Abstract
Objectives
To estimate prevalence and determinants of potentially inappropriate prescribing (PIP) among US older adults using 2012 Beers criteria.
Design
Retrospective cohort study in a random national sample of Medicare beneficiaries.
Setting
2007–2012 fee-for-service Medicare beneficiaries.
Participants
US population aged >65 years with Part A, B and D enrollment in at least 1 month during a calendar year (N=38,250 patients; 1,308,116 observations)
Measurement
We used 2012 Beers criteria to estimate the prevalence of ≥1 PIP within each calendar month and over a 12-month period using data on diagnoses or conditions present in the previous 12 months. To account for the dependence of multiple monthly observations of a single person when estimating 95% confidence intervals (CI) we used generalized estimating equations. We used logistic regression to identify independent determinants of PIP.
Results
The point-prevalence of PIP decreased from 37.6% (95%CI: 37.0–38.1) in 2007 to 34.2% (95%CI: 33.6–34.7) in 2012, with a statistically significant 2% (95%CI: 1–3%) decline per year assuming a linear trend. One year period-prevalence declined from 64.9% in 2007 to 56.6% in 2012. The strongest predictor of PIP was the number of drugs dispensed. Individuals aged 70 years or older and those seen by a geriatrician were less likely to receive PIP.
Conclusion
From 2007 to 2012, the prevalence of PIP in US older adults decreased according to 2012 Beers criteria but remains high, still affecting a third each month and more than a half over 12 months. The number of dispensed prescription could be used to target future interventions.
Keywords: potentially inappropriate prescribing, Beers criteria, older adults, database study, pharmacoepidemiology, Medicare
INTRODUCTION
Aging is associated with the development of multiple chronic diseases and with increasing use of long-term prescription medications to treat these conditions. Potentially Inappropriate Prescribing (PIP) is defined as the use of drugs that have a high risk of adverse drug events (ADE) relative to their potential benefit, particularly when safer or more effective alternative therapies are available for the same condition (1). Studies evaluating the consequences of PIP in older adults have demonstrated that PIP leads to an increased risk for adverse clinical outcomes, jeopardizing the therapeutic objectives (2–6). PIP is considered a major public health problem, given its negative impact on health outcomes, hospitalizations, healthcare utilization, cost and mortality (2–6).
Increasing interest in safer and more effective treatment in older adults has led to the development of prescribing guidelines that support clinical decisions when choosing therapies. Among these, the 2003 Beers criteria are arguably the most widely used in clinical practice and research (7–10). Criticism of the 2003 Beers criteria led to a major revision in 2012 (11,12). The 2012 Beers criteria established an explicit list of unsafe drugs and drug combinations that should be avoided, and also includes a list of drug-disease interactions (DDI) where the use of some drugs should be avoided in patients with these diseases. Additionally, the 2012 revision included a list of drugs that should be used with caution in older adults (11).
A recently published study using a subset of 2012 Beers criteria documented an annual PIP prevalence of 42.6% in US community-dwelling older adults (13). To our knowledge, there are no published studies examining the prevalence of PIP using the complete version of the 2012 Beers criteria. Therefore, we examined the PIP point-prevalence and 12-month period prevalence in older adults using the 2012 Beers criteria, and determined time-trends and factors associated with PIP.
METHODS
Using a random sample of Medicare fee-for-service claims and enrollment data, we constructed a cohort containing one record per Medicare beneficiary per month between 2007 and 2012 in which they utilized their Medicare Part D benefit and were continuously enrolled in Medicare Parts A and B for the 12 months prior.
PIP was defined according to 2012 Beers criteria, based on the list of medications and medication classes deemed (11) to be inappropriate for use in older patients. The operational definition of PIP for this study used all categories of inappropriate prescribing (except for insulin dosed on a sliding scale) and the list of drugs to be used with caution included in the 2012 Beers criteria.
We defined drug classes based on Anatomical Therapeutic Chemical (ATC) codes and a list of generic names. We then used an ATC to national drug code (NDC) crosswalk and searched generic names to identify all Part D claims for each drug class identified in the 2012 Beers criteria. Daily dose was estimated based on number of pills dispensed, strength, and days supplied and was used when the medication’s inappropriate usage definition was defined by excess dosage. Long-term use was defined as more than one month of use based on either a dispensing of a refill or prescriptions with >30 days of supply. Potential DDI was defined by examining diagnosis codes from Part A & B claims during the 12 months preceding the month of the prescription fill.
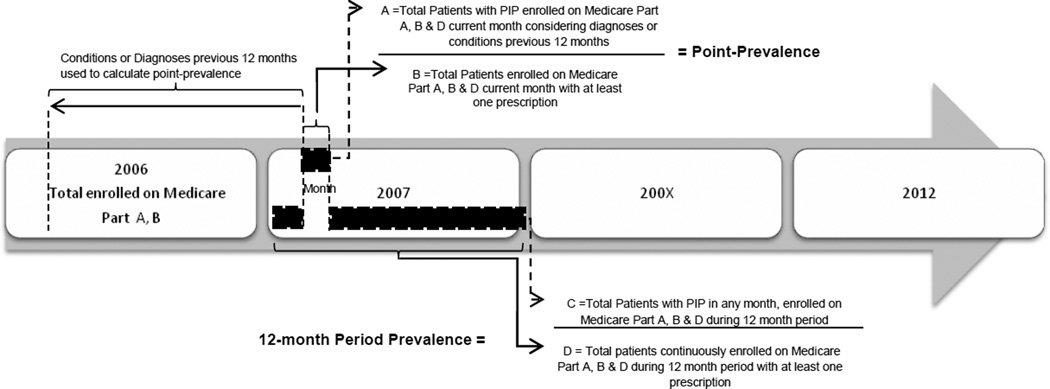
We defined the point-prevalence of PIP as the total number of older adults who filled 1 or more inappropriate prescription divided by the total number of older adults with at least one prescription during the calendar month (Figure 1). We defined the 12-month period prevalence as the number of older adults with PIP in at least one month during the calendar year divided by the total number of adults with at least one prescription during the calendar year.
Figure 1.
Study design
In order to compare the most common PIP according to 2003 Beers criteria and 2012 Beers criteria we performed additional analyses using the full list of drugs and conditions mentioned for each version.
For each person, we also defined the following potential risk factors for PIP: individual characteristics (age, sex, race, region and medical conditions mentioned in Charlson Comorbidity Index), and health care utilization (number of distinct generic drugs filled each month, and number of emergency department visits, outpatient visits, and hospital admissions and physician specialties encountered during the previous 12 months).
We used logistic models and generalized estimating equations (GEE) with an independent correlation structure to account for the dependence of multiple monthly observations of a single person to estimate 95% confidence intervals (CI). We then fit bivariable and multivariable models to examine independent determinants of PIP, as measured using point prevalence. All analyses were performed using SAS version 9.3 (SAS Institute, Inc., Cary, NC).
The study’s protocol was approved by the Institutional Review Board of the Gillings School of Global Public Health, University of North Carolina at Chapel Hill, North Carolina, USA.
RESULTS
The study sample included 1,308,116 observations from 38,250 patients. The mean ± SD age was 77.5±7.8 years (38.2% of them were octogenarians), 65.9% were women, and 84.9% were white. (Table 1) The most common Charlson diagnoses or conditions during the previous 12 months were chronic pulmonary disease (35.5%) and diabetes mellitus without complications (35.0%). Polypharmacy defined as use of ≥ 5 drugs was found in 38.6% of the patients and 8.6% of the sample was taking 10 or more drugs (mean 4.2 ± 3.6). During the previous 12 months, 34.3% and 23.4% of patients had at least one emergency room visit or required hospitalization, respectively.
Table 1.
Sample Characteristics of US Older Adults between 2007 and 2012
| Characteristic | 2007 N=210,878 (23,000 benes) % |
2008 N=212,813 (23,188 benes) % |
2009 N=213,759 (23,214 benes) % |
2010 N=214,356 (23,469 benes) % |
2011 N=221,730 (24,420 benes) % |
2012 N=234,580 (26,062 benes) % |
Overall N=1,308,116 (38,250 benes) % |
|---|---|---|---|---|---|---|---|
| Sex, Male | 32.5 | 33.0 | 33.7 | 34.5 | 34.9 | 35.8 | 34.1 |
| Age, mean(SD) | 77.6(7.7) | 77.6(7.8) | 77.6(7.9) | 77.5(7.9) | 77.4(7.9) | 77.3(7.8) | 77.5(7.8) |
| Age Group (years) | |||||||
| 66–69 | 18.0 | 17.6 | 18.1 | 18.1 | 18.6 | 18.7 | 18.2 |
| 70–74 | 22.4 | 23.1 | 23.2 | 23.8 | 24.2 | 25.0 | 23.6 |
| 75–79 | 21.2 | 20.3 | 19.9 | 19.4 | 19.4 | 19.6 | 20.0 |
| 80–84 | 18.1 | 18.5 | 17.9 | 17.7 | 17.2 | 16.3 | 17.6 |
| 85+ | 20.3 | 20.5 | 20.9 | 21.0 | 20.7 | 20.3 | 20.6 |
| Race | |||||||
| White | 84.8 | 85.0 | 85.0 | 84.9 | 84.7 | 84.8 | 84,9 |
| Afro-American | 8.8 | 8.2 | 8.2 | 8.2 | 8.4 | 8.4 | 8.4 |
| Asian | 2.3 | 2.4 | 2.4 | 2.6 | 2.5 | 2.4 | 2.4 |
| Hispanic | 2.6 | 2.6 | 2.6 | 2.6 | 2.5 | 2.3 | 2.5 |
| Other | 1.1 | 1.3 | 1.3 | 1.3 | 1.4 | 1.4 | 1.3 |
| NAN | 0.4 | 0.4 | 0.4 | 0.4 | 0.3 | 0.3 | 0.4 |
| Unknown | 0.1 | 0.1 | 0.1 | 0.1 | 0.2 | 0.3 | 0.1 |
| Region | |||||||
| South | 39.5 | 39.3 | 39.3 | 39.3 | 39.3 | 39.1 | 39.3 |
| North Central | 25.3 | 24.8 | 24.6 | 24.5 | 24.4 | 23.9 | 24.6 |
| Northeast | 18.4 | 18.8 | 18.6 | 18.5 | 18.4 | 19.3 | 18.7 |
| West | 16.5 | 16.8 | 17.2 | 17.5 | 17.6 | 17.4 | 17.2 |
| Unknown | 0.3 | 0.3 | 0.3 | 0.2 | 0.2 | 0.2 | 0.3 |
| Charlson Comorbidities * | |||||||
| Chronic Pulmonary Disease | 35.0 | 35.0 | 35.4 | 35.8 | 36.1 | 35.7 | 35.5 |
| DM without complications | 33.4 | 33.9 | 34.7 | 35.5 | 36.0 | 36.1 | 35.0 |
| Peripheral Vascular Disease | 21.6 | 21.4 | 22.0 | 22.6 | 22.4 | 22.0 | 22.0 |
| CVD | 19.5 | 19.4 | 19.6 | 19.9 | 19.5 | 19.2 | 19.5 |
| CHF | 19.9 | 19.0 | 18.8 | 18.7 | 18.3 | 17.4 | 18.7 |
| Cancer | 14.0 | 13.9 | 14.1 | 14.7 | 14.9 | 14.9 | 14.4 |
| Renal Disease | 9.7 | 10.2 | 11.4 | 12.8 | 13.9 | 14.5 | 12.2 |
| DM with chronic complications | 10.2 | 10.6 | 11.2 | 11.8 | 11.9 | 12.3 | 11.3 |
| Dementia | 8.4 | 8.4 | 8.7 | 8.7 | 8.5 | 8.3 | 8.5 |
| MI | 6.2 | 5.9 | 5.9 | 6.1 | 6.1 | 6.3 | 6.1 |
| Connective Tissue/Rheumatic Disease | 5.1 | 5.3 | 5.7 | 5.6 | 5.7 | 5.8 | 5.5 |
| Mild Liver Disease | 4.4 | 4.6 | 4.7 | 4.8 | 5.0 | 4.9 | 4.7 |
| Peptic Ulcer Disease | 2.7 | 2.4 | 2.3 | 2.3 | 2.3 | 2.1 | 2.3 |
| Metastatic Carcinoma | 1.8 | 1.7 | 1.6 | 1.7 | 1.8 | 1.8 | 1.7 |
| Paraplegia and Hemiplegia | 0.7 | 0.7 | 0.7 | 0.7 | 0.7 | 0.6 | 0.7 |
| Moderate/Severe Liver Disease | 0.3 | 0.3 | 0.4 | 0.3 | 0.5 | 0.4 | 0.4 |
| AIDS/HIV | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Healthcare Utilization * | |||||||
| Prescription Drug Use** | |||||||
| Polypharmacy (≥ 5 drugs) | 39.8 | 39.8 | 39.3 | 38.9 | 37.9 | 36.7 | 38.6 |
| # of Rx Fills in Month | |||||||
| 1–2 | 32.7 | 33.1 | 33.8 | 34.3 | 35.2 | 36.6 | 34.3 |
| 3–4 | 27.5 | 27.1 | 26.9 | 26.8 | 26.8 | 26.7 | 27.1 |
| 5–9 | 31.1 | 31.0 | 30.6 | 30.2 | 29.4 | 28.6 | 30.0 |
| 10+ | 8.7 | 8.8 | 8.8 | 8.7 | 8.5 | 8.1 | 8.6 |
| # of Rx Fills, mean(SD) | 4.3(3.6) | 4.2(3.6) | 4.1(3.6) | 4.1(3.6) | 4.0(3.6) | 3.9(3.6) | 4.2(3.6) |
| Any Outpatient Office Visits | 92.9 | 92.8 | 93.0 | 93.6 | 93.9 | 93.8 | 93.3 |
| # Outpatient Office Visits, mean(SD) | 8.4(7.3) | 8.3(7.4) | 8.5(7.4) | 8.9(7.7) | 9.2(7.9) | 9.1(7.9) | 8.8(7.6) |
| Any Emergency Visit | 34.6 | 33.5 | 34.3 | 34.2 | 34.5 | 34.7 | 34.3 |
| # of Emergency Visits, mean(SD) | 0.7(1.5) | 0.7(1.5) | 0.7(1.5) | 0.7(1.6) | 0.7(1.6) | 0.7(1.6) | 0.7(1.5) |
| Any Hospital Admission | 24.8 | 23.5 | 23.9 | 23.2 | 23.0 | 22.0 | 23.4 |
| # of Hospital Admissions, mean(SD) | 0.5(1.3) | 0.5(1.2) | 0.5(1.2) | 0.5(1.2) | 0.5(1.2) | 0.5(1.1) | 0.5(1.2) |
| # of Prescribers, mean(SD) | 1.6(0.9) | 1.6(0.9) | 1.6(0.9) | 1.6(0.9) | 1.6(0.9) | 1.6(0.9) | 1.6(0.9) |
| # of Prescribers in Month | |||||||
| 1 | 58.7 | 58.4 | 57.9 | 57.6 | 57.1 | 56.5 | 57.7 |
| 2 | 27.1 | 27.0 | 27.3 | 27.4 | 27.6 | 27.7 | 27.4 |
| 3+ | 14.2 | 14.5 | 14.8 | 15.0 | 15.3 | 15.9 | 15.0 |
| # of Prescriber Specialties, mean(SD) | 1.4(0.7) | 1.4(0.7) | 1.4(0.7) | 1.4(0.7) | 1.4(0.7) | 1.4(0.7) | 1.4(0.7) |
| # of Prescribers Specialties in Month | |||||||
| 1 | 65.3 | 65.1 | 64.7 | 64.7 | 64.0 | 63.6 | 64.5 |
| 2 | 27.0 | 27.2 | 27.7 | 27.8 | 28.4 | 28.7 | 27.8 |
| 3+ | 7.7 | 7.8 | 7.6 | 7.5 | 7.7 | 7.7 | 7.7 |
| Prescriber Specialty * | |||||||
| GP/FP/Internist | 81.1 | 81.1 | 83.0 | 84.4 | 85.5 | 85.7 | 83.6 |
| Geriatrician | 1.5 | 2.4 | 2.4 | 2.4 | 2.3 | 2.4 | 2.4 |
| Other | 42.9 | 44.5 | 48.2 | 49.8 | 52.4 | 55.0 | 49.0 |
Benes: beneficiaries; * in 12 months prior; ** in the current month; NAN: North American Native; Rx: prescription; SD: Standard Deviation; #: Number; GP: General Practitioner; FP: Family Practice; DM: Diabetes Mellitus; CVD: Cerebrovascular Disease; CHF: Congestive Heart Failure; MI: Myocardial Infarction; AIDS/HIV: Acquired Immunodeficiency Syndrome/Human Immunodeficiency Virus infection.
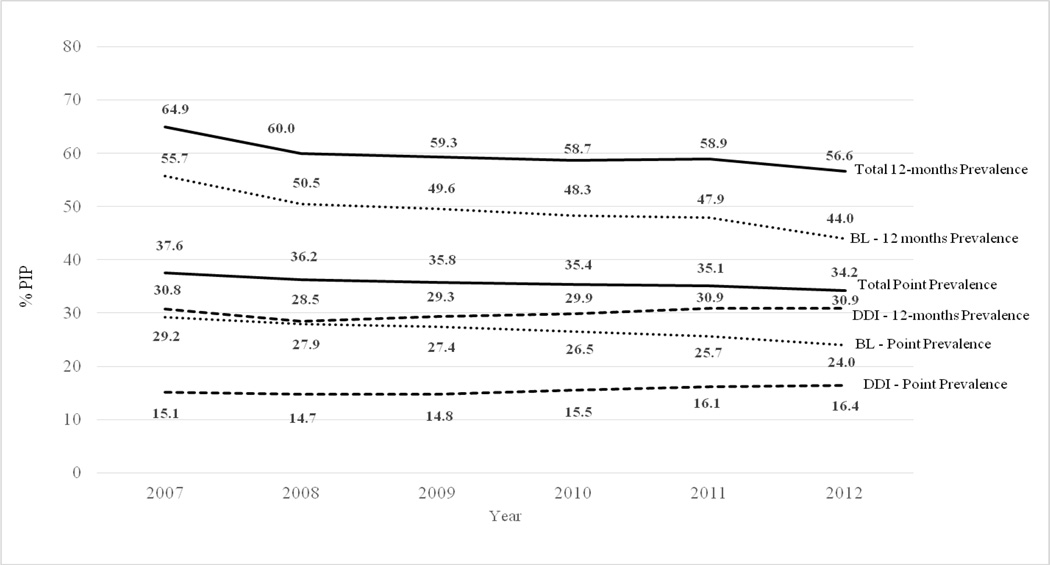
The point-prevalence of PIP decreased from 37.6% (95%CI 37.0–38.1) in 2007 to 34.2% (95%CI 33.6–34.7) in 2012, (Figure 2) with a statistically significant 2% (95%CI: 1–3%) decline per year from 2007 through 2012 assuming a linear trend. The 12-month period prevalence of PIP decreased from 64.9% (95%CI 64.1–65.8) in 2007 to 56.6% (95%CI 55.9–57.4) in 2012. In 2012, DDI accounted for 16.4% of the point prevalence of PIP and 30.9% of the 12-month prevalence.
Figure 2.
Point-Prevalence and 12-Month Prevalence of Potentially Inappropriate Prescribing among US Medicare Older Adults between 2007 and 2012 According to 2012 Beers Criteria
PIP: Potentially Inappropriate Prescribing; Point prevalence: defined as PIP prevalence in the current month; 12-month prevalence: defined as PIP prevalence in the previous 12 months. BL: Beers List; defined as potentially inappropriate prescriptions based on drug choice, dosage or duration of use. DDI: Drug-Disease Interaction; defined as potentially inappropriate prescriptions based on drug-disease interactions. Precision (95%CI) of all estimates within +/− 1 percentage point.
The most frequent PIP according to 2012 Beers criteria based on drug choice or dosing were digoxin in doses >0.125 mg/day (5.0%), glyburide (2.8%) and estrogen (2.6%). (Table 2) The most frequent PIP among DDI criteria were medications inducing or worsening delirium (5.4%), followed by drugs inducing fall and fractures (4.9%) such as anticholinergics and sedatives. In contrast, using the 2003 Beers criteria, the most common PIP based on drug choice or dosing criteria were propoxyphene (2.4%), oral estrogen (2.1%) and clonidine (2.1%); similar to 2012 Beers criteria, anticholinergics and psychotropic drugs use among patients with cognitive impairment were the most frequent DDI criteria detected.
Table 2.
The 10 Most Common Potentially Inappropriate Prescribing Based on Drug Choice or Dosing and Drug-Disease Interaction, Detected Between 2007–2012 According to Beers Criteria 2003 and 2012
| 2012 Beers Criteria | 2003 Beers Criteria | ||
|---|---|---|---|
| Potentially Inappropriate drug choice or dosing |
PIP (%) | PIP (%) | |
| First | Digoxin doses >0.125 mg/d (5.0%) | Propoxyphene (2.4%) | |
| Second | Glyburide (2.8%) | Estrogen oral (2.1%) | |
| Third | Estrogen with or without progestins (2.6%) | Clonidine (2.1%) | |
| Fourth | Spironolactone >25 mg/d (2.4%) | Amitriptyline (1.7%) | |
| Fifth | Amitriptyline (1.8%) | Doxazosin (1.6%) | |
| Drug-Disease Interaction | |||
| First | Delirium - All TCAs, Acths, BZD, chlorpromazine, corticosteroids, H2-receptors antagonists, meperidine, sedative hypnotics, thioridazine (5.4%) | Cognitive Impairment- Barbiturates, Acths, Antispasmodics and muscle relaxants, CNS stimulants (2.0%) | |
| Second | History of falls or fractures - Anticonvulsants, antipsychotics, BZD, non-BZD hypnotics, TCA, SSRIs (4.9%) | Chronic Constipation- CCBs, Acths, and TCAs (1.1%) | |
| Third | Dementia and cognitive impairment – Acth, BZD, H2-receptors antagonists, zolpidem, antipsychotics, chronic and as-needed use (4.2%) | Blood clotting disorders or receiving anticoagulant therapy – NSAIDs, aspirin, dipyridamole, ticlopidine, clopidogrel (1.0%) | |
| Fourth | Heart Failure – NSAIDs and COX-2 inhibitors, nondihydropyridine CCBs (diltiazem, verapamil), pioglitazone, rosiglitazone, cilostazol, dronedarone (3.3%) | Stress Urinary Incontinence – Alpha blockers, Acths, TCAs, long acting BZD (0.6%) | |
| Fifth | Syncope – AChEIs, Peripheral alpha blockers (doxazosin, Prazosin, Terazosin), Tertiary TCAs, Chlorpromazine, thioridazine, and olanzapine (2.1%) | Arrhythmias - TCAs (0.4%) | |
Acths: Anticholinergics; CNS: Central Nervous System; TCA: tricyclic antidepressant; CCB: calcium channel blocker; BZD: Benzodiazepines; SSRI: Selective Serotonin Reuptake Inhibitor; NSAIDs: non-steroidal anti-inflammatory drugs; COX: cyclooxygenase; AChEI: acetylcholinesterase inhibitor.
Several factors were associated with PIP in multivariable analyses. (Table 3) We report patient characteristics and health care utilization factors associated with PIP. The factor most strongly associated with PIP was the number of drugs (OR 7.51; 95%CI 7.09–7.94 for 10+ drugs vs 1–2). Other independent predictors of PIP included patient characteristics such as female sex (OR 1.12; 95%CI 1.07–1.17), residence in the western and southern regions of the country, and medical conditions such as congestive heart failure (OR 1.96; 95%CI 1.88–2.04) and dementia (OR 1.77; 95%CI 1.68–1.87). Having at least one emergency room visit during the previous 12 months (OR 1.23; 95%CI 1.19–1.26), and having more than 1 prescriber in a given month (OR 1.09; 95% CI 1.03–1.16 for 3+ prescribers vs 1) were also associated with an increased risk of PIP. An increased risk of PIP was seen among older adults with a higher number of emergency room visits and outpatient office visits during previous 12 months, and a higher number of prescriptions filled and prescribers in a given month.
Table 3.
Factors Associated with Potentially Inappropriate Prescribing among US Medicare Older Adults According to 2012 Beers Criteria.
| Characteristic | Level | N | % with PIP | ORc (95% IC) | ORa (95% IC) |
|---|---|---|---|---|---|
| Year | 2007 * | 210,878 | 37.6 | 1 | 1 |
| 2008 | 212,813 | 36.2 | 0.94 (0.93–0.96) | 0.94 (0.93–0.96) | |
| 2009 | 213,759 | 35.7 | 0.92 (0.90–0.95) | 0.92 (0.90–0.95) | |
| 2010 | 214,356 | 35.4 | 0.91 (0.89–0.93) | 0.91 (0.89–0.94) | |
| 2011 | 221,730 | 35.1 | 0.90 (0.88–0.93) | 0.92 (0.89–0.94) | |
| 2012 | 234,580 | 34.2 | 0.86 (0.84–0.89) | 0.90 (0.87–0.93) | |
| Patient Characteristics | |||||
| Age Group (year) | 66–69 * | 237,936 | 33.4 | 1 | 1 |
| 70–74 | 309,311 | 32.1 | 0.94 (0.90–0.98) | 0.90 (0.86–0.94) | |
| 75–79 | 261,074 | 34.4 | 1.04 (0.99–1.10) | 0.89 (0.84–0.94) | |
| 80–84 | 230,185 | 37.2 | 1.18 (1.12–1.25) | 0.92 (0.87–0.97) | |
| 85+ | 269,610 | 41.7 | 1.42 (1.35–1.50) | 0.94 (0.89–1.00) | |
| Sex | Male * | 446,165 | 32.9 | 1 | 1 |
| Female | 861,951 | 37.1 | 1.21 (1.16–1.26) | 1.12 (1.07–1.17) | |
| Race/Ethnicity | White * | 1,110,223 | 35.3 | 1 | 1 |
| African-American | 109,337 | 39.5 | 1.19 (1.12–1.27) | 0.93 (0.86–1.00) | |
| Hispanic | 33,035 | 38.1 | 1.13 (1.01–1.26) | 0.83 (0.74–0.93) | |
| Asian | 31,875 | 33.8 | 0.93 (0.83–1.04) | 0.83 (0.74–0.94) | |
| Non American Native | 4,713 | 38.1 | 1.13 (0.85–1.50) | 0.85 (0.64–1.13) | |
| Other | 17,160 | 32.6 | 0.88 (0.74–1.06) | 0.92 (0.76–1.11) | |
| Unknown | 1,773 | 33.6 | 0.93 (0.60–1.44) | 0.75 (0.45–1.23) | |
| Region | Northeast * | 244,361 | 34.1 | 1 | 1 |
| South | 514,112 | 37.2 | 1.14 (1.08–1.20) | 1.17 (1.10–1.24) | |
| West | 224,873 | 36.7 | 1.12 (1.05–1.19) | 1.28 (1.20–1.37) | |
| North-Central | 321,371 | 33.9 | 0.99 (0.93–1.05) | 1.03 (0.97–1.10) | |
| Charlson Comorbidities | |||||
| Myocardial Infarction | No | 1,228,373 | 35.1 | 1 | 1 |
| Yes | 79,743 | 44.9 | 1.51 (1.43–1.59) | 0.77 (0.72–0.81) | |
| CHF | No * | 1,063,952 | 30.7 | 1 | 1 |
| Yes | 244,164 | 57.5 | 3.07 (2.95–3.18) | 1.96 (1.88–2.04) | |
| Peripheral Vascular Disease | No * | 1,020,341 | 33.2 | 1 | 1 |
| Yes | 287,775 | 44.3 | 1.60 (1.54–1.65) | 0.98 (0.94–1.02) | |
| Cerebrovascular Disease | No * | 1,052,687 | 33.3 | 1 | 1 |
| Yes | 255,429 | 45.4 | 1.67 (1.61–1.72) | 1.05 (1.01–1.09) | |
| Dementia | No * | 1,196,587 | 33.5 | 1 | 1 |
| Yes | 111,529 | 58.8 | 2.83 (2.70–2.97) | 1.77 (1.68–1.87) | |
| Chronic Pulmonary Disease | No * | 843,744 | 31.9 | 1 | 1 |
| Yes | 464,372 | 42.5 | 1.58 (1.53–1.63) | 0.97 (0.94–1.01) | |
| Connective
Tissue/Rheumatic Disease |
No * | 1,235,615 | 35.3 | 1 | 1 |
| Yes | 72,501 | 42.5 | 1.36 (1.27–1.45) | 1.04 (0.97–1.11) | |
| Peptic Ulcer Disease | No * | 1,277,442 | 35.4 | 1 | 1 |
| Yes | 30,674 | 48.7 | 1.74 (1.62–1.86) | 1.23 (1.14–1.33) | |
| Mild Liver Disease | No * | 1,246,407 | 35.3 | 1 | 1 |
| Yes | 61,709 | 42.2 | 1.34 (1.26–1.42) | 1.05 (0.99–1.12) | |
| DM without complications | No * | 850,741 | 31.8 | 1 | 1 |
| Yes | 457,375 | 42.8 | 1.60 (1.55–1.66) | 1.12 (1.07–1.16) | |
| Paraplegia and Hemiplegia | No * | 1,298,889 | 35.5 | 1 | 1 |
| Yes | 9,227 | 54.4 | 2.16 (1.88–2.48) | 1.20 (1.03–1.40) | |
| Renal Disease | No * | 1,149,109 | 34.2 | 1 | 1 |
| Yes | 159,007 | 46.4 | 1.66 (1.59–1.74) | 0.90 (0.86–0.95) | |
| DM with chronic complications | No * | 1,159,769 | 34.1 | 1 | 1 |
| Yes | 148,347 | 48.1 | 1.79 (1.71–1.88) | 1.02 (0.96–1.08) | |
| Cancer | No * | 1,119,446 | 35.9 | 1 | 1 |
| Yes | 188,670 | 34.1 | 0.92 (0.88–0.97) | 0.89 (0.85–0.94) | |
| Moderate or
Severe Liver Disease |
No * | 1,303,360 | 35.6 | 1 | 1 |
| Yes | 4,756 | 55.7 | 2.27 (1.86–2.78) | 1.43 (1.16–1.78) | |
| Metastatic Carcinoma | No * | 1,285,287 | 35.6 | 1 | 1 |
| Yes | 22,829 | 37.6 | 1.09 (1.00–1.19) | 0.96 (0.87–1.05) | |
| AIDS/HIV | No * | 1,306,918 | 35.7 | 1 | 1 |
| Yes | 1,198 | 40.3 | 1.22 (0.80–1.85) | 0.90 (0.60–1.36) | |
| Healthcare utilization | |||||
| Prescription Drug Use | |||||
| # of Prescription Fills in Month | |||||
| 1–2* | 440,632 | 18.4 | 1 | 1 | |
| 3–4 | 348,296 | 30.2 | 1.92 (1.87–1.96) | 1.76 (1.71–1.81) | |
| 5–9 | 401,440 | 48.6 | 4.18 (4.05–4.32) | 3.38 (3.25–3.50) | |
| 10+ | 117,748 | 72.4 | 11.59 (11.01–12.21) | 7.51 (7.09–7.94) | |
| Polypharmacy (≥5 drugs) | No * | 788,928 | 23.6 | 1 | - |
| Yes | 519,188 | 54.0 | 3.79 (3.69–3.90) | - | |
| Any Outpatient Office Visits | None * | 87,134 | 42.4 | 1 | 1 |
| 1+ | 1,220,982 | 35.2 | 0.74 (0.70–0.78) | 1.01 (0.94–1.07) | |
| None * | 87,134 | 42.4 | 1 | 1 | |
| # of Outpatient Office Visits | 1–6 | 529,284 | 31.0 | 0.61 (0.58–0.65) | 0.98 (0.92–1.04) |
| 7–12 | 388,015 | 34.4 | 0.71 (0.67–0.76) | 1.00 (0.93–1.06) | |
| 13+ | 303,683 | 43.4 | 1.04 (0.98–1.11) | 1.13 (1.05–1.63) | |
| Any Emergency Visit | None * | 859,550 | 30.3 | 1 | 1 |
| 1+ | 448,566 | 45.9 | 1.95 (1.90–2.00) | 1.23 (1.19–1.26) | |
| # of Emergency Visits | None * | 859,550 | 30.3 | 1 | 1 |
| 1 | 240,605 | 40.3 | 1.55 (1.51–1.59) | 1.15 (1.12–1.19) | |
| 2–5 | 186,957 | 51.2 | 2.41 (2.33–2.49) | 1.33 (1.28–1.38) | |
| 6+ | 21,004 | 63.9 | 4.07 (3.76–4.41) | 1.48 (1.35–1.63) | |
| Any Hospital Admission | None * | 1,002,331 | 31.6 | 1 | 1 |
| 1+ | 305,785 | 49.1 | 2.09 (2.03–2.14) | 0.97 (0.87–1.09) | |
| # of Prescribers in Month | 1 * | 754,195 | 30.2 | 1 | 1 |
| 2 | 358,150 | 38.8 | 1.46 (1.43–1.50) | 1.07 (1.03–1.12) | |
| 3+ | 195,771 | 50.9 | 2.39 (2.31–2.47) | 1.06 (1.00–1.12) | |
| # of
Prescriber Specialties in Month |
1 * | 844,217 | 31.3 | 1 | 1 |
| 2 | 363,642 | 41.0 | 1.53 (1.49–1.56) | 0.96 (0.91–1.01) | |
| 3+ | 100,257 | 53.4 | 2.52 (2.42–2.63) | 1.00 (0.92–1.08) | |
| Prescriber Specialty | |||||
| Geriatrician Prescriber | No * | 1,276,492 | 35.5 | 1 | 1 |
| Yes | 31,624 | 43.5 | 1.40 (1.27–1.54) | 0.89 (0.79–0.99) | |
| GP/FP/Internist Prescriber | No * | 284,470 | 31.5 | 1 | 1 |
| Yes | 1,023,646 | 36.8 | 1.27 (1.22–1.31) | 0.99 (0.95–1.04) | |
| Other Specialty Prescriber | No * | 749,063 | 33.2 | 1 | 1 |
| Yes | 559,053 | 39.0 | 1.28 (1.25–1.32) | 1.08 (1.03–1.12) |
PIP: Potentially Inappropriate Prescribing; ORc: Odds Ratio Crude; ORa: Odds Ratio Adjusted; GP: General Practitioner; NAN: North American Native; DM: Diabetes Mellitus; CHF: Congestive Heart Failure, AIDS/HIV: Acquired immune deficiency syndrome/Human immunodeficiency virus; FP: Family Practitioner; 95% CI: 95% confidence intervals; *: reference group;
Older age and Asian or Hispanic race/ethnicity were associated with lower rates of PIP in adjusted analyses. People with at least one claim prescribed by a geriatrician were less likely to have a PIP (OR 0.89; 95%CI 0.79–0.99).
DISCUSSION
This study provides evidence that PIP remains very common for older people in the United States. Every month, among older adults who filled at least one prescription in the month, one in three received a drug for which the potential harms outweigh the potential benefits. Only a small reduction in PIP has occurred since 2007. We also found that more than 50% of US older adults being treated with prescription medication received at least one PIP during a calendar year. This highlights the importance of a clear PIP prevalence definition for interpretation in pharmacoepidemiological studies, and the cumulative nature of this risk to older adults.
This study is the first to apply the complete version of 2012 Beers criteria to a nationally representative population. A recent systematic review of PIP reported 19 studies produced in 5 countries, and none used the 2012 Beers criteria (14). Further, most previously published studies have modified the 2003 Beers criteria to exclude items that depend on dosage, use frequency or diagnoses (15) or have used subsets of the 2012 criteria (13,16,17).
Our estimates of the prevalence of PIP using 2012 Beers criteria are generally higher than previously published studies, which have reported inappropriate medication use in 14–45.5% of community-dwelling older adults in the US (13–15). Additionally, we noted that the risk for receiving a PIP decreases with increasing age. Thus, our findings are different from previous studies that data on community-dwelling older people suggest that PIP was associated with advancing age in most studies (14). This difference may be explained by the inclusion of DDI in our definition of PIP, or different Medicare drug coverage and drug availability during the study period. Our findings are consistent with previous studies in US suggesting that western and southern regions are more likely to receive a PIP (23).
A recent study by Davidoff et al. (13) used the Medical Expenditure Panel Survey (MEPS) and estimated an annual prevalence of a subset of the 2012 Beers criteria (i.e., 36 medication classes that older adults should avoid) from 2006–2010 ranging from 46% from 2006–2007 to 41% from 2009–2010; lower than our reported estimates for the same subset of medication classes of 56% in 2007 to 48% in 2010. A reason for these discrepant estimates may be due to differing methods of prescription medication capture. The MEPS relies upon self-report of medication use via interviews using medicine bottles and receipts, while our analyses drew upon prescription dispensing records, which are not subject to the same potential for underreporting (10). In addition to the subset of 34 medication classes evaluated by Davidoff et al., we also examined the prevalence of inappropriate medications use among older adults due to DDIs, providing the first complete evaluation of the 2012 Beers criteria using 52 medication classes.
This study is consistent with other data showing a trend of PIP decreasing over time (18,19). However, the relatively high number of drugs taken, and the use of anticholinergic and psychotropic drugs remained common among PIP users. Therefore, strategies to improve quality of care in older adults should focus strategies to reduce total medications (20) as well as anticholinergic burden and psychotropic use specifically (21). A component of successful practice change would likely be implementation of pharmacogeriatric training. Geriatricians were less likely to prescribe a PIP, and they are better trained on pharmacogeriatrics and PIP consequences. In our study we also found that a previous emergency visit was also a predictor of PIP. However, special considerations should be taken into account in the emergency setting; in light of the risk of ADEs after the emergency attention, including a comprehensive medication reconciliation process and selecting safer alternatives during the emergency room visit may enhance the quality and safety of health care and reduce the incidence of ADEs.
A lower prevalence of PIP according to 2003 Beers criteria was found; mainly due to the older version of the Beers criteria include a shorter list of drugs and drug-disease interactions that should be avoided in the older people than 2012 Beers criteria. We also found a decrease of prevalence of PIP according to 2003 Beers criteria in 2011 and 2012, which can be explained because propoxyphene and their combinations were removed from the US market in 2010. (Appendix Table A1 to A4).
It is not surprising that the prevalence of PIP varied between criteria used, clinical setting and the operational PIP definition used. For instance, the medical literature rarely distinguishes between point-prevalence (e.g., in a given month) and 12-month period prevalence (e.g., over a 12 months period). Consistent study methods across pharmacoepidemiological prevalence studies have the potential to enhance the value of this research by allowing comparison between research findings. However, since overlapping lists of the most common PIP still remain frequently prescribed, future interventions to improve health of older patients could optimally focus on the list of common PIP, such as high dose digoxin, glyburide, anticholinergics, psychotropics, and older medications such as propoxyphene, doxazosin and amitriptyline.
Given the consistency of findings across the time and criteria used, the evidence already available of the adverse drug effect listed in these criteria are not enough or crystal clear to change clinical practice for a more safe and better tolerate pharmacotherapy in older adults. Therefore, additional studies evaluating the consequences of adverse effects and benefits of specific drugs should provide a concise message to the physicians and health care providers about potential risk and alternatives of treatment in case of PIP.
Our study has some limitations. First, if patients have alternative sources of prescription coverage or over-the-counter drug utilization (such as some antihistamines or NSAIDs), our estimates might underestimate the real burden of PIP. Several categories of medications were excluded from the Medicare Part D coverage, such as benzodiazepines and barbiturates, some of which are listed in the Beers criteria. Second, while claims data overcome issues of recall bias and provide nearly complete information on prescription drug use, medical status and healthcare utilization can be underrepresented in a database; therefore, the PIP prevalence may be underestimated. Finally, and most importantly, there are no data on the reasons why certain prescription choices were made by a specific clinician for a specific patient. It should be kept in mind that not all PIPs can be avoided; sometimes the benefits of a medication outweigh the risks. Moreover, we cannot be certain that the drugs prescribed and dispensed were actually consumed. It should also be noted that the Beers criteria only address potential over prescribing, while not addressing potential under prescribing or use of duplicate drug classes (11,12). The last may underestimate the PIP prevalence.
Despite these limitations, our prevalence data improves on prior research. Previous studies rarely contained information on drug dosage or patient disease conditions, and thus more often underestimate PIP related to underlying disease, and fail to report excessive dosage or duration (10,13,14). In contrast, our study included diseases or conditions, doses and duration of medication use allowing better estimation of PIP prevalence.
Screening tools such as 2012 Beers criteria may be used to detect potential risks and support medical decision-making in clinical practice. This is a tool for continued improvements in patient safety, when used in quality improvement interventions for geriatric prescribing. This tool also permits a comprehensive understanding of the epidemiology of drug related problems for broader public health purposes. Prescribing guidelines are not meant to supersede the clinical judgement of the prescriber and are not intended as absolute contraindications. The Beers criteria are intended to serve as guidance to reduce risk and prevent harm when using medications in older adults. They therefore allow us to assess the quality of prescribing in clinical practice at the population level (12).
In conclusion, one in three older adults monthly and one in two older adults yearly are exposed to a PIP in the US according to the 2012 Beers criteria, with a slight decrease in the PIP prevalence between 2007 and 2012. Factors associated with PIP such as patient characteristics (3 or more prescriptions filled in a given month, female sex and western and southern regions), and health care utilization (an emergency visit during the previous 12 months) provide clues on how to improve the quality of drug prescribing among older adults. These factors also allow us to identify patients at highest risk for PIP. Further research is needed to quantify the effects of PIP on the risk of ADEs such as delirium, falls and fractures, health cost and frailty.
Supplementary Material
Acknowledgments
The database infrastructure used for this project was funded by the Pharmacoepidemiology Gillings Innovation Lab (PEGIL) for the Population-Based Evaluation of Drug Benefits and Harms in Older US Adults (GIL 200811.0010), the Center for Pharmacoepidemiology, Department of Epidemiology, UNC Gillings School of Global Public Health; the CER Strategic Initiative of UNC’s Clinical Translational Science Award (UL1TR001111); the Cecil G. Sheps Center for Health Services Research, UNC; and the UNC School of Medicine.
Funding: MECESUP UCH0811- Chile, R01 AG023178 from the National Institute on Aging (NIA).
TS receives investigator-initiated research funding and support as Principal Investigator (R01 AG023178) and Co-Investigator (R01 AG042845) from the National Institute on Aging (NIA), and as Co-Investigator (R01 CA174453) from the National Cancer Institute (NCI) at the National Institutes of Health (NIH), and as Principal Investigator from the Patient Centered Outcomes Research Institute (PCORI). Dr. Stürmer does not accept personal compensation of any kind from any pharmaceutical company, though he receives salary support from the Center for Pharmacoepidemiology (current members: GlaxoSmithKline, UCB BioSciences, Merck) and research support from pharmaceutical companies (Amgen, Merck) to the Department of Epidemiology, University of North Carolina at Chapel Hill.
Dr. Jonsson Funk receives investigator-initiated research funding and salary support as Principal Investigator from the Agency for Healthcare Research and Quality (AHRQ, K02 HS017950) and the National Institutes of Health (NIH), National Heart Lung and Blood Institute (NHLBI, R01 HL118255); as a Co-Investigator on grant awards from the NIH National Institute on Aging (NIA, R01 AG023178), the NIH National Center for Advancing Translational Sciences (NCATS, 1UL1TR001111) and Co-Investigator of a Pilot Project from the Patient Centered Outcomes Research Institute (PCORI, 1IP2PI000075). Dr Jonsson Funk does not accept personal compensation of any kind from any pharmaceutical company, though she receives salary support from the Center for Pharmacoepidemiology (current members: GlaxoSmithKline, UCB BioSciences, Merck) and salary and research support from AstraZeneca to the Department of Epidemiology, University of North Carolina at Chapel Hill.
Dr. Lund does not accept personal compensation of any kind from any pharmaceutical company, though she receives salary support and research support from the PhRMA Foundation for a Research Starter Award to the Department of Epidemiology, University of North Carolina at Chapel Hill.
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Agency for Healthcare Research and Quality.
Footnotes
Disclosure: MJ, VP and LH none to disclose.
Author Contribution: Dr. Jirón and V. Pate had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Jirón, Stürmer
Acquisition of data: Stürmer, Jonsson Funk
Analysis and interpretation of data: Pate, Jirón, Stürmer, Hanson, Jonsson Funk, Lund
Drafting of the manuscript: Jirón
Critical revision of the manuscript for important intellectual content: Jirón, Hanson, Stürmer, Jonsson Funk, Pate, Lund
Statistical analysis: Pate
Obtained funding: Stürmer
Administrative, technical, or material support: Jirón, Pate, Stürmer
Study supervision: Stürmer
REFERENCES
- 2.Beers MH, Ouslander JG, Rollingher I, et al. Explicit criteria for determining inappropriate medication use in nursing home residents. Arch Intern Med. 1991;151:1825–1832. [PubMed] [Google Scholar]
- 3.Hamilton H, Gallagher P, Ryan C, et al. Potentially inappropriate medications defined by STOPP criteria and the risk of adverse drug events in older hospitalized patients. Arch Intern Med. 2011;171:1013–1019. doi: 10.1001/archinternmed.2011.215. [DOI] [PubMed] [Google Scholar]
- 4.Klarin I, Wimo A, Fastbom J. The association of inappropriate drug use with hospitalization and mortality. A population study of the very old. Drugs Aging. 2005;22:69–82. doi: 10.2165/00002512-200522010-00005. [DOI] [PubMed] [Google Scholar]
- 5.Lau DT, Kasper JD, Potter DEB, et al. Hospitalization and death associated with potentially inappropriate medication prescriptions among elderly nursing home residents. Arch Intern Med. 2005;165:68–74. doi: 10.1001/archinte.165.1.68. [DOI] [PubMed] [Google Scholar]
- 6.Cahir C, Fahey T, Teeling M, et al. Potentially inappropriate prescribing and cost outcomes for older people: A national population study. Br J Clin Pharmacol. 2010;69:543–552. doi: 10.1111/j.1365-2125.2010.03628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fu AZ, Liu GG, Christensen DB. Inappropriate medication use and health outcomes in the elderly. J Am Soc Geriatr. 2004;52:1934–1939. doi: 10.1111/j.1532-5415.2004.52522.x. [DOI] [PubMed] [Google Scholar]
- 8.Beers MH, Ouslander JG, Rollingher I, et al. Explicit criteria for determining inappropriate medication use in nursing home residents. Arch Intern Med. 1991;151:1825–1832. [PubMed] [Google Scholar]
- 9.Anderson GM, Beers MH, Kerluke K. Auditing prescription practice using explicit criteria and computerized drug benefit claims data. J Eval Clin Pract. 1997;3:283–294. doi: 10.1046/j.1365-2753.1997.t01-1-00005.x. [DOI] [PubMed] [Google Scholar]
- 10.Fick DM, Cooper JW, Wade WE, et al. Updating the Beers Criteria for potentially inappropriate medication use in older adults. Arch Intern Med. 2003;163:2716–2724. doi: 10.1001/archinte.163.22.2716. [DOI] [PubMed] [Google Scholar]
- 11.Goulding MR. Inappropriate medication prescribing for elderly ambulatory care patients. Arch Intern Med. 2004;164:305–312. doi: 10.1001/archinte.164.3.305. [DOI] [PubMed] [Google Scholar]
- 12.Campanelli C. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Soc Geriatr. 2012;60:616–631. doi: 10.1111/j.1532-5415.2012.03923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Mahony, Gallagher P. Inappropriate prescribing in the older population: Need for a new criteria. Age Ageing. 2008;37:138–141. doi: 10.1093/ageing/afm189. [DOI] [PubMed] [Google Scholar]
- 14.Davidoff A, Miller E, Sarpong E, et al. Prevalence of potentially inappropriate medication use in older adults using the 2012 Beers Criteria. J Am Geriatr Soc. 2015;63:486–500. doi: 10.1111/jgs.13320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guaraldo L, Cano F, Damasceno G, et al. Inappropriate medication use among the elderly: A systematic review of administrative databases. BMC Geriatr. 2011;11:79. doi: 10.1186/1471-2318-11-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanlon JT, Schmader KE, Ruby CM, et al. Suboptimal prescribing in older inpatients and outpatients. J Am Geriatr Soc. 2001;49:200–209. doi: 10.1046/j.1532-5415.2001.49042.x. [DOI] [PubMed] [Google Scholar]
- 17.Zuckerman IH, Langenberg P, Baumgarten M, et al. Inappropriate drug use and risk of transition to nursing homes among community-dwelling older adults. Med Care. 2006;44:722–730. doi: 10.1097/01.mlr.0000215849.15769.be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fick DM, Mion LC, Beers MH, et al. Health outcomes associated with potentially inappropriate medication use in older adults. Res Nurs Health. 2008;31:42–51. doi: 10.1002/nur.20232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zimmermann T, Kaduszkiewicz H, van den Bussche H, et al. Potentially inappropriate medication in elderly primary care patients: A retrospective, longitudinal analysis. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2013;56:941–949. doi: 10.1007/s00103-013-1767-5. [DOI] [PubMed] [Google Scholar]
- 20.Price SD, Holman CDJ, Sanfilippo FM, et al. Are older Western Australians exposed to potentially inappropriate medications according to the Beers Criteria? A 13-year prevalence study. Aust J Ageing. 2014;33:E39–E48. doi: 10.1111/ajag.12136. [DOI] [PubMed] [Google Scholar]
- 21.Scott I, Hilmer S, Reeve E, et al. Reducing inappropriate polypharmacy. JAMA Intern Med. 2015;175:827–834. doi: 10.1001/jamainternmed.2015.0324. [DOI] [PubMed] [Google Scholar]
- 22.Gray S, Anderson M, Dublin S, et al. Cumulative use of strong anticholinergics and incident dementia. JAMA Intern Med. 2015;175:401–407. doi: 10.1001/jamainternmed.2014.7663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Opondo D, Eslami S, Visscher S, et al. Inappropriateness of medication prescriptions to elderly patients in the primary care setting: A systematic review. PLoS One. 2012;7:e43617. doi: 10.1371/journal.pone.0043617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Curtis L, Østbye T, Sendersky V, et al. Inappropriate prescribing for elderly Americans in a large outpatient population. Arch Intern Med. 2004;164:1621–1625. doi: 10.1001/archinte.164.15.1621. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.