Abstract
Purpose
The purpose of this study is to assess the utility of positron emission tomography (PET) for predicting recurrence among patients with T1-T2/N1 breast cancer who were treated with mastectomy.
Materials and Methods
Of 712 consecutive patients with T1-T2/N1 breast cancer treated during 2003-2012, 109 had undergone preoperative 18F-fluorodeoxyglucose/PET and were included. Metabolic (maximum standardized uptake value [SUVmax]), volumetric (metabolic tumor volume [MTV]), and combined (total lesion glycolysis [TLG]) indices were measured. The resulting values were analyzed and compared with clinical outcome.
Results
At the median follow-up of 46.7 months, the 3-year relapse-free survival (RFS) rate was 95.2%. SUVmax (area under curve, 0.824) was more useful than MTV or TLG as a means of identifying patients at high risk for any recurrence. In multivariate analysis, SUVmax remained an independent risk factor for RFS (p=0.006). Using the method of Contal and O’Quigley, a SUVmax threshold of 5.36 showed the best predictive performance. The PET-based high-risk group (≥ 5.36 in either breast or nodes) had more T1c-T2, high-grade, hormone-receptor negative, and invasive ductal carcinoma tumors than the low-risk group (< 5.36 in both breast and nodes). The prognosis was much worse when high SUVmax (≥ 5.36) was detected in nodes (p < 0.001). In the no-radiotherapy cohort, the PET-based high-risk group had increased risk of locoregional recurrence when compared to the low-risk group (p=0.037).
Conclusion
High SUVmax on preoperative PET showed association with elevated risk of locoregional recurrence and any recurrence. Pre-treatment PET may improve assessments of recurrence risk and clarify indications for post-mastectomy radiotherapy in this subset of patients.
Keywords: Breast neoplasms, Positron-emission tomography, Recurrence, Prognosis, Mastectomy
Introduction
The results of meta-analyses by the Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) showed that postmastectomy radiotherapy (PMRT) reduced the risks of recurrence and cancer death [1]. Updated studies confirmed benefits of PMRT in patients with N1 disease, irrespective of the use of systemic therapy and axillary dissection [2,3]. When using modern treatment approaches, accurate identification of high-risk patients for PMRT while sparing the remaining patients from potentially unnecessary and costly therapy, and continuing to provide them with excellent local control rates is imperative [4,5]. However, the indications for PMRT are still determined on a case-by-case basis, and definitive indications have varied significantly across institutions and changed over time. Thus, the development of a practical prognostic indicator for recurrence is required to optimize the application of PMRT for this controversial situation.
18F-Fluorodeoxyglucose/positron emission tomography (18F-FDG/PET), which detects the enhanced glycolysis of tumors, has emerged as a useful imaging tool in staging, evaluation of treatment response, and predicting the prognosis of breast cancer [6]. In Korea, 18F-FDG/PET staging is reimbursed for patients who are newly diagnosed with breast cancer; as a result, its use has increased for both locally advanced and early-stage breast cancers. Here, we sought to evaluate the potential utility of 18F-FDG/PET for predicting prognosis in a group of T1-T2/N1 breast cancer patients who underwent mastectomy, and we assessed the question of whether this PET-based high-risk group was at increased risk of locoregional recurrence after treatment without PMRT, who might be potential candidates for adjuvant radiation therapy.
Materials and Methods
1. Patients
A total of 712 consecutive patients who underwent mastectomy and were diagnosed with pathologic T1-T2/N1 breast cancer at a single institution between 2003 and 2012 were identified. Of these 712 patients, the 109 who underwent preoperative 18F-FDG/PET scanning were included in the current study. At our institution, 18F-FDG/PET staging was first offered to patients with early breast cancer in November 2003. An increase in systemic staging with 18F-FDG/PET was observed in our study cohort during the study period (2003-2009: n=46/361, 14% vs. 2010-2012: n=63/114, 55%).
2. Treatment
All patients underwent modified radical mastectomy. Sentinel lymph node (SLN) sampling was performed in all patients, except those (19%) who had biopsy-confirmed or suspicious axillary nodal metastasis on PET imaging. If the frozen section of the SLN was positive, the patient underwent completion of axillary dissection (median, 12 nodes; range, 4 to 34 nodes). Although PMRT was delivered at the physician’s discretion, it was generally performed for patients with high-risk features (mainly based on the number of positive lymph nodes). Adjuvant systemic therapy was selected after discussion with the patient’s medical oncologist.
3. 18F-FDG/PET
18F-FDG/PET scans were performed using a dedicated PET/computed tomography (CT) scanner (Discovery STE, GE Healthcare, Little Chalfont, UK, or Biograph TruePoint 40, Siemens Healthcare, Erlangen, Germany). The mean time interval from PET scan to mastectomy was 10±9 days. The detailed protocols for measurement of blood glucose concentration, determination of injected 18F-FDG quantity, low-dose and contrast enhanced CT and PET scans, and PET data reconstruction have all been described previously [7]. Semiquantitative and volumetric measurements of maximum standardized uptake value (SUVmax), mean SUV (SUVmean), metabolic tumor volume (MTV), and total lesion glycolysis (TLG) of whole body tumors were performed with the PETedge tool that is available in MIMvista software (MIMvista Corp., Cleveland, OH), according to the protocol of Liao et al. [8]. After contouring the tumor using the PETedge tool, volumes of interest (VOIs) were automatically produced by spatial derivatives to locate the tumor surface. The estimated VOIs were manually adjusted using a 2-D “ball” contouring tool. In this study, SUVmax was the maximum SUVmax of all tumors either in breast tissue or in the axillary area. TLG was calculated as follows: TLG=SUVmean×MTV. MTV and TLG were computed after summing the corresponding values of all tumors. 18F-FDG/PET was administered at the clinical discretion of the treating physician during the study period.
4. Statistical analysis
The primary end-point of our study was relapse-free survival (RFS), defined as the length of time from the date of mastectomy to any type of relapse. RFS was estimated using the Kaplan-Meier method. Comparisons of PET parameters according to clinical parameters or any recurrence (locoregional recurrence and distant metastasis as first site of failure) were performed using t tests. One-way analysis of variance (ANOVA) with a post-hoc Bonferroni’s correction was used for multiple comparisons. Receiver operating characteristics (ROC) analyses were performed to determine which parameter was most useful for predicting disease recurrence risk. Univariate and multivariate analyses of disease-free survival (DFS) were performed using Cox’s proportional hazards regression method to assess whether the PET parameter retained statistical significance after adjusting for known clinicopathologic variables. Clinically relevant variables were selected, and multivariate analysis was performed using backwards elimination. The method of Contal and O’Quigley was used to determine the cut-off point for the PET parameter, to allow objective dichotomization [9]. Using this method, the optimal cut-off point is determined by setting it to the point that maximizes the model likelihood. Because all possible cut-off points are assessed, an adjustment is applied to the p-value to control for type I error. Next, to identify the subgroup of patients who may benefit from PMRT, a secondary analysis was performed, excluding patients who underwent PMRT (no-PMRT cohort). The level of statistical significance was set at 0.05. All statistical analyses were performed using SPSS ver. 20.0 (IBM SPSS Statistics, IBM Co., Armonk, NY) or SAS (SAS Institute Inc., Cary, NC).
Results
1. Patient, tumor, and treatment characteristics
The median follow-up period was 46.7 months (range, 14 to 127 months). Data on patient, tumor, and treatment characteristics are summarized in Table 1. The median age of patients was 49 years (range, 27 to 92 years). The mean tumor size was 2.0±0.9 cm (range, 0.4 to 4.5 cm). SLN sampling was performed in 87 patients (81%), with a median of 3 (range, 1 to 7) identified. Axillary dissections were performed in all patients with a median of 12 (range, 4 to 34) total nodes dissected. Adjuvant systemic therapy was administered in most patients (105 of 109, 96%) as follows: chemotherapy (n=94, 86%) and endocrine therapy (n=58, 54%). PMRT was delivered to 34% of the patients.
Table 1.
Patient, tumor, and treatment characteristics
| Characteristic | No. (%) (n=109) |
|---|---|
| Age (yr) | |
| < 45 | 29 (27) |
| ≥ 45 | 80 (73) |
| Histology | |
| IDC | 100 (92) |
| Non-IDC | 9 (8) |
| T stage | |
| T1 | 61 (56) |
| T1a | 2 (2) |
| T1b | 15 (14) |
| T1c | 44 (40) |
| T2 | 48 (44) |
| LVI | |
| Negative | 102 (94) |
| Positive | 7 (6) |
| No. of positive lymph nodes | |
| 1 | 69 (63) |
| 2 | 29 (27) |
| 3 | 11 (10) |
| Percentage of positive lymph nodes | |
| < 25 | 103 (94) |
| ≥ 25 | 6 (6) |
| ECE | |
| Negative | 100 (92) |
| Positive | 9 (8) |
| Histological grade | |
| 1 | 21 (20) |
| 2 | 60 (57) |
| 3 | 25 (24) |
| Estrogen receptor status | |
| Negative | 25 (23) |
| Positive | 84 (77) |
| Progesterone receptor status | |
| Negative | 48 (44) |
| Positive | 61 (56) |
| HER2 overexpression | |
| Negative | 73 (69) |
| Positive | 33 (31) |
| Systemic chemotherapy and/or hormone therapy | 105 (96) |
| Chemotherapy | 94 (86) |
| AC±T | 75 (69) |
| CMF | 14 (13) |
| Unspecified | 5 (5) |
| Hormone therapy | 58 (53) |
| Post-mastectomy radiotherapy | |
| No | 72 (66) |
| Yes | 37 (34) |
IDC, invasive ductal carcinoma; LVI, lymphovascular invasion; ECE, extracapsular extension; AC, adriamycin cyclophosphamide; T, paclitaxel docetaxel; CMF, cyclophosphamide methotrexate 5-fluorouracil.
2. Semiquantitative analysis of PET parameters
Most patients (97.2%) had 18F-FDG–avid tumors in the primary site, and 22 patients (20.2%) had 18F-FDG–avid tumors in axillary nodes. For whole body tumors (WT), the average SUVmax, MTV, and TLG was 4.79±3.07, 7.07±5.97 mL, and 20.22±26.0, respectively. For 18F-FDG–avid tumors in breast tissue (n=106), the average SUVmax, MTV, and TLG was 4.75±3.01, 5.92±5.02 mL, and 17.49±23.48, respectively. For 18F-FDG–avid tumors in axillary nodes (n=22), the average SUVmax, MTV, and TLG was 4.70±3.38, 4.09±4.14 mL, and 12.00±16.13, respectively.
Significant correlations were observed among three parameters (TLGWT vs. MTVWT, Spearman’s rho=0.882; TLGWT vs. SUVmax WT, rho=0.718; MTVWT vs. SUVmax WT, rho=0.574; all p < 0.001). Differences in SUVmax WT, MTVWT, and TLGWT according to clinicopathologic parameters are shown in Table 2. A significantly higher mean SUVmax WT was observed for invasive ductal carcinoma (IDC) tumors compared with non-IDC tumors (p < 0.001). Significantly higher mean values of SUVmax WT, MTVWT, and TLGWTwere observed in T2, high-grade (G3), estrogen receptor (ER)–negative, or progesterone receptor (PR)–negative, tumors than in T1, non-high grade (G1/2), ER+, or PR+ tumors, respectively (all p < 0.05). In addition, as T stage increased, SUVmax of breast, axillary nodes, and WT were increased and more FDG-avid tumors were found in axillary nodes (T1a, 0%; T1b, 13.3%; T1c, 20.5%; T2, 22.9%) (Table 3).
Table 2.
Comparisons of PET indices in whole body tumors according to clinicopathologic parameters (n=109)
| Variable | SUVmax | p-value | MTV (mL) | p-value | TLG | p-value |
|---|---|---|---|---|---|---|
| Age (yr) | ||||||
| < 45 | 3.99±2.44 | 0.099 | 5.15±3.81 | 0.042 | 13.84±13.92 | 0.038 |
| ≥ 45 | 5.09±3.24 | 7.77±6.46 | 22.54±28.90 | |||
| Histology | ||||||
| IDC | 5.04±3.07 | < 0.001 | 7.22±5.75 | 0.409 | 21.19±26.52 | 0.196 |
| Non-IDC | 2.01±1.08 | 5.49±8.32 | 9.47±16.79 | |||
| T stage | ||||||
| T1 | 4.12±2.29 | 0.014 | 5.40±5.14 | 0.001 | 12.17±13.29 | < 0.001 |
| T2 | 5.65±3.70 | 9.19±6.33 | 30.46±33.71 | |||
| LVI | ||||||
| Negative | 4.75±3.10 | 0.570 | 7.01±6.00 | 0.671 | 19.90±26.39 | 0.551 |
| Positive | 5.42±2.84 | 8.04±5.92 | 24.97±20.37 | |||
| No. of positive LNs | ||||||
| 1 | 4.43±2.59 | 0.101 | 6.22±5.23 | 0.051 | 15.84±17.40 | 0.020 |
| 2, 3 | 5.43±3.71 | 8.54±6.90 | 27.79±35.37 | |||
| Positive LNs (%) | ||||||
| < 25 | 4.82±3.10 | 0.695 | 7.17±5.98 | 0.507 | 20.34±26.25 | 0.852 |
| ≥ 25 | 4.31±2.89 | 5.49±6.10 | 18.28±23.14 | |||
| ECE | ||||||
| Negative | 4.81±3.08 | 0.869 | 6.97±5.61 | 0.546 | 19.20±21.59 | 0.532 |
| Positive | 4.63±3.25 | 8.23±9.52 | 31.57±56.55 | |||
| Histological grade | ||||||
| 1, 2 | 3.97±2.59 | < 0.001 | 6.04±5.02 | 0.004 | 14.05±16.20 | 0.002 |
| 3 | 7.73±2.85 | 10.93±7.40 | 42.04±38.88 | |||
| Estrogen receptor status | ||||||
| Negative | 6.66±4.03 | 0.008 | 9.50±6.19 | 0.020 | 33.72±31.19 | 0.014 |
| Positive | 4.24±2.50 | 6.35±5.75 | 16.21±22.96 | |||
| Progesterone receptor status | ||||||
| Negative | 5.52±3.53 | 0.028 | 9.22±7.00 | 0.001 | 29.37±32.93 | 0.002 |
| Positive | 4.22±2.55 | 5.39±4.38 | 13.03±15.74 | |||
| HER2 overexpression | ||||||
| Negative | 4.73±2.64 | 0.818 | 6.77±5.38 | 0.538 | 17.44±18.66 | 0.237 |
| Positive | 4.90±3.98 | 7.63±7.10 | 25.64±37.16 |
PET, positron emission tomography; SUVmax, maximum standardized uptake value; MTV, metabolic tumor volume; TLG, total lesion glycolysis; IDC, invasive ductal carcinoma; LVI, lymphovascular invasion; LN, lymph node; ECE, extracapsular extension.
Table 3.
Comparisons of PET indices in breast, lymph node, and WT according to T classification (n=109)
| T stage | Breast |
Lymph node |
WT SUVmax | ||
|---|---|---|---|---|---|
| Non-avid | SUVmax | Non-avid | SUVmax | ||
| T1a | 0/2 (0) | NA | 2/2 (100) | NA | NA |
| T1b | 1/15 (6.7) | 1.84±0.39 | 13/15 (86.7) | 3.00±2.80 | 3.55±2.03 |
| T1c | 0/44 (0) | 4.80±1.97 | 35/44 (79.5) | 3.64±2.15 | 5.59±2.00 |
| T2 | 2/46 (4.2) | 8.45±4.49 | 37/48 (77.1) | 5.87±4.06 | 8.94±4.38 |
Values are presented as number (%) or mean±standard deviation. PET, positron emission tomography; WT, whole body tumors; SUVmax, maximum standardized uptake value; NA, not applicable.
3. Recurrence
At the time of analysis, six patients (6%) had experienced any recurrences, three presented with locoregional recurrence and three presented with distant metastasis. Six patients (6%) died, either with (n=2) or without (n=4) disease progression. The 3-year RFS and overall survival (OS) rates were 95.2% (95% confidence interval [CI], 91.1% to 99.3%) and 91.8% (95% CI, 84.7% to 98.9%), respectively. The relationship between SUV results and tumor recurrence was examined; patients with any recurrence had higher levels of SUVmax WT, MTVWT, and TLGWT at baseline than those without any recurrence (Fig. 1A). Similar levels of SUVmax WT, MTVWT, and TLGWT were observed between patients who failed locoregionally or distally (all p=0.001; ANOVA with Bonferroni correction) (Fig. 1B).
Fig. 1.
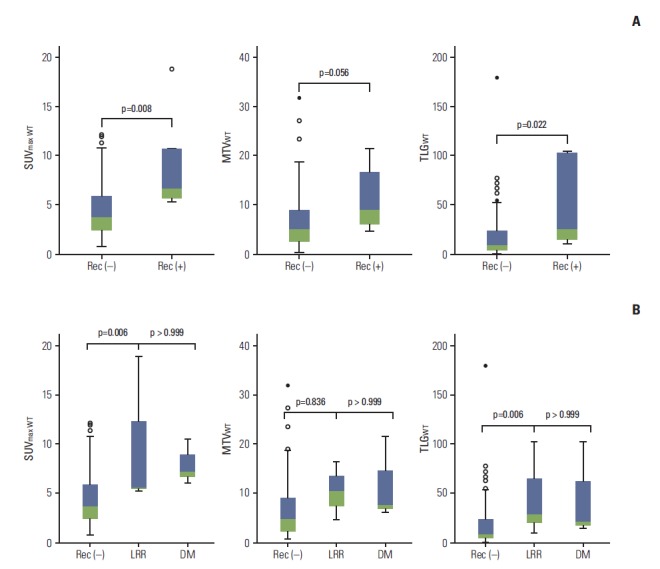
Box plots of maximum standardized uptake value (SUVmax WT), metabolic tumor volume (MTVWT), and total lesion glycolysis (TLGWT), as shown for any recurrence (Rec) (A) and locoregional recurrence (LRR) and distant metastasis (DM) (B).
4. Usefulness of PET for predicting recurrence
ROC curves were generated for SUVmax WT, MTVWT, and TLGWT (Fig. 2). In the ROC analyses, the areas under the curves (AUCs) were 0.824 (95% CI, 0.715 to 0.932) for SUVmax, 0.733 (95% CI, 0.574 to 0.892) for MTV, and 0.778 (95% CI, 0.629 to 0.928) for TLG. These results indicated that SUVmax WT was the most useful index for predicting patients at high risk of developing any recurrence. The relationship of SUVmax WT with the time of any recurrence was also assessed after adjusting for all available clinicopathological variables. In univariate analysis, increased SUVmax WT (considered as a continuous variable) showed significant association with poorer RFS (p=0.001). The significance of this relationship with RFS was retained in the stepwise multivariate regression analysis (p=0.004) (Table 4).
Fig. 2.
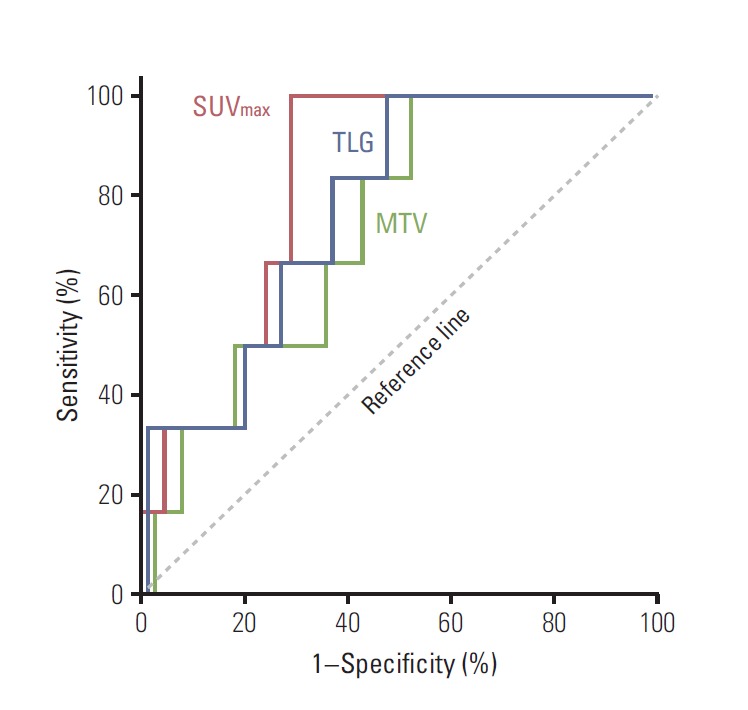
Receiver-operating characteristics curves for maximum standardized uptake value (SUVmax), total lesion glycolysis (TLG), and metabolic tumor volume (MTV) of whole body tumors.
Table 4.
Stepwise univariate and multivariate analyses using Cox’s regression method for disease-free survival in 109 patients
| Variable | Univariate analysis |
Multivariate analysisa) |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | |
| Age (≥ 45 yr vs. < 45 yr) | 0.63 | 0.12-3.48 | 0.597 | 0.14 | 0.01-2.24 | 0.165 |
| T stage (T1 vs. T2) | 0.46 | 0.08-2.55 | 0.373 | NI | - | |
| No. of positive LNs (1 vs. 2, 3) | 0.77 | 0.14-4.22 | 0.761 | 0.19 | 0.01-3.74 | 0.276 |
| Histological grade (1, 2 vs. 3) | 0.63 | 0.12-3.44 | 0.593 | 0.02 | 0.00-0.73 | 0.032 |
| Estrogen receptor status (positive vs. negative) | 0.16 | 0.03-0.86 | 0.033 | 0.04 | 0.00-0.60 | 0.02 |
| Progesterone receptor status (positive vs. negative) | 0.76 | 0.15-3.76 | 0.734 | NI | - | - |
| HER2 overexpression (positive vs. negative) | 1.19 | 0.22-6.51 | 0.844 | NI | - | - |
| Post-mastectomy radiotherapy (yes vs. no) | 0.39 | 0.05-3.41 | 0.396 | 0.06 | 0.00-2.58 | 0.141 |
| 18F-FDG-avidity in axillary nodes (avid vs. non-avid) | 4.30 | 0.87-21.29 | 0.074 | NI | - | - |
| SUVmax (as continuous) | 1.43 | 1.15-1.78 | 0.001 | 1.77 | 1.19-2.62 | 0.004 |
HR, hazard ratio; CI, confidence interval; LN, lymph node; 18F-FDG, 18F-fluorodeoxyglucose; SUVmax, maximum standardized uptake value.
Variables were entered into the multivariate regression model in a stepwise method if p < 0.20 and were removed at any point if p-value was > 0.20.
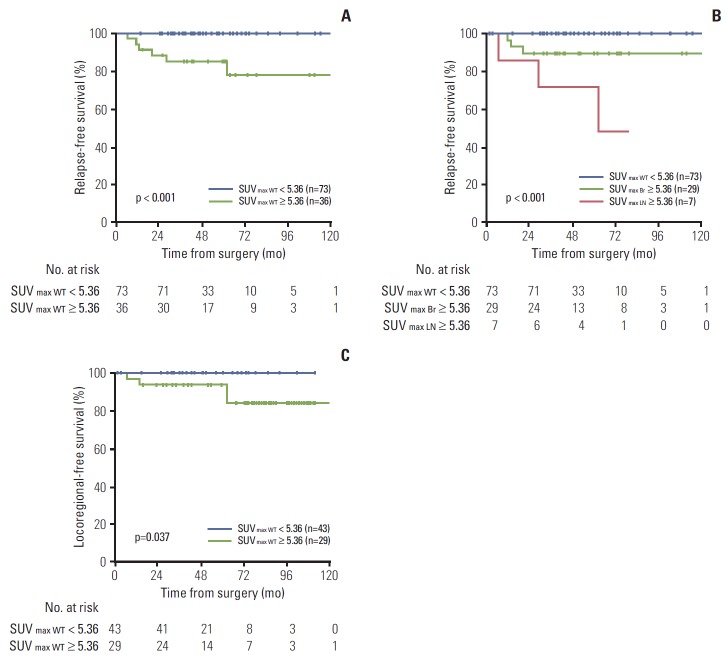
The method of Contal and O’Quigley was also used to objectively determine a cut-off point for SUVmax WT [9]. According to this method, the best predicted clinical performance was a threshold of 5.36, which provided a sensitivity, specificity, positive predictive value, and negative predictive value of 100%, 71%, 17%, and 100%, respectively. The patients were then divided into two groups (high- vs. low-risk) according to this cut-off point, and the characteristics of patients with SUVmax WT ≥ 5.36 were compared to those of patients with SUVmax < 5.36. PET-based high-risk patients (SUVmax WT ≥ 5.36) had more T1c-T2 (vs. T1a-T1b), high-grade, ER/PR negative, and IDC (vs. non-IDC) tumors than lowrisk patients (SUVmax WT < 5.36) (all p < 0.05). Three-year RFS was 85.4% in the PET-based high-risk group compared with 100% in the low-risk group (p < 0.001) (Fig. 3A). The prognosis was much worse when high SUVmax (≥ 5.36) was detected in axillary lymph nodes (p < 0.001) (Fig. 3B).
Fig. 3.
Relapse-free survival rates in the entire cohort (n=109) (A, B) and locoregional recurrence-free survival (C) in the no post-mastectomy radiotherapy cohort (n=72), according to high (≥ 5.36) and low (< 5.36) maximum standardized uptake value (SUVmax) of whole body tumors (WT) (SUVmax WT), the primary tumor (Br) (SUVmax Br), or the axillary lymph nodes (LN) (SUVmax LN).
5. Locoregional recurrence in the no-PMRT cohort
In the no-PMRT cohort (n=72), an increased risk of locoregional recurrence was observed for the PET-based high-risk group, compared with the PET-based low-risk group (3-year locoregional recurrence-free survival, 92.9% vs. 100%; p=0.037) (Fig. 3C). However, age, T stage, number of positive lymph nodes, histologic grade, ER/PR/HER2 status, 18F-FDG–avidity in axillary nodes, and other factors did not show significant association with risk of locoregional recurrence.
Discussion
In this study, we have described the efficacy of PET for identifying patients with T1-T2/N1 breast cancer with a high risk of disease recurrence after mastectomy and standard systemic therapy. We found an association of higher SUVmax WT with both poorer RFS in the entire cohort and a higher risk of locoregional recurrence in the no-PMRT cohort. The SUVmax cut-off value that best predicted prognosis was 5.36. Compared with tumors in the PET-defined low-risk group, tumors in the high-risk group were more likely to have the following characteristics: T1c-T2 (vs. T1a-T1b) stage, high grade, ER/PR negativity, and IDC (vs. non-IDC). In conjunction with conventional risk stratification that relies on multiple factors, preoperative 18F-FDG/PET information may enable identification of high-risk groups with enhanced accuracy. We hypothesize that once these select groups are identified, they may represent the population of T1-2/N1 breast cancer patients who might benefit from PMRT.
Previous retrospective studies have investigated the usefulness of PET in breast cancer, with findings similar to ours [10-14]. Osborne et al. [10] reported that ER– tumors had higher 18F-FDG-uptake than ER+ tumors (median SUVmax, 8.5 vs. 4.0). A study by Mavi et al. [11] reported that ER status alone (rather than PR or HER2 status) had an effect on FDG uptake (F=9.126, p < 0.01). Baba et al. [12] reported correlation of high SUVmaxwith larger tumor size, higher grade, and triple-negative tumors, as well as poor prognosis (5-year DFS: ≥ 4.16 [n=32], 80% vs. < 4.16 [n=28], 100%). Song et al. [13] reported that nodal SUVmax was the only determinant of DFS among well-known clinical variables in N1-3 patients (n=65), similar to our finding that high SUVmax in lymph nodes was associated with worse RFS than high SUVmax in the primary tumor. Nakajima et al. [14] reported association of MTV and TLG with tumor size, number of positive nodes, nodal ratio, nuclear grade, ER status, and triple negative status. Nakajima et al. [14] found that larger MTV was an independent risk factor for locoregional recurrence-free survival and DFS. In comparison with our cohort of patients, the cohort considered by Nakajima et al. [14] had different characteristics, including a higher proportion of T2 disease (63% vs. 44%), more biologically aggressive behavior (SUVmax, MTV, and TLG; median 7.2, 31.7, and 73.4 vs. mean 4.8, 7.1, 20.2), and lesser use of chemotherapy (62.4% vs. 86%). These differences may have increased the importance of MTV in the study by Nakajima et al. [14] as compared with our own. However, both studies found that the AUC of SUVmax was high (> 0.80), and the consistency of these findings should be highlighted.
Compared with other studies, our study had the following strengths: (1) the cohort consisted of a homogenous group of patients with pathologic T1-T2/N1 disease, who were treated with mastectomy and current standard adjuvant therapy. In addition, (2) we did not limit the investigation to metabolic (SUVmax) values, but also assessed volumetric (MTV) values and the combination of both indices (in the form of TLG). The relationships between these indices, RFS, and locoregional recurrence were then evaluated. Finally, (3) the analyses focused on an issue that is currently relevant in clinical practice.
From a cost-effectiveness perspective, it is not clear whether PET should be offered to all patients with T1-T2/N1 disease, in order to identify those at high risk of recurrence, and accordingly indicate PMRT. If our results are confirmed in other series, cost-effectiveness will present another clinical issue. Currently, National Comprehensive Cancer Network (NCCN) experts recommend against the routine use of PET in early breast cancer because of high false-negative rates in small or low grade breast tumors, low sensitivity for detection of nodal metastasis, low prior probability of distant metastasis, and (mainly) cost-ineffectiveness [15]. However, recent studies (including studies with prospective designs) have raised the possibility that PET may have a role in stage II breast cancer [15-19]. The following studies reported relevant results: Groves et al. [17] found that PET impacted cancer management in 16% of patients with stage II disease, Groheux et al. [15] reported that PET modified staging in 5.5% of patients with stage IA disease and 13% of patients with stage IB disease, and the Ontario Clinical Oncology Group Study reported that PET could influence surgical care by avoiding unnecessary SLN biopsies [16].
Our study has several limitations, including those that are inherent to retrospective analyses. Because of the small number of events (recurrences), it was not possible to perform a more complete statistical analysis. Longer follow-up is needed to clarify the relationship between 18F-FDG uptake and dormancy/late recurrence. Although the 5-year OS did not differ significantly according to the use of PET in our cohort (n=712; 5-year OS rate, 93.1% with PET vs. 91% without PET; p=0.412), it is possible that selection bias in our study sample resulted in a better prognosis in the PET group, affecting the results in terms of the cut-off value.
Conclusion
In conclusion, we found that a high SUVmax (≥ 5.36) was associated with an increased risk of locoregional recurrence, as well as any recurrence. Patients with high SUVmax in lymph node involvement appeared to have a much worse prognosis than those with high SUVmax in primary tumors. However, these findings are purely hypothesis generating; confirmatory studies with larger cohorts and longer follow-up periods are necessary before PET-defined risk assessment can be routinely adopted for early breast cancer. Efforts to define the optimal cut-off values of PET parameters are also necessary.
Acknowledgments
We thank Hye Sun Lee, MS, Department of Biostatistics, Severance Hospital, for statistical consultation and analysis of the data.
Footnotes
Conflict of interest relevant to this article was not reported.
References
- 1.Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans V, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 2.EBCTCG (Early Breast Cancer Trialists' Collaborative Group) McGale P, Taylor C, Correa C, Cutter D, Duane F, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383:2127–35. doi: 10.1016/S0140-6736(14)60488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poortmans P. Postmastectomy radiation in breast cancer with one to three involved lymph nodes: ending the debate. Lancet. 2014;383:2104–6. doi: 10.1016/S0140-6736(14)60192-6. [DOI] [PubMed] [Google Scholar]
- 4.Wallgren A, Bonetti M, Gelber RD, Goldhirsch A, Castiglione-Gertsch M, Holmberg SB, et al. Risk factors for locoregional recurrence among breast cancer patients: results from International Breast Cancer Study Group Trials I through VII. J Clin Oncol. 2003;21:1205–13. doi: 10.1200/JCO.2003.03.130. [DOI] [PubMed] [Google Scholar]
- 5.Taghian A, Jeong JH, Mamounas E, Anderson S, Bryant J, Deutsch M, et al. Patterns of locoregional failure in patients with operable breast cancer treated by mastectomy and adjuvant chemotherapy with or without tamoxifen and without radiotherapy: results from five National Surgical Adjuvant Breast and Bowel Project randomized clinical trials. J Clin Oncol. 2004;22:4247–54. doi: 10.1200/JCO.2004.01.042. [DOI] [PubMed] [Google Scholar]
- 6.Langer A. A systematic review of PET and PET/CT in oncology: a way to personalize cancer treatment in a cost-effective manner? BMC Health Serv Res. 2010;10:283. doi: 10.1186/1472-6963-10-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yun M, Lim JS, Noh SH, Hyung WJ, Cheong JH, Bong JK, et al. Lymph node staging of gastric cancer using (18)F-FDG PET: a comparison study with CT. J Nucl Med. 2005;46:1582–8. [PubMed] [Google Scholar]
- 8.Liao S, Penney BC, Wroblewski K, Zhang H, Simon CA, Kampalath R, et al. Prognostic value of metabolic tumor burden on 18F-FDG PET in nonsurgical patients with non-small cell lung cancer. Eur J Nucl Med Mol Imaging. 2012;39:27–38. doi: 10.1007/s00259-011-1934-6. [DOI] [PubMed] [Google Scholar]
- 9.Contal C, O'Quigley J. An application of changepoint methods in studying the effect of age on survival in breast cancer. Comput Stat Data Anal. 1999;30:253–70. [Google Scholar]
- 10.Osborne JR, Port E, Gonen M, Doane A, Yeung H, Gerald W, et al. 18F-FDG PET of locally invasive breast cancer and association of estrogen receptor status with standardized uptake value: microarray and immunohistochemical analysis. J Nucl Med. 2010;51:543–50. doi: 10.2967/jnumed.108.060459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mavi A, Cermik TF, Urhan M, Puskulcu H, Basu S, Yu JQ, et al. The effects of estrogen, progesterone, and C-erbB-2 receptor states on 18F-FDG uptake of primary breast cancer lesions. J Nucl Med. 2007;48:1266–72. doi: 10.2967/jnumed.106.037440. [DOI] [PubMed] [Google Scholar]
- 12.Baba S, Isoda T, Maruoka Y, Kitamura Y, Sasaki M, Yoshida T, et al. Diagnostic and prognostic value of pretreatment SUV in 18F-FDG/PET in breast cancer: comparison with apparent diffusion coefficient from diffusion-weighted MR imaging. J Nucl Med. 2014;55:736–42. doi: 10.2967/jnumed.113.129395. [DOI] [PubMed] [Google Scholar]
- 13.Song BI, Lee SW, Jeong SY, Chae YS, Lee WK, Ahn BC, et al. 18F-FDG uptake by metastatic axillary lymph nodes on pretreatment PET/CT as a prognostic factor for recurrence in patients with invasive ductal breast cancer. J Nucl Med. 2012;53:1337–44. doi: 10.2967/jnumed.111.098640. [DOI] [PubMed] [Google Scholar]
- 14.Nakajima N, Kataoka M, Sugawara Y, Ochi T, Kiyoto S, Ohsumi S, et al. Volume-based parameters of 18F-fluorodeoxyglucose positron emission tomography/computed tomography improve disease recurrence prediction in postmastectomy breast cancer patients with 1 to 3 positive axillary lymph nodes. Int J Radiat Oncol Biol Phys. 2013;87:738–46. doi: 10.1016/j.ijrobp.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Groheux D, Giacchetti S, Espie M, Vercellino L, Hamy AS, Delord M, et al. The yield of 18F-FDG PET/CT in patients with clinical stage IIA, IIB, or IIIA breast cancer: a prospective study. J Nucl Med. 2011;52:1526–34. doi: 10.2967/jnumed.111.093864. [DOI] [PubMed] [Google Scholar]
- 16.Pritchard KI, Julian JA, Holloway CM, McCready D, Gulenchyn KY, George R, et al. Prospective study of 2-[(1) (8)F]fluorodeoxyglucose positron emission tomography in the assessment of regional nodal spread of disease in patients with breast cancer: an Ontario clinical oncology group study. J Clin Oncol. 2012;30:1274–9. doi: 10.1200/JCO.2011.38.1103. [DOI] [PubMed] [Google Scholar]
- 17.Groves AM, Shastry M, Ben-Haim S, Kayani I, Malhotra A, Davidson T, et al. Defining the role of PET-CT in staging early breast cancer. Oncologist. 2012;17:613–9. doi: 10.1634/theoncologist.2011-0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riedl CC, Slobod E, Jochelson M, Morrow M, Goldman DA, Gonen M, et al. Retrospective analysis of 18F-FDG PET/CT for staging asymptomatic breast cancer patients younger than 40 years. J Nucl Med. 2014;55:1578–83. doi: 10.2967/jnumed.114.143297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Groheux D, Moretti JL, Baillet G, Espie M, Giacchetti S, Hindie E, et al. Effect of (18)F-FDG PET/CT imaging in patients with clinical Stage II and III breast cancer. Int J Radiat Oncol Biol Phys. 2008;71:695–704. doi: 10.1016/j.ijrobp.2008.02.056. [DOI] [PubMed] [Google Scholar]