Abstract
Background
Current available tools for assessing high cardiovascular risk (HCR) often require measurements not available in resource-limited settings in low- and middle-income countries (LMICs). There is a need to assess HCR using a pragmatic evidence-based approach.
Objective
To report the prevalence of HCR in ten LMIC areas in Africa, Asia and South America, and to investigate the profiles and correlates of HCR.
Methods
Cross-sectional analysis using data from the NHLBI-UHG Centers of Excellence. HCR was defined as history of heart disease/heart attack, history of stroke, older age (≥50 years for men and ≥60 for women) with history of diabetes, or older age with systolic blood pressure ≥160 mmHg. Prevalence estimates were standardized to the WHO World Standard Population.
Results
A total of 37,067 subjects aged ≥35 years were included; 53.7% were women and mean age was 53.5 (±12.1) years. The overall age-standardized prevalence of HCR was 15.4% (95% CI: 15.0-15.7), ranging from 8.3% (India, Bangalore) to 23.4% (Bangladesh). Among men, the prevalence was 1.7% for the younger age group (35-49 years) and 29.1% for the older group (≥50); among women, 3.8% for the younger group (35-59 years) and 40.7% for the older group (≥60). Among the older group, measured systolic blood pressure ≥160 mmHg (with or without other conditions) was the most common criterion for having HCR, followed by diabetes. The proportion of having met more than one criterion was nearly 20%. Age, education, and body mass index were significantly associated with HCR. Cross-site differences existed and were attenuated after adjusting for age, sex, education, smoking, and body mass index.
Conclusions
The prevalence of HCR in ten LMIC areas was generally high. This study provides a starting point to define targeted populations that may benefit from interventions combining both primary and secondary prevention strategies.
Keywords: Developing Countries, High risk, Prevalence, Resource-Limited Settings, Cardiovascular Disease
Introduction
Cardiovascular diseases (CVDs), along with its risk factors, are a major global health issue. In 2010, ischemic heart disease and stroke accounted for one in four deaths worldwide [1]. In addition, high blood pressure, smoking, and high body mass index (BMI) were top causes of Disability-Adjusted Life-Years globally [2]. Furthermore, these estimates have increased in the last decades [1-3], particularly in low- and middle-income countries (LMICs) [4-6].
Risk assessment based on total risk instead of single risk factors has become a key component of prevention strategies in many clinical guidelines [7-12]. Such strategies allow the identification of those most likely to benefit from interventions while avoiding over-treatment in those with low risk, thus are likely to be cost-effective in resource-limited settings. Unfortunately, most available risk prediction tools for CVDs require laboratory-based measures that are not easily available in resource-limited areas in LMICs [13-16]. Some non-laboratory based assessment tools have been developed and as compared with more sophisticated methods, had reasonable prediction power for cardiovascular events and mortality [15, 17, 18]. However, most prior tools were developed for the purpose of predicting events and thus excluded patients with existing CVD already. These patients are at very high risk of disease recurrence [19-23], and require acute clinical treatments and follow-up while people who do not have such diseases but are at high risk of developing CVDs do not. Nevertheless, there are common sets of essential pharmaceutical and lifestyle interventions that apply to both groups. Therefore, from a public health and implementation point of view, particularly considering practical field conditions for community-wide activities and at the primary care level, we need a simple measure that can combine both patients with existing CVDs and those at high absolute risk of developing them into one indicator of high cardiovascular risk (HCR). Simplified and pragmatic approaches to define HCR are needed to curb the rising epidemic of CVDs, particularly to inform future primary and secondary prevention strategies.
We have developed and validated in China [24] a practical tool to assess HCR based on age, sex, disease history (heart disease, stroke, and diabetes) and measurement of blood pressure only, making it easy to accommodate and implement in resource-limited settings. With minimal training, this tool can be adopted at the primary care level by healthcare workers or even volunteers. In this study, we used existing cross-sectional data from study sites in ten LMICs in Africa, Asia, and South America to assess the prevalence of HCR among adults according to this evidence-based yet pragmatically-defined assessment tool. We also examined the components and profiles of HCR and its socio-demographic and lifestyle correlates.
Materials and Methods
Data Source, Country Selection and Study Population
This study is a cross-sectional analysis using data from the NHLBI-UHG Centers of Excellence program [25]. The countries fulfilling the following criteria were included: 1) population-based sample; 2) having data available for all of the following variables: age, gender, measured systolic blood pressure, personal history of diabetes, personal history of stroke and personal history of heart disease/heart attack. Only subjects with complete data on these variables were included. Moreover, this study only included subjects aged ≥35 years because the prevalence of HCR was relatively low in younger subjects.
According to the above criteria, data from seven centers with samples from ten countries in three world regions were included in the analysis: Africa (South Africa), Asia (Bangladesh, China, India and Pakistan) and South America: Argentina (Bariloche and Marcos Paz), Chile (Temuco), Peru (Lima, Tumbes, Puno) and Uruguay (Pando-Barros Blancos) (Table 1). In each country, participants from selected urban and/or rural study sites were surveyed according to standardized protocols. Survey instruments and methods were similar but not identical across studies, as each setting had further questions based on their particular needs and objectives; information for this study was collected in a similar fashion. Details about each study design, sampling methods, and procedures have been published elsewhere [25-30].
Table 1. Characteristics of datasets included in the analyses.
| Africa | Asia | South America | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Center of Excellence | South Africa | Bangladesh | China | India (Bangalore) | India (New Delhi) | Argentina | Peru | |||
| Country | South Africa | Bangladesh | China | India | India | Pakistan | Argentina | Chile | Uruguay | Peru |
| Survey Year | 2008-2009 | 2011-2012 | 2012 | 2011-2012 | 2010-2011 | 2010-2011 | 2010-2011 | 2010-2011 | 2010-2011 | 2012-2012 |
| Study settings | Cape town | Dhaka, Chandpur | Liaoning, Hebei, Shanxi, Shaanxi, Ningxia | Tamil Nadu, Karnataka, Sevagram | Chennai, Delhi | Karachi | Bariloche, Marcos Paz | Temuco | Pando-Barros Blancos | Lima, Tumbes, Puno |
| Rural or Urban | Urban | Both | Rural | Rural | Urban | Urban | Urban | Urban | Urban | Both |
| Subjects ≥35 years | 752 | 3,760 | 5,298 | 8,616 | 8,736 | 2,681 | 3,982 | 1,940 | 1,579 | 3,621 |
| Subjects Included | 691 | 3,756 | 5,293 | 8,616 | 6,299 | 2,380 | 3,941 | 1,940 | 1,579 | 2,572 |
| Age Range | 35-81 | 40-106 | 35-94 | 35-101 | 35-94 | 35-97 | 35-79 | 35-77 | 35-76 | 35-92 |
| Definition of Heart Disease | Heart Attack | Heart Disease | Heart Disease | Heart Attack | Heart Attack | Heart Attack | Both | Both | Both | Heart Disease |
| Measurements for blood pressure | 1 | 3 | 1 | 3 | 2 | 2 | 1 | 1 | 1 | 3 |
Definition and Components of High Cardiovascular Risk
Study subjects were defined as HCR if they meet one or more of the following criteria:
Personal history of heart disease or heart attack.
Personal history of stroke (including all types but not including transient ischemic attack).
Older age (men aged ≥ 50 years or women aged ≥ 60 years) and personal history of diabetes (including all types).
Older age (men aged ≥ 50 years or women aged ≥ 60 years) and systolic blood pressure ≥160 mmHg.
These criteria were chosen based on the available evidence linking them with the absolute risk of developing cardiovascular outcomes in ten years [24]. Subjects with personal history of cardiovascular disease, namely heart disease/attack or stroke, are at high risk of recurrence regardless of age. Other factors such as diabetes and high blood pressure among older adults increase the absolute risk of cardiovascular outcomes [31-34]. Previous studies have suggested higher cardiovascular risk for men at a younger age than women [35-37], thus the age threshold is different for men and women.
The four criteria were assessed in a similar fashion following standardized procedures across studies. The first criterion was self-report on physician-diagnosed personal history of either heart disease and/or heart attack (Table 1). The presence of stroke (any type) and diabetes (type 1 or 2) was based on self-reported diagnosis too. Self-reported diagnosis was collected with questionnaires developed for each study setting and applied in the local language. Blood pressure was measured with standard procedures across countries. In general, participants had to rest between 5 and 30 minutes before blood pressure was assessed, and where there were more than one blood pressure measurement subjects rested between 30 seconds and 20 minutes. In addition, blood pressure was measured with an automated monitor, electronic sphygmomanometer, or standard aneroid sphygmomanometer. For this study, whenever there was more than one blood pressure record, we used the average of all available measurements. Since we aimed to study HCR, not hypertension per se., we did not considered as high blood pressure a systolic blood pressure reading of 140-159 mmHg. If we used such threshold, even with the age and sex criteria, the absolute risk of cardiovascular events would not reach 10% in 10 years. Therefore, a higher cut-off point of 160 mmHg was used. Diastolic blood pressure was not used because prior literature demonstrating that diastolic blood pressure was not as predictive of risks as systolic blood pressure, especially among older people [38-41]. In addition, we did not include diastolic blood pressure for simplicity in implementation – a main goal of this definition. In a similar fashion, medication use was not considered in this definition. It is worth reiterating that this definition of HCR aims to 1) be pragmatic to aid easy identification at the primary care and community level; 2) be holistic to include existing CVDs and multiple factors to capture absolute risks; and 3) to provide evidence for future primary and secondary prevention of CVDs.
We assessed the prevalence of each component (criteria) in the HCR definition as well as the profile of HCR. For the older group (men ≥50 and women ≥60), they could have up to four components while for the younger age group (men 35-49 and women 35-59 years old), they could only have up to two components: having heart disease and/or stroke.
Independent Variables
Other variables included age (as a continuous variable and in ten-year groups), gender (male and female), study site, smoking status (current, former, none), education (none, any school – one to 11 years of schooling, university/higher – 12 or more years of schooling), and BMI (under/normal weight [BMI<25], overweight [BMI ≥25 & <30], and obesity [BMI ≥30]).
Study Samples
The number of subjects eligible for the study as well as the final number included in the analysis are shown in Table 1. Overall, there were 40,965 subjects aged 35 years or older in the selected study settings. After removing observations with missing values in the variables included in the HCR definition, almost all subjects in Argentina (99.0%), Bangladesh (99.9%), Chile (100%), China (99.9%), India, Bangalore (100%), and Uruguay (100%) were included in the analysis. However, there were fewer subjects included from other settings: 91.9% in South Africa, 88.8% in Pakistan, 72.1% in India, New Delhi, and 71.0% in Peru.
Statistical Procedures
Results were stratified by gender, age group, and site. For age stratification, we used two categories: younger group (men aged 35-49 and women aged 35-59) and older group (men aged ≥ 50 and women aged ≥ 60). Unless otherwise noted, results on prevalence of HCR were standardized according to the WHO World Standard Population based on the world average population between 2000-2025 [42]. Age standardization was conducted within the same broad (younger or older) age group.
We first reported proportions for categorical variables as well as means and standard deviations for numerical variables. Proportions and 95% confidence intervals (95% CI) were used to show the distribution of each component of HCR, and the profile of HCR, i.e., subjects meeting only one, two, three or all four criteria for HCR. Regression models – crude and adjusted – were constructed to assess the strength of the association between HCR and sociodemographic and health variables (age, sex, study site, education, smoking, and BMI status). We used generalized linear models with Poisson family and log link, including robust standard errors. The association estimates are presented as prevalence ratios (PR) and 95% CI [43, 44]. The statistical analyses were conducted with STATA 13 (StataCorp, College Station, TX, USA) by the first author and independently verified with SAS 9.3 (SAS Institute Inc. Cary, NC, United States) by another author.
Ethics
Each individual study received its own Institutional Review Board approval. Informed consent was obtained from all participants before any data were collected. We used pooled and de-identified data to conduct this study.
Results
Population Characteristics
Overall, there were slightly more women (53.7%) than men. The mean age was 53.5 (±12.1) years (Table 2). Mean systolic blood pressure was 129.5 (±22.3) mmHg. Regarding education, 19.2% reported no education and 65.5% had achieved some school-based education, but not university level or higher. For the overall sample, 28.8% were overweight and 17.7% were obese. Between-site variations were large for all variables.
Table 2. Characteristics of the study population.
| Africa | Asia | South America | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Overall | South Africa | Bangladesh | China | India (Bangalore) | India (New Delhi) | Pakistan | Argentina | Chile | Peru | Uruguay | |
| N=37,067 | N=691 | N=3,756 | N=5,293 | N=8,616 | N=6,299 | N=2,38 0 | N=3,941 | N=1,94 0 | N=2,57 2 | N=1,57 9 | |
| Variables in the High Cardiovascular Definition | |||||||||||
|
| |||||||||||
| Sex | |||||||||||
| Males | 46.4 | 36.0 | 45.7 | 47.4 | 48.0 | 48.7 | 47.4 | 48.4 | 46.0 | 48.4 | 48.5 |
| Age | 53.5 ± 12.1 | 50.1 ± 10.4 | 53.7 ± 10.3 | 63.0 ± 10.0 | 51.4 ± 12.8 | 48.8 ± 10.5 | 48.5 ± 10.8 | 50.6 ± 10.4 | 50.0 ± 10.4 | 55.4 ± 12.5 | 51.9 ± 11.0 |
| Age Categories | |||||||||||
| 35-44 | 27.4 | 35.5 | 20.1 | 5.7 | 34.8 | 40.0 | 40.9 | 35.6 | 40.1 | 23.1 | 31.9 |
| 45-54 | 27.3 | 33.7 | 40.9 | 11.6 | 26.0 | 32.9 | 31.0 | 30.0 | 29.1 | 25.8 | 29.0 |
| 55-64 | 24.1 | 19.8 | 22.1 | 38.0 | 18.8 | 17.5 | 17.8 | 22.3 | 18.7 | 26.5 | 22.6 |
| 65-74 | 16.5 | 9.8 | 12.1 | 32.5 | 14.9 | 7.5 | 8.2 | 11.7 | 11.7 | 16.1 | 15.4 |
| ≥75 | 4.8 | 1.2 | 4.8 | 12.2 | 5.6 | 2.1 | 2.1 | 0.4 | 0.4 | 8.6 | 1.1 |
| Heart Disease/Attack | 3.5 | 5.6 | 13.0 | 5.8 | 0.2 | 1.2 | 1.4 | 2.1 | 2.4 | 4.3 | 3.8 |
| Stroke | 2.4 | 3.9 | 4.8 | 7.6 | 0.5 | 0.8 | 1.4 | 0.9 | 0.9 | 0.9 | 1.9 |
| Diabetes | 11.2 | 12.0 | 13.2 | 12.6 | 4.1 | 19.2 | 13.7 | 7.0 | 11.2 | 8.0 | 11.8 |
| Systolic Blood Pressure ≥160 (mmHg) | 9.6 | 12.3 | 6.2 | 24.7 | 6.4 | 7.9 | 7.3 | 6.2 | 6.0 | 2.5 | 8.1 |
| Systolic Blood Pressure (mmHg) | 129.5 ± 22.3 | 131.5 ± 24.3 | 122.4 ± 26.5 | 145.9 ± 22.5 | 125.4 ± 20.1 | 130.2 ± 19.0 | 126.4 ± 20.1 | 127.5 ± 18.7 | 126.0 ± 19.3 | 118.9 ± 17.3 | 129.6 ± 20.5 |
|
| |||||||||||
| Other Variables | |||||||||||
|
| |||||||||||
| Smoking History | N=37,019 | N=690 | N=3,747 | N=5,293 | N=8,616 | N=6,299 | N=2,380 | N=3,933 | N=1,938 | N=2,572 | N=1,551 |
| Current | 33.6 | 25.8 | 58.7 | 28.8* | 51.7 | 22.5 | 27.5 | 28.6 | 30.7 | 12.1 | 30.2 |
| Former | 21.0 | 10.4 | 9.0 | 71.2 | 2.9 | 2.6 | 1.8 | 26.5 | 23.5 | 33.3 | 27.3 |
| None | 45.5 | 63.8 | 32.3 | 45.4 | 74.9 | 70.8 | 44.9 | 45.8 | 54.6 | 42.6 | |
| Education | N=36,983 | N=691 | N=3,756 | N=5,284 | N=8,616 | N=6,299 | N=2,380 | N=3,906 | N=1,922 | N=2,572 | N=1,557 |
| None | 19.2 | 12.7 | 0.0 | 26.5 | 43.0 | 15.2 | 30.5 | 0.9 | 0.4 | 6.6 | 0.6 |
| Any school | 65.5 | 87.3 | 47.6 | 73.4 | 55.1 | 66.4 | 55.8 | 76.4 | 63.5 | 73.8 | 87.9 |
| University/Higher | 15.3 | 0.0 | 52.4 | 0.2 | 1.9 | 18.4 | 13.7 | 22.6 | 36.1 | 19.6 | 11.5 |
| Body Mass Index | N=26,490 | N=691 | N=3,756 | N=5,291 | N=8,615 | N=4,941 | N=1,795 | N=3,933 | N=1,939 | N=2,572 | N=1,572 |
| Under/Normal Weight | 43.6 | 31.0 | 72.6 | 60.7 | 84.0 | 45.6 | 41.5 | 26.0 | 20.0 | 28.4 | 28.6 |
| Overweight | 33.9 | 20.4 | 20.6 | 32.7 | 13.1 | 34.4 | 35.8 | 38.3 | 45.4 | 43.8 | 34.5 |
| Obese | 22.5 | 48.6 | 6.8 | 6.5 | 2.9 | 20.1 | 22.7 | 35.8 | 35.0 | 27.8 | 37.0 |
Results are presented as percentages or as mean ± standard deviation.
Did not ask for non-smokers.
Age-Standardized Prevalence of High Cardiovascular Risk
Across all the study sites, 16.4% (6,071 out of 37,067) subjects met the HCR criteria. The age-standardized prevalence of HCR was 15.4% (15.0-15.7) and varied across sites from 8.3% in Bangalore, India to 23.4% in Bangladesh (Figure 1). Prevalence of HCR for the younger age group was lower than 10% for all except Bangladeshi women. For the older age group, the prevalence was above 20% for most and above 30% for two thirds of the groups in our study.
Figure 1. Age-Standardized Prevalence of High Cardiovascular Risk by Gender, Age Group, and Study Site.
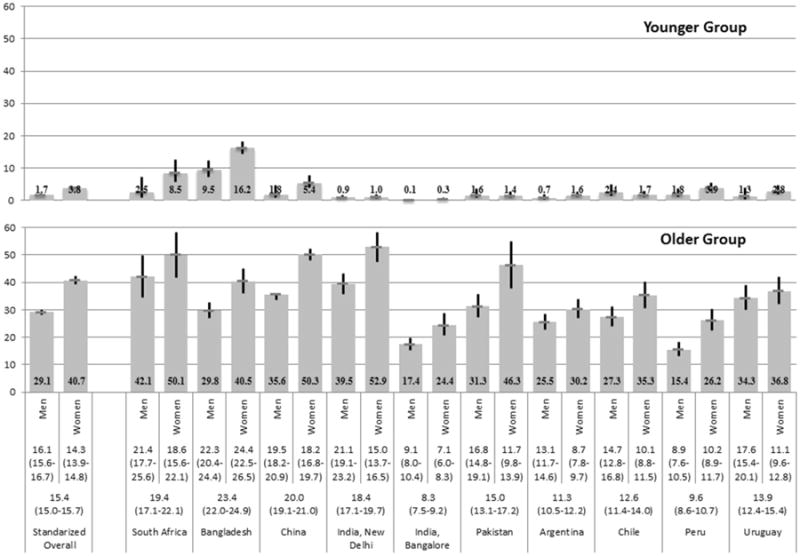
Numbers above the study site represent the overall and sex-specific age-standardized prevalence of high cardiovascular risk and 95% CI.
Prevalence of Components of High Cardiovascular Risk
Table 3 presents the frequency of each HCR component, overall and stratified by age, sex and site. In the younger group, the proportion of having had heart disease was higher than that of stroke. In the older group, the most frequent criterion was having systolic blood pressure over 160 mmHg (13.6% for men vs. 20.5% for women), followed by diabetes (12.3% for men vs. 17.5% for women). In general, women had higher prevalence of each component than men for both age groups. Of note, the four components were not mutually exclusive and participants could have more than one condition.
Table 3. Prevalence of each Cardiovascular Risk Component (Prevalence + 95% CI).
| Younger Group | Older Group | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Heart Disease | Stroke | Heart Disease | Stroke | Diabetes | SBP≥160 | ||||
| Crude | Men | N=6,910 | 1.4 (1.1-1.7) | 0.6 (0.5-0.8) | N=10,269 | 4.6 (4.2-5.0) | 4.0 (3.7-4.4) | 12.3 (11.7-12.9) | 13.6 (13.0-14.3) |
| Overall | Women | N=13,176 | 2.8 (2.6-3.1) | 1.1 (0.9-1.3) | N=6,712 | 5.5 (5.0-6.1) | 4.1 (3.7-4.6) | 17.5 (16.6-18.4) | 20.5 (20.0-21.5) |
| Standardized | Men | N=6,910 | 1.4 (1.1-1.7) | 0.6 (0.5-0.8) | N=10,269 | 4.6 (4.2-5.1) | 4.2 (3.8-4.6) | 12.2 (11.5-12.9) | 14.1 (13.3-14.8) |
| Overall | Women | N=13,176 | 3.0 (2.7-3.3) | 1.1 (1.0-1.3) | N=6,712 | 6.0 (5.3-6.8) | 4.3 (3.8-4.9) | 18.1 (17.0-19.3) | 23.2 (21.9-24.5) |
|
| |||||||||
| South Africa | Men | N=134 | 1.5 (0.4-5.5) | 1.0 (0.2-6.0) | N=115 | 7.2 (3.9-12.7) | 7.3 (4.3-12.1) | 9.6 (5.8-15.5) | 27.2 (20.0-35.9) |
| Women | N=358 | 4.9 (3.0-8.6) | 5.0 (2.8-8.6) | N=84 | 12.9 (7.9-20.3) | 1.8 (0.5-6.4) | 25.0 (18.8-32.5) | 25.4 (18.6-33.6) | |
| Bangladesh | Men | N=618 | 8.6 (6.5-11.3) | 2.6 (1.6-4.0) | N=1,097 | 12.9 (10.9-15.1) | 7.0 (5.6-8.8) | 12.8 (10.9-14.9) | 8.1 (6.6-9.8) |
| Women | N=1,606 | 14.0 (12.4-15.8) | 4.0 (3.1-5.1) | N=435 | 14.6 (12.0-17.7) | 7.0 (4.9-9.8) | 19.0 (15.6-22.9) | 18.1 (14.8-21.9) | |
| China | Men | N=234 | 0.4 (0.1-2.4) | 1.4 (0.4-4.3) | N=2,276 | 4.2 (3.4-5.2) | 8.6 (7.5-9.9) | 8.3 (7.3-9.5) | 22.2 (20.5-23.9) |
| Women | N=466 | 3.6 (2.4-5.3) | 1.8 (1.0-3.6) | N=2,317 | 7.3 (6.3-8.4) | 7.5 (6.4-8.6) | 20.3 (18.6-22.0) | 31.8 (29.9-33.8) | |
| India, New Delhi | Men | N=1,741 | 0.6 (0.3-1.3) | 0.4 (0.2-1.0) | N=1,314 | 4.2 (2.9-6.1) | 1.9 (1.3-2.8) | 26.0 (21.7-30.7) | 14.7 (13.0-16.4) |
| Women | N=2,704 | 0.6 (0.2-1.4) | 4.6 (0.2-1.0) | N=540 | 4.4 (2.9-6.5) | 1.0 (0.5-2.0) | 39.3 (33.5-45.4) | 22.8 (16.9-30.0) | |
| India, Bangalore | Men | N=1,994 | 0.1 (0.0-0.4) | 0.0 (0.0-0.4) | N=2,138 | 0.2 (0.1-0.8) | 1.8 (1.1-2.9) | 7.0 (5.6-8.7) | 10.4 (8.7-12.3) |
| Women | N=3,217 | 0.2 (0.1-0.7) | 0.1 (0.0-0.3) | N=1,267 | 0.7 (0.2-3.4) | 0.8 (0.3-2.0) | 4.3 (3.1-6.0) | 19.8 (16.3-23.9) | |
| Pakistan | Men | N=593 | 1.0 (0.3-3.1) | 0.6 (0.2-1.8) | N=539 | 4.1 (2.6-6.3) | 1.8 (1.0-3.3) | 19.1 (15.3-23.5) | 12.2 (9.0-16.3) |
| Women | N=1,086 | 0.4 (0.1-1.0) | 1.0 (0.5-2.1) | N=162 | 0.4 (0.1-1.6) | 3.5 (1.3-9.0) | 28.7 (21.1-37.8) | 24.3 (16.2-34.8) | |
| Argentina | Men | N=545 | 0.6 (0.2-1.5) | 0.1 (0.0-1.0) | N=1,026 | 4.8 (3.6-6.4) | 1.6 (1.0-2.6) | 10.7 (8.7-13.1) | 12.2 (10.3-14.4) |
| Women | N=1,621 | 1.1 (0.6-1.7) | 0.6 (0.4-1.1) | N=749 | 2.3 (1.4-3.7) | 1.4 (0.7-2.6) | 13.8 (11.2-16.8) | 16.8 (14.3-19.7) | |
| Chile | Men | N=367 | 1.7 (0.8-3.7) | 1.0 (0.3-3.2) | N=552 | 4.3 (2.9-6.3) | 1.2 (0.6-2.5) | 15.1 (12.5-18.2) | 12.0 (9.5-15.0) |
| Women | N=662 | 1.3 (0.7-2.3) | 0.6 (0.3-1.5) | N=359 | 4.5 (2.6-7.7) | 1.3 (0.5-3.1) | 21.1 (17.1-25.8) | 16.1 (12.9-20.0) | |
| Peru | Men | N=447 | 1.6 (0.8-3.1) | 0.4 (0.1-1.7) | N=797 | 4.0 (2.9-5.6) | 0.7 (0.3-1.6) | 8.5 (6.7-10.6) | 3.8 (2.7-5.3) |
| Women | N=883 | 3.5 (2.5-4.8) | 0.5 (0.2-1.1) | N=445 | 8.2 (5.9-11.2) | 2.2 (1.2-3.9) | 14.0 (11.1-17.5) | 6.8 (4.9-9.4) | |
| Uruguay | Men | N=237 | 0.7 (0.2-2.8) | 0.6 (0.1-3.6) | N=415 | 8.3 (6.1-11.2) | 2.9 (1.7-5.1) | 13.6 (10.6-17.4) | 17.2 (13.7-21.3) |
| Women | N=573 | 1.9 (1.1-3.3) | 1.1 (0.5-2.6) | N=354 | 6.5 (4.4-9.4) | 4.9 (3.0-8.0) | 22.5 (18.4-27.1) | 11.3 (8.4-15.0) | |
High cardiovascular risk defined as personal history of heart disease or heart attack, personal history of stroke, older age (men aged ≥ 50 years or women aged ≥ 60 years) and personal history of diabetes, or older age and systolic blood pressure ≥ 160 mmHg; therefore, for younger age group (men 35-49 and women 35-59 years old), there are only two components, while for the older group (men >=50 and women >=60) there are four components.
Profile of High Cardiovascular Risk
Table 4 depicts the proportions of subjects meeting only one, two, three or all four criteria for HCR, stratified by age, sex and site. Most subjects met only one criterion, both in the younger (86.9% men and 92.1% women) and older (81.9% men and 77.3% women) groups. While very few subjects met three or four criteria, the proportion of having two conditions was sizable in the older group (15.7% for men and 19.2% for women).
Table 4. Prevalence of Having One or More Components of High Cardiovascular Risk (Prevalence + 95% CI).
| Younger Group | Older Group | |||||
|---|---|---|---|---|---|---|
| Having only one | Having two | Having only one | Having only two | Having only three or all four | ||
| Crude Overall | Men | 86.9 (79.5-91.9) | 13.1 (8.1-20.5) | 81.9 (80.5-83.3) | 15.7 (14.5-17.1) | 2.3 (1.9-3.0) |
| Women | 92.1 (89.3-94.2) | 7.9 (5.8-10.7) | 77.3 (75.7-78.9) | 19.2 (17.7-20.8) | 3.4 (2.8-4.2) | |
| Standardized | Men | 90.3 (85.2-93.7) | 9.7 (6.3-14.8) | 82.2 (80.7-83.7) | 15.6 (14.2-17.1) | 2.1 (1.7-2.7) |
| Overall | Women | 92.9 (89.6-95.2) | 7.1 (4.8-10.4) | 77.2 (75.4-78.9) | 19.5 (17.8-21.3) | 3.3 (2.6-4.2) |
|
| ||||||
| South Africa | Men | 100.0 | 0.0 (0.0-0.0) | 85.5 (76.1-91.5) | 12.1 (6.3-21.9) | 2.5 (0.8-7.4) |
| Women | 88.3 (83.5-91.9) | 11.7 (8.1-16.5) | 79.0 (68.1-86.9) | 16.1 (9.0-27.3) | 4.9 (1.8-12.7) | |
| Bangladesh | Men | 84.2 (74.2-90.7) | 15.9 (9.3-25.8) | 71.6 (66.5-76.2) | 21.2 (17.0-26.0) | 7.3 (5.0-10.5) |
| Women | 88.6 (84.1-92.0) | 11.4 (8.0-15.9) | 64.1 (57.3-70.3) | 30.0 (23.9-36.9) | 5.9 (3.3-10.5) | |
| China | Men | 100.0 | 0.0 (0.0-0.0) | 81.9 (78.9-84.6) | 15.9 (13.4-18.8) | 2.2 (1.5-3.3) |
| Women | 100.0 | 0.0 (0.0-0.0) | 73.2 (70.6-75.7) | 21.8 (19.5-24.4) | 5.0 (3.9-6.4) | |
| India, New Delhi | Men | 81.5 | 18.5 | 81.5 (77.3-85.1) | 18.4 (14.8-22.79 | 0.1 (0.0-0.4) |
| Women | 100.0 | 0.0 (0.0-0.0) | 81.0 (74.9-86.0) | 16.3 (11.8-22.1) | 2.7 (1.8-4.0) | |
| India, Bangalore | Men | 52.2 | 47.8 | 90.5 (86.3-93.5) | 9.5 (6.5-13.7) | 0.0 (0.0-0.0) |
| Women | 100.0 | 0.0 (0.0-0.0) | 92.9 (87.6-96.0) | 7.1 (4.0-12.3) | 0.1 (0.0-0.7) | |
| Pakistan | Men | 100.0 | 0.0 (0.0-0.0) | 84.7 (79.4-88.9) | 11.3 (7.6-16.5) | 4.0 (2.6-6.0) |
| Women | 96.0 (77.9-99.4) | 4.0 (0.6-22.1) | 75.7 (67.8-82.3) | 24.3 (17.7-32.3) | 0.0 (0.0-0.0) | |
| Argentina | Men | 100.0 | 0.0 (0.0-0.0) | 87.6 (83.3-90.9) | 11.5 (8.3-15.8) | 0.9 (0.4-2.2) |
| Women | 95.9 (88.5-98.6) | 4.1 (1.4-11.5) | 85.2 (79.3-89.6) | 14.3 (10.0-20.2) | 0.5 (0.1-3.1) | |
| Chile | Men | 86.9 | 13.1 | 85.1 (79.2-89.6) | 13.7 (9.4-19.4) | 1.2 (0.3-4.5) |
| Women | 94.4 (76.0-98.9) | 5.6 (1.1-24.0) | 77.5 (68.1-84.8) | 20.9 (13.7-30.3) | 1.7 (0.5-5.7) | |
| Peru | Men | 84.5 | 15.5 | 90.0 (85.8-93.1) | 5.4 (2.9-9.9) | 4.6 (3.4-6.1) |
| Women | 100.0 | 0.0 (0.0-0.0) | 82.6 (75.6-87.9) | 16.4 (11.2-23.2) | 1.1 (0.3-4.0) | |
| Uruguay | Men | 100.0 | 0.0 (0.0-0.0) | 75.4 (68.2-81.4) | 22.6 (16.9-29.4) | 2.1 (0.7-6.2) |
| Women | 88.5 | 11.5 | 78.5 (71.1-84.4) | 20.3 (14.6-27.6) | 1.2 (0.3-5.2) | |
High cardiovascular risk defined as personal history of heart disease or heart attack, personal history of stroke, older age (men aged ≥ 50 years or women aged ≥ 60 years) and personal history of diabetes, or older age and systolic blood pressure ≥ 160 mmHg; therefore, for younger age group (men 35-49 and women 35-59 years old), there are only two components, while for the older group (men >=50 and women >=60) there are four components.
Correlates of High Cardiovascular Risk
Table 5 shows the crude and adjusted prevalence ratio for associated factors and HCR. In multivariable models, subjects in the oldest age group, compared to the youngest individuals, had much higher prevalence of HCR: PR= 19.01 (95% CI 16.10-22.44). Relative to men, women had 21% lower prevalence of HCR. Likewise, those at the highest educational level had 23% lower prevalence. There were large variations across study sites: compared to India (New Delhi), some countries had much higher and others had lower prevalence of HCR. Variations across study sites became smaller after adjusting for other variables.
Table 5. Crude and Adjusted Prevalence Ratio for Associated Factors and High Cardiovascular Risk.
| High Cardiovascular Risk | |||
|---|---|---|---|
| Crude PR (95% CI) | Adjusted PR (95% CI)* | ||
| Age | N=37,067 | N=34,977 | |
| 35-44 | 1 | 1 | |
| 45-54 | 4.33 (3.69-5.10) | 3.79 (3.21-4.48) | |
| 55-64 | 14.03 (12.06-16.33) | 11.44 (9.78-13.38) | |
| 65-74 | 21.49 (18.49-24.98) | 17.79 (15.22-20.80) | |
| ≥75 | 23.07 (19.71-27.01) | 19.01 (16.10-22.44) | |
| Sex | N=37,067 | N=34,977 | |
| Male | 1 | 1 | |
| Female | 0.85 (0.81-0.89) | 0.79 (0.75-0.83) | |
| Education | N=36, 983 | N=34,977 | |
| None | 1 | 1 | |
| Any school | 0.90 (0.85-0.95) | 0.96 (0.91-1.02) | |
| University/Higher | 0.72 (0.67-0.79) | 0.77 (0.70-0.84) | |
| Smoking | N=37,019 | N=34,977 | |
| Current | 1 | 1 | |
| Former | 2.26 (2.15-2.40) | 1.25 (1.17-1.33) | |
| None | 0.88 (0.83-0.93) | 1.13 (1.05-1.21) | |
| BMI | N=35,105 | N=34,977 | |
| Under/Normal Weight | 1 | 1 | |
| Overweight | 1.33 (1.27-1.40) | 1.42 (1.35-1.50) | |
| Obesity | 1.35 (1.27-1.43) | 1.71 (1.60-1.83) | |
| Site | N=37,067 | N=34,977 | |
| Argentina | 1.09 (0.99-1.21) | 0.62 (0.56-0.68) | |
| Bangladesh | 1.70 (1.56-1.86) | 1.62 (1.46-1.80) | |
| Chile | 1.25 (1.10-1.41) | 0.69 (0.61-0.77) | |
| China | 3.02 (2.81-3.25) | 1.22 (1.11-1.35) | |
| India, New Delhi | 1 | 1 | |
| India, Bangalore | 0.59 (0.54-0.65) | 0.52 (0.47-0.58) | |
| Pakistan | 0.89 (0.78-1.02) | 0.84 (0.73-0.97) | |
| Peru | 0.88 (0.77-0.99) | 0.48 (0.42-0.54) | |
| Uruguay | 1.50 (1.33-1.70) | 0.78 (0.69-0.88) | |
| South Africa | 1.39 (1.17-1.66) | 1.11 (0.94-1.31) | |
Adjusted for all variables listed.
Discussion
Main Findings
Among the study populations 35 years and above from selected sites in ten LMICs, the overall age-standardized prevalence of HCR was 15.4% (15.0-15.7), ranging from 8.3% to 23.4%. Among men, the prevalence was 1.7% for the younger age group (35-49 years) and 29.1% for the older group (≥50); among women, 3.8% for 35-59 years and 40.7% for those ≥60 years. Among the older group, measured systolic blood pressure ≥160 mmHg (with or without other conditions) was the most common HCR criterion, followed by diabetes. The proportion of having met more than one criterion was nearly 20%. Age, education, and BMI were significantly associated with HCR. Large cross-site differences existed and were attenuated after adjusting for age, sex, education, smoking, and BMI.
Rational for the High Cardiovascular Risk Definition
In this study, HCR was pragmatically defined to include both patients with existing CVDs and individuals at high risk of developing them. Identification of HCR was based on age, sex, disease history, and measurement of systolic blood pressure only to be suitable for resource-limited settings. Several risk assessment tools have been developed based on different populations [9, 45]. Simplified versions without laboratory tests have also been developed and tested in resource-limited areas, showing satisfactory results [15, 17, 18]. These risk assessment tools focus on prediction of first cardiovascular events. However, according to the high recurrence rate, people with medical history should also be considered as high risk population for risk management [46]. This is why medical history of heart disease and stroke was included in this assessment tool, besides diabetes and high blood pressure, which are two established risk factors for CVD. Estimates based on this definition is easier to obtain than more complex lab-based tools and will provide a composite measure of high-risk population needing intervention. The reliability and validity of our assessment tool have been tested in a previous study in China [24]. The concordance rate between this assessment tool and the gold standard in predicting 10-year absolute risk of having a new or recurrent cardiovascular event is 92.9%. Compared with the gold standard, the sensitivity is 77.2%, the specificity is 98.5%, the positive predictive value is 94.7%, and the negative predictive value is 92.5% [24]. The high specificity shows that individuals identified as non-HCR by our definition are indeed not high-risk while the lower sensitivity implies that this definition misses some people who are high-risk. When resources are limited and the prevalence of HCR is high, the latter is not as serious a concern as false identification.
Interpretation of Results in Light of Previous Evidence on High Cardiovascular Risk
A gender difference was suggested by our results. For each component of HCR, women had higher prevalence in both younger and older groups. These findings were likely a result of the higher cut-off point for women than men in the definition. On the other hand, high prevalence of cardiovascular risk in women was also reported by other studies [47-49]. The higher prevalence of diabetes among females was also indicated in previous research [50, 51].
Most previous studies found that risk factors for CVD tend to cluster among the same individuals. For example, a study in China showed that the prevalence of clustering of cardiovascular disease risk factors (≥2 of hypertension, diabetes, dyslipidemia, or overweight) was 36% [52]. Also, a study in eight African countries and six countries in the Middle East found a similar pattern; their highest frequency was for subjects with two or three risk factors [53]. In our study, most people were defined as HCR by meeting only one criterion while a sizable proportion had two conditions. Clustering of high-risk components was lower than other studies because our definition included existing CVD and the cut-off for systolic blood pressure was set at 160 mmHg not the typical 140 mmHg. Given the high prevalence of hypertension and a lower cut-off point, a larger proportion of people would be identified as HCR. Identifying a larger group of people with risks lower than in our definition may mean less cost-effective or feasible intervention for resource-constrained areas.
Age and obesity were associated with higher prevalence of HCR, as expected. Compared with current smokers, former smokers and non-smokers had higher prevalence of HCR. This result is surprising and reverse causality is a possible explanation for the higher prevalence among former smoker. Higher education was associated with lower prevalence of HCR in multivariable model. This result is consistent with other studies. Gupta et al. reported that people with low or middle educational status were at greater cardiovascular risk than their peers with higher education [54]. No causal inference can be drawn from our cross-sectional study; nevertheless, our results provide further evidence for the role of improving education in reducing health risks.
Although our study was not designed to make cross-countries comparisons, there are interesting findings that need to be further studied. Study settings in the South American region (Argentina, Chile, Peru and Uruguay) had lower age-standardized prevalence of HCR, relative to the other study settings both in Asia and Africa. This finding might reflect differences in the epidemiological transition stage that these settings are in. It could also be due to late diagnosis or more effective management of the risk factors included in our HCR definition.
Strengths and Limitations
The NHLBI-UHG Centers of Excellence database provides us with a unique opportunity to conduct a multi-country study with a considerable sample size. All studies in this program were conducted according to international standards, which is one of the strengths of our study. It also has several important limitations. First, our assessment tool was only validated in China, but not in other LMICs. Each component in the definition, however, has been well-established by many previous studies and it is reasonable to assume that this definition would apply to other countries. Our HCR definition included age-dependent criteria (diabetes and systolic blood pressure for the older group only), which is consistent with previous research on differential influences of risk factors on absolute cardiovascular risk by age. However, such an age-dependent definition could limit comparison of differences in HCR by age. Second, since the studies were conducted in ten countries, heterogeneity of study design and differences in variable definition is a potential limitation. In addition, information about personal history of heart disease, stroke and diabetes in the HCR definition relied on self-reports as not all data datasets had information on verifications by physician diagnoses, medical records, or other more reliable sources. Third, data for each country came from selected urban and/or rural sites only and were not nationally representative, precluding us from making cross-national comparisons. We, therefore, have restricted the presentation and discussion of our findings to ten study areas instead of ten countries per se. Nevertheless, our study was based on diverse study populations across a large number of settings in LMICs and can provide initial indications on the pressing global health issue of HCR.
Conclusions and Implications
The prevalence of HCR across selected study sites in ten LMICs was generally high and a sizable proportion of people with HCR had more than one condition. Our study results highlight the large burden of HCR in LMICs. They also call for urgent actions for larger scale screening and intervention strategies for HCR management in these areas. The HCR assessment tool was designed with scalability and sustainability in mind. With such a tool as the starting point, guideline-based yet simplified intervention strategy incorporating both primary and secondary prevention and management of CVDs have been developed [30]. When successfully implemented, these high-risk strategies have the potential to substantially reduce the risk of CVDs and related costs and consequences. Future studies can evaluate whether these strategies are suitable for the local contexts in different LMICs and are cost-effective in resource-poor settings.
Highlights.
A simple evidence-based high cardiovascular risk (HCR) assessment is needed.
Overall prevalence of HCR in the diverse study settings was 16.4%.
Age-standardized prevalence of HCR ranged from 8.3% to 23.4%.
Nearly 20% of people with HCR met more than one criterion.
Older age and higher body mass index were positively associated with HCR.
Acknowledgments
The authors are thankful to Westat for the assistance merging the datasets and conducting the initial literature review. The authors are grateful to the participants in each study site for their collaboration. The authors want to thank the field teams and data managers in each study for their remarkable work.
Funding: Individual studies were funded in whole or in part with Federal funds from the United States National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract numbers HHSN268200900029C (Argentina), HHSN26820900032C (Bangladesh), HHSN268200900027C (China), HHSN268200900025C (India, Bangalore), HHSN268200900026C (India, New Delhi), HHSN268200900033C (Peru).
Role of the funding source: The funders had no role in study design, data collection, analysis, interpretation, or writing of the report.
Footnotes
Conflict of Interest: The authors report no relationships that could be construed as a conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Rodrigo M. Carrillo-Larco, CRONICAS Center of Excellence for Chronic Diseases, Universidad Peruana Cayetano Heredia, Av. Armendariz 497, Miraflores, Lima 18, Peru
J. Jaime Miranda, CRONICAS Center of Excellence for Chronic Diseases, Universidad Peruana Cayetano Heredia, Av. Armendariz 497, Miraflores, Lima 18, Peru; Department of Medicine, School of Medicine, Universidad Peruana Cayetano Heredia, Av. Honorio Delgado 430, Urb. Ingeniería, San Martín de Porras, Lima 31, Peru.
Xian Li, The George Institute for Global Health at Peking University Health Science Center, Suite 1801, Tower B, Horizon Tower, No. 6 Zhichun Road, Haidian District, Beijing 100088, China.
Chendi Cui, Department of Epidemiology, University of Pittsburgh Graduate School of Public Health, DeSoto street 130, Pittsburgh, Pennsylvania 15261, USA.
Xiaolin Xu, Global Heath Research Center, Duke Kunshan University, No. 8 Duke Avenue, Kunshan, Jiangsu Province 215347, China.
Mohammed Ali, Centre for Chronic Disease Control, Plot No 47, Sector 44, 122002, Gurgaon, Haryana, India.
Alam Dewan S., Centre for Control of Chronic Diseases (CCCD), International Centre for Diarrheal Disease Research, Bangladesh (icddr,b), 68 Shaheed Tajuddin Ahmed Sharani, Mohakhali, Dhaka 1212, Bangladesh.
Thomas A. Gaziano, Brigham & Women's Hospital, Harvard School of Public Health, Harvard University, Cambridge, MA, USA; Division of Cardiovascular Medicine Brigham & Women's Hospital, Boston, USA
Rajeev Gupta, Fortis Escorts Hospital, Jaipur 302017 India; Academic and Research Unit, Rajasthan University of Health Sciences, Jaipur 302033 India.
Vilma Irazola, Centro de Excelencia en Salud Cardiovascular para el Cono Sur (CESCAS), Institute for Clinical Effectiveness and Health Policy (IECS), Dr. Emilio Ravignani 2024, C1414CPV, Buenos Aires, Argentina.
Naomi S. Levitt, Chronic Disease Initiative for Africa (CDIA), c/o Department of Medicine, Faculty of Health Sciences, University of Cape Town, Private Bag x3, Observatory 7935, Cape Town, South Africa; Division of Diabetic Medicine and Endocrinology, Department of Medicine, Faculty of Health Sciences, University of Cape Town, Private Bag x3, Observatory 7935, Cape Town, South Africa
Dorairaj Prabhakaran, Public Health Foundation of India, Plot No 47, Sector 44, 122002, Gurgaon, Haryana, India; Centre for Chronic Disease Control, Plot No 47, Sector 44, 122002, Gurgaon, Haryana, India.
Adolfo Rubinstein, Centro de Excelencia en Salud Cardiovascular para el Cono Sur (CESCAS), Institute for Clinical Effectiveness and Health Policy (IECS), Dr. Emilio Ravignani 2024, C1414CPV, Buenos Aires, Argentina.
Krisela Steyn, Chronic Disease Initiative for Africa (CDIA), c/o Department of Medicine, Faculty of Health Sciences, University of Cape Town, Private Bag x3, Observatory 7925, Cape Town, South Africa.
Nikhil Tandon, Centre for Chronic Disease Control, Plot No 47, Sector 44, 122002, Gurgaon, Haryana, India.
Denis Xavier, St. John's Medical College and Research Institute, Koramangala post, Bangalore, 560 034, India.
Yangfeng Wu, The George Institute for Global Health at Peking University Health Science Center, Suite 1801, Tower B, Horizon Tower, No. 6 Zhichun Road, Haidian District, Beijing 100088, China; Peking University School of Public Health and Clinical Research Institute, #38, Xueyuanlu Rd., Haidian District, Beijing 100191, China.
Lijing L. Yan, The George Institute for Global Health at Peking University Health Science Center, Suite 1801, Tower B, Horizon Tower, No. 6 Zhichun Road, Haidian District, Beijing 100088, China; Global Heath Research Center, Duke Kunshan University, No. 8 Duke Avenue, Kunshan, Jiangsu Province 215347, China
References
- 1.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray CJ, Lopez AD. Measuring the global burden of disease. N Engl J Med. 2013;369(5):448–57. doi: 10.1056/NEJMra1201534. [DOI] [PubMed] [Google Scholar]
- 3.Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385(9963):117–71. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization - WHO. Noncommunnicable Diseases. Available http://www.who.int/mediacentre/factsheets/fs355/en/
- 5.Yusuf S, Rangarajan S, Teo K, Islam S, Li W, Liu L, et al. Cardiovascular risk and events in 17 low-, middle-, and high-income countries. N Engl J Med. 2014;371(9):818–27. doi: 10.1056/NEJMoa1311890. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization (WHO) Global Status Report on Noncommunicable Diseases 2014. 2014 URL: http://apps.who.int/iris/bitstream/10665/148114/1/9789241564854_eng.pdf?ua=1.
- 7.Otgontuya D, Oum S, Buckley BS, Bonita R. Assessment of total cardiovascular risk using WHO/ISH risk prediction charts in three low and middle income countries in Asia. BMC Public Health. 2013;13:539. doi: 10.1186/1471-2458-13-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mendis S, Lindholm LH, Anderson SG, Alwan A, Koju R, Onwubere BJ, et al. Total cardiovascular risk approach to improve efficiency of cardiovascular prevention in resource constrain settings. J Clin Epidemiol. 2011;64(12):1451–62. doi: 10.1016/j.jclinepi.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D'Agostino RB, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S49–73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 10.Ndindjock R, Gedeon J, Mendis S, Paccaud F, Bovet P. Potential impact of single-risk-factor versus total risk management for the prevention of cardiovascular events in Seychelles. Bull World Health Organ. 2011;89(4):286–95. doi: 10.2471/BLT.10.082370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jackson R. Guidelines on preventing cardiovascular disease in clinical practice. BMJ. 2000;320(7236):659–61. doi: 10.1136/bmj.320.7236.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Backer G, Ambrosioni E, Borch-Johnsen K, Brotons C, Cifkova R, Dallongeville J, et al. European guidelines on cardiovascular disease prevention in clinical practice. Third Joint Task Force of European and Other Societies on Cardiovascular Disease Prevention in Clinical Practice. Eur Heart J. 2003;24(17):1601–10. doi: 10.1016/s0195-668x(03)00347-6. [DOI] [PubMed] [Google Scholar]
- 13.Gersh BJ, Sliwa K, Mayosi BM, Yusuf S. Novel therapeutic concepts: the epidemic of cardiovascular disease in the developing world: global implications. Eur Heart J. 2010;31(6):642–8. doi: 10.1093/eurheartj/ehq030. [DOI] [PubMed] [Google Scholar]
- 14.D'Agostino RB, Sr, Grundy S, Sullivan LM, Wilson P. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286(2):180–7. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- 15.Nordet P, Mendis S, Duenas A, de la Noval R, Armas N, de la Noval IL, et al. Total cardiovascular risk assessment and management using two prediction tools, with and without blood cholesterol. MEDICC Rev. 2013;15(4):36–40. doi: 10.37757/MR2013V15.N4.9. [DOI] [PubMed] [Google Scholar]
- 16.Hajifathalian K, Ueda P, Lu Y, Woodward M, Ahmadvand A, Aguilar-Salinas CA, et al. A novel risk score to predict cardiovascular disease risk in national populations (Globorisk): a pooled analysis of prospective cohorts and health examination surveys. Lancet Diabetes Endocrinol. 2015;3(5):339–55. doi: 10.1016/S2213-8587(15)00081-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaziano TA, Young CR, Fitzmaurice G, Atwood S, Gaziano JM. Laboratory-based versus non-laboratory-based method for assessment of cardiovascular disease risk: the NHANES I Follow-up Study cohort. Lancet. 2008;371(9616):923–31. doi: 10.1016/S0140-6736(08)60418-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaziano TA, Pandya A, Steyn K, Levitt N, Mollentze W, Joubert G, et al. Comparative assessment of absolute cardiovascular disease risk characterization from non-laboratory-based risk assessment in South African populations. BMC Med. 2013;11:170. doi: 10.1186/1741-7015-11-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Estol CJ, Bath PM, Gorelick PB, Cotton D, Martin RH. Differences in ischemic and hemorrhagic recurrence rates among race-ethnic groups in the PRoFESS secondary stroke prevention trial. Int J Stroke. 2014;9(Suppl A100):43–7. doi: 10.1111/ijs.12269. [DOI] [PubMed] [Google Scholar]
- 20.Zia E, Engstrom G, Svensson PJ, Norrving B, Pessah-Rasmussen H. Three-year survival and stroke recurrence rates in patients with primary intracerebral hemorrhage. Stroke. 2009;40(11):3567–73. doi: 10.1161/STROKEAHA.109.556324. [DOI] [PubMed] [Google Scholar]
- 21.Hong KS, Yegiaian S, Lee M, Lee J, Saver JL. Declining stroke and vascular event recurrence rates in secondary prevention trials over the past 50 years and consequences for current trial design. Circulation. 2011;123(19):2111–9. doi: 10.1161/CIRCULATIONAHA.109.934786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walsh T, Donnelly T, Carew S, C OC, R OR, Lyons D. Stroke unit care: recurrence, mortality and institutionalisation rates-a four year follow-up study. Ir J Med Sci. 2008;177(2):135–9. doi: 10.1007/s11845-007-0110-2. [DOI] [PubMed] [Google Scholar]
- 23.Cabral NL, Muller M, Franco SC, Longo A, Moro C, Nagel V, et al. Three-year survival and recurrence after first-ever stroke: the Joinville stroke registry. BMC Neurol. 2015;15(1):70. doi: 10.1186/s12883-015-0317-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X, Liu T, Zhang J, Yan L, Sun J, Hao Z, et al. Evaluating a simplified method for identifying high-risk individuals for cardiovascular diseases in the resource-constrained rural areas of China. Zhonghua Liu Xing Bing Xue Za Zhi. 2014;35(9):981–4. [PubMed] [Google Scholar]
- 25.National Heart, Lung, and Blood Institute - NIH. UnitedHealth and NHLBI Collaborating Centers of Excellence. Available: http://www.nhlbi.nih.gov/about/org/globalhealth/centers/
- 26.Fathima FN, Joshi R, Agrawal T, Hegde S, Xavier D, Misquith D, et al. Rationale and design of the Primary pREvention strategies at the community level to Promote Adherence of treatments to pREvent cardiovascular diseases trial number (CTRI/2012/09/002981) Am Heart J. 2013;166(1):4–12. doi: 10.1016/j.ahj.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miranda JJ, Bernabe-Ortiz A, Smeeth L, Gilman RH, Checkley W. Addressing geographical variation in the progression of non-communicable diseases in Peru: the CRONICAS cohort study protocol. BMJ Open. 2012;2(1):e000610. doi: 10.1136/bmjopen-2011-000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nair M, Ali MK, Ajay VS, Shivashankar R, Mohan V, Pradeepa R, et al. CARRS Surveillance study: design and methods to assess burdens from multiple perspectives. BMC Public Health. 2012;12:701. doi: 10.1186/1471-2458-12-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rubinstein AL, Irazola VE, Poggio R, Bazzano L, Calandrelli M, Lanas Zanetti FT, et al. Detection and follow-up of cardiovascular disease and risk factors in the Southern Cone of Latin America: the CESCAS I study. BMJ Open. 2011;1(1):e000126. doi: 10.1136/bmjopen-2011-000126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yan LL, Fang W, Delong E, Neal B, Peterson ED, Huang Y, et al. Population impact of a high cardiovascular risk management program delivered by village doctors in rural China: design and rationale of a large, cluster-randomized controlled trial. BMC Public Health. 2014;14:345. doi: 10.1186/1471-2458-14-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Banerjee C, Moon YP, Paik MC, Rundek T, Mora-McLaughlin C, Vieira JR, et al. Duration of diabetes and risk of ischemic stroke: the Northern Manhattan Study. Stroke. 2012;43(5):1212–7. doi: 10.1161/STROKEAHA.111.641381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Inoue R, Ohkubo T, Kikuya M, Metoki H, Asayama K, Obara T, et al. Stroke risk in systolic and combined systolic and diastolic hypertension determined using ambulatory blood pressure. The Ohasama study. Am J Hypertens. 2007;20(10):1125–31. doi: 10.1016/j.amjhyper.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 33.Peters SA, Huxley RR, Woodward M. Comparison of the sex-specific associations between systolic blood pressure and the risk of cardiovascular disease: a systematic review and meta-analysis of 124 cohort studies, including 1.2 million individuals. Stroke. 2013;44(9):2394–401. doi: 10.1161/STROKEAHA.113.001624. [DOI] [PubMed] [Google Scholar]
- 34.Antikainen R, Jousilahti P, Tuomilehto J. Systolic blood pressure, isolated systolic hypertension and risk of coronary heart disease, strokes, cardiovascular disease and all-cause mortality in the middle-aged population. J Hypertens. 1998;16(5):577–83. doi: 10.1097/00004872-199816050-00004. [DOI] [PubMed] [Google Scholar]
- 35.Anand SS, Islam S, Rosengren A, Franzosi MG, Steyn K, Yusufali AH, et al. Risk factors for myocardial infarction in women and men: insights from the INTERHEART study. Eur Heart J. 2008;29(7):932–40. doi: 10.1093/eurheartj/ehn018. [DOI] [PubMed] [Google Scholar]
- 36.Becker RC. Cardiology patient page. Heart attack and stroke prevention in women. Circulation. 2005;112(17):e273–5. doi: 10.1161/CIRCULATIONAHA.105.551341. [DOI] [PubMed] [Google Scholar]
- 37.Mikkola TS, Gissler M, Merikukka M, Tuomikoski P, Ylikorkala O. Sex differences in age-related cardiovascular mortality. PLoS One. 2013;8(5):e63347. doi: 10.1371/journal.pone.0063347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vishram JK, Borglykke A, Andreasen AH, Jeppesen J, Ibsen H, Jorgensen T, et al. Impact of age on the importance of systolic and diastolic blood pressures for stroke risk: the MOnica, Risk, Genetics, Archiving, and Monograph (MORGAM) Project. Hypertension. 2012;60(5):1117–23. doi: 10.1161/HYPERTENSIONAHA.112.201400. [DOI] [PubMed] [Google Scholar]
- 39.Wang JG, Staessen JA, Franklin SS, Fagard R, Gueyffier F. Systolic and diastolic blood pressure lowering as determinants of cardiovascular outcome. Hypertension. 2005;45(5):907–13. doi: 10.1161/01.HYP.0000165020.14745.79. [DOI] [PubMed] [Google Scholar]
- 40.Leonetti G, Cuspidi C, Facchini M, Stramba-Badiale M. Is systolic pressure a better target for antihypertensive treatment than diastolic pressure? J Hypertens Suppl. 2000;18(3):S13–20. [PubMed] [Google Scholar]
- 41.Strandberg TE, Pitkala K. What is the most important component of blood pressure: systolic, diastolic or pulse pressure? Curr Opin Nephrol Hypertens. 2003;12(3):293–7. doi: 10.1097/00041552-200305000-00011. [DOI] [PubMed] [Google Scholar]
- 42.World Health Organization. Age Standardization of Rates: A New WHO Stardard. 2001 URL: http://www.who.int/healthinfo/paper31.pdf.
- 43.Barros AJ, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol. 2003;3:21. doi: 10.1186/1471-2288-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coutinho LM, Scazufca M, Menezes PR. Methods for estimating prevalence ratios in cross-sectional studies. Rev Saude Publica. 2008;42(6):992–8. [PubMed] [Google Scholar]
- 45.Conroy RM, Pyorala K, Fitzgerald AP, Sans S, Menotti A, De Backer G, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003;24(11):987–1003. doi: 10.1016/s0195-668x(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 46.Wilson PW, D'Agostino R, Sr, Bhatt DL, Eagle K, Pencina MJ, Smith SC, et al. An international model to predict recurrent cardiovascular disease. Am J Med. 2012;125(7):695–703 e1. doi: 10.1016/j.amjmed.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 47.Oluyombo R, Olamoyegun MA, Olaifa O, Iwuala SO, Babatunde OA. Cardiovascular risk factors in semi-urban communities in southwest Nigeria: Patterns and prevalence. J Epidemiol Glob Health. 2015;5(2):167–74. doi: 10.1016/j.jegh.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Petrea RE, Beiser AS, Seshadri S, Kelly-Hayes M, Kase CS, Wolf PA. Gender differences in stroke incidence and poststroke disability in the Framingham heart study. Stroke. 2009;40(4):1032–7. doi: 10.1161/STROKEAHA.108.542894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sivenius J, Tuomilehto J, Immonen-Raiha P, Kaarisalo M, Sarti C, Torppa J, et al. Continuous 15-year decrease in incidence and mortality of stroke in Finland: the FINSTROKE study. Stroke. 2004;35(2):420–5. doi: 10.1161/01.STR.0000110220.63212.59. [DOI] [PubMed] [Google Scholar]
- 50.de Munter JS, Agyemang C, van Valkengoed IG, Bhopal R, Stronks K. Sex difference in blood pressure among South Asian diaspora in Europe and North America and the role of BMI: a meta-analysis. J Hum Hypertens. 2011;25(7):407–17. doi: 10.1038/jhh.2010.77. [DOI] [PubMed] [Google Scholar]
- 51.Yang SH, Dou KF, Song WJ. Prevalence of diabetes among men and women in China. N Engl J Med. 2010;362(25):2425–6. author reply 6. [PubMed] [Google Scholar]
- 52.Gao B, Zhang L, Wang H. Clustering of Major Cardiovascular Risk Factors and the Association with Unhealthy Lifestyles in the Chinese Adult Population. PLoS One. 2013;8(6):e66780. doi: 10.1371/journal.pone.0066780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alsheikh-Ali AA, Omar MI, Raal FJ, Rashed W, Hamoui O, Kane A, et al. Cardiovascular risk factor burden in Africa and the Middle East: the Africa Middle East Cardiovascular Epidemiological (ACE) study. PLoS One. 2014;9(8):e102830. doi: 10.1371/journal.pone.0102830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gupta R, Kaul V, Agrawal A, Guptha S, Gupta VP. Cardiovascular risk according to educational status in India. Prev Med. 2010;51(5):408–11. doi: 10.1016/j.ypmed.2010.08.014. [DOI] [PubMed] [Google Scholar]


