Abstract
Mutations in GDP-mannose pyrophosphorylase B (GMPPB), a catalyst for the formation of the sugar donor GDP-mannose, were recently identified as a cause of muscular dystrophy resulting from abnormal glycosylation of α-dystroglycan. In this series, we report nine unrelated individuals with GMPPB-associated dystroglycanopathy. The most mildly affected subject has normal strength at twenty-five years, while three severely affected children presented in infancy with intellectual disability and epilepsy. Muscle biopsies of all subjects are dystrophic with abnormal immunostaining for glycosylated α-dystroglycan. This cohort, together with previously published cases, allows preliminary genotype-phenotype correlations to be made for the emerging GMPPB common variants c.79G>C (p.D27H) and c.860G>A (p.R287Q). We observe that c.79G>C (p.D27H) is associated with a mild limb-girdle muscular dystrophy phenotype, while c.860G>A (p.R287Q) is associated with a relatively severe congenital muscular dystrophy typically involving brain development. Sixty-six percent of GMPPB families to date have one of these common variants.
Keywords: GMPPB, dystroglycanopathy, limb-girdle muscular dystrophy, congenital muscular dystrophy, congenital myasthenic syndrome
Mutations in GMPPB (MIM #615320) encoding GDP-mannose pyrophosphorylase B (GMPPB) have recently been shown to cause muscular dystrophy resulting from hypoglycosylation of α-dystroglycan [Carss et al., 2013]. Related disorders of α-dystroglycan O-mannosylation (referred to as dystroglycanopathies) are known to cause a wide spectrum of clinical disorders ranging from mild limb-girdle muscular dystrophy to severe congenital muscular dystrophy with central nervous system and eye involvement [Godfrey et al., 2007; Bonnemann et al., 2014]. To date there are few genetically verified individuals with GMPPB-associated dystroglycanopathy reported in literature; these individuals have a wide range of phenotypes, similar to the other dystroglycanopathies [Carss et al., 2013; Raphael et al., 2014; Belaya et al., 2015; Cabrera-Serrano et al., 2015]. Most recently, variants in GMPPB were reported to cause congenital myasthenic syndrome [Belaya et al., 2015].
Alpha-dystroglycan is a highly glycosylated protein integral to maintaining structural stability in striated muscle, supporting a variety of functions in myelinating Schwann cells, and anchoring radial glia endfeet during brain development [Moore et al., 2002; Saito et al., 2003]. In all these sites, α-dystroglycan links the extracellular matrix to the cell surface membrane through its interactions with the transmembrane β-dystroglycan and a variety of extracellular matrix proteins [Barresi and Campbell, 2006]. Defects in the glycosylation of α-dystroglycan reduce its binding to laminin and other extracellular ligands, compromising the function of the dystrophin-glycoprotein complex and other proteins associated with α-dystroglycan [Barresi and Campbell, 2006]. There are currently 18 genes known to be involved in the proper glycosylation of α-dystroglycan, with GMPPB being among the most recently identified [Carss et al., 2013]. GMPPB facilitates the formation of the sugar donor GDP-mannose and pyrophosphate from mannose-1-phosphate and GTP, a very early step in the glycosylation pathway. GDP-mannose is used directly for N-glycosylation or converted to dolichol-phosphate mannose (Dol-P-Man) through the Dol-P-Man synthase enzyme complex. Dol-P-Man serves as a reagent for N-glycosylation, O-mannosylation, C-mannosylation, and glycophosphatidylinositol anchor formation [Carss et al., 2013]. While GMPPB functions early in the glycosylation pathway, transferrin isoelectric focusing, a screening test for N-glycosylation defects seen in the congenital disorders of glycosylation, is normal in those GMPPB-associated dystroglycanopathy individuals who have been tested [Carss et al., 2013; Raphael et al., 2014].
Here we describe the clinical, genetic, and pathological findings in nine unrelated individuals with compound heterozygous variants in GMPPB. All unreported individuals known to the authors with GMPPB variants thought to be pathogenic were included in this case series. Clinical data were collected through retrospective chart review by the primary clinician. Diagnostic genetic testing was done through Prevention Genetics (RefSeq NM_013334.2) or the University of Iowa Department of Pathology (P4, RefSeq NM_013334.3), and de-identified reports were reviewed for all cases. Case clinical features are summarized in Table 1. Each were assigned a phenotype according to the system proposed by Godfrey et al [Godfrey et al., 2007], ranging in this series from limb-girdle muscular dystrophy (LGMD) to congenital muscular dystrophy with cerebellar involvement (CMD-CRB). All individuals had elevated maximal serum creatine kinase (CK) levels ranging from 1,331 to 7,250 (CK during episodes of rhabdomyolysis not included) and dystrophic muscle pathology. Compound heterozygous variants or a single heterozygous variant in GMPPB were found in each individual.
Table 1.
Summary of GMPPB case clinical data.
| Case | P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 |
|---|---|---|---|---|---|---|---|---|---|
| Gender | M | M | M | M | F | F | F | F | M |
|
Age at presentation |
17 years | 17 years | 15 years | 23 years | 5 years | 9 months | infancy | 7 months | birth |
| Current age (yr) | 50 | 26 | 32 | 25 | 20 | 5 | 15 | 13 | 3 |
|
GMPPB mutation - allele 1 |
c.79G>C (p.D27H) |
c.79G>C (p.D27H) |
c.79G>C (p.D27H) |
c.79G>C (p.D27H) |
c.721C>T (p.P241S)a |
c.860G>A (p.R287Q) |
c.860G>A (p.R287Q) |
c.860G>A (p.R287Q) |
c.94C>T (p.P32S)a |
|
GMPPB mutation - allele 2 |
c.1069G>A (p.V357I) |
c.1069G>A (p.V357I) |
c.760G>A (p.V254M)a |
c.402+1G>Aa |
c.1034T>C (p.V345A)a |
c.656T>C (p.I219T) |
c.395C>G (p.S132C)a |
c.859C>T (p.R287W)a |
not yet identified |
|
Prenatal findings |
none | none | none | none | none | lost twin to hemorrhage |
none | none | decreased fetal movements; intrauterine growth restriction |
|
Presenting signs and symptoms |
muscle weakness |
exercise induced myalgia, weakness, myoglobinuria |
muscle weakness; waddling gait |
incidental hyperCKemia with routine bloodwork; myoglobinuria |
muscle weakness |
developmental delay; hypotonia |
developmental delay; unusual movement patterns |
microcephaly; developmental delay; seizures; torticollis |
microcephaly; developmental delay; micrognathia; hypotonia; dysphagia; hypoxia |
|
Maximal Motor Ability |
weight lifting; sports |
weight lifting; sports |
manual labor; junior high sports |
weight lifting; manual labor |
walks independently |
run; climb stairs |
walks independently; feeds orally |
able to sit; crawl | unable to sit independently |
|
Cognitive function |
normal intelligence |
normal intelligence |
normal intelligence |
normal intelligence |
moderate- severe intellectual disability; verbal |
moderate- severe intellectual disability; verbal |
severe intellectual disability, verbal |
severe intellectual disability; non- verbal |
severe intellectual disability; non- verbal |
| Epilepsy | no | no | no | no | no | no | yes | yes | yes |
| Ophthalmologic | none | none | none | none | none | strabismus; no saccadic movements or smooth pursuits |
none | optic atrophy; strabismus |
optic atrophy; strabismus |
| Cardiac | ischemic heart disease |
normal | normal | 1st degree AV block |
normal | normal | normal | normal | bicuspid aortic valve |
|
Respiratory function |
normal | normal | normal | normal | moderate restrictive lung disease |
normal | normal | normal | nocturnal desaturation |
|
Maximum CK (IU/L) |
1,331 | 7,250b | 3,693 | 5,900 | 7,112 | 2,867 | 6,908 | 2,789 | 6,158 |
| Other | none | none | none | none | neuromuscular scoliosis; sensorineural hearing loss |
none | self- stimulatory behavior |
neuromuscular scoliosis; gastrostomy tube |
gastrostomy tube; self-stimulatory behavior |
| Brain MRI | ND | ND | ND | ND | normal | normal | normal | ventriculomegaly; prominent cortical sulci, cerebellar hypoplasia |
ventriculomegaly; hypomyelination; cerebellar hypoplasia |
|
Diagnostic Category |
LGMD | LGMD | LGMD | LGMD | LGMD-MR | CMD-MR | CMD-MR | CMD-CRB | CMD-CRB |
Footnotes:
= novel mutation;
= 52,010 during an episode of rhabdomyolysis; CMD-CRB = congenital muscular dystrophy with cerebellar involvement; CMD-MR = congenital muscular dystrophy with mental retardation; LGMD = limb-girdle muscular dystrophy; LGMD-MR = limb-girdle muscular dystrophy with mental retardation; ND = not done; DNA mutation numbering is based on cDNA sequence.
All cases were submitted to the LOVD database (http://www.dmd.nl/nmdb/home.php?select_db=GMPPB).
P1, P2, and P3 each presented in their mid-to-late teens with inability to keep up with their peers and proximal weakness. P2 presented only with exercise induced myalgias, weakness, and myoglobinuria with recovery to normal strength. They are now ages fifty, twenty-six, and thirty-two years old, respectively. All three individuals continue to walk independently and have normal cognitive and cardiopulmonary function.
P4, now twenty-five years old, presented at age twenty-three after routine blood work showed elevated transaminases. Follow up CK was greater than 5,000 U/L. He has normal strength with infrequent episodes of exercise-induced myoglobinuria. He is cognitively normal and has a first degree AV block and low normal left ventricular systolic function (EF estimated at 50–55%).
P5, now twenty years old, presented at five years old with inability to run or climb stairs. She is no longer ambulatory and developed scoliosis requiring surgery. Her pulmonary function is slowly declining with a restrictive pattern. Echocardiogram is normal but with a gradually declining ejection fraction. She has cognitive disability and requires speech therapy. She has sensorineural hearing loss.
P6, now five years old, presented at nine months with hypotonia, global developmental delay, and strabismus. She is now able to walk independently and run but is ataxic. On exam she has mild proximal weakness and full strength distally. She has intellectual disability but is able to communicate in sentences. She had obstructive sleep apnea that improved after a tonsillectomy, her cardiopulmonary function is otherwise normal.
P7 is a fifteen year old female who presented in infancy with motor and language delays and unusual movement patterns. She walked at sixteen months and continues to walk independently. She has a very mild proximal weakness which has been stable over time. She developed a seizure disorder at eight years of age and is verbal but with significant intellectual disability. Serum transferrin isoelectric focusing was normal.
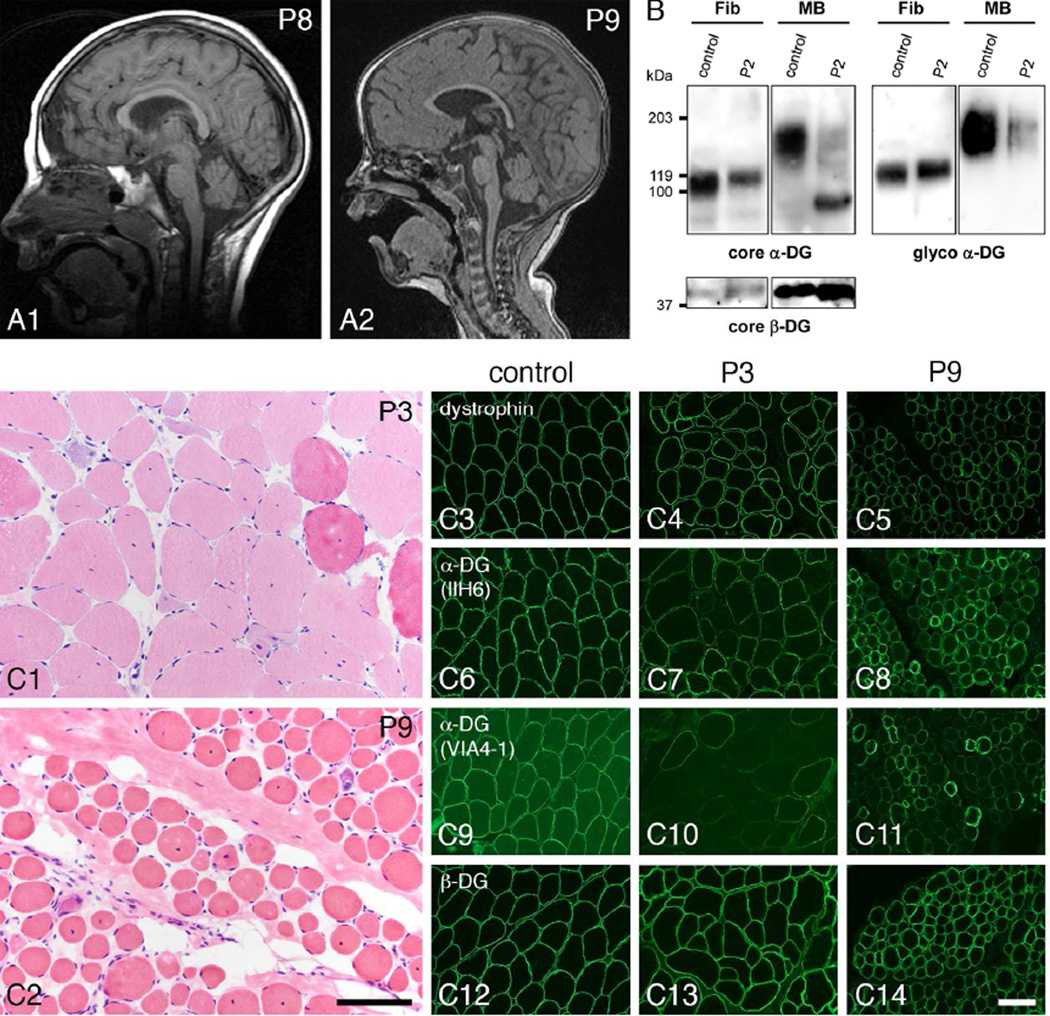
P8 is a thirteen year old female who presented at eight months of age with microcephaly, hypotonia, global developmental delay, and infantile spasms. She remained non-verbal, reportedly crawled briefly at one and a half years but lost that skill by two years; she is unable to sit without support and has cortical visual impairment and strabismus. She fed orally but required a gastrostomy tube at five years for dietary supplementation while she continued to take food by mouth. She developed neuromuscular scoliosis requiring spinal fusion. Brain MRI at age 3 years showed mild cerebellar hypoplasia (Figure 1A).
Figure 1.
A: Sagittal T1 brain MRIs showing cerebellar hypoplasia in P8 at 3 years (A1) and P9 at 19 months (A2). B: Alpha- and β-dystroglycan were evaluated by western blot in case P2. WGA enriched homogenates of cultured skin fibroblasts (Fib) and muscle biopsy (MB) were blotted with the core α-dystroglycan antibody, Shp5 (core α-DG) and the glycoepitope dependent α-dystroglycan antibody, IIH6 (glyco α-DG). Subject muscle shows lower molecular weight, hypoglycosylated α-DG with Shp5 and a decreased amount of functional, properly glycosylated α-DG with IIH6 as compared to control. In contrast, subject fibroblasts appear similar to control. An antibody against core β-dystroglycan, core β-DG (AP83) was used to assess loading. C: These photomicrographs illustrate the muscle biopsy histopathology and immunostaining abnormalities of representative individuals with GMPPB variants. The H&E images show dystrophic pathology in LGMD case P3 (C1) and CMD-CRB case P9 (C2). Muscle biopsies were taken at 15 and 2 years of age respectively. Immunofluorescence images from the same two muscle biopsies show reduced staining for α-dystroglycan with two different glycoepitope-dependent antibodies, IIH6 and VIA4-1. A control muscle biopsy is shown in panels C3, 6, 9, and 12. P3 muscle is shown in panels C4, 7, 10, and 13. P9 muscle is shown in panels C5, 8, 11, and 14. Dystrophin, C3–5; α-dystroglycan (IIH6), C6–8; α-dystroglycan (VIA4-1), C9–11; β-dystroglycan, C12–14. The scale bar in panel C2 equals 100 µm in panels C1 and C2; the scale bar in panel C14 equals 100 µm in panels C3–14.
P9, now three years old, presented prenatally with intrauterine growth restriction and decreased fetal movements. He was hypotonic, in respiratory distress at birth, and continues to require overnight oxygen support. He never fed orally, requiring gavage or gastrostomy feeds. He developed complex partial seizures at twenty-two months of age controlled with levetiracetam. He is non-verbal, is unable to sit independently, and has cortical visual impairment, strabismus, and displays self-injurious and self-stimulatory behaviors. Brain MRI at age 19 months showed cerebellar hypoplasia (Figure 1A). Serum transferrin isoelectric focusing was normal. An extended dystroglycanopathy genetic testing panel identified a single variant in GMPPB that is predicted to be pathogenic; the panel was otherwise normal. Based on phenotype, CK, muscle biopsy, and normal sequencing for other known dystroglycanopathy genes, we propose that a second variant in GMPPB is present, but not identifiable with commercially available testing.
Muscle biopsies of all cases were reviewed at The University of Iowa. Frozen sections from each biopsy were evaluated by hematoxylin and eosin (H&E) staining and by immunofluorescence as reported previously [Moore et al., 2006]. The antibodies used for the immunostaining or western blotting reported here include IIH6 and VIA4-1 anti-glyco-α-dystroglycan, Shp5 anti-α-dystroglycan core protein, 7D11 anti-β-dystroglycan, and ab15277 anti-dystrophin antibodies. Biopsies displayed dystrophic features of varying severity including myonecrosis and regeneration, increased internally placed nuclei, increased fiber size variation, and endomysial fibrosis in the more severe cases (Figure 1C). Immunofluorescence evaluation using IIH6 and VIA4-1 antibodies against glycosylated α-dystroglycan showed mosaic patterns of decreased staining, while dystrophin and β-dystroglycan appeared normal (Figure 1C). Wheat germ agglutinin (WGA) preparations from pooled cryosections were utilized for western blots of α-dystroglycan and β-dystroglycan using published protocols [Michele et al., 2002]. Skeletal muscle WGA preparations from P2 showed reduced signal for glycosylated α-dystroglycan and a reduction in molecular weight for core α-dystroglycan compared to controls (Figure 1B). The glycosylation status of α-dystroglycan in subject fibroblast cultures was evaluated with western blots using WGA enriched cell lysates according to published protocols [Willer et al., 2012]. Western blot analysis of WGA preparations of cultured skin fibroblasts from P2 showed no significant difference in α-dystroglycan glycosylation compared to control fibroblasts (Figure 1B). Western blot analysis of the muscle biopsy (P1) and fibroblast cultures (P3, P4, P7, P8, and P9) from other cases yielded results similar to P2 (data not shown).
In our nine cases, six GMPPB variants were previously reported as pathogenic while five variants are novel. Based on clinical phenotypes and immunofluorescence demonstrating decreased glycosylation of α-dystroglycan in muscle we propose that these additional variants are also pathogenic. In silico analysis of the novel variants using SIFT, Mutation-Taster, and PolyPhen2 produced the following predictions: Two novel variants [c.395C>G (p.S132C), and c.94C>T (p.P32S)] are predicted pathogenic by all three programs, whereas the two novel variants in P4 [c.721C>T (p.P241S), c.1034T>C (p.V345A)] were predicted pathogenic by one of the three programs [Ng and Henikoff, 2003; Adzhubei et al., 2010; Schwarz et al., 2014]. Sequence alignment of these novel variants are shown in Supp. Figure S1. The final novel variant in P4 (c.402+1G>A) alters a highly conserved position in the intron 4 splice donor site. Sequencing of GMPPB in the parents of P7 confirmed heterozygous carrier status for each parent, providing further evidence for the pathogenicity of the novel variant c.395C>G (p.S132C).
These individuals together with previously reported cases show a remarkable range in phenotypic severity, from a minimally symptomatic adult at age twenty-five (P4) to infants with congenital muscular dystrophy including cerebellar involvement (P8, P9). They share features typical of other dystroglycanopathies including serum CK levels greater than five times normal, evidence of myonecrosis and regeneration on muscle biopsy, and abnormal α-dystroglycan glycosylation by immunostaining and western blots of muscle. Electrophysiological testing for myasthenia was not completed in our cases. In contrast to skeletal muscle, the α-dystroglycan glycosylation defect in cultured fibroblasts of affected individuals is absent or very mild (Figure 2B) [Carss et al., 2013].
To date, all but 6 of the reported 41 individuals with GMPPB variants have been compound heterozygotes, making it difficult to confirm genotype-phenotype correlations. However, two variants have been seen frequently enough in GMPPB compound heterozygotes to provide initial insight: c.79G>C (p.D27H) and c.860G>A (p.R287Q). 68% of individuals (28 of 41) and 66% of the families (21 of 32) reported to date, including the present series, have one of these proposed common variants [Carss et al., 2013; Raphael et al., 2014; Belaya et al., 2015; Bharucha-Goebel et al., 2015; Cabrera-Serrano et al., 2015]. Based on data available by the NHLBI Exome Sequencing Project, the mean allelic frequency for the c.79G>C (p.D27H) and c.860G>A (p.R287Q) variants in European Americans is 0.12% and 0.02%, respectively. For comparison, the c.826C>A common pathogenic variant in FKRP (MIM #606596), another dystroglycanopathy-associated gene, has a mean allelic frequency of 0.07% in European Americans. The c.79G>C (p.D27H) variant has been found in 17 individuals from 12 unrelated families [Carss et al., 2013; Belaya et al., 2015; Bharucha-Goebel et al., 2015; Cabrera-Serrano et al., 2015]. These individuals present with hyperCKemia, limb-girdle pattern weakness or muscle pain sometime after age 10 (16 of 17), are able to walk or run independently (17 of 17), and most have normal intelligence (12 of 17, the remaining 5 have borderline intelligence [2 of 5] or intellectual disability [3 of 5]). Cataracts (3 of 14), abnormal electrocardiogram (3 of 14), and epilepsy (1 of 14), are uncommon in reported individuals with this variant. Thus, the c.79G>C (p.D27H) variant is associated with a relatively mild phenotype among individuals with GMPPB-associated dystroglycanopathy. In contrast, the c.860G>A (p.R287Q) variant, which has been seen in 11 individuals from 9 unrelated families [Carss et al., 2013; Raphael et al., 2014; Belaya et al., 2015; Cabrera-Serrano et al., 2015], is associated with a congenital muscular dystrophy phenotype. These individuals have delayed motor developmental (10 of 11) and intellectual disability (10 of 11). Most achieve independent ambulation (8 of 11). Results of brain MRI are reported for eight individuals and five are abnormal with cerebellar hypoplasia. Other variable clinical features in the c.860G>A cases include epilepsy (6 of 9) and strabismus (5 of 9). Compound heterozygous individuals with both the c.79G>C (p.D27H) and c.860G>A (p.R287Q) variants have yet to be reported.
Previous studies have examined the subcellular localization of selected GMPPB variants in C2C12 myoblasts [Carss et al., 2013; Belaya et al., 2015]. Normal cytoplasmic localization of GMPPB was seen in the c.79G>C (p.D27H) variant cells whereas cytoplasmic aggregation of GMPPB was seen in cells with the c.860G>A (p.R287Q) variant. The c.79G>C (p.D27H) variant is located within the nucleotidyl transferase domain of GMPPB, suggesting that it may reduce the efficacy of the enzyme’s catalytic function to a milder degree respective to other pathogenic variants. The cytoplasmic aggregation of GMPPB in c.860G>A (p.R287Q) variant cells suggests that protein misfolding and subsequent reduction in cellular GMPPB activity may be responsible for the relatively severe phenotype seen in individuals with this variant. Like the c.860G>A (p.R287Q) variant, GMPPB aggregation was also seen in c.95C>T (p.P32L) variant cells, a variant that has been seen in a total of five cases in literature (Carss et al P5–6; Cabrera-Serrano et al P1–2, 8) [Carss et al., 2013; Cabrera-Serrano et al., 2015]. The single novel variant identified in our P9 (c.94C>T, p.P32S), makes a predicted serine substitution in the same codon altered by the pathogenic c.95C>T (p.P32L) variant, further supporting the predicted pathogenicity of the c.94C>T (p.P32S) variant in our P9. Interestingly, four of the six individuals with cerebellar hypoplasia have GMPPB variants leading to amino acid substitutions in this highly conserved proline at codon 32 (P9 reported here, Carss et al P5 and P6, and Cabrera-Serrano et al P8) [Carss et al., 2013; Cabrera-Serrano et al., 2015].
GMPPB-associated dystroglycanopathies present with a wide phenotypic spectrum and the current cases, together with those previously published, allow preliminary genotype-phenotype observations for the emerging common pathogenic variants c.79G>C (p.D27H) and c.860G>A (p.R287Q). Muscle biopsy with immunostaining for glycosylated α-dystroglycan can suggest a dystroglycanopathy, or support the diagnosis when novel variants are identified. Unlike several other dystroglycanopathy genotypes [Willer et al., 2012], fibroblast cultures show only subtle, if any, defects in α-glycosylation status [Carss et al., 2013]. Additional experience with the current cohort of cases and the identification of additional cases will be required to more completely understand genotype-phenotype relationships and the natural history of GMPPB-associated dystroglycanopathy. Management remains symptomatic, however, a subset of individuals with GMPPB variants may respond to medications used to treat congenital myasthenic syndromes [Belaya et al., 2015].
Supplementary Material
Acknowledgments
The work presented here was supported in part by the Iowa Wellstone Muscular Dystrophy Cooperative Research Center, U54-NS053672 (BSJ, TW, MOC, KPC, SAM, and KDM), CTSA: U54-TR001013, and MDA grant 238219 (KPC and TW). KPC is an investigator of the Howard Hughes Medical Institute. Muscle biopsy immunostaining was performed by Terese Nelson. Carrie Stephan coordinated research subject visits. Figures were assembled with the assistance of Joel Carl. BSJ and KDM had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The authors would like to thank the NHLBI GO Exome Sequencing Project and its ongoing studies which produced and provided exome variant calls for comparison: the Lung GO Sequencing Project (HL-102923), the WHI Sequencing Project (HL-102924), the Broad GO Sequencing Project (HL-102925), the Seattle GO Sequencing Project (HL-102926) and the Heart GO Sequencing Project (HL-103010).
TLW was employed by Prevention Genetics, LLC. KPC received speaker fees from Pfizer and Alexion pharmaceuticals. SAM performs fee for service muscle biopsy assays for Sarepta Therapeutics. KDM is a consultant for aTyr pharma and is a site principal investigator for industry sponsored trials for Sarepta Therapeutics, Horizon pharma, Shire ViroPharma, Eli Lilly, Pfizer, and Biomarin.
Footnotes
Authorship:
BSJ, first author, was primarily responsible for compiling data and writing the manuscript. KDM, corresponding author, and SAM are joint last authors and were responsible for project conception and data collection. DS, TM, MS, VAS collected clinical information. TW, MOC, TLW and KPC contributed data, technical, and scientific expertise. All authors contributed to critical review and revision of the manuscript.
Potential Conflicts of Interest:
There are no direct conflicts with the work reported here.
BSJ, TW, DS, MOC, TM, MS, and VAS report no disclosures relevant to this manuscript.
References
- NHLBI GO Exome Sequencing Project (ESP) Exome Variant Server. http://evs.gs.washington.edu/EVS/ [Google Scholar]
- Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barresi R, Campbell KP. Dystroglycan: from biosynthesis to pathogenesis of human disease. Journal of cell science. 2006;119:199–207. doi: 10.1242/jcs.02814. [DOI] [PubMed] [Google Scholar]
- Belaya K, Rodriguez Cruz PM, Liu WW, Maxwell S, McGowan S, Farrugia ME, Petty R, Walls TJ, Sedghi M, Basiri K, Yue WW, Sarkozy A, et al. Mutations in GMPPB cause congenital myasthenic syndrome and bridge myasthenic disorders with dystroglycanopathies. Brain : a journal of neurology. 2015 doi: 10.1093/brain/awv185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharucha-Goebel DX, Neil E, Donkervoort S, Dastgir J, Wiggs E, Winder TL, Moore SA, Iannaccone ST, Bonnemann CG. Intrafamilial variability in GMPPB-associated dystroglycanopathy: Broadening of the phenotype. Neurology. 2015 doi: 10.1212/WNL.0000000000001440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnemann CG, Wang CH, Quijano-Roy S, Deconinck N, Bertini E, Ferreiro A, Muntoni F, Sewry C, Beroud C, Mathews KD, Moore SA, Bellini J, et al. Diagnostic approach to the congenital muscular dystrophies. Neuromuscular disorders : NMD. 2014;24:289–311. doi: 10.1016/j.nmd.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera-Serrano M, Ghaoui R, Ravenscroft G, Johnsen RD, Davis MR, Corbett A, Reddel S, Sue CM, Liang C, Waddell LB, Kaur S, Lek M, et al. Expanding the phenotype of GMPPB mutations. Brain : a journal of neurology. 2015;138:836–844. doi: 10.1093/brain/awv013. [DOI] [PubMed] [Google Scholar]
- Carss KJ, Stevens E, Foley AR, Cirak S, Riemersma M, Torelli S, Hoischen A, Willer T, van Scherpenzeel M, Moore SA, Messina S, Bertini E, et al. Mutations in GDP-mannose pyrophosphorylase B cause congenital and limb-girdle muscular dystrophies associated with hypoglycosylation of alpha-dystroglycan. American journal of human genetics. 2013;93:29–41. doi: 10.1016/j.ajhg.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey C, Clement E, Mein R, Brockington M, Smith J, Talim B, Straub V, Robb S, Quinlivan R, Feng L, Jimenez-Mallebrera C, Mercuri E, et al. Refining genotype phenotype correlations in muscular dystrophies with defective glycosylation of dystroglycan. Brain : a journal of neurology. 2007;130:2725–2735. doi: 10.1093/brain/awm212. [DOI] [PubMed] [Google Scholar]
- Michele DE, Barresi R, Kanagawa M, Saito F, Cohn RD, Satz JS, Dollar J, Nishino I, Kelley RI, Somer H, Straub V, Mathews KD, et al. Post-translational disruption of dystroglycan-ligand interactions in congenital muscular dystrophies. Nature. 2002;418:417–422. doi: 10.1038/nature00837. [DOI] [PubMed] [Google Scholar]
- Moore SA, Saito F, Chen J, Michele DE, Henry MD, Messing A, Cohn RD, Ross-Barta SE, Westra S, Williamson RA, Hoshi T, Campbell KP. Deletion of brain dystroglycan recapitulates aspects of congenital muscular dystrophy. Nature. 2002;418:422–425. doi: 10.1038/nature00838. [DOI] [PubMed] [Google Scholar]
- Moore SA, Shilling CJ, Westra S, Wall C, Wicklund MP, Stolle C, Brown CA, Michele DE, Piccolo F, Winder TL, Stence A, Barresi R, et al. Limb-girdle muscular dystrophy in the United States. Journal of neuropathology and experimental neurology. 2006;65:995–1003. doi: 10.1097/01.jnen.0000235854.77716.6c. [DOI] [PubMed] [Google Scholar]
- Ng PC, Henikoff S. SIFT: Predicting amino acid changes that affect protein function. Nucleic Acids Res. 2003;31:3812–3814. doi: 10.1093/nar/gkg509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raphael AR, Couthouis J, Sakamuri S, Siskind C, Vogel H, Day JW, Gitler AD. Congenital muscular dystrophy and generalized epilepsy caused by GMPPB mutations. Brain research. 2014;1575:66–71. doi: 10.1016/j.brainres.2014.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito F, Moore SA, Barresi R, Henry MD, Messing A, Ross-Barta SE, Cohn RD, Williamson RA, Sluka KA, Sherman DL, Brophy PJ, Schmelzer JD, et al. Unique role of dystroglycan in peripheral nerve myelination, nodal structure, and sodium channel stabilization. Neuron. 2003;38:747–758. doi: 10.1016/s0896-6273(03)00301-5. [DOI] [PubMed] [Google Scholar]
- Schwarz JM, Cooper DN, Schuelke M, Seelow D. MutationTaster2: mutation prediction for the deep-sequencing age. Nat Methods. 2014;11:361–362. doi: 10.1038/nmeth.2890. [DOI] [PubMed] [Google Scholar]
- Willer T, Lee H, Lommel M, Yoshida-Moriguchi T, de Bernabe DB, Venzke D, Cirak S, Schachter H, Vajsar J, Voit T, Muntoni F, Loder AS, et al. ISPD loss-of-function mutations disrupt dystroglycan O-mannosylation and cause Walker-Warburg syndrome. Nature genetics. 2012;44:575–580. doi: 10.1038/ng.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.