Abstract
Brain–computer interface (BCI) technology might contribute to rehabilitation of motor function. This speculation is based on the premise that modifying the EEG will modify behavior, a proposition for which there is limited empirical data. The present study asked whether learned modulation of pre-motor sensorimotor rhythm (SMR) activity can affect motor performance in normal human subjects.
Eight individuals first performed a joystick-based cursor-movement task with variable warning periods. Targets appeared randomly on a video monitor and subjects moved the cursor to the target and pressed a select button within 2 sec. SMR features in the pre-movement EEG that correlated with performance speed and accuracy were identified. The subjects then learned to increase or decrease these features to control a two-target BCI task. Following successful BCI training, they were asked to increase or decrease SMR amplitude in order to initiate the joystick task.
After BCI training, pre-movement SMR amplitude was correlated with performance in subjects with initial poor performance: lower amplitude was associated with faster and more accurate movement. The beneficial effect on performance of lower SMR amplitude was greater in subjects with lower initial performance levels. These results indicate that BCI-based SMR training can affect a standard motor behavior. They provide a rationale for studies that integrate such training into rehabilitation protocols and examine its capacity to enhance restoration of useful motor function.
Introduction
Brain activity produces electrical signals that are detectable on the scalp (i.e., electroencephalographic activity (EEG)), on the cortical surface (i.e., electrocorticographic activity (ECoG)), or within the brain (i.e., neuronal activity or local field potentials (LFPs)). Brain-computer interfaces (BCIs) translate specific features of these signals into outputs that allow the user to act on the world without the participation of peripheral nerves and muscles (Wolpaw et al., 2002). BCI research has used various features to provide a variety of communication and control options (Wolpaw and Wolpaw 2012 for review).
EEG sensorimotor rhythms (SMRs) have been used successfully as features for BCI communication and control (e.g., Wolpaw et al., 1991; Pfurtscheller et al., 1993; Kostov and Polak, 2000; Wolpaw and McFarland, 2004; Yuan and He, 2014). SMRs comprise activity in alpha (8-12 Hz) and beta (18-30 Hz) frequency ranges that is recorded over central scalp locations (i.e., over sensorimotor cortex) and is affected by movement or movement imagery (Chatrian, 1976; Pfurtscheller and Neuper, 1997; Pfurtscheller and Lopes da Silva, 1999; McFarland et al., 2000). Furthermore, active movement of specific body parts (i.e., hands or feet) is preceded and accompanied by specific scalp foci of SMR desynchronization (i.e., decrease in amplitude) (Pfurtscheller and McFarland (2012) for review).
SMR BCIs have been used to bypass the site of a lesion that prevents adequate motor control (e.g., Rohm et al., 2013). BCI technology might also be used to improve rehabilitation of sensorimotor function after strokes or other disorders (Dobkin, 2007; Daly and Wolpaw, 2008; Daly and Sitaram, 2012; Ang and Guan, 2013). Given the association of SMRs with normal movement and movement imagery, BCI-based SMR training might be particularly effective. Several studies have used concurrent SMRs to assist movement (e.g., by controlling an orthosis or functional electrical stimulation (FES) of paretic limb muscles) with modest success (Buch et al., 2008; Ramos-Murguialday et al., 2013; Young et al., 2014). The rational for this approach is that the more normal sensory feedback associated with the improved movement should promote beneficial plasticity through Hebbian mechanisms (Wang et al, 2010). Another approach uses SMRs to support motor imagery training in stroke patients with motor deficits (Prasad et al., 2010; Morone et al., 2015).
A number of studies have explored the use of neurofeedback protocols as a means of altering sensorimotor rhythms in users without disabilities (Egner and Gruzlier, 2001 and 2004; Rasey et al., 1996; Vernon et al., 2003). The intent of these studies was to show that altering SMR would also alter behavior. The neurofeedback approach provides users with feedback for altering SMR in a single direction for an extended period of time. As noted by Vernon (2005), an implicit assumption underlying neurofeedback is that the training procedure will lead to long-term changes in the EEG outside of the training context, which will be associated with changes in behavior. Vernon (2005) concludes that evidence for these assumptions is generally lacking. For example, Egner et al. (2004) found that healthy participants learning to enhance low beta (11.7-14.6 Hz) at Cz did not show the expected increase in this activity when tested after training. Boulay et al., 2011 showed that SMR modulation within the context of a simple reaction time task produced reaction times that were shorter when subjects reduced pre-reaction SMR amplitude as compared to when they increased pre-reaction SMR amplitude. This design relied on bi-directional control of task-appropriate SMR activity and shows that SMRs reflect brain activity important in the preparation for movement.
The present study explored a different strategy for using BCI technology to improve motor performance. Based on the fact that SMR desynchronization is associated with preparation for movement (Pfurtscheller and McFarland 2012), we hypothesized that learned regulation of pre-movement SMR amplitude would affect the subsequent movement. To test this hypothesis, we evaluated the impact of pre-movement SMR regulation on the performance of a center-out joystick-based cursor movement task in a three-phase within-subject study. Phase 1 identified a pre-movement SMR feature (i.e., amplitude in a given frequency range at a given location) that correlated with movement performance. Phase 2 trained the subject to increase or decrease that feature. Phase 3 assessed the impact on performance of pre-movement increase or decrease in the SMR feature.
Methods
Subjects
The subjects were 8 healthy adults (5 men and 3 women; ages 24-56; all right-handed) with no previous BCI experience. All gave informed consent for the study, which was reviewed and approved by the New York State Department of Health Institutional Review Board.
The subject sat in a reclining chair facing a 94x53-cm video screen 1.5 m away. EEG was recorded with 9-mm tin electrodes embedded in a cap (ElectroCap, Inc.) at 64 scalp locations according to the modified 10-20 system of Sharbrough et al. (1991). The electrodes were referenced to the right ear; and their signals were amplified and digitized at 256 Hz by g.USB amplifiers. BCI operation and data collection were supported by the BCI2000 platform (Schalk et al., 2004; Schalk and Mellinger 2011). Each subject completed 2-3 one-hr sessions per week for a total of 15-21 sessions spanning 6-10 weeks.
Study Protocol
Figure 1 illustrates the three-phase study protocol. Phase 1 identified a pre-movement SMR feature that correlated with performance on a joystick task. Phase 2 trained the subject to increase or decrease the amplitude of this feature. Phase 3 combined both elements to evaluate the impact of pre-movement SMR feature control on performance of the joystick task.
Figure 1.
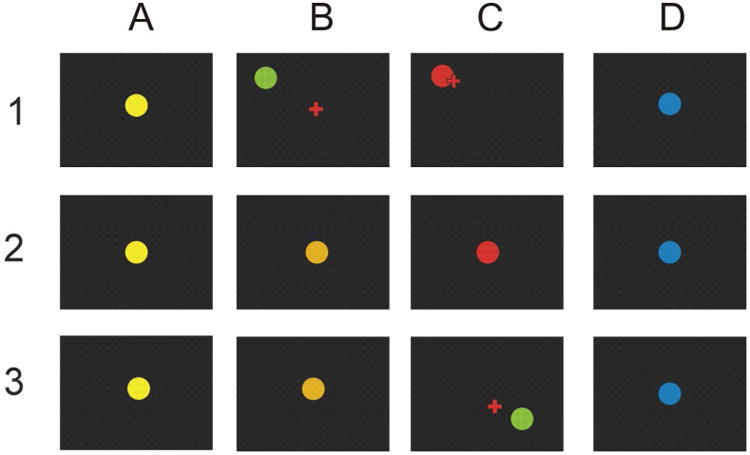
The three phases of the study. Phase 1: Identification of a pre-movement SMR feature correlated with performance. A. A yellow or blue target appears in the center of the screen. B. After 1-7 sec, the target appears at a random location and a cursor appears in the center. C. The subject uses the joystick to move the cursor to the target and then presses the select button, at which point the target turns red if the cursor is in contact with the target. The screen then goes blank for 1 sec prior to the next trial. If the cursor does not reach the target within 2 sec, the screen simply goes blank for 1 sec prior to the next target. D. The next trial begins. Phase 2: Training of the SMR feature identified in Phase 1. A. A yellow or blue target appears in the center of the screen, cueing SMR feature reduction or increase, respectively (see text). B. Target color changes as the SMR feature amplitude approaches criterion. C. Red signals success and the screen goes blank; if the criterion is not reached, the screen simply goes blank. D. One sec later, the next trial begins. Phase 3: consisting of Phase 2 followed by Phase 1. A. A yellow or blue target appears in the center of the screen, cueing SMR feature reduction or increase, respectively (see text). B. Target color changes as the SMR feature amplitude approaches criterion. C. If and when the criterion is reached, the joystick task is initiated with the target appearing at a random location and the cursor appearing in the center. As with phase 1, the subject uses the joystick to move the cursor to the target and then presses the select button, at which point the target turns red for 0.5 sec and the screen then goes blank for 1 sec prior to the next trial. If the cursor does not reach the target within 2 sec, the screen simply goes blank for 1 sec prior to the next trial. D. The next trial begins.
In Phase 1 (Figure 1, row 1), the subject used a joystick with the dominant hand (i.e., always right) to move a cursor to contact a target on the screen. The target was a blue or yellow 5.1-cm. diameter circle that initially appeared in the center of the screen (A). After a variable period of time (1-7 sec) the target turned green and was placed at a random position 12.2-24.4 cm. from the center (B). Simultaneously, a 2-cm plus-shaped cursor appeared at the center. The subject's task was to move the cursor to the target and press the select button located on top of the joystick handle as quickly as possible to acquire the target. If the task was completed in 2 sec., the target turned red for 1 sec (C). Then the screen was blank for one sec. before the start of the next trial (D). If the task was not completed in 2 sec., the screen simply went blank for one sec before the next trial started. Each of the four daily Phase-1 sessions consisted of 8 3-min runs separated by one-min breaks. Due to variations in response speed, this resulted in a range of 189-204 trials/session and a range of 776-801 total trials in all 4 sessions for each subject. EEG signals from locations over sensorimotor cortex in the one sec immediately before the move instruction was analyzed as described below to determine which pre-movement SMR feature correlated most strongly with task performance. An SMR feature was defined as amplitude in a specific frequency band (e.g., 11-13 Hz) at a specific location (e.g., C3). The subjects then moved on to Phase 2 of the study.
In Phase 2 (Figure 1, row 2), feature-dependent color change served as feedback as the subject learned to change the amplitude of the SMR feature identified in Phase 1. For each trial, the color of the 5.1-cm target in the center of the screen indicated the desired direction of change (A). Yellow- and blue-target trials were randomly interspersed; each comprised 50% of the trials. In a yellow-target trial, the subject was asked to maintain the SMR feature amplitude below a criterion value for one sec. As the one-sec running average of SMR amplitude approached the criterion value, the target gradually turned from yellow to orange and finally to bright red for 0.5 sec upon success (B, C). In a blue-target trial, the subject was asked to maintain the SMR feature amplitude above a criterion value for one sec. As the one-sec running average of SMR amplitude approached the criterion value, the target gradually turned from blue to purple to bright red for 0.5 sec upon success. The screen then went blank for one sec before the next trial started (D). If the subject did not reach the criterion within 4 sec, or reached the wrong criterion, the screen went blank for 1.5 sec before the next trial. Over 5-10 daily Phase-2 training sessions (each consisting of 8 3-min runs separated by one-min breaks), the subjects improved their SMR feature control; for the final session they satisfied the criterion within 4 sec on 69-94% of the trials. They then moved on to Phase 3.
In Phase 3 (Figure 1, row 3), the subject performed two-stage trials. The first stage was like Phase 2: The yellow or blue target appeared (A) and the subject had 6 sec (B) to decrease (yellow) or increase (blue) the SMR feature amplitude to a criterion level. Achievement of this criterion initiated the second stage (C). The second stage was like Phase 1: a green target appeared at a random position 12.2-24.4 cm. from the center. Simultaneously, a 2-cm plus-shaped cursor appeared at the center. The subject's task was to move the cursor to the target and press the select button as quickly as possible. If the task was completed in 2 sec., the target turned red for 1 sec. Then the screen was blank for one sec. before the start of the next trial. If the task was not completed in 2 sec, or the fire button was pressed when the cursor did not contact the target, the screen went blank for 1 sec. The first Phase-3 session started with two Phase-2 warm-up trials and was followed by 6 3-min runs separated by 1-min breaks. The next three phase-3 sessions consisted of 8 3-min runs separated by 1-min breaks. This resulted in a range of 64-204 Phase-3 trials/session and a range of 436-620 total Phase-3 trials over all 4 sessions for each subject. Thus, in Phase 3, the subject had to increase or decrease the pre-movement SMR feature in order to initiate the motor (i.e., joystick) task. We assessed the relationships between the amplitude of this pre-movement feature and the parameters of the subsequent motor performance.
Feature Selection
From the Phase-1 data, we determined for each subject the correlations between task performance and pre-movement SMR features. Task performance was characterized by: the latency from the move stimulus (i.e., the simultaneous appearance of the target at a random position and the appearance of the cursor (Fig. 1, Row 1, B)) to the start of movement (i.e., movement latency); the correlation between horizontal and vertical movement (i.e., how straight the movement was as an index of skilled movement (Lee et al, 2013)); the movement time; the total time (i.e., the total time from the move stimulus to target selection), and the final error in pixels between the cursor and the target center (i.e., movement error).
EEG signals from the 64 scalp electrodes were re-referenced according to a Laplacian transform that had an SMR-appropriate spatial frequency range (i.e., 6-cm inter-electrode spacing; McFarland et. al. 1997), and was then subjected to a 16th-order autoregressive spectral analysis (McFarland and Wolpaw, 2008). Amplitudes for 3-Hz-wide spectral bands from 9-24 Hz were computed for 400-msec sliding windows that were updated every 50 msec. Next, each potential SMR feature (i.e., amplitudes for 3-Hz bands from 9 to 24 Hz for electrodes C3 and CP3 (i.e., contralateral sensorimotor cortex)) for the one sec immediately prior to movement onset served as the dependent variable in a multiple regression model that had the following task-performance parameters as the independent variables: movement latency; linearity of movement (measured as r2 for horizontal vs. vertical movement); total movement time; and final error (FE) from the center of the target. Thus, this multiple regression predicted specific SMR features, as the vector of Yi over trials, from the matrix Xij of j task-performance parameters over i trials according to:
| (1) |
The EEG feature achieving the largest r2 value was then used as the SMR feature for training and testing in Phases 2 and 3. In 4 subjects, the SMR feature used comprised the sum of two SMR features (one in the mu frequency range (12 or 13 Hz) and one in the beta range (22 or 24 Hz) (see Table 1) that had nearly equal r2 values.
Table 1. SMR Features and Phase-2 and Phase-3 Accuracies for the Individual Subjects.
| Subject | SMR Location | SMR Center Frequency (Hz) | Phase-2 Training Sessions | Phase-2 Final Accuracy (%) | Phase-3 Final Accuracy (%) |
|---|---|---|---|---|---|
| A | C3 | 12+24 | 7 | 81.1 | 70.5 |
| B | C3 | 21 | 10 | 93.0 | 83.3 |
| C | C3 | 13+22 | 10 | 74.4 | 67.1 |
| D | C3 | 18 | 7 | 66.5 | 69.1 |
| E | CP3 | 15 | 7 | 86.0 | 62.4 |
| F | CP3 | 21 | 5 | 84.9 | 68.3 |
| G | C3 | 12+24 | 10 | 95.3 | 85.8 |
| H | C3 | 12+24 | 10 | 86.4 | 89.4 |
SMR Control
Online SMR control during Phases 2 and 3 was assessed with the same Laplacian transform and autoregressive spectral analysis described above. The resulting feature was then normalized according to:
| (2) |
where a is an estimate of the mean and b is an estimate of the standard deviation of the signal, S (McFarland et al., 2006). The value of C was integrated as a running average over one sec and was updated every 50 ms. The result was displayed as feedback in the form of a continuous color change proportional to the distance to a threshold value that served as the criterion for a completed trial. This criterion was typically 0.85 standard deviations above (blue targets) or below (yellow targets) the mean value for the signal.
Results
The SMR features identified in Phase 1 were specific to the individual subjects. In Phase 2, the subject learned to control the feature; this control was focused topographically and spectrally to the location and frequency band of the SMR feature. In Phase 3, voluntary modulation of this SMR feature had effects on performance that varied across subjects. In general, subjects with the poorest baseline performance on the joystick task showed the greatest improvement with decrease of the pre-movement SMR feature. Table 1 shows for each subject: the SMR feature identified in Phase 1; the number of Phase-2 training sessions; the success rate for the final Phase-2 session; and the success rate for Phase-3 sessions (i.e., the percent of trials in which the pre-movement SMR feature reached criterion and initiated the motor task).
Phase 1
During Phase 1, subjects performed the center-out joystick-based cursor movement task. We found pre-movement SMR features that predicted subsequent motor performance. The best SMR feature and the parameter(s) of motor performance that it predicted varied across subjects. Furthermore, the relationships among the several measures of motor performance also varied across subjects.
Figure 2 shows the trajectory of a single Phase-1 trial from Subject E. Figure 2 also lists the values of the movement parameters for this specific trial. In this trial, the subject initially moved the cursor vertically, and then later refined the trajectory to more accurately approach the target. Overall, on 85.8% of the trials for all subjects in Phase 1, the first movement was toward the target in one dimension only, not in both. This bias was highly significant by a χ2 analysis (p < 0.0001). In contrast, the actual target position did not show any significant bias for requiring movement in just one direction, as would be expected given random target placement. On average, the first movement occurred at 502.4 msec, the first 2-dimensional movement occurred at 644.2 msec (i.e., moving simultaneously in both dimensions), and movement was finished at 1290.6 msec. Thus, on average, subjects initially made a course movement followed after a brief delay by a correction in the trajectory.
Figure 2.
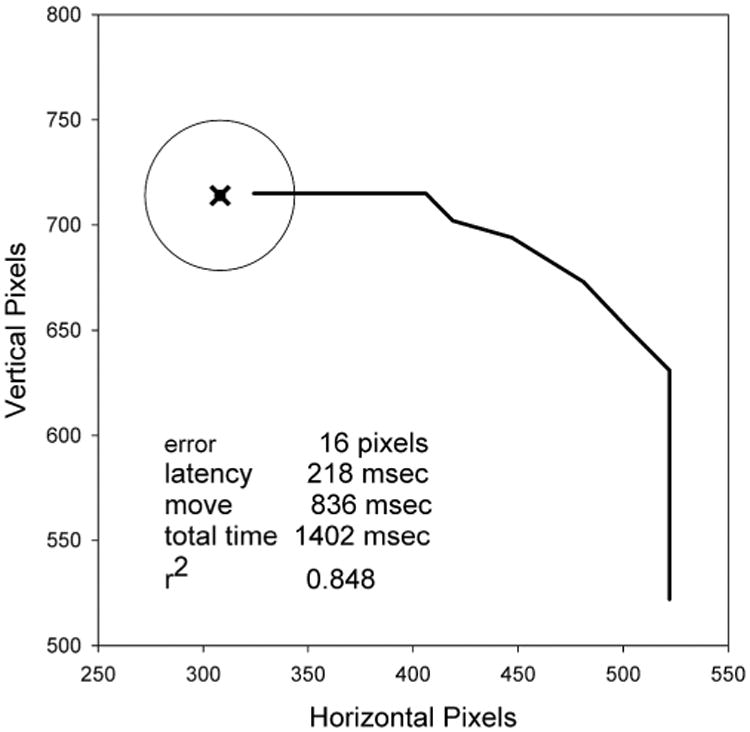
rajectory of a single trial. The x marks the center of the target which is represented by a circle. Note that the subject starts at the lower right with a vertical movement and then corrects the trajectory at two points. A summary of performance metrics for this trial is shown in the lower left corner. Note that only the upper-left quadrant of the screen is shown in the figure.
There were many significant correlations among the movement parameters and the SMR feature in the Phase-1 data from individual subjects. The specific patterns varied across subjects, as illustrated in Table 2. It shows for the Phase-1 data of two subjects univariate correlations among final error, movement latency, movement linearity (r2), and amplitudes in the mu and beta frequency ranges at the location of the selected SMR. The subjects differ both in the correlations among their movement parameters and the correlations between these parameters and the SMR features. For example, for subject E final error is not significantly correlated with movement time while it is positively correlated with linearity. In contrast, for subject H the final error is negatively correlated with movement time but is not significantly correlated with linearity. For subject E beta activity is positively correlated with movement latency and negatively correlated with movement time. In contrast, for subject H beta activity is negatively correlated with movement latency and not significantly correlated with movement time. Thus, as suggested by Cesqui et al. (2012), our subjects reached different but similarly successful solutions to this motor task.
Table 2.
Phase-1 Correlations in two subjects. Movement parameters include: final error between target center and cursor center at the time of the button press; movement latency; movement time; and r2 between horizontal and vertical movement (i.e., linearity of the movement trajectory). SMR parameters include the amplitudes in the mu and beta frequency bands at the selected location (Table 1).
| A. Subject E | |||||
|---|---|---|---|---|---|
| Measure | Error | Latency | Time | r2 | Mu |
| Latency | 0.24** | - | |||
| Time | 0.02 | -0.27** | - | ||
| r2 | 0.33** | -0.09* | -0.20** | - | |
| Mu | -0.10* | 0.09* | -0.07 | 0.06 | - |
| Beta | -0.06 | 0.17** | -0.17** | 0.05 | 0.62** |
| B. Subject H | |||||
| Measure | Error | Latency | Time | r2 | Mu |
| Latency | 0.62** | - | |||
| Time | -0.10* | -0.05 | - | ||
| r2 | -0.04 | -0.09* | -0.31** | - | |
| Mu | 0.00 | -0.02 | 0.01 | 0.08* | - |
| Beta | -0.07 | -0.09* | -0.03 | 0.16** | 0.60** |
p < 0.05
p < 0.0001
Phase 2
During Phase 2, the subjects learned to modulate (i.e., increase or decrease) the SMR feature identified in Phase 1. Figure 3 shows for each subject the topography and spectrum of the correlation between target condition (i.e., SMR decrease (SMRlow) or increase (SMRhigh)) and the SMR feature for the last three Phase-2 training sessions. All eight subjects acquired topographically and spectrally focused SMR control. The sharp topographical and spectral foci of control rule out contamination by non-EEG artifacts (e.g., electromyographic (EMG) or electrooculographic (EOG) signals).
Figure 3.
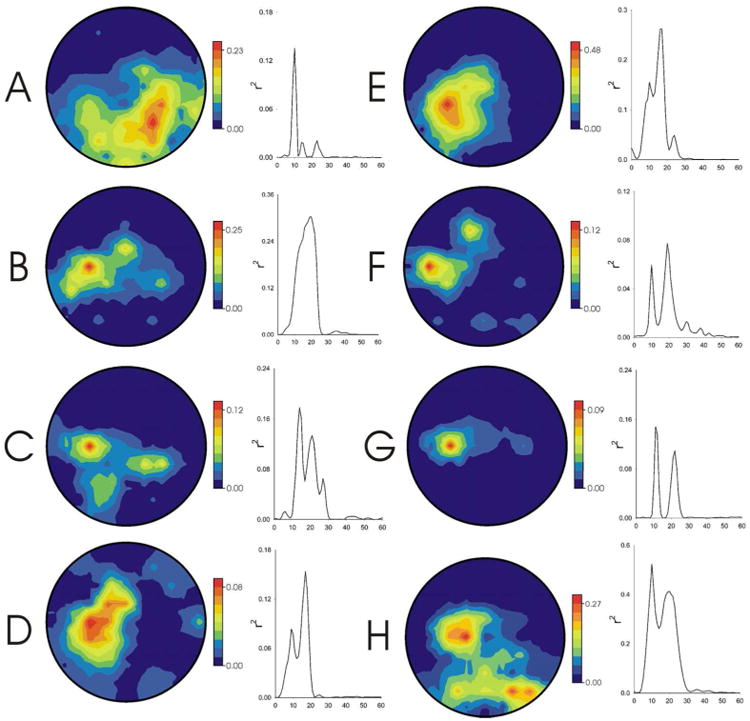
opography (nose at top) and spectrum of the correlation between SMR feature amplitude and the SMR control condition (i.e., SMRhigh or SMRlow) for each subject for the last Phase-2 session. Note that control is focused topographically and spectrally over sensorimotor cortex.
Phase 3
In phase 3 we examined the impact of pre-movement control of the SMR feature on performance of the joystick-based motor task. We found that the effects of SMR feature control on motor performance varied across subjects. There was a tendency for SMR control to have more impact on performance in subjects in whom performance was less good.
Figure 4 summarizes the Phase-3 results for each subject and all the subjects together. It shows the amplitude of the pre-movement SMR feature and the performance parameters for SMRhigh and SMRlow trials and indicates significant differences between SMRhigh and SMRlow trials in these measures. As expected, every subject showed a significant difference in SMR amplitude between the SMRhigh and SMRlow conditions. This SMR difference was generally present in both the mu and beta frequency ranges. The impact of pre-movement SMR amplitude on performance varied markedly across subjects and across performance parameters. While pre-movement SMR reduction was associated with significantly less final error (i.e., less difference between the final cursor position and the center of the target), the effects on other performance parameters varied. SMR reduction reduced movement latency and total time in 3 subjects, increased movement time in one, and increased linearity of movement in one.
Figure 4.
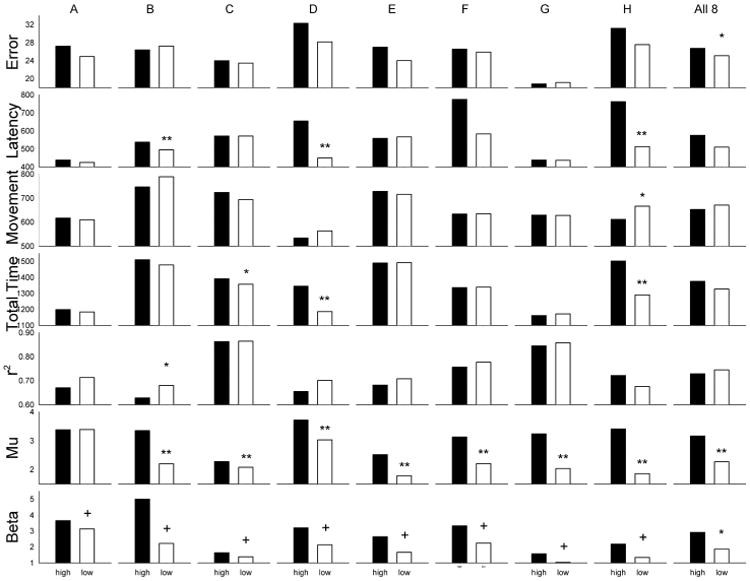
Summary of Phase-3 performance. Means for SMRhigh and SMR low trials during Phase-3 performance are presented for each subject (A through H) and for the entire group. Movement error is the final difference in pixels between the center of the target and the center of the cursor. Movement latency is the time in msec between the target appearance and the first joystick movement. Movement time is the time in msec between the start of joystick movement and target selection. The total time is the time in msec between target appearance and target selection. R2 is the squared correlation between cursor movement in the horizontal and vertical planes (i.e., the linearity of the movement). Mu and Beta are the amplitudes (in μV) in these respective frequency bands at the location of the selected SMR feature. * indicates that the means differed with p<0.05 and ** that they differed with p<0.01 by F-test.
To further clarify the impact of pre-movement SMR amplitude and inter-subject variation in this impact, we performed an ANOVA with the interaction of SMR amplitude with subject as the effect of interest and SMR amplitude by subject by 3-min run as an estimate of error (i.e., the intra-subject variability across runs). We found significant interactions between SMR amplitude and subject for: movement latency (df= 7/21, F= 40.68, p < 0.0001); total time (df= 7/21, F= 18.84, p < 0.0001), and r2 (df= 7/21, F= 2.38, p < 0.0232). For each of these parameters, SMR amplitude significantly affected performance in some subjects but not in others; and there was no significant group effect of SMR amplitude. In contrast, for movement error, the group mean effect of SMR amplitude was significant (df = 1/7, F= 6.41, p < 0.0391) but the interaction of SMR amplitude with subjects and significant effects for individual subjects were not.
ANOVA of effects in individual subjects was based on data from individual trials within subjects. For some measures (e.g., r2) values were missing for trials not completed. For subject B, movement latency was significantly shorter (df= 1/567, F= 26.61, p < 0.0001) and r2 was significantly larger (df= 1/558, F= 6.26m, p < 0.0126) on SMRlow trials. For subject C, total time was significantly shorter (df= 1/421, F= 4.32, p < 0.0382) on SMRlow trials. For subject D, movement latency was significantly shorter (df= 1/443, F= 60.01, p < 0.0001) and total time was significantly less (df= 1/443, F= 1/443, F= 35.80, p < 0.0001) on SMR low trials. For subject H, movement latency was significantly shorter (df= 1/455, F= 77.53, p < 0.0001), total time was significantly less (df= 1/455, F= 58.76, p < 0.0001), and movement time was significantly less (df= 1/371, F= 6.35, p < 0.0121) on SMRlow trials.
To further evaluate the inter-subject differences in the impact of pre-movement SMR amplitude on performance, we examined the correlations between the impact of SMR amplitude on the performance parameters and the values of these measures for SMRhigh trials. We used the performance measures for SMRhigh trials because, as previous studies indicate (Boulay et al, 2012; Gilbertson et al., 2005) performance is usually inversely correlated with SMR level. Figure 5 shows these correlations between SMRhigh performance and the SMRhigh/SMRlow performance difference. For movement error and movement latency, the improvement for SMRlow trials was greater when SMRhigh performance was less good. At the suggestion of a reviewer we also evaluated these measures with Kendall's Tau which resulted in the effects of movement error being significant (p < 0.01) while movement latency was not. Overall, these findings suggests that SMR training has greater impact on individuals with initially lower levels of performance.
Figure 5.
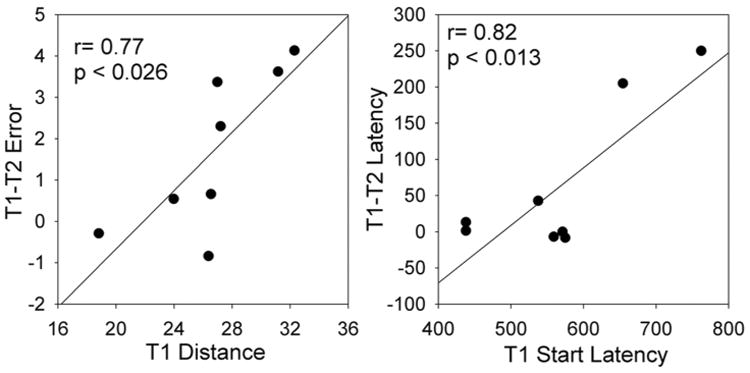
Correlations of several performance parameters in the SMRhigh condition with the differences in these performance parameters between the SMRhigh and SMRlow conditions. Note that poorer SMRhigh performance is associated with greater improvement in the SMRlow condition.
Discussion
There is considerable interest in the potential use of BCI technology for rehabilitation (Dobkin, 2007; Daly and Wolpaw, 2008; Daly and Sitaram, 2012; Ang and Guan, 2013). However exploration of the possible alternative strategies has been limited. For example, most of the recent work has used SMR control to generate movement (i.e., via muscle stimulation or orthotic assistance) (see Ang and Guan, 2013 for a review). In contrast, the present study evaluated SMR control as a means to improve preparation for movement. To the extent that motor dysfunction is associated with poor motor preparation, training pre-movement SMR could prove efficacious. The results of the present study support this possibility.
The present study
In Phase 1 of the present study, EEG signals were recorded while subjects performed a joystick-based task in which they moved a cursor to a target and then pressed a select button. In Phase 2, they were trained to modulate a pre-movement SMR feature that correlated with the performance of this motor task. In Phase 3, they modulated this SMR feature in order to initiate trials of the joystick-based motor task. The effects that pre-movement EEG feature modulation had on their performance in Phase 3 were subject-specific. These subject-specific effects depended in part on the subject's baseline level of performance: the positive impact of pre-movement SMR reduction was greater in subjects with poorer initial performance.
Studies of human motor performance typically focus on group effects, although individuals show consistent idiosyncratic patterns in their motor performances (Cesqui et al., 2012; Golenia et al., 2014). This inter-subject variability may be the result of individual-specific self-organizing processes that may be sub-optimal, but adequate (Cesqui et al., 2012). In the present study, we examined both the group effects and the individual effects of pre-movement SMR level. The typical design for repeated-measures within-subjects analysis uses the treatment by subjects interaction as an estimate of error (i.e., individual differences are error). To evaluate the significance of the treatment by subjects' interaction, we use the treatments by subjects by blocks interaction, which represents the consistency of subject-specific effects over time. This analysis is analogous to that of generalizability theory (Crocker and Algina, 1986), which is designed to evaluate the reliability of individual differences at different points in time. We have employed this type of analysis previously to evaluate individual differences in BCI control (e.g., McFarland et al., 2003). This application of statistical methods to reveal individual differences is likely to be particularly important for the development of effective BCI-based rehabilitation therapies. The effects of such therapies are likely to differ greatly across people with different disorders and with functional impairments that differ in nature, severity, and underlying mechanisms.
On a cautionary note, Table 4 has 40 comparisons which would lead to an expected false positive rate of 2 (at p < 0.05). Of the 8 significant effects in individual subjects reported there, 3 are p < 0.05 and 5 are p < 0.01. This exceeds the false discovery rate, but those comparisons that are just at p < 0.05 should probably be viewed with more caution. All but one of the significant effects reported in Table 4 are associated with a significant SMR amplitude by subject interaction.
Our previous study (Boulay et al., 2011) of SMR modulation with a simple reaction time task found that reaction times were shorter when subjects reduced pre-reaction SMR amplitude. This finding implied that SMRs reflect brain activity important in the preparation for movement. The present study extends this results to a more complex motor task that requires movement initiation, accurate movement, and selection. Its results begin to reveal the relationships between pre-movement SMRs and specific measures of task performance.
The joystick-based aiming task used in the present experiment is clearly more complex than the simple reaction time task of Boulay et al. (2011). For example, as shown in Figure 2 for Subject E, SMR level was positively correlated with movement latency (ML) and negatively correlated with movement time (MT); when SMR was lower, the subject started to move more quickly but then moved more slowly. The figure shows how, in one trial, this subject made an initial coarse (i.e., one-dimensional) movement toward the target and then moved with more accurate two-dimensional control. This tendency towards initial coarse movement followed by more refined movement control is consistent with Woodsworth and Schlosberg's (1963) suggestion that aiming movements consist of an initial ballistic phase followed by a later feedback-driven phase. More recently, Rand and Shimansky (2013) have modeled reaching movements in terms of progressively refined control. The progressive refinement of motor control could be due in part to the accumulation of spatial position information over time as suggested by Zimmermann et al. (2013). Given the time requirements of the present task (i.e., completion within 2 sec), the subject may have initiated movement before fully localizing the target and programming the movement. This tendency may have accounted for the negative correlation between movement latency and movement time for subject E shown in Table 2; longer pre-movement planning may enable shorter movement time. However, subjects might differ in their movement strategies with some allowing more time for planning prior to movement. Different movement strategies could account, in part, for the subject-dependent pattern of results observed in the present study. As discussed by Rugy et al. (2014), movement control processes are distributed over sensorimotor networks at multiple levels. The resulting movement may be locally optimal, but not globally optimal. Individual differences in the relative emphasis on these various control processes could account for the different individual patterns of motor performance. In this regard, it is notable that lesions in different nodes of the motor control network result in different motor deficits (Battaglia-Mayer et al., 2014).
There are several limitations to the results of this pilot study. The sample size is small and only 2 of 8 participants improved their performance after training. In addition, we used healthy subjects to evaluate a procedure that would ultimately be intended to aid recovery of individuals with motor impairments. Part of the problem with using healthy subjects is that the potential for improvements in this population is limited. Also, the brain networks used by healthy subjects may not be intact in patients who have had stroke or other forms of pathology.
SMR-based methods for motor rehabilitation
There are at least two ways in which SMR training might be used to facilitate motor rehabilitation (Daly and Wolpaw 2008). The first strategy is to operantly condition patients to produce more normal SMR activity with the expectation that this will produce more normal movement (e.g., Rozelle and Budzynski, 1995). The second is to use SMR activity to control a device (e.g., an orthosis) that assists attempted movements with the expectation that the more normal sensory feedback provided by the improved movement will induce beneficial activity-dependent plasticity in the CNS (Buch et al., 2008s). In addition, SMR training has been used to facilitate the known beneficial effects of motor imagery on stroke recovery (Prasad et al, 2010; Morone et al., 2015).
Recent studies have explored the second strategy (Ang and Guan (2013) for review). For example, these studies used SMR signals to control an orthosis (Buch et al., 2008) or functional electrical stimulation (Young et al., 2014) that moved a patient's paretic hand. The rational for this approach is that pairing movement with kinesthetic feedback should promote plasticity through Hebbian mechanisms (Wang et al., 2010). Ramos-Murguialday et al. (2013) found that conducting this type of training prior to conventional physiotherapy improved the outcome. In general, these studies report modest positive effects.
The present study explores the first strategy: normalizing movement-associated EEG features. The present study focuses on control of pre-movement SMR; its goal is to modulate the preparation for movement, and to thereby affect the subsequent movement performance. The implication of our approach is that subjects can learn, through feedback, to improve their preparation to respond. This sequential approach – SMR control followed by movement – contrasts with the parallel dual-task approach of other studies, in which the subject is asked to exert SMR control and to produce actual movement at the same time (e.g., Boulay et al., 2011; Ramos-Murguialday et al., 2013). Such dual tasks may interfere with each other (Pashler, 1994); they may be particularly ambiguous and difficult for people with strokes or other disorders that affect cerebral function.
The effectiveness of the pre-movement SMR modulation paradigm depends on whether the brain activity that produces pre-movement SMR affects the subsequent motor behavior. SMR desynchronization is, in fact, a correlate of motor preparation (Pfurtscheller and McFarland, 2012), which is a distributed process engaging all levels of the nervous system, from premotor and motor cortex to subcortical and spinal centers (Cohen et al., 2010). SMRs, particularly in the beta frequency range, are thought to reflect inhibition (Pfurtscheller and McFarland, 2012). This view is consistent with observations that beta-range SMRs decrease prior to movement and increase afterward (Pfurtscheller et al., 1997 & 2005). Learned SMR desynchronization increases motor cortex excitability, as reflected by motor evoked potentials (Pichiorri et al., 2011). These observations provide a rational for using SMR training in motor rehabilitation; methods that increase motor system excitability (referred to as “priming”) are recommended for motor rehabilitation (Pomeroy et al., 2011). However, one potential difficulty might be that subjects do not always produce the desired pattern of results. For example, although our subject A was given feedback based on channel C3, inspection of Figure 3 indicates greatest modulation over the right side of the scalp.
The paradigm of the present study targeted for modulation pre-movement SMR features that were related to the subsequent motor performance (i.e., joystick operation) and were focused both topographically and spectrally. Thus, the paradigm incorporated the effector-specificity and task-dependence of SMRs, both of which are highly relevant to the design of effective rehabilitation protocols. Previous SMR conditioning studies have generally treated these rhythms as reflections of global function (e.g., Hammer et al., 2011); and thus they did not employ spatial filtering methods that focus on SMR features originating in specific areas. Such spatial filtering is important for taking full advantage of the fact that SMR desynchronization is topographically specific to the limb involved in movement or movement imagery (Pfurtscheller and McFarland, 2011). Furthermore, individuals are capable of simultaneously controlling at least three distinct SMR rhythms within the context of a BCI task (McFarland et al., 2010). Recognition and engagement of this specificity is likely to be a key requirement for effective SMR-based rehabilitation therapies, although considerable work remains to be done before this approach is ready for clinical application.
Conclusions
Training protocols that teach people to modulate SMR features associated with motor performance constitute a promising new approach to motor rehabilitation for people with strokes and other disorders. Most studies up to the present have trained SMR modulation during movement. This study trained modulation of SMR features associated with pre-movement preparation and examined the impact of this modulation on the subsequent motor task in normal subjects. In general, pre-movement SMR decrease was associated with better performance; the nature and extent of improvement varied greatly across individuals. Improvement was significantly greater in those with poorer initial performance. These results suggest that pre-movement SMR training might enhance recovery of motor function for people with strokes or other disorders.
Acknowledgments
This work was supported by NIH grants HD30146 (NCMRR, NICHD), EB00856 (NIBIB & NINDS), and 1P41EB018783 (NIBIB).
References
- Ang KK, Guan C. Brain-computer interface in stroke rehabilitation. Journal of Computing Science and Engineering. 2013;7:139–146. [Google Scholar]
- Battaglia-Mayer A, Buiatti T, Caminiti R, Ferraina S, Lacquaniti F, Shallice T. Correction and suppression of reaching movements in the cerebral cortex: Physiological and neuropsychological aspects. Neuroscience and Biobehavioral Reviews. 2014;42:232–251. doi: 10.1016/j.neubiorev.2014.03.002. [DOI] [PubMed] [Google Scholar]
- Berger H. Uber das electrenkephalogramm des menchen. Archives of Psychiatrie and Nervenkrankheiten. 1929;87:527–570. [Google Scholar]
- Boulay CB, Sarnacki WA, Wolpaw JR, McFarland DJ. Trained modulation of sensorimotor rhythms can affect reaction time. Clinical Neurophysiology. 2011;122:1820–1826. doi: 10.1016/j.clinph.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buch E, Weber C, Cohen LG, Braun C, Bimyan MA, Ard T, Mellinger J, Caria A, Soekadar S, Fourkas A, Birbaumer N. Think to move: a neuromagnetic brain-computer interface (BCI) system for chronic stroke. Stroke. 2008;39:910–917. doi: 10.1161/STROKEAHA.107.505313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesqui B, d'Avella A, Portone A, Lacquaniti F. Catching a ball at the right tima and place: Individual factors matter. PLoS ONE. 2012;7:e31770. doi: 10.1371/journal.pone.0031770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatrian GE. The mu rhythm. In: Remond A, editor. Handbook of electroencephalography and clinical neurophysiology: the EEG of the waking adult. Amsterdam: Elsevier; 1976. pp. 46–69. [Google Scholar]
- Cohen O, Sherman E, Zinger N, Perlmoter S, Prut Y. Getting ready to move: transmitted information in the corticospinal pathway during preparation for movement. Current Opinion in Neurobiology. 2010;20:696–703. doi: 10.1016/j.conb.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker L, Algina J. Introduction to classical and modern test theory. New York: Harcourt Brace; 1986. [Google Scholar]
- Daly JJ, Sitaram R. BCI Therapeutic applications for improving brain function. In: Wolpaw RR, Wolpaw EW, editors. Brain-Computer Interfaces: Principles and Practice. New York: Oxford University Press; 2012. pp. 351–362. [Google Scholar]
- Daly JJ, Wolpaw JR. Brain-computer interfaces in neurological rehabilitation. Lancet Neurology. 2008;7:1032–1043. doi: 10.1016/S1474-4422(08)70223-0. [DOI] [PubMed] [Google Scholar]
- De Rugy A, Loeb GE, Carroll TJ. Muscle coordination is habitual rather than optimal. Journal of Neuroscience. 2012;32:7384–7391. doi: 10.1523/JNEUROSCI.5792-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobkin BH. Brain-computer interface technology as a tool to augment plasticity and outcomes for neurological rehabilitation. Journal of Physiology (London) 2007;579:637–642. doi: 10.1113/jphysiol.2006.123067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donchin E, Spencer KM, Wijesinghe R. The mental prosthesis: assessing the speed of a P300-based brain-computer interface. IEEE Transactions on Rehabilitation Engineering. 2000;8:174–179. doi: 10.1109/86.847808. [DOI] [PubMed] [Google Scholar]
- Egner T, Gruzelier JH. Learned self-regulation of EEF frequency components affects attention and event-related potentials in humans. Neuroreport. 2001;12:4155–4159. doi: 10.1097/00001756-200112210-00058. [DOI] [PubMed] [Google Scholar]
- Egner T, Gruzelier JH. EEG biofeedback of low beta band components: frequency-specific effects on variables of attention and event-related potentials. Clinical Neurophysiology. 2004;115:131–139. doi: 10.1016/s1388-2457(03)00353-5. [DOI] [PubMed] [Google Scholar]
- Gilbertson T, Lalo E, Doyle L, Di Lazzaro V, Cioni B, Brwon P. Existing motor state is favored at the expense of new movement during 13-35 Hz oscillatory synchrony in the human corticospinal system. Journal of Neuroscience. 2005;25:7771–7779. doi: 10.1523/JNEUROSCI.1762-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golenia L, Schoemaker MM, Mouton LJ, Bongers RM. Individual differences in learning a novel discrete motor task. PLoS ONE. 9:e112806. doi: 10.1371/journal.pone.0112806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer BU, Colbert AP, Brown KA, Ilioi EC. Neurofeedback for insomnia: a pilot study of z-score SMR and individualized protocols. Applied Psychophysiology and Biofeedback. 2011;36:251–264. doi: 10.1007/s10484-011-9165-y. [DOI] [PubMed] [Google Scholar]
- Kostov A, Polak M. Parallel man-machine training in development of EEG-based cursor control. IEEE Transactions on Rehabilitation Engineering. 2000;8:203–204. doi: 10.1109/86.847816. [DOI] [PubMed] [Google Scholar]
- Lee YH, Wu SK, Liu YP. Performance of remote target pointing hand movements in a 3D environment. Human Movement Science. 2013;32:511–526. doi: 10.1016/j.humov.2012.02.001. [DOI] [PubMed] [Google Scholar]
- McFarland DJ, Krusienski DJ, Wolpaw JR. Brain-computer interface signal processing at the Wadsworth Center: mu and sensorimotor beta rhythms. In: Neuper C, Klimesch W, editors. Progress in Brain Research. Vol. 159. 2006. pp. 411–419. [DOI] [PubMed] [Google Scholar]
- McFarland DJ, McCane LM, David SV, Wolpaw JR. Spatial filter selection for EEG-based communication. Electroencephalography and Clinical Neurophysiology. 1997;103:386–394. doi: 10.1016/s0013-4694(97)00022-2. [DOI] [PubMed] [Google Scholar]
- McFarland DJ, Miner LA, Vaughan TM, Wolpaw JR. Mu and beta rhythm topographies during motor imagery and actual movement. Brain Topography. 2000;12:177–186. doi: 10.1023/a:1023437823106. [DOI] [PubMed] [Google Scholar]
- McFarland DJ, Sarnacki WA, Wolpaw JR. Brain-computer interface (BCI) operation: optimizing information transfer rates. Biological Psychology. 2003;63:237–251. doi: 10.1016/s0301-0511(03)00073-5. [DOI] [PubMed] [Google Scholar]
- McFarland DJ, Sarnacki WA, Wolpaw JR. Electroencephalographic (EEG) control of three-dimensional movement. Journal of Neural Engineering. 2010;7:036007. doi: 10.1088/1741-2560/7/3/036007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland DJ, Wolpaw JR. Sensorimotor rhythm-based brain-computer interface (BCI): model order selection for autoregressive spectral analysis. Journal of Neural Engineering. 2008;5:155–162. doi: 10.1088/1741-2560/5/2/006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellinger J, Schalk G. Using BCI 2000 in BCI Research. In: Graimann B, Pfurtscheller G, Allison B, editors. Brain-Computer Interfaces: Revolutionizing Human-Computer Interaction. Berlin: Springer; 2011. pp. 259–280. [Google Scholar]
- Morone G, Pisotta I, Pichiorri F, Kleih S, Paolucci S, Molinari M, Cincotti F, Kubler A, Mattia D. Proof of principle of a brain-computer interface approach to support poststroke arm rehjabilitation in hospitalized patients: design, acceptability, and usability. Archives of Physical Medicine and Rehabilitation. 2015;96(suppl 1):S71–S78. doi: 10.1016/j.apmr.2014.05.026. [DOI] [PubMed] [Google Scholar]
- Neidermeyer E. The normal EEG of the waking adult. In: Neidermeyer E, Lopes da Silva FH, editors. Elencephalography: basic principles, clinical applications, and related fields. 4Th. Baltimore, MD: Williams and Wilkins; 1999. pp. 149–173. [Google Scholar]
- Pashler H. Dual-task interference in simple tasks: data and theory. Psychological Bulletin. 1994;116:220–244. doi: 10.1037/0033-2909.116.2.220. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Flotzinger D, Kalcher J. Brain-computer interface- a new communication device for handicapped persons. Journal of Microcomputer Applications. 1993;16:293–299. [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clinical Neurophysiology. 1999;110:1842–1857. doi: 10.1016/s1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, McFarland DJ. BCI signal processing: feature translation. In: Wolpaw JR, Wolpaw EW, editors. Brain-Computer Interfaces: Principles and Practice. New York: Oxford University Press; 2012. pp. 147–163. [Google Scholar]
- Pfurtscheller G, Neuper C. Motor imagery activates primary sensorimotor area in humans. Neuroscience Letters. 1997;239:65–68. doi: 10.1016/s0304-3940(97)00889-6. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Neuper C, Brunner C, Lopes da Silva F. Beta rebound after different types of motor imagery in man. Neuroscience Letters. 2005;378:156–159. doi: 10.1016/j.neulet.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Stancak A, Edlinger G. On the existence of different types of central beta rhythms below 30 Hz. Electroencephalography and Clinical Neurophysiology. 1997;102:316–325. doi: 10.1016/s0013-4694(96)96612-2. [DOI] [PubMed] [Google Scholar]
- Pichiorri F, De Vico Fallani F, Cincotti F, Babiloni F, Molinari M, Kleih SC, Neuper C, Kubler A, Mattia D. Sensorimotor thythm-based brain-computer interface training: the impact on motor cortical responsiveness. Journal of Neural Engineering. 2011;8:0205020. doi: 10.1088/1741-2560/8/2/025020. [DOI] [PubMed] [Google Scholar]
- Pomeroy V, Aglioti SM, Mark VW, McFarland D, Stinear C, Wolf SL, Corbetta M, Fizpatrick SM. Neurological principles and rehabilitation of action disorders: rehabilitation interventions. Neurorehabilitation and Neural Repair. 2011;25(5 Suppl):33S–43S. doi: 10.1177/1545968311410942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad G, Herman P, Coyle D, McDonough S, Crosbie J. Applying a brain-computer interface to support motor imagery practice in people with stroke for upper limb recovery: a feasibility study. Journal of NeuroEngineering and Rehabilitation. 2010;7:60. doi: 10.1186/1743-0003-7-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Murguialday A, Broetz D, Rea M, Laer L, Yilmaz O, Brasil FL, Liberati G, Curado MR, Garcia-Cossio E, Cho W, Agostini M, Soares E, Soekadar S, Caria A, Cohen LG, Birbaumer N. Brain-machine interface in chronic stroke rehabilitation: a controlled study. Annals of Neurology. 2013;74:100–108. doi: 10.1002/ana.23879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasey HW, Lubar JF, McIntyre A, Zoffuto AC, Abbot PL. EEG Biofeedback for the enhancement of attentional processing in normal college students. Journal of Neurotherapy. 1996;1:15–21. [Google Scholar]
- Rand MK, Shimansky YP. Two-phase strategy of neural control for planar reaching movements: I. XY coordination variability and its relation to end-point variability. Experimental Brain Research. 2013;225:55–73. doi: 10.1007/s00221-012-3348-5. [DOI] [PubMed] [Google Scholar]
- Rohm M, Schneiders CM, Kreilinger A, Kaiser V, Muller-Putz GR, Rupp R. Hybrid brain-computer interfaces and hybrid neuroprotheses for restoration of upper limb functions in individuals with high-level spinal cord injury. Artificial Intelligence in Medicine. 2013;59:133–142. doi: 10.1016/j.artmed.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Rozelle GR, Budzynski TH. Neurotherapy for stroke rehabilitation: a single case study. Biofeedback and Self Regulation. 1995;20:211–228. doi: 10.1007/BF01474514. [DOI] [PubMed] [Google Scholar]
- Schalk G, McFarland DJ, Hinterberger T, Birbaumer N, Wolpaw JR. BCI2000: a general-purpose brain-computer interface (BCI) system. IEEE Transactions on Biomedical Engineering. 2004;51:1034–1043. doi: 10.1109/TBME.2004.827072. [DOI] [PubMed] [Google Scholar]
- Sharbrough F, Chatrian CE, Lesser RP, Luders H, Nuwer M, Picton TW. American Electroencephalographic Society guidelines for standard electrode position nomenclature. Journal of Clinical Neurophysiology. 1991;8:200–202. [PubMed] [Google Scholar]
- Vernon DJ. Can neurofeedback training enhance performance? An evaluation of the evidence with implications for future research. Applied Psychophysiology and Biofeedback. 2005;30:347–364. doi: 10.1007/s10484-005-8421-4. [DOI] [PubMed] [Google Scholar]
- Vernon D, Egner T, Cooper N, Compton T, Neilands C, Sheri A, Gruzelier J. The effect of training distinct neurofeedback protocols on aspects of cognitive performance. International Journal of Psychophysiology. 2003;47:75–85. doi: 10.1016/s0167-8760(02)00091-0. [DOI] [PubMed] [Google Scholar]
- Wang W, Collinger JL, Perez MA, Tyler-Kabara EC, Cohen LC, Birbaumer N, Brose SW, Schwartz AB, Boinger ML, Weber DJ. Neural interface technology for rehabilitation: exploring and promoting neuroplasticity. Physical Medicine and Rehabilitation Clinics of North America. 2010;21:157–178. doi: 10.1016/j.pmr.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpaw JR, Birbaumer N, McFarland DJ, Pfurtscheller G, Vaughan TM. Brain-computer interfaces for communication and control. Clinical Neurophysiology. 2002;113:767–791. doi: 10.1016/s1388-2457(02)00057-3. [DOI] [PubMed] [Google Scholar]
- Wolpaw JR, McFarland DJ. Control of a two-dimensional movement signal by a non-invasive brain-computer interface. Proceedings of the National Academy of Science. 2004;51:17849–17854. doi: 10.1073/pnas.0403504101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolpaw JR, McFarland DJ, Neat GW, Forneris CA. An EEG-based brain-computer interface for cursor control. Electroencephalography and Clinical Neurophysiology. 1991;78:252–259. doi: 10.1016/0013-4694(91)90040-b. [DOI] [PubMed] [Google Scholar]
- Woodsworth RS, Schlosberg H. Experimental Psychology. 3rd. New York: Holt, Rinehart and Winston; 1963. [Google Scholar]
- Yuan H, He B. Brain-computer interfaces using sensorimotor rhythms: current state and future perspectives. IEEE Transactions on Biomedical Engineering. 2014;61:1425–1434. doi: 10.1109/TBME.2014.2312397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann E, Morrone MC, Burr DC. Spatial position information accumulates steadily over time. Journal of Neuroscience. 2013;33:18396–18401. doi: 10.1523/JNEUROSCI.1864-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]


