Abstract
The recent spread of chikungunya virus to the Western Hemisphere, together with the ongoing Ebola epidemic in West Africa, have highlighted the importance of international collaboration in the detection and management of disease outbreaks. In response to this need, the Global Virus Network (GVN) was formed in 2011. The GVN is a coalition of leading medical virologists in 34 affiliated laboratories in 24 countries, who collaborate to share their resources and expertise. The GVN supports research, promotes training for young scientists, serves as a technical resource for governments, businesses and international organizations, facilitates international scientific cooperation, and advocates for funding and evidence-based public policies. In response to the spread of chikungunya, the GVN formed a task force to identify research gaps and opportunities, including models of infection and disease, candidate vaccines and antivirals, epidemiology and vector control measures. Its members also serve as authoritative sources of information for the public, press, and policy-makers. This article forms part of a symposium in Antiviral Research on “Chikungunya discovers the New World”.
Keywords: Global Virus Network, Chikungunya, Emerging virus, Vector-borne, Arbovirus
1. Introduction
The recent emergence of chikungunya virus (CHIKV) in the Western Hemisphere and its rapid spread in the Caribbean and South America have highlighted the importance of international scientific collaboration in the detection and management of disease outbreaks. In response to this need, the Global Virus Network (GVN) was formed in 2011. The GVN is a coalition of leading medical virologists in 34 affiliated laboratories in 24 countries, who collaborate to share their resources and expertise. The GVN supports research, promotes training for young scientists, serves as a technical resource for governments, businesses and international organizations, facilitates international scientific cooperation, and advocates for funding and evidence-based public policies.
In response to the spread of chikungunya, the GVN formed a task force to identify research gaps and opportunities, including models of infection and disease, candidate vaccines and antivirals, epidemiology and vector control measures. Its members also serve as authoritative sources of information for the public, press and policy-makers. This article reviews the history of the GVN and its response to the appearance of a novel mosquito-borne virus in the Western Hemisphere.
2. The Global Virus Network
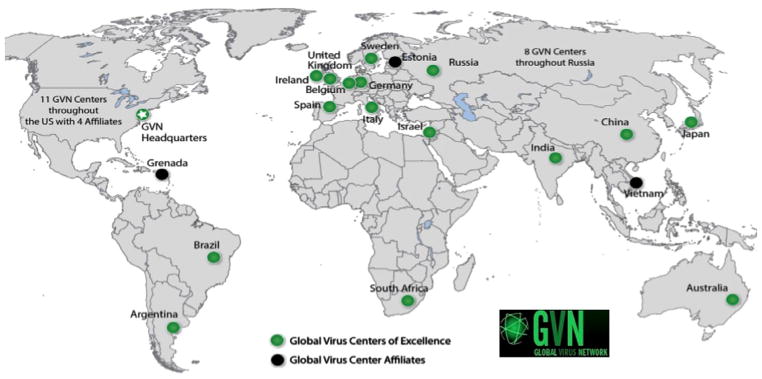
It is not immediately obvious that there is any connection between the writings of Irish novelist James Joyce and the phenomenon of emerging viruses, but in April 2009, Robert Gallo, a pioneer in the field of human retroviruses, was awarded the James Joyce Medal by the Literary & Historical Society at University College Dublin (UCD)—the first life scientist to be so honored. He then gave a lecture entitled, “Viruses, Epidemics and Prospects for Their Control.” It may be that his audience—steeped in the myths and histories of famine and infectious disease—gave little forethought to the idea of controlling mysterious viruses and preventing deadly epidemics. Gallo, on the other hand, posited in his lecture that viruses could run amok and take down society as we know it. Surprised when bold audience members asked him following his lecture, “What are you going to do about it?” Gallo, together with William Hall at UCD and the late Reinhard Kurth at the Robert Koch Institute in Berlin, set about organizing an international consortium of virologists who study human viral diseases to serve as a readily available source of technical and scientific expertise, which would become the Global Virus Network (see Fig. 1).
Fig. 1.
The global reach of the Global Virus Network. For information about Centers of Excellence, Center Affiliates and participating scientists, go to http://gvn.org/.
Historically, the world has been ill-prepared to deal with global disease outbreaks. The influenza pandemic of 1918–19, polio epidemics of the 1950s, and the AIDS crisis beginning in the 1980s are recent reminders of such events and the ad hoc political and medical responses to them. Previous pandemics and outbreaks often have overwhelmed public health authorities because of a lack of basic scientific knowledge about the nature of emerging viral pathogens, the lack of preventive vaccines and therapeutic drugs, and a lack of emergency planning at national and international levels. What Gallo, Hall and Kurth envisioned was an international consortium of world-class facilities, each directed by a recognized expert in human viral diseases who was already involved in global outreach activities (Zakaib, 2011). Their proposed network would promote the investigation of viruses that are emerging as potential epidemic threats, and viruses implicated in human diseases of unknown etiology. It would promote education programs for young scientists, serve as a resource for governments and organizations faced with viral threats, facilitate international scientific cooperation, and advocate for the more research and training in medically-oriented virology.
In March 2011, thirty of the world’s leading virologists gathered in Washington, D.C. to pledge their support for an international coalition of virology institutions, ready to act in times of viral outbreaks, and committed to advancing knowledge of pathogenic viruses. GVN was registered as a non-profit organization shortly thereafter, and in October 2012, the Board of Directors appointed the first President, Sharon Hrynkow, a senior executive and global health leader with twenty years’ experience at the National Institutes of Health and the U.S. Department of State, to turn that vision into a reality.
Today, the GVN is comprised of 34 Centers of Excellence in 24 nations (Table 1). Centers are led by world-class virologists who have deep expertise in two or three areas of virology, strong publication records, a commitment to building technical and scientific capacities in resource-poor countries, and a commitment to supporting the GVN infrastructure. In addition, there are four recently designated “Affiliate Centers” (Estonia, Grenada, Nigeria, and Vietnam). There is no formal application for Center of Excellence membership. Proposals are sent to members of the GVN Scientific Leadership Group—a subset of all Center Directors—for review and voting. If a proposed center is not considered fully formed and stand-alone, it may be designated an Affiliate Center, linked to one or more existing Centers. Also, some Centers represent only one institution, while others may be a consortium of several local facilities.
Table 1.
GVN Centers of Excellence and associated laboratories. Each center has a director who has made significant contributions to the literature; high productivity in terms of publications; has served as a regional resource for research, diagnostics and treatment; programs that interact with other centers in the region, and outreach programs with less developed countries. Current Center Directors are listed at: http://gvn.org/members.
| Centers of Excellence | |
| Argentina | IBBM – National University of La Plata |
| Australia | Peter Doherty Institute for Infection & Immunity, University of Melbourne |
| Belgium | Northern Europe Consortium, Gembloux Agro-Biotech |
| Brazil | Fundación Oswaldo Cruz (FIOCRUZ) – Rio de Janeiro |
| China | Chinese Consortium – Chinese Centers for Disease Control |
| Germany | Robert Koch Institute Berlin; Technical University of Munich; Philipp University Marburg |
| India | Amrita Institute of Medical Sciences, Kerala |
| Ireland | University College Dublin |
| Israel | Tel Aviv University |
| Italy | Italian Consortium – University of Verona |
| Japan | National Institute of Infectious Diseases (NIID-Tokyo) |
| Russia | Moscow Center for HIV/AIDS Prevention and Treatment |
| South Africa | National Institute for Communicable Diseases, Johannesburg |
| Spain | Centro de Biología Molecular Severo Ochoa (CBMSO), Madrid; Centre de Recerca en Sanitat Animal (CReSA), Barcelona |
| Sweden | Scandinavian-Baltic Consortium, Karolinska Institute |
| U.K. | MRC-University of Glasgow, Scotland; The Pirbright Institute, Surrey |
| USA | Maryland: Institute of Human Virology at the University of Maryland School of Medicine; Johns Hopkins Bloomberg School of Public Health; J. Craig Venter Institute, Rockville New York: Icahn School of Medicine at Mt. Sinai; University of Rochester Medical Center; University of Buffalo California: University of California San Francisco; Scripps Research Institute Colorado: Colorado State University, Fort Collins Michigan: University of Michigan Pennsylvania: University of Pittsburgh Cancer Institute Texas: UTMB Institute for Human Infections and Immunity, Galveston National Laboratory |
| Affiliated sites | |
| Estonia | University of Tartu |
| Grenada, W.I. | St. George’s University |
| Nigeria | Institute of Human Virology–Nigeria |
| Vietnam | Ireland Vietnam Blood-Borne Virus Initiative, National Institute of Hygiene and Epidemiology, Hanoi |
3. The chikungunya task force
In early 2014, the Scientific Leadership Group drafted a list of priority action areas for the GVN. They recognized that with limited resources, GVN should focus its early efforts in order to have a scientific and public impact. One of the priorities selected was chikungunya virus (CHIKV), which was timely, as it was then beginning to spread across the Caribbean. It was clear that collaborations and partnerships across the GVN Centers, and in some countries that had been grappling with CHIKV for decades, likely would speed progress in drug and vaccine development and other public health measures.
On World Health Day 2014, the theme of which was vector-borne diseases, the GVN announced its chikungunya task force (Table 2) and outlined its plans to:
Table 2.
The Global Virus Network’s task force on chikungunya virus.
| Members | Home institution | Email address |
|---|---|---|
| Task force co-chairs | ||
| John Fazakerley, PhD | Pirbright Institute, U.K. | john.fazakerley@pirbright.ac.uk |
| Marc Lecuit, MD, PhD | Institut Pasteur in Paris | marc.lecuit@pasteur.fr |
| Scott Weaver, PhD | UTMB in Galveston, TX | sweaver@utmb.edu |
| Task force members | ||
| Sazaly Abu Bakar, PhD | University of Malaya | sazaly@um.edu.my |
| Simon Cauchemez, PhD | Institut Pasteur in Paris | simon.cauchemez@pasteur.fr |
| James Crowe, MD | Vanderbilt University, TN | james.crowe@vanderbilt.edu |
| Xavier de Lamballerie, PhD | Aix Marseille Université | xavier.de-lamballerie@univ-amu.fr |
| Anna-Bella Failloux, PhD | Institut Pasteur in Paris | anna-bella.failloux@pasteur.fr |
| Matthew Frieman, PhD | U. Maryland Sch. Med., Baltimore, MD | mfrieman@som.umaryland.edu |
| Diane Griffin, PhD | Johns Hopkins University, Baltimore | griffin@jhsph.edu |
| William Hall, PhD | University College Dublin, Ireland | william.hall@ucd.ie |
| William Klimstra, PhD | University of Pittsburgh | klimstra@cvr.pitt.edu |
| Peter Liljestrom, PhD | Karolinska Institutet, Sweden | Peter.Liljestrom@ki.se |
| Jean Lim, PhD | Mt. Sinai Hospital, NY | jean.lim@mssm.edu |
| Cal Macpherson, PhD | St. George’s Univ., Grenada | cmacpherson@sgu.edu |
| Andres Merits, PhD | University of Tartu, Estonia | andres.merits@ut.ee |
| Lisa Ng, PhD | Singapore Immunology Network, SIgN | lisa_ng@immunol.a-star.edu.sg |
| Kenneth Olson, PhD | Colorado State University | kenneth.olson@colostate.edu |
| Janusz Paweska, DVM | NICD, South Africa | januszp@nicd.ac.za |
| Ann Powers, PhD | CDC, Atlanta | akp7@cdc.gov |
| Kate Ryman, PhD | University of Pittsburgh | ryman@cvr.pitt.edu |
| E.Sreekumar, PhD | RG Centre for Biotech, Kerala, India | esreekumar@rgcb.res.in |
| Andreas Suhrbier, PhD | QIMR Berghofer, Australia | Andreas.Suhrbier@qimrberghofer.edu.au |
| Mary Wilson, MD | Harvard SPH, Boston | mewilson@hsph.harvard.edu |
| In-Kyu Yoon, MD | AFRIMS, Thailand | yooni@afrims.org |
review the state of the science, including viral pathogenesis, chronic arthralgia, antiviral drugs, point-of-care diagnostics, and vaccine constructs and trials;
identify potential funding sources to support international collaborative research and train the next generation of researchers in arbovirology;
provide expertise and recommendations to journalists, policy-makers and public health officials; and
advocate for increased research and effective public health measures against the virus and its mosquito vectors.
The task force is chaired by Scott Weaver at the University of Texas Medical Branch Galveston, John Fazakerley at the Pirbright Institute in the U.K., and Marc Lecuit at the Institut Pasteur in Paris.
4. Chikungunya: emergence and basic features
4.1. Global spread of the virus
Since its discovery in Tanzania more than fifty years ago, CHIKV has been responsible for significant outbreaks in Thailand (1962–64), the Democratic Republic of the Congo (1999–2000), Kenya (2004), the French island of Réunion (2005–2006), and India (2005–2008) (Weaver and Forrester, 2015; Thiberville et al., 2013a,b). The Réunion outbreak resulted in approximately 272,000 cases and 254 deaths; the outbreak in India resulted in 1.4 million suspected cases (Mavalankar et al., 2008). Travelers returning from Africa and Réunion also introduced the virus into Italy where the first autochthonous transmissions were observed in 2007.
On 19 December 2013, two confirmed cases of locally acquired chikungunya were reported on the French island of Martinique in the Caribbean. The World Health Organization declared this the first time local transmission of the virus had been detected in the Americas. CHIKV is maintained in a zoonotic cycle that may spill into urban cycles every 40–50 years to cause pandemics. It last emerged in the Caribbean in 1827, when it was thought to be dengue (Halstead, 2015). Since late 2013, the virus has spread westward across the Caribbean and into Central and South America. By March 2015, the Pan American Health Organization (PAHO) reported almost 1.3 million suspected and confirmed cases in the Caribbean and the Americas (PAHO data). In the U.S., the Centers for Disease Control and Prevention (CDC) reported almost 3500 cases in early 2015, with eleven locally-acquired cases in Florida.
4.2. Clinical manifestations
Chikungunya typically presents as a rapid-onset febrile illness, associated with arthralgia, myalgia and rash. The onset of fever coincides with viremia, and the intensity of the acute infection correlates with that of viremia (Thiberville et al., 2013a,b). Joint pain is usually symmetric and localized in both the upper and lower limbs with the large joints being almost invariably symptomatic (Staikowsky et al., 2009).
Rash also may occur, but also is seen in other arboviral diseases such as dengue. Less common, non-specific signs and symptoms include lymphadenopathy, pruritus and digestive abnormalities. Severe disease can manifest as encephalopathy and encephalitis, myocarditis, hepatitis and multi-organ failure (Farnon et al., 2008; Lemant et al., 2008). These rare forms can be fatal, and typically arise in patients with underlying medical conditions. Hemorrhagic complications are rare, if they exist at all, and should therefore lead to the consideration of alternate diagnoses, such as a co-infection with dengue.
Neonates are also at risk for severe infection associated with neurological signs. Whereas fetal infection appears to be extremely rare, infection of neonates born to viremic mothers and contaminated during birth can reach 50%, leading to severe disease and encephalopathy in half the cases, and resulting in long-term neurological sequelae. Young children also tend to develop severe disease.
4.3. Pathogenesis
CHIKV can be cultivated in a wide variety of cell lines. In vivo cell tropism has been investigated in rodents and nonhuman primates (Labadie et al., 2010), as well as in human tissue samples (Couderc et al., 2008). In immunocompetent mice, CHIKV targets fibroblasts in the dermis around the injection site, and is rapidly controlled by type-1 interferon responses. In neonatal mice and mice partially or completely deficient in type-1 interferon signaling, the virus disseminates, leading to viremia, a burst of replication in the liver, and intense replication in muscle, joint, and skin fibroblasts (Couderc et al., 2008; Schilte et al., 2010). This tropism seems to mirror that observed in human biopsies, although a detailed analysis of infected human tissues has not been performed (see forthcoming review by Lum and Ng in this symposium).
In laboratory animals, CHIKV also disseminates to the central nervous system (Couderc et al., 2008), yet in contrast to encephalitogenic alphaviruses, it is not known to target brain microvessel endothelial cells or to infect neurons. Experimental infection of pregnant animals, as well as investigation of human placentas from viremic mothers, have shown that, in contrast to other alphaviruses, CHIKV does not directly infect trophoblastic cells, but is likely transmitted to neonates via maternal–fetal blood exchange during delivery.
Whereas myeloid cells do not seem to contribute significantly to viral replication at the early stage of infection, interactions of CHIKV with monocytes and macrophages may play an important role in the inflammatory responses at the acute and chronic phases of disease. Whether persistent CHIKV replication and/or lack of antigenic clearance contribute to chronic arthralgic symptoms requires further studies with animal models and human samples.
4.4. Antiviral drugs
There are no licensed drugs to limit CHIKV replication and improve clinical outcome, and only standard antipyretic and antalgic therapies are available for symptomatic treatment. The major burden of CHIKV infection results not only from the high attack rate and severity of acute infections, but also from chronic joint pain. This can manifest as persistent or relapsing arthralgia, which may be associated with arthritis and mimic rheumatoid arthritis in up to 50% of patients (Schilte et al., 2013). Chronic arthralgia can lead to persistent incapacitation, requiring long-term treatment with nonsteroidal anti-inflammatory and immunosuppressive drugs such as methotrexate, although their safety and efficacy have yet to be demonstrated in clinical trials.
Off-label use of other FDA-approved drugs in a therapeutic manner has been proposed and is under consideration. In animal models of CHIKV infection, prophylaxis with CHIKV IgG or CHIKV-specific monoclonal antibodies was found to be protective (Couderc et al., 2009), suggesting that antibody-based therapies may be a promising disease prevention strategy for individuals who are at risk for severe CHIKV infection.
The study of other CHIKV-specific antivirals is in very early stages (see forthcoming review by Abdelnabi et al. in this symposium). In cell-based screens of compounds against CHIKV infection, a number of drugs with antiviral activity have been identified, some of which target distinct steps in the CHIKV replication cycle. These include chloroquine (Khan et al., 2010) and chlorpromazine (Pohjala et al., 2011), which affect virus entry. Harringtonine and homoharringtonine (Kaur et al., 2013) have been found to affect viral protein translation. Others, including trigocherriolide A (Bourjot et al., 2014), ribavirin (Albulescu et al., 2014), interferon-alpha (Schilte et al., 2010), apigenin and silybin (Pohjala et al., 2011), affect virus replication. More extensive preclinical evaluation of these, and other identified drugs, in animal models of CHIKV disease are necessary before they are proposed for use in humans.
Immune and inflammatory responses to CHIKV infection may also contribute to pathogenesis (Morrison, 2014). Thus, modulation of the immune response to the infection by immune response modulating drugs may also be a viable disease prevention strategy. For example, treatment of mice with bindarit, an inhibitor of MCP-1 synthesis, reduced the severity of CHIKV-induced musculoskeletal tissue inflammation and injury (Chen et al., 2015; Rulli et al., 2011). A deeper understanding of the viral and host factors that contribute to acute and chronic CHIKV disease, both in animal models and in patients, are essential to inform the development of host-targeted therapeutics.
4.5. Vaccines
Because all CHIKV strains essentially fall into a single serotype, with only minor antigenic differences, and there is no evidence for immune enhancement of infection by pre-existing immunity, the virus represents a relatively straightforward target for vaccine development. A variety of approaches have been used for the development of CHIKV vaccines, including non-infectious (Mallilankaraman et al., 2011) and infectious DNA vaccines (Tretyakova et al., 2014), virus-like particles, and inactivated virus (Harrison et al., 1971). Replication-competent forms include: rationally attenuated alphavirus chimeras (Wang et al., 2008) and deletion mutants (Hallengard et al., 2014); a vesicular stomatitis-vectored vaccine (Chattopadhyay et al., 2013); and an internal ribosome entry site-modified CHIKV strain licensed to Takeda Pharmaceuticals (Plante et al., 2011) and shown to be stably attenuated, safe and efficacious in cynomolgus macaques (Roy et al., 2014).
All of these live, attenuated vaccines are efficacious after a single dose. To date, three CHIKV vaccines have progressed to clinical trials. The strain 181/clone25, developed by the U.S. Army in the 1980s (Levitt et al., 1986), proved highly immunogenic but mildly reactogenic in Phase II trials (Edelman et al., 2000). Further development of this vaccine has not been reported. A VLP vaccine produced by expression of the CHIKV structural proteins in vertebrate cells demonstrated efficacy in preclinical studies after multiple doses given to mice or Rhesus macaques (Akahata et al., 2010). Phase 1 clinical studies showed strong immunogenicity after 2–3 doses (Chang et al., 2014). Currently this vaccine is not licensed to a commercial partner. A measles virus-vectored vaccine licensed to Themis Bioscience GmbH generates murine-protective immunity after a single dose (Brandler et al., 2013) and 100% human seroconversion after 2 doses, with no evidence of interference from prior measles virus immunity (Ramsauer et al., 2015).
4.6. Diagnosis
Although several of these vaccines appear very promising for human use, final development and licensure also will present financial and regulatory challenges. The size of the future market for a CHIKV vaccine is difficult to estimate because of the sporadic nature of outbreaks and their tendency to occur in global regions with limited financial resources. This sporadic and unpredictable nature of CHIKV circulation, as well as the lack of affordable, point-of-care diagnostics to estimate incidence, will make the selection of a suitable site for clinical efficacy trials challenging. However, this latter problem could be overcome if efficacy data in animal models such as nonhuman primates, which appear to faithfully reproduce human-like disease, are accepted for licensure.
A major challenge to research on CHIKV and to the control of outbreaks is the limited diagnostic capabilities in many regions where outbreaks occur. For example, of the more than 1.2 M suspected cases in the Americas since the fall of 2013, only 2% have been confirmed by laboratory diagnostics (PAHO data). The combination of RT-PCR during early stages of infection and IgM ELISA later—after the appearance of symptoms and signs—is capable of diagnosing the majority of human infections. However, implementation of these assays is currently limited by the high cost of commercial test kits, limited local capabilities in many endemic countries for implementing in-house tests, including antigen and control sample production. A few rapid, point-of-care tests are available, but their performance is generally lower than that of traditional platforms.
Because distinguishing clinically CHIKV infection from dengue, malaria and other acute febrile illnesses in the absence of laboratory diagnostics can be challenging, improvement in diagnostic capabilities could have dramatic impacts on several fronts: (1) optimal patient management, including reducing the counterproductive use of antibiotics, antimalarials and other drugs that have no effect on CHIKV infection; (2) timely detection of cases imported into CHIKV-free regions where public health measures including patient education and vector control can reduce the risk of initiating of the transmission cycle; (3) identification of optimal sites for clinical research, including trials of vaccines and therapeutics, as well as evaluation the efficacy of vector control and other public health measures in controlling disease and spread.
4.7. The future
CHIKV continues to spread through the Western Hemisphere, creating new challenges to disease prevention, diagnosis, surveillance, and patient care (Weaver and Forrester, 2015). Vector control programs remain notoriously weak throughout much of Latin America and the Caribbean. Surveillance is hampered by a lack of funds and inadequate diagnostics. There are few available laboratory or point-of-care diagnostics, and many of these serology- and PCR-based assays have yet to be validated. Serious, post-infection, chronic arthralgia remains poorly understood with few effective treatment options for patients. Several candidate vaccine constructs exist, but they need to demonstrate safety and efficacy in Phase I and II trials.
Deaths from CHIKV rarely have been reported. During the Réunion Island outbreak in 2005–2006, however, excess deaths attributed either directly or indirectly to CHIKV infection suggested a case fatality rate of approximately one per thousand cases (Josseran et al., 2006). The financial cost of that same outbreak was estimated to be $48 million (€44 million), with direct medical costs estimated at $98 (€90) per outpatient and $2161 (€2000) per inpatient (Soumahoro et al., 2011). As CHIKV continues its advance through the virgin territories of the Caribbean and the Americas similar studies of “excess deaths” and “cost-of-illness” are likely to appear in the literature. In addition, some media outlets in the Caribbean already have reported national estimates of economic losses from chikungunya due to employee absences for medical care (Gammon, 2014). To date, cost-of-illness analyzes and reports of economic losses have focused on the acute phases of CHIKV infection; patients with persistent, post-infection arthritis also are likely to be a significant and long-term source of economic losses and public health burdens.
The GVN and its task force of international experts are committed to advancing knowledge about CHIKV infections and preventive measures through global cooperation and partnerships. Currently, GVN is working to mobilize resources to convene international scientific meetings, support research training for young medical virologists, and provide a venue for greater public health advocacy.
For more information about the chikungunya task force or the GVN in general, visit the web site at http://gvn.org/. For information about the Centers or Excellence and participating in GVN activities, please contact Sharon Hrynkow, PhD, GVN President.
References
- Akahata W, Yang ZY, Andersen H, Sun S, Holdaway HA, Kong WP, Lewis MG, Higgs S, Rossmann MG, Rao S, Nabel GJ. A virus-like particle vaccine for epidemic Chikungunya virus protects nonhuman primates against infection. Nat Med. 2010;16:334–338. doi: 10.1038/nm.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albulescu IC, Tas A, Scholte FE, Snijder EJ, van Hemert MJ. An in vitro assay to study chikungunya virus RNA synthesis and the mode of action of inhibitors. J Gen Virol. 2014;95:2683–2692. doi: 10.1099/vir.0.069690-0. [DOI] [PubMed] [Google Scholar]
- Bourjot M, Leyssen P, Neyts J, Dumontet V, Litaudon M. Trigocherrierin A, a potent inhibitor of chikungunya virus replication. Molecules. 2014;19:3617–3627. doi: 10.3390/molecules19033617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandler S, Ruffie C, Combredet C, Brault JB, Najburg V, Prevost MC, Habel A, Tauber E, Despres P, Tangy F. A recombinant measles vaccine expressing chikungunya virus-like particles is strongly immunogenic and protects mice from lethal challenge with chikungunya virus. Vaccine. 2013;31:3718–3725. doi: 10.1016/j.vaccine.2013.05.086. [DOI] [PubMed] [Google Scholar]
- Chang LJ, Dowd KA, Mendoza FH, Saunders JG, Sitar S, Plummer SH, Yamshchikov G, Sarwar UN, Hu Z, Enama ME, Bailer RT, Koup RA, Schwartz, Akahata W, Nabel GJ, Mascola JR, Pierson TC, Graham BS, Ledgerwood JE The V.R.C.S.T. Safety and tolerability of chikungunya virus-like particle vaccine in healthy adults: a phase 1 dose-escalation trial. Lancet. 2011;384(9959):2046–2052. doi: 10.1016/S0140-6736(14)61185-5. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay A, Wang E, Seymour R, Weaver SC, Rose JK. A chimeric vesiculo/alphavirus is an effective alphavirus vaccine. J Virol. 2013;87:395–402. doi: 10.1128/JVI.01860-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Foo SS, Taylor A, Lulla A, Merits A, Hueston L, Forwood MR, Walsh NC, Sims NA, Herrero LJ, Mahalingam S. Bindarit, an inhibitor of monocyte chemotactic protein synthesis, protects against bone loss induced by chikungunya virus infection. J Virol. 2015;89:581–593. doi: 10.1128/JVI.02034-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couderc T, Chretien F, Schilte C, Disson O, Brigitte M, Guivel-Benhassine F, Touret Y, Barau G, Cayet N, Schuffenecker I, Després P, Arenzana-Seisdedos F, Michault A, Albert ML, Lecuit M. A mouse model for Chikungunya: young age and inefficient type-I interferon signaling are risk factors for severe disease. PLoS Pathog. 2008;4(2):e29. doi: 10.1371/journal.ppat.0040029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couderc T, Khandoudi N, Grandadam M, Visse C, Gangeux N, Bagot S, Prost JF, Lecuit M. Prophylaxis and therapy for Chikungunya virus infection. J Infect Dis. 2009;200:516–523. doi: 10.1086/600381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman R, Tacket CO, Wasserman SS, Bodison SA, Perry JG, Mangiafico JA. Phase II safety and immunogenicity study of live chikungunya virus vaccine TSI-GSD-218. Am J Trop Med Hyg. 2000;62:681–685. doi: 10.4269/ajtmh.2000.62.681. [DOI] [PubMed] [Google Scholar]
- Farnon EC, Sejvar JJ, Staples JE. Severe disease manifestations associated with Acute chikungunya virus infection. Crit Care Med. 2008;36(9):2682–2683. doi: 10.1097/CCM.0b013e3181843d94. [DOI] [PubMed] [Google Scholar]
- Gammon K. The cost of chikungunya to Jamaica. [accessed on April 7, 2015];Jamaica Observer. 2014 Oct 20; < www.jamaicaobserver.com/columns/The-cost-of-chikungunya-to-Jamaica_17772229>.
- Hallengard D, Kakoulidou M, Lulla A, Kummerer BM, Johansson DX, Mutso M, Lulla V, Fazakerley JK, Roques P, Le Grand R, Merits A, Liljeström P. Novel attenuated Chikungunya vaccine candidates elicit protective immunity in C57BL/6 mice. J Virol. 2014;88:2858–2866. doi: 10.1128/JVI.03453-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead SB. Reappearance of chikungunya, formerly called dengue, in the Americas. Emerg Infect Dis. 2015;21:557–561. doi: 10.3201/eid2104.141723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison VR, Eckels KH, Bartelloni PJ, Hampton C. Production and evaluation of a formalin-killed Chikungunya vaccine. J Immunol. 1971;107:643–647. [PubMed] [Google Scholar]
- Josseran L, Paquet C, Zehgnoun A, Caillere N, le Tertre A, Solet JL, Ledrans M. Chikungunya disease outbreak, Reunion Island. Emerg Infect Dis. 2006;12(2):1994–1995. doi: 10.3201/eid1212.060710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur P, Thiruchelvan M, Lee RC, Chen H, Chen KC, Ng ML, Chu JJ. Inhibition of chikungunya virus replication by harringtonine, a novel antiviral that suppresses viral protein expression. Antimicrob Agents Chemother. 2013;57:155–167. doi: 10.1128/AAC.01467-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M, Santhosh SR, Tiwari M, Lakshmana Rao PV, Parida M. Assessment of in vitro prophylactic and therapeutic efficacy of chloroquine against Chikungunya virus in vero cells. J Med Virol. 2010;82:817–824. doi: 10.1002/jmv.21663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labadie K, Larcher T, Joubert C, Mannioui A, Delache B, Brochard P, Guigand L, Dubreil L, Lebon P, Verrier B, de Lamballerie X, Suhrbier A, Cherel Y, le Grand R, Roques P. Chikungunya disease in nonhuman primates involves long-term viral persistence in macrophages. J Clin Invest. 2010;120(3):894–906. doi: 10.1172/JCI40104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemant J, Boisson V, Winer A, Thibault L, André H, Tixier F, Lemercier M, Antok E, Cresta MP, Grivard P, Besnard M, Rollot O, Favier F, Huerre M, Campinos JL, Michault A. Serious acute chikungunya virus infection requiring intensive care during the Reunion Island outbreak in 2005–2006. Crit Care Med. 2008;36(9):2536–2541. doi: 10.1097/CCM.0b013e318183f2d2. [DOI] [PubMed] [Google Scholar]
- Levitt NH, Ramsburg HH, Hasty SE, Repik PM, Cole FE, Lupton HW. Development of an attenuated strain of chikungunya virus for use in vaccine production. Vaccine. 1986;4:157–162. doi: 10.1016/0264-410x(86)90003-4. [DOI] [PubMed] [Google Scholar]
- Mallilankaraman K, Shedlock DJ, Bao H, Kawalekar OU, Fagone P, Ramanathan AA, Ferraro B, Stabenow J, Vijayachari P, Sundaram SG, Muruganandam N, Sarangan G, Srikanth P, Khan AS, Lewis MG, Kim JJ, Sardesai NY, Muthumani K, Weiner DB. A DNA vaccine against chikungunya virus is protective in mice and induces neutralizing antibodies in mice and nonhuman primates. PLoS Negl Trop Dis. 2011;5:e928. doi: 10.1371/journal.pntd.0000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavalankar D, Shastri P, Bandyopadhyay T, Parmar J, Ramani KV. Increased mortality rate associated with chikungunya epidemic, Ahmedabad, India. Emerg Infect Dis. 2008;14(3):412–415. doi: 10.3201/eid1403.070720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison TE. Reemergence of chikungunya virus. J Virol. 2014;88:11644–11647. doi: 10.1128/JVI.01432-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plante K, Wang E, Partidos CD, Weger J, Gorchakov R, Tsetsarkin K, Borland EM, Powers AM, Seymour R, Stinchcomb DT, Osorio JE, Frolov I, Weaver SC. Novel chikungunya vaccine candidate with an IRES-based attenuation and host range alteration mechanism. PLoS Pathog. 2011;7:e1002142. doi: 10.1371/journal.ppat.1002142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohjala L, Utt A, Varjak M, Lulla A, Merits A, Hola T, Tammela P. Inhibitors of alphavirus entry and replication identified with a stable Chikungunya replicon cell line and virus-based assays. PLoS One. 2011;6:e28923. doi: 10.1371/journal.pone.0028923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsauer K, Schwameis M, Firbas C, Mullner M, Putnak RJ, Thomas SJ, Despres P, Tauber E, Jilma B, Tangy F. Immunogenicity, safety, and tolerability of a recombinant measles-virus-based chikungunya vaccine: a randomised, double-blind, placebo-controlled, active-comparator, first-in-man trial. Lancet Infect Dis. 2015;15(5):519–527. doi: 10.1016/S1473-3099(15)70043-5. [DOI] [PubMed] [Google Scholar]
- Roy CJ, Adams AP, Wang E, Plante K, Gorchakov R, Seymour RL, Vinet-Oliphant H, Weaver SC. Chikungunya vaccine candidate is highly attenuated and protects nonhuman primates against telemetrically monitored disease following a single dose. J Infect Dis. 2014;209:1891–1899. doi: 10.1093/infdis/jiu014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rulli NE, Rolph MS, Srikiatkhachorn A, Anantapreecha S, Guglielmotti A, Mahalingam S. Protection from arthritis and myositis in a mouse model of acute chikungunya virus disease by bindarit, an inhibitor of monocyte chemotactic protein-1 synthesis. J Infect Dis. 2011;204:1026–1030. doi: 10.1093/infdis/jir470. [DOI] [PubMed] [Google Scholar]
- Schilte C, Couderc T, Chretién F, Sourisseau M, Gangneux N, Guivel-Benhassine F, Kraxner A, Tschopp J, Higgs S, Michault A, Arenzana-Seisdedos F, Colonna M, Peduto L, Schwartz O, Lecuit M, Albert ML. Type I IFN controls chikungunya virus via its action on non-hematopoietic cells. J Exp Med. 2010;207(2):429–442. doi: 10.1084/jem.20090851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilte C, Staikowsky F, Couderc T, Madec Y, Carpentier F, Kassab S, Albert ML, Lecuit M, Michault A. Chikungunya virus-associated long-term arthralgia: a 36-month prospective longitudinal study. PLoS Negl Trop Dis. 2013;7(3):e2137. doi: 10.1371/journal.pntd.0002137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soumahoro MK, Boelle PY, Gauzere BA, Atsou K, Pelat C, Lambert B, La Ruche G, Gastellu-Etchegorry M, Renault P, Sarazin M, Yazdanpanah Y, Flahault A, Malvy D, Hanslik T. The chikungunya epidemic on La Réunion Island in 2005–2006: a cost-of-illness study. PLoS Negl Trop Dis. 2011;5(6):e1197. doi: 10.1371/journal.pntd.0001197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staikowsky F, Talarmin F, Grivard P, Souab A, Schuffenecker I, le Roux K, Lecuit M, Michault A. Prospective study of Chikungunya virus acute infection in the Island of La Reunion during the 2005–2006 outbreak. PLoS One. 2009;4(10):e7603. doi: 10.1371/journal.pone.0007603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiberville SD, Boisson V, Gaudart J, Simon F, Flahault A, de Lamballerie X. Chikungunya fever: a clinical and virological investigation of outpatients on Reunion Island, South-West Indian Ocean. PLoS Negl Trop Dis. 2013a;7(1):e2004. doi: 10.1371/journal.pntd.0002004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiberville SD, Moyen N, Dupuis-Maguiraga L, Nougairede A, Gould EA, Roques P, de Lamballerie X. Chikungunya fever: epidemiology, clinical syndrome, pathogenesis and therapy. Antiviral Res. 2013b;99(3):345–370. doi: 10.1016/j.antiviral.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretyakova I, Hearn J, Wang E, Weaver S, Pushko P. DNA vaccine initiates replication of live attenuated chikungunya virus in vitro and elicits protective immune response in mice. J Infect Dis. 2014;209:1882–1890. doi: 10.1093/infdis/jiu114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E, Volkova E, Adams AP, Forrester N, Xiao SY, Frolov I, Weaver SC. Chimeric alphavirus vaccine candidates for chikungunya. Vaccine. 2008;26:5030–5039. doi: 10.1016/j.vaccine.2008.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver SC, Forrester NL. Chikungunya: evolutionary history and recent epidemic spread. Antiviral Res. 2015;120:32–39. doi: 10.1016/j.antiviral.2015.04.016. [DOI] [PubMed] [Google Scholar]
- Zakaib GD. Virologists form Global Virus Response Network. [accessed March 23, 2015];Nature News Blog. 2011 Mar 3; [Google Scholar]