Abstract
Background
The lung has a highly regulated system of innate immunity to protect itself from inhaled microbes and toxins. The first line of defense is mucociliary clearance, but if invaders overcome this, inflammatory pathways are activated. Toll-like receptors (TLRs) are expressed on the airway epithelium. Their signaling initiates the inflammatory cascade and leads to production of inflammatory cytokines such as IL-6 and IL-8. We hypothesized that airway epithelial insults, including heavy alcohol intake or smoking, would alter the expression of TLRs on the airway epithelium
Methods
Bronchoscopy with bronchoalveolar lavage and brushings of the airway epithelium was performed in otherwise healthy subjects who had normal chest radiographs and spirometry. A history of alcohol use disorders (AUDs) was ascertained using the Alcohol Use Disorders Identification Test (AUDIT), and a history of cigarette smoking was also obtained. Age, gender and nutritional status in all groups were similar. We used real-time PCR to quantitate TLR1-TLR9 and enzyme-linked immune assay (ELISA) to measure TNFα, IL-6 and IL-8.
Results
Airway brushings were obtained from 26 non-smoking/non-AUD subjects; 28 smoking/non-AUD subjects; 36 smoking/AUD subjects; and 17 non-smoking/AUD subjects. We found that TLR2 is upregulated in AUD subjects, compared to non-smoking/non-AUD subjects, and correlated with their AUDIT scores. We also measured a decrease in TLR4 expression in AUD subjects that correlated with AUDIT score. IL-6 and IL-8 were also increased in bronchial washings from AUD subjects.
Conclusions
We have previously demonstrated in normal human bronchial epithelial (NHBE) cells that in vitro alcohol exposure upregulates TLR2 though a NO/cGMP/PKG dependent pathway, resulting in upregulation of inflammatory cytokine production after gram-positive bacterial product stimulation. Our current translational study confirms that TLR2 is also upregulated in humans with AUDs.
Keywords: human, toll-like receptors, cytokines, airway epithelium
INTRODUCTION
Problem drinking that becomes severe is given the medical diagnosis of alcohol use disorder or AUD, which can be identified using the Alcohol Use Disorder Identification Test (AUDIT). AUDs have long been recognized as a risk factor for pneumonia with Benjamin Rush first publishing this association in 1810. Careful study in more recent history also demonstrates an increased risk of developing pneumonia in those with AUDs (Fernandez-Sola et al., 1995, Samokhvalov et al., 2010), as well as an increased severity of illness and mortality (Naucler et al., 2013). Alcohol likely exerts these effects in a multitude of ways. Alcohol has profound effects on nearly all host defenses of the lung, including mucociliary clearance (Wyatt and Sisson, 2001), airway inflammation (Bailey et al., 2009), macrophage function (Goral and Kovacs, 2005), and alveolar barrier function (Moss et al., 1996).
The airway epithelium plays a role in neutrophil recruitment during pneumococcal pneumonia through its production of chemokines and inflammatory cytokines (Yamamoto et al., 2014). Toll-like receptors (TLRs) are important initiators of the inflammatory response in the airway epithelium (Sha et al., 2004). The human airway epithelium expresses TLR 1–10, pattern recognition receptors that initiate the inflammatory cascade to combat infecting pathogens. Each TLR recognizes a different pathogen-associated molecular pattern ligand (Table 1). TLR2 recognizes lipoprotein components of the gram-positive bacterial cell wall. It dimerizes with either TLR1 or TLR6 to recognize either triacyl lipoprotein (TLR1) or diacyl lipoprotein (TLR6). The most common cause of community-acquired pneumonia is Streptococcus pneumoniae, a gram-positive organism that signals through TLR2. TLR4 is also expressed in the airway epithelium, where it responds to lipopolysaccharide (LPS), a component of the gram-negative cell wall.
Table 1.
Summary of TLR ligands and their origins.
| Ligand | Origin of the ligand | |
|---|---|---|
| TLR1 | Lipoprotein | Bacteria (Gram positive) |
| TLR2 | Triacyl lipoprotein | Bacteria (Gram positive) |
| TLR3 | Double stranded RNA | Viruses |
| TLR4 | LPS | Bacteria (gram negative) |
| TLR5 | Flagellin | Bacteria |
| TLR6 | Diacyl lipoprotein | Bacteria (gram positive) |
| TLR7 | Single stranded RNA | Viruses & bacteria |
| TLR8 | Single stranded RNA | Viruses & bacteria |
| TLR9 | Unmethylated CpG DNA |
Viruses & bacteria |
We previously demonstrated in an in vitro model that alcohol exposure alters the expression of TLRs in the airway epithelium (Bailey et al., 2009). After alcohol exposure, an increase in TLR2 mRNA and protein was observed. The upregulation of TLR2 corresponds to an exaggerated inflammatory response to components of the gram-positive cell wall (Bailey et al., 2009). Subsequently, we discovered that the upregulation of TLR2 by alcohol is related to alcohol-triggered nitric oxide production in the airway epithelial cell, which stimulated cGMP and PKG (Bailey et al., 2010).
To determine whether TLR2 was also upregulated in vivo in the human condition, we measured TLRs 1–9 expression in airway brushings from never smokers and subjects with AUDs. Because those with AUDs also frequently smoke (Walton, 1972), we also measured TLR expression in smokers with and without AUDs. Based on our in vitro model, we hypothesized that human subjects with AUDs would express higher levels of TLR2 mRNA in their airway epithelium, resulting in higher levels of airway cytokines.
MATERIALS AND METHODS
Subject screening, recruitment, and enrollment
The institutional review boards at all participating sites approved this study, and all subjects provided written informed consent before their participation. Subjects with AUDs were recruited between 2008 and 2014 from the Denver Comprehensive Addictions Rehabilitation and Evaluation Services (Denver CARES) center, an inpatient detoxification facility affiliated with Denver Health and Hospital System in Denver, CO. Subjects with AUDs were eligible if they met the following 3 criteria: 1) an Alcohol Use Disorders Identification Test (AUDIT) score of ≥8 (Saunders et al., 1993); 2) used alcohol within the 7 days before enrollment, and 3) 21 yrs old, or older. AUD subjects were excluded from participation if they had any of the following comorbidities: 1) Liver disease (history of cirrhosis, total bilirubin ≥ 2.0 mg/dl, or serum albumin ≤3.0); 2) history of gastrointestinal bleeding; 3) heart disease (history of myocardial infarction, severe valvular dysfunction or ejection fraction < 50%.); 4) renal disease (end-stage renal disease requiring dialysis, or a serum creatinine ≥2 mg/dl); 5) lung disease (abnormal chest radiograph or spirometry (forced vital capacity (FVC) or forced expiratory volume in 1 second (FEV1) <75% predicted)); 6) illicit drug use (defined as a urine drug screen positive for cocaine, opiates, or methamphetamines); 7) diabetes mellitus; 8) inability to provide informed consent; 9) human immunodeficiency virus (HIV) positive; 10) pregnancy; or 11) abnormal nutritional status, based on serum albumin and weight loss history over the prior 6 months (White et al., 2012).
Subjects without AUDs (controls) were recruited from the University of Colorado’s Anschutz Medical Campus in Aurora, CO, and via print advertisements in the Denver metropolitan area. Screening of potential control subjects focused on matching these control subjects to AUD subjects in terms of age, sex, and smoking history. Cigarette-smoking history was assessed by self-report. Pack-year history was calculated in current and former smokers (for both AUD subjects and controls). Time since cessation was also recorded for former smokers.
Eligible subjects who provided informed consent were admitted to the University of Colorado Hospital Clinical and Translational Research Center (CTRC) for bronchoscopy. Subjects were sedated using standard conscious sedation protocols as previously described (Hunninghake et al., 1979, Burnham et al., 2013). Briefly, the bronchoscope was wedged into a sub-segment of either the right middle lobe or the lingula. Three to four 50-ml aliquots of sterile, room temperature 0.9% saline were sequentially instilled and recovered with gentle aspiration. The first aspirated aliquot, representing the “bronchial component” was centrifuged to remove cells and was utilized in experiments for the present investigation. A sterile cytology brush was then used to collect brushings from the left and right mainstem bronchi. Brushes were transferred immediately after collection into microfuge tubes containing RNAlater (Sigma-Aldrich St. Louis, MO). Both the brushings and BAL fluid were stored at −80°C until RNA extraction could be performed.
RNA extraction and real-time PCR
RNA was extracted using the MagMax kit (Life Technologies, Carlsbad, CA) according to the manufacturer’s protocol. RNA quantity and quality were analyzed using a spectrophotometer (NanodropD-1000, Wilmington, DE). Only RNA samples with a 260:280 ratio of >1.8 were used. Total cDNA was synthesized using the Taqman real-time PCR kit (Applied Biosystems) according to the manufacturer’s directions using 50ng of RNA template and random hexamers (2.5 µM). Quantitative Real-time PCR was performed using an ABI Prism 7400 sequence detection system (Applied Biosystems). The reactions were run as a duplex, with the gene of interest containing a FAM probe and the endogenous control 18S ribosomal RNA containing a VIC/Tamra probe. The reaction mixture consisted of a 1X cocktail of Taqman universal master mix (Applied Biosystems) containing primers for both the gene of interest (TLR1-9) and the endogenous control gene 18S Ribosomal RNA. The following primer/probe sets for Toll-like receptors 1 through 9 were selected for study: (TLR1: Hs00413978; TLR2: Hs00152932; TLR3: Hs00152933; TLR4: Hs00152939; TLR5: Hs00152825; TLR6: Hs00271977; TLR7: Hs01933259; TLR8: Hs00152972; TLR9: Hs00370913; Applied Biosystems). Reactions were performed in duplicate. The PCR conditions were 2 min at 50°C, 10 min at 95°C, followed by 40 cycles of 15s at 95°C, and 1 min at 60°C. Each transcript level was normalized to ribosomal RNA using the cycle threshold value (Ct) using the ΔΔCt method (Livak and Schmittgen, 2001). Data is reported as the average Ct normalized to ribosomal RNA.
IL-6, IL-8 and TNFα enzyme-linked immunosorbant assays (ELISA)
IL-6, IL-8 and TNFα were measured in the acellular bronchial lavage fluid using a standard sandwich ELISA as described previously (Wyatt et al., 2010). For IL-6 and IL-8 measurements, microtiter plates were coated with the appropriate capture antibody in Voller’s carbonate buffer (pH 9.6) overnight at 4°C. The anti-human IL-8 antibody (R&D Systems, Minneapolis, MN) was diluted 1:500; IL-6 antibody (R&D Systems) was diluted 1:1000. The plates were washed and recombinant IL-6 or IL-8 standards were applied along with 200µl of samples in duplicate for 2 hours. The plates were washed and the “bridge” antibody was applied, either (rabbit) anti-human IL-8 antibody diluted 1:500 (Rockland, Gilberstville, PA) or IL-6 antibody (R&D Systems) diluted 1:1000 for 1 hour. The plates were washed and then developed using a peroxidase substrate consisting of 10 ng/ml orthophenylenediamine (Sigma-Aldrich), and 0.003% H2O2 in distilled water. The TNFα measurements were made in the same way with the following exceptions: The capture antibody was anti-human TNFα (2 µg/mL), the secondary “bridge” antibody was biotinylated (rabbit) anti-human TNF-α (200 ng/mL). Detection was performed using steptavidin-HRP (1:200, R&D Systems). Total protein levels in the fluid was also measured using the Bradford assay (BioRad, Hercules, CA).
Statistical analysis
All statistical analysis was performed using GraphPad PRISM (Graph Pad, La Jolla, CA). We used non-parametric one-way ANOVAs (Kruskal-Wallis) to compare the 4 groups, followed by Dunn’s multiple comparison’s test. A p value of <0.05 was considered significant. Linear regression was used to evaluate TLR2, TLR4 and cytokine values against AUDIT scores.
RESULTS
Demographics
Subject and control demographic data are summarized in Table 2. We recruited 36 smokers with AUDs and 28 smoker controls, and 17 non-smokers with AUDs and 26 non-smoker controls. The mean age was similar in all groups (p=0.13). The majority of subjects in each group were male (70%–90%), consistent with the increased male prevalence of AUDs (Nolen-Hoeksema and Hilt, 2006). There were no differences in sex between the groups (p=0.15). In terms of race, the groups were equally balanced with a diverse population represented. Mean AUDIT scores were substantially higher in the AUD groups consistent with the presence of alcohol abuse and dependence among subjects with AUDs (Saunders et al., 1993). The non-smoking groups were never smokers, with a mean pack-year history of 0. The smoking groups were all current smokers. The mean body mass index (BMI) was not statistically significantly different between the groups. There were subtle differences in lung function between the groups, with smokers having a slightly lower forced expiratory volume in 1 second (FEV1) compared to non-smokers. There was no difference in FEV1 between the 2 smoking groups (p>0.05).
Table 2.
Demographics of each group. FEV1 Forced expiratory volume in one second
| Nonsmokers n=26 |
Nonsmokers with AUD n=17 |
Smokers n=28 |
Smokers with AUD n=36 |
||
|---|---|---|---|---|---|
| Age (Mean ± STD) | 39.1 ± 8.6 | 44.5 ± 6.3 | 41.6± 6.7 | 42.1± 6.7 | p=0.13 |
| Sex (Male (n. %)) |
19 (73.1%) | 12 (70.5%) | 20 (71.4%) | 33 (91.6%) | p=0.15 |
| Race (n, %) | p=0.22 | ||||
| -Caucasian | 13 (52%) | 7 (43.7%) | 13 (48.1%) | 13 (36.1%) | |
| -African American | 4 (16%) | 2 (12.5%) | 5 (18%) | 5 (13.9%) | |
| -Native American | 0 | 4 (25%) | 0 | 6 (16.7%) | |
| -Hispanic | 9 (36%) | 4 (25%) | 7 (25%) | 10 (27.8%) | |
| -Asian | 0 | 0 | 1 (3.7%) | 0 | |
| -Pacific Islander | 0 | 0 | 0 | 1 (2.8%) | |
| -Did not answer | 0 | 0 | 2 (7.4%) | 1 (2.8%) | |
| Audit Score (Mean ± STD) | 2.72 ± 1.9 | 29.1 ± 8.6 | 2.5± 2.2 | 28.3 ± 7.5 | |
| Pack-years smoked (Mean ± STD) |
0 | 0 | 16.3 ± 13.5 | 12.3 ± 10.7 | |
| BMI (Mean ± STD) | 28.2 ± 5.2 | 26.5 ± 4.7 | 27.5 ± 4.8 | 25.2 ± 3.2 | p=0.07 |
| FEV1 (% ± STD) | 101.4 ± 14.4 | 106.9 ± 11.2 | 93.48 ± 12.5 | 95.9 ± 11.4 | p=0.003 |
TLR expression
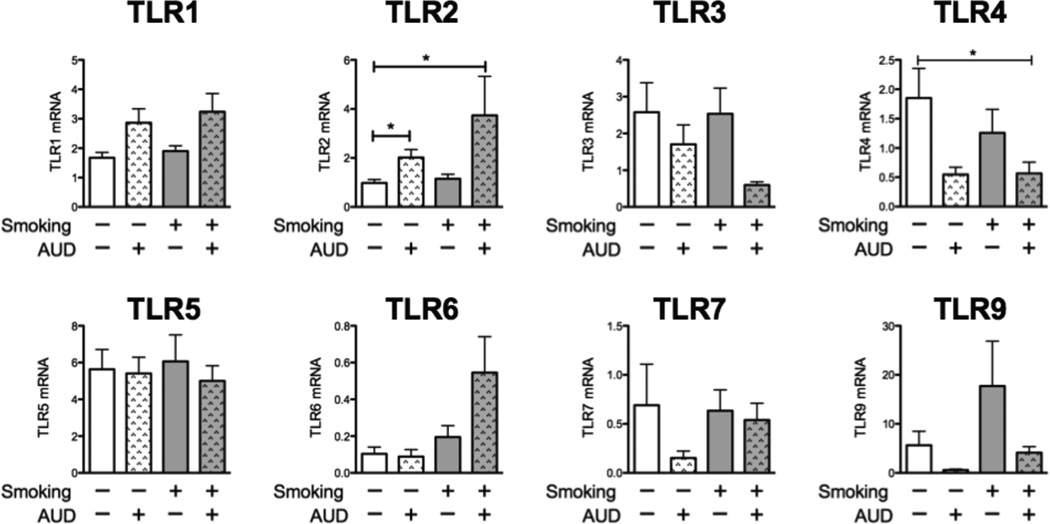
TLR1-9 expression was measured in the airway brushings of all subjects (Figure 1). The data is reported as Ct normalized to ribosomal RNA. TLR8 was measured, but was undetectable in all samples. For most TLRs, there were no statistically significant differences between the groups. We did, however, see a doubling of TLR2 in the non-smokers with AUDs (p<0.05) compared to non-smoking controls, and a near tripling in smokers with AUDs (p<0.05). TLR2 expression was not, however, significantly different between the non-smokers with AUDs and smokers with AUDs. We also observed a decrease in TLR4 in smokers with AUDs compared to non-smoking controls (p<0.05). We also saw trends towards increased expression of TLR2’s binding partners, TLR1 and TLR6, among subjects with AUDs, particularly among AUD subjects who smoked.
Figure 1. TLR2 is increased in AUDs and TLR4 is decreased.
Airway brushings were obtained from 1. Nonsmokers (white bars, n=26), 2. Non-Smokers with AUD’s (dappled white bars, n=17), 3. Smokers without AUD (gray bars, n=28) and 4. Smokers with AUD’s (gray dappled bars, n=36). Data are reported as Ct normalized to 18S ribosomal RNA. Error bars represent standard error of the mean. *p<0.05
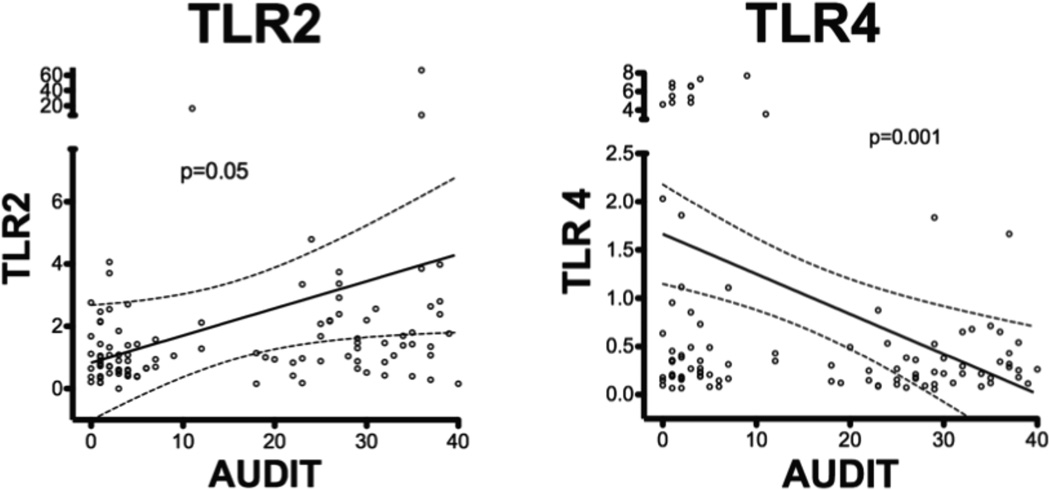
To determine if there was an association between the severity of AUDs and TLR expression, we compared AUDIT scores with mRNA expression of TLR2 and TLR4 (Figure 2). We found that as AUDIT score increased, TLR2 expression also increased (n= 107, p=0.05) while TLR4 expression diminished significantly (n=107, p=0.001). Relationships between AUDIT scores and other measured TLRs were also examined, and found to be not significantly associated.
Figure 2. TLR2 increases with increasing AUDIT score, while TLR4 decreases with increasing AUDIT score.
TLR2 and TLR4 levels were plotted against AUDIT score and the linear regression curve (solid line) was plotted with the 95% confidence intervals (Dashed lines). TLR 2 increased with increasing AUDIT score (n=107, p=0.05), while TLR4 decreased with increasing AUDIT score (n=107, p=0.001).
Airway Cytokines
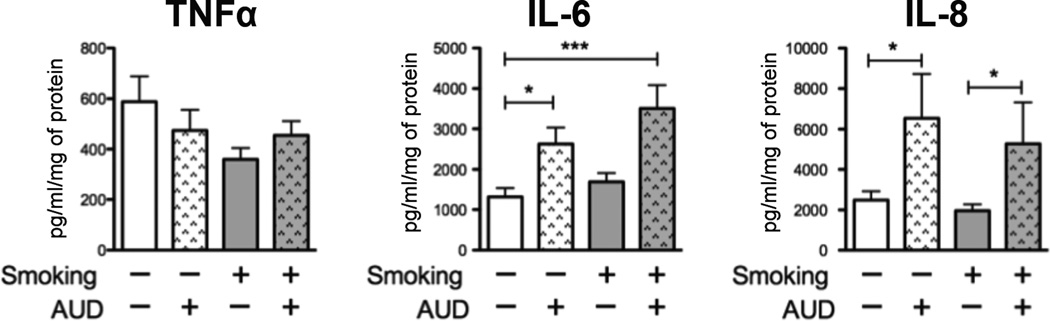
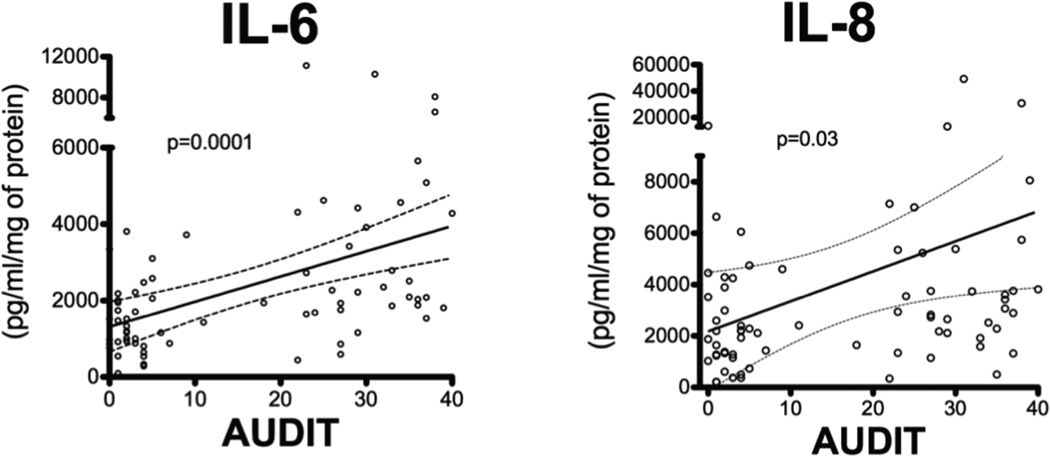
To determine whether the upregulation of TLR2 had inflammatory consequences in the airways, we measured inflammatory cytokines present in the bronchial component of the BAL. Bronchial BAL was available for 19 non-smokers, 18 smokers, 14 non-smokers with AUD, and 23 smokers with AUDs. We measured an increase in both IL-6 and IL-8 among subjects with AUDs, independent of smoking (Figure 3). Like TLR2 expression, the IL-6 (Figure 4) and IL-8 production (Figure 4) was directly related to the AUDIT score (n=72). Linear regression for TNFα was also examined and found to be insignificant. There were no significant differences in total protein between the groups (p=0.54).
Figure 3. IL-6 and IL-8 are increased in the bronchial component of the BAL in those with AUD’s.
TNFα, IL-6 and IL-8 were measured in the bronchial component of the BAL from 19 non-smokers (white bars), 18 smokers (grey bars), 14 non-smokers with AUDs (white dappled bars), and 23 smokers with AUDs (grey dappled bars). We measured increased IL-6 and IL-8 in those with AUDs, independent of smoking. (* p<0.05, *** p<0.001).
Figure 4. Increases in IL-6 and IL-8 correlate with increasing AUDIT score.
IL-6 and IL-8 were plotted against AUDIT scores. The linear regression line (solid line) was plotted with the 95% confidence intervals (dashed line). There was a significant correlation with both IL-6 and IL-8 and AUDIT score (n=72).
DISCUSSION
Alcohol abuse has long been recognized to contribute to increasing incidence and severity of pneumonia. The airway epithelium plays an important role in directing the innate immune response to infection (Sadikot et al., 2006). Little is known about how alcohol intake alters airway epithelial innate immunity. The experiments described here help us understand the potential relationship between alcohol consumption, TLR expression and airway inflammation. We observed that TLR2 is upregulated and TLR4 is downregulated in the airway epithelium of those with AUDs. Smoking in the absence of AUDs did not appreciably influence TLR2 expression; however, when combined with AUDs, it tended to increase expression still further, although these changes were not statistically significant. Changes in TLR expression correlated linearly with individual AUDIT scores, a validated measure of alcohol intake/addiction. Importantly, increased quantities of IL-6 and IL-8 were measured in the bronchial component of the BAL of those with AUDs, suggesting the potential for alterations in TLR2 expression to impact inflammatory state in the airways.
The initiation and resolution of inflammation of the airway epithelium is delicately balanced. While it is possible that a blunted inflammatory response could contribute to increased susceptibility for lung infection, it is also possible that increased baseline inflammation, as observed in these investigations, could lead to worsened lung injury and suboptimal airway repair. This scenario could result in the greater severity of pneumonia typical of patients with AUDs.
TLR2 and alcohol
The effect of alcohol intake and inflammation is complex, depending both on the chronicity and quantity of alcohol intake. Our finding of elevated TLR2 in the airway epithelium in those with AUDs is consistent with our previous work in an in vitro model of alcohol exposure in human airway epithelial cells. In that model, we measured an increase in TLR2 mRNA and protein after 24–48 hours of 50–100mM alcohol exposure (Bailey et al., 2009). The cells that were pretreated with alcohol had a more vigorous response to the TLR2 agonist, peptidoglycan (Bailey et al., 2009). We later demonstrated that the upregulation of TLR2 in the airway epithelium is related to the increase in nitric oxide signaling and PKG activity (Bailey et al., 2010). Although we initially developed this in vitro model to understand the lung’s acute response to alcohol exposure, it in fact recapitulates chronic alcohol intake in the case of TLR2 expression.
The upregulation of TLR2 by alcohol is not unique to the airway epithelium. Lieber-DeCarli alcohol feeding of mice for 10 days is also associated with upregulation of TLR2 in the liver (Gustot et al., 2006). There is also evidence that TLR2 signaling is enhanced by alcohol in microglial cells (Fernandez-Lizarbe et al., 2013), and that TLR2 expression is upregulated in the brains of humans with AUD’s (Crews et al., 2013).
TLR4 and alcohol
Our investigations also suggest that TLR4 expression is diminished in the airways of subjects with AUDs. The significance of this alcohol-triggered decrease in TLR4 in the airway epithelium is unknown. The airway epithelium already has a blunted response to TLR4 agonists due to its relatively low expression and its lack of MD-2 (Ohnishi et al., 2007). There is evidence, however, that TLR4 mutations in the alveolar epithelium can contribute to hyporesponsiveness to TLR4 ligands such as endotoxin (Breslau et al., 1996). Acute alcohol exposure in murine models has also been shown to dampen TLR4 signaling in macrophages at the level of lipid raft formation, CD14 (Szabo et al., 2007), as well as ERK signaling (Goral and Kovacs, 2005). In contrast, chronic alcohol exposure in human monocytes has been shown to increase TNFα release in response to LPS (Mandrekar et al., 2009). Likewise, in the brain, alcohol has been shown to activate TLR4 in glial cells and contribute to neuroinflammation (Pascual et al., 2011).
Increased inflammatory cytokines in those with AUDs
Our finding of increased IL-6 and IL-8 in the bronchial component of that BAL was initially surprising, because most in vitro models of airway epithelial alcohol exposure do not conclusively demonstrate differences in basal cytokine production with alcohol treatment alone (Raju et al., 2013, Bailey et al., 2009), but rather, after stimulation with bacterial products. It is worthwhile noting that human airways in vivo, such as those we examined, are continuously exposed to bacterial products through breathing, while in vitro models remain sterile, which may explain our observations. Our previous work also shows that increased RANTES (Regulated upon Activation Normal T cell Expressed and Secreted) also correlates with AUDIT scores. Both IL-8 and RANTES are dependent on NFκB signaling, which can be activated by TLR2 in the airway epithelium (Berube et al., 2009).
Our results related to IL-8 differ from those previously reported by Rennard et al. (Mio et al., 1997), who report upregulation of IL-8 in the context of smoking. The two studies differ in that alcohol history was not elicited in the Rennard cohort, and we know that smokers are 3 times more likely to abuse alcohol (Breslau et al., 1996). Our cohort was also larger; 26 nonsmokers and 28 smokers, compared to 10 and 12, respectively, in the Rennard study. It is also important to note that in our study, the smokers had a 12–16 pack-year history of smoking, and their daily smoking was limited due to inpatient treatment for AUDs.
Limitations
Although intriguing, our study is not without limitations. Our sample size is relatively small. However, it utilizes matched controls and is one of the largest to examine the role AUDs play in pulmonary inflammation, particularly the airway epithelium. Although we did observe a relationship between AUDs and TLR2, as well as pro-inflammatory cytokine expression, we cannot prove that AUDs were causal in this observation. The relationships we observed, however, are consistent with our prior in vitro work and add additional support to our argument. Further, our assessment of these parameters in healthy subjects, matched closely on the basis of age, smoking and gender should provide more validity to our observations.
We were limited by the fact that we were only able to measure TLR expression using real-time PCR. Although protein levels would further support our contentions, the quantity of protein required to do so is limited by approved research techniques in our center, and may not be safe in asymptomatic research subjects. Despite these obstacles, the correlations we observed between TLR2 with pro-inflammatory cytokine proteins helps to support the possibility that mRNA expression of TLRs may influence airways inflammation.
Finally, although our subjects were primarily men, they are representative of the US population with AUDs; additional investigations will be necessary to compare gender differences in outcome variables we reported (Fillmore et al., 1997).
Conclusions
In a well-characterized cohort of subjects with AUDs and controls without evidence of pulmonary disease, a history of AUDs was associated with enhanced TLR2 expression, in conjunction with increased IL-6 and IL-8 present in bronchial fluid, independent of smoking history. In contrast, AUDs were associated with diminished TLR4 expression in these subjects. These data suggest that alcohol abuse and dependence have the potential to influence the inflammatory state in the airways, and may be one factor that contributes to severe pneumonia with poorer outcomes among those with AUD.
Acknowledgments
K08AA019503 (KLB), R24AA019661 (ELB & TAW), R01AA008769 (JHS).
REFERENCES
- Bailey KL, Sisson JH, Romberger DJ, Robinson JE, Wyatt TA. Alcohol up-regulates TLR2 through a NO/cGMP dependent pathway. Alcohol Clin Exp Res. 2010;34:51–56. doi: 10.1111/j.1530-0277.2009.01065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey KL, Wyatt TA, Romberger DJ, Sisson JH. Alcohol functionally upregulates Toll-like receptor 2 in airway epithelial cells. Alcohol Clin Exp Res. 2009;33:499–504. doi: 10.1111/j.1530-0277.2008.00862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berube J, Bourdon C, Yao Y, Rousseau S. Distinct intracellular signaling pathways control the synthesis of IL-8 and RANTES in TLR1/TLR2, TLR3 or NOD1 activated human airway epithelial cells. Cell Signal. 2009;21:448–456. doi: 10.1016/j.cellsig.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Breslau N, Peterson E, Schultz L, Andreski P, Chilcoat H. Are smokers with alcohol disorders less likely to quit? Am J Public Health. 1996;86:985–990. doi: 10.2105/ajph.86.7.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham EL, Kovacs EJ, Davis CS. Pulmonary cytokine composition differs in the setting of alcohol use disorders and cigarette smoking. American Journal of Physiology-Lung Cellular and Molecular Physiology. 2013;304:L873–L882. doi: 10.1152/ajplung.00385.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Qin L, Sheedy D, Vetreno RP, Zou J. High mobility group box 1/Toll-like receptor danger signaling increases brain neuroimmune activation in alcohol dependence. Biol Psychiatry. 2013;73:602–612. doi: 10.1016/j.biopsych.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Lizarbe S, Montesinos J, Guerri C. Ethanol induces TLR4/TLR2 association, triggering an inflammatory response in microglial cells. J Neurochem. 2013;126:261–273. doi: 10.1111/jnc.12276. [DOI] [PubMed] [Google Scholar]
- Fernandez-Sola J, Junque A, Estruch R, Monforte R, Torres A, Urbano-Marquez A. High alcohol intake as a risk and prognostic factor for community-acquired pneumonia. Arch Intern Med. 1995;155:1649–1654. doi: 10.1001/archinte.1995.00430150137014. [DOI] [PubMed] [Google Scholar]
- Fillmore KM, Golding JM, Leino EV, Motoyoshi M, Shoemaker C, Terry H, Ager CR, Ferrer HP. Patterns and trends in women's and men's drinking. 1997 [Google Scholar]
- Goral J, Kovacs EJ. In vivo ethanol exposure down-regulates TLR2-, TLR4-, and TLR9-mediated macrophage inflammatory response by limiting p38 and ERK1/2 activation. J Immunol. 2005;174:456–463. doi: 10.4049/jimmunol.174.1.456. [DOI] [PubMed] [Google Scholar]
- Gustot T, Lemmers A, Moreno C, Nagy N, Quertinmont E, Nicaise C, Franchimont D, Louis H, Deviere J, Le Moine O. Differential liver sensitization to toll-like receptor pathways in mice with alcoholic fatty liver. Hepatology. 2006;43:989–1000. doi: 10.1002/hep.21138. [DOI] [PubMed] [Google Scholar]
- Hunninghake GW, Gadek JE, Kawanami O, Ferrans VJ, Crystal RG. Inflammatory and immune processes in the human lung in health and disease: evaluation by bronchoalveolar lavage. Am J Pathol. 1979;97:149–206. [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mandrekar P, Bala S, Catalano D, Kodys K, Szabo G. The opposite effects of acute and chronic alcohol on lipopolysaccharide-induced inflammation are linked to IRAK-M in human monocytes. J Immunol. 2009;183:1320–1327. doi: 10.4049/jimmunol.0803206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mio T, Romberger DJ, Thompson AB, Robbins RA, Heires A, Rennard SI. Cigarette smoke induces interleukin-8 release from human bronchial epithelial cells. Am J Respir Crit Care Med. 1997;155:1770–1776. doi: 10.1164/ajrccm.155.5.9154890. [DOI] [PubMed] [Google Scholar]
- Moss M, Bucher B, Moore FA, Moore EE, Parsons PE. The role of chronic alcohol abuse in the development of acute respiratory distress syndrome in adults. JAMA. 1996;275:50–54. [PubMed] [Google Scholar]
- Naucler P, Darenberg J, Morfeldt E, Ortqvist A, Henriques Normark B. Contribution of host, bacterial factors and antibiotic treatment to mortality in adult patients with bacteraemic pneumococcal pneumonia. Thorax. 2013;68:571–579. doi: 10.1136/thoraxjnl-2012-203106. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Hilt L. Possible contributors to the gender differences in alcohol use and problems. The Journal of General Psychology. 2006;133:357–374. doi: 10.3200/GENP.133.4.357-374. [DOI] [PubMed] [Google Scholar]
- Ohnishi T, Muroi M, Tanamoto K. The lipopolysaccharide-recognition mechanism in cells expressing TLR4 and CD14 but lacking MD-2. FEMS Immunol Med Microbiol. 2007;51:84–91. doi: 10.1111/j.1574-695X.2007.00281.x. [DOI] [PubMed] [Google Scholar]
- Pascual M, Balino P, Alfonso-Loeches S, Aragon CM, Guerri C. Impact of TLR4 on behavioral and cognitive dysfunctions associated with alcohol-induced neuroinflammatory damage. Brain Behav Immun. 2011;25(Suppl 1):S80–S91. doi: 10.1016/j.bbi.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Raju S, Painter R, Bagby G, Nelson S, Wang G. Response of Differentiated Human Airway Epithelia to Alcohol Exposure and Klebsiella pneumoniae Challenge. Medical Sciences. 2013;1:2–19. doi: 10.3390/medsci1010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadikot RT, Zeng H, Joo M, Everhart MB, Sherrill TP, Li B, Cheng DS, Yull FE, Christman JW, Blackwell TS. Targeted immunomodulation of the NF-kappaB pathway in airway epithelium impacts host defense against Pseudomonas aeruginosa. J Immunol. 2006;176:4923–4930. doi: 10.4049/jimmunol.176.8.4923. [DOI] [PubMed] [Google Scholar]
- Samokhvalov A, Irving H, Rehm J. Alcohol consumption as a risk factor for pneumonia: a systematic review and meta-analysis. Epidemiology and infection. 2010;138:1789–1795. doi: 10.1017/S0950268810000774. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction (Abingdon, England) 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Sha Q, Truong-Tran AQ, Plitt JR, Beck LA, Schleimer RP. Activation of airway epithelial cells by toll-like receptor agonists. Am J Respir Cell Mol Biol. 2004;31:358–364. doi: 10.1165/rcmb.2003-0388OC. [DOI] [PubMed] [Google Scholar]
- Szabo G, Dolganiuc A, Dai Q, Pruett SB. TLR4, ethanol, and lipid rafts: a new mechanism of ethanol action with implications for other receptor-mediated effects. J Immunol. 2007;178:1243–1249. doi: 10.4049/jimmunol.178.3.1243. [DOI] [PubMed] [Google Scholar]
- Walton RG. Smoking and alcoholism: a brief report. Am J Psychiatry. 1972;128:1455–1456. doi: 10.1176/ajp.128.11.1455. [DOI] [PubMed] [Google Scholar]
- White JV, Guenter P, Jensen G, Malone A, Schofield M Academy of N, Dietetics Malnutrition Work G, Force ASPENMT, Directors ASPENBo. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition) J Acad Nutr Diet. 2012;112:730–738. doi: 10.1016/j.jand.2012.03.012. [DOI] [PubMed] [Google Scholar]
- Wyatt TA, Sisson JH. Chronic ethanol downregulates PKA activation and ciliary beating in bovine bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2001;281:L575–L581. doi: 10.1152/ajplung.2001.281.3.L575. [DOI] [PubMed] [Google Scholar]
- Wyatt TA, Slager RE, Heires AJ, DeVasure JM, VonEssen SG, Poole JA, Romberger DJ. Sequential activation of protein kinase C isoforms by organic dust is mediated by tumor necrosis factor. American journal of respiratory cell and molecular biology. 2010;42:706. doi: 10.1165/rcmb.2009-0065OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Ahyi AN, Pepper-Cunningham ZA, Ferrari JD, Wilson AA, Jones MR, Quinton LJ, Mizgerd JP. Roles of lung epithelium in neutrophil recruitment during pneumococcal pneumonia. Am J Respir Cell Mol Biol. 2014;50:253–262. doi: 10.1165/rcmb.2013-0114OC. [DOI] [PMC free article] [PubMed] [Google Scholar]