Abstract
Objectives
Hodgkin’s lymphoma (HL) is one of the most common cancers among young adults. We investigated the time trends for HL among the 20–44 age group in the USA by gender to identify the potential factors accounting for the incidence trends.
Methods
Using data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results program for 1973–2010, we conducted age–period–cohort modeling to evaluate birth cohort patterns on incidence trends of HL over time.
Results
For all races combined, the age-adjusted incidence patterns were similar to that of whites. The birth cohort patterns for whites and all races were similar, but the patterns differed according to gender. Specifically, except for the 1970–1975 birth cohort, all other birth cohorts showed an increasing birth cohort trend for females. Conversely, there was a decreasing cohort trend in males beginning in the 1960 birth cohort regardless of the assumptions of the period effect.
Conclusion
The established risk factors for HL can seemingly not explain the gender disparities of the cohort pattern, which necessitates further analytical epidemiological studies to explore the risk factors for this disease with respect to potential differences by gender and by histological subtype.
Keywords: Hodgkin’s lymphoma, Incidence rate, Age–period–cohort effect, Descriptive epidemiology
Introduction
Hodgkin’s lymphoma (HL) is one of the most common cancers among young adults [1], but there are few established risk factors for this disease in this demographic group. Previous studies evaluating the descriptive epidemiology of HL have reported a continuing increase worldwide in the incidence of HL in young adults [2-5]. In particular, a study from Israel reported a sharp rise in the incidence of HL in young adults from 1960 to 2005 [2]. The highest incidence was observed in the 20–24 year age group, and it was 6.6 per 100,000 per year for men during the years 1997–2005 and 9.13 per 100,000 per year for women during the period 1988–1996. A study in Singapore has also showed a continuous increase in the incidence of HL for the young age groups for both men (the incidence rates increased annually by 7.0 and 3.4 % in the age groups 15–19 and 20–24 years, respectively) and women (the incidence rates increased annually by 13.7 and 12.2 % in the age groups 15–19 and 20–24 years, respectively) between 1968 and 2004 [3]. An analysis of age- and sex-specific trends of HL incidence in three Nordic countries revealed a significant increase with an annual rate of change of 2.2 % in the incidence of nodular sclerosis, the most common subtype of classical HL, over the period 1978–1997 for adolescents and young adults [4]. The increases were higher in young women compared with males and, in contrast, the incidence of HL significantly decreased in individuals >40 years old [4]. Further, a study in Canada that conducted an age–period–cohort (APC) analysis of HL showed a significant increase in incidence in Canadian females aged 10–29 and in Canadian males aged 10-24 from 1970 to 1995 and found evidence that period and birth cohort effects were responsible for these incidence trends [5].
Our earlier descriptive study showed a rapid increase in the incidence of HL among young adults (age 20–44 group) in both males and females in Connecticut from 1935 to 1992 [6] and predicted based on the birth cohort patterns and drift analysis that the increase in HL incidence would likely stabilize in males but continue to increase in females in the coming years [6]. Several recent studies of other populations in the USA have mainly compared the HL incidence patterns by race and have focused on the survival rate of the disease [7, 8]. In addition, an evaluation of lymphoma incidence patterns in the USA using SEER 12 data suggested stable incidence rates of HL overall over the period 1992–2001, but the temporal trends varied by subtype and demographic group and results for young adults were not presented separately [9].
It is therefore currently unknown if the incidence rate of HL is still increasing among young adults in the USA and if it is to what extent the birth cohort effect could explain the observed incidence patterns. In order to evaluate these trends, the current study was designed to explore time trends and APC patterns of HL incidence between 1973 and 2010 in the USA, with an emphasis on incidence patterns in young adults aged between 20 and 44 years old.
Materials and methods
Data source
We obtained data from the SEER program, which was released in April 2013 and based on the November 2012 submission results (SEER*Stat Database: Incidence—SEER 9 Regs Research Data, Nov 2012 Sub (1973–2010), Single Ages to 85+, Katrina/Rita Population Adjustment). Overall age-adjusted incidence rates for whites and all races combined as well as sex-specific age-adjusted incidence rates were examined for 1973 through 2010 from the SEER 9 registries (Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, Utah). The SEER 9 data account for approximately 12 % of the US population.
A total of 27,282 HL cases (ICD-O-3 9650-9655, 9659, 9661-9667) were included in these nine registries. HL cases were divided into classical HL (CHL, ICD-O-3 9650–9655 9661–9667) and nodular lymphocyte predominant HL (NLHL, ICD-O-3 9659). A total of 26,520 cases (97.21 %) were classical HL. 86.8 % cases were white, and 51.7 % cases were 20–44 years old.
Data analysis
Age-adjusted incidence rates were calculated using SEER*Stat (8.0.4) by gender and for age groups 20–44, with rates adjusted to the 2000 US standard population. Joinpoint analyses were conducted using Joinpoint version 4.0.4 by weighted least squares regression of the natural logarithms of the age-adjusted incidence rates. The data were also presented by calendar year and by cohort year of birth in order to explore the secular trends and the potential birth cohort patterns. The APC analysis based on a log-linear Poisson regression model was conducted for the 20–44 year old age group. Age, period, and cohort models were based on five-year age intervals between 20 and 44, and eight time period intervals (1973–1974, 1975–1979, 1980–1984, 1985–1989, 1990–1994, 1995–2000, 2000–2004, 2005–2010). Because of the nonidentifiability problem (cohort = year–age), the independent effects of age, period, and cohort cannot be evaluated [10]. Since the true value of the period slope is unknown, the cohort effect can be evaluated by constraining the period effect to different assumptions (i.e., parameter values βp = 0, −0.005, or 0.005), where βp = 0 represents a slope of zero, βp = −0.005 indicates that the period slope was decreasing and βp = 0.005 denotes that the period slope was increasing during the study period. For the APC analyses, the effects are reported as the log rate ratios relative to the reference group. The reference groups were the 40–44 year old group for age, the 2005–2010 calendar years for period, and the median year of birth (i.e., 1985) for cohort. All models were fit using SAS (version 9.3). The significance level was set at 0.05 for a two-sided test.
Results
A total of 14,113 newly diagnosed cases of HL between the ages of 20 and 44 were reported to the SEER Project from 1973 to 2010. Of these, 12,082 (85.6 %) were white and 1,442 (10.2 %) were black. The white cases included 6,607 (54.7 %) males and 5,475 (45.3 %) females. Of whites, 11,833 (97.9 %) cases were classical HL.
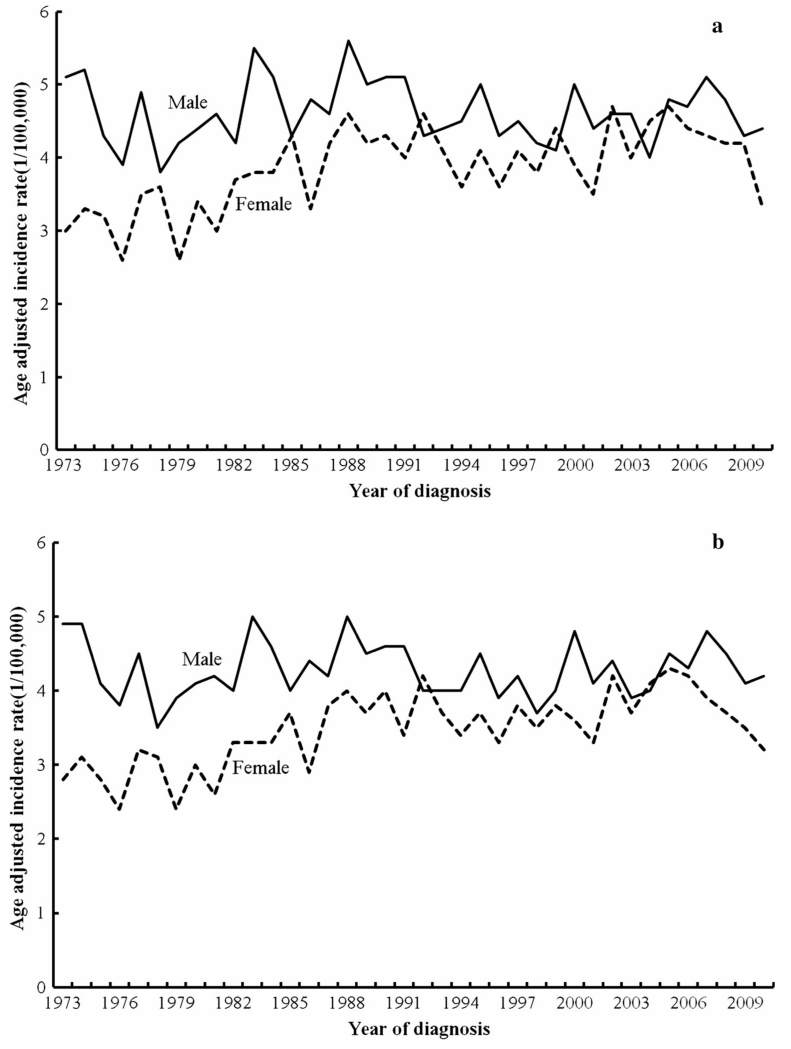
The age-adjusted incidence rates of HL by gender for the 20–44 age group are presented in Fig. 1. For white cases, the age-adjusted incidence rates for 20–44 year old males were higher than that of the 20–44 year old females before 2003, and then, the two rates started to converge between 2003 and 2007. Among white males, the age-adjusted incidence rates fluctuated between a low of 3.8/100,000 in 1978 and a high of 5.6/100,000 in 1988. The rates appeared to level off at around 4.5/100,000 over time. However, there was no joinpoint for incidence rates in white males between 1973 and 2010, with an annual percent change of −0.1 % (95 % CI: 0.4 to +0.2 %) over 1973–2010. Among white females, the rates increased from about 3.0/100,000 in 1973 to around 4.6/100,000 in 1988 and remained stable in subsequent years, except for a sharp decrease in 2010 (3.3/100,000). There was one joinpoint at 1988 for incidence rates in white females, as the rate increased by 2.4 % (95 % CI: +1.0 to +3.8 %) per year from 1973 to 1988 and then stabilized from 1988 to 2010 with an annual percent change of −0.02 % (95 % CI: −0.7 to +0.6 %).
Fig. 1.
Age-adjusted incidence rates of Hodgkin lymphoma by gender for age group 20–44 only (a for whites, b for all races)
For all races combined, the age-adjusted incidence patterns were similar to that of whites (Fig. 1). In all race groups, a decline in the age-adjusted incidence rates was apparent in females beginning in the mid-2000s, while the rates in males declined slightly in the mid-2000s before fluctuating in the most recent years. There was no joinpoint for incidence rates in males between 1973 and 2010. For females, there was one joinpoint at 2006 as the incidence rate increased by 1.1 % (95 % CI: +0.7 to +1.5 %) per year from 1973 to 2006 and began to decrease by 5.8 % (95 % CI: −13.6 to +2.6 %) per year from 2006 to 2010. However, this decrease in female incidence rates beginning in 2006 was not statistically significant.
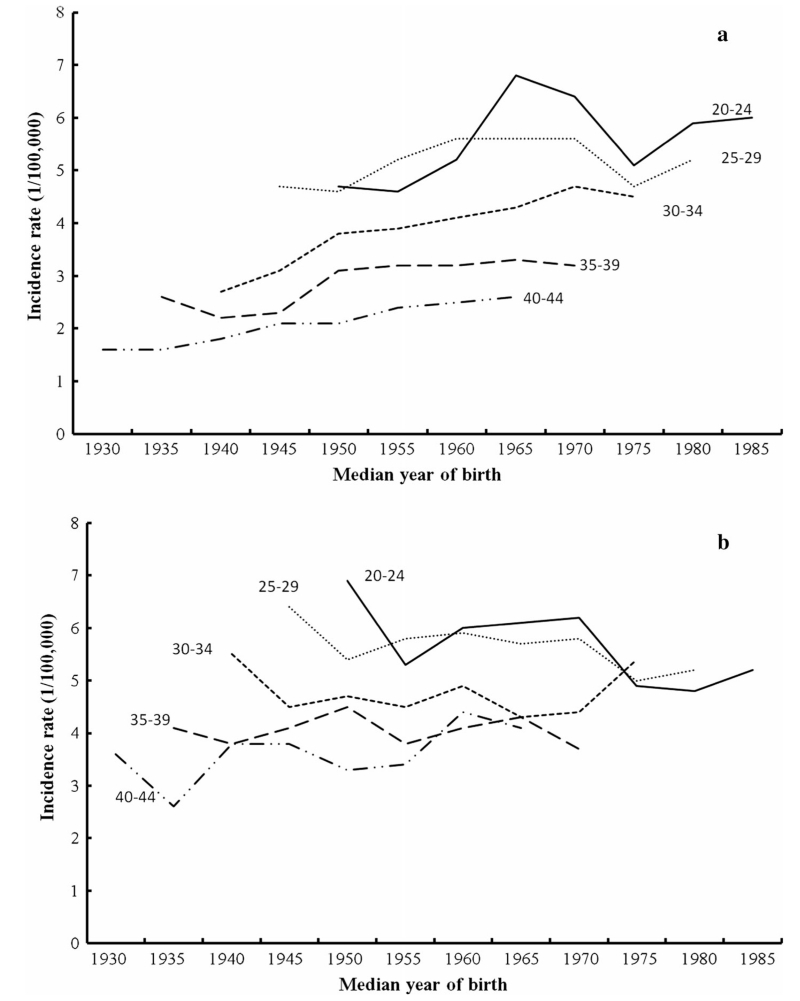
The age-specific incidence rates of HL for whites aged 20–44 by median year of birth are shown in Fig. 2. For white females (Fig. 2a), the incidence rates of HL in the more recent birth cohorts were much higher than that of the earlier cohorts for all age groups between 20 and 44. The incidence rates of HL for white females aged 30–34 had the largest rise, and it increased from 2.7 cases per 100,000 in the 1940 birth cohort to 4.5 cases per 100,000 in the 1975 birth cohort. White females aged 20–24 had the highest incidence rate of all age groups and it increased from 4.0 cases per 100,000 in the 1950 birth cohort to 6.8 cases per 100,000 in the 1965 birth cohort and decreased to 6.0 cases per 100,000 in the 1985 birth cohort. For white males (Fig. 2b), the incidence rate of HL in the successive birth cohorts leveled off or decreased in the age groups between 20 and 40. Conversely, the incidence rates of HL for white males aged 40–44 increased from 3.6 cases per 100,000 in the 1930 birth cohort to 4.1 cases per 100,000 in the 1965 birth cohort.
Fig. 2.
Cohort age curves of HL for whites in age group 20–44 by gender (a female, b male)
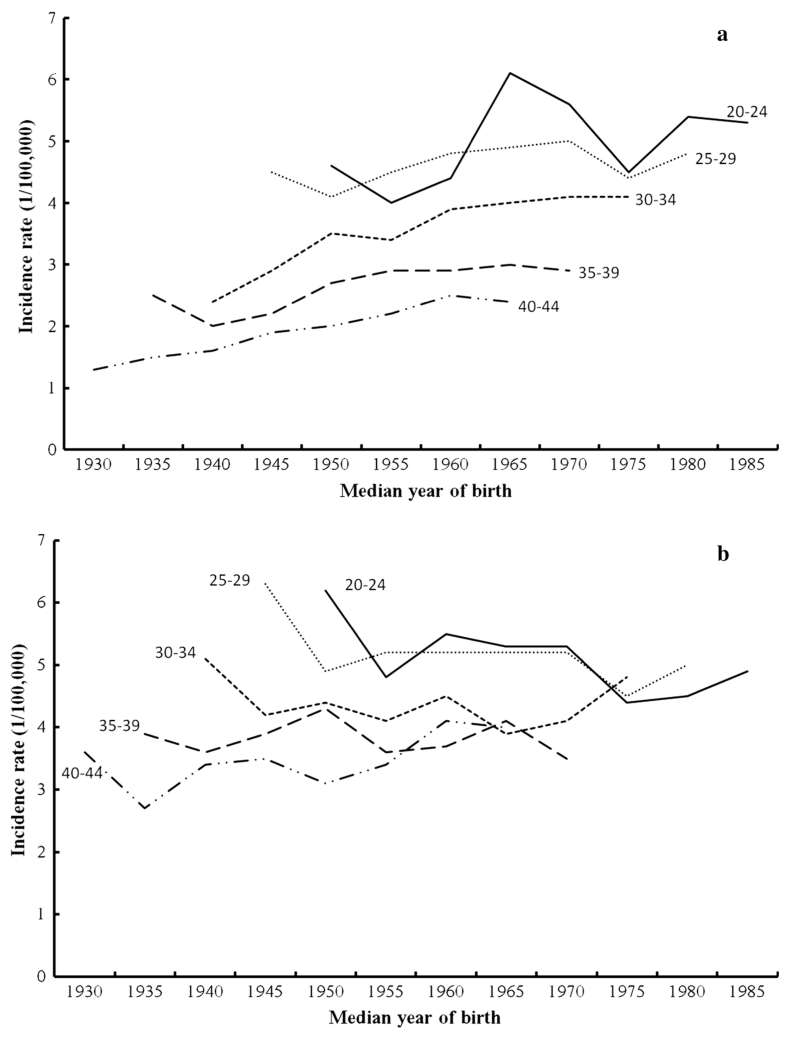
These patterns in both males and females aged 20–44 were very similar in analyses evaluating all racial groups combined (Fig. 3). For females of all races, rates were similarly higher in the more recent birth cohorts in all age groups (Fig. 3a). A decline in rates in successive birth cohorts for males of all races was apparent for all age groups except 40–44 year olds, in whom rates increased from 3.6 cases per 100,000 in the 1930 birth cohort to four cases per 100,000 in the 1965 birth cohort. It was further observed that the sudden change in the incidence slope occurred from 1975 for all age groups for both males and females, while the slopes became much less steeper after 1985 (Figs. 2, 3).
Fig. 3.
Cohort age curves of HL for all races in age group 20–44 by gender (a female, b male)
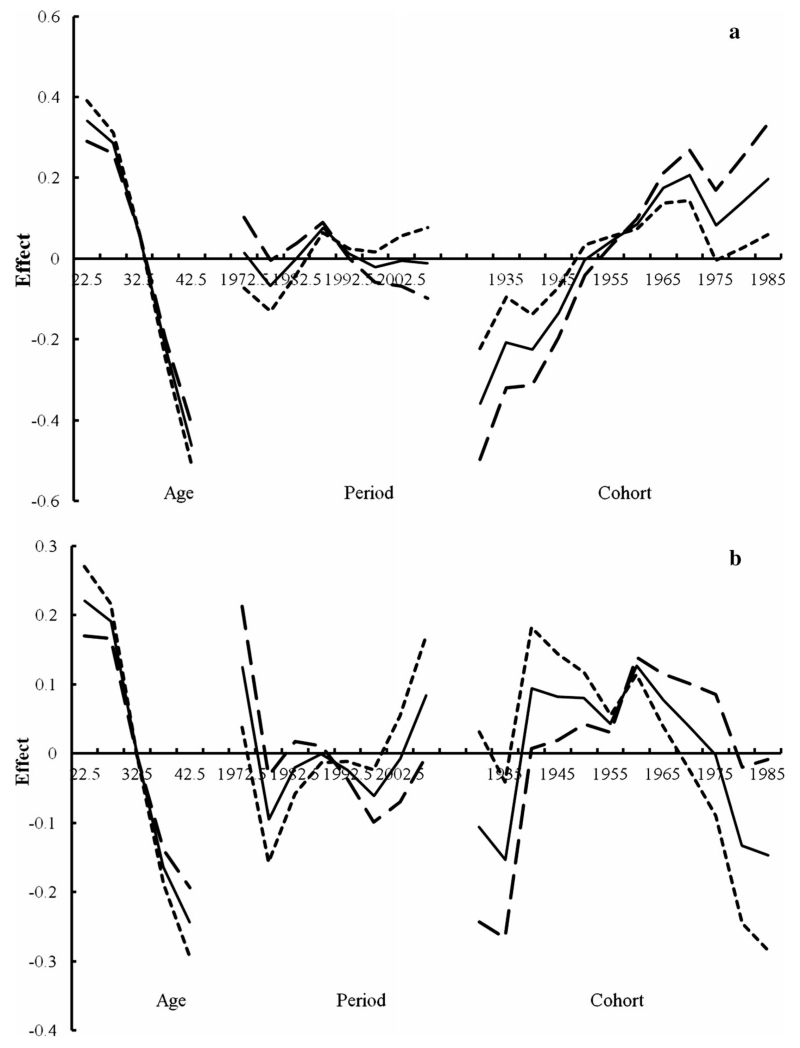
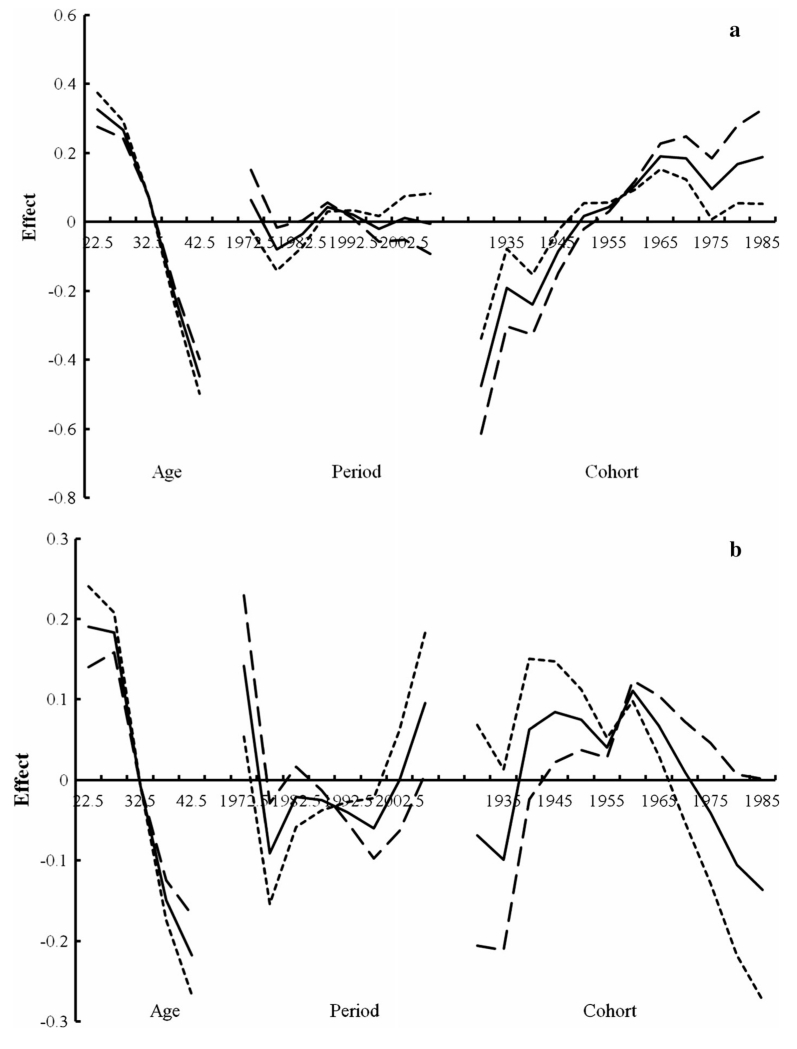
Figures 4, 5 present the results from the APC modeling by gender for whites and all races, respectively, using three different assumptions for the period slope (βp = 0, −0.005, or 0.005), where the solid line is based on an assumption without period slope. The birth cohort patterns for whites and all races were similar, but the patterns differed according to gender. Specifically, an increasing birth cohort trend was observed for females, except for the 1970–1975 birth cohort, for each of the three different assumptions for the period slope (Figs. 4a, 5a). Conversely, there was a decreasing cohort trend in males beginning in the 1960 birth cohort regardless of the assumptions of the period effect (Figs. 4b, 5b). The diverging patterns in the cohort trend by gender were particularly apparent around 1975–1979 based on evaluation of the age–period curves (data not shown). Moreover, the period effect patterns were similar in both males and females before 1997, whereas after 1997 the effects differed by gender in that a noticeable period effect was observed in males but not in females (Figs. 4, 5).
Fig. 4.
Age–period–cohort modeling for HL in whites by gender (a female, b male; solid line βp = 0, dotted line βp = −0.005, long dashed line βp = 0.005)
Fig. 5.
Age–period–cohort modeling for HL in all races by gender (a female, b male; solid line βp = 0, dotted line βp = −0.005, long dashed line βp = 0.005)
Discussion
The results of our earlier study of HL incidence in the state of Connecticut from 1935 to 1992 suggested that the incidence of this malignancy may likely stabilize in males and continue to increase in females in the coming years [6]. Given limited evidence concerning incidence patterns of HL in young adults in the USA, and the potential for birth cohort effects in explaining these trends, we conducted an APC analysis using data from SEER from 1973 to 2010 and observed that the age-adjusted incidence rate for young adults aged 20–44 continued to increase before 1988 and then began to stabilize after 1988 in females. However, our more comprehensive analyses based on SEER data that evaluated age-specific incidence patterns and birth cohort patterns confirmed our earlier observations in the state of Connecticut and suggest important gender differences in the time trends of HL among young adults in the USA.
The diverging direction of the cohort effect for men and women suggests that there were possible gender-related changes in exposure to risk factors for HL after 1975–1979 in particular. Epidemiologic studies conducted over the past few decades have identified several risk factors that are associated with this disease, including infection with the mononucleosis associated Epstein–Barr virus (EBV) and HIV, as well as socioeconomic status and having a family history [11]. Other factors, including occupational exposure to chemicals and reproductive factors such as parity, have also been reported in some studies although these associations have not been consistent [12-16].
EBV is a ubiquitous virus that infects more than 90 % of the population worldwide and is strongly associated with HL, as between 20 and 50 % of HL tumors are EBV positive in Western countries [17]. Several previous studies have evaluated gender differences in the prevalence of EBV infection. A cross-sectional study among young college students showed that the prevalence of EBV seropositivity was significantly greater among women (79.2 %) than among men (67.4 %) [18]. A case–case study also showed that EBV-positive HL appears to be more common in males [19]. Besides EBV, HIV-positive individuals have a significantly increased risk of HL [20]. A population-based study in the San Francisco Bay Area reported that HIV-related HL contributed to an elevation in the regional HL incidence rates, especially in young adults [21]. However, it is unlikely that infection with viral agents can explain the increasing birth cohort trends in females but not in males, particularly since the rate of new HIV infections in North America and Western and Central Europe has been relatively stable between 2001 and 2011 [22].
While gender-related changes in the prevalence of exposure to an environmental or lifestyle risk factor beginning around 1975 could potentially explain the observed birth cohort effects for HL incidence, few such factors have been strongly associated with HL in epidemiologic studies [23], making the trends difficult to interpret. A recent meta-analysis showed that ever smoking was associated with increased risk for HL [24]. However, during 1965-2010, an overall decrease was observed in the prevalence of cigarette smoking among adults for both genders and all races [25, 26]. Therefore, it is unlikely that the effect of smoking could explain the opposite gender-specific cohort effect trends. A previous meta-analysis has also indicated that obesity is positively associated with risk of HL [27], and some epidemiologic evidence has suggested effect modification between body size and risk of HL according to gender [28]. However, the results of the National Health and Nutrition Examination Survey suggested that obesity did not have a significant trend among women from 1999 to 2008 [29] and the prevalence of obesity in 2009–2010 did not show a significant change compared with 2003–2008 [30].
Epidemiologic studies have suggested that long-term use of low-dose aspirin, but not other NSAIDs, reduces HL risk [31, 32]. However, regular acetaminophen use was associated with the increased risk of HL [31]. Studies have showed that there are important racial/ethnic disparities in the prevalence of aspirin use [33], and women are less likely than men to use aspirin regularly among adults with coronary heart disease in the USA [34]. Thus, the association between HL and aspirin use is unlikely to explain why the cohort effect of females was increasing after the 1975 birth cohort.
A previous APC analysis of HL incidence that was conducted in Canada similarly observed significant increases in incidence among young adults from 1970 to 1995 and suggested that childbearing-related reproductive factors may explain the rising rates in young females aged 10–29 [5]. While reproductive factors are not considered a well-established risk factor for HL, as some recent data suggest no significant association [35], other epidemiologic data have suggested an inverse association for HL with increasing parity and earlier age of first child birth [36]. It is thus plausible that the societal shift of having fewer children and later pregnancies among women in the USA over the last two decades [25, 37] could at least partially explain the increasing birth cohort trends in females; however, additional studies will be needed to explore this possibility.
Our study has several limitations. First, we selected SEER 9 Regs Research Data as the data source in order to obtain a longer time interval for the analyses. This selection may limit the external validity of our study since the results may not reflect the diversity of the US population as a whole. Second, given that 86 % of the HL cases in the SEER database were of white race, we were unable to evaluate the APC trends among young adults of other race groups separately due to the small case numbers. However, we note that the incidence trends observed for whites were very similar to those observed for all racial groups combined. Third, it is likely that there was some misclassification of non-Hodgkin and Hodgkin lymphomas [38]. However, no evidence suggests that there were gender disparities in the occurrence of this disease misclassification, and therefore, this may not be an important factor when considering the different cohort effects according to gender. Finally, the lack of individual risk factor data and the complexity of the etiology of this disease in relation to the observed birth cohort patterns limited the inferences that could be made from our study results.
In conclusion, our analysis of HL incidence from 1973 to 2010 using data from SEER suggests that the birth cohort trend is still increasing for females in the USA, whereas for males there was a decreasing cohort trend beginning in the 1960 birth cohort. The few established risk factors that have been identified for HL can seemingly not explain the gender disparities of the cohort pattern, which necessitates further analytical epidemiological studies to explore the risk factors for this disease with respect to potential differences by gender and by histological subtype.
Acknowledgments
This work was partly supported by Fogarty training Grants D43TW 008323 and D43TW 007864-01 from the National Institutes of Health.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Contributor Information
Cairong Zhu, West China School of Public Health, Sichuan University, Chengdu 610041, China; Department of Environmental Health Sciences, Yale School of Public Health, Yale University, 60 College Street, LEPH 442, New Haven, CT 06520-8034, USA.
Bryan A. Bassig, Department of Environmental Health Sciences, Yale School of Public Health, Yale University, 60 College Street, LEPH 442, New Haven, CT 06520-8034, USA
Kunchong Shi, Department of Environmental Health Sciences, Yale School of Public Health, Yale University, 60 College Street, LEPH 442, New Haven, CT 06520-8034, USA.
Peter Boyle, International Prevention Research Institute, Lyon, France.
Huan Guo, Department of Environmental Health Sciences, Yale School of Public Health, Yale University, 60 College Street, LEPH 442, New Haven, CT 06520-8034, USA; School of Public Health, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, Hubei, China.
Tongzhang Zheng, Department of Environmental Health Sciences, Yale School of Public Health, Yale University, 60 College Street, LEPH 442, New Haven, CT 06520-8034, USA.
References
- 1.Ries LAG. SEER survival monograph. U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute; Bethesda, MD: 2007. Cancer survival among adults: U.S. SEER program 1988–2001 patient and tumor characteristics. [Google Scholar]
- 2.Ariad S, Lipshitz I, Benharroch D, Gopas J, Barchana M. A sharp rise in the incidence of Hodgkin’s lymphoma in young adults in Israel. Isr Med Assoc J. 2009;11(8):453–455. [PubMed] [Google Scholar]
- 3.Hjalgrim H, Seow A, Rostgaard K, Friborg J. Changing patterns of Hodgkin lymphoma incidence in Singapore. Int J Cancer. 2008;123(3):716–719. doi: 10.1002/ijc.23504. [DOI] [PubMed] [Google Scholar]
- 4.Hjalgrim H, Askling J, Pukkala E, Hansen S, Munksgaard L, Frisch M. Incidence of Hodgkin’s disease in Nordic countries. Lancet. 2001;358(9278):297–298. doi: 10.1016/S0140-6736(01)05498-8. [DOI] [PubMed] [Google Scholar]
- 5.Liu S, Semenciw R, Waters C, Wen SW, Mao Y. Time trends and sex patterns in Hodgkin’s disease incidence in Canada, 1970–1995. Can J Public Health. 2000;91(3):188–192. doi: 10.1007/BF03404269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen YT, Zheng T, Chou MC, Boyle P, Holford TR. The increase of Hodgkin’s disease incidence among young adults: experience in Connecticut, 1935–1992. Cancer. 1997;79(11):2209–2218. [PubMed] [Google Scholar]
- 7.Evens AM, Antillon M, Aschebrook-Kilfoy B, Chiu BC. Racial disparities in Hodgkin’s lymphoma: a comprehensive population-based analysis. Ann Oncol. 2012;23(8):2128–2137. doi: 10.1093/annonc/mdr578. [DOI] [PubMed] [Google Scholar]
- 8.Shenoy P, Maggioncalda A, Malik N, Flowers CR. Incidence patterns and outcomes for Hodgkin lymphoma patients in the United States. Adv Hematol. 20112011:725219. doi: 10.1155/2011/725219. doi:10.1155/2011/725219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS. Lymphoma incidence patterns by WHO subtype in the United States, 1992–2001. Blood. 2006;107(1):265–276. doi: 10.1182/blood-2005-06-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holford TR. Monitoring the health of populations statistical principles and methods for public health surveillance. Oxford Univerity Press; Oxford: 2003. [Google Scholar]
- 11.Cartwright RA, Watkins G. Epidemiology of Hodgkin’s disease: a review. Hematol Oncol. 2004;22(1):11–26. doi: 10.1002/hon.723. [DOI] [PubMed] [Google Scholar]
- 12.Glaser SL, Clarke CA, Nugent RA, Stearns CB, Dorfman RF. Reproductive factors in Hodgkin’s disease in women. Am J Epidemiol. 2003;158(6):553–563. doi: 10.1093/aje/kwg198. [DOI] [PubMed] [Google Scholar]
- 13.Morton LM, Wang SS, Richesson DA, Schatzkin A, Hollenbeck AR, Lacey JV. Reproductive factors, exogenous hormone use and risk of lymphoid neoplasms among women in the National Institutes of Health-AARP Diet and Health Study Cohort. Int J Cancer. 2009;124(11):2737–2743. doi: 10.1002/ijc.24248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miligi L, Costantini AS, Benvenuti A, Kriebel D, Bolejack V, Tumino R, Ramazzotti V, Rodella S, Stagnaro E, Crosignani P, Amadori D, Mirabelli D, Sommani L, Belletti I, Troschel L, Romeo L, Miceli G, Tozzi GA, Mendico I, Vineis P. Occupational exposure to solvents and the risk of lymphomas. Epidemiology. 2006;17(5):552–561. doi: 10.1097/01.ede.0000231279.30988.4d. [DOI] [PubMed] [Google Scholar]
- 15.Zahm SH, Hoover RN, Fraumeni JF., Jr Hodgkin’s disease and parity. Int J Cancer. 1995;62(3):362–363. doi: 10.1002/ijc.2910620322. [DOI] [PubMed] [Google Scholar]
- 16.Glaser SL. Reproductive factors in Hodgkin’s disease in women: a review. Am J Epidemiol. 1994;139(3):237–246. doi: 10.1093/oxfordjournals.aje.a116990. [DOI] [PubMed] [Google Scholar]
- 17.Flavell KJ, Murray PG. Hodgkin’s disease and the Epstein-Barr virus. Mol Pathol. 2000;53(5):262–269. doi: 10.1136/mp.53.5.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crawford DH, Swerdlow AJ, Higgins C, McAulay K, Harrison N, Williams H, Britton K, Macsween KF. Sexual history and Epstein-Barr virus infection. J Infect Dis. 2002;186(6):731–736. doi: 10.1086/342596. [DOI] [PubMed] [Google Scholar]
- 19.Chang ET, Zheng T, Lennette ET, Weir EG, Borowitz M, Mann RB, Spiegelman D, Mueller NE. Heterogeneity of risk factors and antibody profiles in epstein-barr virus genome-positive and -negative hodgkin lymphoma. J Infect Dis. 2004;189(12):2271–2281. doi: 10.1086/420886. [DOI] [PubMed] [Google Scholar]
- 20.Carbone A, Gloghini A, Serraino D, Spina M. HIV-associated Hodgkin lymphoma. Curr Opin HIV AIDS. 2009;4(1):3–10. doi: 10.1097/COH.0b013e32831a722b. [DOI] [PubMed] [Google Scholar]
- 21.Glaser SL, Clarke CA, Gulley ML, Craig FE, DiGiuseppe JA, Dorfman RF, Mann RB, Ambinder RF. Population-based patterns of human immunodeficiency virus-related Hodgkin lymphoma in the Greater San Francisco Bay Area, 1988–1998. Cancer. 2003;98(2):300–309. doi: 10.1002/cncr.11459. [DOI] [PubMed] [Google Scholar]
- 22.UNAIDS Global fact sheet 2012. 2012 http://www.unaids.org/en/resources/campaigns/20121120_globalreport2012/factsheet/
- 23.Maggioncalda A, Malik N, Shenoy P, Smith M, Sinha R, Flowers CR. Clinical, molecular, and environmental risk factors for Hodgkin lymphoma. Adv Hematol. 20112011:736261. doi: 10.1155/2011/736261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sergentanis TN, Kanavidis P, Michelakos T, Petridou ET. Cigarette smoking and risk of lymphoma in adults: a comprehensive meta-analysis on Hodgkin and non-Hodgkin disease. Eur J Cancer Prev. 2013;22(2):131–150. doi: 10.1097/CEJ.0b013e328355ed08. [DOI] [PubMed] [Google Scholar]
- 25.Frieden TR. Forward: CDC health disparities and inequalities report—United States, 2011. MMWR Surveill Summ. 2011;60(uppl):1–2. [PubMed] [Google Scholar]
- 26.King B, Dube S, Kaufmann R, Shaw L, Pechacek T. Vital signs: current cigarette smoking among adults aged >=18 years-United States, 2005–2010 (Reprinted from MMWR, vol 60, pg 1207–1212, 2011) JAMA. 2011;306(17):1857–1860. [Google Scholar]
- 27.Larsson SC, Wolk A. Body mass index and risk of non-Hodgkin’s and Hodgkin’s lymphoma: a meta-analysis of prospective studies. Eur J Cancer. 2011;47(16):2422–2430. doi: 10.1016/j.ejca.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 28.Li Q, Chang ET, Bassig BA, Dai M, Qin Q, Gao Y, Zhang Y, Zheng T. Body size and risk of Hodgkin’s lymphoma by age and gender: a population-based case–control study in Connecticut and Massachusetts. Cancer Causes Control. 2013;24(2):287–295. doi: 10.1007/s10552-012-0100-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303(3):235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 30.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307(5):491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 31.Chang ET, Zheng T, Weir EG, Borowitz M, Mann RB, Spiegelman D, Mueller NE. Aspirin and the risk of Hodgkin’s lymphoma in a population-based case–control study. J Natl Cancer Inst. 2004;96(4):305–315. doi: 10.1093/jnci/djh038. [DOI] [PubMed] [Google Scholar]
- 32.Chang ET, Froslev T, Sorensen HT, Pedersen L. A nationwide study of aspirin, other non-steroidal anti-inflammatory drugs, and Hodgkin lymphoma risk in Denmark. Br J Cancer. 2011;105(11):1776–1782. doi: 10.1038/bjc.2011.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanchez DR, Diez Roux AV, Michos ED, Blumenthal RS, Schreiner PJ, Burke GL, Watson K. Comparison of the racial/ethnic prevalence of regular aspirin use for the primary prevention of coronary heart disease from the multi-ethnic study of atherosclerosis. Am J Cardiol. 2011;107(1):41–46. doi: 10.1016/j.amjcard.2010.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Opotowsky AR, McWilliams JM, Cannon CP. Gender differences in aspirin use among adults with coronary heart disease in the United States. J Gen Intern Med. 2007;22(1):55–61. doi: 10.1007/s11606-007-0116-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Costas L, Casabonne D, Benavente Y, Becker N, Boffetta P, Brennan P, Cocco P, Foretova L, Maynadie M, Staines A, Kane E, Nieters A, de Sanjose S. Reproductive factors and lymphoid neoplasms in Europe: findings from the EpiLymph case–control study. Cancer Causes Control. 2012;23(1):195–206. doi: 10.1007/s10552-011-9869-6. [DOI] [PubMed] [Google Scholar]
- 36.Kravdal O, Hansen S. Hodgkin’s disease: the protective effect of childbearing. Int J Cancer. 1993;55(6):909–914. doi: 10.1002/ijc.2910550606. [DOI] [PubMed] [Google Scholar]
- 37.Mathews TJ, Hamilton BE. Mean age of mother, 1970–2000. Natl Vital Stat Rep. 2002;51(1):1–13. [PubMed] [Google Scholar]
- 38.Glaser SL, Swartz WG. Time trends in Hodgkin’s disease incidence: the role of diagnostic accuracy. Cancer. 1990;66(10):2196–2204. doi: 10.1002/1097-0142(19901115)66:10<2196::aid-cncr2820661026>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]