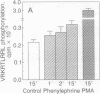
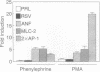
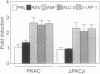
Abstract
A cultured myocardial cell model was used to examine the role of protein kinase C-dependent pathways in the transcriptional activation of two cardiac muscle genes [myosin light chain 2 (MLC-2) and atrial natriuretic factor (ANF)] during alpha-adrenergic receptor-mediated hypertrophy. Phorbol ester (phorbol 12-myristate 13-acetate) and the alpha-adrenergic agonist phenylephrine both activate protein kinase C (PKC) and induce 4- to 5-fold increases in the expression of MLC-2 and ANF promoter/luciferase reporter genes with little effect on Rous sarcoma virus/luciferase or minimal prolactin promoter/luciferase genes. To further assess the role of PKC in cardiac gene regulation, PKC expression vectors encoding constitutively activated PKC-alpha or PKC-beta, or a catalytically inactive PKC, were transiently cotransfected with the cardiac promoter/luciferase constructs. Cotransfection of either activated PKC-alpha or PKC-beta cDNA induces the expression of MLC-2 and ANF promoter/luciferase genes and of a reporter gene responsive to the transcription factor AP-1. The Rous sarcoma virus/luciferase and minimal prolactin promoter/luciferase genes are not concomitantly induced by cotransfectin with the PKC genes, indicating specificity of the transcriptional effect. The finding that activated PKC increases cardiac gene transcription suggests that activation of this enzyme may be a proximal signal for coregulation of two cardiac genes, MLC-2 and ANF, during the course of myocardial cell hypertrophy.
Full text
PDF
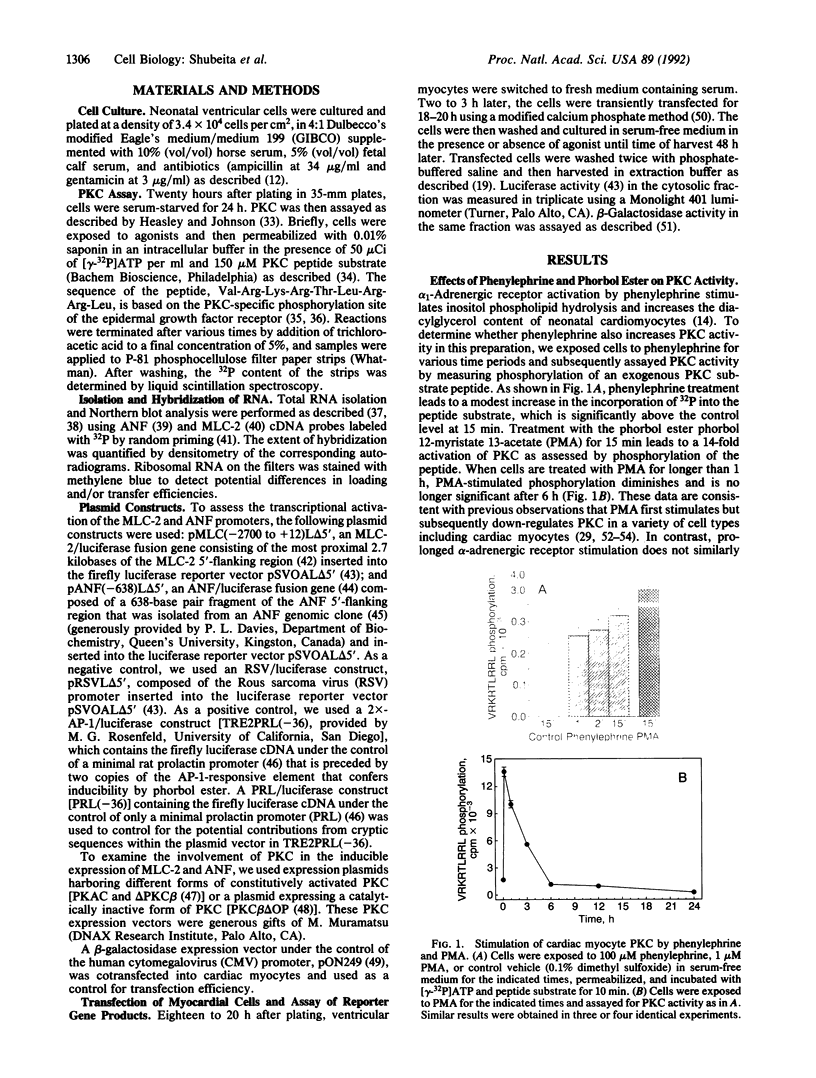

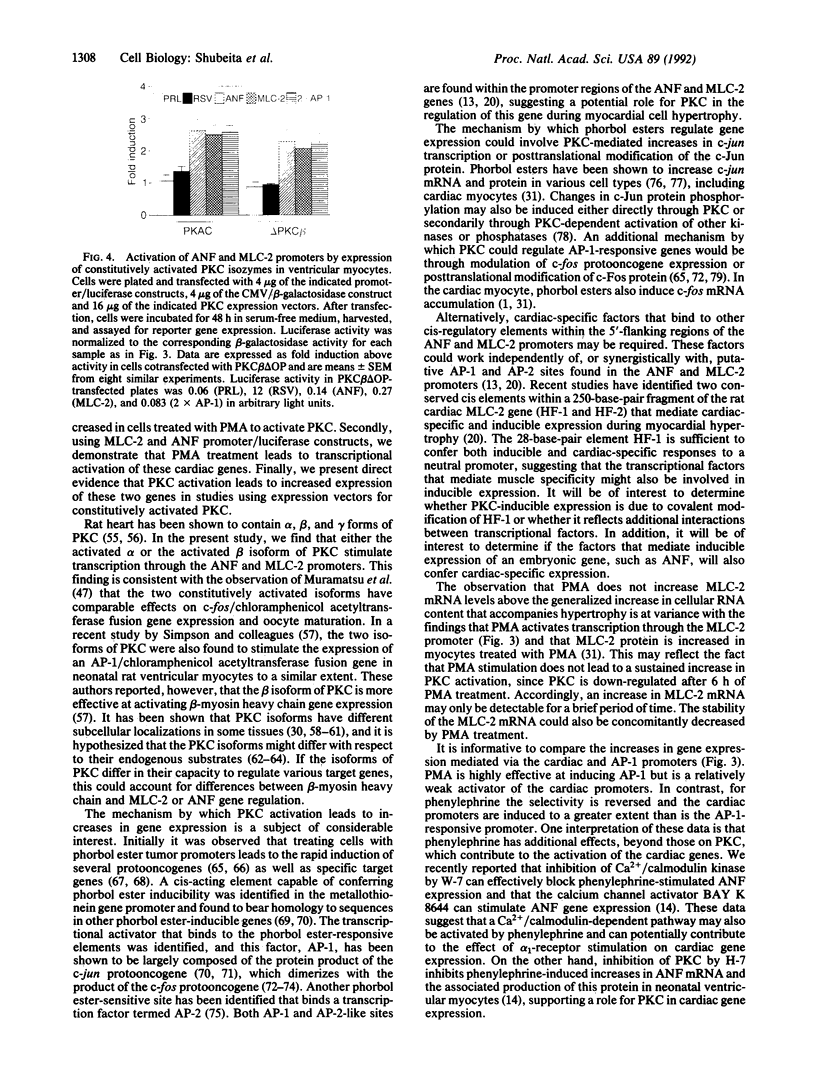

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen I. S., Cohen N. M., Dhallan R. S., Gaa S. T., Lederer W. J., Rogers T. B. Angiotensin II increases spontaneous contractile frequency and stimulates calcium current in cultured neonatal rat heart myocytes: insights into the underlying biochemical mechanisms. Circ Res. 1988 Mar;62(3):524–534. doi: 10.1161/01.res.62.3.524. [DOI] [PubMed] [Google Scholar]
- Angel P., Hattori K., Smeal T., Karin M. The jun proto-oncogene is positively autoregulated by its product, Jun/AP-1. Cell. 1988 Dec 2;55(5):875–885. doi: 10.1016/0092-8674(88)90143-2. [DOI] [PubMed] [Google Scholar]
- Argentin S., Nemer M., Drouin J., Scott G. K., Kennedy B. P., Davies P. L. The gene for rat atrial natriuretic factor. J Biol Chem. 1985 Apr 25;260(8):4568–4571. [PubMed] [Google Scholar]
- Baker K. M., Singer H. A., Aceto J. F. Angiotensin II receptor-mediated stimulation of cytosolic-free calcium and inositol phosphates in chick myocytes. J Pharmacol Exp Ther. 1989 Nov;251(2):578–585. [PubMed] [Google Scholar]
- Barber J. R., Verma I. M. Modification of fos proteins: phosphorylation of c-fos, but not v-fos, is stimulated by 12-tetradecanoyl-phorbol-13-acetate and serum. Mol Cell Biol. 1987 Jun;7(6):2201–2211. doi: 10.1128/mcb.7.6.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishopric N. H., Kedes L. Adrenergic regulation of the skeletal alpha-actin gene promoter during myocardial cell hypertrophy. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2132–2136. doi: 10.1073/pnas.88.6.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishopric N. H., Simpson P. C., Ordahl C. P. Induction of the skeletal alpha-actin gene in alpha 1-adrenoceptor-mediated hypertrophy of rat cardiac myocytes. J Clin Invest. 1987 Oct;80(4):1194–1199. doi: 10.1172/JCI113179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohmann D., Bos T. J., Admon A., Nishimura T., Vogt P. K., Tjian R. Human proto-oncogene c-jun encodes a DNA binding protein with structural and functional properties of transcription factor AP-1. Science. 1987 Dec 4;238(4832):1386–1392. doi: 10.1126/science.2825349. [DOI] [PubMed] [Google Scholar]
- Boyle W. J., Smeal T., Defize L. H., Angel P., Woodgett J. R., Karin M., Hunter T. Activation of protein kinase C decreases phosphorylation of c-Jun at sites that negatively regulate its DNA-binding activity. Cell. 1991 Feb 8;64(3):573–584. doi: 10.1016/0092-8674(91)90241-p. [DOI] [PubMed] [Google Scholar]
- Brown J. H., Buxton I. L., Brunton L. L. Alpha 1-adrenergic and muscarinic cholinergic stimulation of phosphoinositide hydrolysis in adult rat cardiomyocytes. Circ Res. 1985 Oct;57(4):532–537. doi: 10.1161/01.res.57.4.532. [DOI] [PubMed] [Google Scholar]
- Cherrington J. M., Mocarski E. S. Human cytomegalovirus ie1 transactivates the alpha promoter-enhancer via an 18-base-pair repeat element. J Virol. 1989 Mar;63(3):1435–1440. doi: 10.1128/jvi.63.3.1435-1440.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien K. R., Knowlton K. U., Zhu H., Chien S. Regulation of cardiac gene expression during myocardial growth and hypertrophy: molecular studies of an adaptive physiologic response. FASEB J. 1991 Dec;5(15):3037–3046. doi: 10.1096/fasebj.5.15.1835945. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Chiu R., Boyle W. J., Meek J., Smeal T., Hunter T., Karin M. The c-Fos protein interacts with c-Jun/AP-1 to stimulate transcription of AP-1 responsive genes. Cell. 1988 Aug 12;54(4):541–552. doi: 10.1016/0092-8674(88)90076-1. [DOI] [PubMed] [Google Scholar]
- Cochet C., Gill G. N., Meisenhelder J., Cooper J. A., Hunter T. C-kinase phosphorylates the epidermal growth factor receptor and reduces its epidermal growth factor-stimulated tyrosine protein kinase activity. J Biol Chem. 1984 Feb 25;259(4):2553–2558. [PubMed] [Google Scholar]
- Davis R. J., Czech M. P. Tumor-promoting phorbol diesters cause the phosphorylation of epidermal growth factor receptors in normal human fibroblasts at threonine-654. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1974–1978. doi: 10.1073/pnas.82.7.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunnmon P. M., Iwaki K., Henderson S. A., Sen A., Chien K. R. Phorbol esters induce immediate-early genes and activate cardiac gene transcription in neonatal rat myocardial cells. J Mol Cell Cardiol. 1990 Aug;22(8):901–910. doi: 10.1016/0022-2828(90)90121-h. [DOI] [PubMed] [Google Scholar]
- Fabbro D., Mazurek N., Borner C., Conscience J. F., Erne P. Role of protein kinase C (PKC) in short- and long-term cellular responses: inhibition of agonist-mediated calcium transients and down-regulation of PKC. J Cardiovasc Pharmacol. 1988;12 (Suppl 5):S73–S79. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fournier A., Hardy S. J., Clark K. J., Murray A. W. Phorbol ester induces differential membrane-association of protein kinase C subspecies in human platelets. Biochem Biophys Res Commun. 1989 Jun 15;161(2):556–561. doi: 10.1016/0006-291x(89)92635-1. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Heasley L. E., Johnson G. L. Regulation of protein kinase C by nerve growth factor, epidermal growth factor, and phorbol esters in PC12 pheochromocytoma cells. J Biol Chem. 1989 May 25;264(15):8646–8652. [PubMed] [Google Scholar]
- Heathers G. P., Evers A. S., Corr P. B. Enhanced inositol trisphosphate response to alpha 1-adrenergic stimulation in cardiac myocytes exposed to hypoxia. J Clin Invest. 1989 Apr;83(4):1409–1413. doi: 10.1172/JCI114030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson S. A., Spencer M., Sen A., Kumar C., Siddiqui M. A., Chien K. R. Structure, organization, and expression of the rat cardiac myosin light chain-2 gene. Identification of a 250-base pair fragment which confers cardiac-specific expression. J Biol Chem. 1989 Oct 25;264(30):18142–18148. [PubMed] [Google Scholar]
- Henderson S. A., Xu Y. C., Chien K. R. Nucleotide sequence of full length cDNAs encoding rat cardiac myosin light chain-2. Nucleic Acids Res. 1988 May 25;16(10):4722–4722. doi: 10.1093/nar/16.10.4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrich C. J., Simpson P. C. Differential acute and chronic response of protein kinase C in cultured neonatal rat heart myocytes to alpha 1-adrenergic and phorbol ester stimulation. J Mol Cell Cardiol. 1988 Dec;20(12):1081–1085. doi: 10.1016/0022-2828(88)90588-3. [DOI] [PubMed] [Google Scholar]
- Hocevar B. A., Fields A. P. Selective translocation of beta II-protein kinase C to the nucleus of human promyelocytic (HL60) leukemia cells. J Biol Chem. 1991 Jan 5;266(1):28–33. [PubMed] [Google Scholar]
- Huang F. L., Arora P. K., Hanna E. E., Huang K. P. Proteolytic degradation of protein kinase C in the phorbol ester-induced interleukin-2 secreting thymoma cells. Arch Biochem Biophys. 1988 Dec;267(2):503–514. doi: 10.1016/0003-9861(88)90057-4. [DOI] [PubMed] [Google Scholar]
- Huang K. P., Huang F. L., Nakabayashi H., Yoshida Y. Biochemical characterization of rat brain protein kinase C isozymes. J Biol Chem. 1988 Oct 15;263(29):14839–14845. [PubMed] [Google Scholar]
- Imagawa M., Chiu R., Karin M. Transcription factor AP-2 mediates induction by two different signal-transduction pathways: protein kinase C and cAMP. Cell. 1987 Oct 23;51(2):251–260. doi: 10.1016/0092-8674(87)90152-8. [DOI] [PubMed] [Google Scholar]
- Imbra R. J., Karin M. Metallothionein gene expression is regulated by serum factors and activators of protein kinase C. Mol Cell Biol. 1987 Apr;7(4):1358–1363. doi: 10.1128/mcb.7.4.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaki K., Sukhatme V. P., Shubeita H. E., Chien K. R. Alpha- and beta-adrenergic stimulation induces distinct patterns of immediate early gene expression in neonatal rat myocardial cells. fos/jun expression is associated with sarcomere assembly; Egr-1 induction is primarily an alpha 1-mediated response. J Biol Chem. 1990 Aug 15;265(23):13809–13817. [PubMed] [Google Scholar]
- Izumo S., Nadal-Ginard B., Mahdavi V. Protooncogene induction and reprogramming of cardiac gene expression produced by pressure overload. Proc Natl Acad Sci U S A. 1988 Jan;85(2):339–343. doi: 10.1073/pnas.85.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaibuchi K., Fukumoto Y., Oku N., Takai Y., Arai K., Muramatsu M. Molecular genetic analysis of the regulatory and catalytic domains of protein kinase C. J Biol Chem. 1989 Aug 15;264(23):13489–13496. [PubMed] [Google Scholar]
- Karin M., Haslinger A., Heguy A., Dietlin T., Cooke T. Metal-responsive elements act as positive modulators of human metallothionein-IIA enhancer activity. Mol Cell Biol. 1987 Feb;7(2):606–613. doi: 10.1128/mcb.7.2.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariya K., Karns L. R., Simpson P. C. Expression of a constitutively activated mutant of the beta-isozyme of protein kinase C in cardiac myocytes stimulates the promoter of the beta-myosin heavy chain isogene. J Biol Chem. 1991 Jun 5;266(16):10023–10026. [PubMed] [Google Scholar]
- Katz A., Kahana C. Transcriptional activation of mammalian ornithine decarboxylase during stimulated growth. Mol Cell Biol. 1987 Jul;7(7):2641–2643. doi: 10.1128/mcb.7.7.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikkawa U., Kishimoto A., Nishizuka Y. The protein kinase C family: heterogeneity and its implications. Annu Rev Biochem. 1989;58:31–44. doi: 10.1146/annurev.bi.58.070189.000335. [DOI] [PubMed] [Google Scholar]
- Knowlton K. U., Baracchini E., Ross R. S., Harris A. N., Henderson S. A., Evans S. M., Glembotski C. C., Chien K. R. Co-regulation of the atrial natriuretic factor and cardiac myosin light chain-2 genes during alpha-adrenergic stimulation of neonatal rat ventricular cells. Identification of cis sequences within an embryonic and a constitutive contractile protein gene which mediate inducible expression. J Biol Chem. 1991 Apr 25;266(12):7759–7768. [PubMed] [Google Scholar]
- Komuro I., Katoh Y., Kaida T., Shibazaki Y., Kurabayashi M., Hoh E., Takaku F., Yazaki Y. Mechanical loading stimulates cell hypertrophy and specific gene expression in cultured rat cardiac myocytes. Possible role of protein kinase C activation. J Biol Chem. 1991 Jan 15;266(2):1265–1268. [PubMed] [Google Scholar]
- Kosaka Y., Ogita K., Ase K., Nomura H., Kikkawa U., Nishizuka Y. The heterogeneity of protein kinase C in various rat tissues. Biochem Biophys Res Commun. 1988 Mar 30;151(3):973–981. doi: 10.1016/s0006-291x(88)80461-3. [DOI] [PubMed] [Google Scholar]
- Kruijer W., Cooper J. A., Hunter T., Verma I. M. Platelet-derived growth factor induces rapid but transient expression of the c-fos gene and protein. Nature. 1984 Dec 20;312(5996):711–716. doi: 10.1038/312711a0. [DOI] [PubMed] [Google Scholar]
- Leach K. L., Powers E. A., Ruff V. A., Jaken S., Kaufmann S. Type 3 protein kinase C localization to the nuclear envelope of phorbol ester-treated NIH 3T3 cells. J Cell Biol. 1989 Aug;109(2):685–695. doi: 10.1083/jcb.109.2.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. R., Henderson S. A., Reynolds R., Dunnmon P., Yuan D., Chien K. R. Alpha 1-adrenergic stimulation of cardiac gene transcription in neonatal rat myocardial cells. Effects on myosin light chain-2 gene expression. J Biol Chem. 1988 May 25;263(15):7352–7358. [PubMed] [Google Scholar]
- Long C. S., Ordahl C. P., Simpson P. C. Alpha 1-adrenergic receptor stimulation of sarcomeric actin isogene transcription in hypertrophy of cultured rat heart muscle cells. J Clin Invest. 1989 Mar;83(3):1078–1082. doi: 10.1172/JCI113951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar S., Rossi M. W., Fujiki T., Phillips W. A., Disa S., Queen C. F., Johnston R. B., Jr, Rosen O. M., Corkey B. E., Korchak H. M. Protein kinase C isotypes and signaling in neutrophils. Differential substrate specificities of a translocatable calcium- and phospholipid-dependent beta-protein kinase C and a phospholipid-dependent protein kinase which is inhibited by long chain fatty acyl coenzyme A. J Biol Chem. 1991 May 15;266(14):9285–9294. [PubMed] [Google Scholar]
- Meidell R. S., Sen A., Henderson S. A., Slahetka M. F., Chien K. R. Alpha 1-adrenergic stimulation of rat myocardial cells increases protein synthesis. Am J Physiol. 1986 Nov;251(5 Pt 2):H1076–H1084. doi: 10.1152/ajpheart.1986.251.5.H1076. [DOI] [PubMed] [Google Scholar]
- Mochly-Rosen D., Henrich C. J., Cheever L., Khaner H., Simpson P. C. A protein kinase C isozyme is translocated to cytoskeletal elements on activation. Cell Regul. 1990 Aug;1(9):693–706. doi: 10.1091/mbc.1.9.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu M., Kaibuchi K., Arai K. A protein kinase C cDNA without the regulatory domain is active after transfection in vivo in the absence of phorbol ester. Mol Cell Biol. 1989 Feb;9(2):831–836. doi: 10.1128/mcb.9.2.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C., Albert V. R., Elsholtz H. P., Lu L. I., Rosenfeld M. G. Activation of cell-specific expression of rat growth hormone and prolactin genes by a common transcription factor. Science. 1988 Mar 18;239(4846):1400–1405. doi: 10.1126/science.2831625. [DOI] [PubMed] [Google Scholar]
- Poggioli J., Sulpice J. C., Vassort G. Inositol phosphate production following alpha 1-adrenergic, muscarinic or electrical stimulation in isolated rat heart. FEBS Lett. 1986 Oct 6;206(2):292–298. doi: 10.1016/0014-5793(86)80999-1. [DOI] [PubMed] [Google Scholar]
- Qu Y., Torchia J., Phan T. D., Sen A. K. Purification and characterization of protein kinase C isozymes from rat heart. Mol Cell Biochem. 1991 May 15;103(2):171–180. doi: 10.1007/BF00227484. [DOI] [PubMed] [Google Scholar]
- Rauscher F. J., 3rd, Voulalas P. J., Franza B. R., Jr, Curran T. Fos and Jun bind cooperatively to the AP-1 site: reconstitution in vitro. Genes Dev. 1988 Dec;2(12B):1687–1699. doi: 10.1101/gad.2.12b.1687. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Pena A., Rozengurt E. Disappearance of Ca2+-sensitive, phospholipid-dependent protein kinase activity in phorbol ester-treated 3T3 cells. Biochem Biophys Res Commun. 1984 May 16;120(3):1053–1059. doi: 10.1016/s0006-291x(84)80213-2. [DOI] [PubMed] [Google Scholar]
- Rogue P., Labourdette G., Masmoudi A., Yoshida Y., Huang F. L., Huang K. P., Zwiller J., Vincendon G., Malviya A. N. Rat liver nuclei protein kinase C is the isozyme type II. J Biol Chem. 1990 Mar 5;265(7):4161–4165. [PubMed] [Google Scholar]
- Rosenthal N. Identification of regulatory elements of cloned genes with functional assays. Methods Enzymol. 1987;152:704–720. doi: 10.1016/0076-6879(87)52075-4. [DOI] [PubMed] [Google Scholar]
- Schiaffino S., Samuel J. L., Sassoon D., Lompré A. M., Garner I., Marotte F., Buckingham M., Rappaport L., Schwartz K. Nonsynchronous accumulation of alpha-skeletal actin and beta-myosin heavy chain mRNAs during early stages of pressure-overload--induced cardiac hypertrophy demonstrated by in situ hybridization. Circ Res. 1989 May;64(5):937–948. doi: 10.1161/01.res.64.5.937. [DOI] [PubMed] [Google Scholar]
- Schwartz K., de la Bastie D., Bouveret P., Oliviéro P., Alonso S., Buckingham M. Alpha-skeletal muscle actin mRNA's accumulate in hypertrophied adult rat hearts. Circ Res. 1986 Nov;59(5):551–555. doi: 10.1161/01.res.59.5.551. [DOI] [PubMed] [Google Scholar]
- Sei C. A., Irons C. E., Sprenkle A. B., McDonough P. M., Brown J. H., Glembotski C. C. The alpha-adrenergic stimulation of atrial natriuretic factor expression in cardiac myocytes requires calcium influx, protein kinase C, and calmodulin-regulated pathways. J Biol Chem. 1991 Aug 25;266(24):15910–15916. [PubMed] [Google Scholar]
- Shubeita H. E., McDonough P. M., Harris A. N., Knowlton K. U., Glembotski C. C., Brown J. H., Chien K. R. Endothelin induction of inositol phospholipid hydrolysis, sarcomere assembly, and cardiac gene expression in ventricular myocytes. A paracrine mechanism for myocardial cell hypertrophy. J Biol Chem. 1990 Nov 25;265(33):20555–20562. [PubMed] [Google Scholar]
- Sills M. N., Xu Y. C., Baracchini E., Goodman R. H., Cooperman S. S., Mandel G., Chien K. R. Expression of diverse Na+ channel messenger RNAs in rat myocardium. Evidence for a cardiac-specific Na+ channel. J Clin Invest. 1989 Jul;84(1):331–336. doi: 10.1172/JCI114158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson P. C., Long C. S., Waspe L. E., Henrich C. J., Ordahl C. P. Transcription of early developmental isogenes in cardiac myocyte hypertrophy. J Mol Cell Cardiol. 1989 Dec;21 (Suppl 5):79–89. doi: 10.1016/0022-2828(89)90774-8. [DOI] [PubMed] [Google Scholar]
- Simpson P., McGrath A., Savion S. Myocyte hypertrophy in neonatal rat heart cultures and its regulation by serum and by catecholamines. Circ Res. 1982 Dec;51(6):787–801. doi: 10.1161/01.res.51.6.787. [DOI] [PubMed] [Google Scholar]
- Simpson P. Norepinephrine-stimulated hypertrophy of cultured rat myocardial cells is an alpha 1 adrenergic response. J Clin Invest. 1983 Aug;72(2):732–738. doi: 10.1172/JCI111023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson P. Stimulation of hypertrophy of cultured neonatal rat heart cells through an alpha 1-adrenergic receptor and induction of beating through an alpha 1- and beta 1-adrenergic receptor interaction. Evidence for independent regulation of growth and beating. Circ Res. 1985 Jun;56(6):884–894. doi: 10.1161/01.res.56.6.884. [DOI] [PubMed] [Google Scholar]
- Smeal T., Angel P., Meek J., Karin M. Different requirements for formation of Jun: Jun and Jun: Fos complexes. Genes Dev. 1989 Dec;3(12B):2091–2100. doi: 10.1101/gad.3.12b.2091. [DOI] [PubMed] [Google Scholar]
- Steinberg S. F., Kaplan L. M., Inouye T., Zhang J. F., Robinson R. B. Alpha-1 adrenergic stimulation of 1,4,5-inositol trisphosphate formation in ventricular myocytes. J Pharmacol Exp Ther. 1989 Sep;250(3):1141–1148. [PubMed] [Google Scholar]
- Trejo J., Brown J. H. c-fos and c-jun are induced by muscarinic receptor activation of protein kinase C but are differentially regulated by intracellular calcium. J Biol Chem. 1991 Apr 25;266(12):7876–7882. [PubMed] [Google Scholar]
- Trilivas I., McDonough P. M., Brown J. H. Dissociation of protein kinase C redistribution from the phosphorylation of its substrates. J Biol Chem. 1991 May 5;266(13):8431–8438. [PubMed] [Google Scholar]
- Waspe L. E., Ordahl C. P., Simpson P. C. The cardiac beta-myosin heavy chain isogene is induced selectively in alpha 1-adrenergic receptor-stimulated hypertrophy of cultured rat heart myocytes. J Clin Invest. 1990 Apr;85(4):1206–1214. doi: 10.1172/JCI114554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock E. A., White L. B., Smith A. I., McLeod J. K. Stimulation of phosphatidylinositol metabolism in the isolated, perfused rat heart. Circ Res. 1987 Nov;61(5):625–631. doi: 10.1161/01.res.61.5.625. [DOI] [PubMed] [Google Scholar]
- Zhu H., Garcia A. V., Ross R. S., Evans S. M., Chien K. R. A conserved 28-base-pair element (HF-1) in the rat cardiac myosin light-chain-2 gene confers cardiac-specific and alpha-adrenergic-inducible expression in cultured neonatal rat myocardial cells. Mol Cell Biol. 1991 Apr;11(4):2273–2281. doi: 10.1128/mcb.11.4.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivin R. A., Condra J. H., Dixon R. A., Seidah N. G., Chrétien M., Nemer M., Chamberland M., Drouin J. Molecular cloning and characterization of DNA sequences encoding rat and human atrial natriuretic factors. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6325–6329. doi: 10.1073/pnas.81.20.6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]