Abstract
Cholangiocarcinoma with paraneoplastic dermatomyositis (DM) is extremely rare, and the whole body positron emission tomography-computed tomography (PET-CT) finding of paraneoplastic DM is rarely reported. We report a 66-year-old woman with metastatic cholangiocarcinoma, initially presented with bilateral proximal muscle uptake on PET-CT without clinical muscle symptoms. The initial interpretation of the high muscle uptake was metastasis to the muscles. However, while awaiting for chemotherapy, muscle weakness evolved and rapidly progressed. The level of creatine phosphokinase was significantly elevated. Electromyography revealed moderate myopathy, and a muscle biopsy showed degenerating myofibers with variable sizes. The diagnosis of paraneoplastic dermatomyositis was made. This case highlights that, although rare, paraneoplastic dermatomyositis can be present with cholangiocarcinoma. Also, muscle inflammation can precede the clinical muscle symptoms, and paraneoplastic DM should be considered as a possible differential diagnosis in the assessment of cancer patients who present with abnormal muscle tracer uptake in PET-CT scans.
Keywords: Dermatomyositis, Cholangiocarcinoma, Positron-emission tomography
Introduction
Dermatomyositis (DM), as an idiopathic inflammatory myopathy, is associated with increased risk of malignant disease [1]. Non-Hodgkin’s lymphoma and ovarian, lung, gastric, colorectal, and pancreatic cancers are all commonly associated with DM [2]. Cholangiocarcinoma with paraneo-plastic DM is extremely rare [3-5], and the whole body positron emission tomography-computed tomography(PET-CT) finding of paraneoplastic DM is rarely reported. In this report, we describe a rare case of paraneoplastic DM in a patient with cholangiocarcinoma, showing strong diffuse proximal muscle uptake in a whole body PET-CT scan before any muscle symptoms developed.
Case Report
A 66-year-old woman with a history of hypertension experienced general weakness, anorexia, and poor oral intake over a 2-month period. She had not consumed any alcohol, and her physical examination indicated no abnormalities. Laboratory tests showed elevated levels of aspartate aminotransferase and alanine aminotransferase, which were 419 IU/mL and 176 IU/mL, respectively. Tests for antibodies against hepatitis B surface antigen, hepatitis C virus, and human immunodeficiency were negative. The patient had serum carbohydrate antigen 19-9 levels of 9,446 U/mL (reference range, 0 to 37 U/mL), alpha-fetoprotein levels of 27.52 ng/mL (reference range, 0.89 to 8.78 ng/mL), and carcinoembryonic antigen levels of 2.4 ng/mL (reference range, 0 to 5 ng/mL). A computed tomography (CT) scan of the abdomen and pelvis revealed multiple masses in lobes of the liver, peritoneal nodules, and multiple enlarged lymph nodes in the retrocrural, aortocaval, paraaortic, and external iliac areas. Magnetic resonance imaging (MRI) of the patient’s liver indicated multiple, gradually enhancing peripheral masses in both lobes of the liver; the largest of these masses measured 7.5 cm. A biopsy of the liver mass indicated cholangiocarcinoma (Fig. 1A). Consistent with a diagnosis of cholangiocarcinoma, immunostaining for cytokeratin 19 was positive (Fig. 1B) and staining for alphafetoprotein was negative (not shown).
Fig. 1.
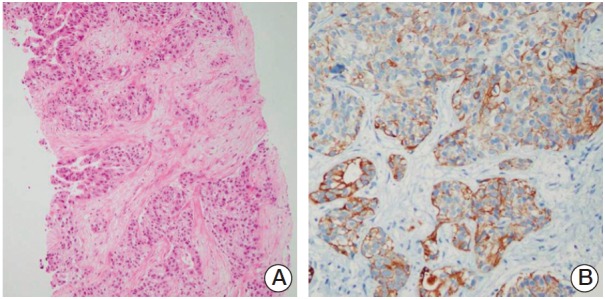
Photomicrographs of the liver biopsy. (A) The liver biopsy indicated adenocarcinoma (H&E staining, ×100). (B) The tumor cells were positive for cytokeratin 19 (×200).
While awaiting chemotherapy, the patient developed muscle weakness in her lower extremities, which progressed rapidly; she was unable to ambulate 10 days later. She denied any myalgia. Upon neurological examination, it was found that the flexion power of both hips was decreased to grade III, and knee flexion was reduced to grade IV. In the distal muscle groups, muscle strength was intact. The patient had bluish-purple skin lesions on her upper eyelids, and erythematous papules over her knuckles (Gottron’s sign). An MRI of her spine showed no evidence of cord compression. Interestingly, a whole body PET-CT scan (Briograph mCT 40, Siemens Medical Solutions, Knoxville, TN), which was performed a week before the onset of muscle weakness, indicated diffuse [18F]-fluorodeoxyglucose (FDG) uptake in the skin and whole muscles, in addition to both of the hepatic lobes, lymph nodes, lung, peritoneum, and L3 vertebra (Figs. 2A and 3A). Symmetrically proximal and bilateral FDG uptake was primarily in the shoulders, upper arms, back, hips, and thigh muscles. The patient had elevated creatine phosphokinase (CK) levels of 7,748 IU/L (reference range, 20 to 270 IU/L), lactic dehydrogenase levels of 1,833 IU/L (reference range, 100 to 225 IU/L), and aldolase levels of 32.6 U/L (reference range, below 7.6 U/L). An antinuclear antibody test was positive at 1:320. An anti-Jo-1 antibody test was negative. Electromyography revealed moderate myopathy. A muscle biopsy taken from the patient’s upper right thigh showed degenerating and regenerating myofibers with variable sizes, endomysial fibrosis, and fat ingrowth, but no evidence of vasculitis or tumor cell infiltrates (Fig. 4). The patient was diagnosed with cholangiocarcinoma with paraneoplastic DM, and treatment with high-dose corticosteroids and chemotherapy consisting of gemcitabine, and cisplatin was initiated. After two cycles of chemotherapy, CT and whole body PET-CT scans showed a marked reduction in cancer burden. FDG uptake in the skin and muscles was resolved (Figs. 2B and 3B). The patient’s skin lesions almost completely disappeared, and her CK levels normalized. However, the muscle weakness improved gradually.
Fig. 2.
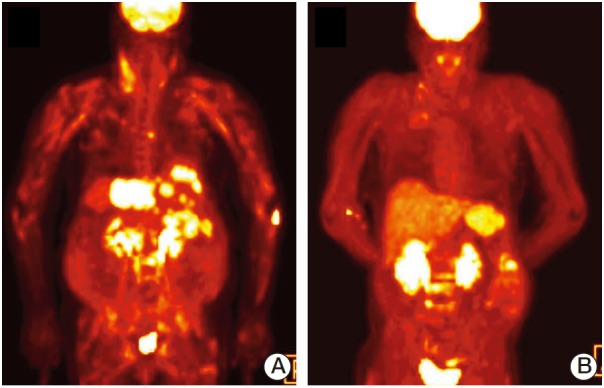
Representative [18F]-fluorodeoxyglucose (FDG) positron emission tomography–computed tomography (PET-CT) images of the patient. Whole-body PET-CT was performed using FDG, scanning started 60 to 90 minutes after tracer injection, and images obtained in transverse, coronal, and sagittal planes were reconstructed. (A) Baseline maximum intensity projection (MIP) image. (B) MIP image after two cycles of chemotherapy.
Fig. 3.
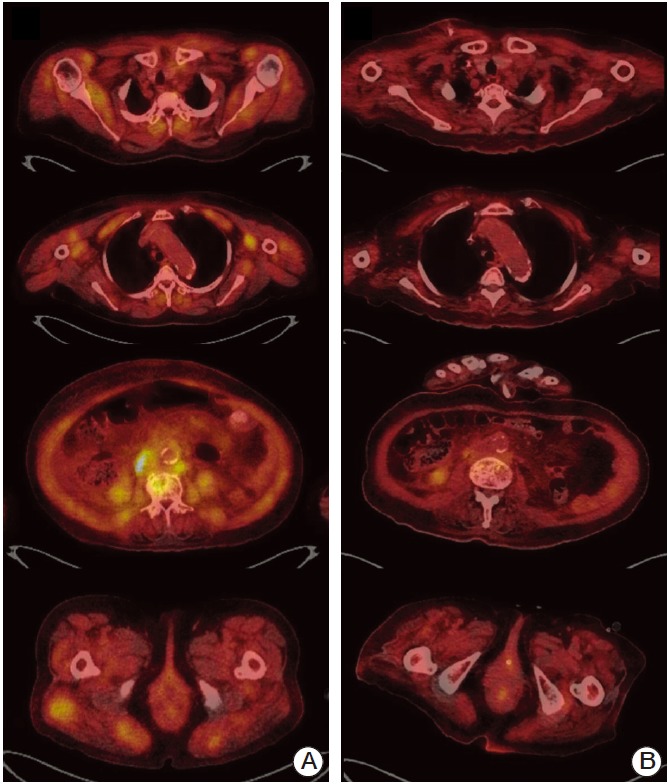
Results of baseline and post-treatment examinations. (A) Baseline whole-body [18F]-fluorodeoxyglucose (FDG) positron emission tomography–computed tomography (PET-CT) scan showed diffuse increased uptake in the proximal muscle groups, including the shoulders, upper arms, back, hips, and thighs, in addition to both of the hepatic lobes, lymph nodes, lung, peritoneum, and L3 vertebra. (B) Whole body FDG PET-CT scan after two cycles of chemotherapy demonstrated a marked reduction in cancer burden and muscle uptake.
Fig. 4.
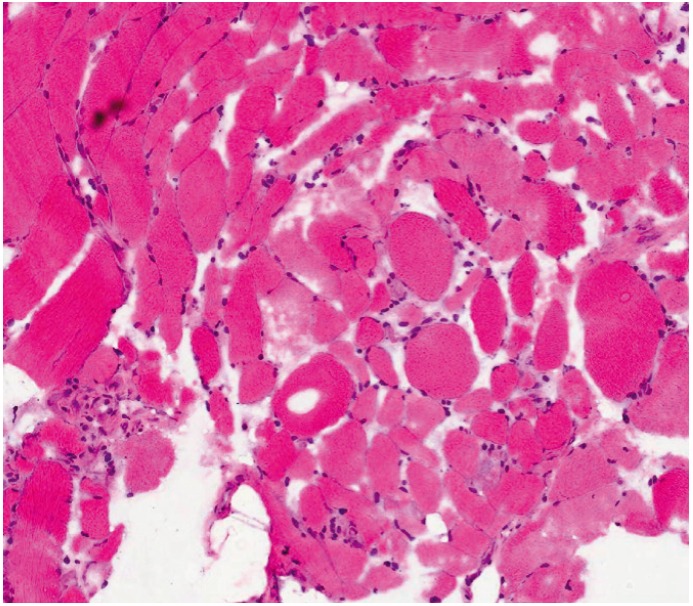
Photomicrograph of muscle biopsy. Atrophic myofibers and degenerating and regenerating myofibers of variable sizes are shown (H&E staining, ×400). There was no evidence of vasculitis or tumor cell infiltrates.
Discussion
Cholangiocarcinoma with paraneoplastic DM is extremely rare [3-5], and biopsy-proven cholangiocarcinoma in an established DM patient has only been reported by Knowles et al. [3]. The whole body PET-CT findings of paraneoplastic DM are also rare, even though PET-CT is frequently used as a tool for detecting and staging of malignant tumors. The principle underlying this use of PET-CT is that cancer cells use more energy than normal tissues, and therefore, take up and retain more glucose than noncancerous cells [6]. In general, skeletal muscles show no significant uptake of the tracer in a well-prepared patient; however, high muscle uptake of FDG, due to highly metabolically active cells, may be observed in association with inflammatory conditions, such as musculoskeletal infection, vasculitis, and myositis [7]. Pathologic FDG uptake can also be seen in primary muscle tumors, metastases to muscles, and other uncommon malignant process, e.g., muscle lymphoma [8]. Interestingly, diffuse FDG uptake in the bilateral proximal muscles of our patient appeared before the onset of muscle weakness, and our initial interpretation of the high muscle uptake was metastasis to the muscles from the underlying cholangiocarcinoma. This case indicates that muscle inflammation can precede the onset of clinical DM symptoms. As such, paraneoplastic DM should be considered as a possible diagnosis in cancer patients with abnormal tracer muscle uptake in PET-CT scans.
Recent studies have suggested that DM might arise as an autoimmune response against cancer which cross-reacts with regenerating muscle cells [9,10]. Casciola-Rosen et al. [9] have reported that myositis-specific autoantigens are expressed in both tumor cells and undifferentiated myoblasts, which indicate that these cells express similar proteins, and that these proteins can serve as autoantigens. Kaji et al. [10] have previously identified a novel autoantibody (anti-155/140 antibody), which is highly specific for paraneoplastic DM. In this context, the successful treatment of underlying malignant disease might improve the clinical course of myositis because it decreases tumor proteins, which serve as autoantigens; indeed, improvement of DM was observed after cancer treatment [11,12]. However, our patient did not show any significant improvement in muscle strength following the treatment of underlying malignancy. One possible explanation for this is that a high tumor burden decreases relatively slowly with cytotoxic chemotherapy. Another explanation could be that the patient’s rapid myositis progression and refractoriness to therapy was indicative of autoimmune antibodies that are reactive against the signal recognition particle (SRP). Anti-SRP antibodies are detected in ~5% of patients with idiopathic inflammatory myopathy, and the presence of anti-SRP antibodies is frequently associated with acute onset severe myopathy. Acute onset severe myopathy is associated with muscle enzyme elevation and systemic features, including dysphagia, and has been shown to be refractory to standard treatments [13]. B Cell depletion, using rituximab, has been used in patients with refractory DM, and good results have also been reported in the treatment of anti-SRP myopathy [14]. In such cases, perivascular muscle fiber necrosis and endomysial fibrosis, with little or no inflammatory cell infiltrates, are frequently found [15]. In the present case, anti-SRP myopathy was considered as a potential diagnosis, given the rapid progression of muscle weakness, relatively high levels of CK, endomysial fibrosis with little inflammatory cell infiltration, and refractoriness to treatment. However, the laboratory test for anti-SRP was not available; hence, the investigation did not proceed further.
Paraneoplastic DM, although rare, can occur with a cholangiocarcinoma, and inflammatory infiltrates observed using PET scans can precede the onset of clinical muscle sympoms. Once identified, paraneoplastic DM could be treated with steroids, as well as chemotherapy. This case illustrates the importance of considering paraneoplastic DM as a possible differential diagnosis in the assessment of cancer patients who present with abnormal tracer muscle uptake in PET-CT scans. In such cases, attention should be paid to the development of muscle weakness.
Acknowledgments
This study was supported by grant no 04-2013-0850 from the SNUH Research Fund and by a grant from the National R&D Program for Cancer Control, Ministry of Health and Welfare, Republic of Korea (1120310).
Footnotes
Conflict of interest relevant to this article was not reported.
References
- 1.Targoff IN. Dermatoniyositis and polymyositis. Curr Probl Dermatol. 1991;3:134–80. [Google Scholar]
- 2.Hill CL, Zhang Y, Sigurgeirsson B, Pukkala E, Mellemkjaer L, Airio A, et al. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet. 2001;357:96–100. doi: 10.1016/S0140-6736(00)03540-6. [DOI] [PubMed] [Google Scholar]
- 3.Knowles BP, Corcoran NM, Usatoff V. Reading the signs: occult metastatic cholangiocarcinoma detected by full-body screening in dermatomyositis. ANZ J Surg. 2007;77:1026–7. doi: 10.1111/j.1445-2197.2007.04307.x. [DOI] [PubMed] [Google Scholar]
- 4.Choi SH, Kim HS, You MR, Kim MW. A case of dermatomyositis associated with infiltrative intrahepatic cholangiocarcinoma. J Korean Rheum Assoc. 2010;17:76–80. [Google Scholar]
- 5.Horie Y, Yamada M, Nakai K, Kawasaki H, Hirayama C, Matsui K, et al. Combined hepatocellular-cholangiocarcinoma associated with dermatomyositis. J Gastroenterol Hepatol. 1989;4:101–4. doi: 10.1111/j.1440-1746.1989.tb00812.x. [DOI] [PubMed] [Google Scholar]
- 6.Warburg O. On the origin of cancer cells. Science. 1956;123:309–14. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 7.Al-Nahhas A, Jawad AS. PET/CT imaging in inflammatory myopathies. Ann N Y Acad Sci. 2011;1228:39–45. doi: 10.1111/j.1749-6632.2011.06016.x. [DOI] [PubMed] [Google Scholar]
- 8.Mummadi V, Arabi M, Jakubowski E, Bou-Assaly W, Geatti O, Gross M, et al. Abnormal 18F-fluorodeoxyglucose (FDG) uptake in muscle tumors on PET-CT: a pictorial atlas of pathologic findings. J Nucl Med. 2010;51(Suppl 2):1090. [Google Scholar]
- 9.Casciola-Rosen L, Nagaraju K, Plotz P, Wang K, Levine S, Gabrielson E, et al. Enhanced autoantigen expression in regenerating muscle cells in idiopathic inflammatory myopathy. J Exp Med. 2005;201:591–601. doi: 10.1084/jem.20041367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaji K, Fujimoto M, Hasegawa M, Kondo M, Saito Y, Komura K, et al. Identification of a novel autoantibody reactive with 155 and 140 kDa nuclear proteins in patients with dermatomyositis: an association with malignancy. Rheumatology (Oxford) 2007;46:25–8. doi: 10.1093/rheumatology/kel161. [DOI] [PubMed] [Google Scholar]
- 11.Yoshinaga A, Hayashi T, Ishii N, Ohno R, Watanabe T, Yamada T. Successful cure of dermatomyositis after treatment of nonseminomatous testicular cancer. Int J Urol. 2005;12:593–5. doi: 10.1111/j.1442-2042.2005.01105.x. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi F, Tsuta K, Nagaoka T, Miyamoto H, Saito Y, Amano H, et al. Successful resection of dermatomyositis associated with thymic carcinoma: report of a case. Surg Today. 2008;38:245–8. doi: 10.1007/s00595-007-3601-x. [DOI] [PubMed] [Google Scholar]
- 13.Gunawardena H, Betteridge ZE, McHugh NJ. Myositis-specific autoantibodies: their clinical and pathogenic significance in disease expression. Rheumatology (Oxford) 2009;48:607–12. doi: 10.1093/rheumatology/kep078. [DOI] [PubMed] [Google Scholar]
- 14.Noss EH, Hausner-Sypek DL, Weinblatt ME. Rituximab as therapy for refractory polymyositis and dermatomyositis. J Rheumatol. 2006;33:1021–6. [PubMed] [Google Scholar]
- 15.Hengstman GJ, ter Laak HJ, Vree Egberts WT, Lundberg IE, Moutsopoulos HM, Vencovsky J, et al. Anti-signal recognition particle autoantibodies: marker of a necrotising myopathy. Ann Rheum Dis. 2006;65:1635–8. doi: 10.1136/ard.2006.052191. [DOI] [PMC free article] [PubMed] [Google Scholar]


