Abstract
Background
The recommendation by the American Society of Transplantation for annual trivalent inactivated influenza vaccination greater than 3 to 6 months post-kidney transplantation provides a unique opportunity to test the in vivo impact of immunosuppression on recall T- and B-cell responses to influenza vaccination.
Methods
This study took advantage of recent breakthroughs in the single-cell quantification of human peripheral blood B-cell responses to prospectively evaluate both B- and T-cell responses to the seasonal (2010 and 2011) influenza vaccine in 23 stable renal transplant recipients and 22 healthy controls.
Results and Conclusion
The results demonstrate that the early B-cell response to influenza vaccination, quantified by the frequency of influenza-specific antibody-secreting cells (ASC) in peripheral blood, was significantly reduced in stable transplant recipients compared to healthy controls. The magnitude of the seroresponse and the rate of sero-conversion were also blunted. The influenza-specific interferon-gamma (IFNγ) T-cell response was significantly reduced in transplant recipients; however, there was no correlation between the magnitude of the influenza-specific IgG ASC and IFNγ responses. The induction of memory T- and B-cell responses to influenza vaccination supports the recommendation to vaccinate while the blunted responses demonstrate the efficacy of immunosuppression in controlling memory responses individual transplant recipients.
Keywords: Influenza vaccine, Plasma cells, B cells, T cells, Kidney transplantation, Immunosuppression
Following numerous reports in the past decade confirming that the annual trivalent inactivated influenza vaccine is safe and generally well tolerated in transplant patients, there has been a greater adoption of the Centers for Disease Control and Prevention (CDC) influenza vaccination guidelines to vaccinate solid-organ transplant recipients (1–6). Nonetheless, concerns regarding the efficacy of vaccination in transplant recipients on chronic immunosuppression (7–13) as well as the possibility that the vaccination could trigger rejection episodes (14–16) are most frequently cited as reasons to not vaccinate (4). Vaccine responses are assessed by hemagglutination-inhibition or microneutralization antibody titers post-immunization (17, 18), and there is considerable variability in the reported overall seroconversion rates in transplant recipients, which could be a result of heterogeneity in the individual response to the vaccine, immunosuppression regimen, or both (3, 13, 19–22).
It has become clear that the administration of the trivalent inactivated influenza vaccine in healthy volunteers triggers a rapid activation of memory influenza-specific B cells, leading to their proliferation and differentiation into plasmablasts within secondary lymphoid organs (23, 24). After 6 to 8 days, a large number of CD19+ CD3−, CD20low CD27highCD38high plasmablasts are detected in the blood by flow cytometry, as well as influenza-specific ASCs by ELISPOT assay (24–26). These plasmablasts emerge rapidly from memory B cells and contribute to the pool of long-lived plasma cells that locate in the bone marrow (24). We hypothesize that these newer approaches for quantifying the recall B-cell response at a single-cell resolution allow for a more direct quantification of the memory B-cell response to influenza vaccination and complements routine assessments of neutralizing antibody titers (27). Furthermore, the easy quantification of this response to influenza vaccination will allow an assessment of the extent to which standard immunosuppression inhibits a recall B-cell response in individual renal transplant recipients (24).
In this study, the B cell and antibody responses to influenza vaccine was quantified in stable kidney transplant recipients and matched healthy controls. Because the influenza vaccine also induces T-cell responses that have been shown to contribute to viral clearance (28–30), we also assessed the magnitude of the recall influenza-specific T-cell responses. Collectively, these studies quantify the magnitude of the cellular and humoral response to influenza vaccination in stable kidney transplant recipients, and at the same time provide insights into the efficacy of immunosuppression in blunting these responses.
RESULTS
Clinical Patient Data
The demographic data of study subjects that received the trivalent inactivated influenza vaccine are summarized in Table 1. Because the time posttransplantation can affect the level of immunosuppression and immune responses blunted in patients with poor kidney function, the transplant study population was restricted to patients greater than or equal to 6 (6–72) months posttransplantation, with serum creatinine less than or equal to 1.5 mg/dL, on standard triple immunosuppression with no escalation in immunosuppression in the 6 months before vaccination. The control group was matched in race, age, and gender, but had significantly lower serum creatinine levels and higher glomerular filtration rates. Transplant and control subjects received a single dose of the 2010 or 2011 inactivated influenza vaccine, which were identical in both years.
TABLE 1.
Demographics of study population
| Transplant (n=22)a | Control (n=21)a | ||
|---|---|---|---|
| Age, yr | 43.6±11.1 | 45.0±12.6 | P>0.05 |
| Male | 7 | 6 | |
| Female | 15 | 15 | |
| African American | 56% | 50% | |
| Hispanic | 17% | 14% | |
| Other | 26% | 36% | |
| Creatinine, mg/dL | 1.12±0.14 | 0.85±0.18 | P<0.05 |
| GFR, mL/min/1.73 m2 | 57.7±7.5 | 82.7±12.0 | P<0.05 |
| Time from transplant, mo | 28±20 | N/A | |
| Type of graft, % | |||
| Cadaveric | 41% | ||
| Living related | 27% | ||
| Living unrelated | 32% | N/A | |
| Percent with 2nd transplant (%) | 27% | N/A | |
| Immunosuppression dose | |||
| Tacrolimus <3 mg/d | 36% | ||
| Tacrolimus 3–6 mg/d | 32% | ||
| Tacrolimus >6 mg/d | 33% | ||
| Tacrolimus trough, ng/mL | 6.8±2.4 | N/A | |
| Induction therapy | |||
| Basiliximab/daclizumab | 39% | ||
| Thymoglobulin | 61% | N/A | |
| Cause of kidney failure | |||
| Hypertension | 36% | ||
| Diabetes | 27% | ||
| FSGS | 14% | ||
| SLE | 4% | ||
| PCKD | 5% | ||
| Unknown | 14% | N/A | |
Values are given as mean±SD.
FSGS, focal segmental glomerulosclerosis; PCKD, polycystic kidney disease; SLE, systemic lupus erythematosus.
Reduced Influenza-Specific B-Cell Responses in Stable Kidney Transplant Recipients
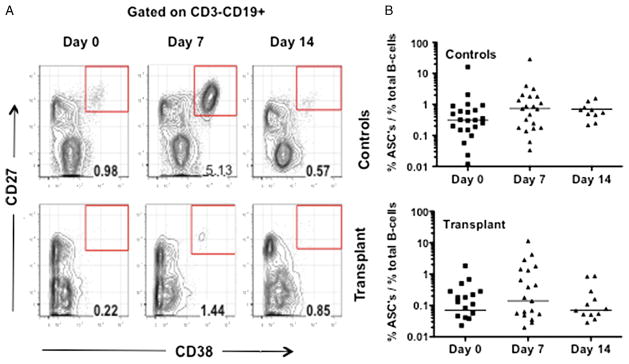
The plasmablast response was first evaluated, identified as CD3−CD19+CD27+CD38+ cells, in the peripheral blood of transplant and controls at 0, 7, and 14 or 28 days after influenza vaccination (Fig. 1A). Overall, the percentage of plasmablasts on day 0 in transplant recipients was significantly lower than controls (P=0.001). Both groups had modestly increased plasmablast percentages on day 7 post-immunization, consistent with previous observations (24), although these increases were not significantly different from day 0 or day 14 (Fig. 1D). The percentage of plasmablasts on day 7 in transplant recipients was significantly lower than controls (P=0.05), and the median increase in the percentage of plasmablasts at day 7 post-vaccination tended to be lower in the transplant group compared to the control (0.09% vs. 0.26%); however, this difference was not significant (P>0.05). Possible explanations for these observations could be variability and the lack of specificity of the measured plasmablast response, thus the influenza-specific ASC IgG response was further evaluated using an ELISPOT assay (Fig. 2).
FIGURE 1.
Total plasmablast response to influenza vaccination. A, gating strategy for the plasmablast (CD3−CD19+ CD27+CD38+) response to influenza vaccination. B, data are presented as percentage of total CD3−CD19+ B cells, on the indicated days post-immunization for healthy controls (N=21) and transplant recipients (N=22). Bars indicate the median, and significant differences were determined by Kruskal-Wallis test.
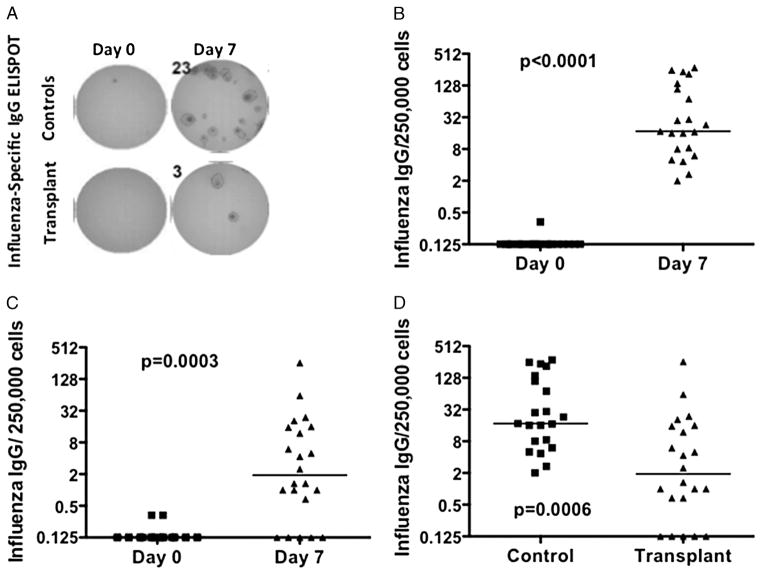
FIGURE 2.
Influenza-specific ASC response to influenza vaccination. A, quantification of the frequency of influenza-specific IgG secreting cells (ASC) by ELISPOT. Data are presented as the frequency of influenza-specific ASC on day 0 and 7 for controls (B) or transplant recipients (C), or the increase (day 7 subtracted from day 0 baseline) (D). Each symbol represents data from an individual (controls N=21; transplant recipients N=22). Bars represent the median, and statistical significance was determined by Wilcoxon signed rank test (B and C) or Mann-Whitney test (D).
Peak ASC response in peripheral blood mononuclear cells (PBMC) was detected on day 7 post-vaccination and returned to baseline by day 14 or 28 post-vaccination (Fig. 2; data not shown). Statistically significant increases in influenza-specific ASC IgG response was observed in both transplant and control cohorts on day 7 post-vaccination, with a median increase of 1.9/250,000 PBMC in transplant recipients and a median increase of 17.3/250,000 PBMC in the controls (Fig. 2A–C). The magnitude of influenza-specific ASC response from day 0 to 7 post-vaccination in the transplant recipients was significantly reduced 9.1-fold compared to the controls (P=0.0006), thus demonstrating the extent maintenance immunosuppression inhibits the rapid generation of ASC from pre-existing memory B cells. The heterogeneity of the ASC responses is noteworthy with 6.3% of transplant recipients having responses above the median and 32% having responses above the first quartile of controls.
Reduced Influenza-Specific Antibody Responses in Stable Kidney Transplant Recipients
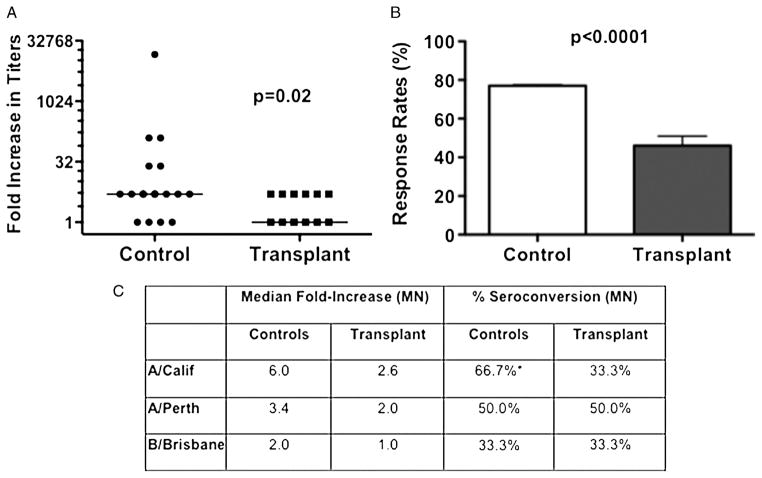
The cellular ASC response has been shown to predict the circulating antibody response, thus we next assessed the serum antibody responses to influenza vaccination (24). Day 0 and day 28 post-vaccination serum samples were tested against the all three viral strains used in the vaccine in an ELISA assay, and the fold increase in titers was determined (Fig. 3). Consistent with the ASC data, the fold increase in influenza-specific IgG titers was significantly reduced in the transplant compared to control populations, with a 5-fold median increase in the controls and a 1-fold median increase in the transplant population (Fig. 3A). The percentage of individuals that achieved greater than or equal to a 4-fold increase in antibody titer from baseline was also significantly reduced in transplant recipients (46.2%) compared to the controls (77.8%) (Fig. 3b). Finally, microneutralization assays were performed with a cohort of sera from patients and controls vaccinated in 2011, and a trend towards reduced percentages of seroconversion (≥4-fold increase between pre-and post-vaccination titers) to A/California/7/09-like (H1N1) virus was observed in the transplant group (Fig. 3C). This observation could reflect the superior immunogenicity of the A/California/7/09-like (H1N1) virus or a greater memory to this virus. No differences were observed with the standard HAI assay (data not shown), likely reflecting the small sample size as well as the superior sensitivity of the micro-neutralization assay.
FIGURE 3.
Anti-influenza IgG seroresponse and seroconversion following influenza vaccination. A, quantification of the anti-influenza IgG seroresponse by ELISA. Data represent the increase in the geometric mean titers (GMT; bar indicates median increase) from day 0 to day 14/28, with transplant patients (N=13) exhibiting significantly reduced responses compared to healthy controls (N=18). B, the response rate (%), which refers to the percentage of subjects with a 4-fold increase in titers on day 14/28 post-vaccination, was also significantly different between the two groups. C, anti-influenza titers were quantified by microneutralization (MN) assays to A/California/7/09-like (H1N1) (A/Calif), A/Perth/16/2009-like (H3N2) (A/Perth), and B/Brisbane/60/2008-like (B/Brisb) influenza virus. Data are presented as the median fold increase and percent seroconversion, which is defined as the percentage with a 4-fold increase in titers at day 28 post-vaccination. Data are from transplant patients (N=6) or controls (N=12) vaccinated in 2011. Statistical significance was determined by Mann-Whitney test (A) or unpaired t test (B) or two-way ANOVA with a Bonferroni post hoc test (C).
Reduced Influenza-Specific IFNγ Response in Stable Kidney Transplant Recipients
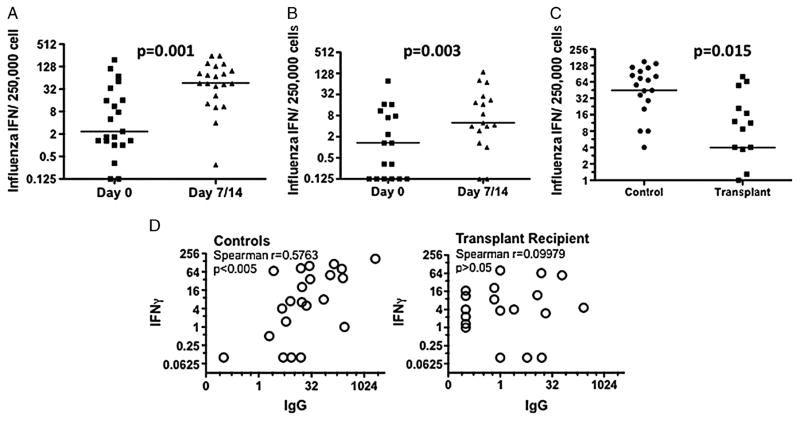
A major component of cell-mediated immunity to influenza vaccine comprises memory CD4+Th1 and CD8+ T cells, which secrete IFNγ and TNF> upon re-exposure to influenza vaccination (31, 32). In this study, T-cell responses to influenza vaccine were quantified with an IFNγ ELISPOT assay using peripheral blood collected at day 0 and 7 or 14 post-vaccination. Controls and transplant recipients had comparably low frequencies of influenza-specific, IFNγ-producing cells in the peripheral blood before vaccination and was significantly increased on day 7 or 14 (Fig. 4A,B). The frequency of influenza-specific IFNγ-producing cells significantly increased from a median of 2.3 to 46/250,000 PBMC and from 1.3 to 5/250,000 PBMC for the controls and transplant recipients, respectively (Fig. 4C). The overall response was significantly reduced in transplant recipients compared to healthy controls, with controls exhibiting a median 44.7-fold increase and transplant recipients a 4.0-fold increase in the frequency of IFNγ-producing cells. A considerable variation was noted in the IFNγ response in transplant recipients, with 18% having responses above the median and 47% above the first quartile of controls. There was a lack of correlation between the magnitude of the influenza-specific IFNγ and the ASC or antibody response in individual transplant patients (Fig. 4D, data not shown), arguing for an independent suppression of influenza-specific T- and B-cell responses by maintenance immunosuppression.
FIGURE 4.
Quantification of the anti-influenza IFNγ response by ELISPOT assays on day 0 and days 7 or 14 post-influenza vaccination. Both controls (A; N=21) and transplant patients (B; N=17) had a significant response to influenza vaccine; however, the magnitude of the response was significantly decreased in transplant recipients (C). Data are presented as medians and statistical significance was determined by Wilcoxon paired rank test (A and B) and Mann-Whitney test (C). Statistically significant correlation between the influenza-specific IFNγ and IgG response (measured by ELISPOT) in healthy controls (right), but no significant correlation in transplant recipients (left).
DISCUSSION
In vitro experiments with human PBMC can provide insights into the potential impact of immunosuppression on the human immune response; however, it is challenging to extrapolate to the in vivo situation. Animal models permit in vivo studies, but species-specific differences in pharmacokinetics, drug metabolism, and dosing make it difficult to accurately extrapolate observations to transplant patients that have considerable genetic variation and experience different immunosuppressive regimens. This study took advantage of the CDC and the American Society of Transplantation guidelines for influenza vaccination of solid-organ transplant recipients to quantify the induced B- and T-cell responses in individual kidney transplant recipients, and comparing their responses to age- and race-matched healthy controls. Importantly, because practically every person has been exposed to influenza infection or vaccination, the response to influenza vaccination arises predominantly from memory B and T cells (24, 33). Thus, this study took advantage of this unique opportunity to assess the immunogenicity of influenza vaccine in transplant patients and to determine the extent to which maintenance immunosuppression in stable renal transplant recipients controls recall B- and T-cell responses.
Calcineurin-based immunosuppression was predicted to be effective at controlling naive and memory T-cell responses because of their ability to inhibit the calcium/calcineurin/nuclear factor of activated T cells signaling downstream of the T-cell receptor that is necessary for the activation of both naive and memory T cells (34, 35). The observation that the influenza-specific IFNγ T response, which predominantly reflects recent induction from memory T cells, was significantly blunted in transplant recipients thus revealed the extent to which maintenance immunosuppression blunts the memory T-cell response to influenza vaccination—namely ~10-fold reduction in median responses compared to healthy controls. Despite significant inhibition, the magnitude of the resulting IFNγ response in individual transplant recipients demonstrated wide variability, with some transplant recipients having responses that were comparable to that observed in healthy controls. This latter observation could be the result of lower efficacy of immunosuppression or individual propensity to be high IFNγ responders to the influenza vaccine or both.
The serological response has been used to provide a clinically relevant estimate of vaccine immunogenicity and protection from live infection (3, 17, 18). A significant reduction was observed in the overall antibody response to the vaccine and seroconversion rates in transplant recipients compared to healthy controls. Because increases in antibody responses could reflect increases in the frequencies of ASC or antibody secretion by individual ASCs, it is reasoned that examination of the plasmablast and ASC responses would provide a more direct measure of the vaccine response and the effect of immunosuppression. Furthermore, it is currently unclear whether current immunosuppression regimens can inhibit memory B-cell differentiation into plasma cells. Thus, this study provides, for the first time, evidence that maintenance immunosuppression suppressed by 9.1-fold the median influenza-specific ASC IgG response in the transplant recipients compared to healthy controls. While this study was not designed to determine whether suppression of the ASC response was a result of indirect effects on inhibiting T-cell help or to direct inhibition of the memory B cells, the lack of correlation between the magnitude of the influenza-specific IFNγ, and ASC and antibody responses in individual transplant patients (Fig. 4D), argues for an independent suppression of influenza-specific T- and B-cell responses by maintenance immunosuppression. Furthermore, there was no correlation between the ASC, or IFNγ response, and the time of transplantation, nor was there an effect of type of induction with thymoglobulin or IL-2 receptor blockade (data no shown). Finally, it was observed that there was no statistically significant correlation between the early plasmablast or influenza-specific ASC response and the antibody response measured on day 14 or day 28 post-vaccination. This observation is similar to the report by Nakaya et al. (36) with a larger cohort of healthy controls where only a modest correlation was observed, thus leading the authors to conclude that a more robust correlate of immunogenicity is required.
In the transplant study cohort, serum tacrolimus levels at enrollment ranged from 4.7 to 14.7 ng/mL, but neither the influenza-specific IFNγ nor IgG ASC responses correlated with the trough concentrations or dose (data not shown). Previous studies have identified mycophenolate mofetil (MMF) to be most effective in reducing serological responses to influenza vaccination (3, 7–12). However, MMF doses in the transplant cohort were relatively consistent, ranging from 1,000 to 1,500 mg/day, thus future studies involving a larger cohort of patients receiving a larger range of doses of MMF will allow us to more fully determine whether MMF doses higher than in stable renal transplant recipients correlate with the magnitude of the influenza-specific ASC response. Finally, all transplant recipients received corticosteroids that have broad effects on non-lymphoid and lymphoid cells. Few influenza vaccination studies on the effect of corticosteroids have been reported, but the small sample sizes and the use of corticosteroids in combination with other immune-modulating drugs, as in the case of this study of transplant recipients, make it difficult to isolate the effects of corticosteroid on influenza vaccination responses (37).
There are a number of limitations of this study that can be remedied in future studies. First, despite the determination of statistically significant reduction in the cellular responses between the transplant patients versus the healthy controls, the overall study population is small especially for the neutralizing antibody responses measured at day 28 post-vaccination. Second, the immunological assays quantify detectable responses elicited by the vaccination, but they may not be sufficient to prevent influenza infection, and the clinically relevant assessment of protection or susceptibility to live influenza infection was not investigated. Third, the cellular responses in transplant recipients and healthy controls allow for a direct assessment of the effect of triple immunosuppression; however, the study lacks power to assess the contribution of each drug. Finally, this study did not assess the generation of long-term memory B and T cells as well as plasma cells, nor did it analyze patients at early (<6 months) post-transplantation where the levels of immunosuppression are considerably higher and more variable.
In summary, this study reveals the extent to which chronic maintenance immunosuppression in stable kidney transplant recipients blunts the recall response to influenza vaccine. While larger studies are needed for definitive statistical proof of efficacy of vaccination or immunosuppression, this study was able to demonstrate that the majority of transplant recipients had significantly reduced ASC, IFNγ, and serological responses to influenza vaccination. In particular, this study clarifies the extent to which maintenance triple immunosuppression blunts memory T-cell responses as well as memory B-cell differentiation into ASC in transplant recipients. This study raises the intriguing question of whether the magnitude of immune response to influenza vaccine can be extrapolated to alloimmune responses and whether individuals with near normal T or B responses, or both, to influenza vaccine are inadequately immunosuppressed and more likely to develop alloantibodies or succumb to late graft failure. Conversely, it would be equally important to test whether individuals with minimal immune responses to influenza vaccine are over-immunosuppressed and more likely to succumb to opportunistic infections.
MATERIALS AND METHODS
Study Design
The Institutional Review Board (IRB) of The University of Chicago (Chicago, IL) approved this project, and informed consent was obtained from all study participants. The CDC did not have access to personal identifiers for the study, thus their IRB review was not required under the US federal regulatory guidance. Kidney transplant recipients were recruited from the University of Chicago renal transplant clinic from October 2010 thru December 2011 with the following inclusion criteria: aged 18 to 65 years; greater than or equal to 6 months posttransplantation with organs from deceased or living donors; serum creatinine less than or equal to 1.5 mg/dL; on triple immunosuppression regimen consisting of tacrolimus (1–12 mg/day), steroids (5 mg/day), and an antiproliferative agent, MMF (1,000–1,500 mg/day) or mycophenolate sodium (720–1,440 mg/day); and without escalation in immunosuppression regimen for any reason in the previous 6-month period. One patient received sirolumus (1 mg/day) instead of tacrolimus. All patients received thymoglobulin (61%) or basiliximab-daclizumab (39%) as induction therapy. Estimated glomerular filtration rate was determined using the four-variable Modification of Diet in Renal Disease Study equation. Healthy controls, aged 18 to 65 years, were recruited during the same period and included family members of enrolled transplant recipients and individuals from the University of Chicago healthcare community. Exclusion criteria for both groups included previous receipt of the 2010 or 2011 influenza vaccine in that same year, known anaphylactic reactions to eggs, or acute febrile illness in the week before enrollment.
Enrolled subjects participated in a total of three study visits. The first visit consisted of informed consent, demographic and clinical data collection, blood collection, and administration of the influenza vaccine available at the University of Chicago medical center (2010–2011: FLUVIRIN—Novartis Vaccines and Diagnostics Limited, Liverpool, UK; 2011–2012: Fluzone—Sanofi Pasteur, Swiftwater, PA, USA). The influenza vaccine composition was identical for both years, comprising A/California/7/2009 (H1N1)-like, A/Perth/16/2009-like (H3N2), and B/Brisbane/60/2008-like viruses. In follow-up visits on days 7 and 14 (for 2010) or 28 (for 2011), post-vaccination blood and serum samples were collected. There were no significant side effects of the influenza vaccine, beyond what was anticipated such as sore arm and low-grade fever, observed in the transplant patients or controls.
Cell and Serum Isolation
Peripheral blood mononuclear cells were enriched using Rosette Sep human total lymphocyte enrichment cocktail (Stemcell Technologies) and lymphocyte separation media (Mediatech). Cells were resuspended in complete media (RPMI-1640+10% FCS+penicillin, streptomycin, L-glutamine, HEPES buffer [1 M], and 50 μM β-mercaptoethanol). Cells were used on the same day of collection. Serum was isolated from fresh blood and frozen at −80°C for later use.
Antibody Secreting Cell Responses by Flow Cytometry
Peripheral blood mononuclear cells were incubated with the following antibodies: anti-CD3 FITC, anti-CD19 PE-Cy7, anti-CD27 PE (Invitrogen) as well as anti-CD38 AF 647 and anti-CD20 PacBlue (Biolegend). Antibody secreting cells were gated as CD19+ CD3−, CD20low and then sub-gated as CD27highCD38high (LSRII-Blue), and data was analyzed using FlowJo software.
ELISPOT and ELISA
To enumerate the number of IgG-secreting or IFNγ-producing influenza-specific antibody secreting cells, 96-well ELISPOT filter plates (Millipore, MAHA N4510) were coated with either the influenza vaccine (FLUVIRIN or Fluzone) or mouse anti-human IFNγ (Mabtech). PBMC in a dilution series was incubated for 12 to 14 hours for ASC and 48 hours for IFNγ ELISPOT assays. Stimulation with the influenza vaccine or phytohemagglutinin (Sigma) was included for the IFNγ ELISPOT assay. To visualize spots, goat anti-human IgG (Fcγ) biotin, mouse anti-human IFNγ biotin (Mabtech), and avidin-horseradish peroxidase (HRP) conjugate (BD Pharmingen) and 3 amino-9 ethylcarbozole substrate (Sigma) were used, and spots were enumerated using an automated ELISPOT counter (Cellular Technologies Ltd.).
Antibody responses were measured using influenza-specific ELISA performed similar to the ELISPOT assay. Briefly, plates were coated with the influenza vaccine, and serum was added in serial dilution and detected with anti-human IgG biotin, avidin-HRP conjugate, and tetramethylbenzidine substrate (eBioscience). Absorbencies were measured at OD450 on a microplate reader (Invitrogen).
Serological Assays
The microneutralization assay was performed at the CDC as previously described (38, 39). Sera were heat inactivated at 56°C for 30 minutes and tested in serial 2-fold dilutions starting at a 1:10 dilution. MN titers were expressed as the reciprocal of the highest dilution of serum that gave greater than or equal to 50% neutralization. Sera that yielded titers less than the starting dilution of 1:10 were reported as a titer of 5.
Statistics
Statistical analyses (described in context) were performed using GraphPad Prism. Mann-Whitney U test was used for continuous variables with non-normal distribution, Wilcoxon signed-rank test was used for paired data, non-parametric Spearman test was used to determine correlation, and unpaired t test or two-way ANOVA with a Bonferroni post hoc test for non-continuous unpaired data.
Acknowledgments
The authors thank Michael D. Decker, MD, MPH, Sanofi Pasteur USA for the gift of the 2010 and 2011 Fluzone Influenza vaccines that were used only in the in vitro stimulations. The authors acknowledge the assistance of Dr. Patrick Cunningham and transplant coordinators, Josefa Sutor, Marybeth McNamara, and Roseanne Sweda, in the recruitment of kidney transplant recipients to this study.
This work was supported in part by grants from the NIH, P01AI097113, R01AI083452, and R01AI072630, to A.S.C.
Footnotes
M.C. designed the clinical study, performed the experiments and data analysis, and co-wrote the article. W.J.C., A.D., and M.J. assisted with the design of the clinical study and patient recruitment. S.A., P.C.W., and R.S. helped with the experimental assays. A.S.C. designed and supervised the study, performed the data analysis, and co-wrote the article. Y.B., V.V., and J.M.K. performed the HAI and microneutralization assays.
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the CDC.
The authors declare no conflicts of interest.
References
- 1.Avery RK. Influenza vaccines in the setting of solid-organ transplantation: are they safe? Curr Opin Infect Dis. 2012;25:464. doi: 10.1097/QCO.0b013e328355a79b. [DOI] [PubMed] [Google Scholar]
- 2.Danzinger-Isakov L, Kumar D. Guidelines for vaccination of solid organ transplant candidates and recipients. Am J Transplant. 2009;9(Suppl 4):S258. doi: 10.1111/j.1600-6143.2009.02917.x. [DOI] [PubMed] [Google Scholar]
- 3.Kumar D, Blumberg EA, Danziger-Isakov L, et al. Influenza vaccination in the organ transplant recipient: review and summary recommendations. Am J Transplant. 2011;11:2020. doi: 10.1111/j.1600-6143.2011.03753.x. [DOI] [PubMed] [Google Scholar]
- 4.Chon WJ, Kadambi PV, Harland RC, et al. Changing attitudes toward influenza vaccination in U.S. Kidney transplant programs over the past decade. Clin J Am Soc Nephrol. 2010;5:1637. doi: 10.2215/CJN.00150110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perez-Romero P, Aydillo TA, Perez-Ordonez A, et al. Reduced incidence of pneumonia in influenza-vaccinated solid organ transplant recipients with influenza disease. Clin Microbiol Infect. 2012;18:E533. doi: 10.1111/1469-0691.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lawal A, Basler C, Branch A, et al. Influenza vaccination in orthotopic liver transplant recipients: absence of post administration ALT elevation. Am J Transplant. 2004;4:1805. doi: 10.1111/j.1600-6143.2004.00564.x. [DOI] [PubMed] [Google Scholar]
- 7.Cordero E, Aydillo TA, Perez-Ordonez A, et al. Deficient long-term response to pandemic vaccine results in an insufficient antibody response to seasonal influenza vaccination in solid organ transplant recipients. Transplantation. 2012;93:847. doi: 10.1097/TP.0b013e318247a6ef. [DOI] [PubMed] [Google Scholar]
- 8.Cordero E, Manuel O. Influenza vaccination in solid-organ transplant recipients. Curr Opin Organ Transplant. 2012;17:601. doi: 10.1097/MOT.0b013e3283592622. [DOI] [PubMed] [Google Scholar]
- 9.Ott U, Sauerbrei A, Lange J, et al. Serological response to influenza A H1N1 vaccine (Pandemrix(R)) and seasonal influenza vaccine 2009/2010 in renal transplant recipients and in hemodialysis patients. Med Microbiol Immunol. 2012;201:297. doi: 10.1007/s00430-012-0231-8. [DOI] [PubMed] [Google Scholar]
- 10.Crespo M, Collado S, Mir M, et al. Efficacy of influenza A H1N1/2009 vaccine in hemodialysis and kidney transplant patients. Clin J Am Soc Nephrol. 2011;6:2208. doi: 10.2215/CJN.02160311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Birdwell KA, Ikizler MR, Sannella EC, et al. Decreased antibody response to influenza vaccination in kidney transplant recipients: a prospective cohort study. Am J Kidney Dis. 2009;54:112. doi: 10.1053/j.ajkd.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blumberg EA, Albano C, Pruett T, et al. The immunogenicity of influenza virus vaccine in solid organ transplant recipients. Clin Infect Dis. 1996;22:295. doi: 10.1093/clinids/22.2.295. [DOI] [PubMed] [Google Scholar]
- 13.Candon S, Thervet E, Lebon P, et al. Humoral and cellular immune responses after influenza vaccination in kidney transplant recipients. Am J Transplant. 2009;9:2346. doi: 10.1111/j.1600-6143.2009.02787.x. [DOI] [PubMed] [Google Scholar]
- 14.Schaffer SA, Husain S, Delgado DH, et al. Impact of adjuvanted H1N1 vaccine on cell-mediated rejection in heart transplant recipients. Am J Transplant. 2011;11:2751. doi: 10.1111/j.1600-6143.2011.03743.x. [DOI] [PubMed] [Google Scholar]
- 15.Katerinis I, Hadaya K, Duquesnoy R, et al. De novo anti-HLA antibody after pandemic H1N1 and seasonal influenza immunization in kidney transplant recipients. Am J Transplant. 2011;11:1727. doi: 10.1111/j.1600-6143.2011.03604.x. [DOI] [PubMed] [Google Scholar]
- 16.Danziger-Isakov L, Cherkassky L, Siegel H, et al. Effects of influenza immunization on humoral and cellular alloreactivity in humans. Transplantation. 2010;89:838. doi: 10.1097/TP.0b013e3181ca56f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beyer WE, Palache AM, Luchters G, et al. Seroprotection rate, mean fold increase, seroconversion rate: which parameter adequately expresses seroresponse to influenza vaccination? Virus Res. 2004;103:125. doi: 10.1016/j.virusres.2004.02.024. [DOI] [PubMed] [Google Scholar]
- 18.Katz JM, Hancock K, Xu X. Serologic assays for influenza surveillance, diagnosis and vaccine evaluation. Expert Rev Anti Infect Ther. 2011;9:669. doi: 10.1586/eri.11.51. [DOI] [PubMed] [Google Scholar]
- 19.Opelz G, Dohler B. Lymphomas after solid organ transplantation: a collaborative transplant study report. Am J Transplant. 2004;4:222. doi: 10.1046/j.1600-6143.2003.00325.x. [DOI] [PubMed] [Google Scholar]
- 20.Bhorade SM, Stern E. Immunosuppression for lung transplantation. Proc Am Thorac Soc. 2009;6:47. doi: 10.1513/pats.200808-096GO. [DOI] [PubMed] [Google Scholar]
- 21.Samaniego M, Becker BN, Djamali A. Drug insight: maintenance immunosuppression in kidney transplant recipients. Nat Clin Pract Nephrol. 2006;2:688. doi: 10.1038/ncpneph0343. [DOI] [PubMed] [Google Scholar]
- 22.Eckerle I, Rosenberger KD, Zwahlen M, et al. Serologic vaccination response after solid organ transplantation: a systematic review. PloS One. 2013;8:e56974. doi: 10.1371/journal.pone.0056974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li GM, Chiu C, Wrammert J, et al. Pandemic H1N1 influenza vaccine induces a recall response in humans that favors broadly cross-reactive memory B cells. Proc Natl Acad Sci U S A. 2012;109:9047. doi: 10.1073/pnas.1118979109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wrammert J, Smith K, Miller J, et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008;453:667. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sasaki S, Sullivan M, Narvaez CF, et al. Limited efficacy of inactivated influenza vaccine in elderly individuals is associated with decreased production of vaccine-specific antibodies. J Clin Invest. 2011;121:3109. doi: 10.1172/JCI57834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wrammert J, Koutsonanos D, Li GM, et al. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J Exp Med. 2011;208:181. doi: 10.1084/jem.20101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Srinivas TR, Kaplan B, Meier-Kriesche HU. Mycophenolate mofetil in solid-organ transplantation. Expert Opin Pharmacother. 2003;4:2325. doi: 10.1517/14656566.4.12.2325. [DOI] [PubMed] [Google Scholar]
- 28.Bot A, Bot S, Bona CA. Protective role of gamma interferon during the recall response to influenza virus. J Virol. 1998;72:6637. doi: 10.1128/jvi.72.8.6637-6645.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bot A, Bot S, Garcia-Sastre A, et al. Protective cellular immunity against influenza virus induced by plasmid inoculation of newborn mice. Dev Immunol. 1998;5:197. doi: 10.1155/1998/50472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forrest BD, Pride MW, Dunning AJ, et al. Correlation of cellular immune responses with protection against culture-confirmed influenza virus in young children. Clin Vaccine Immunol. 2008;15:1042. doi: 10.1128/CVI.00397-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Avetisyan G, Ragnavolgyi E, Toth GT, et al. Cell-mediated immune responses to influenza vaccination in healthy volunteers and allogeneic stem cell transplant recipients. Bone Marrow Transplant. 2005;36:411. doi: 10.1038/sj.bmt.1705064. [DOI] [PubMed] [Google Scholar]
- 32.Deng Y, Jing Y, Campbell AE, et al. Age-related impaired type 1 T cell responses to influenza: reduced activation ex vivo, decreased expansion in CTL culture in vitro, and blunted response to influenza vaccination in vivo in the elderly. J Immunol. 2004;172:3437. doi: 10.4049/jimmunol.172.6.3437. [DOI] [PubMed] [Google Scholar]
- 33.Faenzi E, Zedda L, Bardelli M, et al. One dose of an MF59-adjuvanted pandemic A/H1N1 vaccine recruits pre-existing immune memory and induces the rapid rise of neutralizing antibodies. Vaccine. 2012;30:4086. doi: 10.1016/j.vaccine.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 34.Tsuda K, Yamanaka K, Kitagawa H, et al. Calcineurin inhibitors suppress cytokine production from memory T cells and differentiation of naive T cells into cytokine-producing mature T cells. PloS One. 2012;7:e31465. doi: 10.1371/journal.pone.0031465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pearl JP, Parris J, Hale DA, et al. Immunocompetent T-cells with a memory-like phenotype are the dominant cell type following antibody-mediated T-cell depletion. Am J Transplant. 2005;5:465. doi: 10.1111/j.1600-6143.2005.00759.x. [DOI] [PubMed] [Google Scholar]
- 36.Nakaya HI, Li S, Pulendran B. Systems vaccinology: learning to compute the behavior of vaccine induced immunity. Wiley Interdiscip Rev Syst Biol Med. 2012;4:193. doi: 10.1002/wsbm.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kunisaki KM, Janoff EN. Influenza in immunosuppressed populations: a review of infection frequency, morbidity, mortality, and vaccine responses. Lancet Infect Dis. 2009;9:493. doi: 10.1016/S1473-3099(09)70175-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kendal AP, Pereira MS, Skehel JJ. Concepts and Procedures for Laboratory-Based Influenza Surveillance. Atlanta, GA: U. S. Department of Health and Human Services, Public Health Service, Centers for Disease Control; 1982. [Google Scholar]
- 39.Rowe T, Abernathy RA, Hu-Primmer J, et al. Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J Clin Microbiol. 1999;37:937. doi: 10.1128/jcm.37.4.937-943.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]