Abstract
Predicting ecosystem functioning at large spatial scales rests on our ability to scale up from local plots to landscapes, but this is highly contingent on our understanding of how functioning varies through space. Such an understanding has been hampered by a strong experimental focus of biodiversity–ecosystem functioning research restricted to small spatial scales. To address this limitation, we investigate the drivers of spatial variation in multitrophic energy flux—a measure of ecosystem functioning in complex communities—at the landscape scale. We use a structural equation modelling framework based on distance matrices to test how spatial and environmental distances drive variation in community energy flux via four mechanisms: species composition, species richness, niche complementarity and biomass. We found that in both a tropical and a temperate study region, geographical and environmental distance indirectly influence species richness and biomass, with clear evidence that these are the dominant mechanisms explaining variability in community energy flux over spatial and environmental gradients. Our results reveal that species composition and trait variability may become redundant in predicting ecosystem functioning at the landscape scale. Instead, we demonstrate that species richness and total biomass may best predict rates of ecosystem functioning at larger spatial scales.
Keywords: β-diversity, niche complementarity, energy flux, functional diversity, litter invertebrates, multitrophic
1. Introduction
Global declines in biodiversity resulting from anthropogenic disturbance have stimulated widespread concern over the associated loss of ecosystem functioning and services provided by natural systems [1,2]. In the past two decades, a considerable effort has been made to understand the mechanisms that drive rates in ecosystem functioning, with an especially large focus on the importance of biodiversity [1,3,4]. Most of this research has emerged from experimental studies that attempt to directly link species richness with ecosystem processes at the local scale. Yet, owing to the largely experimental nature of the research that has developed in this field so far, little is known about the mechanisms driving landscape-scale patterns in ecosystem functioning of complex, natural ecosystems [1,4–6].
The importance of spatial context in biodiversity and ecosystem functioning (BEF) research has been increasingly realized in recent years [7,8]. For example, France & Duffy [9] demonstrated that metacommunity structure and dispersal were highly important for maintaining rates and temporal stability of productivity. This and many other studies [3], however, focused primarily on the role of species richness for determining ecosystem functioning in a relatively simple system limited to very few species and trophic levels. In comparison, forest litter invertebrate communities harbour remarkably high numbers of species that span many trophic levels and yield highly complex food webs [10,11] that are directly related to important ecosystem services [12]. This raises the question of how ecosystem functioning of multitrophic communities varies across space in terrestrial forest ecosystems where species turnover is relatively high [13]. Incorporating such high levels of diversity is challenging, if not impossible, for manipulative BEF experiments, thus calling for the implementation of landscape-level field research that extends beyond correlative analyses and rather tries to identify causal mechanisms [1,4]. A major hindrance to the implementation of this approach has been the difficulty of directly linking measured ecosystem functions (such as decomposition, predation and herbivory rates) with multitrophic species assemblages that are sampled at the landscape level. In this study, we overcome this limitation by analytically calculating energy fluxes among biomass pools via trophic interactions in natural litter macroinvertebrate communities [11].
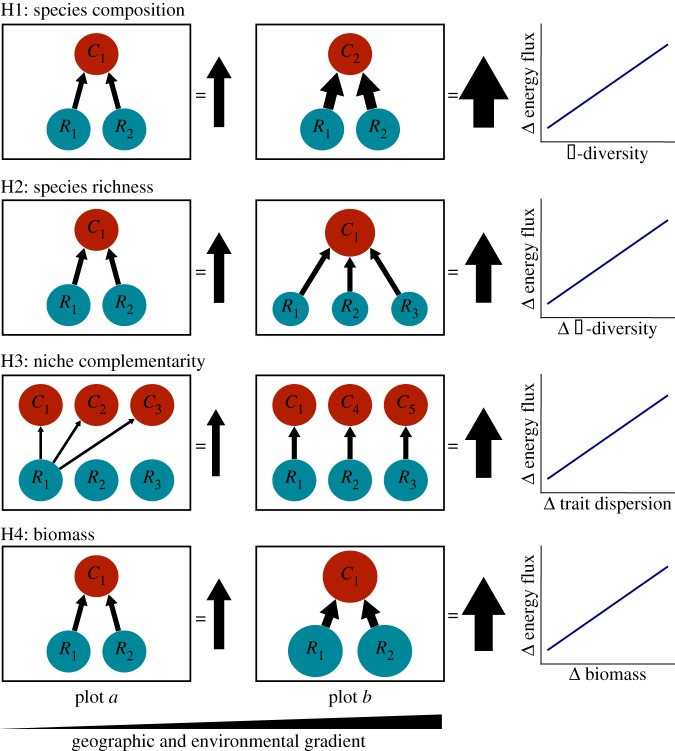
Using structural equation modelling, we address four hypotheses of how multitrophic ecosystem functioning of litter macroinvertebrate communities can vary over spatial and environmental gradients in a temperate and a tropical ecosystem. First, the composition of species might affect energy fluxes [14] owing to particular attributes of species that confer especially important effects on resource uptake (figure 1, H1). Thus, turnover of species among communities (β-diversity) should scale positively with dissimilarity in community energy fluxes (figure 1, H1). Second, community energy flux might scale with species richness (α-diversity) owing to concurrent variability in resource heterogeneity (figure 1, H2); [15]. This would occur as the addition of resource species allows consumers to increase their resource uptake because of an increase in available resource niches. In this case, we would expect that dissimilarity among locales in α-diversity should be positively correlated with dissimilarity of community energy fluxes (figure 1, H2). Third, we hypothesize that community energy fluxes scale positively with functional diversity (hereafter functional dispersion), because a wider range of traits should allow consumers to feed on a wider range of resource species. Specifically, given sufficient resource heterogeneity [15], a larger range of traits among consumers should reduce competition over shared resources owing to increased resource partitioning in more functionally diverse consumer assemblages, allowing for the complementary use of resources (figure 1, H3) [16–18]. Therefore, if niche complementarity determines rates of functioning, then we would expect that dissimilarity of functional dispersion should scale positively with dissimilarity of community energy fluxes (figure 1, H3). Finally, rates of energy flux may be purely biomass-dependent, regardless of the species assemblage, because greater species biomass inherently increases energetic demands and, thus, energy flux among biomass pools. In such a case, we would expect a positive relationship between dissimilarity in community biomass and community energy fluxes (figure 1, H4).
Figure 1.
Graphical representation of the four proposed mechanisms (H1–H4) that drive spatial variation in ecosystem functioning. Hypothetical examples illustrate how each mechanism would drive variation in energy flux between two spatially and environmentally distinct sampling plots (a,b). Blue and red circles represent biomass-weighted (circle size) resource and consumer populations, respectively, with Ri and Ci denoting different resource and consumer species. Black arrows within the example communities indicate hypothetical energy fluxes between resources and their consumers, with the overall sum of energy flux indicated to the right of each hypothetical community. Graphs to the right of the example communities indicate the hypothesized relationship between turnover in the measured community attribute used to test the hypothesis and turnover in energy flux.
In this study, we disentangle the relative contributions of β-diversity, α-diversity, functional dispersion and total biomass on community energy fluxes in temperate and tropical litter macroinvertebrate communities. Using standardized sampling, we compared litter invertebrate communities across two extensive spatial and environmental gradients in managed German forests (48 plots) and plantation agriculture and agroforests in Sumatra, Indonesia (32 plots) to assess the relative roles of spatial and environmental distance in determining community assembly, and how these processes give rise to variation in ecosystem functioning. In doing so, we disentangle potential mechanisms that mediate variation in ecosystem functioning across landscapes and determine how geographical distance can be used to predict ecosystem functioning at large spatial scales.
2. Methods
(a). Study sites
To disentangle the mechanisms responsible for driving variation in ecosystem functioning at the landscape scale, we compared plots across two spatially extensive study regions comprising two landscapes in Sumatra, Indonesia and three landscapes in Germany (electronic supplementary material, figure S1). Plots in both regions were established across a range of land use management intensities. In Indonesia, plots were located in each of four land use types: primary degraded forest, jungle rubber agroforests, monoculture rubber and monoculture oil palm, replicated four times in each of two spatially independent landscapes located near Bukit Duabelas National Park and Harapan Rainforest, making a total of 32 plots (see [11,19] for details of the study design). In Germany, plots were located in each of four land use types of varying management intensity: unmanaged beech forest, 70-year-old managed beech forest, 30-year-old managed beech forest and intensively managed coniferous forest. These plots were replicated four times in each of three spatially independent landscapes in the Swabian Alb Biosphere Reserve, the Hainich National Park and the Schorfheide-Chorin Biosphere Reserve, making a total of 48 plots (see [20] for details of the study design). Within each region, the plots covered a large geographical range, with interplot distance ranging from 0.1 to 90 km in Indonesia and from 0.3 to 630 km in Germany. The Indonesian land use systems probably cover a greater range of management intensity than the German systems owing to generally higher material inputs and harvesting of, for example, oil palm plantations [19]. Thus, direct quantitative comparisons between the two study regions should be made with some caution. Nevertheless, by using a suite of measured environmental parameters to quantify environmental distance in comparable units among sampling sites, such potential regional biases can be overcome.
(b). Animal sampling and measurements
In both regions, macroinvertebrates were sampled from the leaf litter using litter sieves. In Indonesia, a total of three 1 m2 subsamples were taken at each plot (50 × 50 m) between October and November 2012; and in Germany, a total of four 0.25 m2 subsamples were taken from each plot (100 × 100 m) in spring 2011. Leaf litter from each subsample was removed from the surface of the ground down to the soil and placed in a coarse mesh sieve, from which all visible invertebrates were hand collected and stored in ethanol. Collected specimens were identified to higher taxonomic groupings (classes, orders or families where possible) and then further identified to species (see electronic supplementary material, table S1 for more details on sampled invertebrates). In cases where species identification was not possible (predominantly in the Indonesian samples), specimens where identified to morphospecies based on consistent morphological characteristics. The number of species per plot was recorded, and the dissimilarity among plots in α-diversity was then calculated as the log response ratio (LRR) between values, from which we compiled a dissimilarity matrix for each region. Furthermore, we calculated Jaccard dissimilarities among plots and compiled dissimilarity matrices from these values to quantify dissimilarity in species composition across each region.
Specimens were assigned to trophic groups based on a combination of taxonomy, morphology and information from the literature. The body lengths (mm) of all collected individuals were measured and then converted to live body masses (mg) using allometric equations from the literature (see [11] for details and sources of allometric equations). In addition, we assessed the mobility of collected specimens based on whether the individual was winged, legged or both. If the specimen was a wingless juvenile but was known to have a winged life stage, it was allocated to a winged category. We also recorded whether or not each specimen was eusocial based on taxonomy. Further details describing the justifications for trait selection and their assignment can be found in [21]. Finally, individual metabolic rates were calculated for all collected individuals with regression equations using body masses, temperatures measured at each plot, and taxonomic group [22].
Total community biomass was calculated by summing body masses from all individuals collected at each plot. We then compiled dissimilarity matrices based on LRRs of total biomass values among all plots in each region. As a way of quantifying heterogeneity in the functional roles of individuals present in each plot (i.e. to estimate the potential for niche complementarity in sampled communities), we calculated functional dispersion of communities [16,23] from four measured traits: trophic group, body mass, mobility and eusociality. Specifically, functional dispersion calculates the mean distance of species to the community trait–mean centroid weighted by their relative abundances [23]. Functional dispersion was calculated using the FD package in R [24]. Dissimilarity matrices were also compiled from LRRs between functional dispersion values at each plot for each region.
To measure ecosystem functioning in a way that incorporates all sampled trophic levels into a single variable and that can be easily quantified across large spatial scales, we analytically assessed community energy flux [11] for all 80 sampled communities across both regions. To do so, we used the formula
where F is the total flux of energy into the biomass pool of a given trophic level; ea is the diet-specific assimilation efficiency of a given trophic group [25]; X is the summed metabolic rates of all individuals within a trophic group in a given community; and L is the loss of energy from a given biomass pool to higher trophic levels owing to predation [11]. We then summed together fluxes among all trophic levels to obtain a community level measure of energy flux, and compiled dissimilarity matrices from LRRs between total community energy flux values within each region.
(c). Quantifying spatial and environmental distance
Pairwise spatial distances among plots were calculated as great circle distances in kilometres separately for each study region. To quantify environmental distance, a total of 15 measured environmental parameters were used to characterize the 80 plots across both study regions: mean soil moisture content, mean soil temperature, soil pH, litter depth and 11 different elements measured from the leaf litter (C, N, P, Al, Ca, Fe, K, Mg, Mn, Na and S). Soil moisture content (%) and soil temperature (°C) were recorded hourly using soil sensors placed at 30 cm depth in the soil within each plot. Soil pH was analysed in a 1 : 4 soil-to-water ratio at the Indonesian plots [26] and a 1 : 10 soil-to-solution (CaCl) ratio for the German plots [20]. Leaf litter samples were collected at each of the 80 research plots and the amounts (mg) of 11 different elements in leaf litter dry mass were analysed (see [20] for details).
To select the environmental parameters that are most important for explaining variation in BEF of the sampled litter macroinvertebrate communities, we employed two steps. First, we ran a non-metric multidimensional scaling ordination based on Jaccard dissimilarities of all macroinvertebrate communities. Using the envfit function in the vegan package in R [24], we then performed a permutational vector fitting analysis of all 15 environmental parameters as raw and additionally log-transformed variables in the ordination (for both the temperate and tropical data). Only the vectors that yielded α < 0.05 from the permutation tests were retained and then standardized by subtracting their means and dividing by their standard deviations. In cases where both the logged and untransformed variables were significant, we selected the variable with the highest R2-value (see electronic supplementary material, table S2 for details). Finally, we ran a principal component analysis with these retained variables, selected the site scores of the first three principal components (74.75% and 78.11% variation contained in the first three axes for the Indonesian and German data, respectively), and calculated dissimilarities among all plots as Euclidean distances.
(d). Constructing path models based on distance matrices
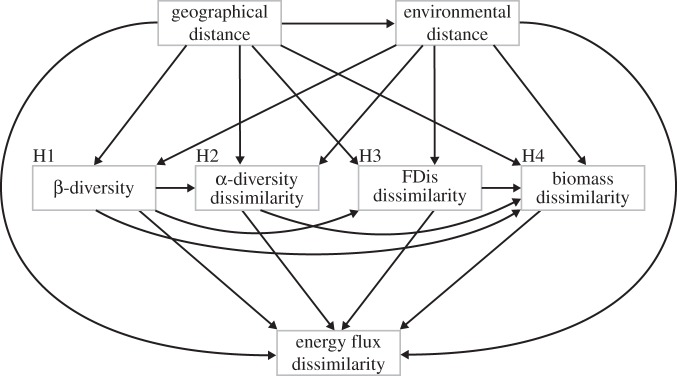
In order to disentangle the roles of different components of biodiversity from pure biomass effects in driving variation in ecosystem functioning across environmental and spatial gradients (figure 1), we employed the use of multiple regression on distance matrices (MRMs) using the ecodist package in R [24] within a path modelling framework. Specifically, MRM regresses a response matrix on any number of explanatory matrices composed of dissimilarities or distances, allowing for the inference of how differently measured multi- or univariate variables might influence each other across environmental and spatial distances [27]. Previous studies have proven path modelling to be a highly effective tool for disentangling the complex causal relationships among environmental change, community attributes and ecosystem functioning [28,29]. Here, we use Shipley's [30] d-separation method of generalized causal path analysis, as this method is highly flexible for using a variety of model types, such as MRMs in this case.
To construct the path model, we identified the basis set BU of independence claims that were implied by our hypothetical causal model (figure 2). The independence claims in BU describe the pi probability that variable pairs (Xi, Xj) are independent conditional on the variable set Z, which is a direct cause of either Xi or Xj. The combined pi of the full model was calculated as
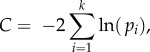 |
and the C value was then compared with a chi-square (χ2) distribution with 2k degrees of freedom [30]. The resulting probability, p, indicates whether the data depart significantly from what would be expected under such a causal model. A model is rejected if the resulting p-value is smaller than the specified α-level (in this case, α = 0.05). As such, if p > 0.05, the causal model is considered to provide a good fit to the data [30].
Figure 2.
Diagram of all the possible effects that were tested in the path model framework of this study. Variables in the path models are indicated by grey boxes and represent distance matrices. H1–H4 denote where hypotheses 1–4 (as in figure 1) were tested in the model. ‘FDis' stands for functional dispersion.
The path model was constructed to test how spatial and environmental distance can influence the role of four different mechanisms through which biodiversity can drive spatial variation in ecosystem functioning: H1) β-diversity, H2) α-diversity, H3) functional dispersion, and H4) community biomass (figures 1 and 2). In addition, we assumed and tested for effects of β-diversity on α-diversity and functional dispersion, as well as effects of these three variables on community biomass (figure 2). For a full list of the variables used in the path models, see electronic supplementary material, table S3.
All MRM models were performed with 10 000 permutations to ensure stable estimations of the p-values that were used to calculate the C statistic in the path models. To assess the relative importance of environmental versus geographical distance for dissimilarity in species composition, α-diversity, functional dispersion and biomass, as well as the relative importance of these variables for driving dissimilarity in community energy fluxes, we calculated range-standardized coefficients for each predictor variable as recommended by [31]. Specifically, this is a standardization of raw coefficients βxy expressing the effect of x on y, whereby the range-standardized coefficient βstdxy = βxy × (xmax − xmin)/(ymax − ymin), where the max and min values are the largest and smallest calculated dissimilarity values from the distance matrices. This method of coefficient standardization yields dimensionless coefficients that can be interpreted as the proportional change in y across the range of x after controlling for all other predictors in the model. All data preparation and statistical analysis was carried out in R 3.1.3 [24].
3. Results
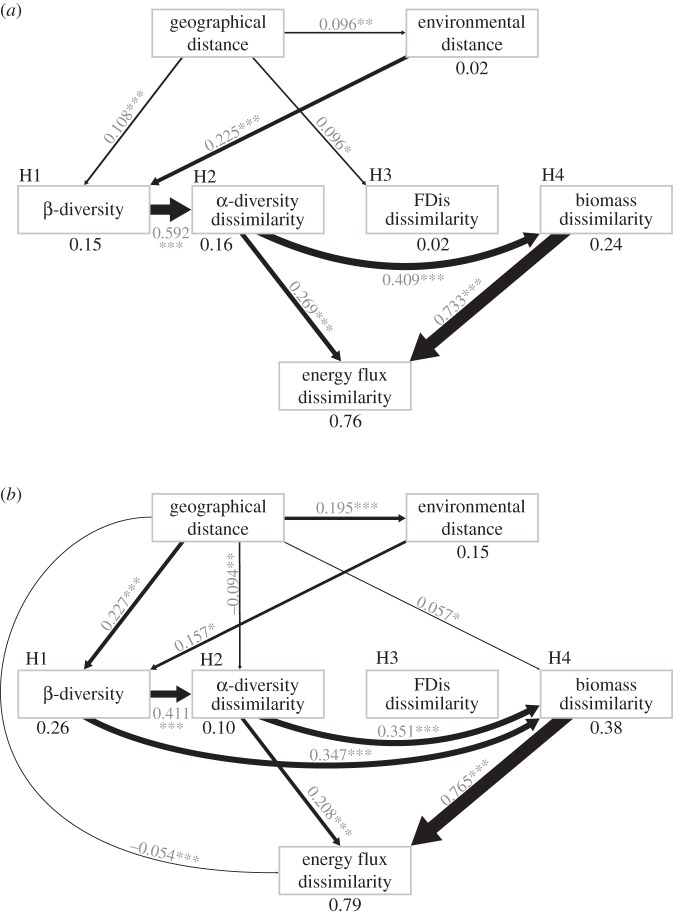
In both the Indonesian and German study regions, geographical and environmental distance played an important role in structuring litter macroinvertebrate communities. The strongest spatial and environmental turnover was observed for species composition (β-diversity), compared with the other diversity variables across the two regions. Across the sampling sites in Indonesia, we found that environmental distance played a larger role than geographical distance in driving β-diversity (figure 3a), with species dissimilarity changing by 23% versus 11% over its measured range (electronic supplementary material, table S4) across the environmental and spatial gradients, respectively. In contrast, geographical distance had a stronger effect on species turnover than environmental distance at the German sites (figure 3b), with a shift in species dissimilarity of 20% compared with 23% (electronic supplementary material, table S4) across the environmental and spatial gradients, respectively. Despite the combined effects of spatial and environmental distance on β-diversity, there was relatively low overall variance in β-diversity explained by these variables in Indonesia (R2 = 0.15), but considerably higher variance explained at the German sites (R2 = 0.26, figure 3). Interestingly, the other measures of macroinvertebrate community structure only responded to geographical distance, with a very weak response of dissimilarity in functional dispersion to geographical distance in Indonesia and similarly weak but significant turnover in α-diversity and biomass across the spatial gradient at the German sites (figure 3).
Figure 3.
Path models constructed from MRM testing for the different mechanisms that determine spatial variation in energy fluxes of litter macroinvertebrate communities across landscapes in (a) Indonesia and (b) Germany. H1–H4 denote hypothesis tests as shown in figure 2. ‘FDis' stands for functional dispersion. Values under each response variable indicate the R2 for each individual MRM. Widths of arrows and adjacent values indicate the range-standardized effect size of each predictor variable. Asterisks denote significance levels: *p < 0.05; **p < 0.01; ***p < 0.001.
The high β-diversity observed across sites in both regions (71–99% and 53–98% species turnover in Indonesia and Germany, respectively), which was partially driven by the spatial and environmental gradients in these sampling regions, was an important determinant of dissimilarity among plots in α-diversity and community biomass (figure 3). Specifically, the overall 29% shift in β-diversity in Indonesia and 45% shift in Germany across their measured range directly drove a respective 59% and 41% change in α-diversity across these study regions. However, the explained variance for turnover in α-diversity was low, with an R2 of 0.16 in Indonesia and only 0.10 in Germany. We found that community biomass also responded with a 35% change across the measured range of β-diversity, but this pattern was only evident at the German sites. Consequently, substantially more variation in biomass turnover could be explained across the German sites (R2 = 0.38) compared with the Indonesian sites (R2 = 0.24).
Interestingly, the emergence of specific and generalizable mechanisms that drive turnover in ecosystem functioning across sites was clear from our path models. In both regions, we found a clear effect of α-diversity and biomass on energy fluxes (figure 3). Specifically, this means that with increasing dissimilarity in the total number of species and total biomass among sampling sites we found a resulting increase in the dissimilarity of energy fluxes of these sites. Across both the Indonesian and German sites, there were relatively similar changes in community energy fluxes across the range of α-diversity (27% in Indonesia and 21% in Germany). This likeness between the two regions in turnover of energy fluxes also held in response to turnover in biomass, with a 73% change in energy flux dissimilarity in Indonesia and a 77% change in Germany across their respective ranges of community biomasses (figure 3 and electronic supplementary material, table S4). At the German sites only, we found a residual effect of geographical distance on energy flux turnover after controlling for all other predictors in the path model, but this effect was extremely weak (only a 5% change) compared with those of α-diversity and biomass (figure 3 and electronic supplementary material, table S4). In addition to the clear detection of mechanisms driving spatial turnover in energy flux, we also found that a high proportion of variation in energy flux turnover was explained by α-diversity and biomass (76% and 79% in Indonesia and Germany, respectively).
4. Discussion
By employing a landscape level approach across a tropical and a temperate region, our study effectively disentangles the potential mechanisms responsible for driving differences in ecosystem functioning across landscapes. We found that both spatial and environmental distance are important for driving turnover in community composition, leading to clear differences among sampling sites in overall rates of energy flux in multitrophic communities of litter macroinvertebrates. Despite some differences in the relative strength of effects on various community attributes (such as α- and β-diversity) and energy fluxes, we found remarkable similarities in the potential mechanisms driving these responses between the Indonesian and German study regions.
Interestingly, we found that environmental distance had a stronger effect on species turnover in the Indonesian communities, whereas geographical distance had a stronger effect in the German communities. These findings seemingly contradict those of Myers et al. [32], who showed that environmental factors played a stronger role in driving species turnover in a temperate compared with a tropical region. Our results may differ because of the different taxa among the two studies, i.e. plants versus litter macroinvertebrates, as dispersal of these two groups is likely to differ considerably, leading to different mechanisms of assembly operating on these organisms. In any case, our findings could provide evidence for greater environmental filtering in the tropical Indonesian communities compared with stronger dispersal-dependent random assembly in the temperate German communities [32]. Alternatively, the larger total geographical extent across the German study region (630 km) could inherently give rise to overall higher species turnover compared with the Indonesian study region (90 km). Nevertheless, the range of environmental distance among plots was highly similar between the Indonesian (0.097–3.862 Euclidean distance) and the German study regions (0.149–3.840 Euclidean distance). Therefore, the 36% stronger standardized effect of environmental distance on β-diversity in Indonesia compared with Germany quite probably indicates that the tropical communities are more subject to environmental filtering than the temperate communities.
We found that turnover in species composition (β-diversity) strongly predicted dissimilarity in total species richness among sampling sites (α-diversity) in both of the study regions. This result could suggest that coexistence of species at the local scale might depend on functional differences among species that allow them to use resources differently [33]. In other words, particular species assemblages are likely to give rise to greater numbers of species in assemblages where antagonistic interactions, such as predation and competition, are weaker. As such, we would expect β-diversity to predict dissimilarity among plots in α-diversity. However, these results should be interpreted with caution because α-diversity and β-diversity are typically highly correlated when turnover is calculated using Jaccard dissimilarities [34]. In both the Indonesian and German study regions, increasing dissimilarity among communities also resulted in greater among-plot dissimilarity in total biomass. These results are confirmative of a multitude of previous studies showing that species richness drives patterns in productivity, especially for primary producer organisms [35,36]. Interestingly, we also found an effect of species composition on community biomass in the German litter communities, suggesting that the combined identities of particular species might be driving varying levels of biomass across these landscapes. For example, the occurrences of particular species that are competitively dominant where particular resources are available are likely to drive locally increased biomass in European forests [37].
Perhaps most strikingly, we found evidence for highly consistent patterns between the tropical and temperate study regions in the mechanisms that had direct effects on spatial variation in ecosystem functioning. In both study regions, we found that turnover in biomass and species richness among plots was highly important for turnover in energy flux. In fact, even the standardized effect size of these variables on energy flux was almost identical between the two regions, with changes in energy flux turnover of 73% in Indonesia versus 77% in Germany in response to biomass turnover, and 27% versus 21% changes in energy flux turnover (in Indonesia and Germany, respectively) in response to α-diversity turnover. These results strongly support the species richness (H2) and biomass (H4) hypotheses. Regarding the species richness hypothesis, it is likely that increasing species richness of potential prey in litter invertebrate communities allows for increased resource exploitation by higher trophic level consumers [38]. As Gamfeldt et al. [38] demonstrated experimentally, this should also result in higher biomasses of consumer species and, thus, overall higher community biomass. As such, dissimilarity among communities in α-diversity should also drive dissimilarity in biomass—a pattern which our path models both strongly support. Furthermore, after holding constant any effects of species richness on energy flux, spatial variation in litter invertebrate biomass still had a very strong effect on spatial variation in energy flux, most likely owing to the strong correlation between total biomass and a community's energetic demand [11]. This indicates that, regardless of any resource diversity effects, total biomass of organisms expectedly plays an important role in determining ecosystem process rates [28].
Although we did not find any direct effects of functional dispersion (H3) or β-diversity (H1) on spatial variation in energy fluxes, this does not necessarily mean that these factors do not play a role in shaping spatial patterns in ecosystem functioning in litter macroinvertebrate communities. On the contrary, our path models indicate that there were indirect effects of species turnover on energy flux via spatial variation in α-diversity, probably resulting from altered patterns in the coexistence of various species in both the tropical and temperate communities [33]. Therefore, although these indirect effects of β-diversity do not lend support to the species composition hypothesis (H1), our results do indicate that there are multiple interacting mechanisms that could be driving the spatial variability of ecosystem functioning in real-world systems. Nevertheless, our results also indicate that a very simplistic set of predictors, i.e. species richness and total biomass, may provide the strongest predictive power for ecosystem functioning at the landscape scale.
5. Conclusion
Our study provides new insights into the mechanisms that probably determine spatial patterns in multitrophic BEF by confirming results across both tropical and temperate landscapes. Despite some minor differences among our two study regions in the possible mechanisms driving spatial variation in ecosystem functioning, we find remarkable similarity from the tropical to temperate systems, indicating that globally consistent and generalizable patterns in BEF relationships at the landscape scale probably exist. These results call for various new avenues of BEF research, such as the extension of landscape level mechanistic tests of BEF relationships to freshwater, marine and other terrestrial ecosystems, as the spatial dynamics of these systems could vary [39]. Moreover, investigating spatial variation in energy flux among trophic levels could shed light on how the trait-dependent loss of biodiversity could lead to the rapid decay of ecosystem services at larger spatial scales [40]. In recent years, the merging of food web ecology and BEF research has moved towards centre stage [41] owing to the enhancement of predictive accuracy with increased ecological complexity. Bringing these merged fields of ecology into the arena of spatial ecology presents an exciting new frontier in the exploration of biodiversity–ecosystem functioning relationships.
Supplementary Material
Acknowledgements
We thank Steffen Mumme, Megawati, Rizky Nazarreta, Keisha Disa Putirama, Rosario Reza Valentino Lasse for assistance in the field and laboratory; Ana Meijide for providing climate data from the Indonesian sites; Falk Hänsel, Spaska Forteva, Stephan Wöllauer and Thomas Nauss for providing climate data from the German sites; Kara Allen for providing soil pH data and Martyna Kowtowska for providing litter depth data for the Indonesian sites. We also thank Jonathan Lefcheck for discussing ideas with us, as well as Helmut Hillebrand and two anonymous reviewers for providing highly constructive comments on earlier versions of this manuscript. In addition, special thanks to Roswitha Ehnes for providing additional metabolic rate regression parameters; as well as to the Indonesian village leaders, local site owners, PT REKI, and Bukit Duabelas National Park for granting us access to their properties.
Data accessibility
All underlying data for this manuscript are archived in the Biodiversity Exploratories Information System (www.bexis.uni-jena.de) and EFForTS-Information System (https://efforts-is.uni-goettingen.de).
Author contributions
A.D.B., P.W. and U.B. conceived and designed the study; A.D.B., M.J. and D.O. collected the data; A.D.B. and P.W. analysed the data; A.D.B. wrote the first draft and all authors contributed substantially to the writing of the final manuscript. All authors gave final approval for publication.
Competing interests
We have no competing interests.
Funding
A.D.B. and M.J. were financed by the Deutsche Forschungsgemeinschaft (DFG) in the framework of the collaborative German–Indonesian research project CRC990. P.W., D.O. and D.H. acknowledge funding in the scope of the BEFmate project from the Ministry of Science and Culture of Lower Saxony. DO additionally acknowledges funding by the DFG Priority Programme 1374 ‘Infrastructure-Biodiversity-Exploratories' (BR 2315/7-2). Fieldwork permits in the Biodiversity-Exploratories were issued by the responsible state environmental offices of Baden-Württemberg, Thüringen and Brandenburg (according to §72 BbgNatSchG). U.B. acknowledges the support of the German Centre for integrative Biodiversity Research (iDiv) Halle-Jena-Leipzig funded by the German Research Foundation (FZT 118).
References
- 1.Cardinale BJ, et al. 2012. Biodiversity loss and its impact on humanity. Nature 486, 59–67. ( 10.1038/nature11148) [DOI] [PubMed] [Google Scholar]
- 2.Díaz S, Fargione J, Chapin FS III, Tilman D. 2006. Biodiversity loss threatens human well-being. PLoS Biol. 4, 1300–1305. ( 10.1371/journal.pbio.0040277) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balvanera P, Pfisterer AB, Buchmann N, He J-S, Nakashizuka T, Raffaelli D, Schmid B. 2006. Quantifying the evidence for biodiversity effects on ecosystem functioning and services. Ecol. Lett. 9, 1146–1156. ( 10.1111/j.1461-0248.2006.00963.x) [DOI] [PubMed] [Google Scholar]
- 4.Tilman D, Isbell F, Cowles JM. 2014. Biodiversity and ecosystem functioning. Annu. Rev. Ecol. Evol. Syst. 45, 471–493. ( 10.1146/annurev-ecolsys-120213-091917) [DOI] [Google Scholar]
- 5.Brose U, Hillebrand H. 2016. Biodiversity and ecosystem functioning in dynamic landscapes. Phil. Trans. R. Soc. B 371, 20150267 ( 10.1098/rstb.2015.0267) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Snelgrove PVR, Thrush SF, Wall DH, Norkko A. 2014. Real world biodiversity–ecosystem functioning: a seafloor perspective. Trends Ecol. Evol. 29, 398–405. ( 10.1016/j.tree.2014.05.002) [DOI] [PubMed] [Google Scholar]
- 7.Godbold JA, Bulling MT, Solan M. 2011. Habitat structure mediates biodiversity effects on ecosystem properties. Proc. R. Soc. B 278, 2510–2518. ( 10.1098/rspb.2010.2414) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chalcraft DR, Williams JW, Smith MD, Willig MR. 2004. Scale dependence in the species-richness–productivity relationship: the role of species turnover. Ecology 85, 2701–2708. ( 10.1890/03-0561) [DOI] [Google Scholar]
- 9.France KE, Duffy JE. 2006. Diversity and dispersal interactively affect predictability of ecosystem function. Nature 441, 1139–1143. ( 10.1038/nature04729) [DOI] [PubMed] [Google Scholar]
- 10.Digel C, Curtsdotter A, Riede JO, Klarner B, Brose U. 2014. Unravelling the complex structure of forest soil food webs: higher omnivory and more trophic levels. Oikos 123, 1157–1172. ( 10.1111/oik.00865) [DOI] [Google Scholar]
- 11.Barnes AD, Jochum M, Mumme S, Haneda NF, Farajallah A, Widarto TH, Brose U. 2014. Consequences of tropical land use for multitrophic biodiversity and ecosystem functioning. Nat. Commun. 5, 5351 ( 10.1038/ncomms6351) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Handa IT, et al. 2014. Consequences of biodiversity loss for litter decomposition across biomes. Nature 509, 218–221. ( 10.1038/nature13247) [DOI] [PubMed] [Google Scholar]
- 13.Basset Y, et al. 2012. Arthropod diversity in a tropical forest. Science 338, 1481–1484. ( 10.1126/science.1226727) [DOI] [PubMed] [Google Scholar]
- 14.Hooper DU, et al. 2005. Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol. Monogr. 75, 3–35. ( 10.1890/04-0922) [DOI] [Google Scholar]
- 15.Tylianakis JM, Rand TA, Kahmen A, Klein A-M, Buchmann N, Perner J, Tscharntke T. 2008. Resource heterogeneity moderates the biodiversity-function relationship in real world ecosystems. PLoS Biol. 6, 947–956. ( 10.1371/journal.pbio.0060122) [DOI] [Google Scholar]
- 16.Gagic V, et al. 2015. Functional identity and diversity of animals predict ecosystem functioning better than species-based indices. Proc. R. Soc. B 282, 20142620 ( 10.1098/rspb.2014.2620) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Striebel M, Behl S, Diehl S, Stibor H. 2009. Spectral niche complementarity and carbon dynamics in pelagic ecosystems. Am. Nat. 174, 141–147. ( 10.1086/599294) [DOI] [PubMed] [Google Scholar]
- 18.Poisot T, Mouquet N, Gravel D. 2013. Trophic complementarity drives the biodiversity–ecosystem functioning relationship in food webs. Ecol. Lett. 16, 853–861. ( 10.1111/ele.12118) [DOI] [PubMed] [Google Scholar]
- 19.Drescher J, et al. 2016. Ecological and socio-economic functions across tropical land use systems after rainforest conversion. Phil. Trans. R. Soc. B 371, 20150275 ( 10.1098/rstb.2015.0275) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ott D, Digel C, Klarner B, Maraun M, Pollierer M, Rall BC, Scheu S, Seelig G, Brose U. 2014. Litter elemental stoichiometry and biomass densities of forest soil invertebrates. Oikos 123, 1212–1223. ( 10.1111/oik.01670) [DOI] [Google Scholar]
- 21.Mumme S, Jochum M, Brose U, Haneda NF, Barnes AD. 2015. Functional diversity and stability of litter-invertebrate communities following land-use change in Sumatra, Indonesia. Biol. Conserv. 191, 750–758. ( 10.1016/j.biocon.2015.08.033) [DOI] [Google Scholar]
- 22.Ehnes RB, Rall BC, Brose U. 2011. Phylogenetic grouping, curvature and metabolic scaling in terrestrial invertebrates. Ecol. Lett. 14, 993–1000. ( 10.1111/j.1461-0248.2011.01660.x) [DOI] [PubMed] [Google Scholar]
- 23.Laliberté E, Legendre P. 2010. A distance-based framework for measuring functional diversity from multiple traits. Ecology 91, 299–305. ( 10.1890/08-2244.1) [DOI] [PubMed] [Google Scholar]
- 24.R Core Team. 2015. R: a language and environment for statistical computing. See http://www.R-project.org/.
- 25.De Ruiter PC, Van Veen JA, Moore JC, Brussaard L, Hunt HW. 1993. Calculation of nitrogen mineralization in soil food webs. Plant Soil 157, 263–273. ( 10.1007/BF00011055) [DOI] [Google Scholar]
- 26.Allen K, Corre MD, Tjoa A, Veldkamp E. 2015. Soil nitrogen-cycling responses to conversion of lowland forests to oil palm and rubber plantations in Sumatra, Indonesia. PLoS ONE 10, e0133325 ( 10.5061/dryad.q20p3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lichstein JW. 2007. Multiple regression on distance matrices: a multivariate spatial analysis tool. Plant Ecol. 188, 117–131. ( 10.1007/s11258-006-9126-3) [DOI] [Google Scholar]
- 28.Barnes AD, Emberson RM, Krell F-T, Didham RK. 2014. The role of species traits in mediating functional recovery during matrix restoration. PLoS ONE 9, e115385 ( 10.1371/journal.pone.0115385) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laliberté E, Tylianakis JM. 2012. Cascading effects of long-term land-use changes on plant traits and ecosystem functioning. Ecology 93, 145–155. ( 10.1890/11-0338.1) [DOI] [PubMed] [Google Scholar]
- 30.Shipley B. 2009. Confirmatory path analysis in a generalized multilevel context. Ecology 90, 363–368. ( 10.1890/08-1034.1) [DOI] [PubMed] [Google Scholar]
- 31.Grace JB. 2006. Structural equation modeling and natural systems. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 32.Myers JA, Chase JM, Jiménez I, Jørgensen PM, Araujo-Murakami A, Paniagua-Zambrana N, Seidel R. 2013. Beta-diversity in temperate and tropical forests reflects dissimilar mechanisms of community assembly. Ecol. Lett. 16, 151–157. ( 10.1111/ele.12021) [DOI] [PubMed] [Google Scholar]
- 33.Kneitel JM, Chase JM. 2004. Trade-offs in community ecology: linking spatial scales and species coexistence. Ecol. Lett. 7, 69–80. ( 10.1046/j.1461-0248.2003.00551.x) [DOI] [Google Scholar]
- 34.Chao A, Chazdon RL, Shen T-J. 2005. A new statistical approach for assessing similarity of species composition with incidence and abundance data. Ecol. Lett. 8, 148–159. ( 10.1111/j.1461-0248.2004.00707.x) [DOI] [Google Scholar]
- 35.Tilman D, Reich PB, Knops J, Wedin D, Mielke T, Lehman C. 2001. Diversity and productivity in a long-term grassland experiment. Science 294, 843–845. ( 10.1126/science.1060391) [DOI] [PubMed] [Google Scholar]
- 36.Cardinale BJ, et al. 2013. Biodiversity simultaneously enhances the production and stability of community biomass, but the effects are independent. Ecology 94, 1697–1707. ( 10.1890/12-1334.1) [DOI] [PubMed] [Google Scholar]
- 37.Salamon J-A, Alphei J, Ruf A, Schaefer M, Scheu S, Schneider K, Sührig A, Maraun M. 2006. Transitory dynamic effects in the soil invertebrate community in a temperate deciduous forest: effects of resource quality. Soil Biol. Biochem. 38, 209–221. ( 10.1016/j.soilbio.2005.04.033) [DOI] [Google Scholar]
- 38.Gamfeldt L, Hillebrand H, Jonsson PR. 2005. Species richness changes across two trophic levels simultaneously affect prey and consumer biomass. Ecol. Lett. 8, 696–703. ( 10.1111/j.1461-0248.2005.00765.x) [DOI] [Google Scholar]
- 39.Soininen J, Lennon JJ, Hillebrand H. 2007. A multivariate analysis of beta diversity across organisms and environments. Ecology 88, 2830–2838. ( 10.1890/06-1730.1) [DOI] [PubMed] [Google Scholar]
- 40.Larsen TH, Williams NM, Kremen C. 2005. Extinction order and altered community structure rapidly disrupt ecosystem functioning. Ecol. Lett. 8, 538–547. ( 10.1111/j.1461-0248.2005.00749.x) [DOI] [PubMed] [Google Scholar]
- 41.Reiss J, Bridle JR, Montoya JM, Woodward G. 2009. Emerging horizons in biodiversity and ecosystem functioning research. Trends Ecol. Evol. 24, 505–514. ( 10.1016/j.tree.2009.03.018) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All underlying data for this manuscript are archived in the Biodiversity Exploratories Information System (www.bexis.uni-jena.de) and EFForTS-Information System (https://efforts-is.uni-goettingen.de).