mechanisms underlying biodiversity–ecosystem functioning relationships—complementarity—is not equally sensitive to nutrient enrichment and drought. Thus, the disruption of complementarity effects appears to be one of the key mechanisms that propagate changes in ecosystem dynamics in natural grasslands affected by chronic resource alterations such as N deposition [43,49,60]. By contrast, pulse disturbances such as drought may not alter the strength of diversity effects and, by extension, their ability to de-stabilize ecosystem functioning [11]. Together these results suggest that, while resource availability strongly impacts biodiversity, the relationship between biodiversity and ecosystem function is largely unaffected by resource availability, and, therefore, that biodiversity is a strong regulator of ecosystem function across a wide range of environmental conditions.
Supplementary Material
Acknowledgements
The authors would like to thank Marten Winter and sDiv staff for their support, Christian Rixen for contributing data, and Kevin Mueller and Wolfgang Weisser for their contributions during our workshop.
Data accessibility
All sources included in this meta-level analysis are listed in the electronic supplementary material.
Authors' contributions
F.I. and N.E. conceived the project; D.C., F.I., P.M., H.B., A.E., C.R., A.W., B.W., J.C., J.G., Y.H., A.H., M.L., S.T.M., A.S.M., S.N., E.S.,M.T., J.vR. and W.W. developed the project during a workshop; C.R., A.S., A.W., B.W., C.B., E.dL., A.H., A.J., J.K., V.L., C.P., H.W.P., P.R., B.S., A.S., D.T. and A.V. contributed experimental data; D.C. assembled and analysed the data with substantial input from F.I., N.E., J.C., P.M. and J.vR.; and D.C., P.M., N.E. and F.I. wrote the paper with input from all authors.
Competing interests
The authors have no competing interests.
Funding
This paper is a joint effort of the STABILITY group funded by sDiv (www.idiv.de/stability), the Synthesis Centre of the German Centre for Integrative Biodiversity Research (iDiv) Halle-Jena-Leipzig (DFG FZT 118). M.L. was supported by the TULIP Laboratory of Excellence (ANR-10-LABX-41).
References
- 1.Barnosky AD, et al. 2012. Approaching a state shift in Earth's biosphere. Nature 486, 52–58. ( 10.1038/nature11018) [DOI] [PubMed] [Google Scholar]
- 2.Cardinale BJ, et al. 2012. Biodiversity loss and its impact on humanity. Nature 486, 59–67. ( 10.1038/nature11148) [DOI] [PubMed] [Google Scholar]
- 3.Brose U, Hillebrand H. 2016. Biodiversity and ecosystem functioning in dynamic landscapes. Phil. Trans. R. Soc. B 371, 20150267 (doi:10.1098.rstb.2015.0267) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hooper DU, et al. 2012. A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature 486, 105–108. ( 10.1038/nature11118) [DOI] [PubMed] [Google Scholar]
- 5.Tilman D, Isbell F, Cowles JM. 2014. Biodiversity and ecosystem functioning. Annu. Rev. Ecol. Evol. Syst. 45, 471–493. ( 10.1146/annurev-ecolsys-120213-091917) [DOI] [Google Scholar]
- 6.Hector A, et al. 1999. Plant diversity and productivity experiments in european grasslands. Science 286, 1123–1127. ( 10.1126/science.286.5442.1123) [DOI] [PubMed] [Google Scholar]
- 7.Lee M, Manning P, Rist J, Power SA, Marsh C. 2010. A global comparison of grassland biomass responses to CO2 and nitrogen enrichment. Phil. Trans. R. Soc. B 365, 2047–2056. ( 10.1098/rstb.2010.0028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith MD, Knapp AK, Collins SL. 2009. A framework for assessing ecosystem dynamics in response to chronic resource alterations induced by global change. Ecology 90, 3279–3289. ( 10.1890/08-1815.1) [DOI] [PubMed] [Google Scholar]
- 9.Reich PB, et al. 2001. Plant diversity enhances ecosystem responses to elevated CO2 and nitrogen deposition. Nature 410, 809–810. ( 10.1038/35071062) [DOI] [PubMed] [Google Scholar]
- 10.Pfisterer AB, Schmid B. 2002. Diversity-dependent production can decrease the stability of ecosystem functioning. Nature 416, 84–86. ( 10.1038/416084a) [DOI] [PubMed] [Google Scholar]
- 11.Isbell F, et al. 2015. Biodiversity increases the resistance of ecosystem productivity to climate extremes. Nature 526, 574–577. ( 10.1038/nature15374) [DOI] [PubMed] [Google Scholar]
- 12.Thakur MP, et al. 2015. Plant diversity drives soil microbial biomass carbon in grasslands irrespective of global environmental change factors. Glob. Change Biol 21, 4076–4085. ( 10.1111/gcb.13011) [DOI] [PubMed] [Google Scholar]
- 13.Wright AJ, et al. 2015. Flooding disturbances increase resource availability and productivity but reduce stability in diverse plant communities. Nat. Commun. 6, 6092 ( 10.1038/ncomms7092) [DOI] [PubMed] [Google Scholar]
- 14.van Ruijven J, Berendse F. 2005. Diversity–productivity relationships: Initial effects, long-term patterns, and underlying mechanisms. Proc. Natl Acad. Sci. USA 102, 695–700. ( 10.1073/pnas.0407524102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suding KN, Collins SL, Gough L, Clark C, Cleland EE, Gross KL, Milchunas DG, Pennings S. 2005. Functional- and abundance-based mechanisms explain diversity loss due to N fertilization. Proc. Natl Acad. Sci. USA 102, 4387–4392. ( 10.1073/pnas.0408648102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartwig UA. 1998. The regulation of symbiotic N2 fixation: a conceptual model of N feedback from the ecosystem to the gene expression level. Persp. Plant Ecol. Evol. Syst. 1, 92–120. ( 10.1078/1433-8319-00054) [DOI] [Google Scholar]
- 17.McKane RB, et al. 2002. Resource-based niches provide a basis for plant species diversity and dominance in arctic tundra. Nature 415, 68–71. ( 10.1038/415068a) [DOI] [PubMed] [Google Scholar]
- 18.Harpole WS, et al. 2011. Nutrient co-limitation of primary producer communities. Ecol. Lett. 14, 852–862. ( 10.1111/j.1461-0248.2011.01651.x) [DOI] [PubMed] [Google Scholar]
- 19.Hautier Y, Niklaus PA, Hector A. 2009. Competition for light causes plant biodiversity loss after eutrophication. Science 324, 636–638. ( 10.1126/science.1169640) [DOI] [PubMed] [Google Scholar]
- 20.Reich PB, Hobbie SE, Lee T, Ellsworth DS, West JB, Tilman D, Knops JMH, Naeem S, Trost J. 2006. Nitrogen limitation constrains sustainability of ecosystem response to CO2. Nature 440, 922–925. ( 10.1038/nature04486) [DOI] [PubMed] [Google Scholar]
- 21.Sterck F, Markesteijn L, Schieving F, Poorter L. 2011. Functional traits determine trade-offs and niches in a tropical forest community. Proc. Natl Acad. Sci. USA 108, 20627. ( 10.1073/pnas.1106950108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oelmann Y, et al. 2011. Plant diversity effects on aboveground and belowground N pools in temperate grassland ecosystems: development in the first 5 years after establishment. Glob. Biogeochem. Cycles 25, GB2014 ( 10.1029/2010GB003869) [DOI] [Google Scholar]
- 23.Reich PB, Tilman D, Isbell F, Mueller K, Hobbie SE, Flynn DFB, Eisenhauer N. 2012. Impacts of biodiversity loss escalate through time as redundancy fades. Science 336, 589–592. ( 10.1126/science.1217909) [DOI] [PubMed] [Google Scholar]
- 24.Cong W-F, van Ruijven J, Mommer L, De Deyn GB, Berendse F, Hoffland E. 2014. Plant species richness promotes soil carbon and nitrogen stocks in grasslands without legumes. J. Ecol. 102, 1163–1170. ( 10.1111/1365-2745.12280) [DOI] [Google Scholar]
- 25.Fornara DA, Tilman D. 2009. Ecological mechanisms associated with the positive diversity–productivity relationship in an N-limited grassland. Ecology 90, 408–418. ( 10.1890/08-0325.1) [DOI] [PubMed] [Google Scholar]
- 26.Hector A, et al. 2010. General stabilizing effects of plant diversity on grassland productivity through population asynchrony and overyielding. Ecology 91, 2213–2220. ( 10.1890/09-1162.1) [DOI] [PubMed] [Google Scholar]
- 27.Van Ruijven J, Berendse F. 2010. Diversity enhances community recovery, but not resistance, after drought. Journal of Ecology 98, 81–86. ( 10.1111/j.1365-2745.2009.01603.x) [DOI] [Google Scholar]
- 28.Mueller KE, Tilman D, Fornara DA, Hobbie SE. 2012. Root depth distribution and the diversity–productivity relationship in a long-term grassland experiment. Ecology 94, 787–793. ( 10.1890/12-1399.1) [DOI] [Google Scholar]
- 29.Dimitrakopoulos PG, Schmid B. 2004. Biodiversity effects increase linearly with biotope space. Ecol. Lett. 7, 574–583. ( 10.1111/j.1461-0248.2004.00607.x) [DOI] [Google Scholar]
- 30.Yachi S, Loreau M. 1999. Biodiversity and ecosystem productivity in a fluctuating environment: the insurance hypothesis. Proc. Natl Acad. Sci. USA 96, 1463–1468. ( 10.1073/pnas.96.4.1463) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tilman D, Downing JA. 1994. Biodiversity and stability in grasslands. Nature 367, 363–365. ( 10.1038/367363a0) [DOI] [Google Scholar]
- 32.Vetter D, Rücker G, Storch I. 2013. Meta-analysis: a need for well-defined usage in ecology and conservation biology. Ecosphere 4, 1–24. ( 10.1890/ES13-00062.1) [DOI] [Google Scholar]
- 33.Koricheva J, Gurevitch J. 2014. Uses and misuses of meta-analysis in plant ecology. J. Ecol. 102, 828–844. ( 10.1111/1365-2745.12224) [DOI] [Google Scholar]
- 34.Loreau M, Hector A. 2001. Partitioning selection and complementarity in biodiversity experiments. Nature 412, 72–76. ( 10.1038/35083573) [DOI] [PubMed] [Google Scholar]
- 35.Cardinale BJ, Matulich KL, Hooper DU, Byrnes JE, Duffy E, Gamfeldt L, Balvanera P, Connor MIO, Gonzalez A. 2011. The functional role of producer diversity in ecosystems. Am. J. Bot. 98, 1–21. ( 10.3732/ajb.1000364) [DOI] [PubMed] [Google Scholar]
- 36.Cardinale BJ, Wright JP, Cadotte MW, Carroll IT, Hector A, Srivastava DS, Loreau M, Weis JJ. 2007. Impacts of plant diversity on biomass production increase through time because of species complementarity. Proc. Natl Acad. Sci. USA 104, 18 123–18 128. ( 10.1073/pnas.0709069104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fargione J, Tilman D, Dybzinski R, Lambers JHR, Clark C, Harpole WS, Knops JMH, Reich PB, Loreau M. 2007. From selection to complementarity: shifts in the causes of biodiversity–productivity relationships in a long-term biodiversity experiment. Phil. Trans. R. Soc. B 274, 871–876. ( 10.1098/rspb.2006.0351) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vicente-Serrano SM, et al. 2013. Response of vegetation to drought time-scales across global land biomes. Proc. Natl Acad. Sci. USA 110, 52–57. ( 10.1073/pnas.1207068110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Team RC. 2014. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; (http://cran.r-project.org) [Google Scholar]
- 40.Weigelt A, Weisser WW, Buchmann N, Scherer-Lorenzen M. 2009. Biodiversity for multifunctional grasslands: equal productivity in high-diversity low-input and low-diversity high-input systems. Biogeosciences 6, 1695–1706. ( 10.5194/bg-6-1695-2009) [DOI] [Google Scholar]
- 41.Nyfeler D, Huguenin-Elie O, Suter M, Frossard E, Connolly J, Lüscher A. 2009. Strong mixture effects among four species in fertilized agricultural grassland led to persistent and consistent transgressive overyielding. J. Appl. Ecol. 46, 683–691. ( 10.1111/j.1365-2664.2009.01653.x) [DOI] [Google Scholar]
- 42.Soliveres S, et al. 2016. Locally rare species influence grassland ecosystem multifunctionality. Phil. Trans. R. Soc. B 371, 20150269 (doi:10.1098.rstb.2015.0269) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Isbell F, Reich PB, Tilman D, Hobbie SE, Polasky S, Binder S. 2013. Nutrient enrichment, biodiversity loss, and consequent declines in ecosystem productivity. Proc. Natl Acad. Sci. USA 110, 11 911–11 916. ( 10.1073/pnas.1310880110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Allan E, et al. 2015. Land use intensification alters ecosystem multifunctionality via loss of biodiversity and changes to functional composition. Ecol. Lett. 18, 834–843. ( 10.1111/ele.12469) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sala OE, et al. 2000. Global biodiversity scenarios for the year 2100. Science 287, 1770–1774. ( 10.1126/science.287.5459.1770) [DOI] [PubMed] [Google Scholar]
- 46.Strecker T, Barnard RL, Niklaus PA, Scherer-Lorenzen M, Weigelt A, Scheu S, Eisenhauer N. 2015. Effects of plant diversity, functional group composition, and fertilization on soil microbial properties in experimental grassland. PLoS ONE 10, e0125678 ( 10.1371/journal.pone.0125678) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fargione J, Tilman D. 2005. Niche differences in phenology and rooting depth promote coexistence with a dominant C4 bunchgrass. Oecologia 143, 598–606. ( 10.1007/s00442-005-0010-y) [DOI] [PubMed] [Google Scholar]
- 48.Oelmann Y, Richter AK, Roscher C, Rosenkranz S, Temperton VM, Weisser WW, Wilcke W. 2011. Does plant diversity influence phosphorus cycling in experimental grasslands? Geoderma 167–168, 178–187. ( 10.1016/j.geoderma.2011.09.012) [DOI] [Google Scholar]
- 49.Hautier Y, Tilman D, Isbell F, Seabloom EW, Borer ET, Reich PB. 2015. Anthropogenic environmental changes affect ecosystem stability via biodiversity. Science 348, 336–340. ( 10.1126/science.aaa1788) [DOI] [PubMed] [Google Scholar]
- 50.La Pierre KJ, Blumenthal DM, Brown CS, Klein JA, Smith MD. 2016. Drivers of variation in aboveground net primary productivity and plant community composition differ across a broad precipitation gradient. Ecosystems 19, 1–13. ( 10.1007/s10021-015-9949-7) [DOI] [Google Scholar]
- 51.Tilman D, Lehman CL, Bristow CE. 1998. Diversity–stability relationships: statistical inevitability or ecological consequence? Am. Nat. 151, 277–282. ( 10.1086/286118) [DOI] [PubMed] [Google Scholar]
- 52.Verheyen K, Bulteel H, Palmborg C, Olivié B, Nijs I, Raes D, Muys B. 2008. Can complementarity in water use help to explain diversity–productivity relationships in experimental grassland plots? Oecologia 156, 351–361. ( 10.1007/s00442-008-0998-x) [DOI] [PubMed] [Google Scholar]
- 53.Reich PB. 2014. The world-wide ‘fast–slow’ plant economics spectrum: a traits manifesto. J. Ecol. 102, 275–301. ( 10.1111/1365-2745.12211) [DOI] [Google Scholar]
- 54.Craine JM, Ocheltree TW, Nippert JB, Towne EG, Skibbe AM, Kembel SW, Fargione JE. 2013. Global diversity of drought tolerance and grassland climate-change resilience. Nat. Clim. Change 3, 63–67. ( 10.1038/nclimate1634) [DOI] [Google Scholar]
- 55.Choat B, et al. 2012. Global convergence in the vulnerability of forests to drought. Nature 491, 752–755. ( 10.1038/nature11688) [DOI] [PubMed] [Google Scholar]
- 56.Wright SJ, et al. 2010. Functional traits and the growth-mortality trade-off in tropical trees. Ecology 91, 3664–3674. ( 10.1890/09-2335.1) [DOI] [PubMed] [Google Scholar]
- 57.Ziter C, Bennett EM, Gonzalez A. 2014. Temperate forest fragments maintain aboveground carbon stocks out to the forest edge despite changes in community composition. Oecologia 176, 893–902. ( 10.1007/s00442-014-3061-0) [DOI] [PubMed] [Google Scholar]
- 58.Fraser LH, et al. 2012. Coordinated distributed experiments: an emerging tool for testing global hypotheses in ecology and environmental science. Front. Ecol. Environ. 11, 147–155. ( 10.1890/110279) [DOI] [Google Scholar]
- 59.Grace JB, et al. 2016. Integrative modelling reveals mechanisms linking productivity and plant species richness. Nature 529, 390–393. ( 10.1038/nature16524) [DOI] [PubMed] [Google Scholar]
- 60.Smith M, et al. 2015. Global environmental change and the nature of aboveground net primary productivity responses: insights from long-term experiments. Oecologia 177, 935–947. ( 10.1007/s00442-015-3230-9) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All sources included in this meta-level analysis are listed in the electronic supplementary material.