Abstract
This manuscript summarizes the proceedings of the symposium entitled, “Stress, Palatable Food and Reward”, that was chaired by Drs. Linda Rinaman and Yvonne Ulrich-Lai at the 2014 Neurobiology of Stress Workshop held in Cincinnati, OH. This symposium comprised research presentations by four neuroscientists whose work focuses on the biological bases for complex interactions among stress, food intake and emotion. First, Dr. Ulrich-Lai describes her rodent research exploring mechanisms by which the rewarding properties of sweet palatable foods confer stress relief. Second, Dr. Stephanie Fulton discusses her work in which excessive, long-term intake of dietary lipids, as well as their subsequent withdrawal, promotes stress-related outcomes in mice. Third, Dr. Mark Wilson describes his group’s research examining the effects of social hierarchy-related stress on food intake and diet choice in group-housed female rhesus macaques, and compared the data from monkeys to results obtained in analogous work using rodents. Lastly, Dr. Gorica Petrovich discusses her research program that is aimed at defining cortical–amygdalar–hypothalamic circuitry responsible for curbing food intake during emotional threat (i.e., fear anticipation) in rats. Their collective results reveal the complexity of physiological and behavioral interactions that link stress, food intake and emotional state, and suggest new avenues of research to probe the impact of genetic, metabolic, social, experiential, and environmental factors.
Keywords: Anorexia, Anxiety, Comfort food, Depression, HPA Axis, Obesity
Introduction
Acute or chronic exposure to stress (defined as an ongoing or anticipated threat to homeostasis or well-being) evokes a constellation of physiological and behavioral responses that markedly alter metabolic and behavioral state in humans and experimental animals (Dallman et al., 2003). Stress-induced activation of the neuroendocrine hypothalamic-pituitary-adrenal (HPA) axis increases glucocorticoid synthesis and glucose availability to fuel the metabolic demands of other physiological and behavioral stress responses. Glucocorticoids also regulate the accumulation and storage of body fat, and can increase appetite, food intake, and body weight gain. Acute and chronic exposure to stress can alter both the quantity and quality of calories consumed, and stress-induced alterations in food intake and energy balance can interact with emotional state (Epel et al., 2001). However, although functional associations among stress, food intake/energy balance, and emotion are readily apparent, mechanisms linking these outcomes are poorly understood. Compelling experimental evidence supports the view that stress can either increase or decrease caloric intake, and numerous reports indicate that chronic stress exposure can promote either obesity or anorexia within certain dietary environments.
Stressors impact energy balance and affective state in a manner that depends on a multitude of biological and environmental factors, including temporal, genetic, social, contextual, species-specific, sex-dependent, nutritional, developmental, metabolic, and experience-dependent elements. This profound complexity offers unique opportunities for researchers to selectively manipulate biological and environmental conditions in order to probe the functional underpinnings of causal relationships of interest. In this Symposium, four researchers present their experimental work investigating a variety of linkages between stress, food choice and intake, body weight gain, and emotional behavior. The collective work of these investigators spans a broad array of biologically distinct models and approaches, and their findings highlight key similarities and differences in the complex relationship between stress and food intake that depend on both biological and environmental contexts.
In the second section of this Symposium report ("Stress relief by palatable food reward"), Dr. Yvonne Ulrich-Lai presents findings by her research group exploring the ability of "comfort foods" to reduce physiological and behavioral stress responses in rats. In her experimental paradigm, rats that receive twice-daily limited access to 30% sucrose solution display no significant changes in body weight or adiposity, but display markedly attenuated behavioral and physiological stress responsiveness. The stress-reducing effect of sweet "comfort food" access does not depend on the nutritional properties of sucrose, but appears to require reward signaling through central neural circuits that promote synaptic remodeling within the basolateral nucleus of the amygdala.
In the third section of this report ("Emotional consequences of prolonged high-fat feeding"), Dr. Stephanie Fulton presents her laboratory's findings using an experimental model in which mice consuming a high-fat diet (HFD) become obese and display related metabolic derangements that serve to generate a state of stress. These diet-induced obese mice are characterized by a depressive-like behavioral phenotype and a hyper-responsive HPA stress axis. Further, when their HFD diet is replaced with standard chow (in an experimental "dieting" condition), the mice display behavioral and endocrine anxiety-like behavior accompanied by increased motivation (operant responding) for palatable foods. Dr. Fulton also presents evidence that saturated fats in the diet produce a larger effect than unsaturated fats to alter neural processes of emotion and reward.
In the fourth part of this Symposium report ("Stress-induced eating"), Dr. Mark Wilson summarizes research using socially housed, non-human primates (female rhesus macaques) to explore the biological bases of stress-induced "emotional" over-eating. His research program also considers the role of social status and history of adverse experience on stress-induced eating. Dr. Wilson compares and contrasts data from rodent and monkey studies to make the important point that changes in food intake after stress exposure depend critically on history of stress, the type of food available for consumption, and the sex of the animal, and he further suggests that stress-induced reductions in central dopamine signaling, possibly mediated through central corticotropin releasing factor type 1 receptor (CRF-R1) activation, may provide a common thread for increased eating and other goal-directed behaviors as a consequence of stress exposure.
While the initial sections of this Symposium report focus on the neurobiological substrates of increased food intake after stress, the final section ("Stress-induced anorexia"), prepared by Dr. Gorica Petrovich, considers the opposite side of the coin: what are the neural substrates of stress-induced anorexia? To probe the underlying circuits, Dr. Petrovich's research group uses a rat model of conditioned fear in order to suppress food intake in hungry rats during exposure to danger cues that also increase anxiety-like behavior. Dr. Petrovich reports striking sex differences in experimental outcomes, such that female rats take much longer to extinguish their conditioned "fear anorexia" compared to males. Brain regions that are critical for feeding suppression by conditioned fear cues include the prefrontal cortex, central amygdala, and lateral hypothalamus, and she points out that selective neural signaling through the basolateral vs. central amygdala may comprise a biological "fear-or-feeding" switch.
Stress relief by palatable food reward - Yvonne Ulrich-Lai
‘Comfort food’
For most people, stress influences both the amount and types of food that they eat. For example, approximately 35–60% of people report eating more total calories when they are experiencing stress, whereas approximately 25–40% of people report eating less (Epel et al., 2004, Oliver and Wardle, 1999, Weinstein et al., 1997). Furthermore, for many people stress alters food selection towards eating a greater proportion of calories from highly-palatable foods (i.e., tasty, calorically-dense foods containing high amounts of sugars, other carbohydrates, and/or fats) (Groesz et al., 2012, Kim et al., 2013, Laugero et al., 2011, McCann et al., 1990, Oliver and Wardle, 1999, Tryon et al., 2013), and this shift can even occur in many people that reduce their total caloric intake during stress (Oliver and Wardle, 1999). This altered food selection is often referred to as eating ‘comfort food’ - a term that reflects the idea that palatable food intake reduces stress responses, thereby providing a potential means for people to ‘self-medicate’ for stress relief. Consistent with this idea, the intake of palatable and/or high carbohydrate food is associated with improved mood, decreased perceived stress and reduced plasma cortisol concentration in people (Anderson et al., 1987, Barr et al., 1999, de Castro, 1987, Deuster et al., 1992, Dube et al., 2005, Fernandez et al., 2003, Gibson, 2006, Lieberman et al., 1986, Utter et al., 1999), particularly those with high stress-proneness (Markus et al., 2000).
Similar changes in palatable food intake are observed in rodents during stress. When rats are offered both a highly-palatable food (e.g., lard, sucrose) and a less palatable food (e.g., chow), they choose to eat a large proportion of their daily calories as the highly-palatable food (la Fleur et al., 2014, Packard et al., 2014, Pecoraro et al., 2004, Zeeni et al., 2013). When these rodents are then exposed to chronic stress, palatable food intake is generally preserved whereas chow intake is reduced, resulting in a greater proportion of their daily food intake being derived from the palatable food source (Packard et al., 2014, Pecoraro et al., 2004). Moreover, elevated palatable food intake in rodents is associated with reduced stress indices, including anxiety-related behaviors, stress-induced learned helplessness, post-stress plasma corticosterone concentration, and reactions to pain and distress (Bell et al., 2002, Francolin-Silva et al., 2006, la Fleur et al., 2005, Minor and Saade, 1997, Prasad and Prasad, 1996, Shide and Blass, 1989, Strack et al., 1997, Suchecki et al., 2003). Based on evidence reviewed by Dr. Fulton (see next section), such relief may be transitory/temporary in the face of continued stress exposure, which generally leads to increased HPA activation that, in turn, may sustain palatable food intake and promote obesity. Taken together, however, results from human and rodent studies strongly suggest that palatable food intake blunts acute stress responses, although, the mechanisms by which such ‘comfort’ foods engender stress relief are largely unknown. In order to explore these mechanisms, we first developed and characterized a paradigm of limited exposure to a sucrose drink, as described below.
Limited sucrose intake (LSI) paradigm reduces stress responses
To explore the mechanisms by which moderate palatable food intake dampens stress, we have developed a limited sucrose intake (LSI) “snacking” model, in which adult male rats with ad libitum chow and water available are given additional brief (30 min) twice-daily access to a small amount (4 ml) of 30% sucrose solution (or water as a control) for 2–4 weeks (Ulrich-Lai et al., 2010, Ulrich-Lai et al., 2007). The rats rapidly begin to drink the sucrose solution, but not the water, with intakes approximating the maximum permitted (8 ml per day, ~9 kcal/d) (Ulrich-Lai et al., 2007). Rats with this LSI modestly decrease chow intake (~9 kcal/d), resulting in no effect on body weight or adiposity (Ulrich-Lai et al., 2007). As a result, this sucrose paradigm allows us to explore the effects of this modest dietary change on stress responses without the additional, and perhaps confounding, effects of increased body weight and adiposity that could alone influence stress systems (see later section by Dr. Fulton).
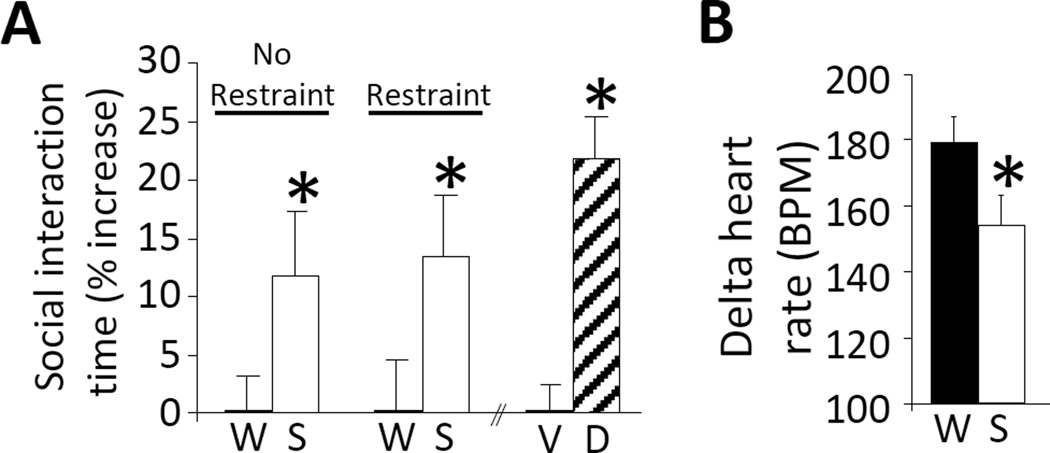
A history of LSI blunts several types of behavioral and physiological responses to stress. For example, LSI lessens indices of anxiety-related behaviors (Figure 1) in social interaction, elevated-plus maze, and open field tests of behavioral anxiety (Ulrich-Lai et al., 2010). In addition, both CRF mRNA expression in the hypothalamic paraventricular nucleus (PVN) (Ulrich-Lai et al., 2007) and HPA axis hormonal responses to a subsequent acute stressor (e.g., restraint) are decreased after 14 days of LSI (Figure 2A,B) (Christiansen et al., 2011, Ulrich-Lai et al., 2010, Ulrich-Lai et al., 2011, Ulrich-Lai et al., 2007). The tachycardic response to restraint is also reduced following LSI (Figure 1B), suggesting that the sympathetic response to acute stress is similarly blunted by sucrose ingestion (Ulrich-Lai et al., 2010). This implies that LSI provides a general stress relief that involves multiple components of the stress response.
Figure 1.
A history of limited sucrose intake reduces behavioral and physiological responses to stress. (A) In a social interaction test of behavioral anxiety, rats with a history of limited sucrose (S) drink spent a greater proportion of time interacting socially with a novel conspecific relative to water (W) controls, regardless of whether they received an acute restraint stress (Restraint) or not (No Restraint) immediately prior to the social interaction test (left). *p < 0.05 vs. water controls. This increased social interaction was similar to that which occurs after treatment with the known anxiolytic diazepam (D) vs. vehicle (V) (right). *p < 0.05 vs. vehicle. (B) The tachycardic response to acute restraint was reduced by limited sucrose drink relative to water controls. *p < 0.05 vs. water controls. Data are shown as mean ± SEM. Reproduced from (Ulrich-Lai et al., 2010).
Figure 2.
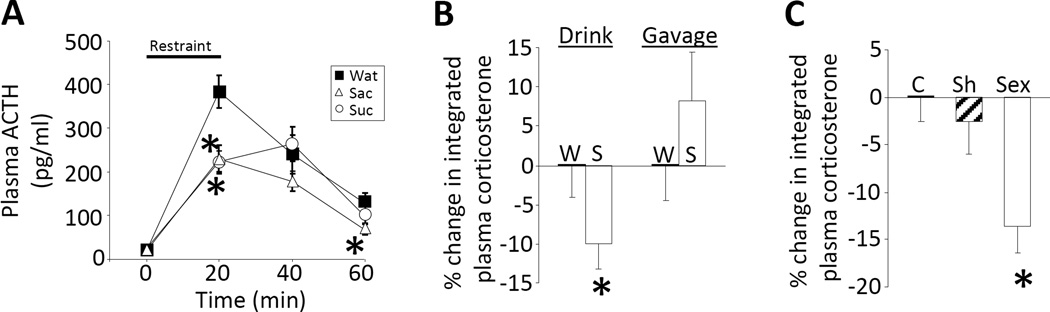
A history of limited sucrose intake reduces hypothalamic-pituitary-adrenal (HPA) axis responses to stress and is largely mediated by the pleasurable and rewarding properties of the sucrose. (A) The plasma adrenocorticotropic hormone (ACTH) response to a 20-min restraint stress is blunted by either limited sucrose (Suc; 30%) or saccharin (Sac; 0.1%; a non-caloric, artificial sweetener) drink intake, suggesting that the pleasurable or rewarding properties of these sweet drinks are sufficient for stress relief. *p < 0.05 vs. Water (Wat). (B) Rats given twice-daily sucrose (S) drink have a reduced integrated (area-under-the-curve) plasma corticosterone response to restraint relative to water-only controls (left). In contrast, those receiving twice-daily orogastric gavage of 4 ml of sucrose vs. water (to replicate many of the post-ingestive effects of the drink while minimizing taste and hedonic properties), do not have a reduced plasma corticosterone response (right), suggesting that sucrose’s hedonic/rewarding properties are necessary. *p < 0.05 vs. Water. (C) Male rats with a history of daily, limited (30 min) access to a sexually-responsive female engaged in sexual activity (Sex) and had a reduced integrated plasma corticosterone response to restraint compared to both undisturbed controls (C) and sham-sex rats (Sh; this group received a female rat in their cages, but the female was enclosed in a wire mesh box that prevented physical interactions), suggesting that other types of natural reward provide similar HPA-dampening. *p < 0.05 vs. control and sham. Data are shown as mean ± SEM. Reproduced from (Ulrich-Lai et al., 2010).
Role of reward in LSI-induced stress relief
Brain reward pathways are largely responsible for processing information related to the motivation, expectation and pursuit of pleasurable experiences. Palatable food intake is a naturally rewarding behavior, and it is possible that sucrose ingestion may constrain stress system activation, at least in part, via its actions on brain reward circuitry. We addressed this possibility by determining the extent to which the taste, hedonic, and/or rewarding properties of sucrose contribute to its stress-relieving effects. The non-caloric sweetener saccharin is highly-palatable to rats, and was used to replicate many of the hedonic/rewarding properties of sucrose while minimizing the post-ingestive consequences (e.g., macronutrients, calories, osmolarity) (Collier and Novell, 1967, Smith and Sclafani, 2002). Rats given limited saccharin (0.1%) drink intake showed HPA axis-dampening similar to effects of sucrose ingestion (Ulrich-Lai et al., 2010), suggesting that the hedonic/rewarding properties of sucrose may be sufficient for stress relief (Figure 2A). In contrast, twice-daily orogastric gavage of 4 ml of sucrose was used to replicate many of the post-ingestive effects of sucrose while minimizing its taste, hedonic and/or reward properties (though likely not completely preventing reward system activation (Sclafani, 2004)). Rats given twice-daily limited sucrose gavage did not exhibit HPA axis-dampening (Ulrich-Lai et al., 2010), suggesting that the post-ingestive effects of sucrose are not sufficient, and furthermore indicating that its hedonic/rewarding properties are likely necessary (Figure 2B). Lastly, if the naturally pleasurable and rewarding properties of sucrose are mediating stress relief, then another type of naturally rewarding behavior may replicate these effects. In order to test this possibility, male rats were given limited (up to 30 min) intermittent (once daily) access to a sexually-receptive female for two weeks and then restraint-induced plasma corticosterone was measured (Figure 2C). Rats with a history of sexual activity had a lower HPA response to restraint compared to both undisturbed home cage controls and also to sham-sex males (which received a female in their cage, but with the female contained within a wire mesh box so the male could not physically access the female) (Ulrich-Lai et al., 2010). Collectively, this work indicates that in this limited access paradigm, the pleasurable and rewarding properties of palatable food, as well as other types of natural rewards, appear to play a major role in attenuating the response to a stressor.
A pivotal role for the basolateral amygdala (BLA) in reward and stress processing
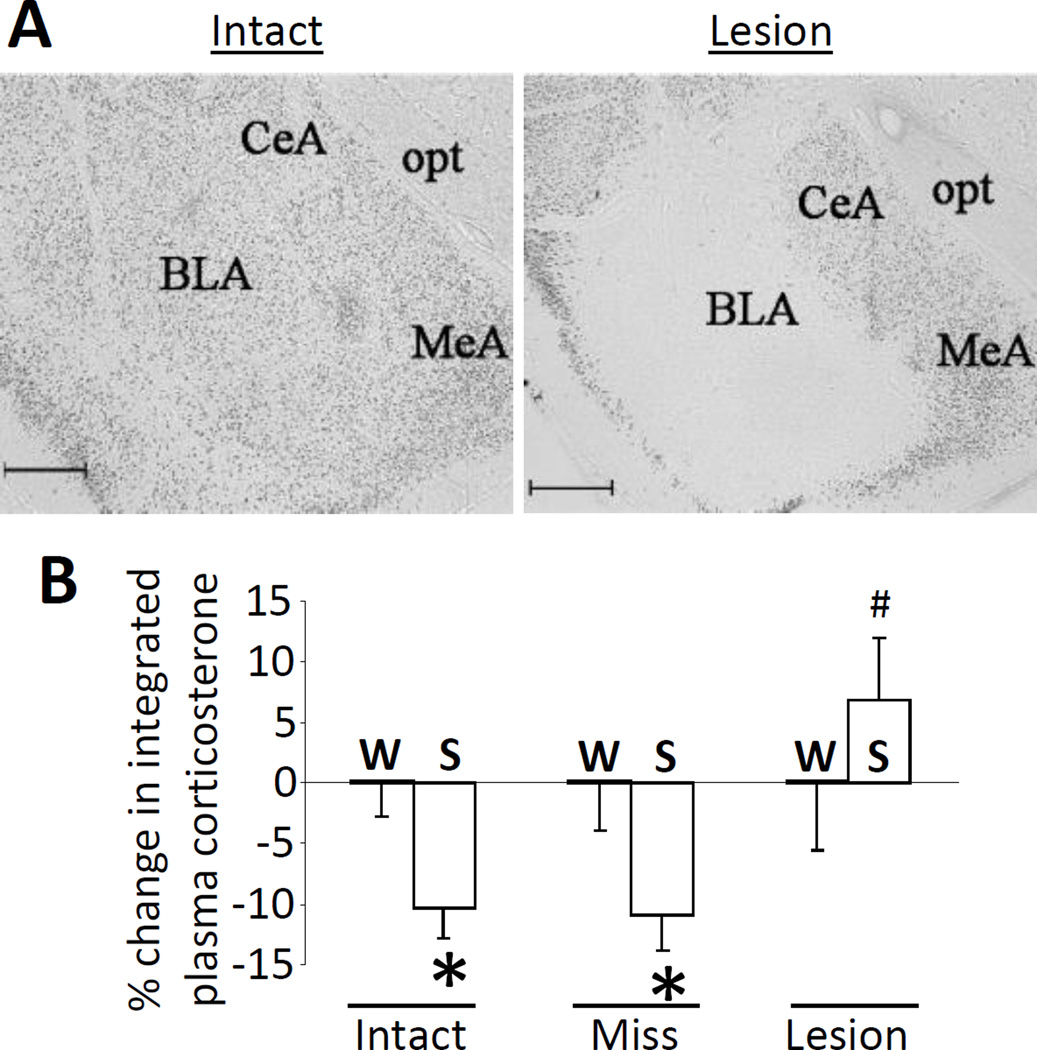
The BLA contributes to brain reward circuitry and is activated by palatable food intake, which increases neuronal firing and cFos expression (Fontanini et al., 2009, Muramoto et al., 1993, Verwey et al., 2007, Yamamoto et al., 1997). Moreover, the BLA is implicated in the control of behavioral and physiological stress responses (Bhatnagar et al., 2004, Coover et al., 1973, Feldman et al., 1983, Goldstein et al., 1996), and stress-induced c-fos mRNA responses in the BLA are reduced by a prior history of LSI (Ulrich-Lai et al., 2007). Taken together, this suggests that the BLA is well-positioned to mediate stress relief by LSI. In order to test this possibility, we assessed whether axon-sparing, ibotenate lesions of the BLA prevent HPA-dampening by sucrose ingestion (Figure 3A,B) (Ulrich-Lai et al., 2010). As a positive control rats given vehicle injection into the BLA, and hence with an intact BLA, showed typical HPA-dampening by LSI. In contrast, LSI did not result in HPA-dampening in rats with confirmed bilateral BLA lesions (using NeuN-immunolabeling to characterize the lesion extent), despite equivalent sucrose drink intake. Lastly, rats with ibotenate lesions that missed the BLA showed typical HPA-dampening by LSI. As a whole, this work suggests that neuronal activity in the BLA is necessary for LSI-mediated stress relief.
Figure 3.
Neuronal activity in the basolateral amygdala (BLA) is necessary for hypothalamic-pituitary-adrenal (HPA) axis-dampening by limited sucrose intake. The plasma corticosterone response to restraint stress was assessed after sucrose (S) vs. water (W) intake in rats with bilateral vehicle-injected, intact BLA (Intact), or bilateral ibotenate infusion that produced lesions that either missed (Miss) or hit (Lesion) the BLA. (A) Representative images of neuron-specific nuclear protein (NeuN)-immunolabeling in the BLA of Intact and Lesion rats. CeA = central amygdala, MeA = medial amygdala, opt = optic tract. Scale bar = 500 um. (B) Sucrose reduced the integrated (area-under-the-curve) plasma corticosterone response to restraint stress (relative to water control rats) in Intact and Miss rats, while this HPA-dampening did not occur in rats with bilateral BLA lesion (Lesion). *p < 0.05 vs. water controls, #p < 0.05 vs. Intact-Sucrose and Miss-Sucrose. Data are shown as mean ± SEM. Reproduced from (Ulrich-Lai et al., 2010).
In order to identify potential intra-BLA mechanisms for stress relief by sucrose, we collected BLA tissue after LSI (vs. water controls) and performed a microarray analysis of mRNA expression (Ulrich-Lai et al., 2010). This procedure identified 145 genes whose expression was altered by LSI. Functional clustering analyses indicated that these genes were significantly enriched in members of the intracellular calcium signaling and long-term potentiation (LTP) pathways, suggestive of synaptic plasticity (Ulrich-Lai et al., 2010). Subsequent neuroanatomical studies showed that LSI increased BLA immunolabeling for synaptophysin (Ulrich-Lai et al., 2010), a marker of pre-synaptic terminals (Tominaga-Yoshino et al., 2008, Wiedenmann and Franke, 1985), indicating an increased density of pre-synaptic terminals in the BLA. Furthermore, LSI increased the number of phospho-cAMP response element-binding protein (pCREB)-, phospho-calcium/calmodulin-dependent protein kinase II alpha (pCamKII)-, and FosB/deltaFosB-positive cells in the BLA (Christiansen et al., 2011, Ulrich-Lai et al., 2010), all of which are post-synaptic indices that are linked with synaptic strengthening and plasticity (Huang et al., 2000, Lisman et al., 2002, Rodrigues et al., 2004, Silva et al., 1998). Taken together, these data indicate that LSI induces synaptic remodeling in the BLA. Consistent with thisinference, both LSI-induced HPA-dampening and elevated BLA FosB/deltaFosB levels persist for at least 21 days following cessation of LSI (Christiansen et al., 2011) – these long-lasting effects of sucrose are suggestive of enduring changes in synaptic remodeling. Collectively, this work indicates that LSI-mediated stress relief may result, at least in part, from sucrose-ingestion induced plasticity and remodeling in the BLA. Future work will be directed towards understanding the phenotype of this plasticity and the defining role that it plays in this process.
Summary
Evidence reviewed in this section indicates that a modest amount of sucrose intake can reduce collective (behavioral, HPA axis and cardiovascular/sympathetic) stress responses, in the absence of clear changes in body weight and adiposity. These stress effects are primarily mediated by the pleasurable and rewarding properties of the sucrose drink, and the BLA, a key reward- and stress-regulatory brain region, is necessary for sucrose-induced stress relief. Lastly, the BLA undergoes synaptic remodeling following sucrose intake, which may enable stress relief to persist beyond the cessation of sucrose intake. Importantly, this work suggests that individuals may not need to consume large amounts of palatable food to achieve relief from acute stress. Moreover, the ability of other types of natural rewards to provide similar effects may be a useful strategy to diminish the contribution of life stress to obesity and other stress-related disorders.
Emotional consequences of prolonged high-fat feeding - Stephanie Fulton
Strong ties exist between the ingestion of foods rich in fat and/or sugar and the modulation of emotional states. As described, palatable food intake can relieve negative emotions by dampening signs of stress and anxiety following exposure to stressors or conditions of chronic unpredictable stress (Finger et al., 2011, Finger et al., 2012, la Fleur et al., 2005, Maniam and Morris, 2010, Pecoraro et al., 2004, Ulrich-Lai et al., 2010). Moreover, individuals experiencing depressed mood show increased preference and consumption of ‘comfort foods' and report the use of these foods as means to alleviate adverse feelings (Macht, 2008). Such positive emotional reactions to tasty, energy-dense food, including their rewarding and hedonic actions, are considered to play a significant role in overeating and the development of obesity (Fulton, 2010). While intake of palatable foods can attenuate negative emotions and mood states on the short term, their excessive consumption leading to increased adipose tissue mass and obesity can heighten vulnerability to depression and anxiety (Hryhorczuk et al., 2013). Mood disorders are now well recognized as a significant risk of obesity and associated metabolic pathologies like type 2 diabetes mellitus. Obesity elevates the odds of depression by about 55% (Luppino et al., 2010) whereas as many as 1 in 3 people with diabetes are estimated to experience depressed mood at a level that diminishes functioning, glycemic control, and increases the risk of diabetic complications (Anderson et al., 2001). Indeed, apart from impairing quality of life and everyday functioning, depressed mood presents additional threats by counteracting adherence to treatment and lifestyle changes and increasing the risk of complications associated with metabolic diseases.
Diet-induced obesity elicits depressive-like behavior
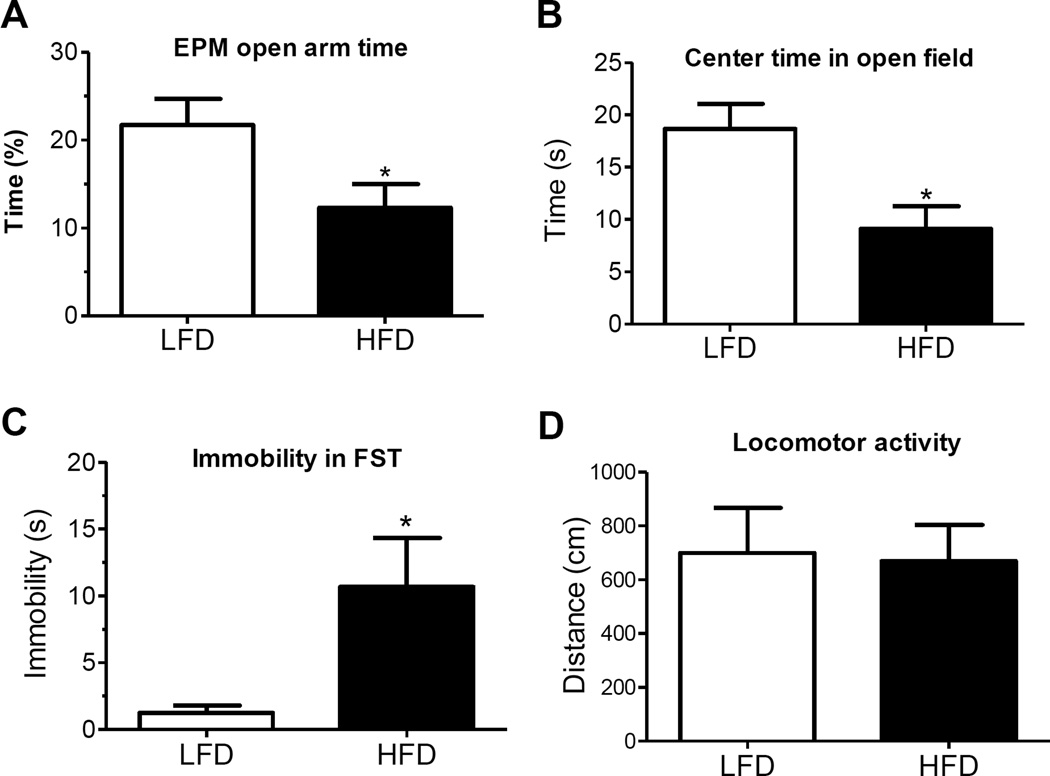
Emerging findings are beginning to uncover the mechanisms that promote depressed mood in obesity, including the contribution of diet and abdominal adiposity. Using the diet-induced obesity (DIO) model, we demonstrated that adult C57Bl6 mice consuming a diet high in trans-fat (as 58% of kcal in diet) for twelve weeks develop depressive-like features characterized by greater immobility in the forced swim task and reduced time spent in open areas of the elevated-plus maze and open field tests (Sharma and Fulton, 2013) (Fig. 4). These behavioral indices of despair and anxiety were accompanied by elevated basal HPA activity and increased corticosterone secretion in response to stress. Study of the neuroadaptive changes in brain nuclei controlling motivation and emotional regulation in response to excess dietary fat is essential to understand the pathogenesis of diet-induced depression. The nucleus accumbens (NAc) is a limbic region that is well-implicated in motivation, food-seeking and the hedonic deficits associated with depression. The NAc is highly interconnected with other brain areas important for mood regulation including prefrontal cortex, hippocampus and amygdala (Russo and Nestler, 2013). The significance of the NAc in the etiology of depression is underscored by the success of deep brain stimulation in the NAc to improve mood ratings in depressed individuals (Bewernick et al., 2012). The well-characterized reduction in brain monoamine levels in depression is posited to be linked to neuroplastic adaptations that involve transcriptional and translational changes generated by signals such as brain-derived neurotrophic factor (BDNF) and CREB (Russo and Nestler, 2013). Depressive-like responses can be triggered by stress-induced increases in pCREB activation and BDNF expression in the NAc whereas antidepressant-like actions are linked to their downregulation in this region (Martinowich et al., 2007, Nestler and Carlezon, 2006). Diet-induced depressive behavior was associated with decreased NAc protein expression of the rate-limiting enzyme for dopamine biosynthesis, tyrosine hydroxylase (TH), and increases in ΔFosB, pCREB and BDNF (Sharma et al., 2013a). Increases in BDNF and pCREB protein levels in the NAc correlated positively with the magnitude of behavioral despair in the forced swim test suggesting that prolonged high-fat feeding may promote depressive symptomatology via the neuroplastic changes elicited by these signaling molecules.
Figure 4. Diet-induced depressive-like behavior.
(A) Percentage of time spent in open arms of the elevated plus maze (EPM) was significantly reduced in high fat diet (HFD)-fed mice as compared to control low fat diet (LFD)-fed mice. (B) Significant increase in time spent in center of open field in HFD mice compared to control LFD mice. (C) Effect of chronic consumption of HFD on depressive behaviour expressed as immobility time in the forced swim test (FST) conducted in a separate cohort of mice. (D) Spontaneous locomotor activity expressed as total distance traveled during 15 min in a metabolism cage in C57Bl6 LFD and HFD mice (n = 6 each group). Mean±SEM *p < 0.05. Figure from (Sharma and Fulton, 2013).
Diets high in saturated fat and relatively low in monounsaturated and polyunsaturated fatty acids (PUFA) may contribute to the pathogenesis of mood disorders during obesity. The consumption of foods rich in saturated and/or trans-fat are associated with risk of depression while a diet consisting of mostly unsaturated fats, such as the Mediterranean diet, is largely protective. Interestingly, elevated dietary consumption of trans-fatty acids, such as that implemented in studies described above, has been shown to reduce brain docosahexaenoic acid, an omega-3 PUFA with described antidepressant actions (Phivilay et al., 2009). Indeed, insufficient dietary PUFA augments depression risk (Peet et al., 1998) whereas increasing dietary omega-3 PUFA has been shown to be protective (Lin and Su, 2007, Moranis et al., 2012, Oddy et al., 2011) and to prevent inflammation-induced depression (Su et al., 2014). Diets rich in saturated fatty acids are associated with increases in overall adiposity and bias visceral fat accumulation (Rosqvist et al., 2014). Adipose accumulation in abdominal stores is more effective than total body fat in predicting the risk of several complications of obesity, including insulin resistance and the metabolic syndrome (Tchernof and Despres, 2013). In a similar manner, central adipose depot mass is a better predictor of developing depression than body weight or body mass index (BMI) (van Reedt Dortland et al., 2013a). Consistent with these findings, Sharma and colleagues recently demonstrated that male adult mice consuming a saturated high-fat diet (50% kcal palm oil) for eight weeks develop greater visceral obesity and metabolic impairments including elevated levels of plasma corticosterone and cytokines along with increased anxiety and depressive-like behaviors as compared to mice consuming an isocaloric monounsaturated fat diet (Sharma and Fulton, 2013). These effects could be related to the actions of saturated fats to stimulate pro-inflammatory signaling. Obesity is characterized by a state of prolonged low-grade inflammation and several gene markers of neuroinflammation are upregulated in the brain of rodents by chronic high-fat feeding (De Souza et al., 2005, Thaler et al., 2012). Correspondingly, several lines of evidence implicate immune responses in the pathophysiology of depression (Miller et al., 2009). In agreement with a role for inflammation, a recent study demonstrated that elevated circulating levels of the pro-inflammatory signal C-reactive protein is a factor accounting for most of the association between depression severity with lipid and obesity levels (van Reedt Dortland et al., 2013b). Collectively, these data suggest that abdominal obesity and associated circulating inflammatory signals contribute to the etiology of depression; however the neural mechanisms involved remain unknown.
Stress, anxiety and craving responses to high-fat diet removal
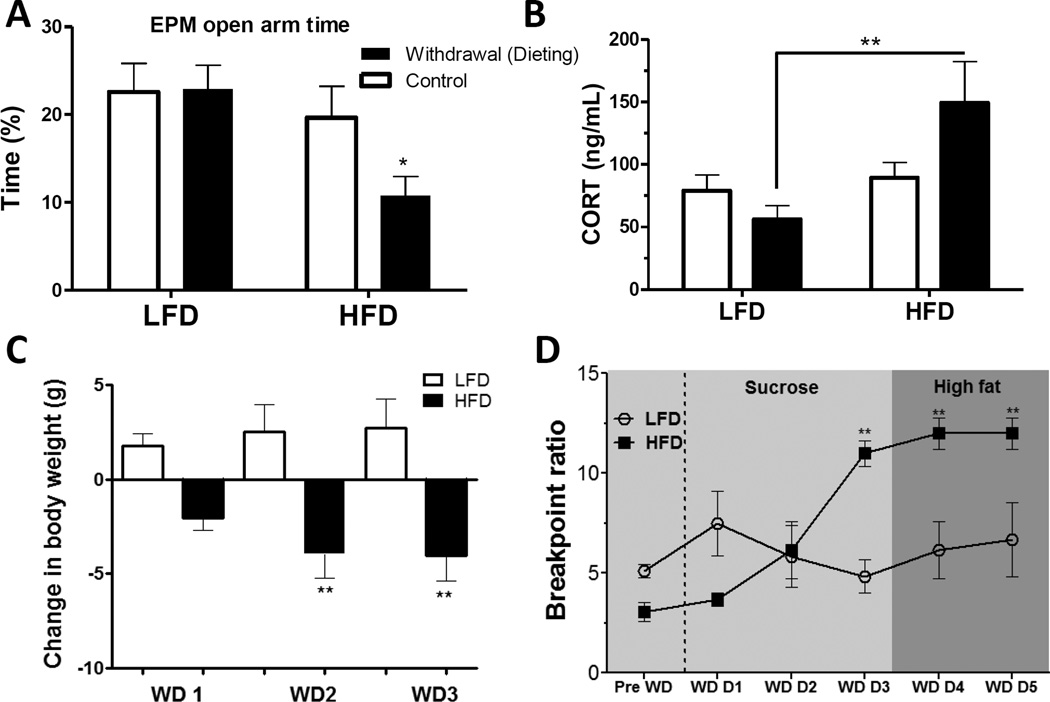
Is the onset of depressive-like behavior induced by prolonged high-fat feeding secondary to metabolic impairments produced by obesity? To begin addressing this question, we examined whether high-fat feeding preceding the development of severe obesity and associated metabolic dysfunction could provoke similar behavioral depressive-like symptomatology and neurobiological changes (Sharma and Fulton, 2013). HFD consumption for 6 weeks resulted in moderate weight gain (~11% increase vs. controls), enhanced stress-induced HPA activation, and sucrose anhedonia (reduced ability to experience pleasure/reward from sucrose drink), but did not increase anxiety-like behavior. Similarly, others have shown that high-fat feeding independent of obesity (using a pair-feeding paradigm) can dampen mesolimbic dopamine reward responses and turnover (Davis et al., 2008). However, returning high-fat fed mice to a normal chow diet (i.e., akin to "dieting") increased anxiety-like behavior (Fig. 5), elevated physiological stress responses (Fig. 5B,C), and greater motivation to access both sucrose and fatty food (Fig. 5D) (Sharma et al., 2013b). Similarly, several groups have shown that removal of a high-fat/sugar diet following prolonged intermittent or regular intake can increase behavioral and physiological signs of depression and anxiety or withdrawal (Avena et al., 2008, Cottone et al., 2009a, Cottone et al., 2009b, Teegarden et al., 2008). Thus, not only can chronic high-fat feeding provoke signs of anhedonia and attenuate mesolimbic dopamine function prior to development of severe metabolic dysfunction, it appears to augment sensitivity to stress, including stress triggered by dieting, which may further perpetuate overeating, weight gain and depressed mood.
Figure 5. High-fat diet (HFD) withdrawal (dieting) increases anxiety, stress and palatable food craving.
(A) Elevated plus maze (EPM) open arm time was significantly reduced in mice withdrawn from HFD as compared to mice withdrawn from low fat diet (LFD). (B) Elevated basal plasma corticosterone (CORT) concentration in HFD withdrawn mice as compared to LFD withdrawn mice. (C) Body weights decreased post-withdrawal in mice subjected to HFD withdrawal (WD: day of withdrawal). (D) Withdrawal from HFD, but not LFD, significantly increased breakpoint thresholds for sucrose rewards (indicating increased motivation for sucrose reward) as of day 3 of withdrawal (WD D3). Breakpoints remained elevated on day 4 and 5 of withdrawal when sucrose rewards were replaced by high-fat food rewards. Mean ± SEM; *p ≤ 0.05, **p ≤ 0.001. Figure from (Sharma et al., 2013b).
In order to isolate potential neurobiological mechanisms contributing to increased anxiety and palatable food craving following dieting, we analyzed protein expression of several neuroplasticity markers in the NAc and amygdala. The 6-week period of high-fat feeding (not associated with anxiety and HPA stimulation) increased pCREB in the BLA and central nucleus of the amygdala (CeA), similar to findings reported for the BLA following the LSI paradigm (Ulrich-Lai et al., 2010) (see previous section), and elevated ΔFosB in the NAc as previously documented (Sharma and Fulton, 2013, Teegarden et al., 2008). However, removal of the HFD and subsequent heightened anxiety and stress responses and elevated motivation for palatable food measured days later were associated with lower pCREB and NAc ΔFosB levels in the amygdala. At the same time, NAc BDNF protein levels increased in response to the dieting intervention, similar to findings in the diet-induced depression model described above. Thus, chronic high-fat feeding prior to development of severe metabolic dysfunction provokes signs of stress, anxiety and food craving in response to dieting in a manner inversely related to neuroplastic adaptations that are associated with stress relief. These neurobiological and behavioral responses to dieting can thus reinstate palatable food-seeking and thereby foster overeating, weight gain and the development of depression that accompanies diet-induced obesity.
Summary
It is increasingly evident that the amount and quality of the nutrients consumed over the long run can impact the neural circuits controlling emotions, motivation and mood. Findings on several fronts tie the development of depressed mood in obese individuals to poor dietary lifestyle, central adipose mass and associated metabolic disturbances. Weight gain and to a greater extent obesity produced by excessive intake of dietary fats can generate depressive-like symptomatology and several neuroplastic adaptations in brain reward circuitry in mice. Chronic high-fat feeding may affect neural processes of emotion and reward via actions on energy metabolism, endocrine function and immunity or perhaps through the direct actions of free fatty acids on the central nervous system. Recent research findings propose the nature of the dietary fat as an important element, with saturated fats being more deleterious than unsaturated fats. Trans- and saturated fats favour central fat deposition and are linked with cardiometabolic and neurological diseases (Barnard et al., 2014, Kanoski and Davidson, 2011, Micha and Mozaffarian, 2010). Advancing our knowledge of the mechanisms contributing to diet-induced depression can be accomplished by building upon our understanding of the metabolic disturbances associated with abdominal obesity, and how they influence central signaling mechanisms that regulate emotion. It also is imperative to understand the neurobehavioral processes by which sensitivity to stressors, like dieting, is enhanced by prolonged high-fat feeding. Improved understanding of these mechanisms may suggest novel ways to avert the self-defeating cycle of depressed mood promoting the intake of comfort foods as a means to achieve stress relief.
Research by Drs. Ulrich-Lai and Fulton also indicates that palatable food intake can have opposing effects on physiological and behavioral responses to stress. Stress responses are decreased in chow-fed rats with a history of intermittent intake of small amounts of sweetened drink (LSI), and this effect appears to be mediated primarily by the rewarding (vs. macronutrient/energetic) properties of the food (see previous section). In contrast, unlimited and prolonged access to high dietary fat in mice increases stress responses in a manner that is modulated by the type of fat. Several key differences between these two palatable food paradigms likely influence the valence of stress effects. These factors include the amount and type of palatable food offered (small amounts of sugar vs. large amounts of fat), the duration of exposure (2 weeks for LSI vs. 12 weeks for HFD), whether the paradigm altered either body weight or adiposity (no effects for LSI vs. increase in body weight with HFD), whether the food was offered as a choice (choice with LSI vs. no choice with HFD), whether food intake was limited (intake limited with LSI vs. unlimited with HFD), as well as the species tested (rat for LSI vs. mouse for HFD). Indeed, some evidence suggests that stress relief associates with increased carbohydrate intake and also with the presence of food choice, whereas stress promotion associates with less dietary carbohydrate, lack of dietary choice, and increased adiposity (Anderson et al., 1987, Barr et al., 1999, de Castro, 1987, Deuster et al., 1992, Markus et al., 2000, Utter et al., 1999). We refer readers to the “Concluding remarks” section for additional discussion of this intriguing topic.
Stress-induced eating - Mark Wilson
Diet-induced obesity (DIO) resulting from the persistent consumption of a calorically dense diet is a well-accepted strategy in animal models to study the consequences of obesity (Hariri and Thibault, 2010, Panchal and Brown, 2011). However, some animals show resistance to developing this phenotype (Archer and Mercer, 2007). While a number of genetic factors could account for this variance in susceptibility to DIO (Levin, 2000, Yazbek et al., 2010), accumulating data indicate a history of adverse experience resulting from chronic stressor exposure may also be important (Dallman et al., 2007, Tamashiro et al., 2011). It must be noted, however, that the effects of stressor exposure on food intake are complex and may be bidirectional with the specific effect dependent on a number of factors such as whether the experience is severe (see later section by Dr. Petrovich), and/or whether it is acute or chronic (Maniam and Morris, 2012). Furthermore, macronutrient content of the diet is also likely important for this directionality, as a large literature from rodent models suggests that stressor exposure to animals fed a typical low calorie, laboratory chow diet produces inappetence (Harris et al., 1998, Tamashiro et al., 2006), an effect reversed by the central administration of a CRF receptor antagonist (Smagin et al., 1999). Indeed, acute central administration of CRF to adult macaques has an immediate and lasting suppressive effects on food intake (Glowa and Gold, 1991). However, an extensive literature in rodent models indicates that chronic exposure to stressors is a mitigating factor in the preference for and consumption of excess calories from high caloric diets containing fats and sugars (Tamashiro et al., 2006, Warne, 2009). These data underscore the importance of considering diet when assessing the consequences of stressor exposure on appetite.
The studies in rodents of stress-induced intake of calorically dense diets are consistent with the notion of comfort food ingestion in people (Adam and Epel, 2007, Epel et al., 2000) (see previous section by Dr. Ulrich-Lai). With the exception of a few reports (Solomon et al., 2011), the majority of studies in rodent models have used males (Tamashiro et al., 2005, Warne, 2009), despite the growing evidence highlighting significant bias towards stress-eating and obesity in women (Grunberg and Straub, 1992, Kitraki et al., 2004, Soulis et al., 2005, Soulis et al., 2007). Socially housed female macaques provide an opportunity to further define mechanisms for stress-induced emotional feeding in women. A large body of data shows that social subordination in established macaque groups produces a number of stress-related phenotypes in females (Michopoulos et al., 2012a), including a dysregulation of the HPA axis (Kaplan et al., 2010, Michopoulos et al., 2012a, Shively et al., 1997a) and an upregulation of a number of proinflammatory genes (Tung et al., 2012). In a dietary environment where a typical low fat, high fiber laboratory chow diet is available (“low caloric diet” or LCD), subordinate female rhesus monkeys consistently show lower body weights and lower serum concentrations of leptin than more dominant group mates (Michopoulos and Wilson, 2011). Employing automated feeding stations that detect food intake by free-feeding individuals in these social groups (Wilson et al., 2008), this reduced body weight in subordinate females is associated with periodic mild inappetence (Michopoulos, 2009) or calorie intake similar to dominant females (Michopoulos et al., 2012a). What is not known is whether there is a status differences in energy expenditure in these socially-housed monkeys.
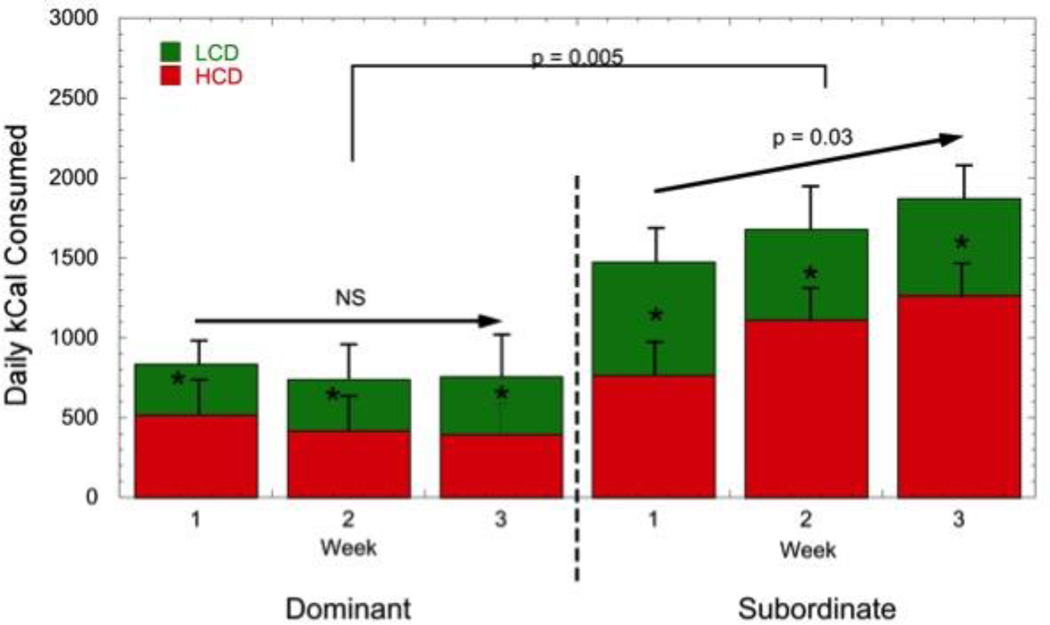
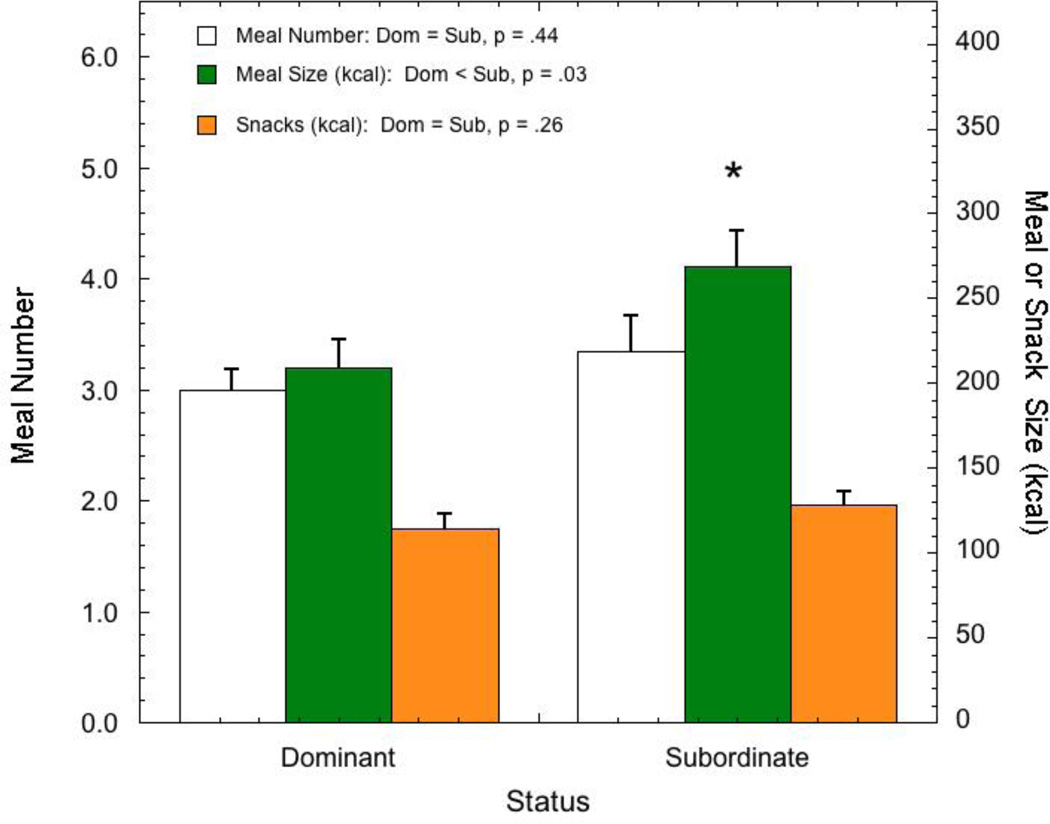
A different pattern emerges when animals are maintained in a rich dietary environment and given the choice between the low calorie, laboratory chow diet and a high fat, high sugar diet. This dietary choice environment, where animals have access to both a LCD and high caloric diet (HCD), best models the human dietary environment, sustains stress-induced consumption of HCDs (Warne, 2009), and promotes obesity more effectively than HCD alone (Rolls et al., 1983). Under these conditions, all animals prefer the HCD (Arce et al., 2010, Michopoulos et al., 2012c). However, dominant females consume a similar number of calories in the rich dietary environment as they do when only LCD is available (Arce et al., 2010, Michopoulos et al., 2012c). In contrast, subordinates consume significantly more calories in this dietary choice environment compared with dominant females (Fig. 6) (Arce et al., 2010, Michopoulos et al., 2012c)). This status difference in caloric intake is the result of increased meal size by subordinates compared with dominant females and is not due to more frequent meals or calories consumed outside of defined meals (snacks) (Fig. 7) (Moore et al., 2013)). Interestingly, these statistically defined meals were significantly more often comprised of calories from both diets rather than either diet alone (Moore et al., 2013). While these studies were short term in nature, focusing on changes in appetite and not the consequences of consuming these diets on body composition, the data nonetheless suggest that female rhesus monkeys provide a model to better understand how psychosocial stressor exposure may account for variance in DIO in females.
Figure 6. Average (± SEM) daily caloric intake for dominant and subordinate female rhesus monkeys from both a low caloric (LCD) and high caloric diet (HCD). Redrawn from (Arce et al., 2010, Michopoulos et al., 2012c).
Figure 7.
Comparison of average (± SEM) meal size (kcal), meal number, and calories from snacks for dominant and subordinate female rhesus monkeys maintained in a dietary environment where both a low caloric (LCD) and high caloric diet (HCD) are available. Redrawn from (Moore et al., 2013).
Possible mechanisms for stress-induced emotional feeding
It is not fully known how chronic stressor exposure increases susceptibility to DIO. One possibility is that subordinate female monkeys may be more sensitive to orexigenic signals. Although acute ghrelin administration increases intake of a LCD in subordinate but not dominant females (Michopoulos et al., 2010), it has no effect on consumption of a HCD. In contrast, a number of studies in rats have confirmed that glucocorticoids dose-dependently increase the intake of diets high in fat and sugar (Dallman et al., 2005, Warne, 2009), supporting the well- established notion that these steroids promote food intake (Sominsky and Spencer, 2014). Although prospective studies assessing the direct effects of stress-like glucocorticoid levels on diet preference and caloric intake have not been performed in monkeys, serum cortisol concentrations following a dexamethasone suppression test positively predict consumption of a HCD but not a LCD in female rhesus monkeys (Michopoulos et al., 2012c). These data suggest that female monkeys with reduced glucocorticoid negative feedback will consume more calories from a palatable, calorically denser diet.
Importantly, the consumption of these palatable HCDs in male rats subsequently reduces the response to acute stressors (Foster et al., 2009, la Fleur et al., 2005, Pecoraro et al., 2004, Ulrich-Lai et al., 2011). However, a different pattern emerges in female monkeys. Both dominant and subordinate females respond similarly to an acute separation from their social group with a robust increase in serum cortisol concentration. However, this response is significantly greater when monkeys are maintained in a rich dietary environment, with access to both a HCD and LCD, compared to a condition when only the LCD is available (Arce et al., 2010, Michopoulos et al., 2012b). These data are consistent with those showing intake of calorically dense foods increases HPA axis activity in rodents (Kamara et al., 1998, Tannenbaum et al., 1997) and in humans (Pasquali et al., 2002). Furthermore, data showing that glucocorticoids are key signals changing food salience and increasing caloric intake (Dallman et al., 2005, Warne, 2009), are consistent with the notion that emotional feeding is sustained by activation of the HPA axis, and that acute stress superimposed on chronic stress may promote continued intake of HCDs. While these observations in female monkeys are similar to those reported by Dr. Fulton in the previous section that HFD consumption enhances stress responsivity, they contrast those summarized above by Dr. Ulrich-Lai clearly showing comfort food intake diminishes the acute response to a stressor in male rats. It is unclear what accounts for these differences in HPA responsivity in animals maintained on palatable diet, but this may include the nature of the stressor or a history of comfort food intake. It is tempting to suggest that these differences may reflect sex differences in HPA axis regulation (Handa et al., 1994, Toufexis and Wilson, 2012, Viau et al., 2005), and in how it is modified by dietary environment; however, results from different paradigms described above by Drs. Ulrich-Lai and Fulton were from studies on male rodents.
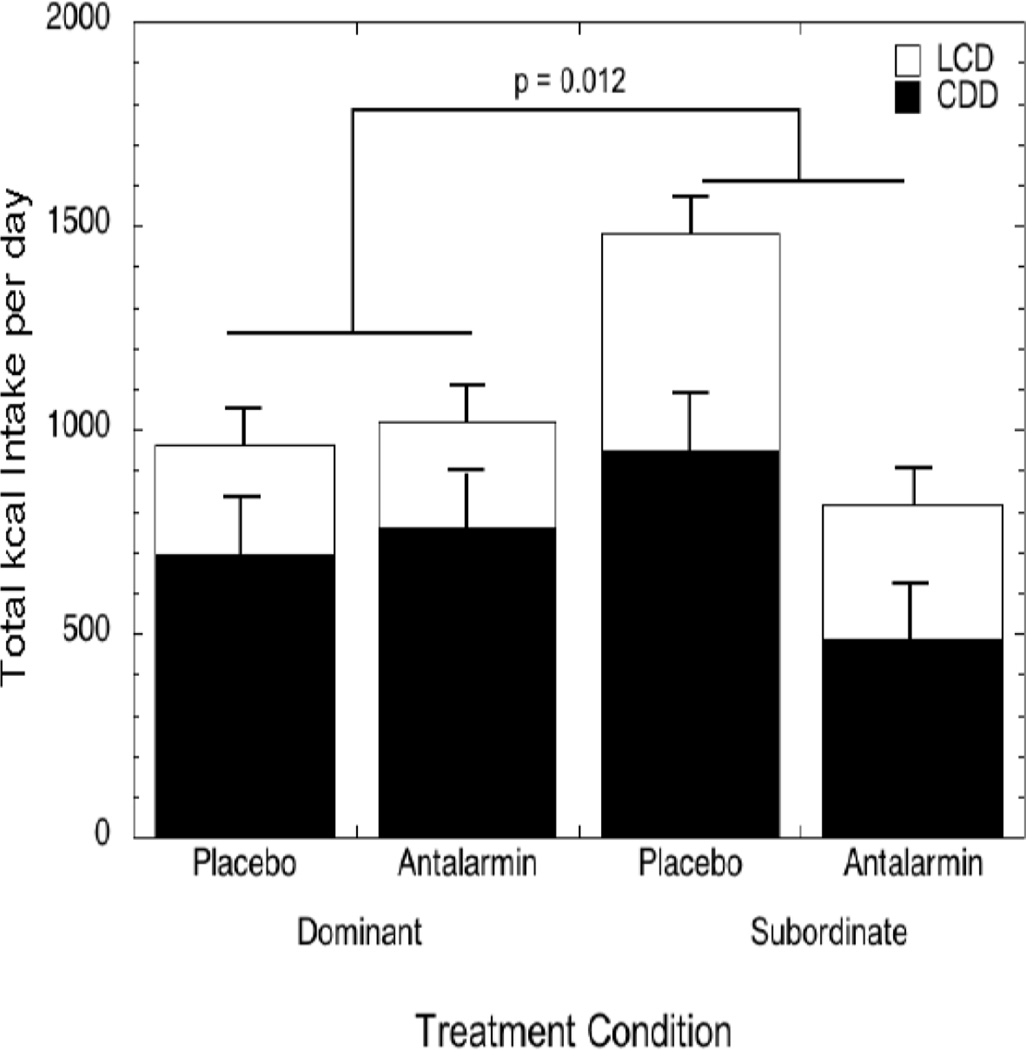
Additional observations from rodents indicate that chronic activation of central CRF-R1 receptors are likely important for stress-induced over consumption of palatable diets. In these rodent models, chronic diet cycling (intermittent dietary restriction of a palatable diet) functions as a mild stressor, and immunohistochemistry reveals elevated CRF-positive cells in the CeA among diet cycled but not control rats (Cottone et al., 2009a, Iemolo et al., 2013). Importantly, microinfusion of a CRF-R1 antagonist, Antalarmin, into the CeA of diet cycled male rats fully blocks hyperphagia of a palatable food with no effect on standard chow intake (Iemolo et al., 2013). Our data in female rhesus monkeys are consistent with these findings: during placebo administration, subordinate females consume significantly more calories than non-subordinate females within a dietary environment that includes a choice between a LCD and HCD. However, acute peripheral administration of Antalarmin significantly reduces average daily caloric intake in subordinate female monkeys to amounts comparable to those consumed by dominant group mates (Fig. 8) (Moore et al., 2015). Because Antalarmin penetrates the blood brain barrier and this dose and route of administration does not affect peripheral cortisol secretion (Herod et al., 2011a, Herod et al., 2011b), the data suggest that activation of central CRF-R1 may be important for sustaining this emotional feeding phenotype across species.
Figure 8.
Average (± SEM) daily caloric intake of both a low caloric (LCD) and high caloric diet (HCD) in dominant and subordinate female rhesus monkeys during two days of placebo and two days of treatment with the corticotropin releasing factore receptor type 1 (CRF-R1) antagonist, Antalarmin. Subordinate but not dominant females showed a significant attenuation in caloric intake during Antalarmin treatment. Reprinted from (Moore et al., 2015).
An emerging hypothesis is that excess food consumption and resulting obesity is a form of addiction involving the same corticostriatal circuitry engaged by drugs of abuse (Tomasi and Volkow, 2013). Assuming this hypothesis is viable, the question remains of what accounts for differential vulnerability to the addictive properties of food. Because chronic stressor exposure, possibly mediated through central CRF signaling, is a risk factor for psychostimulant abuse in people (Sinha and Jastreboff, 2013) or self-administration in experimental animals (Goeders, 2002, Koob and Kreek, 2007), including monkeys (Morgan et al., 2002), it is possible that exposure to chronic stressors increases vulnerability to food addiction (Dallman et al., 2005). As highlighted in previous sections by Dr. Ulrich-Lai and Dr. Fulton, evidence from rodents and humans suggests that changes in dopamine activity are at least part of the mechanism linking stress to comfort food ingestion (Bassareo and Di Chiara, 1999, Martel and Fantino, 1996a, Martel and Fantino, 1996b, Pelchat, 2002, Rada et al., 2005). Based on studies in rodents, it is well established that signals from the stress axis, including glucocorticoids and CRF target dopamine neurons in mesolimbic regions (Ambroggi et al., 2009, Burke and Miczek, 2013, Koob et al., 2014) producing a dysregulation of dopamine neurotransmission (Izzo et al., 2005) that increases the expression of anhedonia and the risk for developing an addictive phenotype (Anisman and Matheson, 2005, Koob and Kreek, 2007, Koob and Le Moal, 2001). This stress-induced dopamine dysfunction has been referred to as a “reward deficiency syndrome”, characterized by reduced dopamine activity, notably reduced availability of dopamine type 2 receptors (D2R) (Blum et al., 1996) that is both predictive of an addictive phenotype (Volkow et al., 2003, Volkow and Wise, 2005) and observed in human obesity (Wang et al., 2001). Whether the imposition of chronic stress in the monkey model of social subordination leads to this reward deficiency syndrome that predicts stress-induced consumption of calorically dense diets remains to be determined. We do know that social subordination in female macaques produces widespread reduction in D2R binding potential throughout the reward network (Grant et al., 1998, Shively et al., 1997a), which consists of dopamine-rich areas, including the striatum and prefrontal cortex, and is critical for regulating reward- and goal-directed behaviors and responses (Haber and Knutson, 2010). Studies of obese humans indicate disrupted connectivity within cortico-striatal pathways (Tomasi and Volkow, 2013). Preliminary data suggest that social subordination in female macaques reduces functional connectivity within the cingulo-opercular/limbic network, which includes the NAc, amygdala, and prefrontal cortex and has been linked to stimulus salience (Godfrey, 2013). Because stressor exposure affects structural and functional connectivity of the prefrontal cortex and other regions regulating reward (Govindan et al., 2010, Kaufman and Charney, 2001, Sanchez et al., 1998, Teicher et al., 2002), it is possible that exposure to chronic stress compromises dopamine modulation of prefrontal regulation of the striatum, increasing vulnerability for food addiction.
Summary
Identifying the mechanisms by which chronic stress increases the preference for and consumption of palatable, calorically dense foods may provide insights into possible treatment strategies. While the studies in female macaques show many similarities to studies of male rodents, more investigation is needed to determine if sex differences in stress responsivity lead to differences in vulnerabilities to food addiction as they do for psychostimulant addiction (Bobzean et al., 2014). Given that a history of stressor exposure can have lasting effects on biological signaling and behavior (Zorrilla et al., 2014), an important question is whether the alleviation of chronic stress – by experimentally manipulating social rank so that formerly subordinate females are now dominant (Shively et al., 1997b) - reverses the preference for, and consumption of palatable, calorically dense diets. Such a finding would be critically important for people attempting to make life style changes to improve their health. The use of female macaque monkeys provides a translational animal model for women that complements the use of other animal models to understand the causal relationship between chronic stress and risk for obesity.
Stress-induced anorexia - Gorica Petrovich
Stress has profound and multifaceted effects on food intake and body weight, which often are stimulatory (see previous section) (Meye and Adan, 2014, Tamashiro et al., 2011). Fear of anticipated stress, however, inhibits food intake (Cannon, 1915, Schachter et al., 1968), and sustained stress, fear, and anxiety have been linked to prolonged cessation of eating and anorexia nervosa (Hotta et al., 1999, Kaye, 2008, Klein and Walsh, 2004, Vallés et al., 2000). Anticipation of aversive events also suppresses food approach and consummatory and instrumental actions towards food (Petrovich, 2013). This suppression of feeding is adaptive and promotes survival when fear is triggered by real danger (i.e., avoiding imminent danger takes priority over feeding), but could become maladaptive when sustained fear halts feeding under energy-depleted states, such as in anorexia nervosa. The neural mechanisms controlling the balance between hunger and fear are therefore important in normal function and in disease. Nevertheless, the brain substrates mediating anorexigenic effects of stress anticipation (i.e., fear) on food consumption have been largely unexplored. Here, a behavioral model for short-term fear anorexia1 will be described, along with functional and anatomical evidence that a prefrontal-amygdala-hypothalamic circuitry is critical.
Fear Anorexia
The fear anorexia model utilizes conditioned fear cues to inhibit feeding in hungry rats. A fear cue in these preparations is a signal that predicts danger, a tone or environment (context) previously associated with mild, electric foot-shocks through Pavlovian conditioning, based on well-known preparations (Fendt and Fanselow, 1999, LeDoux, 2000, Maren, 2001). After conditioning, fear cues can potently inhibit feeding in hungry rats (Petrovich and Lougee, 2011, Petrovich et al., 2009, Reppucci et al., 2013). These effects could be induced by discrete (tone) or contextual conditioned cues (i.e., by placing animals in an aversive environment; conditioned context).
During the feeding tests, conditioned fear cues also induce freezing behavior, a species-typical defense response for rodents, characterized by complete immobility, except as necessitated for breathing (Blanchard and Blanchard, 1969, Fanselow, 1984). Importantly, cessation of feeding in the conditioned fear anorexia model is not simply a product of freezing behavior. Rats do not halt eating simply because they are immobile; rather, anorexia and freezing are two separate behavioral outputs driven by the same fear cue (Petrovich and Lougee, 2011, Petrovich et al., 2009, Reppucci et al., 2013). Furthermore, fear anorexia and freezing are supported by dissociable amygdalar substrates. The CeA, but not the BLA, is critical for fear cue anorexia, while both are necessary for fear cue driven freezing behavior (Petrovich et al., 2009).
This animal model may be informative about aspects of eating dysregulation in humans. Particularly, the potential contribution of fear in the maintenance of low food intake in anorexia nervosa (AN) has been hypothesized (Petrovich and Lougee, 2011). AN is a complex eating disorder characterized by relentless maintenance of extremely low body weight through restricted eating, sometimes combined with excessive physical activity and purging (American Psychiatric Association, 2013, Kaye, 2008, Klein and Walsh, 2004, Treasure et al., 2010). Restricted food intake is maintained persistently, despite emaciation, and despite serious health consequences that can be fatal. The drive to block intake of calories under these circumstances is paradoxical and occurs despite physiological hunger signals—high ghrelin and low leptin baseline circulating concentrations (Jimerson and Wolfe, 2006, Kaye, 2008) and increased central amounts of the potent orexigenic neuropeptide, neuropeptide Y (Kaye, 2008).
An intense and persistent fear of weight gain (including fear of food and eating), despite being underweight, is a key symptom and a diagnostic criterion of AN (American Psychiatric Association, 2013). Anxiety, typically associated with sustained fear, is also highly prevalent in AN. Furthermore, individuals with AN have aberrant processing within the key brain fear network regions (the amygdala and medial prefrontal cortex) (Giordano et al., 2001, Seeger et al., 2002, Takano et al., 2001, Uher et al., 2004). The underlying fear in AN, and associated over-activity within the fear network, therefore could be a critical facilitator of halted eating. Nevertheless, fear effects on food consumption under chronic food restriction and low body weight remain to be established.
Sex differences in fear anorexia
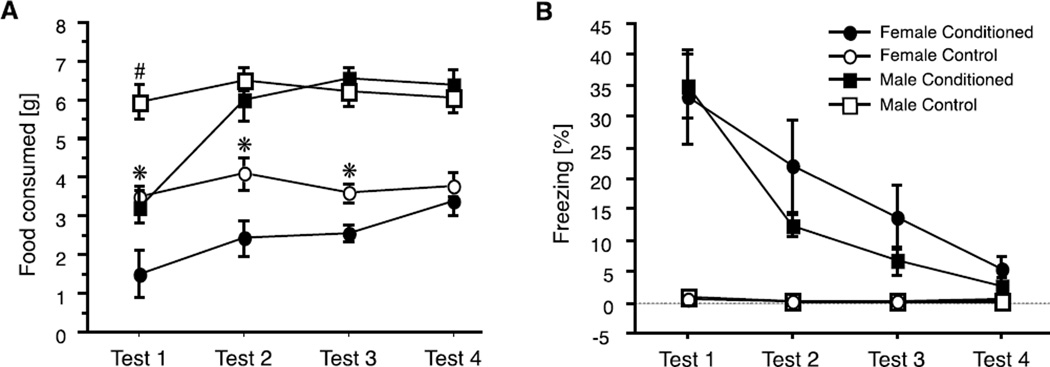
A recent study compared learning and feeding behavior of adult male and female rats in the fear anorexia paradigm to determine whether there were sex differences (Fig. 9A) (Petrovich and Lougee, 2011). Males and females acquired fear cues similarly well, indicated by freezing behavior during tests (Fig. 9B). The extent of fear anorexia was also similar for the two sexes during first of four tests. A pronounced sex difference, however, emerged during extinction: females showed sustained inhibition of feeding compared to males. Male rats extinguished this fear anorexia during the second test, when rats in the conditioned and control groups ate similar, large amounts of food. It took females in the conditioned group two additional tests to reach the consumption levels of the controls (Fig. 9A) (Petrovich and Lougee, 2011). These findings suggest a biological susceptibility of the female sex potentially relevant to AN, a disease that afflicts women disproportionately more than men (see discussion on fear and AN, above). This model provides a novel framework for investigation into sex differences in the control of feeding and the underlying brain substrates.
Figure 9.
(A) Food consumption (mean ± SEM) of male and female rats during tests with fear cue presentations. During training, rats in the conditioned groups received tones paired with footshocks, while rats in the control groups received tones but no shocks. Tests were conducted on separate days, under acute food deprivation (no footshocks given during tests). Significant differences (p < 0.05) between Conditioned and Control groups are indicated by * for females and by the # for males. (B) Conditioned freezing behavior during food consumption tests. Graphs show percentages (mean ± SEM) of the total time spent freezing during the tests. Adapted from (Petrovich and Lougee, 2011), with permission.
Prefrontal-amygdala-hypothalamic circuitry
Extensive work over the last two decades has focused on determining the brain substrates underlying fear and its behavioral sequelae (LeDoux, 2012, Seymour and Dolan, 2008), and similar efforts have been directed towards examining the central substrates of hunger, food intake, and energy balance (Berthoud, 2007, Morton et al., 2006). How fear and hunger compete, however, has not been adequately addressed, and the underlying brain substrates are not well understood. Yet, knowing when to eat and when to halt feeding and attend to danger is essential for survival, and dysfunction of this system could lead to disease.
Recent work has begun to identify components of a fear anorexia neural network. One of the key structures in fear acquisition and behavioral expression, the CeA, was found to be also critical for fear anorexia (Petrovich et al., 2009). Neurotoxic, bilateral lesions of the CeA eliminated decreased consumption during tests with fear cues, and Fos induction in the CeA during similar tests corresponded with the amounts of food consumed (Petrovich et al., 2009, Reppucci and Petrovich, 2014). The circuitry through which the CeA acts to inhibit food intake has not been delineated, but connectional and functional evidence strongly implicate the lateral hypothalamus (LHA). The LHA has been historically associated with the initiation of feeding (Elmquist et al., 1999), and recently was conceptualized as a critical integrator of physiological and environmental feeding signals (Berthoud and Münzberg, 2011, Petrovich, 2013). Indeed, the LHA is crucial for cue induced feeding, a preparation that requires integration between physiological and environmental feeding signals (Petrovich, 2013). Cue induced feeding is driven by food cues and occurs independently of hunger, and the LHA is an integral part of its critical forebrain network, together with the BLA and ventromedial prefrontal cortex (vmPFC) (Petrovich, 2013). The BLA is also necessary for stress relief by palatable food reward and a site of plasticity in the LSI paradigm, described by Dr. Ulrich-Lai in a previous section. Fear anorexia, similar to cue induced feeding, requires competition with physiological hunger/satiety signals and the amygdala; however, the amygdalar component for fear anorexia is the CeA, instead of the BLA.
The CeA is anatomically well positioned to influence LHA processing, via direct and indirect pathways (Petrovich et al., 2001, Yoshida et al., 2006). Its targets within the LHA include the suprafornical region, which is important for ingestive behavior (Hahn and Swanson, 2010), and contains neurons that express an orexigenic peptide orexin/hypocretin (de Lecea et al., 1998, Nakamura et al., 2009, Sakurai et al., 1998) with demonstrated importance in arousal, reward processing, and anticipatory feeding motivation (Petrovich et al., 2012). In addition, the CeA sends direct pathways to the LHA brainstem targets responsible for feeding suppression, the parabrachial nucleus and the nucleus of the solitary tract (Carter et al., 2013, Hahn and Swanson, 2010, Maniscalco et al., 2013, Swanson and Petrovich, 1998).
Another major telencephalic input to the LHA is from the vmPFC (Petrovich, 2013), an area critical for cognitive processing and executive function, including decision-making in goal-directed behaviors (O'Doherty, 2011). The vmPFC and its connections to the LHA support cue-induced feeding (Petrovich, 2013), and are set up to control other forms of feeding. Stimulation of the vmPFC µ-opioid (Mena et al., 2011) and dopamine receptor (Land et al., 2014) systems elicit robust feeding in sated animals, and these effects depend on the LHA (Mena et al., 2013) and amygdala (Land et al., 2014), respectively. In turn, the vmPFC has access to LHA and CeA processing via direct, topographically organized connections (Hahn and Swanson, 2010, Reppucci and Petrovich, 2012, Swanson and Petrovich, 1998). In particular, the infralimbic cortical area sends direct pathways to the CeA, as well as to its targets within the LHA and brainstem, including the parabrachial nucleus and the nucleus of the solitary tract (Hahn and Swanson, 2010). This connectional pattern would allow the vmPFC to simultaneously control the CeA and its outputs, which would be an efficient way to regulate feeding under imminent stress.
Summary
Dissociable amygdala substrates participate in stimulatory and inhibitory control of food intake. The BLA, but not CeA, is critical for food cue driven feeding (Petrovich, 2013) and is recruited during initial cue-food learning (Cole et al., 2013), while the CeA, but not BLA, is critical for fear cue inhibition of feeding (Petrovich et al., 2009). Both are connected with the vmPFC and LHA. The vmPFC-BLA-LHA system mediates food cue stimulatory effects on feeding, while a parallel system formed by the CeA, vmPFC and LHA is well positioned to support fear cue inhibitory effects on feeding. Future work should help in determining how these two systems might interact in the control of food intake and specific neurochemical mediators recruited to control a "fear-or-feeding" switch.
Concluding Remarks
This research symposium, and each of the contributing authors' research programs, shines a spotlight on the complex physiological and behavioral interactions that link stress, food intake and emotion. Each researcher has his or her own distinct perspective, working hypotheses, experimental models, and scientific schema that drive their interest in these issues, but their cumulative efforts promote an increased appreciation and understanding of the myriad biological problems to be addressed.
Effects of palatable food intake on stress responses
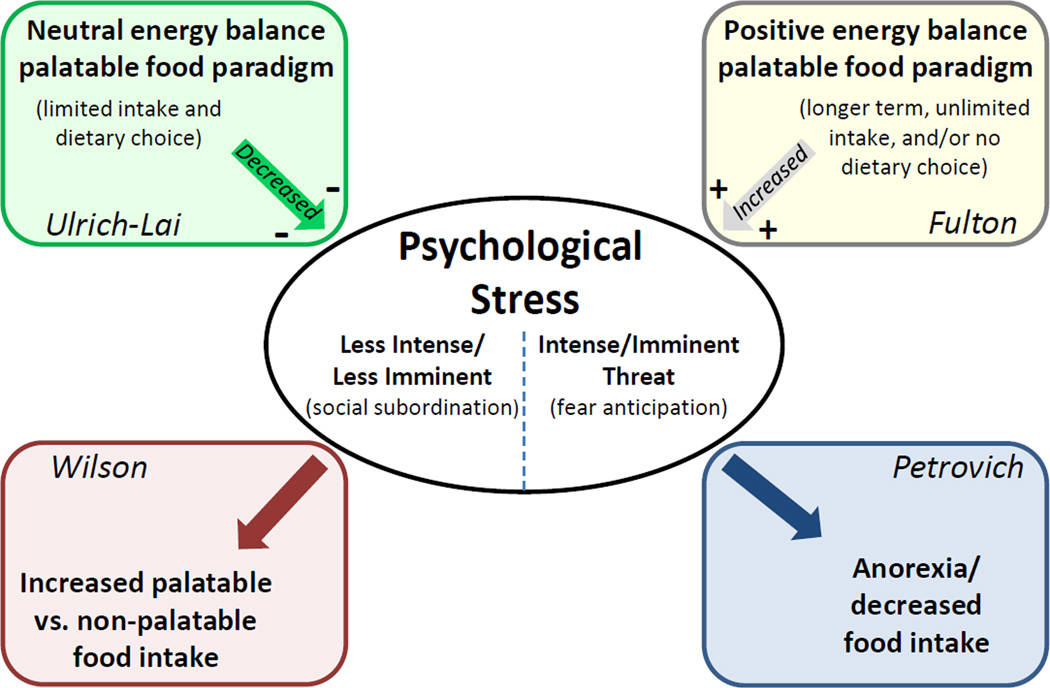
As summarized in Figure 10, Dr. Ulrich-Lai has applied a rat model incorporating stable energy balance, access to limited amounts of palatable (sucrose) diet, and dietary choice to demonstrate improved stress coping via "comfort food" exposure. Conversely, Dr. Fulton's research uses mice that have become overweight and obese after unlimited, long term access to a diet high in fat/sugar, without dietary choice, which promotes increased physiological and behavioral stress responsiveness. Collectively, these and other research findings indicate that highly palatable food intake can have opposing effects on stress-regulatory systems, with effect direction dependent upon several factors. First, consistent with the work of Dr. Susanne la Fleur’s group (la Fleur et al., 2005), dietary choice may promote reduced stress responses, whereas lack of dietary choice may promote increased stress responses. However, Dr. Wilson’s approach also provides a dietary choice to female rhesus monkeys and the results suggest a facilitation of the stress response under these dietary conditions. Second, the stress-regulatory effects of palatable foods may vary with macronutrient composition. For instance, Dr. Fulton’s work indicates that some dietary fats can foster increased stress responses (see previous section), whereas dietary carbohydrates may preferentially curb stress responses (Anderson et al., 1987, Barr et al., 1999, de Castro, 1987, Deuster et al., 1992, Markus et al., 2000, Utter et al., 1999). Indeed, the high caloric diet used in the studies of rhesus monkeys by Dr. Wilson derived 45% of its calories from saturated fats. Lastly, weight gain and associated metabolic dysfunction secondary to palatable food intake, as occurs following long-term and/or unlimited access to palatable food, may enhance stress responding. Consistent with this inference, increased adiposity is often associated with increased HPA axis tone (Francis et al., 2013, Lawlor et al., 2011, Lu et al., 2014, Ryan et al., 2014). Notably, data from Dr. Ulrich-Lai’s limited sucrose paradigm suggests that it may be possible to prevent these stress-promoting effects by limiting the amount and/or duration of palatable food intake.
Figure 10.
Summary schematic illustrating the complex inter-relationships between stress and ingestive behavior, and highlighting the overall approach and key findings of each symposium speaker.
Effects of stress on food intake
Dr. Wilson has used a non-human primate model (female rhesus monkeys) to examine the role of social stress on dietary choice and intake, and the resulting physiological impact of stress-induced food intake. His group has shown that social subordinates eat more when offered unlimited access to a rich dietary environment (choice between standard chow and a high fat-high sugar diet) than social dominants. In contrast, Dr. Petrovich has used a rat model to examine the neurobiological mechanism by which a more intense and imminent psychological stress, i.e., conditioned fear, promotes hypophagia and anorexia. She has shown that the CeA, likely working with the vmPFC and LHA, is essential for reducing food intake during fear anticipation in otherwise hungry rats. Importantly, the divergent consummatory effects that are observed by these two research groups provide critical insight into the consequences of stress on food intake. For example, the influence of stress on ingestive behavior depends in large part on the type and/or chronicity of stress experienced, with acute, intense and imminent threats of pain/injury being linked with reduced food intake (Bellisle et al., 1990, Popper et al., 1989) (see previous section by Dr. Petrovich), while ongoing social stress is associated with increased food intake (Bartolomucci et al., 2009)(see previous section by Dr. Wilson). Stress effects also vary with the type of food offered, such that highly palatable food options tend to produce stress-overeating when compared to low palatability foods (Bertiere et al., 1984, Pecoraro et al., 2004, Willner et al., 1998)(see section by Dr. Wilson).
Future research priorities
This research symposium closed with an extended group discussion between the contributing authors and workshop participants that was moderated by Dr. Rinaman. This lively discussion focused in large part on highlighting future research priorities for the field. More specifically, the group broached several intriguing questions that are ripe for experimental analysis. A few of these questions are posed below.
How, when, and by what central neural mechanism(s) does palatable food intake transition from promoting stress relief to enhancing stress responsiveness? In other words, how does the apparently adaptive or beneficial effect of consuming rewarding foods become maladaptive and deleterious with regards to stress responsiveness? Important considerations include the type and duration of the stressor, the macronutrient quality of the palatable diet, and subject sex.
What is the role or importance of timing (with regards to stress exposure) and context (e.g., safe/familiar environment vs. novel/challenging environment) in mechanisms that underlie stress-induced overeating vs. stress-induced anorexia?
What is the role of sex hormones in mechanisms that underlie stress-induced overeating vs. stress-induced anorexia? Is female vulnerability to stress-related overeating and stress-related anorexia mediated by a common mechanism?
Do "comfort foods" provide transitory stress relief by blunting activation of the HPA axis and other stress responses, by stimulating dopamine reward or other systems that counteract or compete with stress, or both? Attention needs to be focused on peripheral signals (e.g., glucocorticoids and inflammatory cytokines) and central neuropeptides (e.g., CRF) as mediators for these effects.
Is diet important for the bi-directionality of stress on food intake? For example, can prolonged exposure to stress suppress appetite and food intake even when palatable foods are available? Does dietary choice matter and, if so, how does stress-induced disruption of circuits involving prefrontal regions predict diet choice and consumption.
Is diet-induced obesity linked to a particularly vulnerable stress-responsive phenotype? Are the deleterious effects of stress amplified in individuals who are prone to dietary obesity? Is obesity resistance associated with stress resilience?
As these and other fascinating questions are addressed by ongoing and future work, including thoughtful consideration and integration of data across species and diverse experimental paradigms, the field will move towards an improved understanding of the functional associations and causal links among stress, food intake, and emotion.
Acknowledgments
The collaborative efforts of Drs. Zach Johnson, Mar Sanchez, Donna Toufexis, and Vasiliki Michopoulos were critical for Dr. Wilson’s studies.
Yvonne M. Ulrich-Lai was supported by NIH grants R01 DK091425 (YMU), R03 DK089018 (YMU) and K01 DK078906 (YMU). Stephanie Fulton was supported by a CIHR grant (MOP-123280) and New Investigator salary award. Mark Wilson was supported by NIH grants MH081816 (DT), MH79100 (MW), DK096983 (MW), and ODP51011132. Gorica Petrovich was supported by NIH grant DK085721, and Linda Rinaman was supported by NIH grants DK100685 and MH059911.
Footnotes
In this Section, ‘anorexia’ refers to inhibition of food consumption in general, while ‘anorexia nervosa’ refers to an eating disorder.
Declaration of Interest Statement
The authors report no conflicts of interest.
References Cited
- Adam TC, Epel ES. Stress, eating and the reward system. Physiol Behav. 2007;91:449–458. doi: 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Ambroggi F, Turiault M, Milet A, Deroche-Gamonet V, Parnaudeau S, Balado E, Barik J, van der Veen R, Maroteaux G, Lemberger T, Schutz G, Lazar M, Marinelli M, Piazza PV, Tronche F. Stress and addiction: glucocorticoid receptor in dopaminoceptive neurons facilitates cocaine seeking. Nat Neurosci. 2009;12:247–249. doi: 10.1038/nn.2282. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Press; 2013. [Google Scholar]
- Anderson KE, Rosner W, Khan MS, New MI, Pang SY, Wissel PS, Kappas A. Diet-hormone interactions: protein/carbohydrate ratio alters reciprocally the plasma levels of testosterone and cortisol and their respective binding globulins in man. Life Sci. 1987;40:1761–1768. doi: 10.1016/0024-3205(87)90086-5. [DOI] [PubMed] [Google Scholar]
- Anderson RJ, Freedland KE, Clouse RE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care. 2001;24:1069–1078. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- Anisman H, Matheson K. Stress, depression, and anhedonia: caveats concerning animal models. Neurosci Biobehav Rev. 2005;29:525–546. doi: 10.1016/j.neubiorev.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Arce M, Michopoulos V, Shepard KN, Ha QC, Wilson ME. Diet choice, cortisol reactivity, and emotional feeding in socially housed rhesus monkeys. Physiol Behav. 2010;101:446–455. doi: 10.1016/j.physbeh.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer ZA, Mercer JG. Brain responses to obesogenic diets and diet-induced obesity. Proc Nutr Soc. 2007;66:124–130. doi: 10.1017/S0029665107005356. [DOI] [PubMed] [Google Scholar]
- Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev. 2008;32:20–39. doi: 10.1016/j.neubiorev.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard ND, Bunner AE, Agarwal U. Saturated and trans fats and dementia: a systematic review. Neurobiol Aging 35 Suppl. 2014;2:S65–S73. doi: 10.1016/j.neurobiolaging.2014.02.030. [DOI] [PubMed] [Google Scholar]
- Barr RG, Pantel MS, Young SN, Wright JH, Hendricks LA, Gravel R. The response of crying newborns to sucrose: is it a "sweetness" effect? Physiol Behav. 1999;66:409–417. doi: 10.1016/s0031-9384(98)00294-7. [DOI] [PubMed] [Google Scholar]
- Bartolomucci A, Cabassi A, Govoni P, Ceresini G, Cero C, Berra D, Dadomo H, Franceschini P, Dell'Omo G, Parmigiani S, Palanza P. Metabolic consequences and vulnerability to diet-induced obesity in male mice under chronic social stress. PLoS One. 2009;4:e4331. doi: 10.1371/journal.pone.0004331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassareo V, Di Chiara G. Differential responsiveness of dopamine transmission to food-stimuli in nucleus accumbens shell/core compartments. Neuroscience. 1999;89:637–641. doi: 10.1016/s0306-4522(98)00583-1. [DOI] [PubMed] [Google Scholar]
- Bell ME, Bhargava A, Soriano L, Laugero K, Akana SF, Dallman MF. Sucrose intake and corticosterone interact with cold to modulate ingestive behaviour, energy balance, autonomic outflow and neuroendocrine responses during chronic stress. J Neuroendocrinol. 2002;14:330–342. doi: 10.1046/j.1365-2826.2002.00784.x. [DOI] [PubMed] [Google Scholar]
- Bellisle F, Louis-Sylvestre J, Linet N, Rocaboy B, Dalle B, Cheneau F, L'Hinoret D, Guyot L. Anxiety and food intake in men. Psychosom Med. 1990;52:452–457. doi: 10.1097/00006842-199007000-00007. [DOI] [PubMed] [Google Scholar]
- Berthoud H-R, Münzberg H. The lateral hypothalamus as integrator of metabolic and environmental needs: from electrical self-stimulation to opto-genetics. Physiol Behav. 2011;104:29–39. doi: 10.1016/j.physbeh.2011.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud HR. Interactions between the “cognitive” and “metabolic” brain in the control of food intake. Physiol Behav. 2007;91:486–498. doi: 10.1016/j.physbeh.2006.12.016. [DOI] [PubMed] [Google Scholar]
- Bertiere MC, Sy TM, Baigts F, Mandenoff A, Apfelbaum M. Stress and sucrose hyperphagia: role of endogenous opiates. Pharmacol Biochem Behav. 1984;20:675–679. doi: 10.1016/0091-3057(84)90183-7. [DOI] [PubMed] [Google Scholar]
- Bewernick BH, Kayser S, Sturm V, Schlaepfer TE. Long-term effects of nucleus accumbens deep brain stimulation in treatment-resistant depression: evidence for sustained efficacy. Neuropsychopharmacology. 2012;37:1975–1985. doi: 10.1038/npp.2012.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar S, Vining C, Denski K. Regulation of chronic stress-induced changes in hypothalamic-pituitary-adrenal activity by the basolateral amygdala. Ann N Y Acad Sci. 2004;1032:315–319. doi: 10.1196/annals.1314.050. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Crouching as an index of fear. J Comp Physiol Psychol. 1969;67:370–375. doi: 10.1037/h0026779. [DOI] [PubMed] [Google Scholar]
- Blum K, Sheridan PJ, Wood RC, Braverman ER, Chen TJ, Cull JG, Comings DE. The D2 dopamine receptor gene as a determinant of reward deficiency syndrome. J R Soc Med. 1996;89:396–400. doi: 10.1177/014107689608900711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobzean SA, DeNobrega AK, Perrotti LI. Sex differences in the neurobiology of drug addiction. Exp Neurol. 2014;259:64–74. doi: 10.1016/j.expneurol.2014.01.022. [DOI] [PubMed] [Google Scholar]
- Burke AR, Miczek KA. Stress in adolescence and drugs of abuse in rodent models: Role of dopamine, CRF, and HPA axis. Psychopharmacology (Berl) 2013 doi: 10.1007/s00213-013-3369-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon WB. Bodily changes in pain, hunger, fear and rage. New York: Appleton; 1915. [Google Scholar]
- Carter ME, Soden ME, Zweifel LS, Palmiter RD. Genetic identification of a neural circuit that suppresses appetite. Nature. 2013;503:111–114. doi: 10.1038/nature12596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen AM, Dekloet AD, Ulrich-Lai YM, Herman JP. "Snacking" causes long term attenuation of HPA axis stress responses and enhancement of brain FosB/deltaFosB expression in rats. Physiol Behav. 2011;103:111–116. doi: 10.1016/j.physbeh.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S, Powell DJ, Petrovich GD. Differential recruitment of distinct amygdalar nuclei across appetitive associative learning. Learn Mem. 2013;20:295–299. doi: 10.1101/lm.031070.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier G, Novell K. Saccharin as a sugar surrogate. J Comp Physiol Psychol. 1967;64:401–408. [PubMed] [Google Scholar]
- Coover G, Ursin H, Levine S. Corticosterone and avoidance in rats with basolateral amygdala lesions. J Comp Physiol Psychol. 1973;85:111–122. doi: 10.1037/h0034858. [DOI] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Roberto M, Bajo M, Pockros L, Frihauf JB, Fekete EM, Steardo L, Rice KC, Grigoriadis DE, Conti B, Koob GF, Zorrilla EP. CRF system recruitment mediates dark side of compulsive eating. Proc Natl Acad Sci U S A. 2009a;106:20016–20020. doi: 10.1073/pnas.0908789106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Steardo L, Zorrilla EP. Consummatory, anxiety-related and metabolic adaptations in female rats with alternating access to preferred food. Psychoneuroendocrinology. 2009b;34:38–49. doi: 10.1016/j.psyneuen.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman MF, Akana SF, Pecoraro NC, Warne JP, la Fleur SE, Foster MT. Glucocorticoids, the etiology of obesity and the metabolic syndrome. Curr Alzheimer Res. 2007;4:199–204. doi: 10.2174/156720507780362236. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Pecoraro N, Akana SF, Fleur SEl, Gomez F, Houshyar H, Bell ME, Bhatnagar S, Laugero KD, Manalo S. Chronic stress and obesity: A new view of "comfort food". Proc Natl Acad Sci U S A. 2003;100:11696–11701. doi: 10.1073/pnas.1934666100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman MF, Pecoraro NC, la Fleur SE. Chronic stress and comfort foods: self-medication and abdominal obesity. Brain Behav Immun. 2005;19:275–280. doi: 10.1016/j.bbi.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Davis JF, Tracy AL, Schurdak JD, Tschop MH, Lipton JW, Clegg DJ, Benoit SC. Exposure to elevated levels of dietary fat attenuates psychostimulant reward and mesolimbic dopamine turnover in the rat. Behav Neurosci. 2008;122:1257–1263. doi: 10.1037/a0013111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Castro JM. Macronutrient relationships with meal patterns and mood in the spontaneous feeding behavior of humans. Physiol Behav. 1987;39:561–569. doi: 10.1016/0031-9384(87)90154-5. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza CT, Araujo EP, Bordin S, Ashimine R, Zollner RL, Boschero AC, Saad MJ, Velloso LA. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology. 2005;146:4192–4199. doi: 10.1210/en.2004-1520. [DOI] [PubMed] [Google Scholar]
- Deuster P, Singh A, Hofmann A, Moses F, Chrousos G. Hormonal responses to ingesting water or a carbohydrate beverage during a 2 h run. Med Sci Sports Exerc. 1992;24:72–79. [PubMed] [Google Scholar]
- Dube L, LeBel JL, Lu J. Affect asymmetry and comfort food consumption. Physiol Behav. 2005;86:559–567. doi: 10.1016/j.physbeh.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Elias CF, Saper CB. From lesions to leptin: Hypothalamic control of food intake and body weight. Neuron. 1999;22:221–232. doi: 10.1016/s0896-6273(00)81084-3. [DOI] [PubMed] [Google Scholar]
- Epel E, Jimenez S, Brownell K, Stroud L, Stoney C, Niaura R. Are stress eaters at risk for the metabolic syndrome? Ann N Y Acad Sci. 2004;1032:208–210. doi: 10.1196/annals.1314.022. [DOI] [PubMed] [Google Scholar]
- Epel E, Lapidus R, McEwen B, Brownell K. Stress may add bite to appetite in women: a laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology. 2001;26:37–49. doi: 10.1016/s0306-4530(00)00035-4. [DOI] [PubMed] [Google Scholar]
- Epel ES, McEwen B, Seeman T, Matthews K, Castellazzo G, Brownell KD, Bell J, Ickovics JR. Stress and body shape: stress-induced cortisol secretion is consistently greater among women with central fat. Psychosom Med. 2000;62:623–632. doi: 10.1097/00006842-200009000-00005. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. What is conditioned fear? Trends Neurosci. 1984;7:460–462. [Google Scholar]
- Feldman S, Siegel RA, Conforti N. Differential effects of medial forebrain bundle lesions on adrenocortical responses following limbic stimulation. Neuroscience. 1983;9:157–163. doi: 10.1016/0306-4522(83)90053-2. [DOI] [PubMed] [Google Scholar]
- Fendt M, Fanselow MS. The neuroanatomical and neurochemical basis of conditioned fear. Neurosci Biobehav Rev. 1999;23:743–760. doi: 10.1016/s0149-7634(99)00016-0. [DOI] [PubMed] [Google Scholar]
- Fernandez M, Blass EM, Hernandez-Reif M, Field T, Diego M, Sanders C. Sucrose attenuates a negative electroencephalographic response to an aversive stimulus for newborns. J Dev Behav Pediatr. 2003;24:261–266. doi: 10.1097/00004703-200308000-00007. [DOI] [PubMed] [Google Scholar]
- Finger BC, Dinan TG, Cryan JF. High-fat diet selectively protects against the effects of chronic social stress in the mouse. Neuroscience. 2011;192:351–360. doi: 10.1016/j.neuroscience.2011.06.072. [DOI] [PubMed] [Google Scholar]
- Finger BC, Dinan TG, Cryan JF. The temporal impact of chronic intermittent psychosocial stress on high-fat diet-induced alterations in body weight. Psychoneuroendocrinology. 2012;37:729–741. doi: 10.1016/j.psyneuen.2011.06.015. [DOI] [PubMed] [Google Scholar]
- Fontanini A, Grossman SE, Figueroa JA, Katz DB. Distinct subtypes of basolateral amygdala taste neurons reflect palatability and reward. J Neurosci. 2009;29:2486–2495. doi: 10.1523/JNEUROSCI.3898-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster MT, Warne JP, Ginsberg AB, Horneman HF, Pecoraro NC, Akana SF, Dallman MF. Palatable foods, stress, and energy stores sculpt corticotropin-releasing factor, adrenocorticotropin, and corticosterone concentrations after restraint. Endocrinology. 2009;150:2325–2333. doi: 10.1210/en.2008-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis LA, Granger DA, Susman EJ. Adrenocortical regulation, eating in the absence of hunger and BMI in young children. Appetite. 2013;64:32–38. doi: 10.1016/j.appet.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francolin-Silva AL, da Silva Hernandes A, Fukuda MT, Valadares CT, Almeida SS. Anxiolytic-like effects of short-term postnatal protein malnutrition in the elevated plus-maze test. Behav Brain Res. 2006;173:310–314. doi: 10.1016/j.bbr.2006.06.042. [DOI] [PubMed] [Google Scholar]
- Fulton S. Appetite and reward. Front Neuroendocrinol. 2010;31:85–103. doi: 10.1016/j.yfrne.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Gibson EL. Emotional influences on food choice: Sensory, physiological and psychological pathways. Physiol Behav. 2006;89:53–61. doi: 10.1016/j.physbeh.2006.01.024. [DOI] [PubMed] [Google Scholar]
- Giordano GD, Renzetti P, Parodi RC, Foppiani L, Zandrino F, Giordano G, Sardanelli F. Volume measurement with magnetic resonance imaging of hippocampus-amygdala formation in patients with anorexia nervosa. J Endocrinol Invest. 2001;24:510–514. doi: 10.1007/BF03343884. [DOI] [PubMed] [Google Scholar]
- Glowa JR, Gold PW. Corticotropin releasing hormone produces profound anorexigenic effects in the rhesus monkey. Neuropeptides. 1991;18:55–61. doi: 10.1016/0143-4179(91)90164-e. [DOI] [PubMed] [Google Scholar]
- Godfrey J, Kelly, Clare, Zhang, Xiaodong, Castellanos, Xavier, Wilson, Mark, Sanchez MM. Cingulo-Opercular and Limbic Intrinsic Functional Connectivity is Affected by Delayed Puberty and Social Status in Female Rhesus Macaques. Annual Meeting of the Society of Biological Psychiatry (SOBP); May, 2013; San Francisco, CA. 2013. [Google Scholar]
- Goeders NE. Stress and cocaine addiction. J Pharmacol Exp Ther. 2002;301:785–789. doi: 10.1124/jpet.301.3.785. [DOI] [PubMed] [Google Scholar]
- Goldstein LE, Rasmusson AM, Bunney BS, Roth RH. Role of the amygdala in the coordination of behavioral, neuroendocrine, and prefrontal cortical monoamine responses to psychological stress in the rat. J Neurosci. 1996;16:4787–4798. doi: 10.1523/JNEUROSCI.16-15-04787.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindan RM, Behen ME, Helder E, Makki MI, Chugani HT. Altered water diffusivity in cortical association tracts in children with early deprivation identified with Tract-Based Spatial Statistics (TBSS) Cereb Cortex. 2010;20:561–569. doi: 10.1093/cercor/bhp122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KA, Shively CA, Nader MA, Ehrenkaufer RL, Line SW, Morton TE, Gage HD, Mach RH. Effect of social status on striatal dopamine D2 receptor binding characteristics in cynomolgus monkeys assessed with positron emission tomography. Synapse. 1998;29:80–83. doi: 10.1002/(SICI)1098-2396(199805)29:1<80::AID-SYN7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Groesz LM, McCoy S, Carl J, Saslow L, Stewart J, Adler N, Laraia B, Epel E. What is eating you? Stress and the drive to eat. Appetite. 2012;58:717–721. doi: 10.1016/j.appet.2011.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunberg NE, Straub RO. The role of gender and taste class in the effects of stress on eating. Health Psychol. 1992;11:97–100. doi: 10.1037//0278-6133.11.2.97. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn JD, Swanson LW. Distinct patterns of neuronal inputs and outputs of the juxtaparaventricular and suprafornical regions of the lateral hypothalamic area in the male rat. Brain Res Rev. 2010;64:14–103. doi: 10.1016/j.brainresrev.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa RJ, Burgess LH, Kerr JE, O'Keefe JA. Gonadal steroid hormone receptors and sex differences in the hypothalamo-pituitary-adrenal axis. Horm Behav. 1994;28:464–476. doi: 10.1006/hbeh.1994.1044. [DOI] [PubMed] [Google Scholar]
- Hariri N, Thibault L. High-fat diet-induced obesity in animal models. Nutr Res Rev. 2010;23:270–299. doi: 10.1017/S0954422410000168. [DOI] [PubMed] [Google Scholar]
- Harris RB, Zhou J, Youngblood BD, Rybkin II, Smagin GN, Ryan DH. Effect of repeated stress on body weight and body composition of rats fed low- and high-fat diets. Am J Physiol. 1998;275:R1928–R1938. doi: 10.1152/ajpregu.1998.275.6.R1928. [DOI] [PubMed] [Google Scholar]
- Herod SM, Dettmer AM, Novak MA, Meyer JS, Cameron JL. Sensitivity to stress-induced reproductive dysfunction is associated with a selective but not a generalized increase in activity of the adrenal axis. Am J Physiol Endocrinol Metab. 2011a;300:E28–E36. doi: 10.1152/ajpendo.00223.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herod SM, Pohl CR, Cameron JL. Treatment with a CRH-R1 antagonist prevents stress-induced suppression of the central neural drive to the reproductive axis in female macaques. Am J Physiol Endocrinol Metab. 2011b;300:E19–E27. doi: 10.1152/ajpendo.00224.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta M, Shibasaki T, Arai K, Demura H. Corticotropin-releasing factor receptor type 1 mediates emotional stress-induced inhibition of food intake and behavioral changes in rats. Brain Res. 1999;823:221–225. doi: 10.1016/s0006-8993(99)01177-4. [DOI] [PubMed] [Google Scholar]
- Hryhorczuk C, Sharma S, Fulton SE. Metabolic disturbances connecting obesity and depression. Front Neurosci. 2013;7:177. doi: 10.3389/fnins.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Martin KC, Kandel ER. Both protein kinase A and mitogen-activated protein kinase are required in the amygdala for the macromolecular synthesis-dependent late phase of long-term potentiation. J Neurosci. 2000;20:6317–6325. doi: 10.1523/JNEUROSCI.20-17-06317.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iemolo A, Blasio A, St Cyr SA, Jiang F, Rice KC, Sabino V, Cottone P. CRF-CRF Receptor System in the Central and Basolateral Nuclei of the Amygdala Differentially Mediates Excessive Eating of Palatable Food. Neuropsychopharmacology. 2013;38:2456–2466. doi: 10.1038/npp.2013.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzo E, Sanna PP, Koob GF. Impairment of dopaminergic system function after chronic treatment with corticotropin-releasing factor. Pharmacol Biochem Behav. 2005;81:701–708. doi: 10.1016/j.pbb.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Jimerson DC, Wolfe BE. Psychobiology of eating disorders. In: Wonderlich A, Mitchell JE, de Zwaan M, Steiger H, editors. Annual Review of Eating Disorders part 2–2006. Oxford: Radcliffe Publishing; 2006. pp. 1–15. [Google Scholar]
- Kamara K, Eskay R, Castonguay T. High-fat diets and stress responsivity. Physiol Behav. 1998;64:1–6. doi: 10.1016/s0031-9384(97)00534-9. [DOI] [PubMed] [Google Scholar]
- Kanoski SE, Davidson TL. Western diet consumption and cognitive impairment: links to hippocampal dysfunction and obesity. Physiol Behav. 2011;103:59–68. doi: 10.1016/j.physbeh.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan J, Chen H, Appt SE, Lees CJ, Franke AA, Berga SL, Wilson M, Manuck SB, Clarkson TB. Impairment of ovarian function and associated health-related abnormalities are attributable to low social status in premenopausal monkeys and not mitigated by a high-isoflavone soy diet. Hum Reprod. 2010;25:3083–3094. doi: 10.1093/humrep/deq288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Charney D. Effects of early stress on brain structure and function: implications for understanding the relationship between child maltreatment and depression. Dev Psychopathol. 2001;13:451–471. doi: 10.1017/s0954579401003030. [DOI] [PubMed] [Google Scholar]
- Kaye W. Neurobiology of anorexia and bulimia nervosa. Physiol Behav. 2008;94:121–135. doi: 10.1016/j.physbeh.2007.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Yang HY, Kim AJ, Lim Y. Academic stress levels were positively associated with sweet food consumption among Korean high-school students. Nutrition. 2013;29:213–218. doi: 10.1016/j.nut.2012.08.005. [DOI] [PubMed] [Google Scholar]
- Kitraki E, Soulis G, Gerozissis K. Impaired neuroendocrine response to stress following a short-term fat-enriched diet. Neuroendocrinology. 2004;79:338–345. doi: 10.1159/000079665. [DOI] [PubMed] [Google Scholar]
- Klein DA, Walsh BT. Eating disorders: clinical features and pathophysiology. Physiol Behav. 2004;81:359–374. doi: 10.1016/j.physbeh.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–1159. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Buck CL, Cohen A, Edwards S, Park PE, Schlosburg JE, Schmeichel B, Vendruscolo LF, Wade CL, Whitfield TW, Jr, George O. Addiction as a stress surfeit disorder. Neuropharmacology. 2014;76 Pt B:370–382. doi: 10.1016/j.neuropharm.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- la Fleur SE, Houshyar H, Roy M, Dallman MF. Choice of lard, but not total lard calories, damps adrenocorticotropin responses to restraint. Endocrinology. 2005;146:2193–2199. doi: 10.1210/en.2004-1603. [DOI] [PubMed] [Google Scholar]
- la Fleur SE, Luijendijk MC, van der Zwaal EM, Brans MA, Adan RA. The snacking rat as model of human obesity: effects of a free-choice high-fat high-sugar diet on meal patterns. Int J Obes (Lond) 2014;38:643–649. doi: 10.1038/ijo.2013.159. [DOI] [PubMed] [Google Scholar]
- Land BB, Narayanan NS, Liu RJ, Gianessi CA, Brayton CE, Grimaldi DM, Sarhan M, Guarnieri DJ, Deisseroth K, Aghajanian GK, DiLeone RJ. Medial prefrontal D1 dopamine neurons control food intake. Nat Neurosci. 2014;17:248–253. doi: 10.1038/nn.3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laugero KD, Falcon LM, Tucker KL. Relationship between perceived stress and dietary and activity patterns in older adults participating in the Boston Puerto Rican Health Study. Appetite. 2011;56:194–204. doi: 10.1016/j.appet.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor DA, Harbord RM, Tybjaerg-Hansen A, Palmer TM, Zacho J, Benn M, Timpson NJ, Smith GD, Nordestgaard BG. Using genetic loci to understand the relationship between adiposity and psychological distress: a Mendelian Randomization study in the Copenhagen General Population Study of 53,221 adults. J Intern Med. 2011;269:525–537. doi: 10.1111/j.1365-2796.2011.02343.x. [DOI] [PubMed] [Google Scholar]
- LeDoux J. Rethinking the emotional brain. Neuron. 2012;73:653–676. doi: 10.1016/j.neuron.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Levin BE. Metabolic imprinting on genetically predisposed neural circuits perpetuates obesity. Nutrition. 2000;16:909–915. doi: 10.1016/s0899-9007(00)00408-1. [DOI] [PubMed] [Google Scholar]
- Lieberman HR, Wurtman JJ, Chew B. Changes in mood after carbohydrate consumption among obese individuals. Am J Clin Nutr. 1986;44:772–778. doi: 10.1093/ajcn/44.6.772. [DOI] [PubMed] [Google Scholar]
- Lin PY, Su KP. A meta-analytic review of double-blind, placebo-controlled trials of antidepressant efficacy of omega-3 fatty acids. J Clin Psychiatry. 2007;68:1056–1061. doi: 10.4088/jcp.v68n0712. [DOI] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- Lu Q, Tao F, Hou F, Zhang Z, Sun Y, Xu Y, Xu S, Zhao Y. Cortisol reactivity, delay discounting and percent body fat in Chinese urban young adolescents. Appetite. 2014;72:13–20. doi: 10.1016/j.appet.2013.09.019. [DOI] [PubMed] [Google Scholar]
- Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BW, Zitman FG. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry. 2010;67:220–229. doi: 10.1001/archgenpsychiatry.2010.2. [DOI] [PubMed] [Google Scholar]
- Macht M. How emotions affect eating: A five-way model. Appetite. 2008;50:1–11. doi: 10.1016/j.appet.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Maniam J, Morris MJ. Palatable cafeteria diet ameliorates anxiety and depression-like symptoms following an adverse early environment. Psychoneuroendocrinology. 2010;35:717–728. doi: 10.1016/j.psyneuen.2009.10.013. [DOI] [PubMed] [Google Scholar]
- Maniam J, Morris MJ. The link between stress and feeding behaviour. Neuropharmacology. 2012;63:97–110. doi: 10.1016/j.neuropharm.2012.04.017. [DOI] [PubMed] [Google Scholar]
- Maniscalco JW, Kreisler AD, Rinaman L. Satiation and stress-induced hypophagia: examining the role of hindbrain neurons expressing prolactin-releasing peptide or glucagon-like peptide 1. Front Neurosci. 2013;6:1–17. doi: 10.3389/fnins.2012.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Markus R, Panhuysen G, Tuiten A, Koppeschaar H. Effects of food on cortisol and mood in vulnerable subjects under controllable and uncontrollable stress. Physiol Behav. 2000;70:333–342. doi: 10.1016/s0031-9384(00)00265-1. [DOI] [PubMed] [Google Scholar]
- Martel P, Fantino M. Influence of the amount of food ingested on mesolimbic dopaminergic system activity: a microdialysis study. Pharmacol Biochem Behav. 1996a;55:297–302. doi: 10.1016/s0091-3057(96)00087-1. [DOI] [PubMed] [Google Scholar]
- Martel P, Fantino M. Mesolimbic dopaminergic system activity as a function of food reward: a microdialysis study. Pharmacol Biochem Behav. 1996b;53:221–226. doi: 10.1016/0091-3057(95)00187-5. [DOI] [PubMed] [Google Scholar]
- Martinowich K, Manji H, Lu B. New insights into BDNF function in depression and anxiety. Nat Neurosci. 2007;10:1089–1093. doi: 10.1038/nn1971. [DOI] [PubMed] [Google Scholar]
- McCann BS, Warnick GR, Knopp RH. Changes in plasma lipids and dietary intake accompanying shifts in perceived workload and stress. Psychosom Med. 1990;52:97–108. doi: 10.1097/00006842-199001000-00008. [DOI] [PubMed] [Google Scholar]
- Mena JD, Sadeghian K, Baldo BA. Induction of hyperphagia and carbohydrate intake by µ-opioid receptor stimulation in circumscribed region of frontal cortex. J Neurosci. 2011;31:3249–3260. doi: 10.1523/JNEUROSCI.2050-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mena JD, Selleck RA, Baldo BA. Mu-opioid stimulation in rat prefrontal cortex engages hypothalamic orexin/hypocretin-containing neurons, and reveals dissociable roles of nucleus accumbens and hypothalamus in cortically driven feeding. J Neurosci. 2013;33:18540–18552. doi: 10.1523/JNEUROSCI.3323-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meye FJ, Adan RA. Feelings about food: the ventral tegmental area in food reward and emotional eating. Trends Pharmacol Sci. 2014;35:31–40. doi: 10.1016/j.tips.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Micha R, Mozaffarian D. Saturated fat and cardiometabolic risk factors, coronary heart disease, stroke, and diabetes: a fresh look at the evidence. Lipids. 2010;45:893–905. doi: 10.1007/s11745-010-3393-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Higgins M, Toufexis D, Wilson ME. Social subordination produces distinct stress-related phenotypes in female rhesus monkeys. Psychoneuroendocrinology. 2012a;37:1071–1085. doi: 10.1016/j.psyneuen.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Loucks T, Berga SL, Rivier J, Wilson ME. Increased ghrelin sensitivity and calorie consumption in subordinate monkeys is affected by short-term astressin B administration. Endocrine. 2010;38:227–234. doi: 10.1007/s12020-010-9378-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Reding KM, Wilson ME, Toufexis D. Social subordination impairs hypothalamic-pituitary-adrenal function in female rhesus monkeys. Horm Behav. 2012b;62:389–399. doi: 10.1016/j.yhbeh.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Shepard KN, Arce M, Whitley JA, Wilson ME. Food History and social status affect food intake in monkeys. Society for the Study of Ingesive Behavior, Portland, OR. 2009;52:848. [Google Scholar]
- Michopoulos V, Toufexis D, Wilson ME. Social stress interacts with diet history to promote emotional feeding in females. Psychoneuroendocrinology. 2012c;37:1479–1490. doi: 10.1016/j.psyneuen.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michopoulos V, Wilson ME. Body weight decreases induced by estradiol in female rhesus monkeys are dependent upon social status. Physiol Behav. 2011;102:382–388. doi: 10.1016/j.physbeh.2010.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor TR, Saade S. Poststress glucose mitigates behavioral impairment in rats in the "learned helplessness" model of psychopathology. Biol Psychiatry. 1997;42:324–334. doi: 10.1016/S0006-3223(96)00467-2. [DOI] [PubMed] [Google Scholar]
- Moore CJ, Johnson ZP, Toufexis D, Wilson ME. Antagonism of CRF Type 1 receptors attenuates caloric intake of free feeding subordinate female rhesus monkeys in a rich dietary environment. J Neuroendocrinol. 2015;27:33–43. doi: 10.1111/jne.12232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore CJ, Lowe J, Michopoulos V, Ulam P, Toufexis D, Wilson ME, Johnson Z. Small changes in meal patterns lead to significant changes in total caloric intake. Effects of diet and social status on food intake in female rhesus monkeys. Appetite. 2013;62:60–69. doi: 10.1016/j.appet.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moranis A, Delpech JC, De Smedt-Peyrusse V, Aubert A, Guesnet P, Lavialle M, Joffre C, Laye S. Long term adequate n-3 polyunsaturated fatty acid diet protects from depressive-like behavior but not from working memory disruption and brain cytokine expression in aged mice. Brain Behav Immun. 2012;26:721–731. doi: 10.1016/j.bbi.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, Nader SH, Buchheimer N, Ehrenkaufer RL, Nader MA. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci. 2002;5:169–174. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- Muramoto K, Ono T, Nishijo H, Fukuda M. Rat amygdaloid neuron responses during auditory discrimination. Neuroscience. 1993;52:621–636. doi: 10.1016/0306-4522(93)90411-8. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Tsumori T, Yokota S, Oka T, Yasui Y. Amygdaloid axons innervate melanin-concentrating hormone and orexin containing neurons in the mouse lateral hypothalamus. Brain Res. 2009;1278:66–74. doi: 10.1016/j.brainres.2009.04.049. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- O'Doherty JP. Contributions of the ventromedial prefrontal cortex to goal-directed action selection. Ann N Y Acad Sci. 2011;1239:118–129. doi: 10.1111/j.1749-6632.2011.06290.x. [DOI] [PubMed] [Google Scholar]
- Oddy WH, Hickling S, Smith MA, O'Sullivan TA, Robinson M, de Klerk NH, Beilin LJ, Mori TA, Syrette J, Zubrick SR, Silburn SR. Dietary intake of omega-3 fatty acids and risk of depressive symptoms in adolescents. Depress Anxiety. 2011;28:582–588. doi: 10.1002/da.20822. [DOI] [PubMed] [Google Scholar]
- Oliver G, Wardle J. Perceived effects of stress on food choice. Physiol Behav. 1999;66:511–515. doi: 10.1016/s0031-9384(98)00322-9. [DOI] [PubMed] [Google Scholar]
- Packard AE, Ghosal S, Herman JP, Woods SC, Ulrich-Lai YM. Chronic variable stress improves glucose tolerance in rats with sucrose-induced prediabetes. Psychoneuroendocrinology. 2014;47:178–188. doi: 10.1016/j.psyneuen.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchal SK, Brown L. Rodent models for metabolic syndrome research. J Biomed Biotechnol. 2011;2011:351982. doi: 10.1155/2011/351982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasquali R, Ambrosi B, Armanini D, Cavagnini F, Uberti ED, Del Rio G, de Pergola G, Maccario M, Mantero F, Marugo M, Rotella CM, Vettor R. Cortisol and ACTH response to oral dexamethasone in obesity and effects of sex, body fat distribution, and dexamethasone concentrations: a dose-response study. J Clin Endocrinol Metab. 2002;87:166–175. doi: 10.1210/jcem.87.1.8158. [DOI] [PubMed] [Google Scholar]
- Pecoraro N, Reyes F, Gomez F, Bhargava A, Dallman MF. Chronic stress promotes palatable feeding, which reduces signs of stress: feedforward and feedback effects of chronic stress. Endocrinology. 2004;145:3754–3762. doi: 10.1210/en.2004-0305. [DOI] [PubMed] [Google Scholar]
- Peet M, Murphy B, Shay J, Horrobin D. Depletion of omega-3 fatty acid levels in red blood cell membranes of depressive patients. Biol Psychiatry. 1998;43:315–319. doi: 10.1016/s0006-3223(97)00206-0. [DOI] [PubMed] [Google Scholar]
- Pelchat ML. Of human bondage: food craving, obsession, compulsion, and addiction. Physiol Behav. 2002;76:347–352. doi: 10.1016/s0031-9384(02)00757-6. [DOI] [PubMed] [Google Scholar]
- Petrovich GD. Forebrain networks and the control of feeding by environmental learned cues. Physiol Behav. 2013;121:10–18. doi: 10.1016/j.physbeh.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Canteras NS, Swanson LW. Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Res Rev. 2001;38:247–289. doi: 10.1016/s0165-0173(01)00080-7. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Hobin MP, Reppucci CJ. Selective Fos induction in hypothalamic orexin/hypocretin, but not melanin-concentrating hormone neurons, by a learned food-cue that stimulates feeding in sated rats. Neuroscience. 2012;224:70–80. doi: 10.1016/j.neuroscience.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Lougee M. Sex differences in fear cue-induced inhibition of feeding: Prolonged effect in female rats. Physiol Behav. 2011;104:996–1001. doi: 10.1016/j.physbeh.2011.06.020. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Ross CA, Mody P, Holland PC, Gallagher M. Central but not basolateral amygdala is critical for control of feeding by aversive conditioned cues. J Neurosci. 2009;29:15205–15212. doi: 10.1523/JNEUROSCI.3656-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phivilay A, Julien C, Tremblay C, Berthiaume L, Julien P, Giguere Y, Calon F. High dietary consumption of trans fatty acids decreases brain docosahexaenoic acid but does not alter amyloid-beta and tau pathologies in the 3xTg-AD model of Alzheimer's disease. Neuroscience. 2009;159:296–307. doi: 10.1016/j.neuroscience.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Popper R, Smits G, Meiselman HL, Hirsch E. Eating in combat: a survey of U.S. Marines. Mil Med. 1989;154:619–623. [PubMed] [Google Scholar]
- Prasad A, Prasad C. Short-term consumption of a diet rich in fat decreases anxiety response in adult male rats. Physiol Behav. 1996;60:1039–1042. doi: 10.1016/0031-9384(96)00135-7. [DOI] [PubMed] [Google Scholar]
- Rada P, Avena NM, Hoebel BG. Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience. 2005;134:737–744. doi: 10.1016/j.neuroscience.2005.04.043. [DOI] [PubMed] [Google Scholar]
- Reppucci CJ, Kuthyar M, Petrovich GD. Contextual fear cues inhibit eating in food-deprived male and female rats Appetite. 2013;69:186–195. doi: 10.1016/j.appet.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Reppucci CJ, Petrovich GD. Neuroanatomical evidence for a topographically organized forebrain network composed of the amygdala, medial prefrontal cortex, and lateral hypothalamus in rats. Washington, DC: Society for Neuroscience; 2012. Program # 283.05. [Google Scholar]
- Reppucci CJ, Petrovich GD. Suppressed Fos induction within the central nucleus of the amygdala corresponds with inhibited feeding in the presence of a fear-cue in male and female rats. Washington, DC: Society for Neuroscience; 2014. Program # 256.10. [Google Scholar]
- Rodrigues SM, Farb CR, Bauer EP, LeDoux JE, Schafe GE. Pavlovian fear conditioning regulates Thr286 autophosphorylation of Ca2+/calmodulin-dependent protein kinase II at lateral amygdala synapses. J Neurosci. 2004;24:3281–3288. doi: 10.1523/JNEUROSCI.5303-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls BJ, Van Duijvenvoorde PM, Rowe EA. Variety in the diet enhances intake in a meal and contributes to the development of obesity in the rat. Physiol Behav. 1983;31:21–27. doi: 10.1016/0031-9384(83)90091-4. [DOI] [PubMed] [Google Scholar]
- Rosqvist F, Iggman D, Kullberg J, Cedernaes J, Johansson HE, Larsson A, Johansson L, Ahlstrom H, Arner P, Dahlman I, Riserus U. Overfeeding polyunsaturated and saturated fat causes distinct effects on liver and visceral fat accumulation in humans. Diabetes. 2014;63:2356–2368. doi: 10.2337/db13-1622. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat Rev Neurosci. 2013;14:609–625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan KK, Mul JD, Clemmensen C, Egan AE, Begg DP, Halcomb K, Seeley RJ, Herman JP, Ulrich-Lai YM. Loss of melanocortin-4 receptor function attenuates HPA responses to psychological stress. Psychoneuroendocrinology. 2014;42:98–105. doi: 10.1016/j.psyneuen.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richarson JA, Kozlowski GP, Wilson S, Arch JRS, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Hearn EF, Do D, Rilling JK, Herndon JG. Differential rearing affects corpus callosum size and cognitive function of rhesus monkeys. Brain Res. 1998;812:38–49. doi: 10.1016/s0006-8993(98)00857-9. [DOI] [PubMed] [Google Scholar]
- Schachter S, Goldman R, Gordon R. Effects of fear, food deprivation, and obesity on eating. J Pers Soc Psychol. 1968;10:91–97. doi: 10.1037/h0026284. [DOI] [PubMed] [Google Scholar]
- Sclafani A. Oral and postoral determinants of food reward. Physiol Behav. 2004;81:773–779. doi: 10.1016/j.physbeh.2004.04.031. [DOI] [PubMed] [Google Scholar]
- Seeger G, Braus DF, Ruf M, Goldberger U, Schmidt MH. Body image distortion reveals amygdala activation in patients with anorexia nervosa -a functional magnetic resonance imaging study. Neurosci Lett. 2002;326:25–28. doi: 10.1016/s0304-3940(02)00312-9. [DOI] [PubMed] [Google Scholar]
- Seymour B, Dolan R. Emotion, decision making, and the amygdala. Neuron. 2008;58:662–671. doi: 10.1016/j.neuron.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Sharma S, Decarie-Spain L, Hryhorczuk C, Alquier T, Fulton S. A saturated but not a monounsaturated high-fat diet increases depressive-like behavior and neuroinflammation. Keystone Symposia: Neuronal Control of Appetite, Metabolism and Weight. Banff, Alberta. 2013a [Google Scholar]
- Sharma S, Fernandes MF, Fulton S. Adaptations in brain reward circuitry underlie palatable food cravings and anxiety induced by high-fat diet withdrawal. Int J Obes (Lond) 2013b;37:1183–1191. doi: 10.1038/ijo.2012.197. [DOI] [PubMed] [Google Scholar]
- Sharma S, Fulton S. Diet-induced obesity promotes depressive-like behaviour that is associated with neural adaptations in brain reward circuitry. Int J Obes (Lond) 2013;37:382–389. doi: 10.1038/ijo.2012.48. [DOI] [PubMed] [Google Scholar]
- Shide DJ, Blass EM. Opioidlike effects of intraoral infusions of corn oil and polycose on stress reactions in 10-day-old rats. Behav Neurosci. 1989;103:1168–1175. doi: 10.1037//0735-7044.103.6.1168. [DOI] [PubMed] [Google Scholar]
- Shively CA, Grant KA, Ehrenkaufer RL, Mach RH, Nader MA. Social stress, depression, and brain dopamine in female cynomolgus monkeys. Ann N Y Acad Sci. 1997a;807:574–577. doi: 10.1111/j.1749-6632.1997.tb51972.x. [DOI] [PubMed] [Google Scholar]
- Shively CA, Laber-Laird K, Anton RF. Behavior and physiology of social stress and depression in female cynomolgus monkeys. Biol Psychiatry. 1997b;41:871–882. doi: 10.1016/S0006-3223(96)00185-0. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Kogan JH, Frankland PW, Kida S. CREB and memory. Annu Rev Neurosci. 1998;21:127–148. doi: 10.1146/annurev.neuro.21.1.127. [DOI] [PubMed] [Google Scholar]
- Sinha R, Jastreboff AM. Stress as a common risk factor for obesity and addiction. Biol Psychiatry. 2013;73:827–835. doi: 10.1016/j.biopsych.2013.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smagin GN, Howell LA, Redmann S, Jr, Ryan DH, Harris RB. Prevention of stress-induced weight loss by third ventricle CRF receptor antagonist. Am J Physiol. 1999;276:R1461–R1468. doi: 10.1152/ajpregu.1999.276.5.R1461. [DOI] [PubMed] [Google Scholar]
- Smith JC, Sclafani A. Saccharin as a sugar surrogate revisited. Appetite. 2002;38:155–160. doi: 10.1006/appe.2001.0467. [DOI] [PubMed] [Google Scholar]
- Solomon MB, Jankord R, Flak JN, Herman JP. Chronic stress, energy balance and adiposity in female rats. Physiol Behav. 2011;102:84–90. doi: 10.1016/j.physbeh.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sominsky L, Spencer SJ. Eating behavior and stress: a pathway to obesity. Front Psychol. 2014;5:434. doi: 10.3389/fpsyg.2014.00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulis G, Kitraki E, Gerozissis K. Early neuroendocrine alterations in female rats following a diet moderately enriched in fat. Cell Mol Neurobiol. 2005;25:869–880. doi: 10.1007/s10571-005-4943-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulis G, Papalexi E, Kittas C, Kitraki E. Early impact of a fat-enriched diet on behavioral responses of male and female rats. Behav Neurosci. 2007;121:483–490. doi: 10.1037/0735-7044.121.3.483. [DOI] [PubMed] [Google Scholar]
- Strack AM, Akana SF, Horsley CJ, Dallman MF. A hypercaloric load induces thermogenesis but inhibits stress responses in the SNS and HPA system. Am J Physiol. 1997;272:R840–R848. doi: 10.1152/ajpregu.1997.272.3.R840. [DOI] [PubMed] [Google Scholar]
- Su KP, Lai HC, Yang HT, Su WP, Peng CY, Chang JP, Chang HC, Pariante CM. Omega-3 Fatty acids in the prevention of interferon-alpha-induced depression: results from a randomized, controlled trial. Biol Psychiatry. 2014;76:559–566. doi: 10.1016/j.biopsych.2014.01.008. [DOI] [PubMed] [Google Scholar]
- Suchecki D, Antunes J, Tufik S. Palatable solutions during paradoxical sleep deprivation: reduction of hypothalamic-pituitary-adrenal axis activity and lack of effect on energy imbalance. J Neuroendocrinol. 2003;15:815–821. doi: 10.1046/j.1365-2826.2003.01067.x. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Petrovich GD. What is the amygdala? Trends Neurosci. 1998;21:323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- Takano A, Shiga T, Kitagawa N, Koyama T, Katoh C, Tsukamoto E, Tamaki N. Abnormal neuronal network in anorexia nervosa studied with I-123-IMP SPECT. Psychiatry Res. 2001;107:45–50. doi: 10.1016/s0925-4927(01)00093-2. [DOI] [PubMed] [Google Scholar]
- Tamashiro KL, Hegeman MA, Sakai RR. Chronic social stress in a changing dietary environment. Physiol Behav. 2006;89:536–542. doi: 10.1016/j.physbeh.2006.05.026. [DOI] [PubMed] [Google Scholar]
- Tamashiro KL, Nguyen MM, Sakai RR. Social stress: from rodents to primates. Front Neuroendocrinol. 2005;26:27–40. doi: 10.1016/j.yfrne.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Tamashiro KL, Sakai RR, Shively CA, Karatsoreos IN, Reagan LP. Chronic stress, metabolism, and metabolic syndrome. Stress. 2011;14:468–474. doi: 10.3109/10253890.2011.606341. [DOI] [PubMed] [Google Scholar]
- Tannenbaum BM, Brindley DN, Tannenbaum GS, Dallman MF, McArthur MD, Meaney MJ. High-fat feeding alters both basal and stress-induced hypothalamic-pituitary-adrenal activity in the rat. Am J Physiol. 1997;273:E1168–E1177. doi: 10.1152/ajpendo.1997.273.6.E1168. [DOI] [PubMed] [Google Scholar]
- Tchernof A, Despres JP. Pathophysiology of human visceral obesity: an update. Physiol Rev. 2013;93:359–404. doi: 10.1152/physrev.00033.2011. [DOI] [PubMed] [Google Scholar]
- Teegarden SL, Nestler EJ, Bale TL. Delta FosB-mediated alterations in dopamine signaling are normalized by a palatable high-fat diet. Biol Psychiatry. 2008;64:941–950. doi: 10.1016/j.biopsych.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP. Developmental neurobiology of childhood stress and trauma. Psychiatr Clin North Am. 2002;25:397–426. doi: 10.1016/s0193-953x(01)00003-x. [DOI] [PubMed] [Google Scholar]
- Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, Zhao X, Sarruf DA, Izgur V, Maravilla KR, Nguyen HT, Fischer JD, Matsen ME, Wisse BE, Morton GJ, Horvath TL, Baskin DG, Tschop MH, Schwartz MW. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest. 2012;122:153–162. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Volkow ND. Striatocortical pathway dysfunction in addiction and obesity: differences and similarities. Crit Rev Biochem Mol Biol. 2013;48:1–19. doi: 10.3109/10409238.2012.735642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga-Yoshino K, Urakubo T, Okada M, Matsuda H, Ogura A. Repetitive induction of late-phase LTP produces long-lasting synaptic enhancement accompanied by synaptogenesis in cultured hippocampal slices. Hippocampus. 2008;18:281–293. doi: 10.1002/hipo.20391. [DOI] [PubMed] [Google Scholar]
- Toufexis D, Wilson ME. Dihydrotestosterone differentially modulates the cortisol response of the hypothalamic-pituitary axis in male and female rhesus macaques, and restores circadian secretion of cortisol in females. Brain Res. 2012;1429:43–51. doi: 10.1016/j.brainres.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treasure J, Claudino AM, Zucker N. Eating disorders. Lancet. 2010;375:583–593. doi: 10.1016/S0140-6736(09)61748-7. [DOI] [PubMed] [Google Scholar]
- Tryon MS, Carter CS, Decant R, Laugero KD. Chronic stress exposure may affect the brain's response to high calorie food cues and predispose to obesogenic eating habits. Physiol Behav. 2013;120:233–242. doi: 10.1016/j.physbeh.2013.08.010. [DOI] [PubMed] [Google Scholar]
- Tung J, Barreiro LB, Johnson ZP, Hansen KD, Michopoulos V, Toufexis D, Michelini K, Wilson ME, Gilad Y. Social environment is associated with gene regulatory variation in the rhesus macaque immune system. Proc Natl Acad Sci U S A. 2012;109:6490–6495. doi: 10.1073/pnas.1202734109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uher R, Murphy T, Brammer MJ, Dalgleish T, Phillips ML, Ng VW, Andrew CM, Williams SCR, Campbell IC, Treasure J. Medial prefrontal cortex activity associated with symptom provocation in eating disorders. Am J Psychiatry. 2004;161:1238–1246. doi: 10.1176/appi.ajp.161.7.1238. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Christiansen AM, Ostrander MM, Jones AA, Jones KR, Choi DC, Krause EG, Evanson NK, Furay AR, Davis JF, Solomon MB, de Kloet AD, Tamashiro KL, Sakai RR, Seeley RJ, Woods SC, Herman JP. Pleasurable behaviors reduce stress via brain reward pathways. Proc Natl Acad Sci U S A. 2010;107:20529–20534. doi: 10.1073/pnas.1007740107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Ostrander MM, Herman JP. HPA axis dampening by limited sucrose intake: reward frequency vs. caloric consumption. Physiol Behav. 2011;103:104–110. doi: 10.1016/j.physbeh.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Ostrander MM, Thomas IM, Packard BA, Furay AR, Dolgas CM, Van Hooren DC, Figueiredo HF, Mueller NK, Choi DC, Herman JP. Daily limited access to sweetened drink attenuates hypothalamic-pituitary-adrenocortical axis stress responses. Endocrinology. 2007;148:1823–1834. doi: 10.1210/en.2006-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utter AC, Kang J, Nieman DC, Williams F, Robertson RJ, Henson DA, Davis JM, Butterworth DE. Effect of carbohydrate ingestion and hormonal responses on ratings of perceived exertion during prolonged cycling and running. Eur J Appl Physiol Occup Physiol. 1999;80:92–99. doi: 10.1007/s004210050563. [DOI] [PubMed] [Google Scholar]
- Vallés A, Martí O, García A, Armario A. Single exposure to stressors causes long-lasting, stress-dependent reduction of food intake in rats. Am J Physiol Reg Int Comp Physiol. 2000;279:R1138–R1144. doi: 10.1152/ajpregu.2000.279.3.R1138. [DOI] [PubMed] [Google Scholar]
- van Reedt Dortland AK, Giltay EJ, van Veen T, Zitman FG, Penninx BW. Longitudinal relationship of depressive and anxiety symptoms with dyslipidemia and abdominal obesity. Psychosom Med. 2013a;75:83–89. doi: 10.1097/PSY.0b013e318274d30f. [DOI] [PubMed] [Google Scholar]
- van Reedt Dortland AK, Vreeburg SA, Giltay EJ, Licht CM, Vogelzangs N, van Veen T, de Geus EJ, Penninx BW, Zitman FG. The impact of stress systems and lifestyle on dyslipidemia and obesity in anxiety and depression. Psychoneuroendocrinology. 2013b;38:209–218. doi: 10.1016/j.psyneuen.2012.05.017. [DOI] [PubMed] [Google Scholar]
- Verwey M, Khoja Z, Stewart J, Amir S. Differential regulation of the expression of Period2 protein in the limbic forebrain and dorsomedial hypothalamus by daily limited access to highly palatable food in food-deprived and free-fed rats. Neuroscience. 2007;147:277–285. doi: 10.1016/j.neuroscience.2007.04.044. [DOI] [PubMed] [Google Scholar]
- Viau V, Bingham B, Davis J, Lee P, Wong M. Gender and puberty interact on the stress-induced activation of parvocellular neurosecretory neurons and corticotropin-releasing hormone messenger ribonucleic acid expression in the rat. Endocrinology. 2005;146:137–146. doi: 10.1210/en.2004-0846. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ. The addicted human brain: insights from imaging studies. J Clin Invest. 2003;111:1444–1451. doi: 10.1172/JCI18533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci. 2005;8:555–560. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, Netusil N, Fowler JS. Brain dopamine and obesity. Lancet. 2001;357:354–357. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- Warne JP. Shaping the stress response: Interplay of palatable food choices, glucocorticoids, insulin and abdominal obesity. Mol Cell Endocrinol. 2009;300:137–146. doi: 10.1016/j.mce.2008.09.036. [DOI] [PubMed] [Google Scholar]
- Weinstein SE, Shide DJ, Rolls BJ. Changes in food intake in response to stress in men and women: psychological factors. Appetite. 1997;28:7–18. doi: 10.1006/appe.1996.0056. [DOI] [PubMed] [Google Scholar]
- Wiedenmann B, Franke WW. Identification and localization of synaptophysin, an integral membrane glycoprotein of Mr 38,000 characteristic of presynaptic vesicles. Cell. 1985;41:1017–1028. doi: 10.1016/s0092-8674(85)80082-9. [DOI] [PubMed] [Google Scholar]
- Willner P, Benton D, Brown E, Cheeta S, Davies G, Morgan J, Morgan M. "Depression" increases "craving" for sweet rewards in animal and human models of depression and craving. Psychopharmacology (Berl) 1998;136:272–283. doi: 10.1007/s002130050566. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Fisher J, Fischer A, Lee V, Harris RB, Bartness TJ. Quantifying food intake in socially housed monkeys: social status effects on caloric consumption. Physiol Behav. 2008;94:586–594. doi: 10.1016/j.physbeh.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Sako N, Sakai N, Iwafune A. Gustatory and visceral inputs to the amygdala of the rat: conditioned taste aversion and induction of c-fos-like immunoreactivity. Neurosci Lett. 1997;226:127–130. doi: 10.1016/s0304-3940(97)00265-6. [DOI] [PubMed] [Google Scholar]
- Yazbek SN, Spiezio SH, Nadeau JH, Buchner DA. Ancestral paternal genotype controls body weight and food intake for multiple generations. Hum Mol Genet. 2010;19:4134–4144. doi: 10.1093/hmg/ddq332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, McCormack S, España RA, Crocker A, Scammell TE. Afferents to the orexin neurons of the rat brain. J Comp Neurol. 2006;494:845–861. doi: 10.1002/cne.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeni N, Daher C, Fromentin G, Tome D, Darcel N, Chaumontet C. A cafeteria diet modifies the response to chronic variable stress in rats. Stress. 2013;16:211–219. doi: 10.3109/10253890.2012.708952. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Logrip ML, Koob GF. Corticotropin releasing factor: A key role in the neurobiology of addiction. Front Neuroendocrinol. 2014;35:234–244. doi: 10.1016/j.yfrne.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]