Abstract
Importance
Hydroxy-methylglutaryl-coenzyme A reductase inhibitors affect several mechanisms underlying acute kidney injury (AKI).
Objective
To test the hypothesis that short-term high-dose perioperative atorvastatin would reduce AKI following cardiac surgery
Design, Setting, Participants
Double-blinded, placebo-controlled, randomized trial of adult cardiac surgery patients conducted November 2009 to October 2014 at Vanderbilt University Medical Center
Intervention
Statin-naïve patients (n=199) were randomly assigned 80mg atorvastatin the day before surgery, 40mg the morning of surgery, and 40mg daily following surgery (n=102) or matching placebo (n=97). Patients using statins prior to study enrollment (n=416) continued their pre-enrollment statin until the day of surgery, were randomly assigned 80mg atorvastatin the morning of surgery and 40mg the morning after (n=206) or matching placebo (n=210), and resumed their statin on postoperative day 2.
Main Outcome
AKI, defined as 0.3 mg/dl rise in serum creatinine within 48 hours of surgery (AKIN criteria)
Results
The DSMB recommended stopping the statin-naïve group due to increased AKI among statin-naïve participants with chronic kidney disease (CKD, estimated glomerular filtration rate <60 ml/min/1.73 m2) receiving atorvastatin and then recommended stopping for futility after 615 participants (median age, 67 years; 188 [30.6%] women, and 202 [32.8%] diabetic) completed the study. Among all participants (n=615), AKI occurred in 64 of 308 participants (20.8%) randomized to atorvastatin versus 60 of 307 participants (19.5%) randomized to placebo (risk ratio [RR], 1.06 [95% CI, 0.78–1.46]; P=0.75). Among statin-naïve participants (n=199), AKI occurred in 22 of 102 (21.6%) receiving atorvastatin versus 13 of 97 (13.4%) receiving placebo (RR, 1.61 [0.86–3.01]; P=0.15), and serum creatinine increased 0.11mg/dl (−0.11 to 0.56) (median [10th to 90th percentile]) in those randomized to atorvastatin versus 0.05 (−0.12 to 0.33) placebo (mean difference, 0.08 mg/dl [95% CI, 0.01–0.15]; P=0.007). Among statin-users (n=416), AKI occurred in 42 of 206 (20.4%) randomized to atorvastatin versus 47 of 210 (22.4%) placebo (RR, 0.91 [0.63–1.32]; P=0.63). In CKD patients (n=179), AKI occurred in 30 of 84 (35.7%) randomized to atorvastatin versus 31 of 95 (32.6%) placebo (RR, 1.09 [0.73–1.65]; P=0.76). In CKD patients naïve to statins (n=36), AKI occurred in 9 of 17 (52.9%) randomized to atorvastatin, versus 3 of 19 (15.8%) placebo (RR, 3.35 [1.12–10.05]; P=0.03), and serum creatinine increased 0.26 (−0.22 to 0.94) versus −0.06 mg/dl (−0.16 to 0.41) (mean difference, 0.28mg/dl [0.02–0.54]; P=0.04). In CKD statin-users (n=143), AKI occurred in 21 of 67 (31.3%) randomized to atorvastatin, versus 28 of 76 (36.8%) placebo (RR, 0.85 [0.54–1.35]; P=0.59).
Conclusions and Relevance
Among patients undergoing cardiac surgery, high-dose perioperative atorvastatin treatment, compared to placebo administration, did not reduce the risk of AKI overall, among patients naive to statins, or patients already using a statin. These results do not support the initiation of statin therapy to prevent AKI following cardiac surgery.
Trial Registration
Clinicaltrials.gov identifier: NCT00791648
Keywords: statin, atorvastatin, AKI, CKD, kidney injury, renal function, kidney, atrial fibrillation, delirium, pneumonia, surgery, cardiac surgery
Introduction
Acute kidney injury (AKI) complicates recovery from cardiac surgery in up to 30% of patients.1 Surgical, anesthetic, and critical care advancements have reduced perioperative mortality but have not reduced the incidence of AKI, and some studies have reported increasing rates of postoperative dialysis.2–4 Recent large cohort studies confirm that even mild postoperative AKI is independently associated with a five-fold increase in in-hospital death.5 A diagnosis of AKI following cardiac surgery is accompanied by higher rates of postoperative arrhythmias, respiratory failure, systemic infection, and myocardial infarction.1,5–7 Treatments are needed to reduce this debilitating postoperative outcome.
Hydroxy-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors (statins) affect several mechanisms underlying postoperative AKI, and statin treatment may affect AKI in patients following cardiac surgery. In a recent observational study of 17,000 cardiac surgery patients, investigators reported a 22% relative risk reduction in AKI associated with preoperative statin treatment in adjusted analyses,8 while investigators of a similar study of 11,000 patients observed no effect.9 These observational studies that compare preoperative statin users to non-users do not adequately test the hypothesis that pre-, intra-, and early post- (peri-) operative statin treatment reduces AKI. It is both unclear if de novo initiation of perioperative statin treatment in patients naïve to statins affects AKI, or if statin continuation or perioperative withdrawal in patients already using statins affects AKI.
We performed the Statin AKI Cardiac Surgery RCT to test the hypothesis that short-term high-dose perioperative atorvastatin reduces AKI following cardiac surgery.
METHODS
Study Design and Patients
The Statin AKI Cardiac Surgery RCT (NCT00791648) was an investigator-initiated, double-blinded, placebo-controlled, randomized clinical trial conducted to test the hypothesis that short-term, high-dose, perioperative atorvastatin treatment reduces AKI following cardiac surgery (see Protocol in the Supplement). Adult patients undergoing elective coronary artery bypass grafting, valvular heart surgery, or ascending aortic surgery at Vanderbilt University Medical Center (VUMC) were eligible for study participation. Patients with prior statin intolerance; acute coronary syndrome, defined as ST or non-ST elevation myocardial infarction with elevated serum troponin concentrations; liver dysfunction, defined as serum transaminase concentrations greater than three times the upper limit of normal (120 U/L), a bilirubin concentration greater than 3 mg/dl, or a diagnosis of cirrhosis; current use of potent CYP3A4 inhibitors including azole antifungals, protease inhibitors, and macrolide antibiotics; current use of cyclosporine; current renal replacement therapy; history of kidney transplant; a requirement for emergency or urgent surgery; or pregnancy were excluded.
The study was approved by the Vanderbilt University Institutional Review Board for Research on Human Subjects and conducted according to the Declaration of Helsinki. All patients provided written informed consent. Anesthetic, surgical, and postoperative management were conducted according to institutional protocols as described in the Supplement.
Intervention
Pre-study statin-naïve patients were randomized to receive 80 mg atorvastatin the day prior to surgery, 40 mg atorvastatin the morning of surgery (at least three hours prior to surgery), and 40 mg daily atorvastatin at 10:00 in the morning following surgery for the duration of hospitalization or to a matching placebo regimen. Pre-study statin-using patients were randomized to receive study drug only on days they otherwise would have not been prescribed a statin under standard of care, as it was deemed unethical to randomize statin-using patients to placebo throughout the perioperative period based on prior observational studies and clinical trials in similar patient populations demonstrating that statin withdrawal may increase AKI.10–13 Therefore pre-study statin-using patients continued their pre-enrollment statin until the day of surgery and resumed their statin on postoperative day 2. On the day of surgery they were randomly assigned 80 mg atorvastatin at least three hours prior to surgery and 40 mg atorvastatin the day after surgery at 10:00 in the morning or to a matching placebo regimen. Statin administration subsequent to study intervention – starting at hospital discharge in pre-enrollment statin-naïve patients and starting on postoperative day two in pre-enrollment statin-using patients – was at the discretion of the treating physician. The intervention in statin-naïve and statin-using patients was designed to be pragmatic, lending itself to maximum generalizability for subsequent cardiac surgery patients. We chose atorvastatin because short-term periprocedural atorvastatin treatment has been reported to reduce arrhythmia following cardiac surgery and myocardial injury following percutaneous coronary intervention studies,14,15 and we chose an 80 mg loading dose and a 40 mg maintenance dose to maximize potential benefit, to limit toxicity, and to fall within recommended daily dosing.
Participants were randomized in permuted blocks of size 2 or 4 to receive oral atorvastatin or matching-placebo within strata defined by pre-study statin use, the presence of chronic kidney disease (CKD, defined as a modified diet in renal disease-calculated estimated glomerular filtration rate [eGFR] less than 60 mL/min/1.73 m2), and by a history of diabetes. The VUMC Investigation Drug Service (IDS) compounded and maintained atorvastatin and placebo capsules identical in size, color, texture, weight, and taste. Using a randomization schedule provided by the study statistician, the IDS assigned intervention to recruited patients, dispensed atorvastatin or matching-placebo study drug, and logged treatment assignment in private records. Investigators, clinicians, patients, and all research personnel were blinded to treatment for the duration of the study.
Outpatient adherence to intervention was monitored by directly questioning patients and family members on the morning of surgery, and inpatient adherence was monitored by reviewing the electronic medication administration record. For intubated patients unable to swallow pills, study drug was mixed with water and administered via oral gastric tube.
Study Outcomes
The primary end point was the diagnosis of AKI according to Acute Kidney Injury Network (AKIN) criteria, specifically a 0.3 mg/dl (26.5 mmol/L) increase in serum creatinine or the initiation of renal replacement therapy within 48 hours of surgery.16 AKIN Stage 1 AKI is defined as an increase of 0.3 mg/dl or 50%; stage 2, a 100% increase; and stage III, a 200% increase or initiation of renal replacement therapy. Baseline creatinine was defined as the most recent creatinine measurement prior to surgery, and serum creatinine was measured in all inpatients on the morning of surgery and no more than seven days prior to surgery in outpatients. Postoperatively, serum creatinine was measured at 2:00 in the morning daily throughout duration of hospitalization. All measurements were performed in the CLIA-certified VUMC clinical laboratory.
Secondary end points included the maximum creatinine increase from baseline to 48 hours following surgery (postoperative day 2), the incidence and duration of intensive care unit (ICU) delirium (incorrectly noted as a co-primary endpoint during editing of study registration), the degree of myocardial injury, the incidence of postoperative atrial fibrillation, the incidence of pneumonia, and the incidence of stroke. Study personnel assessed for delirium once in the morning and once in the afternoon while patients were in the ICU using the Richmond agitation and sedation scale (RASS) and the confusion assessment method for ICU (CAM-ICU).17 We quantified myocardial injury by measuring serum creatine kinase myocardial band (CK-MB) concentrations on postoperative day one in the clinical laboratory. Atrial fibrillation was diagnosed by the patient’s clinical physician and confirmed by research personnel using electrocardiograms and rhythm strips. We defined pneumonia as a positive sputum culture or postoperative pulmonary infiltrate with systemic signs of infection (temperature > 38.6° C or white blood cell count > 12,000/ul) and the use of parenteral antibiotics or documentation of the diagnosis by the patient’s physician. Stroke was defined as any new deficit on neurologic exam and confirmed with radiological evidence. Additional secondary end-points were duration of postoperative mechanical ventilation, duration of ICU stay, and incidence of in-hospital death.
Safety Evaluation
Safety outcomes included acute liver toxicity assessed by measuring aspartate aminotransferase on postoperative day 1, acute muscle toxicity assessed by questioning participants daily for myalgias and measuring serum creatine kinase on postoperative day 1, and adverse events. Adverse events were defined as any untoward medical occurrence in a participant and included postoperative fever, tachycardia, hypotension, vasoplegia, decreased cardiac output, anemia, leukocytosis, and lactic acidosis (see Supplement for specific diagnostic criteria).
The Data and Safety Monitoring Board (DSMB) reviewed patient recruitment practices, safety reporting, and data quality after 30 patients completed study and evaluated interim analyses after 277 patients (the first third of planned enrollment) had completed the study to assess safety of the intervention and after 546 patients (the second third of planned enrollment) had completed the study to assess safety, efficacy, and futility of the intervention. The DSMB made recommendations based on qualitative assessments of the safety, efficacy, and futility of the intervention, detailed in the DSMB charter included in the Supplement. In October 2012 the DSMB recommended study continuation after the first planned interim analysis. In August 2014 following the 2nd interim analyses, the DSMB recommended discontinuation of the recruitment of statin-naïve patients due to evidence of increased risk of AKI in patients randomized to atorvastatin, particularly in patients with preexisting CKD. Following a conditional power analysis for statin-using patients that assumed the true treatment effect among statin-using patients would be consistent with what had been observed in statin-using patients who had completed the study, the DSMB recommended in October 2014 discontinuation of the recruitment of statin-using patients due to futility on the primary end point.
Baseline Characteristics and other Patient Data
Demographic, past medical history, hemodynamic, anesthetic, surgical, cardiopulmonary bypass, transfusion, medication exposure, ICU, and laboratory data were collected on all patients. Race and ethnicity data were determined by patient self-reporting and were collected because African American ancestry is associated with AKI. In addition to safety and clinical care laboratory measurements, a lipid panel was assessed prior to surgery. To assess baseline mental competence all participants were administered a mini-mental state exam and a Trails B test prior to surgery by research personnel.
Statistical Analysis
The analysis was completed according to the Statistical Analysis Plan provided in the Supplement and summarized here. We planned to study 820 patients in order to detect a 30% relative risk reduction of AKI between treatment groups with an assumed AKI incidence of 27.6% in the placebo group,12 a type-1 error probability of 0.05, and 80% power. We summarized demographics, patient characteristics, and safety and efficacy end points with the 50th (10th, 90th) percentiles for continuous variables and with percentages for categorical variables. We used the Pearson chi-square test and the Wilcoxon rank sum test to compare randomized treatment groups regarding categorical and continuous outcomes, respectively. To estimate treatment effects, we used Quasi-Poisson, log-linear regression for binary outcomes with event rates greater than 10% due to ease of interpretation of relative risks and because we designed the study to detect a relative risk reduction, logistic regression for rare binary outcomes due to model stability with event rates less than 10% and close approximation of the odds ratio with the relative risk for rare outcomes,18,19 proportional odds regression for ordered categorical and highly skewed outcomes (e.g., delirium duration and ICU duration), and linear regression for continuous outcomes. The CK-MB, time-to-extubation, creatine kinase, and AST outcomes were log-transformed to improve model fit. In tables, we include absolute differences between treatment groups, derived from model transformations. For dichotomous outcomes, the absolute differences are the estimated differences in proportions between statin and placebo groups. For continuous outcomes, the absolute differences are the estimated differences in the geometric means between statin and placebo groups. For delirium duration and ICU duration outcomes the absolute differences are the differences in the proportions of those in the statin group greater than the median value in the placebo group minus 0.5 (i.e., the proportion of those in the placebo greater than the median value in the placebo group).
Several subgroup analyses were planned prior to initiating the study since treatment effects could vary based on pre-existing statin use and baseline kidney disease: 1) pre-study statin-naïve, 2) pre-study statin-using, 3) CKD, 4) pre-study statin-naïve with CKD, 5) pre-study statin-using with CKD.
In the main text we report the primary unadjusted analyses. In the Supplement, we include propensity score-adjusted analyses to adjust for potential imbalances in the randomization scheme in subgroups of patients and treatment-received analyses. All analyses were performed using R version 3.1.2, and a two-sided significance level of 5% was required to achieve statistical significance.
RESULTS
Patient recruitment, randomization, baseline characteristics, and protocol adherence
Between November 2009 and October 2014, 4466 adult patients presented for coronary bypass, valve, or ascending aorta surgery (Figure 1). Ten percent of these patients had acute coronary syndrome, 3.7% end stage renal disease, 3.1% liver dysfunction, 2.6% were allergic to statins, and 5.5% required emergent or urgent surgery. Of those approached for study participation, approximately 30% did not provide consent. We recruited six hundred and fifty-three patients. Twenty-two were subsequently excluded from study and fourteen withdrew consent prior to randomization. Six hundred seventeen patients were randomized to treatment. One patient withdrew consent following randomization, prior to receiving treatment, and one patient was withdrawn from the study on the day of surgery by his intensivist to administer statin treatment. Thus 615 patients (199 statin-naïve and 416 statin-using) were included in the primary analysis, and 308 of these were randomized to atorvastatin and 307 to placebo. Patient characteristics were well balanced between treatment groups (Table 1). Median patient age was 67 years, 30.6% were female, and 32.8% diabetic. Coronary artery bypass grafting was performed in half of the patients and two thirds of patients received valve surgery. Mean serum LDL concentrations at induction of anesthesia – following treatment initiation but before surgery – were 8.1 (95% CI 2.2 to 14.1, P=0.01) mg/dl lower in patients randomized to atorvastatin versus placebo.
Figure 1. Recruitment, randomization, and follow-up.
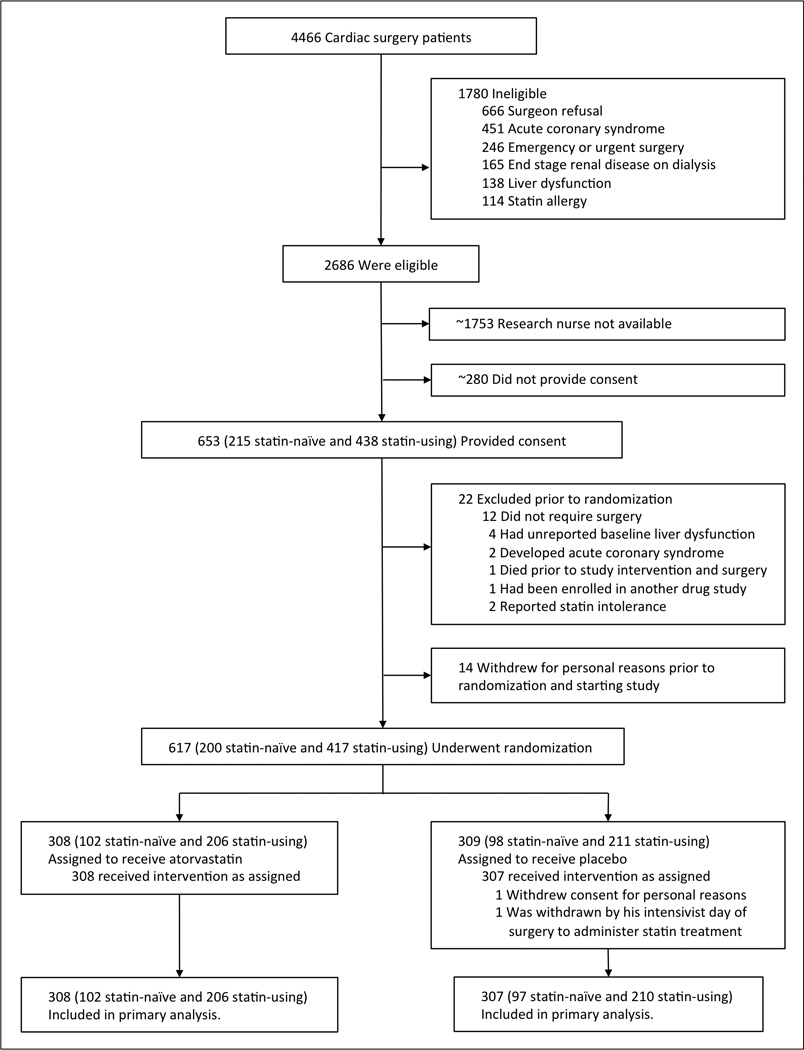
We sought to study three patients each week in order to maximize recruitment but ensure each subject was studied per protocol with the research staff available. We were unable to recruit all eligible patients. Approximately (~) 1753 were not approached, and ~280 did not provide consent.
Table 1.
Baseline and intraoperative patient characteristics. Binary characteristics are reported as No. (%), and continuous characteristics as median (10th percentile to 90th percentile).
| Characteristic | Statin (n = 308) | Placebo (n = 307) |
|---|---|---|
| Age, years | 66 (49 to 81) | 67 (51 to 81) |
| Female | 94 (30.5%) | 94 (30.6%) |
| African American | 11 (3.6%) | 15 (4.9%) |
| Medical history | ||
| Congestive heart failure | 129 (41.9%) | 114 (37.1%) |
| Left ventricular ejection fraction, % | 60 (35 to 60) | 55 (38 to 60) |
| Myocardial infarction | 53 (17.2%) | 57 (18.6%) |
| Atrial fibrillation | 73 (23.7%) | 68 (22.1%) |
| Prior cardiac surgery | 57 (18.5%) | 53 (17.3%) |
| Diabetes | 104 (33.8%) | 98 (31.9%) |
| Body mass index, kg/m2 | 28.0 (22.2 to 36.8) | 27.6 (22.5 to 36.9) |
| Current smoking | 48 (15.6%) | 40 (13.0%) |
| Chronic obstructive pulmonary disease | 36 (11.7%) | 28 (9.1%) |
| Obstructive sleep apnea | 49 (15.9%) | 39 (12.7%) |
| Peripheral vascular disease | 86 (27.9%) | 84 (27.4%) |
| Cerebral vascular accident | 23 (7.5%) | 18 (5.9%) |
| Transient ischemic event | 12 (3.9%) | 6 (2.0%) |
| Charlson Index | 2 (0 to 5) | 2 (0 to 5) |
| Minimental state exam score | 29 (27 to 30) | 29 (26 to 30) |
| Trails B score, seconds | 109 (66 to 206) | 105 (65 to 200) |
| Hemodynamics | ||
| Systolic BP, mmHg | 127 (105 to 155) | 130 (106 to 156) |
| Diastolic BP, mmHg | 69 (54 to 88) | 70 (54 to 88) |
| Heart rate, beats/minute | 71 (57 to 90) | 72 (56 to 93) |
| Cardiac index, liters/min/m2 * | 2.2 (1.6 to 3.0) | 2.2 (1.5 to 3.1) |
| Central venous pressure, mmHg* | 13 (7 to 20) | 13 (7 to 21) |
| Medication use | ||
| Statin | 206 (66.9%) | 210 (68.4%) |
| ACE inhibitor | 88 (28.6%) | 104 (33.9%) |
| Baseline laboratory data | ||
| Creatinine, mg/dl | 1.01 (0.74 to 1.64) | 1.02 (0.74 to 1.56) |
| Glomerular filtration, ml/min/1.73 m2 | 74 (39 to 96) | 71 (37 to 96) |
| Hematocrit, % | 40 (33 to 46) | 40 (32 to 46) |
| AST, u/l | 23 (16 to 35) | 23 (16 to 40) |
| LDL cholesterol, mg/dl* | 68 (42 to 102) | 74 (41 to 113) |
| HDL cholesterol, mg/dl* | 35 (23 to 52) | 37 (26 to 55) |
| Total cholesterol, mg/dl* | 125 (92 to 169) | 132 (97 to 181) |
| Triglycerides, mg/dl* | 98 (49 to 195) | 94 (51 to 183) |
| Procedure characteristics | ||
| CABG surgery | 148 (48.1%) | 153 (49.8%) |
| Number of CABG vessels* | 2 (1 to 4) | 2 (1 to 3) |
| Valve surgery | 200 (64.9%) | 197 (64.2%) |
| Cardiopulmonary bypass use | 218 (70.8%) | 217 (70.7%) |
| Cardiopulmonary bypass time, min*§ | 134 (91 to 240) | 144 (86 to 227) |
Characteristic was assessed in the operating room prior to surgery but after initiation of randomized treatment.
Among patients receiving cardiopulmonary bypass.
BP, blood pressure; ACE, angiotensin-converting enzyme; AST, aspartate aminotransferase; LDL, low density lipoprotein; HDL, high density lipoprotein; CABG, coronary artery bypass grafting
Treatment adherence was achieved in 2356 of 2400 (98.2%) total doses of study drug with 180 of 199 (91.0%) statin-naïve patients receiving all protocol-directed doses and 391 of 416 (94.0%) pre-study statin-using patients receiving all protocol-directed doses. For details, see Supplement.
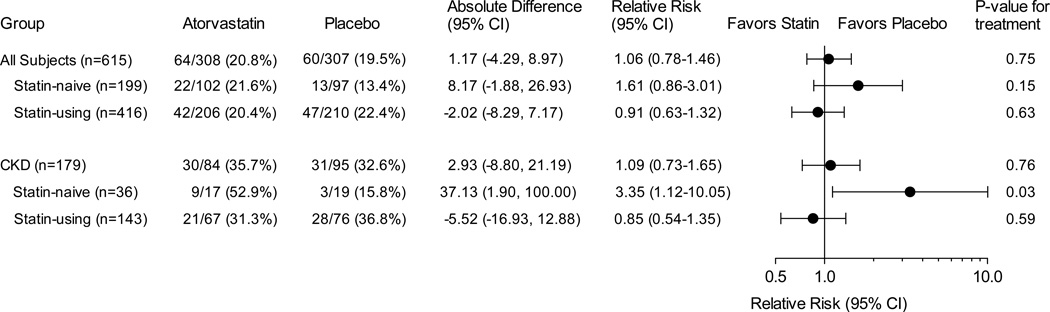
AKI and serum creatinine analyses
Among all patients (n=615), the primary end point (AKI by AKIN criteria) occurred in 64 of 308 participants (20.8%) randomized to atorvastatin and in 60 of 307 participants (19.5%) randomized to placebo (Pearson chi squared P=0.75). Perioperative atorvastatin treatment did not affect risk for AKI in the total cohort (risk ratio [RR], 1.06 [95% CI 0.78–1.46]; Figure 2) or perioperative serum creatinine concentrations. Serum creatinine concentrations increased 0.07 (−0.13 to 0.51) (median [10th to 90th percentile]) mg/dl within the first 48 postoperative hours among participants randomized to atorvastatin, compared to 0.07 (−0.12 to 0.52) mg/dl in participants randomized to placebo (mean difference, −0.01 mg/dl [95% CI, −0.06–0.04]; P=0.89).
Figure 2. Efficacy of treatment to prevent Acute Kidney Injury in all patients and prespecified subgroups.
Absolute differences are the estimated differences in proportions between statin and placebo groups derived from model transformations. Quasi-Poisson, log-linear regression was used to calculate estimates and confidence intervals (CI) and should be interpreted as risk ratios or the relative risk (RR) of treatment for the primary end point, acute kidney injury. P-values correspond to the Pearson chi-square test.
Among pre-study statin-naïve patients (n=199), AKI occurred in 22 of 102 participants (21.6%) randomized to atorvastatin, compared to 13 of 97 participants (13.4%) randomized to placebo (RR, 1.61 [0.86–3.01]; P=0.15), and serum creatinine concentrations increased more within the first 48 postoperative hours among those randomized to atorvastatin (0.11 [-0.11 to 0.56] mg/dl), compared to those randomized to placebo (0.05 [-0.12 to 0.33]; mean difference, 0.08 mg/dl [0.01–0.15]; P=0.007).
Among pre-study statin-using patients (n=416), high-dose perioperative atorvastatin or short-term statin withdrawal did not affect AKI risk. AKI occurred in 42 of 206 pre-study statin-using participants (20.4%) randomized to atorvastatin, compared to 47 of 210 participants (22.4%) randomized to placebo (RR, 0.91 [0.63–1.32]; P=0.63), and postoperative changes in serum creatinine were not different between groups (Table 2).
Table 2.
Secondary outcomes among all patients and pre-study statin-naïve, statin-using, and chronic kidney disease (CKD) prespecified subgroups. We report binary outcomes as No. (%), and continuous outcomes as median (10th percentile to 90th percentile). Estimates and confidence intervals (CI) should be interpreted as risk ratios or relative risks (RR) for dichotomous outcomes, differences in means (diff) for creatinine change outcomes, geometric mean ratios (GM) for CK-MB and time-to-extubation outcomes, and odds ratios (OR) for being in the higher response category across all dichotomizations of delirium duration and ICU duration outcomes. P-values correspond to the Pearson chi-square test for binary outcomes and the Wilcoxon rank sum test for continuous outcomes.
| Patients and Outcome | Statin | Placebo | Absolute Difference (95% CI) |
Estimate (95% CI) | P- value |
|---|---|---|---|---|---|
| All patients | n = 308 | n = 307 | |||
| Stage 2 or 3 AKI* | 10 (3.2%) | 8 (2.6%) | 0.65 (−1.30, 5.51) | RR, 1.25 (0.50, 3.12) | 0.81 |
| 48h creat change, mg/dl† | 0.07 (−0.13 to 0.51) | 0.07 (−0.12 to 0.52) | −0.01 (−0.06, 0.04) | diff, −0.01 (−0.06, 0.04) | 0.89 |
| Delirium‡ | 69 (22.4%) | 75 (24.4%) | −1.95 (−7.56, 5.37) | RR, 0.92 (0.69, 1.22) | 0.56 |
| Delirium duration, days | 0.0 (0.0 to 1.0) | 0.0 (0.0 to 1.5) | −3.48 (−12.50, 5.75) | OR, 0.87 (0.60, 1.26) | 0.45 |
| CK-MB on POD1, ng/ml | 23.0 (6.0 to 81.7) | 23.7 (5.9 to 87.2) | −1.20 (−4.57, 2.88) | GM, 0.95 (0.81, 1.12) | 0.72 |
| Atrial fibrillation | 115 (37.3%) | 103 (33.6%) | 3.70 (−3.36, 12.77) | RR, 1.11 (0.90, 1.38) | 0.38 |
| Stroke | 10 (3.2%) | 7 (2.3%) | 0.98 (−1.04, 5.97) | RR, 1.44 (0.54, 3.83) | 0.60 |
| Pneumonia | 8 (2.6%) | 18 (5.9%) | −3.27 (−4.78, 0) | RR, 0.43 (0.18, 1.00) | 0.05 |
| Time-to-extubation (min) | 465 (240 to 1227) | 450 (225 to 1164) | 0 (−76.82, 99.42) | GM, 1.00 (0.83, 1.22) | 0.47 |
| ICU duration (days) | 3 (2 to 6) | 3 (2 to 6) | −0.51 (−7.47, 6.52) | OR, 0.98 (0.74, 1.30) | 0.88 |
| In-hospital death | 4 (1.3%) | 1 (0.3%) | 0.60 (−0.13, 8.04) | RR, 3.02 (0.56, 30.24) | 0.39 |
| Statin-naïve | n = 102 | n = 97 | |||
| Stage 2 or 3 AKI* | 5 (4.9%) | 2 (2.1%) | 2.90 (−1.11, 23.10) | RR, 2.38 (0.47, 12.00) | 0.44 |
| 48h creat change, mg/dl† | 0.11 (−0.11 to 0.56) | 0.05 (−0.12 to 0.33) | 0.08 (0.01, 0.15) | diff, 0.08 (0.01, 0.15) | 0.007 |
| Delirium‡ | 29 (28.4%) | 25 (25.8%) | 2.58 (−23.99, 19.35) | RR, 1.10 (0.70, 1.75) | 0.76 |
| Delirium duration, days | 0.0 (0.0 to 1.0) | 0.0 (0.0 to 1.5) | 0.98 (−14.10, 15.75) | OR, 1.04 (0.56, 1.92) | 0.91 |
| CK-MB on POD1, ng/ml | 25 (10 to 91) | 27 (13 to 88) | −2.78 (−9.27, 5.87) | GM, 0.91 (0.70, 1.19) | 0.49 |
| Atrial fibrillation | 43 (42.2%) | 28 (28.9%) | 13.29 (0, 32.95) | RR, 1.46 (1.00, 2.14) | 0.05 |
| Stroke | 4 (3.9%) | 5 (5.2%) | −1.25 (−4.11, 8.44) | RR, 0.75 (0.20, 2.88) | 0.74 |
| Pneumonia | 3 (2.9%) | 5 (5.2%) | −2.22 (−4.49, 6.43) | RR, 0.56 (0.13, 2.40) | 0.49 |
| Time-to-extubation (min) | 482 (245 to 1090) | 480 (238 to 1191) | −55.68 (−172.10, 96.17) | GM, 0.89 (0.66, 1.19) | 0.99 |
| ICU duration (days) | 3 (2 to 6) | 3 (2 to 6) | 9.35 (−2.91, 20.67) | OR, 1.46 (0.89, 2.41) | 0.14 |
| In-hospital death§ | 1 (1.0%) | 0 (0.0%) | 1.00 | ||
| Statin-using | n = 206 | n = 210 | |||
| Stage 2 or 3 AKI* | 5 (2.4%) | 6 (2.9%) | −0.44 (−2.15, 5.08) | RR, 0.85 (0.26, 2.75) | 1.00 |
| 48h creat change, mg/dl† | 0.06 (−0.15 to 0.51) | 0.09 (−0.12 to 0.57) | −0.05 (−0.12, 0.02) | diff, −0.05 (−0.12, 0.02) | 0.12 |
| Delirium‡ | 40 (19.4%) | 50 (23.8%) | −4.28 (−10.47, 4.28) | RR, 0.82 (0.56, 1.18) | 0.28 |
| Delirium duration, days | 0.0 (0.0 to 1.0) | 0.0 (0.0 to 1.0) | −6.18 (−17.11, 5.16) | OR, 0.78 (0.49, 1.23) | 0.28 |
| CK-MB on POD1, ng/ml | 22 (5 to 66) | 22 (5 to 78) | −0.86 (−4.70, 3.85) | GM, 0.96 (0.78, 1.18) | 0.90 |
| Atrial fibrillation | 72 (35.0%) | 75 (35.7%) | −0.71 (−8.93, 9.64) | RR, 0.98 (0.75, 1.27) | 0.92 |
| Stroke | 6 (2.9%) | 2 (1.0%) | 2.06 (−0.38, 12.64) | RR, 3.12 (0.62, 15.64) | 0.17 |
| Pneumonia | 5 (2.4%) | 13 (6.2%) | −3.75 (−5.35, 0.46) | RR, 0.38 (0.13, 1.08) | 0.09 |
| Time-to-extubation (min) | 460 (234 to 1236) | 420 (225 to 1071) | 25.72 (−72.89, 154.35) | GM, 1.06 (0.83, 1.36) | 0.42 |
| ICU duration (days) | 3 (2 to 6) | 3 (2 to 7) | −5.25 (−13.29, 3.49) | OR, 0.81 (0.58, 1.15) | 0.24 |
| In-hospital death | 3 (1.5%) | 1 (0.5%) | 0.69 (−0.30, 10.64) | RR, 2.40 (0.39, 24.94) | 0.38 |
| CKD | n = 84 | n = 95 | |||
| Stage 2 or 3 AKI* | 6 (7.1%) | 4 (4.2%) | 2.94 (−2.14, 20.33) | RR, 1.70 (0.49, 5.84) | 0.52 |
| 48h creat change, mg/dl† | 0.09 (−0.26 to 0.73) | 0.07 (−0.25 to 0.83) | −0.01 (−0.14, 0.13) | diff, −0.01 (−0.14, 0.13) | 0.94 |
| Delirium‡ | 28 (33.3%) | 25 (26.3%) | 7.36 (−5.26, 27.88) | RR, 1.28 (0.80, 2.06) | 0.32 |
| Delirium duration, days | 0.0 (0.0 to 1.9) | 0.0 (0.0 to 2.0) | 7.26 (−8.14, 21.67) | OR, 1.34 (0.72, 2.53) | 0.36 |
| CK-MB on POD1, ng/ml | 27 (8 to 68) | 23 (5 to 83) | 1.21 (−5.32, 10.15) | GM, 1.05 (0.78, 1.42) | 0.55 |
| Atrial fibrillation | 31 (36.9%) | 37 (38.9%) | −1.95 (−13.62, 14.78) | RR, 0.95 (0.65, 1.38) | 0.89 |
| Stroke | 2 (2.4%) | 4 (4.2%) | −1.85 (−3.76, 7.80) | RR, 0.55 (0.10, 3.11) | 0.69 |
| Pneumonia | 5 (6.0%) | 7 (7.4%) | −1.39 (−5.52, 9.86) | RR, 0.80 (0.24, 2.61) | 0.77 |
| Time-to-extubation (min) | 552 (272 to 1390) | 530 (246 to 1236) | 164.93 (−34.72, 460.06) | GM. 1.38 (0.92, 2.06) | 0.52 |
| ICU duration (days) | 4 (2 to 7) | 4 (2 to 6) | −0.25 (−12.89, 12.55) | OR, 0.99 (0.59, 1.67) | 0.33 |
| In-hospital death§ | 3 (3.6%) | 0 (0%) | 0.10 | ||
| Statin-naïve with CKD | n = 17 | n = 19 | |||
| Stage 2 or 3 AKI* | 3 (17.6%) | 1 (5.3%) | 12.46 (−3.34, 100.00) | RR, 3.35 (0.37, 30.13) | 0.31 |
| 48h creat change, mg/dl† | 0.26 (−0.22 to 0.94) | −0.06 (−0.16 to 0.41) | 0.28 (0.02, 0.54) | diff, 0.28 (0.02, 0.54) | 0.04 |
| Delirium‡ | 11 (64.7%) | 4 (21.1%) | 43.68 (5.28, 100.00) | RR, 3.07 (1.25, 7.56) | 0.02 |
| Delirium duration, days | 0.5 (0.0 to 1.5) | 0.0 (0.0 to 2.1) | 32.05 (2.61, 44.94) | OR, 4.57 (1.11, 18.77) | 0.04 |
| CK-MB on POD1, ng/ml | 33 (10 to 147) | 24 (11 to 161) | 4.77 (−13.47, 45.16) | GM, 1.17 (0.52, 2.61) | 0.66 |
| Atrial fibrillation | 8 (47.1)% | 3 (10.5%) | 20.79 (−0.42, 87.15) | RR, 2.98 (0.96, 9.30) | 0.03 |
| Stroke§ | 0 (0.0%) | 2 (10.5%) | 0.49 | ||
| Pneumonia§ | 2 (11.8%) | 0 (0.0%) | 0.21 | ||
| Time-to-extubation (min) | 650 (361 to 1809) | 635 (296 to 1202) | 182.26 (−131.98, 691.34) | GM, 1.29 (0.79, 2.10) | 0.67 |
| ICU duration (days) | 5 (3 to 8) | 3 (2 to 6) | 26.08 (−1.55, 41.50) | OR, 3.18 (0.94, 10.76) | 0.07 |
| In-hospital death§ | 1 (5.9%) | 0 (0.0%) | 0.48 | ||
| Statin-using with CKD | n = 67 | n = 76 | |||
| Stage 2 or 3 AKI* | 3 (4.5%) | 3 (3.9%) | 0.51 (−3.00, 17.51) | RR, 1.13 (0.23, 5.49) | 1.00 |
| 48h creat change, mg/dl† | 0.05 (−0.25 to 0.67) | 0.12 (−0.25 to 0.94) | −0.08 (−0.23, 0.07) | diff, −0.08 (−0.23, 0.07) | 0.31 |
| Delirium‡ | 17 (25.4%) | 21 (27.6%) | −2.21 (−12.97, 16.28) | RR, 0.92 (0.53, 1.59) | 0.84 |
| Delirium duration, days | 0.0 (0.0 to 2.0) | 0.0 (0.0 to 1.8) | −1.55 (−18.97, 16.22) | OR, 0.94 (0.45, 1.96) | 0.87 |
| CK-MB on POD1, ng/ml | 27 (8 to 54) | 23 (5 to 76) | 0.47 (−6.05, 9.53) | GM, 1.02 (0.74, 1.41) | 0.76 |
| Atrial fibrillation | 23 (34.3%) | 34 (44.7%) | −10.28 (−21.90, 7.15) | RR, 0.77 (0.51, 1.16) | 0.23 |
| Stroke | 2 (3.0%) | 2 (2.6%) | 0.35 (−2.17, 15.56) | OR, 1.14 (0.16, 8.31) | 1.00 |
| Pneumonia | 3 (4.5%) | 7 (9.2%) | −4.75 (−8.10, 6.66) | OR, 0.46 (0.11, 1.86) | 0.33 |
| Time-to-extubation (min) | 550 (268 to 1194) | 488 (245 to 1285) | 158.26 (−55.39, 510.39) | GM, 1.40 (0.86, 2.29) | 0.62 |
| ICU duration (days) | 3 (2 to 7) | 4 (2 to 6) | −6.82 (−20.42, 7.81) | OR, 0.76 (0.42, 1.37) | 0.36 |
| In-hospital death§ | 2 (3.0%) | 0 (0.0%) | 0.22 |
Stage 2 or 3 AKI was defined according to Acute Kidney Injury Network criteria, specifically a 100% increase in serum creatinine within 48 hours of surgery or initiation of dialysis.
Maximum change within 48 hours of surgery from baseline.
Delirium was defined as any positive confusion assessment method for the ICU exam.
Estimates and confidence intervals were not calculated for outcomes with zero events in the statin or placebo treatment groups.
AKI, acute kidney injury; POD1, postoperative day 1
In the baseline CKD subgroup (n=179 of 615), the incidences of AKI were also similar between treatment groups. Thirty of the 84 participants with CKD randomized to atorvastatin (35.7%) developed AKI, and 31 of the 95 CKD participants randomized to placebo (32.6%) developed AKI (RR, 1.09 [0.73–1.65]; P=0.76). In the statin-naïve CKD subgroup (n=36), AKI occurred in 9 of 17 participants (52.9%) randomized to atorvastatin, compared to 3 of 19 participants (15.8%) randomized to placebo (RR, 3.35 [1.12–10.05]; P=0.03). Postoperative serum creatinine concentrations increased a median of 0.26 mg/dl (−0.22 to 0.94) within the first 48 postoperative hours in patients randomized to atorvastatin, compared to −0.06 mg/dl (−0.16 to 0.41) in patients randomized to placebo (mean difference, 0.28 mg/dl [0.02–0.54]; P=0.04). Among the statin-using CKD subgroup (n=143), AKI occurred in 21 of 67 participants (31.3%) randomized to atorvastatin, compared to 28 of 76 participants (36.8%) randomized to placebo (RR, 0.85 [0.54–1.35]; P=0.59). Postoperative changes in serum creatinine were not different between statin-using CKD patients randomized to atorvastatin or placebo.
In the total cohort, AKI required dialysis in 5 of the 308 (1.6%) patients randomized to atorvastatin compared to 3 of the 307 (1.0%) patients randomized to placebo (P=0.71). Of the 124 cases of AKI in the study, 106 (85.4%) were stage 1, 6 (4.8%) stage 2, and 12 (9.7%) stage 3. There was no observed treatment effect on moderate/severe (Stage 2 or 3) AKI, and treatment effects on moderate/severe AKI were similar to treatment effects on Stage 1, 2, or 3 AKI in the entire cohort and subgroups (Table 2).
Secondary end points and adjusted analyses
Perioperative high-dose atorvastatin treatment did not affect the secondary end points of ICU delirium, atrial fibrillation, myocardial injury, or stroke (Table 2). The incidence of postoperative pneumonia was numerically lower but not significantly different in patients randomized to atorvastatin. This result should be interpreted with caution based on multiple comparisons for secondary end points and risk of type 1 error.
All but one of the 17 strokes was ischemic in nature rather than hemorrhagic. ICU length of stay and all-cause in-hospital mortality were not significantly different between treatment groups.
Propensity-adjusted analyses yielded results that were largely consistent with the intention-to-treat unadjusted analyses, although the evidence for harm of atorvastatin treatment in the statin-naïve patients with preexisting CKD was attenuated (Supplement Table 1).
Safety end points
There was no evidence to suggest that perioperative high-dose atorvastatin treatment increased skeletal muscle or hepatic markers of statin toxicity. Six atorvastatin-treated patients (1.9%) reported proximal muscle myalgias on postoperative day 1, 2, or 3, compared to five placebo-treated patients (1.6%, P=0.77, Table 3). Postoperative day 1 concentrations of creatine kinase and aspartate aminotransferase were also not increased by high-dose short-term perioperative atorvastatin treatment, nor were adverse events (see Supplement for details of adverse events).
Table 3.
Treatment toxicity among all patients and prespecified subgroups. We report the No. (%) of myalgias on any of postoperative day (POD) 1, 2, and 3 exams and the median (10th percentile to 90th percentile) of POD1 plasma concentrations of creatine kinase and aspartate aminotransferase (AST). Estimates and confidence intervals (CI) should be interpreted as risk ratios or relative risks (RR) for myalgias outcomes and geometric mean ratios (GM) for log-transformed creatine kinase and AST outcomes. P-values correspond to the Pearson chi-square test for myalgias and the Wilcoxon rank sum test for creatine kinase and AST.
| Statin | Placebo | Absolute Difference (95% CI) |
Estimate (95% CI) | P-value | |
|---|---|---|---|---|---|
| All patients | n = 308 | n = 307 | |||
| Myalgias | 6 (1.9%) | 5 (1.6%) | 0.31 (−1.02, 4.45) | RR, 1.20 (0.36, 3.96) | 0.77 |
| Creatine kinase on POD1, u/l | 486 (249 to 1093) | 549 (225 to 1455) | −38.08 (−97.91, 21.76) | GM, 0.93 (0.82, 1.04) | 0.29 |
| AST on POD1, u/l | 49 (25 to 105) | 52 (27 to 146) | −5.73 (−10.89, 0.00) | GM, 0.90 (0.81, 1.00) | 0.11 |
| Statin-naïve | n = 102 | n = 97 | |||
| Myalgias | 1 (1.0%) | 2 (2.1%) | −1.10 (−2.01, 7.97) | RR, 0.47 (0.04, 5.22) | 0.53 |
| Creatine kinase on POD1, u/l | 486 (237 to 1178) | 556 (224 to 1631) | −22.07 (−121.39, 99.32) | GM, 0.96 (0.78, 1.18) | 0.73 |
| AST on POD1, u/l | 52 (29 to 119) | 56 (32 to 150) | −5.68 (−14.50, 5.68) | GM, 0.91 (0.77, 1.09) | 0.52 |
| Statin-using | n = 206 | n = 210 | |||
| Myalgias | 5 (2.4%) | 3 (1.4%) | 0.98 (−0.82, 7.98) | RR, 1.72 (0.41, 7.29) | 0.49 |
| Creatine kinase on POD1, u/l | 486 (255 to 1011) | 538 (226 to 1199) | −48.62 (−113.45, 27.01) | GM, 0.91 (0.79, 1.05) | 0.27 |
| AST on POD1, u/l | 46 (24 to 99) | 50 (25 to 142) | −6.03 (−11.50, 0.00) | GM, 0.89 (0.79, 1.00) | 0.14 |
| CKD | n = 95 | n = 84 | |||
| Myalgias | 1 (1.1%) | 2 (2.4%) | −1.04 (−2.28, 11.03) | RR, 0.56 (0.05, 6.31) | 0.60 |
| Creatine kinase on POD1, u/l | 460 (248 to 1141) | 521 (243 to 1076) | −25.54 (−122.57, 91.93) | GM, 0.95 (0.76, 1.18) | 0.65 |
| AST on POD1, u/l | 49 (27 to 93) | 59 (28 to 143) | −8.88 (−17.17, 0.59) | GM, 0.85 (0.71, 1.01) | 0.08 |
| Statin-naïve with CKD | n = 17 | n = 19 | |||
| Myalgias* | 0 (0.0%) | 2 (10.5%) | 0.17 | ||
| Creatine kinase on POD1, u/l | 454 (217 to 2088) | 520 (228 to 1037) | −5.03 (−226.34, 402.38) | GM, 0.99 (0.55, 1.80) | 0.59 |
| AST on POD1, u/l | 55 (28 to 154) | 47 (32 to 148) | 4.36 (−21.78, 47.29) | GM, 1.07 (0.65, 1.76) | 0.91 |
| Statin-using with CKD | n = 67 | n = 76 | |||
| Myalgias* | 1 (1.5%) | 0 (0.0%) | 0.47 | ||
| Creatine kinase on POD1, u/l | 489 (251 to 1086) | 549 (250 to 1090) | −30.75 (−133.26, 97.38) | GM, 0.94 (0.74, 1.19) | 0.85 |
| AST on POD1, u/l | 46 (25 to 80) | 60 (28 to 130) | −11.69 (−19.29, −1.75) | GM, 0.80 (0.67, 0.97) | 0.03 |
Estimates and confidence intervals were not calculated for outcomes with zero events in the statin or placebo treatment groups.
DISCUSSION
This double-blinded, placebo-controlled, randomized clinical trial found no evidence that high-dose perioperative atorvastatin reduces the incidence or severity of AKI following cardiac surgery. In fact, among patients naïve to statins, high-dose perioperative atorvastatin increased serum concentrations of creatinine, and there was some evidence that statin treatment may increase AKI among statin-naïve patients with baseline CKD. Among pre-study statin users, there was no evidence that perioperative statin continuation or withdrawal affected postoperative AKI. Prespecified secondary end points delirium, myocardial injury, atrial fibrillation and stroke were also similar between randomized treatment groups.
Statin treatment presents an attractive therapy to reduce AKI following cardiac surgery based on preclinical studies of statin mechanisms and observational studies of cardiac surgery cohorts. Short-term statin treatment limits vascular superoxide generation,20,21 reduces endothelial dysfunction by restoring endothelial derived nitric oxide synthase activity during hypoxia,22 and attenuates lymphocyte activation.23 These effects decrease inflammation, endothelial dysfunction, and oxidant stress, mechanisms implicated in AKI development,24 and in a study of cardiac surgery patients, three weeks of atorvastatin 20mg reduced peak plasma interleukin-6 and −8 concentrations and neutrophil adhesion.25
Prior studies of statins to reduce AKI following cardiac surgery are limited to a pilot study and numerous observational reports. Prowle et al randomized 100 cardiac surgery patients to four days of atorvastatin 40mg or placebo starting the day prior to surgery and compared peak serum creatinine rise from baseline to postoperative day 5.26 They found no difference in creatinine rise and a marginal but insignificant reduction in AKI rates defined by risk, injury, failure, loss, end-stage (RIFLE) criteria in patients randomized to atorvastatin.27 The majority of observational studies compare patients who are using statins prior to surgery to patients not using statins prior to surgery. The association between preoperative statin use and postoperative AKI is inconsistent, possibly due to selection bias for statin use, variable effects of treatment, and disparate patient populations. Some of these studies reported a lower incidence of AKI among patients using statins preoperatively,8,28–30 while others reported no differences between statin-using and -non-using groups.9,31–34 Few studies have reported statin exposure in the entire perioperative period – specifically the day prior to surgery, the day of surgery, and the first two days following surgery – a period of time when acute inflammation, endothelial dysfunction, and oxidant injury surge and therefore a potential therapeutic window for preventing AKI.
Contrary to our hypothesis, de novo initiation of daily perioperative atorvastatin treatment did not reduce the incidence of AKI or reduce the increase in serum creatinine associated with cardiac surgery. On the contrary, initiation of high-dose atorvastatin in statin-naïve patients increased serum creatinine more from baseline to postoperative day 2 than initiation of placebo, and among statin-naïve patients with CKD, there was evidence that statin treatment may increase AKI. The small number of patients in the statin-naïve CKD subgroup, the wide confidence interval, and the loss of statistical significance in the propensity score-adjusted model are limitations to this finding, but the strength of the signal was high. The mechanism of any deleterious effects is unclear. The kidneys eliminate less than 1% of atorvastatin,35 so any renal toxic effect is unlikely to be related to increased circulating atorvastatin in CKD patients. Since CKD patients are at high risk for AKI,2 any injurious mechanism of de novo atorvastatin initiation may be magnified in CKD patients. A harmful effect of de novo statin treatment on renal function has been previously reported in a different critically ill patient population. The ARDS network recently reported that patients randomized to daily rosuvastatin after being admitted to the ICU with acute respiratory distress syndrome experienced increased renal failure compared to patients randomized to placebo.36 Eighty-five percent of these ARDS network patients were naïve to statins.
Most patients presenting for cardiac surgery, however, are already using statins, and in the current study there was little evidence that continuation or withdrawal from statin treatment on the day of surgery and postoperative day 1 affects AKI. The two-day duration of treatment for pre-study statin users could be a limitation to this finding, but this treatment duration is consistent with the opportunity for intervention in current clinical practice. We deemed a longer period of statin withdrawal unethical based on reports that perioperative statin withdrawal for periods as short as 1–2 days correlates with increased risk of AKI following cardiac or vascular surgery, the observation that 40% of statin-using patients resume statin use within one day of cardiac surgery, and a clinical trial in statin-using percutaneous coronary intervention patients demonstrating that placebo administration on the day of intervention increases myocardial injury compared to atorvastatin (statin continuation) treatment.10,12,13,37 Therefore, to limit risk in statin-using patients randomized to placebo, we did not reduce statin exposure compared to usual care. Also in keeping with the pragmatic nature of the trial, we did not initiate a preoperative treatment in pre-study statin-naïve patients multiple days or weeks before surgery, since many cardiac surgery patients receive surgery soon after consultation with their surgeon, and we deemed it impractical to require patients to return one year after surgery, so the impact of treatment on long-term renal function is unknown.
We acknowledge other limitations. This was not a multicenter study. We diagnosed AKI using AKIN criteria,16 but urine output AKIN criteria were not used, and AKIN criteria are insensitive to AKI that develops late in the postoperative period. The statistical significance for the deleterious effect of treatment on the primary endpoint in the statin-naïve CKD subgroup was lost in the secondary propensity-score adjusted analysis, possibly as a result of type 1 error or lack of power in this subgroup, and the cohort as a whole was at low risk for more severe clinical outcomes such as Stage 2 or 3 AKI, which have been more closely associated with long-term outcomes.
Conclusion
Among patients undergoing cardiac surgery, high-dose perioperative atorvastatin treatment, compared to placebo administration, did not reduce the risk of AKI overall, among patients naive to statins, or patients already using a statin. These results do not support the initiation of statin therapy to prevent AKI following cardiac surgery.
Supplementary Material
Acknowledgments
We would like to thank Anthony DeMatteo, B.S., for technical assistance, Jennifer Morse, M.S., and Misty Hale for data management assistance, and Will Hardeman, B.A., Cleo Carter, B.A., Kiersten Card, Jennifer Morse, M.S., and Damon Michaels, B.S., of the Vanderbilt Department of Anesthesiology’s Perioperative Clinical Research Institute for assisting us in data collection.
Frederic Billings and Jonathan Schildcrout had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Frederic Billings, Jonathan Schildcrout, and Yaping Shi conducted the data analysis.
Dr. Brown reports receiving grants from Shire Pharmaceuticals and New Haven Pharmaceuticals and personal fees from Novartis Pharmaceuticals and Alnylam Pharmaceuticals.
Funding Sources:
National Institutes of Health [K23GM102676, K12ES015855, and UL1RR024975] and the Vanderbilt University Department of Anesthesiology
Data and Safety Monitoring Board:
Mias Pretorius, M.D., M.Sc. (chair); Julia B. Lewis, M.D.; Stephen K. Ball, M.D.; and Leena Choi, Ph.D.
Footnotes
Potential conflicts of interest:
No author reports any relevant conflicts of interest.
REFERENCES
- 1.Rosner MH, Okusa MD. Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol. 2006;1:19–32. doi: 10.2215/CJN.00240605. [DOI] [PubMed] [Google Scholar]
- 2.Cooper WA, O’Brien SM, Thourani VH, et al. Impact of renal dysfunction on outcomes of coronary artery bypass surgery: results from the Society of Thoracic Surgeons National Adult Cardiac Database. Circulation. 2006;113(8):1063–1070. doi: 10.1161/CIRCULATIONAHA.105.580084. [DOI] [PubMed] [Google Scholar]
- 3.Swaminathan M, Shaw AD, Phillips-Bute BG, et al. Trends in acute renal failure associated with coronary artery bypass graft surgery in the United States. Crit Care Med. 2007;35:2286–2291. doi: 10.1097/01.ccm.0000282079.05994.57. [DOI] [PubMed] [Google Scholar]
- 4.Siddiqui NF, Coca SG, Devereaux PJ, et al. Secular trends in acute dialysis after elective major surgery--1995 to 2009. CMAJ. 2012;184(11):1237–1245. doi: 10.1503/cmaj.110895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birnie K, Verheyden V, Pagano D, et al. Predictive models for kidney disease: improving global outcomes (KDIGO) defined acute kidney injury in UK cardiac surgery. Crit Care. 2014;18(6):606. doi: 10.1186/s13054-014-0606-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryckwaert F, Boccara G, Frappier JM, Colson PH. Incidence, risk factors, and prognosis of a moderate increase in plasma creatinine early after cardiac surgery. Crit Care Med. 2002;30:1495–1498. doi: 10.1097/00003246-200207000-00016. [DOI] [PubMed] [Google Scholar]
- 7.Thakar CV, Yared J-P, Worley S, Cotman K, Paganini EP. Renal dysfunction and serious infections after open-heart surgery. Kidney Int. 2003;64(1):239–246. doi: 10.1046/j.1523-1755.2003.00040.x. [DOI] [PubMed] [Google Scholar]
- 8.Layton JB, Kshirsagar AV, Simpson RJ, et al. Effect of statin use on acute kidney injury risk following coronary artery bypass grafting. Am J Cardiol. 2013;111(6):823–828. doi: 10.1016/j.amjcard.2012.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Argalious M, Xu M, Sun Z, Smedira N, Koch CG. Preoperative statin therapy is not associated with a reduced incidence of postoperative acute kidney injury after cardiac surgery. Anesth Analg. 2010;111(2):324–330. doi: 10.1213/ANE.0b013e3181d8a078. [DOI] [PubMed] [Google Scholar]
- 10.Heeschen C, Hamm CW, Laufs U, Snapinn S, Bohm M, White HD. Withdrawal of statins increases event rates in patients with acute coronary syndromes. Circulation. 2002;105:1446–1452. doi: 10.1161/01.cir.0000012530.68333.c8. [DOI] [PubMed] [Google Scholar]
- 11.Di Sciascio G, Patti G, Pasceri V, Gaspardone A, Colonna G, Montinaro A. Efficacy of atorvastatin reload in patients on chronic statin therapy undergoing percutaneous coronary intervention: results of the ARMYDA-RECAPTURE (Atorvastatin for Reduction of Myocardial Damage During Angioplasty) Randomized Trial. J Am Coll Cardiol. 2009;54:558–565. doi: 10.1016/j.jacc.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 12.Billings FT, Pretorius M, Siew ED, Yu C, Brown NJ. Early Postoperative Statin Therapy Is Associated With a Lower Incidence of Acute Kidney Injury After Cardiac Surgery. J Cardiothorac Vasc Anesth. 2010;24(6):913–920. doi: 10.1053/j.jvca.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kor DJ, Brown MJ, Iscimen R, et al. Perioperative statin therapy and renal outcomes after major vascular surgery: a propensity-based analysis. J Cardiothorac Vasc Anesth. 2008;22(2):210–216. doi: 10.1053/j.jvca.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 14.Patti G, Chello M, Candura D, et al. Randomized trial of atorvastatin for reduction of postoperative atrial fibrillation in patients undergoing cardiac surgery: results of the ARMYDA-3 (Atorvastatin for Reduction of MYocardial Dysrhythmia After cardiac surgery) study. Circulation. 2006;114:1455–1461. doi: 10.1161/CIRCULATIONAHA.106.621763. [DOI] [PubMed] [Google Scholar]
- 15.Patti G, Pasceri V, Colonna G, et al. Atorvastatin pretreatment improves outcomes in patients with acute coronary syndromes undergoing early percutaneous coronary intervention: results of the ARMYDA-ACS randomized trial. J Am Coll Cardiol. 2007;49:1272–1278. doi: 10.1016/j.jacc.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 16.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286:2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 18.Wacholder S. Binomial regression in GLIM: estimating risk ratios and risk differences. Am J Epidemiol. 1986;123(1):174–184. doi: 10.1093/oxfordjournals.aje.a114212. [DOI] [PubMed] [Google Scholar]
- 19.Katz KA. The (relative) risks of using odds ratios. Arch Dermatol. 2006;142(6):761–764. doi: 10.1001/archderm.142.6.761. [DOI] [PubMed] [Google Scholar]
- 20.Wassmann S, Laufs U, Baumer AT, et al. HMG-CoA reductase inhibitors improve endothelial dysfunction in normocholesterolemic hypertension via reduced production of reactive oxygen species. Hypertension. 2001;37:1450–1457. doi: 10.1161/01.hyp.37.6.1450. [DOI] [PubMed] [Google Scholar]
- 21.Wagner AH, Kohler T, Ruckschloss U, Just I, Hecker M. Improvement of nitric oxide-dependent vasodilatation by HMG-CoA reductase inhibitors through attenuation of endothelial superoxide anion formation. Arterioscler Thromb Vasc Biol. 2000;20:61–69. doi: 10.1161/01.atv.20.1.61. [DOI] [PubMed] [Google Scholar]
- 22.Laufs U, Fata VL, Liao JK. Inhibition of 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase blocks hypoxia-mediated down-regulation of endothelial nitric oxide synthase. J Biol Chem. 1997;272:31725–31729. doi: 10.1074/jbc.272.50.31725. [DOI] [PubMed] [Google Scholar]
- 23.Kwak B, Mulhaupt F, Myit S, Mach F. Statins as a newly recognized type of immunomodulator. Nat Med. 2000;6:1399–1402. doi: 10.1038/82219. [DOI] [PubMed] [Google Scholar]
- 24.Billings FT4th, Pretorius M, Schildcrout JS, et al. Obesity and oxidative stress predict AKI after cardiac surgery. J Am Soc Nephrol. 2012;23(7):1221–1228. doi: 10.1681/ASN.2011090940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chello M, Patti G, Candura D, et al. Effects of atorvastatin on systemic inflammatory response after coronary bypass surgery. Crit Care Med. 2006;34:660–667. doi: 10.1097/01.CCM.0000201407.89977.EA. [DOI] [PubMed] [Google Scholar]
- 26.Prowle JR, Calzavacca P, Licari E, et al. Pilot double-blind, randomized controlled trial of short-term atorvastatin for prevention of acute kidney injury after cardiac surgery. Nephrology (Carlton) 2012;17(3):215–224. doi: 10.1111/j.1440-1797.2011.01546.x. [DOI] [PubMed] [Google Scholar]
- 27.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christenson JT. Preoperative lipid-control with simvastatin reduces the risk of postoperative thrombocytosis and thrombotic complications following CABG. Eur J Cardiothorac Surg. 1999;15:394–400. doi: 10.1016/s1010-7940(99)00034-2. [DOI] [PubMed] [Google Scholar]
- 29.Tabata M, Khalpey Z, Pirundini PA, Byrne ML, Cohn LH, Rawn JD. Renoprotective effect of preoperative statins in coronary artery bypass grafting. Am J Cardiol. 2007;100:442–444. doi: 10.1016/j.amjcard.2007.03.071. [DOI] [PubMed] [Google Scholar]
- 30.Huffmyer JL, Mauermann WJ, Thiele RH, Ma JZ, Nemergut EC. Preoperative statin administration is associated with lower mortality and decreased need for postoperative hemodialysis in patients undergoing coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth. 2009;23(4):468–473. doi: 10.1053/j.jvca.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 31.Pan W, Pintar T, Anton J, Lee VV, Vaughn WK, Collard CD. Statins are associated with a reduced incidence of perioperative mortality after coronary artery bypass graft surgery. Circulation. 2004;110:II45–II49. doi: 10.1161/01.CIR.0000138316.24048.08. [DOI] [PubMed] [Google Scholar]
- 32.Thielmann M, Neuhäuser M, Marr A, et al. Lipid-lowering effect of preoperative statin therapy on postoperative major adverse cardiac events after coronary artery bypass surgery. J Thorac Cardiovasc Surg. 2007;134(5):1143–1149. doi: 10.1016/j.jtcvs.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 33.Fedoruk LM, Wang H, Conaway MR, Kron IL, Johnston KC. Statin therapy improves outcomes after valvular heart surgery. Ann Thorac Surg. 2008;85(5):1521–1525. doi: 10.1016/j.athoracsur.2008.01.078. discussion 1525–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Molnar AO, Parikh CR, Coca SG, et al. Association between preoperative statin use and acute kidney injury biomarkers in cardiac surgical procedures. Ann Thorac Surg. 2014;97(6):2081–2087. doi: 10.1016/j.athoracsur.2014.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lennernäs H. Clinical pharmacokinetics of atorvastatin. Clin Pharmacokinet. 2003;42(13):1141–1160. doi: 10.2165/00003088-200342130-00005. [DOI] [PubMed] [Google Scholar]
- 36.National Heart, Lung, and Blood Institute ARDS Clinical Trials Network; Truwit JD, Bernard GR, et al. Rosuvastatin for sepsis-associated acute respiratory distress syndrome. N Engl J Med. 2014;370(23):2191–2200. doi: 10.1056/NEJMoa1401520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Di Sciascio G, Patti G, Pasceri V, Gaspardone A, Colonna G, Montinaro A. Efficacy of atorvastatin reload in patients on chronic statin therapy undergoing percutaneous coronary intervention: results of the ARMYDA-RECAPTURE (Atorvastatin for Reduction of Myocardial Damage During Angioplasty) Randomized Trial. J Am Coll Cardiol. 2009;54(6):558–565. doi: 10.1016/j.jacc.2009.05.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.