Abstract
The Oxidative Stress Theory of Aging has had tremendous impact in research involving aging and age-associated diseases including those that affect the nervous system. With over half a century of accrued data showing both strong support for and against this theory, there is a need to critically evaluate the data acquired from common biomedical research models, and to also diversify the species used in studies involving this proximate theory. One approach is to follow Orgel’s second axiom that “evolution is smarter than we are” and judiciously choose species that may have evolved to live with chronic or seasonal oxidative stressors. Vertebrates that have naturally evolved to live under extreme conditions (e.g., anoxia or hypoxia), as well as those that undergo daily or seasonal torpor encounter both decreased oxygen availability and subsequent reoxygenation, with concomitant increased oxidative stress. Due to its high metabolic activity, the brain may be particularly vulnerable to oxidative stress. Here, we focus on oxidative stress responses in the brains of certain mouse models as well as extremophilic vertebrates. Exploring the naturally evolved biological tools utilized to cope with seasonal or environmentally variable oxygen availability may yield key information pertinent for how to deal with oxidative stress and thereby mitigate its propagation of age-associated diseases.
Introduction
The recent death of one of the most influential bio-gerontologists of all time, Dr. Denham Harman (1916–2014), the founding father of the Oxidative Stress Theory of Aging, forces one to reflect on the tremendous impact his paradigm shifting work has had in biomedical research as well as the outstanding issues involving his postulate. Dr. Harman’s theory has enjoyed considerable impact and support as a basis of why and how we age. It also is considered a fundamental component of research concerning cancer, cardiovascular, and neurodegenerative diseases [1, 2]. It has also been ardently embraced in ecological and physiological studies as a mechanistic explanation for the observed life history trade off associated with allocating energy to reproduction or somatic tissue maintenance [3], suggesting ubiquitous importance throughout life’s seasons.
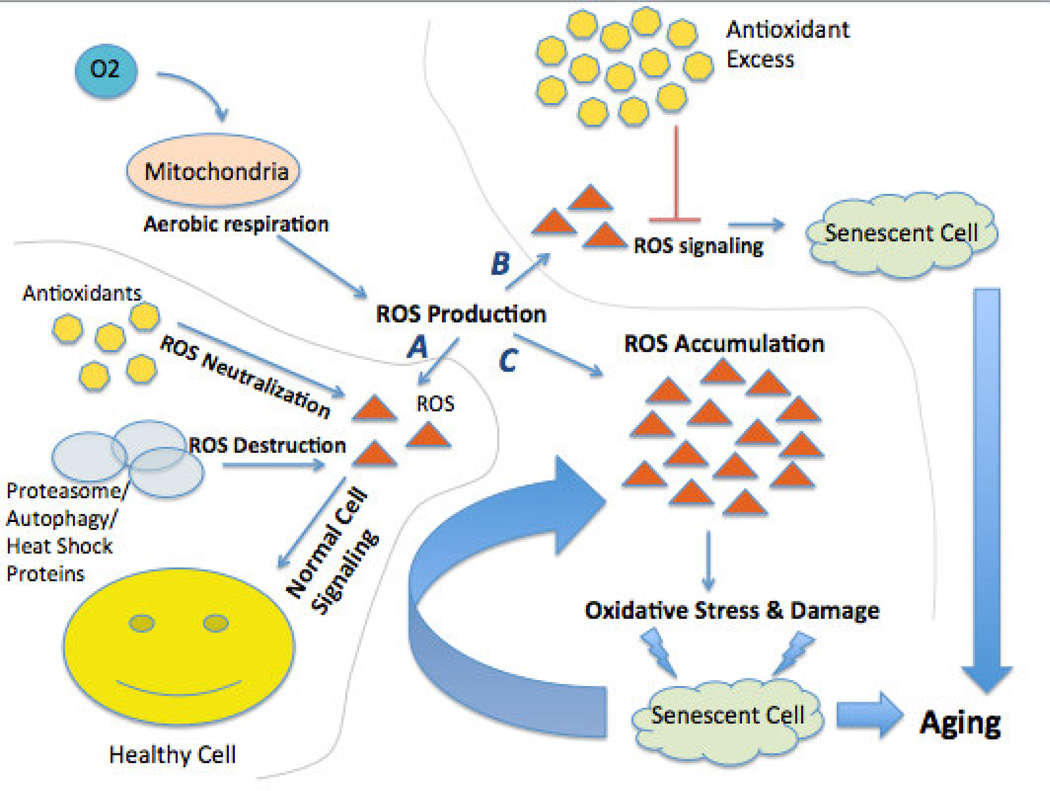
Sixty years ago, Dr. Harman proposed that aging occurs when a cell is no longer able to balance the inevitable creation of reactive oxygen species [ROS] during aerobic metabolism with their neutralization by antioxidants. The irreversible destructive effects of these metabolic byproducts accumulate and cause oxidative damage, cellular degeneration, and functional decline. Additionally, they catalyze the further production of ROS that perpetuates damage and destruction of surrounding cells. Since damaged macromolecules in turn become ROS, oxidative damage becomes a self-propagating insult (Figure 1). If left unchecked, this catalytic chain reaction often coincides with age-associated cumulative damage to cellular macromolecules and organelles. Further, when inadequate repair processes are available, organ functionality decreases and the incidence of age-associated morbidities such as neurodegenerative diseases increases [4–6].
Figure 1. The Oxidative Stress Theory of Aging.
As an inevitable byproduct of aerobic respiration, the mitochondria in a normally functioning cell create reactive oxygen species (ROS). ROS in moderate amounts are beneficial and essential for normal cell signaling and cellular immunity. In a normally functioning cell, antioxidants may adequately neutralize excess ROS. If levels of ROS are unchecked they cause oxidative damage to the cellular constituents (protein, lipids, and DNA). These macromolecules may be protected to some extent by molecular chaperones mechanisms or, if damaged, removed from the cell by either autophagy or the proteasome. Damage that cannot be repaired or removed may accrue in the cell leading to impaired function and cell death. These cellular changes may accrue as the organism gets older, giving rise to the deleterious aging phenotype.
The brain, in particular, is more susceptible to oxidative stress than other organs. Although the brain only accounts for ~2% of body mass it consumes 15–20% of the energy generated in the entire body. The high mass specific metabolic rate is attributed to the high proportion of omega-three polyunsaturated fatty acids (PUFAs) in brain tissue [7]. These phospholipids are highly susceptible to peroxidation. Moreover, brain tissue contains high levels of redox-active iron and copper further enhancing its vulnerability to oxidative stress. Brain also has little potential to replenish damaged cells since it is composed mostly of terminally differentiated neurons and glia. As deaths associated with neurodegenerative diseases continue to increase in prevalence with few, if any, treatment options available, here we have focused the on what is known about oxidative stress in the brain, the impact thereof in cognitive function and the mechanisms of protecting this important organ in vertebrates especially those living in extremely hostile habitats.
The Physiological Role of Reactive Oxygen Species in the Brain
Though oxygen provides the chemical energy essential for life, harnessing this element for metabolic activities carries a high cost due to the formation of highly volatile ROS. ROS are best known for indiscriminately attacking phospholipids, proteins and DNA. If left unchecked these insults can cause substantial damage at the cellular level and eventually affect the organism as a whole. As the central regulator of the entire organism and one of the most metabolically active tissues in the body, the brain must tightly regulate its oxygen handling and redox regulation to preserve somatic health.
In moderate amounts, ROS are essential for normal brain/cell function. Moreover many endogenous cytoprotective molecules are upregulated by ROS, thereby triggering many adaptations to counteract and neutralize oxidative stressors [8]. Physiological ROS levels are extremely critical during brain development. In the developing brain, ROS contribute to healthy neural stem cell proliferation and differentiation and neurons produce high levels of physiologically active ROS to promote these critical functions. This is evidenced by decreased neural stem cell (NSC) proliferation rate and altered neuronal differentiation when neuronal cultures are supplemented with antioxidants [9–11]. Conversely, hypoxia conditioned media enhances NSC proliferation and favors neuronal survival over that of astrocytes [12]. Standard culture atmospheric conditions also influence cell survival, proliferation and fate determination in induced pluripotent stem cell (iPS) neurospheres [13–16]. While neurosphere number and size remain constant regardless of whether they are cultured at normal atmospheric conditions (21% O2) or under hypoxia (1–5% O2), culturing the iPS neurospheres in 1% oxygen increased gene expression of HIF-1A and VEGF and EPOR [10] angiogenic growth factors amenable to neurogenesis and brain development.
In the adult brain, ROS exerts physiological functions through the modulation of redox-sensitive proteins. ROS second messengers, such as nitrous oxide (NO) and carbon monoxide (CO) promote long-term potentiation (LTP) by inducing GMP-regulated glutamate release [17–21]. These reversible reactions alter synaptic plasticity and cellular metabolism through a delicate balance where high concentrations diminish LTP and synaptic signaling, and low concentrations enhance these processes.
Despite an age-associated decrease in CNS metabolic function with age, oxidative stress as measured by altered membrane lipids, oxidized proteins, and damaged DNA increase while natural antioxidant levels become dysregulated even in cognitively healthy individuals [22–24]. Gradually, physiological levels of ROS or reactive nitrogen species (RNS) start to exceed the brain’s innate defenses. When compensatory defenses fail to counteract excess oxidative stress, impairments in neuronal function and cognition occur. The precise mechanisms involved in brain aging that lead to oxidative stress are complex; oxidative damage accrual is not uniform among individuals, or in the various brain regions, or even among cell types within the same specific brain region.
Dealing with Excess ROS in the Brain
As post mitotic cells, neurons require highly effective mechanisms to eliminate oxidative damaged macromolecules. To combat ROS and eradicate damaged macromolecules, neurons utilize highly effective antioxidant defenses and efficient repair and removal mechanisms. Both autophagy and the ubiquitin proteasome system play a pivotal role in the removal of damaged proteins and organelles [25–27]. Chaperone interaction with these degradative machinery and assist in this regard. After mild oxidative stress, chaperones facilitate clearance of damaged macromolecules to prevent neuronal cell death both in vitro [28] and in vivo [29]. Not surprisingly, therefore, when proteasome populations are depleted in mouse forebrain neurons, oxidative damage and ROS generating oxidative stressors increase dramatically [30].
To combat oxidative stress-induced DNA damage (i.e., 8-oxo-7, 8-dihydro-29-deoxyguanosine (8-oxodG) lesions or single strand nicks) cells execute base excision repair (BER) [31]. These repair and damage removal mechanisms decline with age [32] and can lead to cognitive decline [31, 33]. In particular, aged brains exhibit high levels of oxidativly-induced DNA mutations, especially in mitochondrial DNA (mtDNA) [34–37]. mtDNA mutations have exceptionally dire implications in the brain as mtDNA half-life is extremely long even when compared to other post-mitotic tissues (e.g., heart 7 days compared to brain 31 days) [38]. To assess the effects of DNA oxidative damage on longevity, levels of 8-oxodG were compared among six mammals ranging in maximum lifespan (MLS) (3.5 years, mice, to 46 years, horse) [38]. Short-lived species contained higher 8-oxodG than long-lived species; further the ratio between mtDNA and nDNA 8-oxodG levels tended to be lower in long-lived species than in short-lived species, 3 and 9 fold for horse and rat, respectively [38]. Although this study uses phylogenetically diverse species that differ dramatically in body size and other allometrically dependent variables that may confound the data, this study strongly implicates maintenance of brain mtDNA integrity as a strong correlate for longevity. However, it is critical to show that oxidative damage in brain tissue, regardless of whether these are neurons or other brain cells, is linked to altered brain function.
Oxidative Stress and Senescence-accelerated Mouse Models
While technologies for cognitive function assessment and in vivo brain imaging have seen remarkable advancements in the past decade, linking oxidative damage in the brain to specific cognitive and cellular functions is extremely difficult, especially with respect to human aging and neurodegenerative diseases. Animal models allow for better control over variability in genetic and environmental factors, and for in vivo findings to be validated through biochemical and histological assays. Many critical insights into brain oxidative stress have been acquired through a serendipitous outcross of an unknown albino mouse strain with AKR/J mice. AKR/J mice gave rise to several distinct inbred senescence-accelerated mouse (SAM) strains [39], with spontaneous gene mutation(s) that ostensibly impact upon various aspects of aging. The SAMP-10 mouse strain exhibits brain atrophy and neurodegeneration while that of the SAMP-8 develop learning and memory deficits [40, 41]. Several studies using SAMP mice indicate that oxidative stress greatly contributes to their accelerated aging phenotypes and many of these SAMP strains exhibit decreased antioxidant expression [42, 43]. In addition to dysregulation of these antioxidant enzymes, increased caspase-3 and calpain activity are also evident and coincide with cortical astrogliosis and neuron loss [44]. Protease dysregulation and the resulting altered removal of oxidatively damaged cellular components may also contribute to neuronal death in SAMP-8 [45, 46] suggesting numerous mechanisms of accelerated aging in these mice.
In addition to exacerbating normal aging phenotypes, oxidative stress is also involved in pathological brain conditions. Elevated levels of cortical mtDNA deletions have been found in patients with Huntington’s disease [47], Parkinson’s disease [48] and Alzheimer’s disease (AD) [49]. Alzheimer’s pathogenesis is characterized by elevated Aβ and phosphorylated tau, which coincide with neurodegeneration and dementia. Elevated antioxidant expression in AD animal models (Tg2576 or Tg19959) revealed that SOD-2 overexpression reduced Aβ plaque deposition, ameliorated oxidative stress and prevented cognitive decline [50, 51]. A follow-up study using diffusion tensor imaging revealed that white matter tracts were similar between Tg2576 mice with or without SOD-2 overexpression to suggest that SOD-2 was exerting functional, not structural improvements [52]. A separate group used the same Tg2576 AD mouse, but crossed to a mouse overexpressing mitochondria-targeted catalase and found decreased Aβ, oxidative DNA damage and extended lifespan compared to Tg2576 control mice [53]. Collectively these studies show that genetic restoration of antioxidants greatly ameliorates AD-associated pathology and cognitive deficits in transgenic mice. However, supplementation of antioxidants in human clinical trials has had conflicting results and may even be more harmful than beneficial [54–56]. Despite a strong push from the pharmaceutical and neutraceutical industries, many years of study of the potential of antioxidant supplementation an “anti-aging” and “anti-neurodegenerative” therapy, there is no well-authenticated study that unequivocally demonstrates a benefit of antioxidant supplementation in humans or other mammals.
Constraints posed by laboratory studies
Laboratory studies can provide unparalleled opportunities to conduct carefully controlled comprehensive studies and tease out the inconsistent data regarding the Oxidative Stress Theory of Aging. However, laboratory studies come with their own suite of issues, which perhaps may be key in the unsuccessful attempts with antioxidant therapies to treat neurodegenerative/age-associated disorders in human patients. A key question is whether captive care adequately simulates real life situations that may alter molecular responses to oxidative stress and if there is indeed a tradeoff between life history traits and survival in laboratory animals. For example, the high oxygen content in the regulated air exchanges flowing through the animal facility may affect the level of oxidative stress encountered, especially when animals are at rest. Another confounding problem that may compromise oxidative stress studies is disparate food availability, both in quantity and quality, in captive and wild animals. Whereas animals in the wild may commonly encounter periods with limited food availability and may have to spend considerable time and energy foraging, laboratory rodents are kept in small cages that restrict exercise with an ad libitum supply of food. Moreover, while most rodents in the wild rest in thermally buffered burrows or crevices, mice in captivity usually are exposed to the same thermal conditions when resting and active and are chronically cold-stressed. In most animal facilities mice are housed at temperatures (20–24°C) well below thermoneutrality (30–32°C) and thus are required to expend considerable energy on facultative thermogenesis [57, 58]. With no opportunity for physical exercise and little mental stimulation these cold-stressed rodents often consume vast amounts of food relative to animals in the wild and become obese. As a result, they may show very different gene expression to well-exercised animals, and may develop various associated pathological conditions, including reduced cognitive function [59, 60] that may constrain or confound their central nervous system responses to oxidative stress and aging. If these sedentary rodents are subjected to high fat or high sugar diets they may become even more vulnerable to oxidative stress [61] and show marked cognitive impairments [62]. Not surprisingly therefore they may be more responsive to experimental manipulations that reduce oxidative stress or alter these detrimental environmental conditions.
Insights Arising from Comparative Biology Studies ~ the Evolutionary Paradigm
A common general assumption that “below the surface” all species share the same biochemical, molecular and cellular process, so experimental findings based upon laboratory mice and rats would apply to all mammals including humans. Disappointingly, this does not appear to be the case. While we have successfully cured neurodegenerative diseases many times over in mice, many of the promising preclinical findings based upon lab rodents have not translated into effective human treatments [63]. Given that many of the concerns raised in the role of oxidative stress, aging and neurodegeneration commonly arise through studies solely using mice and rats in a tightly controlled laboratory environment, there is a dire need for additional animal models that show different ecophysiological strategies as well as longevity traits. Species that have adapted to extreme environments (e.g., with pronounced seasonal thermal fluctuations or low oxygen) or that are extremely long-lived such as the naked mole-rat [64] may be particularly useful [57, 65–67]. Such unusual, highly adapted species enable one to test the ubiquity of findings observed in traditional laboratory models, and evaluate whether nature has already evolved the appropriate mechanisms to overcome the cellular and molecular challenges posed by oxidative stress.
Lessons from Hypoxia-Tolerant Animals
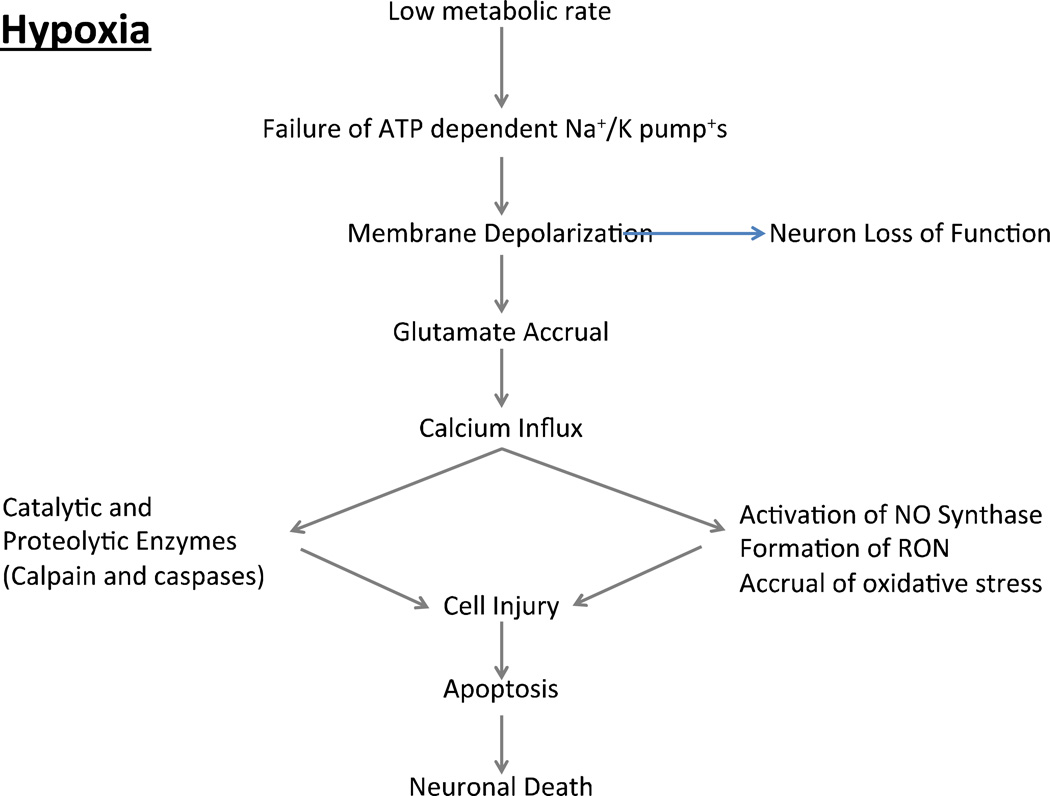
The detrimental effects of brain hypoxia/ischemia have been reported in all vertebrate phyla ranging from fish to mammals [68, 69]. The high mass specific metabolic rates evident in the brains of both poikilothermic and homeothermic species is attributed to the oxygen-dependent maintenance of neuronal plasma membrane ionic gradients essential for normal brain function [70]. When energy substrates are limited as commonly occurs in response to hypoxia, hypoglycemia, or a decline in cerebral perfusion, neurons encounter a critical shortage of energy and the neuronal membrane potential becomes compromised (Figure 2 and 3). The ensuing spontaneous depolarization causes an increase in intracellular calcium, and dysregulated release of neurotransmitters. Particularly devastating is spontaneous glutamate release, which eventually culminates in excitotoxic cell death [71, 72]. Despite the low levels of metabolism associated with hypoxic/ischemic events, the high levels of intracellular cations and ADP increase mitochondrial ROS production (Figure 4). Deleterious levels of ROS are further exacerbated by ischemia-induced RNS (peroxynitrite) formation, and the effects of DNA-repair enzyme PARP-1 inducing NAD+ dysfunction and the concomitant impact upon mitochondrial respiration [71]. Reoxygenation/reperfusion following hypoxia/ischemia events coupled with the acute increase in metabolism also gives rise to considerable oxidative stress (Figure 4). Mitochondria are not the only source of ROS during reoxygenation, rather nicotin-amide adenine dinucleotide phosphate (NADPH)-oxidase (NOX) is a key component, and elevation in NOX often causes considerable neuronal death [73].
Figure 2. A schematic of how hypoxia may induce neuronal death.
Inadequate oxygen availability to meet metabolic demands gives rise to an intracellular energy crisis that compromises ATP-dependent processes in the cell. As a result of the dysfunction of the Na+/K+ pumps the ionic gradients between intracellular and extracellular compartments cannot be maintained, and membrane potential is altered and synaptic function impaired. Membrane depolarization leads to the release of neurotransmitters. The most abundant excitatory neurotransmitter is glutamate, which promotes the opening of calcium channels. The rapid influx of calcium leads to the activation of various degradative pathways as well as to the activation of nitric oxide (NO) synthase and the formation of reactive oxygen and nitrogen species (RON). Increased free radicals and catabolic pathways damage cell membranes, structural proteins and DNA, triggering apoptosis and neuronal death.
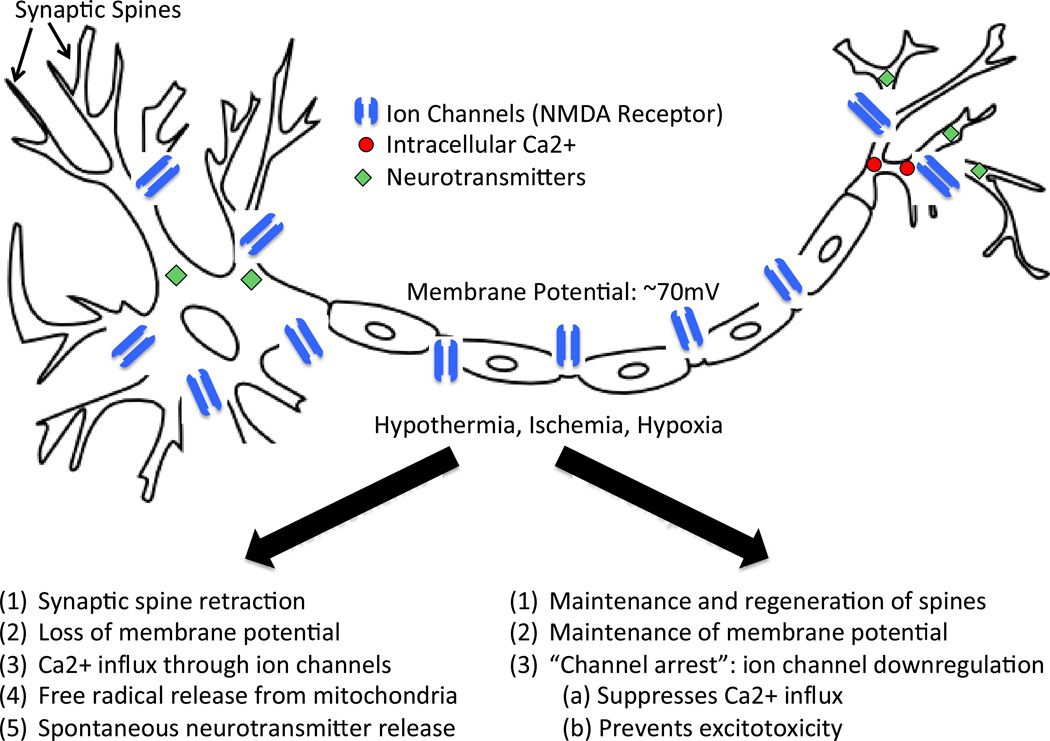
Figure 3. Effects of hypoxia on neurons.
Neurons may undergo hypoxia-induced cell death due to a loss of membrane potential, increased Ca2+ influx and resultant glutamate-mediated excitotoxicity. In contrast, neurons from species that have evolved to live in harsh environments (extremophiles) have evolved mechanisms to preserve neuronal membrane potential. By downregulating ion channel expression and activity, especially NMDA receptor activity, the Ca2+ influx and spontaneous neurotransmitter release is prevented and membrane potential is maintained.
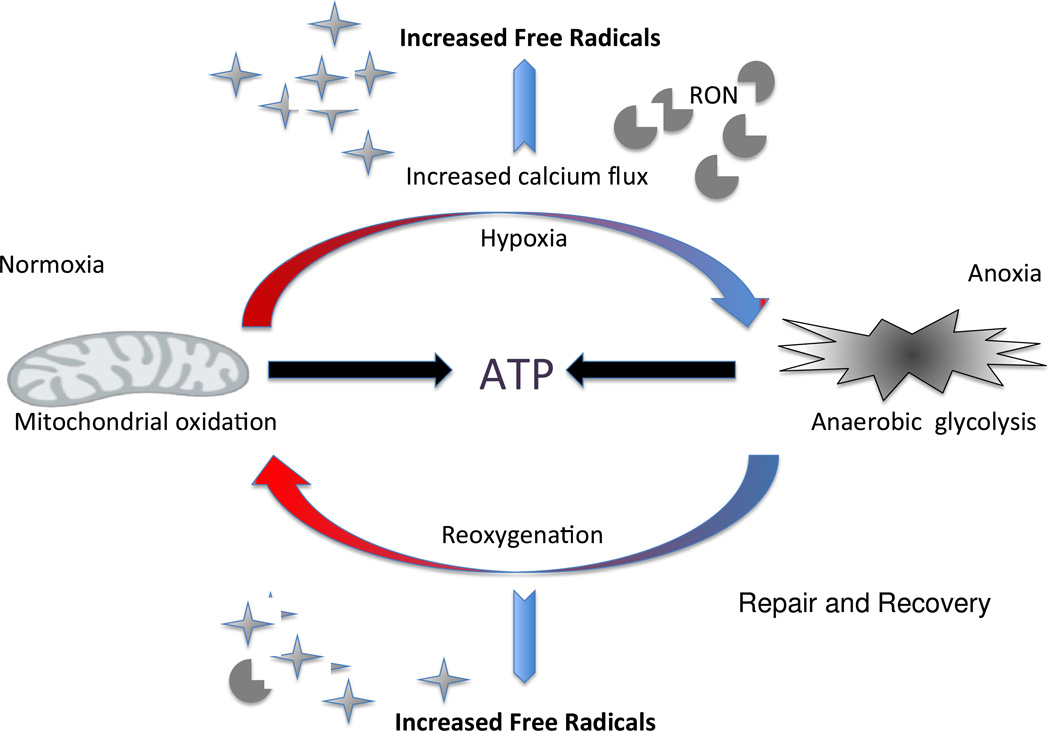
Figure 4. Both hypoxia and reoxygenation induce oxidative stress.
Despite lower metabolic activity during hypoxia and predominant reliance on glycolysis for energy production, altered ion flux and nitric oxide synthase activity give rise to an increase in free radical generation. During periods of reoxygenation and arousal from torpor the increase in metabolic rate leads to an increase in reactive oxygen and reactive nitrogen (RON) formation and concomitant oxidative stress.
Many tissues experience oxygen tension markedly lower than air (21%, 156mmHg); the brain is no exception [74]. In the central nervous system (CNS) PO2 values are highly conserved among mammalian species, but are brain region specific with highest levels in the pia (8%, 60mmHg) and low levels in the midbrain (0.55%, 4.1mmHg) [75]. While certain brain regions are extremely sensitive to oxygen tension (i.e. hippocampal CA1 and cerebellar granule cells [76–78], hypoxia itself is not necessarily detrimental to most brain neurons. This is especially evidenced by studies on hibernating (decreased metabolic rate due to low temperatures), bromating (winter dormancy), estivating (decreased metabolic rate due to high temperatures), and hypoxia and anoxic tolerant vertebrates. Tolerance of extreme variation in oxygen availability is evident in fish, amphibians, turtles, snakes, birds and mammals [69, 79, 80].
Both changes in environmental temperature and hypoxia induce hibernation or brumation in select endothermic and ectothermic species. Both conditions are characterized by decreased activity, metabolic rate and body temperature resulting in reduced cerebral blood flow [80, 81]. These changes coupled with pronounced changes at the cellular level in ion channel concentration, kinase activity and other protective mechanisms contribute to both metabolic depression and the formation free radicals (Figure 4). The sudden tissue oxygen reperfusion during arousal initiates a rapid increase in mitochondrial activity and also results in oxidative stress and ensuing tissue damage [82]. In the case of the brain, this phenomenon is opposite to the physiological conditions during a stroke and rather reflects the damage associated with therapeutic reperfusion. Instead of depleting the oxygen flow to the brain, the brain experiences a dramatic oxygen surge and concomitant oxidative stress. Many species have naturally evolved mechanisms to cope with such extremes in oxygen availability and strikingly hibernating mammals appear particularly tolerant of both hypoxia and reoxygenation induced oxidative stress. For example, in sharp contrast to data from mice, arctic ground squirrels (AGS), Urocitellus parryii, even when euthermic show no signs of neuronal injury following 10 minutes of ischemia [83]. How extremophiles tolerate large swings in oxygen availability and oxidative stress has far reaching application in both aging and age-associated diseases.
Antioxidant Mechanisms
The African lungfish (Protopterus dolloi) is a prime example of an extremophile. Lungfish are found in the hot arid regions of Africa and can survive prolonged droughts lasting several years with no access to food or water by entering a prolonged period of estivation in which they surround themselves in a relatively impermeable mucous cocoon [84]. During estivation, H2O2 detoxification and anti-oxidant protein levels of MnSOD and CuZnSOD (Table 1), enzymes that catalyze superoxide detoxification, are elevated in lungfish brains. These may serve as compensatory mechanisms to cope with the higher levels of 4-hydroxynonenal adducts, a marker for oxidative and nitrative damage that occur during estivation [84] and prevent the accrual of oxidative damage when most repair pathways are barely functioning during this dormancy period.
Table 1. Brain antioxidant defenses in extreme organisms and laboratory mice compared to controls.
All organisms (excluding mice) in this table show oxidative stress/damage resistance in the brain. All show increases in antioxidant defenses except for the 13-lined Ground Squirrel, and the Naked Mole-rat.
| Antioxidant | Animal | Levels |
|---|---|---|
| Superoxide Dismutase (SOD) | 13-lined Ground Squirrels (S. tridecemlineatus) | -- |
| Lung Fish (P.dolloi) | ||
| Glutathinone Peroxidase (GPx) | 13-lined Ground Squirrels | -- |
| Greater Horseshoe Bats (R. ferrumequinum) | ||
| Naked Mole-rats (H. glaber) | ||
| Aging Mice (M. musculus, C57BL6) | ||
| Glutathione Reductase (GR) | 13-lined Ground Squirrels | -- |
| Glutathione (GSH) | European Common Frog (R. temporaria) | |
| Catalase (CAT) | 13-lined Ground Squirrels | |
| Aging Mice | ||
| Thiobituric acid related substances (TBARS) | Painted Turtle (C. picta) | |
| Marsh Frog (R. ridibunda) | ||
signifies there is no change between control and experimental animals
signifies an increase in levels from control to experimental animals
signifies a decrease in levels from control to experimental animals
In response to extreme cold, lower vertebrates in their brumation and hibernating mammals and birds show a wide range of equivocal antioxidant data providing no general consensus as to whether it is the period of hypoxia or reoxygenation that is the most stressful and requiring better antioxidant protection. The general consensus, however, is that these extremophiles show upregulation of the antioxidant armory to combat the oxidative stress associated with either hypoxia or reoxygenation [80]. Very different effects may be evident in blood and the various tissue samples making data interpretation difficult and cautioning investigators on the need to examine more than one tissue or even brain region.
Many extremophilic species express high levels of antioxidants constitutively. For example, studies have shown that hatchlings of painted turtles (Chrysemys picta) have an enhanced antioxidant defense system that allows them to withstand oxidative stress amassed during periods of super-cooling, freezing, or hypoxia [85]. Other species may produce more antioxidants during both brumation or during the arousal period while in the spring and summer have relatively poor antioxidant defenses. Both goldfish and garter snakes fall into this category [86]. Another example is the marsh frog, Rana ridibunda, that upon arousal from its winter dormancy, as metabolic rate increases, markers of both oxidative stress and antioxidants are heightened. Remarkably, within one hour of rewarming when exposed to 20°C, levels of the oxidative stress marker thiobarbituric acid reactive substances [TBARS] decrease by 50% to suggest that these animals have mechanisms for detoxifying ROS and lipid peroxides in the short arousal period [87]. In contrast, the European common frog Rana temporaria, as expected, displays lower oxidative metabolism in the brain during brumation than in the spring during peak metabolic activity. Brains from these frogs collected in the spring display higher levels of GSH antioxidant (Table 1), and increased sulfane sulfur compounds that prevent lipid peroxidation and the production of ROS [88]. This study suggests that oxidative damage protection may be up-regulated in the spring to cope with their more aerobically active period [88] rather than during the period of anoxia. In contrast, the carp, gold fish and leopard frog show higher levels of antioxidants during brumation than when active [69]. During periods of hypoxia, less oxidative damage in brain tissue is evident, and this is attributed to elevated glutathione peroxidase [GPx] whereas SOD and catalase had similar levels during both hypoxia and reoxygenation [89].
Similar equivocal findings are evident in heterothermic birds and mammals. Studies from the hibernating greater horseshoe bat, Rhinolophus ferrumequinum, have demonstrated pronounced global gene differential expression in the brains between hibernating and non-hibernating seasons [90]. Specifically, glutathione peroxidase-3 gene activity (Table 1), which functions in H2O2 detoxification, is increased by 8 fold in the active brain compared to the torpid brain for this horseshoe bat [90]. Trying to elucidate the mechanism by which hibernating squirrels are able to maintain low levels of oxidative stress throughout hibernation and arousal, it was reported that antioxidants rose in the ground squirrel (Citellus citellus) in response to winter conditions [91]. Similarly, the 13-lined ground squirrel (Spermophilus tridecemlineatus), upregulates catalase in both brain and heart tissue [92] (Table 1). During its torpid period, plasma and cerebrospinal fluid ascorbate levels were 3–4 fold greater than during euthermia, whereas brain levels were significantly lower during hibernation than when the squirrels were active [93]. Brain levels of superoxide dismutase, glutathione peroxidase and glutathione reductase were however similar between hibernating and active squirrels [92].
It is likely that species differences in antioxidant responses to hypoxia and reoxygenation in animals undergoing prolonged periods of dormancy may reflect different strategies for dealing with the pervasive problems associated with oxidative stress. Some species constitutively exhibit high levels under non-stressed conditions. Others raise levels as they enter hibernation presumably in anticipation of the oxidative stress they will either encounter during their torpid periods or during the metabolically costly periods of arousal. Still others have transiently raised levels during either arousal or torpor. Despite the accrual of considerable data documenting these comparative differences much work remains to be done before we can fully understand the role of antioxidants in assisting these extremophiles survival in harsh environments, and the mechanisms regulating their expression in the brains of these animals.
Alternative Mechanisms: Regulation of Protein Homeostasis: Chaperones, Proteasomes and Autophagy
Extreme-living species also use other protein homeostatic mechanisms to prevent the accumulation of oxidatively damaged proteins within a cell and aid in the repair and recovery of tissues after hypoxic or reoxygenation events. Molecular chaperones protect proteins from unfolding stressors and also help traffic damaged proteins, preventing them from aggregating or damaging other molecules. The induction of the heat shock protein family of chaperones may be triggered by both oxidative stress and temperature changes [94, 95]. Hibernating ground squirrels show increased levels of heat shock protein (HSP) HSP70 family protein GRP78, and its transcription factor ATF4 [96, 97] (Table 2). High constitutive levels of the molecular chaperone, HSP72 also have been reported in anoxia tolerant turtle species; this phenomena appeared brain specific and was not found in other tissues (Table 2) [98]. In addition, in response to anoxia a number of other HSPs including HSP25/27, HSP40, HSP60, and HSP90 are induced [98–101]. Further, heat shock factor 1 (HSF1) the transcription factor responsible for activating the heat shock response [102] reportedly increases in response to anoxia in these turtles [101]. During this type of oxidative stress, activation of the heat shock response may help maintain protein stability, as well as pre-condition proteins for re-oxygenation, preventing them from accruing damage upon a return to normoxia. In the brain, this could provide neuronal protection against widely varying oxygen states. Further, chaperones such as HSP72 and HSP25 have been shown to aid in the adaptation of protein degradation machinery to the oxidative stress environment to maintain function [28] and stabilize substrates for more efficient degradation [103].
Table 2. Heat Shock Proteins (HSPs) upregulated in the brain tissue of oxidative stress resistant animals in response to oxidative stress.
| Heat Shock Protein | Animal |
|---|---|
| GRP78 | 13-lined Ground Squirrel (S. tridecemlineatus) |
| HSP25/27 | Red-eared Terrapin (T. scripta elegans) |
| Naked Mole-rat (H. glaber) | |
| HSP40 | Red-eared Terrapin |
| Naked Mole-rat | |
| HSP60 | Red-eared Terrapin |
| 13-lined Ground Squirrel | |
| HSP70/72 | Epaulette Shark (H. ocellatum) |
| Red-eared Terrapin | |
| 13-lined Ground Squirrel | |
| Naked Mole-rat | |
| HSP90 | Red-eared Terrapin |
Both autophagy and proteasome activity are upregulated in animals encountering chronic oxidative stress. Autophagy induction prior to, or triggered by, a mild oxidative stress is neuroprotective [104, 105]. Conversely, inhibiting autophagy exacerbates the toxicity of oxidative stress and the levels of oxidative damage (reviewed in [27]). However, neurons require a delicate balance as excessive or chronic upregulation of autophagy promotes neuronal death [106, 107]. Proteasome chymotrypsin-like (ChT-L) activity cleaves proteins at hydrophobic sites and generally is activated in response to oxidative damage [108], thereby preventing the accumulation of damaged proteins to pathological levels. The proteasome, itself, may be a target of oxidative stress [109, 110], and once sufficiently damaged its function is impaired [110]. Generally, this is detrimental to neuronal function as the proteasome is responsible for several aspects of synaptic function, such as spinogenesis [111], presynaptic neurotransmission [112], long-term potentiation [113], synaptic scaling [114], apical dendrite outgrowth/polarization [115, 116], dendritic arborization [117], and synapse formation/elimination [118]. Animals that live with chronic oxidative stress are likely to have evolved mechanisms to protect and maintain proteasome function. For example, the proteasome-associated chaperone HSP70 was induced under anoxic conditions in the cerebellum of the epaulette shark (Hemiscyllium ocellatum) [119]. This hypoxia and anoxia tolerant species also showed positive modulation of proteasome degradation during oxygen deprivation in the cerebellum perhaps to prevent excitotoxicity and protect cerebellar dendrites from damage during these stress events [120].
Upregulated proteasome activity also appears to be the case for the subterranean dwelling naked mole-rat (Heterocephalus glaber) in both liver and brain [121–123]. These rodents commonly encounter periods of extreme hypoxia when resting in nests 8 feet below the surface. When actively excavating foraging burrows, they encounter normoxic atmospheres as their burrows are unsealed and exposed to above ground conditions [124, 125]. The cytosolic fractions of various tissues, including the brain, exhibit the highest levels of oxidative stress [126]. Not only is the activity of proteasomes in this fraction high but naked mole-rat proteasomes in the cytosolic fractions are also extremely resistant to oxidative stressors and other proteasome inhibitors [127]. Proteasome protection against inhibition is not due to intrinsic properties of the naked mole-rat proteasome but rather appears to be due to an extraneous transferable cytosolic factor that also can protect both human and yeast proteasomes from inhibition [127]. This protective factor has among its constituents the molecular chaperones HSP40 and HSP70 [127]. An increase in chaperone expression and proteasome activity may represent one of the mechanisms that sustain brain integrity in extremophiles, thereby, preventing cell death, senescence and neurodegeneration in response to hypoxia and reoxygenation and may also be a key component in the extension of maximum lifespan. Brain HSP60, HSP72, Glucose-Regulated Protein, 78kDa (GRP78), and GRP94 protein expression correlate positively with maximum life span potential when the brains of 13 mammalian and bird species are examined [128]. Together, these brain data suggest that key heat shock chaperones and their concomitant impact upon proteostasis are linked to sustained brain health and species longevity. This also supports our findings linked to the beneficial effects of rapamycin on protein homeostasis in mouse brains [129].
Oxidative stress and the naked mole-rat, tolerance to hypoxia and hyperoxia
Despite high levels of proteolytic degradation and the chaperone mediated removal of damaged proteins, the extremely long-lived naked mole-rat (for review see [125]) exhibits high levels of oxidatively damaged macromolecules evident even in young animals when compared to shorter-lived mice [130]. Ratios of GSH/GSSG reveal a pro-oxidant milieu and levels of antioxidant defenses appear similar to those of mice. Of the brain regions examined, the hippocampus appears the least protected with low levels of HSP70, antioxidant capacity, and proteasome activity [123]. Despite this non-protective profile as well as high levels of beta amyloid (Aβ), a protein causally associated with Alzheimer’s disease [131] and phosphorylated tau [132], there is no overt evidence for neurodegenerative disease even in 30-year-old naked mole-rat brains. Further, antioxidant defenses seemingly do not account for the naked mole-rats’ ability to withstand oxidative damage (Table 1). These contradictory findings suggest that naked mole-rats either utilize other protective mechanisms to maintain brain integrity, like neuregulin-1 [133], or they have evolved mechanisms to utilize high levels of ROS and low oxygen, as occurs in development, throughout life. Several studies comparing naked mole-rat and mouse brain response to differing levels of carbon dioxide and oxygen support this premise. For example, the naked mole-rat is able to recover normal brain function after being exposed to doses of carbon dioxide that are fatal to mice. Hippocampal neurons of the naked mole-rat consistently tolerate oxygen deprivation for over 5 hours, and show no signs of neural degradation even 24 hours after exposure, suggesting evidence of hypoxia tolerance in the adult brain of naked mole-rats [134]. Further, naked mole-rat brains maintain synaptic function much longer than mice in reduced oxygen, and recover from 30-minute periods of no oxygen [124], an experiment that would routinely kill a mouse [135]. The naked mole-rat maintenance of the neonatal, hypoxia tolerant NMDA receptor subunit, GluN2D, may contribute to this extreme hypoxia tolerance [136] and likely is an adaptation to living in environments with variable partial pressures of oxygen.
Novel Organism may help us to redefine the Oxidative Stress Theory of Aging
Molecular oxidation commonly occurs in response to myriad stimuli including environmental stress, injury, infection, poor nutrition, and exposure to various toxins. The level of damage accrued is partly dependent upon the ability of a wide variety of both enzymatic (e.g., superoxide dismutase) and non-enzymatic antioxidants to neutralize the bulk of ROS, and other cytoprotective mechanisms that can detoxify, repair, and eliminate damaged macromolecules, allowing for the survival of aerobic organisms. However, it cannot be overlooked that ROS are necessary components of several cellular pathways such as cellular differentiation, regulation of immunity, autophagy and longevity, and metabolic adaptation to hypoxia [137], and have even been shown to be necessary for the maintenance of adult stem cells in the hippocampus [138]. There is much debate as to whether the Oxidative Damage Theory of Aging is self-explanatory in its current iteration. Certainly, studies with non-traditional model species that have evolved to cope with extreme and variable conditions suggest that this theory is not correct in all of its assumptions; most display higher ROS levels than common model organisms, yet live exponentially longer and healthier lives. Findings in extreme models do not discount the amassed data concerning the responses of traditional short-lived model organisms to oxidative stress. However, results attained from extreme models suggest that more efficient evolved mechanisms and processes to deal with high levels of stress are yet to be discovered. We suggest that utilizing a broader array of model organisms will engender a more thorough understanding of oxidative stress, including detoxification mechanisms. Perhaps, the unique protective or compensatory processes naturally harnessed by extreme species will hold promising therapeutic potential, and they should not be ignored.
Acknowledgments
This work was funded by awards to RB from the NIH/NIA (1R21AG043912), The Glenn Foundation, The Owen Foundation and AFAR, Breakthroughs in Gerontology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Valentina R. Garbarino, Email: garbarino@livemail.uthscsa.edu.
Miranda E. Orr, Email: orrm3@uthscsa.edu.
Karl A. Rodriguez, Email: rodriguezk@uthscsa.edu.
Rochelle Buffenstein, Email: buffenstein@uthscsa.edu.
References
- 1.Harman D. Free radical theory of aging: dietary implications. American Journal of Clinical Nutrition. 1972;25(8):839–843. doi: 10.1093/ajcn/25.8.839. [DOI] [PubMed] [Google Scholar]
- 2.Liochev SI. Reactive oxygen species and the free radical theory of aging. Free Radic Biol Med. 2013;60:1–4. doi: 10.1016/j.freeradbiomed.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 3.Kirkwood TB. Evolution of ageing. Nature. 1977;270(5635):301–304. doi: 10.1038/270301a0. [DOI] [PubMed] [Google Scholar]
- 4.Tamagno E, et al. Oxidative stress activates a positive feedback between the gamma- and beta-secretase cleavages of the beta-amyloid precursor protein. J Neurochem. 2008;104(3):683–695. doi: 10.1111/j.1471-4159.2007.05072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heitzer T, et al. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104(22):2673–2678. doi: 10.1161/hc4601.099485. [DOI] [PubMed] [Google Scholar]
- 6.Bartsch H, Nair J. Chronic inflammation and oxidative stress in the genesis and perpetuation of cancer: role of lipid peroxidation, DNA damage, and repair. Langenbecks Arch Surg. 2006;391(5):499–510. doi: 10.1007/s00423-006-0073-1. [DOI] [PubMed] [Google Scholar]
- 7.Hulbert AJ, et al. Life and death: metabolic rate, membrane composition, and life span of animals. Physiol Rev. 2007;87(4):1175–1213. doi: 10.1152/physrev.00047.2006. [DOI] [PubMed] [Google Scholar]
- 8.Holmstrom KM, Finkel T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat Rev Mol Cell Biol. 2014;15(6):411–421. doi: 10.1038/nrm3801. [DOI] [PubMed] [Google Scholar]
- 9.Tsatmali M, Walcott EC, Crossin KL. Newborn neurons acquire high levels of reactive oxygen species and increased mitochondrial proteins upon differentiation from progenitors. Brain Research. 2005;1040(1–2):137–150. doi: 10.1016/j.brainres.2005.01.087. [DOI] [PubMed] [Google Scholar]
- 10.Tsatmali M, et al. Reactive oxygen species modulate the differentiation of neurons in clonal cortical cultures. Mol Cell Neurosci. 2006;33(4):345–357. doi: 10.1016/j.mcn.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le Belle JE, et al. Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell Stem Cell. 2011;8(1):59–71. doi: 10.1016/j.stem.2010.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai M, et al. Hypoxic Conditioned Medium from Rat Cerebral Cortical Cells Enhances the Proliferation and Differentiation of Neural Stem Cells Mainly through PI3-K/Akt Pathways. PLoS One. 2014;9(11):e111938. doi: 10.1371/journal.pone.0111938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parrinello S, et al. Oxygen sensitivity severely limits the replicative lifespan of murine fibroblasts. Nature Cell Biology. 2003;5(8):741–747. doi: 10.1038/ncb1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldberg MP, Choi DW. Combined oxygen and glucose deprivation in cortical cell culture: calcium-dependent and calcium-independent mechanisms of neuronal injury. Journal of Neuroscience. 1993;13(8):3510–3524. doi: 10.1523/JNEUROSCI.13-08-03510.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morrison SJ, et al. Culture in reduced levels of oxygen promotes clonogenic sympathoadrenal differentiation by isolated neural crest stem cells. Journal of Neuroscience. 2000;20(19):7370–7376. doi: 10.1523/JNEUROSCI.20-19-07370.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung DJ, et al. Effect of hypoxia on generation of neurospheres from adipose tissue-derived canine mesenchymal stromal cells. Vet J. 2014;199(1):123–130. doi: 10.1016/j.tvjl.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 17.Knapp LT, Klann E. Role of reactive oxygen species in hippocampal long-term potentiation: contributory or inhibitory? J Neurosci Res. 2002;70(1):1–7. doi: 10.1002/jnr.10371. [DOI] [PubMed] [Google Scholar]
- 18.Verma A, et al. Carbon monoxide: a putative neural messenger. Science. 1993;259(5093):381–384. doi: 10.1126/science.7678352. [DOI] [PubMed] [Google Scholar]
- 19.O'Dell TJ, et al. Tests of the roles of two diffusible substances in long-term potentiation: evidence for nitric oxide as a possible early retrograde messenger. Proc Natl Acad Sci U S A. 1991;88(24):11285–11289. doi: 10.1073/pnas.88.24.11285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stevens CF, Wang Y. Reversal of long-term potentiation by inhibitors of haem oxygenase. Nature. 1993;364(6433):147–149. doi: 10.1038/364147a0. [DOI] [PubMed] [Google Scholar]
- 21.Zhuo M, et al. Nitric oxide and carbon monoxide produce activity-dependent long-term synaptic enhancement in hippocampus. Science. 1993;260(5116):1946–1950. doi: 10.1126/science.8100368. [DOI] [PubMed] [Google Scholar]
- 22.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408(6809):239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 23.Benzi G, Moretti A. Age- and peroxidative stress-related modifications of the cerebral enzymatic activities linked to mitochondria and the glutathione system. Free Radic Biol Med. 1995;19(1):77–101. doi: 10.1016/0891-5849(94)00244-e. [DOI] [PubMed] [Google Scholar]
- 24.Currais A, Maher P. Functional consequences of age-dependent changes in glutathione status in the brain. Antioxid Redox Signal. 2013;19(8):813–822. doi: 10.1089/ars.2012.4996. [DOI] [PubMed] [Google Scholar]
- 25.Davies KJ. Degradation of oxidized proteins by the 20S proteasome. Biochimie. 2001;83(3–4):301–310. doi: 10.1016/s0300-9084(01)01250-0. [DOI] [PubMed] [Google Scholar]
- 26.Shringarpure R, Grune T, Davies KJ. Protein oxidation and 20S proteasome-dependent proteolysis in mammalian cells. Cell Mol Life Sci. 2001;58(10):1442–1450. doi: 10.1007/PL00000787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee J, Giordano S, Zhang J. Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Biochem J. 2012;441(2):523–540. doi: 10.1042/BJ20111451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grune T, et al. HSP70 mediates dissociation and reassociation of the 26S proteasome during adaptation to oxidative stress. Free Radic Biol Med. 2011;51(7):1355–1364. doi: 10.1016/j.freeradbiomed.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y, et al. Ubiquilin-1 protects cells from oxidative stress and ischemic stroke caused tissue injury in mice. Journal of Neuroscience. 2014;34(8):2813–2821. doi: 10.1523/JNEUROSCI.3541-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elkharaz J, et al. Implications for oxidative stress and astrocytes following 26S proteasomal depletion in mouse forebrain neurones. Biochim Biophys Acta. 2013;1832(12):1930–1938. doi: 10.1016/j.bbadis.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Xu G, et al. Base excision repair, aging and health span. Mech Ageing Dev. 2008;129(7–8):366–382. doi: 10.1016/j.mad.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swain U, Rao KS. Age-dependent decline of DNA base excision repair activity in rat cortical neurons. Mech Ageing Dev. 2012;133(4):186–194. doi: 10.1016/j.mad.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 33.Borgesius NZ, et al. Accelerated age-related cognitive decline and neurodegeneration, caused by deficient DNA repair. Journal of Neuroscience. 2011;31(35):12543–12553. doi: 10.1523/JNEUROSCI.1589-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chomyn A, Attardi G. MtDNA mutations in aging and apoptosis. Biochem Biophys Res Commun. 2003;304(3):519–529. doi: 10.1016/s0006-291x(03)00625-9. [DOI] [PubMed] [Google Scholar]
- 35.Kraytsberg Y, et al. Mutation and intracellular clonal expansion of mitochondrial genomes: two synergistic components of the aging process? Mech Ageing Dev. 2003;124(1):49–53. doi: 10.1016/s0047-6374(02)00169-0. [DOI] [PubMed] [Google Scholar]
- 36.Trifunovic A, et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429(6990):417–423. doi: 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]
- 37.Gross NJ, Getz GS, Rabinowitz M. Apparent turnover of mitochondrial deoxyribonucleic acid and mitochondrial phospholipids in the tissues of the rat. J Biol Chem. 1969;244(6):1552–1562. [PubMed] [Google Scholar]
- 38.Barja G, Herrero A. Oxidative damage to mitochondrial DNA is inversely related to maximum life span in the heart and brain of mammals. FASEB J. 2000;14(2):312–318. doi: 10.1096/fasebj.14.2.312. [DOI] [PubMed] [Google Scholar]
- 39.Takeda T, Hosokawa M, Higuchi K. Senescence-accelerated mouse (SAM): a novel murine model of accelerated senescence. J Am Geriatr Soc. 1991;39(9):911–919. doi: 10.1111/j.1532-5415.1991.tb04460.x. [DOI] [PubMed] [Google Scholar]
- 40.Yagi H, et al. Age-related deterioration of ability of acquisition in memory and learning in senescence accelerated mouse: SAM-P/8 as an animal model of disturbances in recent memory. Brain Research. 1988;474(1):86–93. doi: 10.1016/0006-8993(88)90671-3. [DOI] [PubMed] [Google Scholar]
- 41.Ohta A, et al. Behavioral characteristics of the SAM-P/8 strain in Sidman active avoidance task. Brain Research. 1989;498(1):195–198. doi: 10.1016/0006-8993(89)90421-6. [DOI] [PubMed] [Google Scholar]
- 42.Kim HC, et al. Oxidative damage causes formation of lipofuscin-like substances in the hippocampus of the senescence-accelerated mouse after kainate treatment. Behav Brain Res. 2002;131(1–2):211–220. doi: 10.1016/s0166-4328(01)00382-5. [DOI] [PubMed] [Google Scholar]
- 43.Liu J, Mori A. Age-associated changes in superoxide dismutase activity, thiobarbituric acid reactivity and reduced glutathione level in the brain and liver in senescence accelerated mice (SAM): a comparison with ddY mice. Mech Ageing Dev. 1993;71(1–2):23–30. doi: 10.1016/0047-6374(93)90032-m. [DOI] [PubMed] [Google Scholar]
- 44.Sureda FX, et al. Changes in oxidative stress parameters and neurodegeneration markers in the brain of the senescence-accelerated mice SAMP-8. Exp Gerontol. 2006;41(4):360–367. doi: 10.1016/j.exger.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 45.Farr SA, et al. The antioxidants alpha-lipoic acid and N-acetylcysteine reverse memory impairment and brain oxidative stress in aged SAMP8 mice. J Neurochem. 2003;84(5):1173–1183. doi: 10.1046/j.1471-4159.2003.01580.x. [DOI] [PubMed] [Google Scholar]
- 46.Yasui F, et al. Effects of chronic acetyl-L-carnitine treatment on brain lipid hydroperoxide level and passive avoidance learning in senescence-accelerated mice. Neurosci Lett. 2002;334(3):177–180. doi: 10.1016/s0304-3940(02)01127-8. [DOI] [PubMed] [Google Scholar]
- 47.Horton TM, et al. Marked increase in mitochondrial DNA deletion levels in the cerebral cortex of Huntington's disease patients. Neurology. 1995;45(10):1879–1883. doi: 10.1212/wnl.45.10.1879. [DOI] [PubMed] [Google Scholar]
- 48.Ikebe S, et al. Increase of deleted mitochondrial DNA in the striatum in Parkinson's disease and senescence. Biochem Biophys Res Commun. 1990;170(3):1044–1048. doi: 10.1016/0006-291x(90)90497-b. [DOI] [PubMed] [Google Scholar]
- 49.Corral-Debrinski M, et al. Marked changes in mitochondrial DNA deletion levels in Alzheimer brains. Genomics. 1994;23(2):471–476. doi: 10.1006/geno.1994.1525. [DOI] [PubMed] [Google Scholar]
- 50.Massaad CA, Pautler RG, Klann E. Mitochondrial superoxide: a key player in Alzheimer's disease. Aging (Albany NY) 2009;1(9):758–761. doi: 10.18632/aging.100088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dumont M, et al. Reduction of oxidative stress, amyloid deposition, and memory deficit by manganese superoxide dismutase overexpression in a transgenic mouse model of Alzheimer's disease. FASEB J. 2009;23(8):2459–2466. doi: 10.1096/fj.09-132928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bitner BR, et al. Improvements in a Mouse Model of Alzheimer's Disease Through SOD2 Overexpression are Due to Functional and Not Structural Alterations. Magn Reson Insights. 2012;5:1–6. doi: 10.4137/MRI.S9352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mao P, et al. Mitochondria-targeted catalase reduces abnormal APP processing, amyloid beta production and BACE1 in a mouse model of Alzheimer's disease: implications for neuroprotection and lifespan extension. Hum Mol Genet. 2012;21(13):2973–2990. doi: 10.1093/hmg/dds128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.ADNJ dG. Antioxidants and redox signaling: Internet resources. Antioxid Redox Signal. 2000;2(4):937–940. doi: 10.1089/ars.2000.2.4-937. [DOI] [PubMed] [Google Scholar]
- 55.Linnane AW, Kios M, Vitetta L. Healthy aging: regulation of the metabolome by cellular redox modulation and prooxidant signaling systems: the essential roles of superoxide anion and hydrogen peroxide. Biogerontology. 2007;8:445–467. doi: 10.1007/s10522-007-9096-4. [DOI] [PubMed] [Google Scholar]
- 56.Sanz A, Pamplona R, Barja G. Is the mitochondrial free radical theory of aging intact? Antioxid Redox Signal. 2006;8:582–599. doi: 10.1089/ars.2006.8.582. [DOI] [PubMed] [Google Scholar]
- 57.Buffenstein R, Nelson OL, Corbit KC. Questioning the preclinical paradigm: natural, extreme biology as an alternative discovery platform. Aging (Albany NY) 2014;6(11):913–920. doi: 10.18632/aging.100704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kokolus KM, et al. Baseline tumor growth and immune control in laboratory mice are significantly influenced by subthermoneutral housing temperature. Proc Natl Acad Sci U S A. 2013;110(50):20176–20181. doi: 10.1073/pnas.1304291110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aguiar AS, Jr, et al. Physical exercise improves motor and short-term social memory deficits in reserpinized rats. Brain Research Bulletin. 2009;79(6):452–457. doi: 10.1016/j.brainresbull.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 60.Martin LB, 2nd, Weil ZM, Nelson RJ. Immune defense and reproductive pace of life in Peromyscus mice. Ecology. 2007;88(10):2516–2528. doi: 10.1890/07-0060.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Salmon AB, Richardson A, Perez VI. Update on the oxidative stress theory of aging: does oxidative stress play a role in aging or healthy aging? Free Radic Biol Med. 2010;48(5):642–655. doi: 10.1016/j.freeradbiomed.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Orr ME, et al. Mammalian target of rapamycin hyperactivity mediates the detrimental effects of a high sucrose diet on Alzheimer's disease pathology. Neurobiol Aging. 2014;35(6):1233–1242. doi: 10.1016/j.neurobiolaging.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Woodcock J, Woosley R. The FDA critical path initiative and its influence on new drug development. Annu Rev Med. 2008;59:1–12. doi: 10.1146/annurev.med.59.090506.155819. [DOI] [PubMed] [Google Scholar]
- 64.Buffenstein R. Negligible senescence in the longest living rodent, the naked mole-rat: insights from a successfully aging species. J Comp Physiol B. 2008;178(4):439–445. doi: 10.1007/s00360-007-0237-5. [DOI] [PubMed] [Google Scholar]
- 65.Hochachka PW, Somero GN. Biochemical adaptation : mechanism and process in physiological evolution. New York: Oxford University Press; 2002. p. 466. xi. [Google Scholar]
- 66.Abboud J, Storey KB. Novel control of lactate dehydrogenase from the freeze tolerant wood frog: role of posttranslational modifications. PeerJ. 2013;1:e12. doi: 10.7717/peerj.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ruf T, Geiser F. Daily torpor and hibernation in birds and mammals. Biol Rev Camb Philos Soc. 2014 doi: 10.1111/brv.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lutz PL, Nilsson GE, Prentice H. The Brain Without Oxygen: Causes of Failure Molecular and Physiological Mechanisms for Survival. Dordrecht: Kluwers Press; 2003. [Google Scholar]
- 69.Bickler PE, Buck LT. Hypoxia tolerance in reptiles, amphibians, and fishes: life with variable oxygen availability. Annu Rev Physiol. 2007;69:145–170. doi: 10.1146/annurev.physiol.69.031905.162529. [DOI] [PubMed] [Google Scholar]
- 70.Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973;134(3):707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Doyle KP, Simon RP, Stenzel-Poore MP. Mechanisms of ischemic brain damage. Neuropharmacology. 2008;55(3):310–318. doi: 10.1016/j.neuropharm.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brennan-Minnella AM, Won SJ, Swanson RA. NADPH Oxidase-2: Linking Glucose, Acidosis, and Excitotoxicity in Stroke. Antioxid Redox Signal. 2014 doi: 10.1089/ars.2013.5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Manzanero S, Santro T, Arumugam TV. Neuronal oxidative stress in acute ischemic stroke: sources and contribution to cell injury. Neurochem Int. 2013;62(5):712–718. doi: 10.1016/j.neuint.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 74.Panchision DM. The role of oxygen in regulating neural stem cells in development and disease. J Cell Physiol. 2009;220(3):562–568. doi: 10.1002/jcp.21812. [DOI] [PubMed] [Google Scholar]
- 75.Erecinska M, Silver IA. Tissue oxygen tension and brain sensitivity to hypoxia. Respir Physiol. 2001;128(3):263–276. doi: 10.1016/s0034-5687(01)00306-1. [DOI] [PubMed] [Google Scholar]
- 76.Wang Y, et al. NAD(P)H:quinone oxidoreductase activity is increased in hippocampal pyramidal neurons of patients with Aalzheimer's disease. Neurobiol Aging. 2000;21(4):525–531. doi: 10.1016/s0197-4580(00)00114-7. [DOI] [PubMed] [Google Scholar]
- 77.Wang X, et al. High intrinsic oxidative stress may underlie selective vulnerability of the hippocampal CA1 region. Brain Res Mol Brain Res. 2005;140(1–2):120–126. doi: 10.1016/j.molbrainres.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 78.Wang X, et al. Genomic and biochemical approaches in the discovery of mechanisms for selective neuronal vulnerability to oxidative stress. BMC Neurosci. 2009;10:12. doi: 10.1186/1471-2202-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hochachka PW, Monge C. Evolution of human hypoxia tolerance physiology. Adv Exp Med Biol. 2000;475:25–43. doi: 10.1007/0-306-46825-5_5. [DOI] [PubMed] [Google Scholar]
- 80.Dave KR, et al. Neuroprotection: lessons from hibernators. Comp Biochem Physiol B Biochem Mol Biol. 2012;162(1–3):1–9. doi: 10.1016/j.cbpb.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lutz PL, Nilsson GE. Vertebrate brains at the pilot light. Respir Physiol Neurobiol. 2004;141(3):285–296. doi: 10.1016/j.resp.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 82.Carey HV, Frank CL, Seifert JP. Hibernation induces oxidative stress and activation of NK-kappaB in ground squirrel intestine. J Comp Physiol B. 2000;170(7):551–559. doi: 10.1007/s003600000135. [DOI] [PubMed] [Google Scholar]
- 83.Dave KR, et al. The arctic ground squirrel brain is resistant to injury from cardiac arrest during euthermia. Stroke. 2006;37(5):1261–1265. doi: 10.1161/01.STR.0000217409.60731.38. [DOI] [PubMed] [Google Scholar]
- 84.Page MM, et al. Upregulation of intracellular antioxidant enzymes in brain and heart during estivation in the African lungfish Protopterus dolloi. J Comp Physiol B. 2010;180(3):361–369. doi: 10.1007/s00360-009-0416-7. [DOI] [PubMed] [Google Scholar]
- 85.Baker PJ, Costanzo JP, Lee RE., Jr Oxidative stress and antioxidant capacity of a terrestrially hibernating hatchling turtle. J Comp Physiol B. 2007;177(8):875–883. doi: 10.1007/s00360-007-0185-0. [DOI] [PubMed] [Google Scholar]
- 86.Hermes-Lima M, Zenteno-Savin T. Animal response to drastic changes in oxygen availability and physiological oxidative stress. Comp Biochem Physiol C Toxicol Pharmacol. 2002;133(4):537–556. doi: 10.1016/s1532-0456(02)00080-7. [DOI] [PubMed] [Google Scholar]
- 87.Bagnyokova TV, Storey KB, Lushchak VI. Induction of oxidative stress in Rana ridibunda during recovery from winter hibernation. Journal of Thermal Biology. 2003;28(1):21–28. [Google Scholar]
- 88.Wrobel M. Sulfurtransferase activity and sulfur compound content in Rana temporaria brain following hibernation. Acta Neurobiol Exp (Wars) 2001;61(1):69–72. doi: 10.55782/ane-2001-1385. [DOI] [PubMed] [Google Scholar]
- 89.Lushchak VI, et al. Hypoxia and recovery perturb free radical processes and antioxidant potential in common carp (Cyprinus carpio) tissues. Int J Biochem Cell Biol. 2005;37(6):1319–1330. doi: 10.1016/j.biocel.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 90.Lei M, et al. Comparison of brain transcriptome of the greater horseshoe bats (Rhinolophus ferrumequinum) in active and torpid episodes. PLoS One. 2014;9(9):e107746. doi: 10.1371/journal.pone.0107746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Buzadzic B, et al. Seasonal variation in the antioxidant defense system of the brain of the ground squirrel (Citellus citellus) and response to low temperature compared with rat. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1997;117(2):141–149. doi: 10.1016/s0742-8413(97)00061-3. [DOI] [PubMed] [Google Scholar]
- 92.Page MM, et al. Intracellular antioxidant enzymes are not globally upregulated during hibernation in the major oxidative tissues of the 13-lined ground squirrel Spermophilus tridecemlineatus. Comp Biochem Physiol A Mol Integr Physiol. 2009;152(1):115–122. doi: 10.1016/j.cbpa.2008.09.032. [DOI] [PubMed] [Google Scholar]
- 93.Drew KL, et al. Ascorbate and glutathione regulation in hibernating ground squirrels. Brain Res. 1999;851(1–2):1–8. doi: 10.1016/s0006-8993(99)01969-1. [DOI] [PubMed] [Google Scholar]
- 94.Oksala NK, et al. Natural thermal adaptation increases heat shock protein levels and decreases oxidative stress. Redox Biol. 2014;3:25–28. doi: 10.1016/j.redox.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kim BM, et al. Heavy metals induce oxidative stress and trigger oxidative stress-mediated heat shock protein (hsp) modulation in the intertidal copepod Tigriopus japonicus. Comp Biochem Physiol C Toxicol Pharmacol. 2014;166:65–74. doi: 10.1016/j.cbpc.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 96.Mamady H, Storey KB. Up-regulation of the endoplasmic reticulum molecular chaperone GRP78 during hibernation in thirteen-lined ground squirrels. Mol Cell Biochem. 2006;292(1–2):89–98. doi: 10.1007/s11010-006-9221-8. [DOI] [PubMed] [Google Scholar]
- 97.Mamady H, Storey KB. Coping with the stress: expression of ATF4, ATF6, and downstream targets in organs of hibernating ground squirrels. Arch Biochem Biophys. 2008;477(1):77–85. doi: 10.1016/j.abb.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 98.Prentice HM, et al. The upregulation of cognate and inducible heat shock proteins in the anoxic turtle brain. J Cereb Blood Flow Metab. 2004;24(7):826–828. doi: 10.1097/01.WCB.0000126565.27130.79. [DOI] [PubMed] [Google Scholar]
- 99.Kesaraju S, et al. Modulation of stress proteins and apoptotic regulators in the anoxia tolerant turtle brain. J Neurochem. 2009;109(5):1413–1426. doi: 10.1111/j.1471-4159.2009.06068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Storey KB. Anoxia tolerance in turtles: metabolic regulation and gene expression. Comp Biochem Physiol A Mol Integr Physiol. 2007;147(2):263–276. doi: 10.1016/j.cbpa.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 101.Krivoruchko A, Storey KB. Regulation of the heat shock response under anoxia in the turtle, Trachemys scripta elegans. J Comp Physiol B. 2010;180(3):403–414. doi: 10.1007/s00360-009-0414-9. [DOI] [PubMed] [Google Scholar]
- 102.Abravaya K, et al. The human heat shock protein hsp70 interacts with HSF, the transcription factor that regulates heat shock gene expression. Genes Dev. 1992;6(7):1153–1164. doi: 10.1101/gad.6.7.1153. [DOI] [PubMed] [Google Scholar]
- 103.Bryantsev AL, et al. Regulation of stress-induced intracellular sorting and chaperone function of Hsp27 (HspB1) in mammalian cells. Biochem J. 2007;407(3):407–417. doi: 10.1042/BJ20070195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kiffin R, et al. Activation of chaperone-mediated autophagy during oxidative stress. Molecular Biology of the Cell. 2004;15(11):4829–4840. doi: 10.1091/mbc.E04-06-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ulgherait M, et al. AMPK modulates tissue and organismal aging in a non-cell-autonomous manner. Cell Rep. 2014;8(6):1767–1780. doi: 10.1016/j.celrep.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Li Y, et al. Taurine attenuates methamphetamine-induced autophagy and apoptosis in PC12 cells through mTOR signaling pathway. Toxicol Lett. 2012;215(1):1–7. doi: 10.1016/j.toxlet.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 107.Dong Y, et al. Ascorbic acid ameliorates seizures and brain damage in rats through inhibiting autophagy. Brain Research. 2013;1535:115–123. doi: 10.1016/j.brainres.2013.08.039. [DOI] [PubMed] [Google Scholar]
- 108.Pickering AM, et al. Nrf2-dependent induction of proteasome and Pa28alphabeta regulator are required for adaptation to oxidative stress. J Biol Chem. 2012;287(13):10021–10031. doi: 10.1074/jbc.M111.277145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kiffin R, Bandyopadhyay U, Cuervo AM. Oxidative stress and autophagy. Antioxid Redox Signal. 2006;8(1–2):152–162. doi: 10.1089/ars.2006.8.152. [DOI] [PubMed] [Google Scholar]
- 110.Grimm S, Hohn A, Grune T. Oxidative protein damage and the proteasome. Amino Acids. 2012;42(1):23–38. doi: 10.1007/s00726-010-0646-8. [DOI] [PubMed] [Google Scholar]
- 111.Hamilton AM, et al. Activity-dependent growth of new dendritic spines is regulated by the proteasome. Neuron. 2012;74(6):1023–1030. doi: 10.1016/j.neuron.2012.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Willeumier K, Pulst SM, Schweizer FE. Proteasome inhibition triggers activity-dependent increase in the size of the recycling vesicle pool in cultured hippocampal neurons. Journal of Neuroscience. 2006;26(44):11333–11341. doi: 10.1523/JNEUROSCI.1684-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pavlopoulos E, et al. Neuralized1 activates CPEB3: a function for nonproteolytic ubiquitin in synaptic plasticity and memory storage. Cell. 2011;147(6):1369–1383. doi: 10.1016/j.cell.2011.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hou Q, Gilbert J, Man HY. Homeostatic regulation of AMPA receptor trafficking and degradation by light-controlled single-synaptic activation. Neuron. 2011;72(5):806–818. doi: 10.1016/j.neuron.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hamilton AM, Zito K. Breaking it down: the ubiquitin proteasome system in neuronal morphogenesis. Neural Plast. 2013;2013:196848. doi: 10.1155/2013/196848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vadhvani M, et al. The centrosomal E3 ubiquitin ligase FBXO31-SCF regulates neuronal morphogenesis and migration. PLoS One. 2013;8(2):e57530. doi: 10.1371/journal.pone.0057530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Puram SV, et al. The ubiquitin receptor S5a/Rpn10 links centrosomal proteasomes with dendrite development in the mammalian brain. Cell Rep. 2013;4(1):19–30. doi: 10.1016/j.celrep.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yi JJ, Ehlers MD. Emerging roles for ubiquitin and protein degradation in neuronal function. Pharmacol Rev. 2007;59(1):14–39. doi: 10.1124/pr.59.1.4. [DOI] [PubMed] [Google Scholar]
- 119.Renshaw GM, Warburton J, Girjes A. Oxygen sensors and energy sensors act synergistically to achieve a graded alteration in gene expression: consequences for assessing the level of neuroprotection in response to stressors. Front Biosci. 2004;9:110–116. doi: 10.2741/1206. [DOI] [PubMed] [Google Scholar]
- 120.Dowd WW, et al. Compensatory proteome adjustments imply tissue-specific structural and metabolic reorganization following episodic hypoxia or anoxia in the epaulette shark (Hemiscyllium ocellatum) Physiol Genomics. 2010;42(1):93–114. doi: 10.1152/physiolgenomics.00176.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pérez VI, et al. Protein stability and resistance to oxidative stress are determinants of longevity in the longest-living rodent, the naked mole-rat. Proc Natl Acad Sci USA. 2009;106(9):3059–3064. doi: 10.1073/pnas.0809620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rodriguez KA, et al. Altered composition of liver proteasome assemblies contributes to enhanced proteasome activity in the exceptionally long-lived naked mole-rat. PLoS One. 2012;7(5):e35890. doi: 10.1371/journal.pone.0035890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Edrey YH, et al. Oxidative damage and amyloid-beta metabolism in brain regions of the longest-lived rodents. J Neurosci Res. 2014;92(2):195–205. doi: 10.1002/jnr.23320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Larson J, Park TJ. Extreme hypoxia tolerance of naked mole-rat brain. Neuroreport. 2009;20(18):1634–1637. doi: 10.1097/WNR.0b013e32833370cf. [DOI] [PubMed] [Google Scholar]
- 125.Edrey YH, et al. Successful aging and sustained good health in the naked mole rat: a long-lived model for biogenrontology and biomedical research. ILAR J. 2011;52:41–53. doi: 10.1093/ilar.52.1.41. [DOI] [PubMed] [Google Scholar]
- 126.Jung T, et al. Intracellular distribution of oxidized proteins and proteasome in HT22 cells during oxidative stress. Free Radic Biol Med. 2006;40(8):1303–1312. doi: 10.1016/j.freeradbiomed.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 127.Rodriguez KA, et al. A cytosolic protein factor from the naked mole-rat activates proteasomes of other species and protects these from inhibition. BBA-MOL BASIS DIS. 2014 doi: 10.1016/j.bbadis.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Salway KD, et al. Higher levels of heat shock proteins in longer-lived mammals and birds. Mech Ageing Dev. 2011;132(6–7):287–297. doi: 10.1016/j.mad.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 129.Rodriguez KA, et al. Divergent tissue and sex effects of rapamycin on the proteasome-chaperone network of old mice. Front Mol Neurosci. 2014;7:83. doi: 10.3389/fnmol.2014.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Andziak B, et al. High oxidative damage levels in the longest-living rodent, the naked mole-rat. Aging Cell. 2006;5(6):463–471. doi: 10.1111/j.1474-9726.2006.00237.x. [DOI] [PubMed] [Google Scholar]
- 131.Edrey YH, et al. Amyloid beta and the longest-lived rodent: the naked mole-rat as a model for natural protection from Alzheimer's disease. Neurobiol Aging. 2013;34(10):2352–2360. doi: 10.1016/j.neurobiolaging.2013.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Orr ME, et al. Sustained high levels of neuroprotective, high molecular weight, phosphorylated tau in the longest-lived rodent. Neurobiol Aging. 2014 doi: 10.1016/j.neurobiolaging.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Edrey YH, et al. Sustained high levels of neuregulin-1 in the longest-lived rodents; a key determinant of rodent longevity. Aging Cell. 2012;11(2):213–222. doi: 10.1111/j.1474-9726.2011.00772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nathaniel TI, et al. Tolerance to oxygen nutrient deprivation in the hippocampal slices of the naked mole rats. J Integr Neurosci. 2009;8(2):123–136. doi: 10.1142/s0219635209002149. [DOI] [PubMed] [Google Scholar]
- 135.Arrieta-Cruz I, Pfaff DW, Shelley DN. Mouse model of diffuse brain damage following anoxia, evaluated by a new assay of generalized arousal. Exp Neurol. 2007;205(2):449–460. doi: 10.1016/j.expneurol.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Peterson BL, Park TJ, Larson J. Adult naked mole-rat brain retains the NMDA receptor subunit GluN2D associated with hypoxia tolerance in neonatal mammals. Neurosci Lett. 2012;506(2):342–345. doi: 10.1016/j.neulet.2011.11.042. [DOI] [PubMed] [Google Scholar]
- 137.Sena LA, Chandel NS. Physiological roles of mitochondrial reactive oxygen species. Mol Cell. 2012;48(2):158–167. doi: 10.1016/j.molcel.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dickinson BC, et al. Nox2 redox signaling maintains essential cell populations in the brain. Nat Chem Biol. 2011;7(2):106–112. doi: 10.1038/nchembio.497. [DOI] [PMC free article] [PubMed] [Google Scholar]