Abstract
Objective
To test the efficacy of adjunctive ziprasidone in adults with non-psychotic unipolar major depression experiencing persistent symptoms following 8 weeks of open-label escitalopram.
Method
This was a multi-center, parallel randomized, double-blind, placebo-controlled trial conducted at three academic medical centers in the United States. The participant pool consisted of 139 outpatients with persistent symptoms of major depressive disorder following an 8-week open label trial of escitalopram (phase 1). Subjects were randomized (1:1, n=139) to adjunctive ziprasidone (escitalopram+ziprasidone, n=71) or adjunctive placebo (escitalopram+placebo, n=68), with 8 weekly follow-up assessments. Primary outcome was defined by clinical response according to the 17-item Hamilton Depression Rating Scale (HAMD-17) and determined by a 50% or greater reduction in scale scores. The Hamilton Anxiety Rating scale (HAM-A) and Visual Analogue Scale for Pain were defined a priori as key secondary outcome measures.
Results
Rates of clinical response (35.2% vs. 20.5%, p=0.04) and mean improvement in HAMD-17 total scores (−6.4 ± 6.4 vs. −3.3 ± 6.2, p=0.04) were significantly greater for the escitalopram+ziprasidone group. Several secondary measures of antidepressant efficacy were also in favor of adjunctive ziprasidone. Escitalopram+ziprasidone also resulted in significantly greater improvement in HAM-A, but not Visual Analogue Scale for Pain scores. Ten (14%) patients discontinued escitalopram+ziprasidone due to intolerance versus none for escitalopram+placebo (p<0.01 versus placebo).
Conclusions
Adjunctive ziprasidone, when added to escitalopram, demonstrated antidepressant efficacy in adult patients with major depressive disorder experiencing persistent symptoms following 8 weeks of open-label escitalopram.
Introduction
Despite the increase in the number of medications approved by the U.S. Food and Drug Administration (FDA) for use as monotherapy in patients with major depressive disorder, (1) many patients suffering from depression continue to remain symptomatic despite one or more treatment trials of adequate dose and duration. (2) As a result, achieving remission for many such patients often requires the use of adjunctive treatment strategies including antidepressant augmentation with a variety of pharmacologic and nonpharmacologic agents. (3)
Atypical antipsychotics represent one possible adjunctive therapy for the management of treatment-resistant major depression. (4–6) To date, numerous randomized, double-blind, placebo controlled trials have been published utilizing either aripiprazole, olanzapine, quetiapine, or risperidone as adjunctive therapy for major depressive disorder, (7,8) studies which resulted in their regulatory approval of three of these agents (aripiprazole, olanzapine, and quetiapine) in the U.S. for this indication.
There are several pharmacologic and clinical properties of the atypical antipsychotic agent, ziprasidone, that provide the clinical rationale for testing it as an adjunctive therapy in major depressive disorder. Ziprasidone possesses the highest 5-HT2A/D2 receptor binding affinity ratio of all FDA-approved antipsychotic medications. (9,10) Ziprasidone also acts as a serotonin-1A (5-HT1A)-receptor partial agonist9 and the 5-HT1A partial agonist aripiprazole is approved for adjunctive therapy for major depressive disorder. Ziprasidone has also been shown (in vitro) to inhibit the neuronal uptake of serotonin and norepinephrine via their relevant transporters, with potency comparable to that of the antidepressants imipramine and desipramine, (11) as well as to inhibit the neuronal uptake of dopamine, properties which also distinguish it pharmacologically from the other atypical antipsychotic drugs. Our group had previously conducted and published the results of an open-label ziprasidone augmentation study for treatment-resistant major depression. (12) In that study, 10 of 20 patients (50%) with major depressive disorder who failed to respond to an adequate therapeutic trial of an SSRI had a positive antidepressant response after 6 weeks of treatment with ziprasidone in addition to their SSRI. To our knowledge, these promising but preliminary results have not yet been confirmed in a randomized trial. We now report the results of an 8-week, NIMH-funded, randomized, double-blind, placebo controlled trial of ziprasidone augmentation of the selective serotonin reuptake inhibitor (SSRI) escitalopram for patients with persistent major depressive disorder symptoms despite participation in a prospective, 8-week open-label trial of flexible-dose lead-in treatment with escitalopram. The primary study hypothesis was that adjunctive ziprasidone would demonstrate greater antidepressant effects than adjunctive placebo. Due to its ability to block the reuptake of serotonin and norepinephrine, similar to the antidepressants venlafaxine (13) and duloxetine (14), the two a priori defined key secondary hypotheses were that adjunctive ziprasidone would demonstrate greater anxiolytic effects than adjunctive placebo, as well as a greater effect on somatic pain.
Methods
Study Design
This was an 8-week, randomized, double-blind, parallel-group, placebo controlled trial of ziprasidone augmentation of escitalopram for patients with major depressive disorder experiencing the persistence of symptoms despite an 8-week, prospective, open-label, flexible-dose trial of escitalopram.
The study was conducted at three academic medical centers in the U.S. (Massachusetts General Hospital, University of Alabama, and Vanderbilt University), from July 2008 to October 2013. The study was approved by local institutional review boards (IRB)’s and written informed consent was obtained from all study subjects before any study procedures were carried out. This study was carried out in two phases, beginning with an 8-week single-arm open trial of escitalopram (Phase 1), followed by 8 additional weeks of randomized, double-blind treatment with either adjunctive ziprasidone or placebo (Phase 2). Eligible participants for phase 1 of the study were men or women 18–65 years of age with a primary diagnosis of current major depressive disorder according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria confirmed by the Structured Clinical Interview for DSM-IV (SCID-I/P), (15) and a 16-item Quick Inventory of Depressive Symptomatology-Self Rated scale (QIDS-SR) (16) total score ≥ 10 at screening.
Patients were considered ineligible for participation in the study if they were pregnant, breastfeeding, or lactating women, women of child bearing potential who were not using a medically accepted means of contraception. In addition, patients at high suicide or homicide risk or otherwise deemed unsafe to enroll in the study by the investigator, diagnosed with unstable medical illness, uncontrolled seizure disorder, a history of multiple adverse reactions or an allergy to the study drug, with a DSM-IV diagnosis of a substance use disorder active within the last six months, bipolar disorder (current or past), or any psychotic disorder or psychotic symptoms (current or past) were also excluded. Patients who had received an adequate trial of escitalopram during the current major depressive episode (prior to study entry), or any lifetime trial of ziprasidone were also excluded, as were patients who received an investigational psychotropic drug within 3 months of their screening visit. In addition, patients who had failed more than 3 antidepressant trials of adequate dose and duration during the current major depressive episode were excluded. Examples of adequate dosage of an antidepressant trial included either ≥ 150mg of imipramine (or its tricyclic antidepressant equivalent), ≥ 60mg of phenelzine (or its monoamine oxidase inhibitor equivalent), ≥ 20mg of fluoxetine (or its SSRI-equivalent), ≥ 150mg of bupropion, ≥ 300mg of trazodone (or nefazodone), ≥75mg of venlafaxine, ≥60mg of duloxetine, or ≥ 15mg of mirtazapine. A trial of adequate duration was defined as one during which the patient was on any given antidepressant at an adequate dose for a minimum of 6 weeks. Finally, patients on an antidepressant or antipsychotic medication were excluded from the trial.
Study Procedures
Open-label trial of escitalopram (Phase 1)
Subjects who were found eligible to participate at the screening visit began an 8-week open-label trial of escitalopram, initiated at a daily dose of 10 mg. During Phase 1, subjects returned to the clinic for a total of 8 weekly visits. Clinician rated and self-report scales were administered during each of these visits, including the 17-item Hamilton Depression Scale (HAMD-17), (17) the Clinical Global Impressions- Severity and Improvement scales (CGI-S, CGI-I), (18) and the QIDS-SR. During the first 4 weeks, escitalopram doses could be increased by 10mg increments per week, up to a maximum dose of 30 mg daily, as deemed clinically necessary and appropriate by the treating clinician and with subjects’ consent. All subjects remained on a stable dose after week 4 for the remaining duration of the open-label (Phase 1) and subsequent double-blind trial (Phase 2), unless reductions in daily escitalopram dose were deemed clinically necessary. The minimal acceptable escitalopram dose for the study was 10mg. Patients were permitted to take the following psychotropic concomitant medications, provided that daily doses remained stable throughout Phases 1 and 2 and had been stable for at least two weeks prior to screening: benzodiazepine- or benzodiazepine-like agents, anticonvulsants, lithium, and buspirone.
Double-blind trial of adjunctive ziprasidone versus placebo (Phase 2)
At the end of the open-label trial, subjects who continued to meet DSM-IV criteria for major depressive disorder; had a QIDS-SR score ≥ 10; and did not have abnormal serum potassium or magnesium levels, evidence of untreated hypothyroidism, a positive urine drug screen, or significant cardiac conduction problems such as atrial fibrillation, atrial flutter, atrio-ventricular block, of disqualifying ECG changes (prolonged QTc or QRS intervals) were enrolled in the double-blind phase of the study (Phase 2). The total duration of the second phase of the study was 8 weeks, conducted in weekly visits. All visits during the double-blind trial operated according to a standard manual. (19) In addition to the HAMD-17, CGI, and QIDS-SR, the 14-item Hamilton Anxiety Rating Scale (HAM-A), (20) and Visual Analogue Scale for Pain (21) were administered during all visits.
Patients were randomized to receive adjunctive ziprasidone or placebo throughout the remainder of the study in a 1:1 fashion. A central randomization center used computer generated a list of random numbers to allocate treatments. None of the investigators, study clinicians, clinical raters, or subjects at any of the study sites had access to the randomization list. Ziprasidone (20 mg per capsule) and placebo were in capsule form and identical in appearance. Independent pharmacists prepared ziprasidone and placebo capsules. Study drugs were pre-packed in bottles and consecutively numbered for each patient according to the randomization schedule. All patients were instructed to take one capsule of study medication twice daily with a full meal in addition to continuing on the same dosage of escitalopram that they were on at the end of Phase 1. Following the first Phase 2 visit and throughout the eight subsequent weekly visits, study clinicians could increase a subject’s dose of the study drug in 1-capsule, twice-per-day, weekly increments, yielding a daily possible ziprasidone dosage range of 20–80mg twice daily (40–160mg total daily dose). When deemed appropriate, study clinicians could also lower the dosage of study medication to address intolerable or uncomfortable side effects. Patients unable to tolerate a minimum dosage of 10mg of escitalopram and 20mg of study drug (ziprasidone or placebo) were withdrawn from the study. Compliance was determined at each study visit by pill count, and subjects with less than 80% compliance were withdrawn from the study.
Outcome measures
The a priori defined primary outcome measure for testing of the primary study hypothesis (antidepressant efficacy) was clinical response, defined as a 50% or greater reduction (improvement) from baseline to endpoint in HAMD-17 total score. The HAM-A and Visual Analogue Scale for Pain were selected a priori as the outcome measures of choice to test for the two key secondary hypotheses (anxiolytic and analgesic efficacy) of the study. The QIDS-SR, and CGI were included as secondary outcome measures of antidepressant efficacy. Clinical response for the QIDS-SR, and HAM-A was defined similar to that for the HAMD-17. Remission on the HAMD-17 and HAM-A was defined as a final score of 7 or less, while remission on the QIDS-SR was defined as a final score of 5 or less.
Statistical Analysis
For the calculation of sample size relative to power we selected a targeted treatment effect of a difference in the proportions of responders on the HAMD-17 of 0.22. Specifically, we assumed that approximately 57% of patients receiving adjunctive ziprasidone and 35% of patients receiving adjunctive placebo would meet criteria for response (based on a meta-analysis with the most up-do-date data available at the time7. Thus, we projected that a study involving 8 weekly visits assessing outcome and randomizing 160 patients in a 1:1 fashion to adjunctive ziprasidone- versus adjunctive placebo- would have a power of at least 80% to detect a treatment difference setting alpha at 0.05 (two tailed).
The intent-to-treat dataset including all randomized patients was utilized for all analyses. All subjects were analyzed according to their allocated treatment group. Categorical baseline characteristics were compared between treatment groups with chi-square tests. Continuous baseline data were analyzed via t-test. A mixed effect model with repeated measures approach was used to model the effects of treatment for all efficacy analyses, adjusting for baseline severity. All tests were conducted with a significance level of 0.05 (2-sided), with no adjustments for multiplicity, using STATA SE Version 12 statistical software.
Results
Subject Demographic Characteristics, Clinical Characteristics, and Follow-Up
531 subjects were screened across the three sites, resulting in 458 (86.2%) outpatients meeting eligibility for enrollment in Phase 1 of the study. Of these patients, 311 (67.9%) completed the first phase of the study, of which 139 eligible patients were randomized to double-blind treatment with adjunctive ziprasidone (20–80 mg twice daily) or adjunctive placebo (the others were not eligible either due to premature discontinuation from the study, or not meeting inclusion criteria for the double-blind phase). Mean (sd) HAMD-17 scores for these 139 patients at the initial study visit (baseline of Phase 1) was 20.0 (4.4). Mean (sd) number of historical trials of antidepressants failed during the current episode were 0.94 (0.76). Four (2.8%) of these patients participating in the randomized phase responded to the escitalopram lead-in phase according to the HAMD-17 (50% or greater reduction in scores during phase 1) but were randomized because they met inclusion criteria as stated in the methods section (continued to meet DSM-IV criteria for major depressive disorder and had a QIDS-SR score ≥ 10 by the end of the lead-in). The remainder were non-responders according to the HAMD-17.
Baseline demographic and clinical data for these patients are presented in Table 1. There were no statistically significant differences at baseline between the two treatment groups for any of these variables. Forty-nine (69.0%) adjunctive ziprasidone– and 53 (77.9%) placebo– treated patients completed the trial (p=0.23). Ten (14%) patients discontinued adjunctive ziprasidone due to intolerance (four for anxiety and agitation/akathisia, two because of sedation, one because of insomnia and one due to a QTc interval greater than 500msec at week 2) versus none for placebo (p<0.01 versus placebo). Three (4.2%) patients discontinued adjunctive ziprasidone due to inefficacy, 3 (4.2%) were lost to follow-up, and 6 (8.4%) discontinued adjunctive ziprasidone for various other reasons. Respective numbers for the placebo arm were 1 (1.4%), 7 (10.2%) and 7 (10.2%) (all comparisons with adjunctive ziprasidone were not statistically significant). Mean (sd) daily study pills for patients in the adjunctive ziprasidone and placebo group were 4.9 (2.0) versus 6.3 (2.3), respectively (p<0.01), corresponding to a mean (sd) daily dose of 98mg (40mg) for ziprasidone-treated patients. Corresponding mean ziprasidone doses for responders and remitters, respectively, were 96.0 mg (32.6) and 96.9 mg (32.3).
Table 1.
Demographic and clinical characteristics at baseline of patients with major depression receiving blinded pharmacotherapy with either ziprasidone or placebo augmentation of escitalopram.a
| Ziprasidone + Escitalopram | Placebo + Escitalopram | p- value | |||
|---|---|---|---|---|---|
| (n=71)* | (n=68)** | ||||
| Mean | SD | Mean | SD | ||
| Female sex, n (%) | 49.0 | 69.0 | 49.0 | 72.0 | 0.15 |
| Age, years | 44.7 | 13.8 | 44.2 | 11.9 | 0.81 |
| Escitalopram dose | 21.0 | 5.8 | 19.2 | 3.1 | 0.16 |
| HAMD-17 | 17.6 | 4.9 | 17.3 | 4.4 | 0.74 |
| QIDS-SR | 14.2 | 3.9 | 13.2 | 3.2 | 0.12 |
| CGI-S | 3.8 | 0.8 | 3.8 | 0.6 | 0.80 |
| HAM-A | 13.5 | 4.6 | 13.5 | 4.5 | 0.98 |
| VAS-Pain | 33.1 | 30.2 | 38.9 | 33.5 | 0.28 |
All values are presented as mean (SD), unless otherwise indicated, at the beginning of Phase 2.
N=71 for all variables in the ziprasidone+escitalopram condition.
N=68 for all variables in the placebo+escitalopram condition.
Key: CGI-S = Clinical Global Impression severity subscale; HAM-A = Hamilton Anxiety Rating Scale; HAMD-17 = 17-item Hamilton Depression Rating Scale; QIDS-SR = 16-item self-rated Quick Inventory of Depressive Symptoms; VAS-Pain = Visual Analogue Scale for Pain.
Antidepressant Efficacy
Results for primary and secondary efficacy measures over 8 weeks of double-blind (Phase 2) treatment are presented in Table 2 and Figure 1. Rates of HAMD-17-defined clinical response, the study primary outcome measure, were significantly higher with adjunctive ziprasidone (n=25 [35.2%]) than adjunctive placebo according to mixed effect model with repeated measures analyses (n=14 [20.5%], p=0.04). Adjunctive ziprasidone was superior to placebo on most secondary outcome measures for depression as well according to mixed effect model with repeated measures analyses (Table 2). The standardized between-treatment effect size (Cohen’s d) was 0.53.
Table 2.
Response rates, remission rates, and change in psychopathology and pain measures for patients with major depression receiving blinded pharmacotherapy with either ziprasidone or placebo augmentation of escitalopram.
| Ziprasidone + Escitalopram | Placebo + Escitalopram | p- value | |||
|---|---|---|---|---|---|
| (n=71) | (n=68) | ||||
| N | % | N | % | ||
| HAMD-17 responsea | 25 | 35.2 | 14 (20.5) | 20.5 | 0.04 |
| HAMD-17 remissionb | 27 | 38.0 | 21 (30.8) | 30.8 | 0.32 |
| QIDS-SR responsea | 22 | 30.9 | 9 (13.2) | 13.2 | 0.03 |
| QIDS-SR remissionb | 17 | 23.9 | 7 (10.2) | 10.2 | 0.02 |
| HAM-A responsea | 25 | 35.2 | 7 (10.2) | 10.2 | <0.001 |
| HAM-A remissionb | 32 | 45.0 | 14 (20.5) | 20.5 | <0.01 |
| Mean c | (SD) | Mean c | (SD) | ||
| HAMD-17 change from baseline | −6.4 | 6.4 | −3.3 | 6.2 | 0.04 |
| QIDS-SR change from baseline | −4.5 | 4.7 | −2.0 | 3.2 | 0.03 |
| CGI-S change from baseline | −1.0 | 1.4 | −0.6 | 0.9 | 0.02 |
| CGI-I at study endpoint | 2.4 | 1.2 | 2.9 | 1.2 | 0.06 |
| HAM-A change from baseline | −3.5 | 6.2 | −1.5 | 4.9 | 0.04 |
| VAS-pain change from baseline | −5.1 | 29.8 | −0.8 | 33.1 | 0.46 |
Positive treatment response was defined as a ≥ 50% reduction (improvement) from baseline values.
Remission was defined as a total score of 7 or less on the HAMD-17 or HAM-A (or total score of 5 or less on the QIDS-SR) at study endpoint.
Values reported as mean change from baseline represent mean changes in total scores from baseline to study endpoint.
Key: CGI-S = Clinical Global Impression severity subscale; HAM-A = Hamilton Anxiety Rating Scale; HAMD-17 = 17-item Hamilton Depression Rating Scale; QIDS-SR = 16-item self-rated Quick Inventory of Depressive Symptoms; VAS-Pain = Visual Analogue Scale for Pain.
Figure 1.
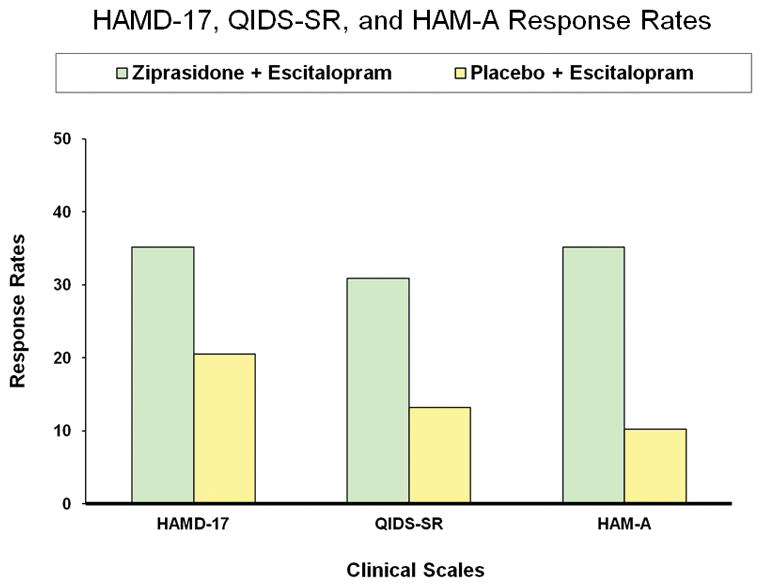
Figure Title: HAMD-17, QIDS-SR, and HAM-A Response Rates; Y-Axis Title: Response Rates; X-Axis Title: Clinical Scales; Footnotes (P values for Ziprasidone+Escitalopram condition): HAMD-17 (p= 0.04 MMRM); QIDS-SR (p=0.03 MMRM); HAM-A (p<0.001 MMRM).
Anxiolytic Efficacy and Effect on Somatic Pain Scores
Adjunctive ziprasidone therapy resulted in significantly greater anxiolytic effects but not greater effects on the Visual Analogue Scale for Pain than placebo, according to mixed effect model with repeated measures analyses than placebo (Table 2). The former included significantly greater baseline to endpoint reduction in HAM-A total scores and significantly higher HAM-A –defined positive response rates with adjunctive ziprasidone than placebo (Table 2).
Study Adverse Events
The most frequently occurring (minimum of 5% frequency in at least one group) treatment- emergent adverse events are shown in Table 3. There were significantly higher rates of somnolence/fatigue, irritability, anxiety/agitation, and muscle twitching in the adjunctive ziprasidone group, as compared with the adjunctive placebo group. Numerically higher rates of treatment-emergent akathisia (self-report) were observed in the adjunctive ziprasidone group than the placebo group; however, these differences occurred at the level of statistical trend. There were no significant between-group differences in reported rates of sexual dysfunction or weight gain. Two serious adverse events (SAEs) occurred during Phase 2 among patients treated with adjunctive ziprasidone (one hospitalization due to treatment-emergent suicidal ideation, and one hospitalization due to a fall), both of which were reviewed in detail and deemed to be unrelated to the study medication. Two SAEs occurred during Phase 2 in the adjunctive placebo group (hospitalizations due to treatment-emergent viral meningitis and pneumonia, respectively).
Table 3.
Frequency of adverse events for patients with major depression receiving blinded pharmacotherapy with either ziprasidone or placebo augmentation of escitalopram.
| Ziprasidone + Escitalopram | Placebo + Escitalopram | p- value | |||
|---|---|---|---|---|---|
| (n=71) | (n=68) | ||||
| N | % | N | % | ||
| Somnolence/fatigue | 24 | 33.8 | 8 | 11.7 | 0.002 |
| Akathisia | 11 | 15.4 | 5 | 7.3 | 0.13 |
| Headaches | 5 | 7.0 | 9 | 13.2 | 0.23 |
| Irritability | 7 | 9.8 | 1 | 1.4 | 0.03 |
| Poor concentration/memory | 6 | 8.4 | 1 | 1.4 | 0.06 |
| Insomnia | 6 | 8.4 | 6 | 8.8 | 0.94 |
| Dizziness | 5 | 7.0 | 3 | 4.4 | 0.51 |
| Anxiety/agitation | 4 | 5.6 | 0 | 0.0 | 0.05 |
| Muscle twitching | 8 | 11.2 | 1 | 1.4 | 0.02 |
| Dry mouth | 7 | 9.8 | 11 | 16.1 | 0.27 |
| Nausea | 3 | 4.2 | 9 | 13.2 | 0.06 |
| Gastrointestinal upset | 8 | 11.2 | 3 | 4.4 | 0.13 |
| Sexual dysfunction | 7 | 9.8 | 5 | 7.3 | 0.60 |
| Diarrhea | 4 | 5.6 | 7 | 10.2 | 0.31 |
| Weight gain | 1 | 1.4 | 4 | 5.8 | 0.16 |
Includes adverse effects that occurred at an incidence rate of 5% of greater in either treatment group.
Discussion
The present work is the first randomized, double-blind, placebo-controlled trial to compare the efficacy of adjunctive ziprasidone, an atypical antipsychotic agent, with adjunctive placebo in outpatients with major depressive disorder. The study employed an 8-week prospective, open lead-in involving the use of flexible doses of the SSRI escitalopram, requiring patients to demonstrate the persistence of depressive symptoms in order to be eligible for randomization in the double-blind phase of the study. The results of this study show adjunctive ziprasidone to have superior antidepressant efficacy to adjunctive placebo in escitalopram-treated patients with major depressive disorder. Statistical significance was demonstrated on the study primary outcome measure (response rates according to the 17-item Hamilton Depression Rating Scale) was well as most secondary measures of antidepressant efficacy (the degree of change on HAMD-17, QIDS-SR, and CGI-S, and QIDS-SR response). The number needed to treat for response according to the HAMD-17 was approximately 7, a similar order of magnitude as other atypical antipsychotic drugs when used as adjuncts to antidepressant treatment of major depressive disorder. (7, 8, 22) Of note is also a very low placebo response rate (mean HAMD-17 score reduction in the placebo group was 3.3 points – one of the lowest ever published in major depressive disorder) which was probably due, in part, to the use of an open-label lead-in with escitalopram. (23) This clearly aided with “signal detection” in this study, (24) although at the expense of efficiency, since only 139 (26.1%) of 531 subjects enrolled were randomized. Alternative designs may have preserved the present effect size with better efficiency. (25)
In parallel, anxiolytic efficacy (a secondary study hypothesis) was also shown as evidenced by significantly greater response rates on the HAM-A among adjunctive ziprasidone- than placebo-treated patients. The number needed to treat for response on the HAM-A was 4. This finding is of clinical relevance since the presence of co-morbid anxiety symptoms in patients with major depressive disorder represents a distinct clinical challenge for patients and clinicians alike. (26–28) The same was not observed with respect to somatic pain, where a statistically significant difference in the reduction of such symptoms between the two treatment arms was not observed. Finally, a greater proportion of patients discontinued adjunctive ziprasidone than placebo in this trial due to intolerance (principally due to sedation or “activation”-type adverse events (anxiety, agitation, insomnia), with a number needed to harm (NNH- intolerance) of 10. This yields a number needed to treat/NNH ratio of 0.7, indicating a favorable benefit relative to risk. Moreover, serious adverse events in the study were equal with ziprasidone and placebo. What was surprising, however, was the number of patients who developed fatigue/somnolence on adjunctive ziprasidone (approximately one in three patients – NNH=4) given that, unlike quetiapine and olanzapine, ziprasidone does not have any appreciable affinity for histaminic or muscarinic receptors. Interestingly enough, a review of quetiapine and olanzapine cites similar rates of somnolence/fatigue as those for ziprasidone in the present trial, (29) while rates of somnolence and fatigue with ziprasidone appear to be higher than those reported with adjunctive aripiprazole. (22)
Several possible limitations should be taken into consideration when interpreting the results of the present study. First, the study was designed to focus on the efficacy of adjunctive ziprasidone when specifically combined with the SSRI escitalopram. Whether results would have been similar or different had we also augmented other antidepressants with ziprasidone remains unclear. Second, the present trial involved a number of inclusion and exclusion criteria. Whether adjunctive ziprasidone is also efficacious among patients who meet such exclusion criteria (i.e. at imminent risk of suicide, with serious or unstable medical illness, the elderly, etc.) is unknown. Third, although the present study enrolled patients who failed anywhere between one and four antidepressant trials (and as a result, its findings are generalizeable to such patients in clinical practice), whether clinicians chose to use it as second, third, fourth or fifth-line treatment cannot be answered with this specific trial design. Ultimately, such decisions need to be made on a case by case basis. Fourth, unlike several other trials focusing on the use of atypical antipsychotic agents in major depressive disorder, (30–32) the present trial did not employ a specific pharmacologic algorithm to address akathisia or insomnia (the introduction of agents with central nervous activity such as propranolol, benzodiazepines, or benztropine in the double-blind phase was not permitted). Whether results with respect to study adherence and tolerability were the use of such agents was permitted or even encouraged during the double-blind phase of the trial would have differed remains unclear. Finally, nearly one in three ziprasidone-treated patients discontinued the study prematurely (an even higher discontinuation rate was noted in an earlier, open-label augmentation trial with a similar dosing regimen (33). Whether better tolerability could have been achieved with a different dosing regimen is also unclear.
In conclusion, in the present study, adjunctive ziprasidone when combined with the SSRI escitalopram demonstrated greater antidepressant efficacy in patients with major depressive disorder versus adjunctive placebo. A statistically significant greater proportion of patients discontinued adjunctive ziprasidone due to intolerance. These results suggest that, similar to other atypical antipsychotic agents, adjunctive ziprasidone can represent a useful treatment option for patients with major depressive disorder.
Acknowledgments
This study was supported by the National Institute for Mental Health (NIMH R01MH081235), Pfizer Inc. (providing free blinded ziprasidone/placebo pills), and Forest Laboratories, Inc. (providing free escitalopram).
Disclosures and Conflicts of Interest
George I. Papakostas: Consultant: Abbott Laboratories, AstraZeneca PLC, Avanir Pharmaceuticals, Brainsway Ltd, Bristol-Myers Squibb Company, Cephalon Inc., Dey Pharma, L.P., Eli Lilly Co., Genentech, Inc, GlaxoSmithKline, Evotec AG, H. Lundbeck A/S, Inflabloc Pharmaceuticals, Janssen Global Services LLC, Jazz Pharmaceuticals, Johnson & Johnson Companies, Novartis Pharma AG, One Carbon Therapeutics, Inc, Otsuka Pharmaceuticals, PAMLAB LLC, Pfizer Inc., Pierre Fabre Laboratories, Ridge Diagnostics (formerly known as Precision Human Biolaboratories), Shire Pharmaceuticals, Sunovion Pharmaceuticals, Takeda Pharmaceutical Company LTD, Theracos, Inc., Wyeth, Inc. Honoraria: Abbott Laboratories, Astra Zeneca PLC, Avanir Pharmaceuticals, Bristol-Myers Squibb Company, Brainsway Ltd, Cephalon Inc., Dey Pharma, L.P., Eli Lilly Co., Evotec AG, GlaxoSmithKline, Inflabloc Pharmaceuticals, Jazz Pharmaceuticals, H. Lundbeck A/S, Novartis Pharma AG, Otsuka Pharmaceuticals, PAMLAB LLC, Pfizer, Pierre Fabre Laboratories, Ridge Diagnostics, Shire Pharmaceuticals, Sunovion Pharmaceuticals, Takeda Pharmaceutical Company LTD, Theracos, Inc., Titan Pharmaceuticals, Wyeth Inc. Research support: AstraZeneca PLC, Bristol-Myers Squibb Company, Forest Pharmaceuticals, the National Institute of Mental Health, PAMLAB LLC, Pfizer Inc., Ridge Diagnostics (formerly known as Precision Human Biolaboratories), Sunovion Pharmaceuticals, Theracos, Inc. Speaker’s bureau (past): BristolMyersSquibb Co and Pfizer, Inc.
Maurizio Fava: Consultant: Abbott Laboratories; Affectis Pharmaceuticals AG; Alkermes, Inc.; Amarin Pharma Inc.; Aspect Medical Systems; AstraZeneca; Auspex Pharmaceuticals; Bayer AG; Best Practice Project Management, Inc.; BioMarin Pharmaceuticals, Inc.; Biovail Corporation; BrainCells Inc; Bristol-Myers Squibb; CeNeRx BioPharma; Cephalon, Inc.; Cerecor; CNS Response, Inc.; Compellis Pharmaceuticals; Cypress Pharmaceutical, Inc.; DiagnoSearch Life Sciences (P) Ltd.; Dinippon Sumitomo Pharma Co. Inc.; Dov Pharmaceuticals, Inc.; Edgemont Pharmaceuticals, Inc.; Eisai Inc.; Eli Lilly and Company; EnVivo Pharmaceuticals, Inc.; ePharmaSolutions; EPIX Pharmaceuticals, Inc.; Euthymics Bioscience, Inc.; Fabre-Kramer Pharmaceuticals, Inc.; Forest Pharmaceuticals, Inc.; GenOmind, LLC; GlaxoSmithKline; Grunenthal GmbH; i3 Innovus/Ingenis; Janssen Pharmaceutica; Jazz Pharmaceuticals, Inc.; Johnson & Johnson Pharmaceutical Research & Development, LLC; Knoll Pharmaceuticals Corp.; Labopharm Inc.; Lorex Pharmaceuticals; Lundbeck Inc.; MedAvante, Inc.; Merck & Co., Inc.; MSI Methylation Sciences, Inc.; Naurex, Inc.; Neuralstem, Inc.; Neuronetics, Inc.; NextWave Pharmaceuticals; Novartis AG;Nutrition 21; Orexigen Therapeutics, Inc.; Organon Pharmaceuticals; Otsuka Pharmaceuticals; Pamlab, LLC.; Pfizer Inc.; PharmaStar; Pharmavite® LLC.; PharmoRx Therapeutics; Precision Human Biolaboratory; Prexa Pharmaceuticals, Inc.; Puretech Ventures; PsychoGenics; Psylin Neurosciences, Inc.;RCT Logic, LLC ( formerly Clinical Trials Solutions, LLC); Rexahn Pharmaceuticals, Inc.; Ridge Diagnostics, Inc.; Roche; Sanofi-Aventis US LLC.; Sepracor Inc.; Servier Laboratories; Schering-Plough Corporation; Solvay Pharmaceuticals, Inc.; Somaxon Pharmaceuticals, Inc.; Somerset Pharmaceuticals, Inc.; Sunovion Pharmaceuticals; Supernus Pharmaceuticals, Inc.; Synthelabo; Takeda Pharmaceutical Company Limited; Tal Medical, Inc.; Tetragenex Pharmaceuticals, Inc.; TransForm Pharmaceuticals, Inc.; Transcept Pharmaceuticals, Inc.; Vanda Pharmaceuticals, Inc.; Grant/Research Support: Abbot Laboratories; Alkermes, Inc.; American Cyanamid;Aspect Medical Systems; AstraZeneca; Avanir Pharmaceuticals; BioResearch; BrainCells Inc.; Bristol-Myers Squibb; CeNeRx BioPharma; Cephalon; Clintara, LLC; Covance; Covidien; Eli Lilly and Company;EnVivo Pharmaceuticals, Inc.; Euthymics Bioscience, Inc.; Forest Pharmaceuticals, Inc.; Ganeden Biotech, Inc.; GlaxoSmithKline; Harvard Clinical Research Institute; Hoffman-LaRoche; Icon Clinical Research; i3 Innovus/Ingenix; Janssen R&D, LLC; Jed Foundation; Johnson & Johnson Pharmaceutical Research & Development; Lichtwer Pharma GmbH; Lorex Pharmaceuticals; Lundbeck Inc.; MedAvante; Methylation Sciences Inc; National Alliance for Research on Schizophrenia & Depression (NARSAD); National Center for Complementary and Alternative Medicine (NCCAM); National Institute of Drug Abuse (NIDA); National Institute of Mental Health (NIMH); Neuralstem, Inc.; Novartis AG; Organon Pharmaceuticals; PamLab, LLC.; Pfizer Inc.; Pharmacia-Upjohn; Pharmaceutical Research Associates., Inc.; Pharmavite® LLC;PharmoRx Therapeutics; Photothera; Reckitt Benckiser; Roche Pharmaceuticals; RCT Logic, LLC (formerly Clinical Trials Solutions, LLC); Sanofi-Aventis US LLC; Shire; Solvay Pharmaceuticals, Inc.; Stanley Medical Research Institute (SMRI); Synthelabo; Wyeth-Ayerst Laboratories; Speakers or Advisory Boards: Adamed, Co; Advanced Meeting Partners; American Psychiatric Association; American Society of Clinical Psychopharmacology; AstraZeneca; Belvoir Media Group; Boehringer Ingelheim GmbH; Bristol-Myers Squibb; Cephalon, Inc.; CME Institute/Physicians Postgraduate Press, Inc.; Eli Lilly and Company; Forest Pharmaceuticals, Inc.; GlaxoSmithKline; Imedex, LLC; MGH Psychiatry Academy/Primedia; MGH Psychiatry Academy/Reed Elsevier; Novartis AG; Organon Pharmaceuticals; Pfizer Inc.; PharmaStar; United BioSource,Corp.; Wyeth-Ayerst Laboratories; Equity Holdings: Compellis; PsyBrain, Inc.; Patent for Sequential Parallel Comparison Design (SPCD), which are licensed by MGH to Pharmaceutical Product Development, LLC (PPD); and patent application for a combination of Ketamine plus Scopolamine in Major Depressive Disorder (MDD); Copyright for the MGH Cognitive & Physical Functioning Questionnaire (CPFQ), Sexual Functioning Inventory (SFI), Antidepressant Treatment Response Questionnaire (ATRQ), Discontinuation-Emergent Signs & Symptoms (DESS), and SAFER; Lippincott, Williams & Wilkins; Wolkers Kluwer; World Scientific Publishing Co. Pte.Ltd.
Lee Baer: Dr. Baer reports no competing interests.
Michaela B. Swee: Michaela Swee reports no competing interests.
Adrienne Jaeger: Adrienne Jaeger reports no competing interests.
William V. Bobo: Grant/Research Support: NIMH, Mayo Foundation.
Richard C. Shelton: Consultant: Bristol-Myers Squibb Company; Cerecor, Inc.; Clintara, LLC; Cyberonics, Inc.; Forest Pharmaceuticals; Janssen Pharmaceutica; Medtronic, Inc.; MSI Methylation Sciences, Inc.; Naurex, Inc.; Pamlab, Inc.; Pfizer, Inc.; Ridge Diagnostics; Shire Pic; Takeda Pharmaceuticals; Grant/Research Support: Alkermes, Inc.; Assurex Health; Avanir Pharmaceuticals, Inc.; Bristol-Myers Squibb; Elan, Corp.; Forest Pharmaceuticals; Janssen Pharmaceutica; Naurex, Inc.; Novartis Pharmaceuticals; Otsuka Pharmaceuticals; Pamlab, Inc.; Takeda Pharmaceuticals.
Footnotes
Clinical Trial Registration: Ziprasidone Augmentation of SSRIs for Patients With Major Depressive Disorder (MDD) That do Not Sufficiently Respond to Treatment With SSRIs; Number: NCT00633399; URL: http://clinicaltrials.gov/show/NCT00633399.
References
- 1.Papakostas GI, Fava M. Monoamine-based Pharmacotherapy. In: Licinio J, Wong ML, editors. Biology of Depression: From Novel Insights to Therapeutic Strategies. 1. Weinheim: Wiley-VCH Verlag; 2005. pp. 87–140. [Google Scholar]
- 2.Papakostas GI, Fava M. Does the probability of receiving placebo influence clinical trial outcome? A meta-regression of double-blind, randomized clinical trials in MDD. Eur Neuropsychopharmacol. 2009 Jan;19(1):34–40. doi: 10.1016/j.euroneuro.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Papakostas GI. Managing partial response or nonresponse: switching, augmentation, and combination strategies for major depressive disorder. J Clin Psychiatry. 2009;70(Suppl 6):16–25. doi: 10.4088/JCP.8133su1c.03. [DOI] [PubMed] [Google Scholar]
- 4.Shelton RC, Papakostas GI. Augmentation of antidepressants with atypical antipsychotics for treatment-resistant major depressive disorder. Acta Psychiatr Scand. 2008;117(4):253–9. doi: 10.1111/j.1600-0447.2007.01130.x. [DOI] [PubMed] [Google Scholar]
- 5.Papakostas GI. Augmentation strategies in the treatment of major depressive disorder. Examining the evidence on augmentation with atypical antipsychotics. CNS Spectr. 2007;12(12 Suppl 22):10–2. doi: 10.1017/s1092852900016023. [DOI] [PubMed] [Google Scholar]
- 6.Papakostas GI. Atypical Antipsychotic Agents for Treatment-Resistant Major Depressive Disorder. Essent Psychopharmacol. 2005;6(4):209–20. [PubMed] [Google Scholar]
- 7.Papakostas GI, Shelton RC, Smith J, Fava M. Augmentation of antidepressants with atypical antipsychotic medications for treatment-resistant major depressive disorder: a meta-analysis. J Clin Psychiatry. 2007;68(6):826–31. doi: 10.4088/jcp.v68n0602. [DOI] [PubMed] [Google Scholar]
- 8.Nelson J, Papakostas G. Atypical antipsychotic augmentation in major depressive disorder: a meta-analysis of placebo-controlled randomized trials. AM J Psychiat. 2009;166(9):980–991. doi: 10.1176/appi.ajp.2009.09030312. [DOI] [PubMed] [Google Scholar]
- 9.Richelson E, Souder T. Binding of antipsychotic drugs to human brain receptors, focus on newer generation compounds. Life Science. 2000;68(1):29–39. doi: 10.1016/s0024-3205(00)00911-5. [DOI] [PubMed] [Google Scholar]
- 10.Tatsumi M, Jansen K, Blakely RD, Richelson E. Pharmacological profile of neuroleptics at human monoamine transporters. Eur J Pharmacol. 1999;368(2–3):277–283. doi: 10.1016/s0014-2999(99)00005-9. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt AW, Lebel A, Howard HR, Jr, Zom SH. Ziprasidone: A novel antipsychotic agent with a unique human receptor binding profile. Eur J Pharmacol. 2001;425:197–201. doi: 10.1016/s0014-2999(01)01188-8. [DOI] [PubMed] [Google Scholar]
- 12.Papakostas GI, Petersen TJ, Nierenberg AA, et al. Ziprasidone augmentation of selective serotonin reuptake inhibitors (SSRIs) for SSRI-resistant major depressive disorder. J Clin Psychiatry. 2004;65:217–21. doi: 10.4088/jcp.v65n0212. [DOI] [PubMed] [Google Scholar]
- 13.Silverstone PH, Salinas E. Efficacy of venlafaxine extended release in patients with major depressive disorder and comorbid generalized anxiety disorder. J Clin Psychiatry. 2001;62(7):523–9. doi: 10.4088/jcp.v62n07a04. [DOI] [PubMed] [Google Scholar]
- 14.Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst Rev. 2014 Jan 3;1:CD007115. doi: 10.1002/14651858.CD007115.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.First BM, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders - Patient Edition (SCID I/P) Biometrics Research Department, New York State Psychiatric Institute; 1995. [Google Scholar]
- 16.Trivedi MH, Rush AJ, Ibrahim HM, et al. The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. Psychol Med. 2004;34(1):73–82. doi: 10.1017/s0033291703001107. [DOI] [PubMed] [Google Scholar]
- 17.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960a;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guy W, editor. ECDEU Assessment Manual for Psychopharmacology, revised. National Institute of Mental Health; Rockville, MD: 1976. DHEW Pub. No. (ADM)76–338. [Google Scholar]
- 19.Fawcett J, Epstein P, Fiester SJ, Elkin I, Autry JH. Clinical management--imipramine/placebo administration manual. NIMH Treatment of Depression Collaborative Research Program. Psychopharmacol Bull. 1987;23(2):309–324. [PubMed] [Google Scholar]
- 20.Hamilton M. The assessment of anxiety states by rating. J Neurol Neurosurg Psychiatry. 1960b;23:50–55. [Google Scholar]
- 21.DeLoach LJ, Higgins MS, Caplan AB, Stiff JL. The visual analog scale in the immediate postoperative period: intrasubject variability and correlation with a numeric scale. Anesth Analg. 1998;86(1):102–6. doi: 10.1097/00000539-199801000-00020. [DOI] [PubMed] [Google Scholar]
- 22.Thase ME, Trivedi MH, Nelson JC, Fava M, Swanink R, Tran QV, Pikalov A, Yang H, Carlson BX, Marcus RN, Berman RM. Examining the efficacy of adjunctive aripiprazole in major depressive disorder: a pooled analysis of 2 studies. Prim Care Companion. J Clin Psychiatry. 2008;10(6):440–7. doi: 10.4088/pcc.v10n0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iovieno N, Papakostas GI. Does the presence of an open-label antidepressant treatment period influence study outcome in clinical trials examining augmentation/combination strategies in treatment partial responders/nonresponders with major depressive disorder? J Clin Psychiatry. 2012 May;73(5):676–83. doi: 10.4088/JCP.11r06978. [DOI] [PubMed] [Google Scholar]
- 24.Iovieno N, Papakostas GI. Correlation between different levels of placebo response rate and clinical trial outcome in major depressive disorder: a meta-analysis. J Clin Psychiatry. 2012;73(10):1300–6. doi: 10.4088/JCP.11r07485. [DOI] [PubMed] [Google Scholar]
- 25.Papakostas GI, Ostergaard S, Iovieno N. The nature of placebo response in clinical studies of major depressive disorder. J Clin Psychiatry. doi: 10.4088/JCP.14r09297. In press. [DOI] [PubMed] [Google Scholar]
- 26.Fava M, Rush AJ, Alpert JE, Balasubramani GK, Wisniewski SR, Carmin CN, Biggs MM, Zisook S, Leuchter A, Howland R, Warden D, Trivedi MH. Difference in treatment outcome in outpatients with anxious versus nonanxious depression: a STAR*D report. Am J Psychiatry. 2008;165(3):342–51. doi: 10.1176/appi.ajp.2007.06111868. [DOI] [PubMed] [Google Scholar]
- 27.Papakostas GI, Clain A, Ameral VE, Baer L, Brintz C, Smith WT, Londborg PD, Glaudin V, Painter JR, Fava M. Fluoxetine-clonazepam cotherapy for anxious depression: an exploratory, post-hoc analysis of a randomized, double blind study. Int Clin Psychopharmacol. 2010;25(1):17–21. doi: 10.1097/YIC.0b013e32833205a4. [DOI] [PubMed] [Google Scholar]
- 28.Papakostas GI, Fan H, Tedeschini E. Severe and anxious depression: combining definitions of clinical sub-types to identify patients differentially responsive to selective serotonin reuptake inhibitors. Eur Neuropsychopharmacol. 2012;22(5):347–55. doi: 10.1016/j.euroneuro.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 29.Citrome L. Adjunctive aripiprazole, olanzapine, or quetiapine for major depressive disorder: an analysis of number needed to treat, number needed to harm, and likelihood to be helped or harmed. Postgrad Med. 2010;122(4):39–48. doi: 10.3810/pgm.2010.07.2174. [DOI] [PubMed] [Google Scholar]
- 30.Marcus RN, McQuade RD, Carson WH, Hennicken D, Fava M, Simon JS, Trivedi MH, Thase ME, Berman RM. The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a second multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychopharmacol. 2008;28(2):156–65. doi: 10.1097/JCP.0b013e31816774f9. [DOI] [PubMed] [Google Scholar]
- 31.Berman RM, Marcus RN, Swanink R, McQuade RD, Carson WH, Corey-Lisle PK, Khan A. The efficacy and safety of aripiprazole as adjunctive therapy in major depressive disorder: a multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2007;68(6):843–53. doi: 10.4088/jcp.v68n0604. [DOI] [PubMed] [Google Scholar]
- 32.Berman RM, Fava M, Thase ME, Trivedi MH, Swanink R, McQuade RD, Carson WH, Adson D, Taylor L, Hazel J, Marcus RN. Aripiprazole augmentation in major depressive disorder: a double-blind, placebo-controlled study in patients with inadequate response to antidepressants. CNS Spectr. 2009;14(4):197–206. doi: 10.1017/s1092852900020216. [DOI] [PubMed] [Google Scholar]
- 33.Dunner DL, Amsterdam JD, Shelton RC, Loebel A, Romano SJ. Efficacy and tolerability of adjunctive ziprasidone in treatment-resistant depression: a randomized, open-label, pilot study. J Clin Psychiatry. 2007;68(7):1071–7. doi: 10.4088/jcp.v68n0714. [DOI] [PubMed] [Google Scholar]


