Abstract
Purpose
The purpose of this study is to evaluate the prognostic significance of the tumor volume reduction rate (TVRR) measured during adaptive definitive radiation therapy (RT) for nasopharyngeal cancer (NPC).
Materials and Methods
We reviewed the RT records of 159 NPC patients treated with definitive RT with or without concurrent chemotherapy between January 2006 and February 2013. Adaptive re-planning was performed in all patients at the third week of RT. The pre- and mid-RT gross tumor volumes (GTVs) of the primary tumor and the metastatic lymph nodes were measured and analyzed for prognostic implications.
Results
After a median follow-up period of 41.5 months (range, 11.2 to 91.8 months) for survivors, there were 43 treatment failures. The overall survival and progression-free survival (PFS) rates at 5 years were 89.6% and 69.7%, respectively. The mean pre-RT GTV, mid-RT GTV, and TVRR were 45.9 cm3 (range, 1.5 to 185.3 cm3), 26.7 cm3 (1.0 to 113.8 cm3), and –41.9% (range, –87% to 78%), respectively. Patients without recurrence had higher TVRR than those with recurrence (44.3% in the no recurrence group vs. 34.0% in the recurrence group, p=0.004), and those with TVRR > 35% achieved a significantly higher rate of PFS at 5 years (79.2% in TVRR > 35% vs. 53.2% in TVRR ≤ 35%; p < 0.001). In multivariate analysis, TVRR was a significant factor affecting PFS (hazard ratio, 2.877; 95% confidence interval, 1.555 to 5.326; p=0.001).
Conclusion
TVRR proved to be a significant prognostic factor in NPC patients treated with definitive RT, and could be used as a potential indicator for early therapeutic modification during the RT course.
Keywords: Tumor volume reduction rate, Nasopharyngeal carcinoma, Radiotherapy, Adaptive therapy, Prognostic factor
Introduction
Radiation therapy (RT) with or without concurrent chemotherapy is an established definitive treatment modality for both early and locally advanced stage nasopharyngeal cancer (NPC). Although it allows favorable oncologic outcomes, there is a wide range of response to RT, highlighting the need to identify patients who might have radiation resistance and high risk of recurrence. Thus, earlier prediction of RT response and consequent long-term prognosis might be a critical issue for tailoring subsequent treatment to increase the chance of a cure in an individual patient.
The TNM staging system is the most commonly accepted staging method for head and neck cancer, and it aids clinicians in determining prognosis and in selecting the most optimal therapeutic modality [1]. The TNM staging system is considered the most important prognostic factor; however, because it is usually based on the size and/or extent of the primary tumor and/or metastatic lymph node, it sometimes does not correlate with actual tumor burden [2]. Although this uni- or bi-dimensional measurement is an important factor, three-dimensional volumetric data have become another important factor that should be considered, particularly when using non-surgical therapies, such as RT or chemotherapy.
Many studies have demonstrated the prognostic value of tumor volume in various cancers, and there is mounting evidence that pretreatment tumor volume and/or residual tumor volume have prognostic value [3-6]. However, few studies investigating changes in tumor volume during RT as a prognostic factor have been reported [7-10]. We previously reported on the prognostic impact of tumor volume reduction rate (TVRR) measured during adaptive RT for oropharyngeal cancer [8].
In regard to the nasopharynx subsite of head and neck cancer, however, TVRR as a prognostic factor has not been reported yet, thus its prognostic role remains uncertain. The aim of this study was therefore to examine the volumetric parameters measured before and during definitive RT to determine possible prognostic implications in NPC patients. To the best of our knowledge, the present study is the first to report on the value of TVRR as an independent prognostic factor in NPC.
Materials and Methods
1. Patient selection
We reviewed the medical records of consecutive NPC patients treated with definitive RT with or without concurrent chemotherapy at Samsung Medical Center between March 2006 and February 2013. To be eligible for the current study, patients were required to have (1) histologically confirmed carcinoma of the nasopharynx, (2) no distant metastasis at the time of initial diagnosis, (3) completed more than 90% of the planned RT course without significant interruption, and (4) available RT plans. The exclusion criteria were (1) other non-squamous cell carcinoma histologic types such as lymphoma and salivary type carcinoma, (2) patients whose primary tumor or neck node was surgically removed or who received induction chemotherapy before RT, and (3) immediate follow-up loss after completion of RT. Consequently, 159 NPC patients were eligible.
Before the initiation of RT, complete medical history taking and physical examination, direct flexible fiberoptic endoscopic examination, computed tomography (CT) scans and magnetic resonance image of the head-and-neck region, and whole-body 18F-fluorodeoxyglucose positron emission tomography (PET) with CT were performed in all patients.
2. Treatment scheme
Each patient underwent CT simulation in the supine position, immobilized by a thermoplastic mask, with a mouthpiece to keep the mouth open. Simulation CT images were obtained at 2.5- to 3.75-mm slice intervals with intravenous contrast enhancement. A second CT simulation was performed in all patients in order to generate an adaptive re-plan after delivery of the median 14 fractions (12 to 17 fractions). All sets of acquired simulation CT images were imported into the Pinnacle3 treatment planning system (ver. 9.2, Royal Phillips Electronics, Miami, FL), and the gross tumor volumes (GTVs) of the primary tumor and the metastatic lymph nodes were manually contoured. The delineation of GTV was based on both clinical examination findings as well as all available diagnostic images.
RT techniques were either 3-dimensional conformal RT (3D-CRT) using 4 MV and/or 10 MV photons from linear accelerator or intensity-modulated radiation therapy (IMRT) using 6 MV photons from helical tomotherapy.
The clinical target volume (CTV) was arbitrarily subdivided into two risk levels: high risk CTV encompassed the immediately adjacent regions to the GTVs and the lymph nodes considered at equivocal risk of metastasis based on size, shape, and PET uptake; and low risk CTV covered the apparently uninvolved lymphatics and more than one station away from the nodal GTV.
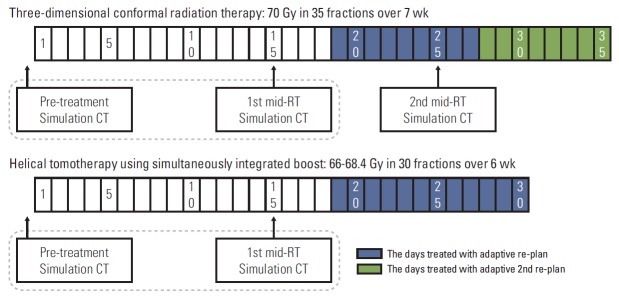
The prescribed radiation dose was differently according to the RT technique. When using 3D-CRT technique, the doses to the GTV, high risk CTV and low risk CTV were 70 Gy in 35 fractions, 54 Gy in 27 fractions, and 36 Gy in 18 fractions, respectively, with 2 Gy per fraction. When using IMRT technique, simultaneous integrated boost (SIB) was adapted, and the dose schedules changed during the study period. In earlier cases, the fraction sizes were 2.2 Gy to the GTV and 2.0 Gy to the CTV throughout the RT course. In later cases, the fraction sizes to the GTV and CTV were 2.2 Gy and 2.0 Gy during the first 18 fractions, and then 2.4 Gy and 2.0 Gy during the following 12 fractions, respectively. As a result, the actually delivered doses to the GTV, high risk and low risk CTVs were 66.0 or 68.4 Gy in 30 fractions, 60 Gy in 30 fractions, and 36 Gy in 18 fractions, respectively. The schemes of the adaptive re-plans and measurements of the GTVs along the RT courses are illustrated in Fig. 1.
Fig. 1.
Scheme of adaptive re-plan for tumor volume measurements along the radiation therapy (RT) course. CT, computed tomography.
In accordance with institutional guidelines, patients with locally advanced disease such as clinical T3 or T4 tumors and/or lymph node metastasis received concurrent chemotherapy with RT, unless they had no contraindication to addition of chemotherapy. Three weeks after completion of RT, adjuvant chemotherapy (1 to 3 cycles of cisplatin+5-fluorouracil) was optionally administered to some NPC patients.
3. Measurement of tumor volume and TVRR
The primary tumor and the metastatic lymph nodes were delineated on both the pre- and mid-RT simulation CT images, and 3-dimensional tumor volumes were calculated on the RT planning system. Examples of the GTV delineation are illustrated in Fig. 2. Changes in the GTV’s between these two CT images were also analyzed. TVRR was defined as the percent (%) reduction of the GTV in relation to the pre-RT GTV, where TVRR=(Pre-RT GTV–Mid-RT GTV)/Pre-RT GTV.
Fig. 2.
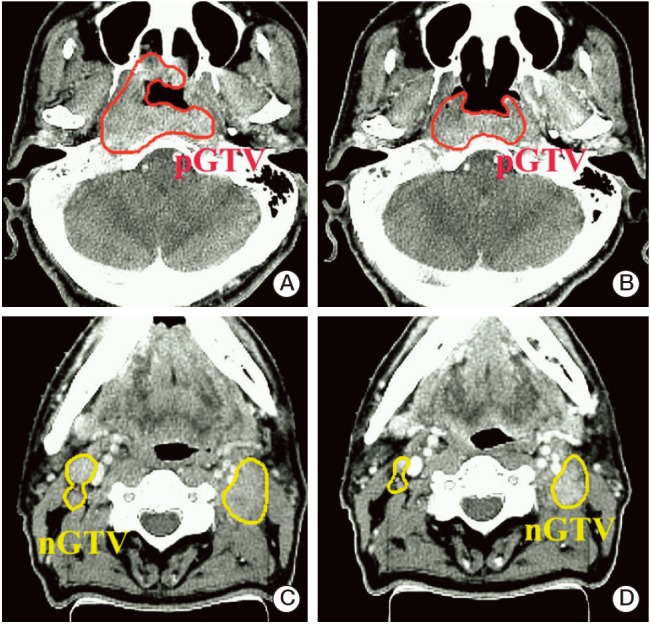
Examples of gross tumor volume (GTV) delineation: GTVs of primary tumor (pGTV) and metastatic lymph node (nGTV) were delineated on computed tomography images taken before radiation therapy (A, C) and during radiation therapy (at 28.6 Gy irradiation) (B, D) in a patient with cT3N2M0 nasopharyngeal cancer. Tumor reduction rate in this case was –40%.
4. Post-treatment follow-up
All patients were asked to visit for follow-up evaluation of disease status on a regular basis. The first evaluation was scheduled at one month of RT completion, and then every 3 months for the first 2 years, every 6 months until 5 years, and annually thereafter. On each visit, a thorough physical examination was performed together with imaging studies alternating between CT of the head-and-neck region and PET-CT.
5. Statistical analysis
The overall survival (OS) duration was measured from the date of RT initiation to the date of death or last follow-up visit, and progression-free survival (PFS) from the date of RT initiation to the date of first recurrence of disease of any type or the last clinical follow-up. The rates of OS and PFS were calculated using Kaplan-Meier estimates with the log-rank test for univariate comparison.
The relationship between the volumetric parameters and clinical outcome was assessed using Mann-Whitney U test. Prognostic factor analyses between tumor volume parameters were performed as both continuous and categorical variables to determine which independently affected prognosis. The optimal thresholds of the continuous variables were determined to be those producing the highest accuracy. The discriminatory performances of the volumetric data were assessed by receiver operating characteristic (ROC) analysis. The log-rank test was used for validation of the determined cut-off values.
Cox proportional hazard regression analysis was used to determine the implications of the potential prognosticators. The statistical tests were two-sided, and a p-value of < 0.05 was considered statistically significant. Factors with p-value of < 0.2 in univariate analysis were included in the multivariate analyses. Variable risk was expressed as a hazard ratio (HR) with a corresponding 95% confidence interval (95% CI). Statistical analysis was performed using SPSS ver. 18.0 (SPSS Inc., Chicago, IL).
Results
1. Patients and treatment characteristics
The characteristics of the patients and the treatment are shown in Table 1. The median age was 51 years, ranging from 17 to 86 years, and 94.3% of the patients were male. Approximately half of the pretreatment clinical T stage was cT1 (77 patients, 44.7%), but the majority of patients (130 patients, 81.8%) had metastatic lymph nodes on initial diagnosis. The distribution of TNM stage based on the American Joint Committee on Cancer (AJCC) seventh edition was stage I in 15 patients (9.4%), II in 39 (34.5%), III in 70 (44.0%), and IVA/B in 35 (22.0%).
Table 1.
Patients’ characteristics
| Characteristic | No. of patients (%) |
|---|---|
| Age (yr) | |
| ≤ 50 | 76 (47.8) |
| > 50 | 83 (52.2) |
| Sex | |
| Male | 150 (94.3) |
| Female | 9 (5.7) |
| History of smoking | |
| Never smoker | 84 (52.8) |
| Current or ex-smoker | 75 (41.2) |
| Clinical T stage | |
| cT1 | 71 (44.7) |
| cT2 | 24 (15.1) |
| cT3 | 36 (22.6) |
| cT4 | 28 (17.6) |
| Clinical N stage | |
| cN0 | 29 (18.2) |
| cN1 | 56 (35.2) |
| cN2 | 67 (42.1) |
| cN3 | 7 (4.4) |
| TNM stage | |
| I | 15 (9.4) |
| II | 39 (34.5) |
| III | 70 (44.0) |
| IVA/B | 35 (22.0) |
| Low neck involvement | |
| No | 130 (81.8) |
| Yes | 29 (18.2) |
| Treatment modality | |
| RT alone | 24 (15.1) |
| CCRT | 135 (84.9) |
| RT fractionation | |
| Conventional | 46 (28.9) |
| Accelerated | 113 (71.1) |
| Adjuvant chemotherapy | |
| No | 104 (65.4) |
| Yes | 55 (34.6) |
RT, radiation therapy; CCRT, concurrent chemoradiation therapy.
Concurrent chemotherapy with RT was administered to 135 patients (84.9%). The median total radiation dose was 68.4 Gy (66 to 72.4 Gy), and 113 patients (71.1%) received IMRT. A cisplatin-based regimen was administered concurrently to all patients: cisplatin 100 mg/m2 every 3 weeks was administered to 111 patients (69.8%); weekly cisplatin 20 mg/m2 to 23 (14.5%); and cisplatin plus docetaxel 20 mg/m2 every 3 weeks to one (0.6%), respectively. After completion of RT, adjuvant chemotherapy with cisplatin plus 5-fluorouracil was administered to 34.6% of patients.
2. Survival outcome
The median follow-up duration for survivors was 41.5 months (range, 11.2 to 91.8 months). Treatment failure of any type was observed in 43 patients (27.0%), and 13 patients died during the follow-up period. The estimated 5-year PFS and OS rates were 69.7% and 89.6%, respectively. As expected, patients with more advanced TNM stage had worse OS and PFS: OS rates at 5 years were 100% in stages I and II, 84.7% in stage III, and 82% in stage IV (p=0.045), respectively. Five-year PFS rates were 100% in stage I, 77% in stage II, 64.0% in stage III, and 58.2% in stage IV disease (p=0.022).
3. Tumor volumetric parameters
The mean volumes of pre-RT and mid-RT GTVs were 45.9 cm3 (range, 1.5 to 185.3 cm3) and 26.7 cm3 (range, 1.0 to 113.8 cm3), respectively. The mean TVRR relative to pre-RT baseline was –41.9%, ranging from –87% to 78%. TVRR did not correlate with the pre-RT GTV (Spearman’s correlation coefficient, 0.041; p=0.511).
In correlation analyses between TNM stage and volumetric parameters, positive correlation was observed between absolute GTVs and TNM stages. We performed a correlation analyses between TNM stage and volumetric parameters. There was a positive correlation between absolute GTVs and TNM stages. The mean±standard deviation (SD) of pre-RT GTV according to each stage was as follows: 12.6±6.3 cm3 in stage I, 29.4±22.5 cm3 in stage II, 50.2±31.0 cm3 in stage III, and 70.0±41.5 cm3 in stage IV (Spearman correlation coefficient, 0.562; p < 0.001). The mean±SD of mid-RT GTV according to each stage was 7.1±4.6 cm3 in stage I, 13.7±8.6 cm3 in stage II, 29.4±20.0 cm3 in stage III, and 44.0±28.3 cm3 in stage IV (Spearman correlation coefficient, 0.583; p < 0.001). TVRR showed a marginally significant correlation with clinical stages: 46.8%±25.1%, 39.1%±25.9%, 30.7%±24.8%, and 30.8%±21.7%, respectively (Spearman correlation coefficient, 0.138; p=0.082).
The results of the correlation analyses of the volumetric parameters according to recurrence status are shown in Table 2. Patients without recurrence tended to show lower pre- and mid-RT GTVs and higher TVRR compared to patients with recurrence.
Table 2.
Comparison analyses of volumetric parameters according to recurrence status
| Parameter | Recurrence, mean (range, cm3) |
p-valuea) | |
|---|---|---|---|
| No | Yes | ||
| Pre-RT GTV | |||
| Primary | 24.2 (1.5 to 93.8) | 32.6 (2.9 to 165.7) | 0.089 |
| Nodal | 16.8 (0.0 to 112.7) | 26.2 (0.0 to 116.6) | 0.022 |
| Total | 40.9 (1.5 to 138.0) | 58.8 (6.0 to 185.3) | 0.004 |
| Mid-RT GTV | |||
| Primary | 14.5 (0.6 to 74.2) | 23.4 (1.6 to 104.5) | 0.006 |
| Nodal | 7.9 (0.0 to 49.8) | 14.7 (0.0 to 84.9) | 0.009 |
| Total | 22.3 (1.0 to 87.9) | 38.0 (5.3 to 113.8) | < 0.001 |
| TVRRb) | |||
| Primary | -43.4 (-93.5 to 3.8) | -28.1 (-86.9 to 51.5) | 0.004 |
| Nodalc) | -46.1 (-85.5 to 98.9) | -38.4 (-83.0 to 139.3) | 0.143 |
| Total | -44.3 (-87.2 to 3.80) | -34.0 (-72.0 to 78.3) | 0.004 |
RT, radiation therapy; GTV, gross tumor volume, TVRR, tumor volume reduction rate.
Calculated by Mann-Whitney test,
Tumor volume reduction rate=(Mid-RT GTV–Pre-RT GTV)/Pre-RT GTV,
Nodal TVRRs were calculated from 130 patients with clinically positive nodes.
4. TVRR as a prognostic factor
As continuous variables, all volumetric parameters showed statistical significance in univariate analysis for PFS (pre-RT GTV [HR, 1.011; 95% CI, 1.004 to 1.018; p=0.03], mid-RT GTV [HR, 1.024; 95% CI, 1.013 to 1.035; p < 0.001], and TVRR [HR, 7.514; 95% CI, 2.777 to 20.329; p < 0.001]), but only TVRR displayed prognostic significance in multivariate analysis (HR, 5.949; 95% CI, 1.090 to 32.467; p=0.038).
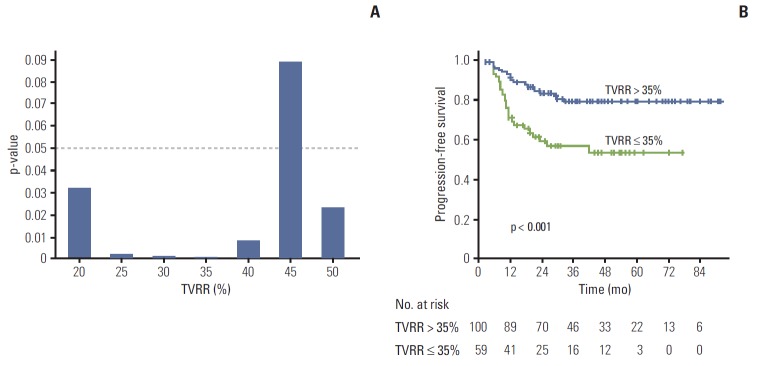
In ROC analysis, the calculated area under the ROC curve of TVRR was 0.647 (p=0.004). A TVRR cut-off value of 35% showed the maximal discriminative power (sensitivity, 66.7%; specificity, 71.2%).
When dichotomized by various TVRR cut-off values, the PFS rate for the higher TVRR group was higher than that of the lower TVRR group (Fig. 3A). The most significantly discriminative power was identified as 35% of TVRR. The PFS rate of patients with TVRR > 35% was 79.2%, while that of those with TVRR ≤ 35% was 53.2% (Fig. 3B).
Fig. 3.
(A) Univariate analyses of various cut-offs of tumor volume reduction rate (TVRR) in the context of predicting progression-free survival. (B) Kaplan-Meier curves of progression-free survival stratified using a 35% TVRR.
Results of univariate and multivariate analyses of the impacts of patient- and treatment-related factors on PFS are shown in Table 3. In multivariate analysis, TVRR was identified as an independently significant prognostic factor for PFS when adjusted for clinical factors (HR, 2.877; 95% CI, 1.555 to 5.326; p=0.001).
Table 3.
Factors affecting progression-free survival in univariate and multivariate analyses
| Characteristic | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| 5-yr PFS (%) | p-value | HR (95% CI) | p-value | |
| Age (≤ 50 yr vs. > 50 yr) | 67.4 vs. 74.5 | 0.451 | 0.861 (0.469-1.583) | 0.631 |
| Sex (male vs. female) | 70.5 vs. 88.9 | 0.539 | 0.506 (0.068-3.753) | 0.506 |
| History of smoking (never vs. current/ex-smoker) | 73.9 vs. 67.3 | 0.385 | - | - |
| Clinical T stage (cT1-2 vs. cT3-4) | 75.6 vs. 64.2 | 0.065 | - | - |
| Clinical N stage (cN0-1 vs. cN2-3) | 77.5 vs. 63.8 | 0.053 | - | - |
| Low neck node involvement (no vs. yes) | 73.5 vs. 60.1 | 0.166 | 1.658 (0.819-3.357) | 0.160 |
| TNM stage (I -II vs. III-IV) | 83.7 vs. 64.4 | 0.008 | 2.036 (0.880-4.708) | 0.097 |
| RT fractionation (conventional vs. accelerated) | 71.1 vs. 71.3 | 0.942 | - | - |
| Adjuvant chemotherapy (no vs. yes) | 71.5 vs. 70.0 | 0.708 | - | - |
| Pre-RT tumor volume (≤ 45 mm3 vs. > 45 mm3) | 77.9 vs. 59.4 | 0.005 | 1.414 (0.730-2.736) | 0.304 |
| Tumor volume reduction rate (> 35% vs. ≤ 35%) | 79.2 vs. 53.2 | < 0.001 | 2.877 (1.555-5.326) | 0.001 |
PFS, progression-free survival; HR, hazard ratio; CI, confidence interval; RT, radiation therapy.
Discussion
In this study, we investigated the usefulness of TVRR measured from simulation CT before and during adaptive RT in patients with NPC. The most significant result of the present study was that TVRR was an independent prognostic factor in terms of predicting PFS in NPC patients treated with definitive RT with or without concurrent chemotherapy.
TVRR during the RT course has recently emerged as a prognostic factor in various malignancies including head and neck cancers. In a previous study, we reported on the prognostic impact of TVRR measured during definitive RT on loco-regional control [8]. With a 35% cut-off value of TVRR, the 3-year loco-regional control rate was 94.4% in patients with higher TVRR, but 72.4% in those with less TVRR. Yang et al. [11] also demonstrated that TVRR was a predictor for local control in patients with oro- and hypopharyngeal cancer treated with IMRT. Hoeben et al. [9], who studied 18F-fluorothymidine PET-CT before and during the course of RT for head and neck squamous cell carcinoma, reported that GTV decrease above median (31%) was associated with significantly better 3-year loco-regional control and disease-free survival. Our results were consistent with those reported in these studies in that TVRR also influenced prognosis in NPC.
The other main finding from the present study was that pre- and mid-RT absolute tumor volumes had less impact on clinical outcomes than TVRR. Several studies have reported association of pre-treatment tumor volume with the probability of tumor control and survival in patients with head-and-neck cancer arising in the subglottic larynx [12], the hypopharynx [13], the oropharynx [5], and the nasopharynx [4]. The present study also showed that pre- and mid-RT GTV were statistically significant in univariate analysis. After adjustment for other volumetric parameters, however, these factors lost their prognostic significance (Table 3). Based on this result, we would speculate that TVRR has a greater prognostic value than absolute tumor volume.
In addition to the prognostic role of TVRR, it could provide additional discriminative efficacy in risk group stratification during the course of definitive RT. Adaptive re-planning during the RT course is an emerging issue being routinely incorporated into clinical practice at many institutions. Variable degrees of volumetric and geometric changes are commonly observed during the RT course in treatment of head and neck cancer [14,15]. Many radiation oncologists recommend repeated CT simulation and subsequent re-planning during the RT course, as the adaptive RT may provide more optimal dose delivery to the target while decreasing the normal tissue toxicities [16-18], and can even improve local control and quality of life [19,20]. Accordingly, adaptive RT has become essential in treatment of head-and-neck cancer patients. In the current clinical setting, volumetric parameters, which could be easily acquired, could provide useful prognostic information. TVRR may have more than just prognostic value, and it could aid clinicians in the individualization of therapeutic strategy. Most tumor responses are evaluated after completion of a definitive RT course, when it might be too late to commence an appropriate therapeutic modification. By predicting recurrence before the completion of definitive treatment, we could anticipate the opportunity for tailoring therapy on an individual basis. A few strategic therapeutic modifications could be considered. An escalation of total radiation dose to a higher level than initially planned might be one example. It can be, more or less, quite easily implemented by using IMRT that employs the SIB technique. A second example is the indicator used in selection of candidates for intensified adjuvant chemotherapy. All of these modifications will hopefully contribute to improving clinical outcomes by early identification of patients who might not benefit from the initial treatment plan.
We generated RT plans, either conventionally fractionated 3D-CRT or dose-painting IMRT, using the shrinking field technique. The initial RT plan was designed for 36 Gy to the low risk CTV, and the subsequent plan was designed without further irradiation to this region. For this reason, the second simulation CT was performed in the third week after the initiation of RT at authors’ institution.
One question that has arisen from the current study involves the optimal timing of the second simulation CT during the RT course. Several studies investigating tumor volume changes during the RT course have been reported. The nodal and primary tumor GTVs were reported to shrink by 1.2%-3% per treatment day on average [14,15], but their regression did not occur at a constant rate. The largest tumor volume changes occurred after the second week of the RT course [21,22] and appeared to be significant after 3-4 weeks of RT, and could have a potential dosimetric impact when highly conformal RT techniques were used [14]. In addition, Wang et al. [18] reported that re-planning for NPC patients before the 25th fraction during IMRT helps to ensure an adequate dose to the target volumes, as well as safely limited doses to the critical normal structures. A speculative conclusion that could be drawn from these studies is that the appropriate timing of the second CT, either for effective adaptive intervention or for assessment of tumor regression, might be longer than 3 weeks after the start of RT. However, if adaptive re-planning is performed in the late phase of the RT course, this advantage might decrease, as there might remain only a few fractions of RT. In the current study, Most re-planning CT scans of patients were obtained between 13 to 15 fractions according to the institutional policy. It appears that this timing is quite reasonable in achieving the benefits of adaptive re-planning. Further studies on this issue are needed.
The current study may have a few weak points. First, because of its retrospective nature, selection bias might have been unavoidable. Second, Epstein-Barr virus (EBV) status, which is known to have prognostic importance in NPC patients [23,24], was not fully addressed. Again, because of the limitations of the retrospective data, tests identifying EBV were conducted in only 26 patients (16.4%). These data were considered insufficient for incorporation into prognostic analysis. Further studies investigating the association of TVRR and EBV might be needed. Finally, there is a possibility of inter- and/or intra-physician variation in GTV measurements. Despite these limitations, the discriminatory performance of the volumetric parameters could be utilized as an indicator for tailoring therapy on an individual patient basis.
Conclusion
The current study shows that TVRR measured during the RT course is a proven independent prognostic factor predicting PFS. TVRR can be easily calculated during the process of adaptive RT by simply comparing the pre- and mid-RT simulation CT images. TVRR can provide not only prognostic information, but can also be a useful indicator for tailoring treatment strategies before the completion of definitive RT.
Footnotes
Conflict of interest relevant to this article was not reported.
References
- 1.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. New York: Springer; 2009. [Google Scholar]
- 2.Ball DL, Fisher R, Burmeister B, Graham P, Joseph D, Penniment M, et al. Stage is not a reliable indicator of tumor volume in non-small cell lung cancer: a preliminary analysis of the Trans-Tasman Radiation Oncology Group 99-05 database. J Thorac Oncol. 2006;1:667–72. [PubMed] [Google Scholar]
- 3.Chen MK, Chen TH, Liu JP, Chang CC, Chie WC. Better prediction of prognosis for patients with nasopharyngeal carcinoma using primary tumor volume. Cancer. 2004;100:2160–6. doi: 10.1002/cncr.20210. [DOI] [PubMed] [Google Scholar]
- 4.Chua DT, Sham JS, Kwong DL, Tai KS, Wu PM, Lo M, et al. Volumetric analysis of tumor extent in nasopharyngeal carcinoma and correlation with treatment outcome. Int J Radiat Oncol Biol Phys. 1997;39:711–9. doi: 10.1016/s0360-3016(97)00374-x. [DOI] [PubMed] [Google Scholar]
- 5.Lok BH, Setton J, Caria N, Romanyshyn J, Wolden SL, Zelefsky MJ, et al. Intensity-modulated radiation therapy in oropharyngeal carcinoma: effect of tumor volume on clinical outcomes. Int J Radiat Oncol Biol Phys. 2012;82:1851–7. doi: 10.1016/j.ijrobp.2011.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang JZ, Mayr NA, Zhang D, Li K, Grecula JC, Montebello JF, et al. Sequential magnetic resonance imaging of cervical cancer: the predictive value of absolute tumor volume and regression ratio measured before, during, and after radiation therapy. Cancer. 2010;116:5093–101. doi: 10.1002/cncr.25260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nam H, Park W, Huh SJ, Bae DS, Kim BG, Lee JH, et al. The prognostic significance of tumor volume regression during radiotherapy and concurrent chemoradiotherapy for cervical cancer using MRI. Gynecol Oncol. 2007;107:320–5. doi: 10.1016/j.ygyno.2007.06.022. [DOI] [PubMed] [Google Scholar]
- 8.Lee H, Ahn YC, Oh D, Nam H, Kim YI, Park SY. Tumor volume reduction rate measured during adaptive definitive radiation therapy as a potential prognosticator of locoregional control in patients with oropharyngeal cancer. Head Neck. 2014;36:499–504. doi: 10.1002/hed.23328. [DOI] [PubMed] [Google Scholar]
- 9.Hoeben BA, Troost EG, Span PN, van Herpen CM, Bussink J, Oyen WJ, et al. 18F-FLT PET during radiotherapy or chemoradiotherapy in head and neck squamous cell carcinoma is an early predictor of outcome. J Nucl Med. 2013;54:532–40. doi: 10.2967/jnumed.112.105999. [DOI] [PubMed] [Google Scholar]
- 10.Yeo SG, Kim DY, Park JW, Oh JH, Kim SY, Chang HJ, et al. Tumor volume reduction rate after preoperative chemoradiotherapy as a prognostic factor in locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2012;82:e193–9. doi: 10.1016/j.ijrobp.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 11.Yang SN, Liao CY, Chen SW, Liang JA, Tsai MH, Hua CH, et al. Clinical implications of the tumor volume reduction rate in head-and-neck cancer during definitive intensity-modulated radiotherapy for organ preservation. Int J Radiat Oncol Biol Phys. 2011;79:1096–103. doi: 10.1016/j.ijrobp.2009.12.055. [DOI] [PubMed] [Google Scholar]
- 12.Mancuso AA, Mukherji SK, Schmalfuss I, Mendenhall W, Parsons J, Pameijer F, et al. Preradiotherapy computed tomography as a predictor of local control in supraglottic carcinoma. J Clin Oncol. 1999;17:631–7. doi: 10.1200/JCO.1999.17.2.631. [DOI] [PubMed] [Google Scholar]
- 13.Pameijer FA, Mancuso AA, Mendenhall WM, Parsons JT, Mukherji SK, Hermans R, et al. Evaluation of pretreatment computed tomography as a predictor of local control in T1/T2 pyriform sinus carcinoma treated with definitive radiotherapy. Head Neck. 1998;20:159–68. doi: 10.1002/(sici)1097-0347(199803)20:2<159::aid-hed10>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 14.Barker JL, Jr, Garden AS, Ang KK, O'Daniel JC, Wang H, Court LE, et al. Quantification of volumetric and geometric changes occurring during fractionated radiotherapy for head-and-neck cancer using an integrated CT/linear accelerator system. Int J Radiat Oncol Biol Phys. 2004;59:960–70. doi: 10.1016/j.ijrobp.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 15.Castadot P, Geets X, Lee JA, Christian N, Gregoire V. Assessment by a deformable registration method of the volumetric and positional changes of target volumes and organs at risk in pharyngo-laryngeal tumors treated with concomitant chemo-radiation. Radiother Oncol. 2010;95:209–17. doi: 10.1016/j.radonc.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Hansen EK, Bucci MK, Quivey JM, Weinberg V, Xia P. Repeat CT imaging and replanning during the course of IMRT for head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2006;64:355–62. doi: 10.1016/j.ijrobp.2005.07.957. [DOI] [PubMed] [Google Scholar]
- 17.Bhide SA, Davies M, Burke K, McNair HA, Hansen V, Barbachano Y, et al. Weekly volume and dosimetric changes during chemoradiotherapy with intensity-modulated radiation therapy for head and neck cancer: a prospective observational study. Int J Radiat Oncol Biol Phys. 2010;76:1360–8. doi: 10.1016/j.ijrobp.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Wang W, Yang H, Hu W, Shan G, Ding W, Yu C, et al. Clinical study of the necessity of replanning before the 25th fraction during the course of intensity-modulated radiotherapy for patients with nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2010;77:617–21. doi: 10.1016/j.ijrobp.2009.08.036. [DOI] [PubMed] [Google Scholar]
- 19.Zhao L, Wan Q, Zhou Y, Deng X, Xie C, Wu S. The role of replanning in fractionated intensity modulated radiotherapy for nasopharyngeal carcinoma. Radiother Oncol. 2011;98:23–7. doi: 10.1016/j.radonc.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Yang H, Hu W, Wang W, Chen P, Ding W, Luo W. Replanning during intensity modulated radiation therapy improved quality of life in patients with nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2013;85:e47–54. doi: 10.1016/j.ijrobp.2012.09.033. [DOI] [PubMed] [Google Scholar]
- 21.Bosmans G, van Baardwijk A, Dekker A, Ollers M, Boersma L, Minken A, et al. Intra-patient variability of tumor volume and tumor motion during conventionally fractionated radiotherapy for locally advanced non-small-cell lung cancer: a prospective clinical study. Int J Radiat Oncol Biol Phys. 2006;66:748–53. doi: 10.1016/j.ijrobp.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 22.Britton KR, Starkschall G, Tucker SL, Pan T, Nelson C, Chang JY, et al. Assessment of gross tumor volume regression and motion changes during radiotherapy for non-small-cell lung cancer as measured by four-dimensional computed tomography. Int J Radiat Oncol Biol Phys. 2007;68:1036–46. doi: 10.1016/j.ijrobp.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 23.Chan AT, Ma BB, Lo YM, Leung SF, Kwan WH, Hui EP, et al. Phase II study of neoadjuvant carboplatin and paclitaxel followed by radiotherapy and concurrent cisplatin in patients with locoregionally advanced nasopharyngeal carcinoma: therapeutic monitoring with plasma Epstein-Barr virus DNA. J Clin Oncol. 2004;22:3053–60. doi: 10.1200/JCO.2004.05.178. [DOI] [PubMed] [Google Scholar]
- 24.Lin JC, Chen KY, Wang WY, Jan JS, Liang WM, Tsai CS, et al. Detection of Epstein-Barr virus DNA in the peripheral-blood cells of patients with nasopharyngeal carcinoma: relationship to distant metastasis and survival. J Clin Oncol. 2001;19:2607–15. doi: 10.1200/JCO.2001.19.10.2607. [DOI] [PubMed] [Google Scholar]