Abstract
Background
The implications of rising obesity for cardiovascular health in low- and middle-income countries (LMICs) has generated much interest, in part because associations between obesity and cardiovascular health appear to vary across ethnic groups.
Objective
We assessed general and central obesity in four regions—Africa, East Asia, South America, and South Asia. We further investigate whether 1) body mass index (BMI) and waist circumference differentially relate to cardiovascular health; and 2) associations between obesity metrics and adverse cardiovascular health vary by region.
Methods
Using baseline anthropometric data collected between 2008 and 2012 from 7 cohorts in 9 countries, we estimated the proportion of participants with general and central obesity using BMI and waist circumference classifications, respectively, by study site. We used Poisson regression to examine the associations (prevalence ratios) of continuously measured BMI and waist circumference with prevalent diabetes and hypertension by sex. Pooled estimates across studies were computed by sex and age.
Results
31,118 participants aged 20 to 79 years were analyzed. General obesity was highest in South Asian cities and central obesity was highest in South America. The proportion classified with general obesity (range 11% to 50%) tended to be lower than the proportion classified as centrally obese (range 19% to 79%). Every standard deviation higher of BMI was associated with 1.65 and 1.60 times higher probability of diabetes and 1.42 and 1.28 times higher probability of hypertension, for men and women respectively, aged 40–69 years. Every standard deviation higher of waist circumference was associated with 1.48 and 1.74 times higher probability of diabetes and 1.34 and 1.31 times higher probability of hypertension, for men and women respectively, aged 40–69 years. Associations of obesity measures with diabetes were strongest in South Africa among men and in South America among women. Associations with hypertension were weakest in South Africa among both men and women.
Conclusions
BMI and waist circumference were both reasonable predictors of prevalent diabetes and hypertension. Across diverse ethnicities and settings, BMI and waist circumference remain salient metrics of obesity that can identify those with increased cardiovascular risk.
INTRODUCTION
Survey data show that mean body mass index (BMI) increased worldwide by 0.4 kg/m2 per decade between 1980 to 2008, corresponding to a doubling of worldwide obesity prevalence from 6% in 1980 to 12% in 2008 [1,2]. Although the global rising trend in BMI has drawn attention to the problem of over nutrition and anthropometric measures of obesity are simple, inexpensive, and feasible tools to classify cardiovascular health in the absence of costly laboratory tests, the implications of excess weight on cardiovascular disease (CVD), events, and mortality remain unclear. For example, several authors argue that the relation between obesity measures and cardiovascular health and mortality varies by ethnic group (e.g., [3–6]). Which metric of obesity (e.g., BMI, waist circumference) best relates to cardiovascular health also remains an issue (e.g. [7–10]).
In the context of low and middle income countries, resolving these concerns is pressing because the bulk of the knowledge base regarding the relation of obesity and cardiovascular health has been generated from cohorts of composed of select ethnic backgrounds (e.g., [11]) and/or populations in high-income settings (e.g., [12]) that are exposed to different environments compared with residents of LMICs. Important contributions regarding diverse populations in LMICs have come from case-control study designs (e.g., [13]) or large-scale pooling projects relying on relatively older data sources [7]. As such, studying different weight-related measures and how they relate to each other across diverse populations and settings using current data contributes to the discourse regarding appropriate indicators for weight monitoring and control.
Using cross-sectional data collected between 2008 and 2012, we report the prevalence of obesity in four regions. Further, we aimed to address two dimensions of controversy in the weight status literature by investigating whether 1) general (BMI) and central (waist circumference) obesity differentially relate to cardiovascular health; 2) adverse cardiovascular health associated with a given level of obesity was consistent across four low- and middle-income settings.
METHODS
Study design and participants
We used recent cross-sectional data (collected in 2008–2009 in South Africa and in 2010–2012 for other countries) from adult populations with objectively assessed height, weight, and waist measurements residing in 9 LMICs in 4 broad geographic regions: Africa, East Asia, South America, and South Asia. The studies have been previously described in detail [14–19]. Briefly, population-based sampling was used to identify and recruit participants ages 18 and older (exact age inclusion varied by study) in a total of 19 sites in South Africa, China, Argentina, Chile, Peru, Uruguay, Bangladesh, India, and Pakistan. The five provinces sampled in the China study were combined into one group because those sites were selected to be homogenous and represent rural locales in northern China. See Table 1 for a list of populations represented in this study. A total of 36,236 individuals were enrolled at baseline across the studies and included in a harmonized dataset following methodology described in this issue [20]. For this analysis, we restricted to men and women aged 20 to 79 years of at baseline (561 excluded) with complete anthropometric assessments (4,487 excluded), for a total study sample of 31,118.
Table 1.
Sample characteristics
| Region | Site | Sample size | Demographics | Anthropometry | Cardiovascular health indicators | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, years (min-max) | Male, % | Any education, % | BMI, kg/m2 | Waist circumference, cm | FPG, mg/dL | HbA1c, % | Self-reported diabetes, % | SBP, mmHg | DBP, mmHg | Hypertension medication, % | |||
| Africa | Cape Town, South Africa1 | 1,088 | 39.9 (22–75) | 36.4% | 88.8% | 28.9±0.2 | 91.2±0.5 | 94.3±1.2 | n/a | 7.6% | 123.6±0.7 | 81.1±0.4 | 18.2% |
| East Asia | Northern China, China2 | 5,666 | 55.5 (20–79) | 51.2% | 81.7% | 24.3±0.1 | n/a | n/a | n/a | 9.1% | 141.4±0.3 | 86.1±0.2 | 25.3% |
| South America | Bariloche, Argentina3 | 1,987 | 48.0 (30–79) | 47.9% | 99.6% | 28.1±0.1 | 93.6±0.3 | 92.3±0.5 | n/a | 5.4% | 125.1±0.4 | 84.9±0.3 | 16.0% |
| Marcos Paz, Argentina3 | 1,973 | 47.4 (33–78) | 50.3% | 98.4% | 29.7±0.2 | 96.6±0.4 | 100.3±0.9 | n/a | 6.5% | 125.8±0.5 | 80.8±0.3 | 21.1% | |
| Temuco, Chile3 | 1,944 | 47.1 (31–77) | 46.5% | 99.6% | 28.9±0.1 | 96.1±0.3 | 98.7±0.7 | n/a | 9.8% | 123.7±0.5 | 80.8±0.3 | 18.5% | |
| Canelones, Uruguay3 | 1,562 | 48.5 (32–76) | 49.1% | 99.6% | 28.2±0.2 | 97.1±0.4 | 94.0±0.6 | n/a | 9.7% | 126.9±0.5 | 81.1±0.3 | 23.2% | |
| Lima, Peru4 | 1,018 | 50.7 (35–79) | 48.4% | 94.9% | 28.4±0.1 | 92.4±0.4 | 97.0±1.0 | 5.8±0.0 | 6.8% | 115.5±0.5 | 72.6±0.4 | 13.5% | |
| Puno (rural), Peru4 | 554 | 51.1 (35–79) | 46.5% | 93.1% | 25.4±0.2 | 85.9±0.5 | 89.3±0.8 | 5.8±0.0 | 2.0% | 116.0±0.7 | 75.3±0.4 | 2.2% | |
| Puno (urban), Peru4 | 546 | 50.8 (35–79) | 48.8% | 97.8% | 28.0±0.2 | 93.0±0.5 | 95.3±1.2 | 5.9±0.0 | 4.0% | 111.8±0.7 | 71.8±0.4 | 10.3% | |
| Tumbes, Peru4 | 973 | 50.2 (35–79) | 49.2% | 96.5% | 28.5±0.2 | 94.4±0.3 | 103.2±1.3 | 6.1±0.0 | 7.6% | 118.9±0.6 | 73.4±0.4 | 19.3% | |
| South Asia | Dhaka, Bangladesh5 | 1,882 | 49.6 (40–79) | 43.4% | 100% | 24.6±0.1 | 86.8±0.3 | n/a | n/a | 19.1% | 125.1±0.7 | 82.9±0.6 | n/a |
| Matlab, Bangladesh | 1,805 | 51.5 (40–79) | 43.6% | 99.9% | 20.8±0.1 | 73.9±0.2 | n/a | n/a | 5.7% | 115.1±0.5 | 73.5±0.3 | n/a | |
| Chennai, India6 | 3,348 | 38.6 (20–79) | 39.8% | 92.4% | 25.4±0.1 | 83.0±0.3 | 106.0±0.9 | 6.2±0.0 | 16.3% | 121.1±0.4 | 80.6±0.3 | 8.5% | |
| New Delhi, India6 | 3,873 | 41.2 (20–79) | 48.2% | 83.3% | 25.1±0.1 | 86.6±0.2 | 111.2±0.7 | 6.2±0.0 | 11.6% | 124.9±0.3 | 83.4±0.2 | 11.5% | |
| Karachi, Pakistan6 | 2,969 | 37.9 (20–79) | 43.5% | 74.3% | 25.0±0.1 | 86.8±0.3 | 101.4±0.7 | 5.9±0.0 | 8.5% | 118.3±0.4 | 79.2±0.2 | 13.1% | |
n/a, Not available.
Notes: All values are means±SE unless noted as percents.
Data sources were as follows.
Peer, N., Steyn, K., Lombard, C., Gwebushe, N. & Levitt, N. A High Burden of Hypertension in the Urban Black Population of Cape Town: The Cardiovascular Risk in Black South Africans (CRIBSA) Study. PLoS ONE 8, (2013).
Yan, L. L. et al. Population impact of a high cardiovascular risk management program delivered by village doctors in rural China: design and rationale of a large, cluster-randomized controlled trial. BMC Public Health 14, 345 (2014).
Rubinstein, A. L. et al. Detection and follow-up of cardiovascular disease and risk factors in the Southern Cone of Latin America: the CESCAS I study. BMJ Open 1, e000126 (2011).
Miranda, J. J. et al. Addressing geographical variation in the progression of non-communicable diseases in Peru: the CRONICAS cohort study protocol. BMJ Open 2, e000610 (2012).
Alam, D., Chowdhury, A., Siddiquee, A. & Ahmed, S. Prevalence and determinants of Chronic Obstructive Pulmonary Disease (COPD) in Bangladesh. COPD J. Chronic Obstr. Pulm. Dis. (In press).
Nair, M. et al. CARRS Surveillance study: design and methods to assess burdens from multiple perspectives. BMC Public Health 12, 701 (2012).
Obesity metrics and definitions
We examined both general and central obesity, defined using BMI (measured as weight in kilograms divided by the square of height in meters) and waist circumference thresholds, respectively. “General obesity” was defined using WHO International thresholds of BMI > 30, and “overweight” was defined as 25 kg/m2 < BMI ≤ 30 kg/m2 [21]. For ethnic-specific general obesity, we modified the thresholds to define BMI > 25 kg/m2 as obese and BMI > 23 kg/m2 as overweight among East and South Asian participants [4]; classification for all other participants followed international thresholds. “Central obesity” was defined using the International Diabetes Federation recommendations of waist circumference > 94 cm in men of Caucasian and African ancestry and > 80 cm in women of all ethnic backgrounds [22]. For East and South Asian men, central obesity was defined waist circumference > 90 cm [22]. We also considered BMI and waist as continuous (linear) indicators of obesity in regression analyses. The China study did not collect waist circumference data and was excluded from analyses of central obesity.
Cardiovascular health indicators
Diabetes and hypertension status were investigated as indicators of cardiovascular health. Diabetes was defined using laboratory-measured fasting blood glucose (FPG) ≥ 126 mg/dL, current use of diabetes medication, or previous diagnosis of diabetes based on self-report. A participant was classified as having diabetes if s/he had any of the three diabetes indicators. Diabetes classification was based solely on self-report in China and Bangladesh, neither of which had objectively measured diabetes indicators. Hypertension was defined as measured systolic blood pressure (SBP) ≥ 140 mmHg or diastolic blood pressure (DBP) ≥ 90 mmHg or taking hypertension medication. Bangladesh did not include questions regarding use of hypertension medication, so hypertension was defined using measured blood pressure only.
Statistical Analysis
The demographic composition and distribution of anthropometry and cardiovascular health indicators was described by site. Proportions obese were reported by site and sex, and were standardized based on the WHO standard population structure [23]. The proportion underweight as well as the proportion obese in each age group (20–34, 35–49, 50–64, and 65–79 years) was also estimated in each site by sex in supplemental analyses. Estimation by age group was restricted to sites with 20 or more participants in the respective age group.
Examination of the relation between deciles of BMI and waist circumference and diabetes and hypertension indicated linear relationships between exposures and outcomes. We thus treated BMI and waist circumference as continuous variables in further analysis. Both obesity measures were standardized to have mean=0 and standard deviation = 1 to facilitate comparisons of effect size estimates associated with each.
We used Poisson regression models with robust variance estimation to estimate the relative prevalence of each cardiovascular health indicator (i.e., diabetes and hypertension) as a function of each obesity measure (i.e., BMI and waist circumference) [24,25]. All models were estimated by sex and adjusted for age in years and a fixed effect for study site, and accounted for clustering of participants within study sites. Models with interaction terms included respective main effects.
We first estimated a pooled prevalence ratio for the overall sample among participants aged 40–69 years, the age group with the best coverage across studies. We next derived the site-specific association by including an interaction term between site (a 14-level categorical variable) and the obesity measure of interest. To test whether any regional patterning existed in variations across sites, we then estimated a model with an interaction term between region (a 4-level categorical variable) and the obesity measure. Generalized score statistics were used to test statistical difference in measures of associations by site and region.
We also estimated age-specific prevalence ratios by sex. Parallel to our approach above, this was done by including an interaction term between age group (a 4-level categorical variable; participant age of 20–34, 35–49, 50–64, or 65–79 years) and obesity measure. To assess linear trends by age group, we included an interaction term between age group treated as a discrete ordinal variable and the respective obesity measure. A p-value <.05 was considered evidence of a linear trend in prevalence ratios by age group.
As a sensitivity analysis, we also estimated each association of interest separately by study and meta-analyzed those results to produce pooled estimates and estimate measures of heterogeneity across sites [26]. Associations were largely the same, and indicated heterogeneity across the study sites (data not shown; available from the first author).
Statistical analysis was performed using SAS 9.4 Software (Cary, NC).
RESULTS
Table 1 describes the demographic, anthropometric, and cardiovascular profile of participants by study site. The ages of available participants varied from study to study. After age-standardizing to the world population, the lowest mean age of 39 years observed in Chennai, India and highest mean age of 56 years observed in northern China. The proportion of men ranged from 36% in Cape Town, South Africa to 51% in northern China, and the proportion with any education ranged from 74% in Karachi, Pakistan to 100% in urban Dhaka, Bangladesh. Mean (SE) BMI and waist circumference was lowest in rural Matlab, Bangladesh (20.8±0.1kg/m2 and 73.9±0.2 cm, respectively), while BMI was highest in Marcos Paz, Argentina (29.7 kg/m2±0.2) and waist circumference was highest in Canelones, Uruguay (97.1 cm ±0.4). FPG ranged from 89.3 mg/dL (Rural Puno, Peru) to 111.2 mg/dL (New Delhi, India). Hba1c was assessed at only 6 sites, and ranged from mean of 5.8 at both Puno sites to 6.2 in New Delhi and Chennai. Self-reported diabetes was lowest in rural Puno (2%) and highest in Dhaka (19.1%). Mean systolic blood pressure was highest in northern China (141 mmHg), and was lowest in urban Puno (112 mmHg). Rural Puno reported the lowest proportion of hypertension medication (2%) and northern China reported the highest (25%).
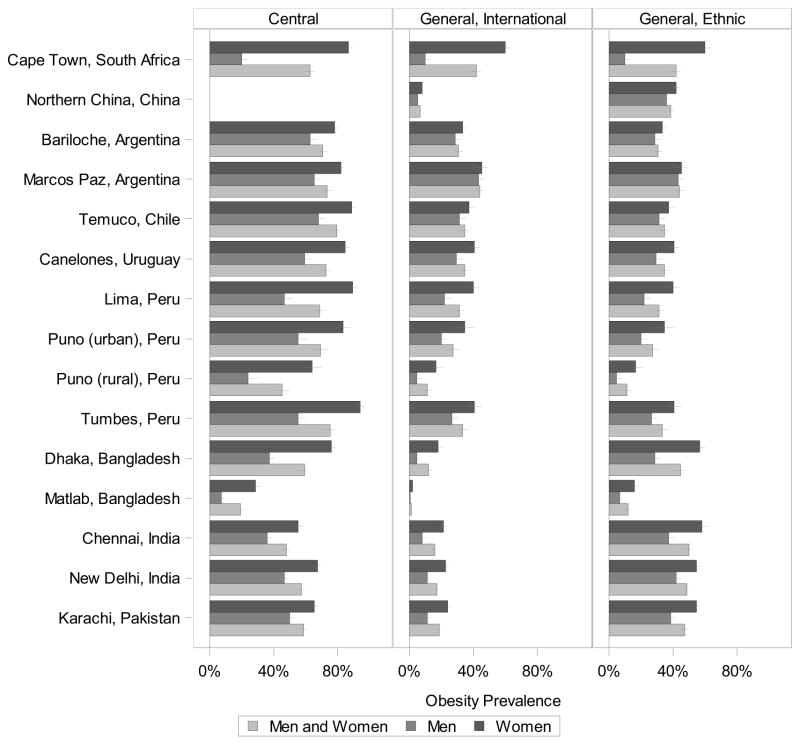
Figure 1 and Supplemental Table 1 report the age-standardized proportion classified as generally obese and centrally obese based on international BMI and waist circumference cut-offs, respectively. For both international and ethnic-specific definitions rural Matlab, Bangladesh had the lowest proportion of general obesity (2% and 12%, respectively). The highest proportion of obesity varied by definition; under the international classification, Marcos Paz, Argentina had the highest obesity (44%), and under the ethnic-specific classification, Chennai, India had the highest obesity (50%). Central obesity was lowest in rural Matlab, Bangladesh (19%) and was highest in Temuco, Chile (79%). In general, the proportion with central obesity was higher than the proportion with general obesity. The proportion of obesity was higher among women compared with men at all sites; in fact, at roughly half the sites, the proportion of obese women was near double that of obese men. Supplemental Table 1 also shows the proportion underweight, which was lower than the proportion obese in most settings. Obesity by age group, sex, and site is reported in Supplemental Table 2.
Figure 1.
Prevalence of central and general obesity by sex. Central obesity is classified based on waist circumference, and general obesity (international and ethnic-specific cut points) is classified based on BMI. The error bars mark the 95% confidence interval of the prevalence.
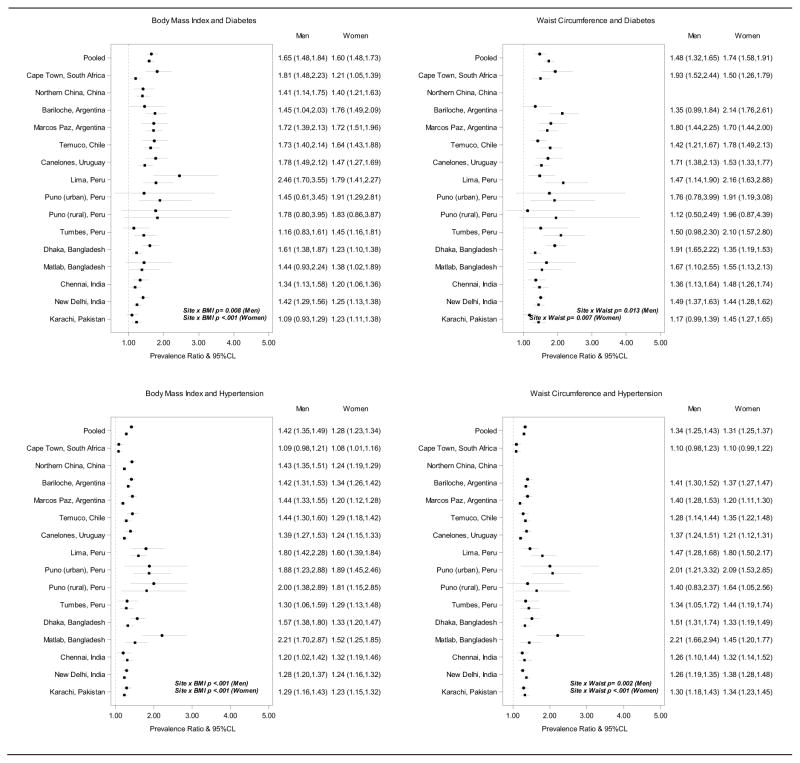
Figure 2, top panel, shows the prevalence ratios (PR) of diabetes by site and sex and for the pooled sample among participants aged 40–69 years. For every 1 SD higher BMI, we noted a 65% and 60% higher pooled prevalence of diabetes for men and women, respectively (top-left panel). The interaction term between site and BMI was statistically significant among men (p=.008) and women (p<.001), and site-specific PR point estimates ranged from 1.09 to 2.46 among men and 1.20 to 1.91 among women. Every 1 SD higher waist circumference was associated with 48% and 74% higher pooled prevalence of diabetes among men and women, respectively (top-right panel). Associations between waist circumference and diabetes also statistically differed across sites (p=.013 among men and p=.007 among women), and site-specific PR point estimates ranged from 1.17 to 1.93 among men and 1.44 to 2.16 among women.
Figure 2.
Associations between BMI, kg/m2, (left panels) and waist circumference, cm, (right panels) and prevalent cardiovascular risk factors among adults aged 40–69 years. The circle marks the prevalence ratio estimate for men and the square the estimate for women; the dashed vertical line shows the null value. BMI and waist circumference were standardized to have mean=0 and SD=1 in order to compare.
Figure 1, bottom panel, shows the PRs of hypertension by site and sex and for the pooled sample among participants aged 40–69 years. Every 1 SD higher BMI was associated a 42% and 28% higher pooled prevalence of hypertension for men and women, respectively. Associations between BMI and hypertension statistically differed across sites (p<.001 for men and women), and site-specific PR point estimates ranged from 1.09 to 2.21 among men and 1.08 to 1.89 among women. Every 1 SD higher waist circumference was associated a 34% and 31% higher pooled prevalence of hypertension for women and men. Site-specific prevalence ratios for hypertension by SD of waist circumference also varied by site (p=.002 for men and p<.001 for women), with point estimates ranging from 1.10 to 2.21 among men and 1.10 to 2.09 among women.
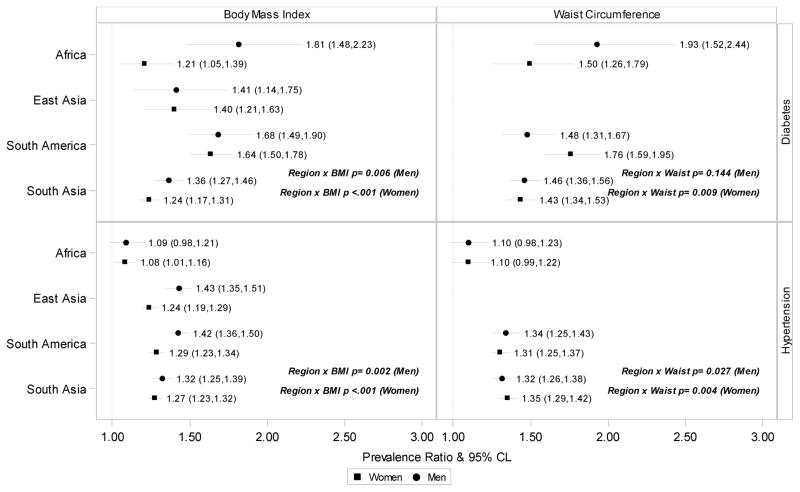
Figure 3 shows prevalence ratios by region. With the exception of the association between waist circumference and diabetes among men, interactions between obesity measures and region were statistically significant. Both BMI and waist circumference associations with diabetes were highest in South Africa among men and highest in South America among women. Associations of both BMI and waist circumference with hypertension, on the other hand, were lowest in South Africa. Associations with hypertension were relatively similar in East Asia, South America, and South Asia.
Figure 3.
Associations of BMI, kg/m2, and waist circumference, cm, with diabetes and hypertension in each region. The dashed vertical line shows the null value.
Figure 4 reports the sex- and age-stratified associations between BMI and waist circumference exposures with prevalent diabetes and hypertension. With respect to the association between obesity measures and diabetes, we observed that associations were generally strongest in the youngest age group but were still substantial and significant in older age groups; there was a linear trend by age for BMI and diabetes among men and a linear trend by age for waist circumference and diabetes among women. PR point estimates were higher for women compared with men at ages 20–34 years for both obesity measures and diabetes, but did not statistically differ in this or any other age group. Similarly, the associations between both obesity measures and hypertension were attenuated in older relative to younger age groups, and we observed a statistically significant linear trend in the PR estimates by age in both men and women. BMI-hypertension associations were slightly stronger in men compared with women in each age group; there was no consistent sex difference in associations between waist circumference and hypertension, however.
Figure 4.
Associations of BMI, kg/m2, and waist circumference, cm, with diabetes and hypertension in each age group. The p-value is from the test for a linear trend across age groups. The dashed vertical line shows the null value.
DISCUSSION
Based on contemporary data collected across geographically and ethnically diverse low- and middle income settings, we examined BMI and waist circumference to assess general and central obesity and investigate their respective associations with diabetes and hypertension. Despite lower BMI in South Asian participants, South Asian cities tended to have the highest proportion of general obesity following ethnic-specific classification, ranging from 45% to 50%. In contrast, central obesity was highest in urban South American sites, ranging from 72% to 79%. At each site, the proportion with central obesity (19% to 79%) was higher than the proportion with general obesity (12% to 50% based on ethnic classifications) with the exception of Chennai, India.
Regarding whether general and central obesity differentially relate to cardiovascular health, we found that the magnitude of pooled PRs for each of diabetes and hypertension did not consistently differ from one another; among men, there was indication that BMI had a stronger association with both risk factors and among women that waist circumference may be a stronger correlate of diabetes. Generally, the PRs for diabetes tended to be larger than the PRs for hypertension. Regarding the consistency of associations across sites, obesity measures were positively associated with diabetes and hypertension at all sites (as expected), but PRs did statistically differ by site.
The tendency for lower proportions of general obesity in rural locations in East Asia, South Asia, and South (with the exception of Hebei, China) is consistent with previous studies [27,28]. The fact that the prevalence of general obesity was greater than 10% even in rural settings, however, may reflect the trend of rising obesity observed even in rural areas across LMICs [27,29]. Similar to previous studies, we found that associations between obesity measures and cardiovascular health indicators were attenuated with older age. For example, in a large study of Asians and Europeans, the associations between BMI and incident diabetes were higher at younger relative to older ages [30].
The regional variations in obesity associations are intriguing but must be interpreted cautiously. The studies we drew upon were not designed to be representative of their respective nations let alone regions, and further investigation is needed to confirm regional/country patterns we observed; all of Africa was represented by a single study in South Africa. In addition to representing diverse ethnic groups, the studies were situated in varying levels of urbanization, and participants are expected to be socioeconomically diverse. Because the studies were not designed to collect comparable measures of socioeconomic status or contextual factors, we were unable to statistically account for heterogeneity in these factors across sites. Furthermore, the age range of participants differed across studies; this may also contribute to the observed regional variation.
Other limitations of our study include the cross-sectional design, so the direction of observed associations cannot be confirmed. Further, the studies were not intended for all of the subgroup analyses – such as age by sex estimates – which we performed. All the same, we used indirect age standardization to minimize any such differences. We lacked laboratory-assessed fasting plasma glucose to classify undiagnosed diabetes status in China and Bangladesh; measurement error may affect the validity of obesity associations with diabetes in those settings and comparability of results with other studies with laboratory assessments. Notwithstanding, a major strength of this study is that Africa and South America data are not often included in multi-ethnic studies of obesity or its cardiovascular correlates. We also use very recent data to describe obesity, which we know to be a dynamic phenomenon at the population-level.
Whilst the burden of NCDs is becoming a higher priority for LMIC health agendas, it is reasonable to acknowledge that the individual settings are accompanied by nuances and challenges, including different pace of socioeconomic development, urbanization and social inequalities across regions. Known exposures that contribute to major risk factors and CV outcomes have been well described, yet, our study adds to the literature that the magnitude of associations appears to differ by place [31]. With the recognition that context matters, it is all the more important to question the geographical and temporal stability of apparently established relationships between obesity and cardiovascular health. Our study is well positioned to provide a clear message on this topic, thus contributing information for evidence-based prioritization of policy in a realistic scenario of scarcity of resources. For example, our data suggest that a large fraction of the diabetes burden among men in South Africa and both men and women in South America is related to obesity. Future investigation using these data may quantify burdens of cardiovascular risk factors associated with obesity across these settings.
In summary, BMI and waist circumference can provide useful information to classify the presence of hypertension and diabetes in the population. We observed that both obesity measures may have larger magnitudes of associations with diabetes than hypertension and that these associations also tended to be higher in the younger age groups. As contemporary data to investigate obesity and biomarkers of cardiovascular health become increasingly available, additional analyses to answer clinically relevant questions may be undertaken.
Supplementary Material
Acknowledgments
This research was supported in part by Federal funds from the United States National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN268200900026C and the United Health Group (Minneapolis, MN, USA). Additional support was received from the Fogarty International Centre and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (grant number 1 D43 HD065249).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants. The Lancet. 2011;377:557–67. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stevens GA, Singh GM, Lu Y, Danaei G, Lin JK, Finucane MM, et al. National, regional, and global trends in adult overweight and obesity prevalences. Popul Health Metr. 2012;10:22. doi: 10.1186/1478-7954-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Y, Copeland WK, Vedanthan R, Grant E, Lee JE, Gu D, et al. Association between body mass index and cardiovascular disease mortality in east Asians and south Asians: pooled analysis of prospective data from the Asia Cohort Consortium. BMJ. 2013;347 doi: 10.1136/bmj.f5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–63. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 5.Deurenberg P, Deurenberg-Yap M, Guricci S. Asians are different from Caucasians and from each other in their body mass index/body fat per cent relationship. Obes Rev. 2002;3:141–6. doi: 10.1046/j.1467-789X.2002.00065.x. [DOI] [PubMed] [Google Scholar]
- 6.Kalk WJ, Joffe BI, Sumner AE. The waist circumference of risk in black South african men is lower than in men of European ancestry. Metab Syndr Relat Disord. 2011;9:491–5. doi: 10.1089/met.2011.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prospective Studies Collaboration. Body-mass index and cause-specific mortality in 9003000 adults: collaborative analyses of 57 prospective studies. The Lancet. 2009;373:1083–96. doi: 10.1016/S0140-6736(09)60318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bodicoat DH, Gray LJ, Henson J, Webb D, Guru A, Misra A, et al. Body Mass Index and Waist Circumference Cut-Points in Multi-Ethnic Populations from the UK and India: The ADDITION-Leicester, Jaipur Heart Watch and New Delhi Cross-Sectional Studies. PLoS ONE. 2014;9:e90813. doi: 10.1371/journal.pone.0090813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crowther NJ, Norris SA. The current waist circumference cut point used for the diagnosis of metabolic syndrome in sub-Saharan African women is not appropriate. PLoS One. 2012;7:e48883. doi: 10.1371/journal.pone.0048883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hou X, Lu J, Weng J, Ji L, Shan Z, Liu J, et al. Impact of Waist Circumference and Body Mass Index on Risk of Cardiometabolic Disorder and Cardiovascular Disease in Chinese Adults: A National Diabetes and Metabolic Disorders Survey. PLoS ONE. 2013;8:e57319. doi: 10.1371/journal.pone.0057319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’Agostino RB, Sr, Grundy S, Sullivan LM, Wilson P for the CHD Risk Prediction Group. Validation of the framingham coronary heart disease prediction scores: Results of a multiple ethnic groups investigation. JAMA. 2001;286:180–7. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- 12.Danesh J, Saracci R, Berglund G, Feskens E, Overvad K, Panico S, et al. EPIC-Heart: the cardiovascular component of a prospective study of nutritional, lifestyle and biological factors in 520,000 middle-aged participants from 10 European countries. Eur J Epidemiol. 2007;22:129–41. doi: 10.1007/s10654-006-9096-8. [DOI] [PubMed] [Google Scholar]
- 13.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–52. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 14.Miranda JJ, Bernabe-Ortiz A, Smeeth L, Gilman RH, Checkley W, Group CCS. Addressing geographical variation in the progression of non-communicable diseases in Peru: the CRONICAS cohort study protocol. BMJ Open. 2012;2:e000610. doi: 10.1136/bmjopen-2011-000610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nair M, Ali MK, Ajay VS, Shivashankar R, Mohan V, Pradeepa R, et al. CARRS Surveillance study: design and methods to assess burdens from multiple perspectives. BMC Public Health. 2012;12:701. doi: 10.1186/1471-2458-12-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rubinstein AL, Irazola VE, Poggio R, Bazzano L, Calandrelli M, Zanetti FTL, et al. Detection and follow-up of cardiovascular disease and risk factors in the Southern Cone of Latin America: the CESCAS I study. BMJ Open. 2011;1:e000126. doi: 10.1136/bmjopen-2011-000126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yan LL, Fang W, Delong E, Neal B, Peterson ED, Huang Y, et al. Population impact of a high cardiovascular risk management program delivered by village doctors in rural China: design and rationale of a large, cluster-randomized controlled trial. BMC Public Health. 2014;14:345. doi: 10.1186/1471-2458-14-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peer N, Steyn K, Lombard C, Gwebushe N, Levitt N. A High Burden of Hypertension in the Urban Black Population of Cape Town: The Cardiovascular Risk in Black South Africans (CRIBSA) Study. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0078567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alam D, Chowdhury A, Siddiquee A, Ahmed S. Prevalence and determinants of Chronic Obstructive Pulmonary Disease (COPD) in Bangladesh. COPD J Chronic Obstr Pulm Dis. doi: 10.3109/15412555.2015.1041101. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Health, Lung, and Blood Institute-UnitedHealth Group Global Health Initiative Investigators. Tackling Heart and Lung Diseases in Low- and Middle-income Countries: Overview of the National Health, Lung, and Blood Institute-UnitedHealth Group Global Health Initiative and Centers of Excellence Program. Glob Heart. 2015 [Google Scholar]
- 21.WHO. WHO | Physical status: the use and interpretation of anthropometry. WHO; 1995. [accessed January 9, 2014]. http://www.who.int/childgrowth/publications/physical_status/en/ [Google Scholar]
- 22.The IDF Consensus Worldwide Definition of the Metabolic Syndrome 2006.
- 23.Ahmad O, Boschi-Pinto C, Lopez A, Murray C, Lozano R, Inoue M. Age Standardisation of Rates: a New WHO Standard. World Health Organization; 2001. [Google Scholar]
- 24.Barros AJ, Hirakata VN. Alternatives for logistic regression in cross-sectional studies: an empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol. 2003;3:21. doi: 10.1186/1471-2288-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spiegelman D, Hertzmark E. Easy SAS Calculations for Risk or Prevalence Ratios and Differences. Am J Epidemiol. 2005;162:199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 26.Takkouche B, Khudyakov P, Costa-Bouzas J, Spiegelman D. Confidence Intervals for Heterogeneity Measures in Meta-analysis. Am J Epidemiol. 2013:kwt060. doi: 10.1093/aje/kwt060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balarajan Y, Villamor E. Nationally Representative Surveys Show Recent Increases in the Prevalence of Overweight and Obesity among Women of Reproductive Age in Bangladesh, Nepal, and India. J Nutr. 2009;139:2139–44. doi: 10.3945/jn.109.112029. [DOI] [PubMed] [Google Scholar]
- 28.Hou X. Urban—Rural Disparity of Overweight, Hypertension, Undiagnosed Hypertension, and Untreated Hypertension in China. Asia Pac J Public Health. 2008;20:159–69. doi: 10.1177/1010539507312306. [DOI] [PubMed] [Google Scholar]
- 29.Jaacks LM, Slining MM, Popkin BM. Recent Underweight and Overweight Trends by Rural–Urban Residence among Women in Low- and Middle-Income Countries. J Nutr. 2015;145:352–7. doi: 10.3945/jn.114.203562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh GM, Danaei G, Farzadfar F, Stevens GA, Woodward M, Wormser D, et al. The Age-Specific Quantitative Effects of Metabolic Risk Factors on Cardiovascular Diseases and Diabetes: A Pooled Analysis. PLoS ONE. 2013;8:e65174. doi: 10.1371/journal.pone.0065174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ebrahim S, Pearce N, Smeeth L, Casas JP, Jaffar S, Piot P. Tackling Non-Communicable Diseases In Low- and Middle-Income Countries: Is the Evidence from High-Income Countries All We Need? PLoS Med. 2013;10:e1001377. doi: 10.1371/journal.pmed.1001377. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.