Abstract
An initial response to whole-body or local exposure of the extremities to cold is a strong vasoconstriction, leading to a rapid decrease in hand and foot temperature. This impairs tactile sensitivity, manual dexterity, and muscle contractile characteristics while increasing pain and sympathetic drive, decreasing gross motor function, occupational performance, and survival. A paradoxical and cyclical vasodilatation often occurs in the fingers, toes, and face, and this has been termed the hunting response or cold-induced vasodilatation (CIVD). Despite being described almost a century ago, the mechanisms of CIVD are still disputed; research in this area has remained largely descriptive in nature. Recent research into CIVD has brought increased standardization of methodology along with new knowledge about the impact of mediating factors such as hypoxia and physical fitness. Increasing mechanistic analysis of CIVD has also emerged along with improved modeling and prediction of CIVD responses. The present review will survey work conducted during this century on CIVD, its potential mechanisms and modeling, and also the broader context of manual function in cold conditions.
Keywords: cold-induced vasodilatation, finger blood flow, manual function, sympathetic nervous system
Abbreviations
- AVA
Arteriovenous anastomoses
- CIVD
Cold-induced vasodilation
- CWI
Cold water immersion
- eNOS
Endothelial nitric oxide synthase
- FIO2
Inspired fraction of oxygen
- NO
Nitric oxide
- RIF
Resistance index of frostbite
- SkBF
Skin blood flow
- Tfdi
First dorsal interosseous muscle temperature
- Tif
Index finger temperature
Introduction
The hands and feet form powerful thermoregulatory regulators in the body, serving as heat radiators and evaporators in hot environments and as thermal insulators in the cold. Taylor et al.1 modeled that each hand and foot can dissipate 150–220 W·m-2 through radiation and convection at rest in an ambient temperature of 27°C, with even greater heat dissipation through sweating. In contrast to this high capacity for heat dissipation, vasoconstriction during hypothermia is sufficiently strong to reduce heat flow to < 0.1 W. Besides being powerful thermoeffectors, the non-hairy, glabrous skin of the extremities can also serve as powerful thermosensors that affect thermoregulatory behavior in a feedforward and feedback fashion.2 Blood flow to the extremities of the hands and feet respond rapidly upon exposure to cold, with a sympathetically-mediated vasoconstriction reducing blood flow to the peripheries in favor of a central pooling of blood in the torso and deep body core. Due to this vasoconstriction and the high surface area-to-volume ratio, the skin temperature of the fingers and toes tends to rapidly and exponentially decrease to a level approaching that of the ambient environment.
Decrements in tactile sensitivity, manual dexterity, and gross motor function of the hands can lead to decreased overall performance in occupational settings, such as in mast and pole workers3 and divers.4 The rapid5 and sustained6,7 impairment of manual performance from local cold exposure can also degrade an individual's ability to operate emergency equipment (e.g., escape hatches, opening flares) or move into a safer situation (e.g., hauling oneself from the water into a liferaft), turning a survivable situation into a critical one.8 Beyond immediate impairment, continued cold exposure and vasoconstriction can also lead to non-freezing cold injuries from reduced nutritional blood flow leading to necrosis and non-freezing cold injuries such as immersion foot,9,10 or cold injuries such as frostbite from cell temperature dropping below the point of freezing and crystallization.11-13 The increased accessibility of outdoor recreational opportunities during winter, along with high altitude expeditions, extends the potential for injury beyond traditional occupational settings.14
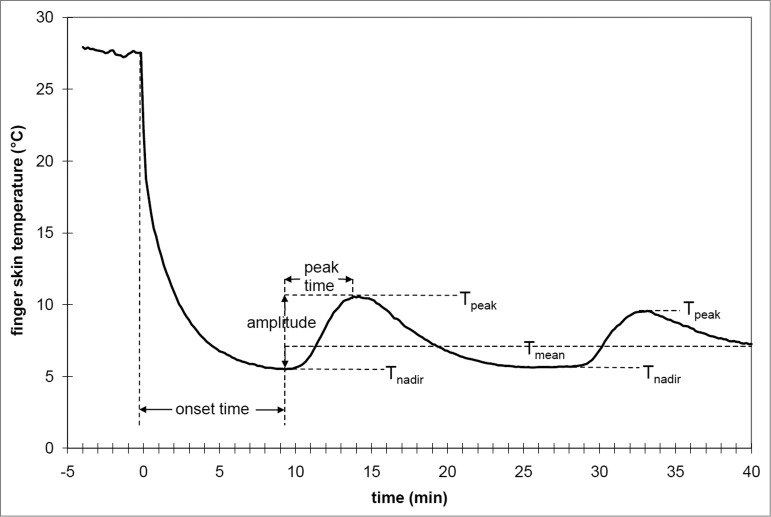
Despite an overall drive for vasoconstriction in the cold, a common observation in the toes and fingertips is that, after a brief period of lowered skin blood flow and temperature, a seemingly paradoxical and temporary increase in blood flow and rewarming occurs. During these episodes, skin temperature can rise by as much as 10ºC, and this fall and rise can occur repeatedly in a cyclic fashion. This pattern of periodic warming was first reported by Lewis,15 and he labeled it the “hunting response” for its apparent oscillatory pattern – this response has also been termed the “cold-induced vasodilation” or CIVD phenomenon.16 In addition to the fingers and toes, CIVD has been observed in the face17 and forearms.18 Whole body thermal status is known to be an important determinant for CIVD prevalence and intensity, with an inverse relationship between CIVD responses and body temperature, along with the lack of any observed CIVD below a threshold body temperature. A stylized “classic” CIVD response is provided in Figure 1, demonstrating the typical responses and measures used to quantify CIVD.
Figure 1.
General schematic of typical CIVD responses and measured variables in finger skin temperature during immersion in cold water. Reprinted from Cheung and Daanen, Microcirculation 2012; 19:65–77. Used with permission.
The mechanisms driving CIVD remain open to debate. However, their anatomical endpoint of action likely revolves around arteriovenous anastomoses (AVA) within the cutaneous microcirculation. AVA are shunts within the skin that permits blood to bypass the capillaries and instead flow directly from the arterioles to venules. The prevalence of AVA at the fingertips and toes provided initial though indirect extrapolation that their relaxation and an increase in local blood flow are what causes CIVD.19 Another indirect argument for the involvement of AVAs in CIVD is that capillary blood flow by itself appears insufficient to match the magnitude of heat loss observed during CIVD.20 More recently, Bergersen21 and Bergersen et al.22-24 used a combination of ultrasound and laser-Doppler measurements across the fingers and other arteries to demonstrate the presence and direct involvement of AVAs in the CIVD response.
While the mechanisms underlying CIVD remain unclear, understanding the nature of CIVD is of important occupational and clinical relevance. Because of the elevated extremity blood flow and temperature, CIVD has generally been presumed to provide a protective function by maintaining local tissue integrity and minimizing the risk of cold injuries. Through this increase of finger temperature, it is also presumed that CIVD can improve manual dexterity in the cold. Comparing research findings has also been hampered by inconsistent methodology in experimental protocols and also quantification of CIVD response. Studies in the field have frustratingly remained largely descriptive, though a greater emphasis on standardization and mechanistic studies have emerged in the past 2 decades.25
The field of CIVD was last comprehensively surveyed by Daanen,16 including the proposal of several potential mechanisms and a call for greater standardization in the reporting of CIVD parameters. Rather than replicate this excellent resource, the current review will focus on the body of work in this field since that publication, along with broadening the scope to the overall theme of extremity response to local cold exposure, including the effects on muscle performance and manual function. A comprehensive review on the adaptations of peripheral circulation to prolonged cold exposure, surveying population data, longitudinal field studies, and laboratory acclimation was recently performed by Cheung and Daanen.25 The present review will therefore not repeat this information, except to note that recent laboratory studies have reported no systematic physiological improvement in extremity response with acclimation.25-32
Epidemiology
The dangers posed by freezing and non-freezing cold injuries have been common knowledge for centuries, owing largely to epidemiological surveys of the military.12 Beyond these specific populations, the increasing popularity and ease of access to high altitude and winter sports has increased the risk of cold exposure injuries to the general and recreational populations.9,14,33 This is highlighted by the prevalence of commercial guided expeditions to Mount Everest and their significant death rate.34 Even at moderate elevations (2800 – 3960 m), ∼37% of mountaineers suffer frostbite injuries each year.35 Notably, the strongest risk factors in this latter report were inappropriate clothing along with lack of knowledge about how to deal with cold and severe weather. Thus, research and better public education appears critical in decreasing the odds for cold injuries in both recreational and occupational groups.
The location of cold injuries vary, dependent on both anatomy and exposure. Most of the research on local cold exposure utilizes the hands or fingers, likely due to ease of access in the lab and especially in the field. However, there is not a direct extrapolation from finger to toe or foot responses, with the risk of freezing and non-freezing cold injuries being at least as prevalent35,36 or else as predominant11 in the feet as compared to hands. The post-injury impact from freezing or non-freezing cold injuries on long-term extremity blood flow is unclear. The traditional view is that a significant risk factor for frostbite is prior cold injury, though systematic cross-sectional studies comparing individuals with and without prior cold injuries are somewhat lacking. Nevertheless, in support of this supposition, Davey et al.37 reported lower basal skin blood flow and skin temperatures in the feet of individuals with prior non-freezing cold injuries, reduced cold sensitivity compared to non-injured individuals, and a slower rate of rewarming. Specifically, cold-sensitive individuals with a prior injury had similar rewarming profiles as an occluded or non-perfused foot model, such that rewarming occurred solely due to passive conductive heat transfer rather than the vasodilatory changes in non-injured individuals. In contrast, Morrison et al.38 reported in a case study that, 40 y post-injury including finger and toe amputations, rates of rewarming did not differ between injured and non-injured digits in one elite mountaineer.
Population Studies
The decreasing isolation of high-altitude and polar populations has reduced the ease of performing population-specific or racial comparison studies. As a result, little additional information concerning racial diversity and variability in CIVD responses has occurred in the past 2 decades. A recent controlled laboratory study compared local hand and feet immersion responses in Caucasian, Asian, and African participants – ethnicity was self-reported, and all were born in the United Kingdom or long term (>7 years) residents.39 Compared to Caucasians, Africans had a higher skin temperature threshold for both the initiation of vasoconstriction and vasodilatation, along with a lower skin blood flow (SkBF) during 8°C hand immersion and a slower rate of rewarming; Asian participants had intermediate responses between those of Caucasians and Africans. In comparing white versus black Africans who were chronically hypertensive to a cold pressor test of hand immersion into ice water, black Africans reported greater pain perception, along with an increased blood pressure and heart rate rise compared to the white Africans, with a strong correlation between pain perception and cardiovascular responses across all subjects.40 It is acknowledged, however, that chronic hypertension may have altered systemic vasomotion compared to non-hypertensive individuals.
Such cross-sectional population studies supports the anecdotal and epidemiological reports of greater risk for cold injuries in particular racial populations regardless of residency. Lee et al.41 reported that tropical indigenes, even 4 y after moving to a more temperate environment of Japan, experienced lower middle finger temperatures during 4°C finger immersion compared to temperate natives. Another source of information concerning peripheral blood flow diversity comes from military epidemiological reports.42 Based upon cold injury assessment of 311 British Army soldiers and broadly categorized as African-American, Pacific Islanders, Gurkhas, and Caucasians, African-Americans had a 30-fold greater risk incidence of compared to Caucasians, while Pacific Islanders had a slightly increased 2.6-fold greater incidence compared to Caucasians; Gurkhas had no abnormal cases of cold injury. Similar data is reported in the American military, with African-American men and women experiencing 4- and 2-fold increases in cold weather injuries compared to Caucasian personnel.43 The genetic or phenotypic contributors to population differences remain unknown. Overall, these studies combine to suggest that population variability in both psychological and physiological responses to cold exposure remain evident, and especially that the psychological response may significantly influence sympathetic drive and cardiovascular responses.
The effect of sex on cold injury risk is unclear. With contact cooling across a range of materials, females had faster rates of fingertip skin temperature cooling than males, suggesting a potentially greater risk for cold injuries in females.44 However, hand volume provided an even stronger correlation with cooling rates, so anthropometric differences may predominate instead.45 A similar finding was reported by Lunt and Tipton46 with females exhibiting faster toe temperature cooling in response to conductive cooling of the soles of the feet; no intra-menstrual cycle differences were evident. Again, the generally larger male foot volume, along with a strong correlation between foot dimension and toe cooling rate, suggest a dominant anthropometric rather than sex effect. Jay and Havenith45 attempted to separate these effects, recruiting males and females with similar hand dimensions. With slow rates of material cooling, sex had no correlation, but index finger volume was the strongest predictor of fingertip temperature cooling rate, and was attributed to a larger thermal inertia from the greater finger volume. However, with fast rates of material cooling, females cooled faster with no anthropometric correlation, and this was suggested to be due to higher starting finger temperature and greater epidermal insulation in males.45 Overall, as many thermophysiological laboratory studies have relied predominantly on male participants, further work is required to determine whether physiological sex-related differences exist in response to local cold exposure.
Another population group that is of both mechanistic and practical interest for research is the elderly. The ability to maintain thermal homeostasis in both the heat and cold is impaired with age, and appears to be above and beyond any effect from differences in body composition or physical fitness and activity. Older (>70 y) individuals had decreased vasoconstrictor ability and defense of esophageal temperature during mild cold stress than anthropometrically-matched younger (∼23 y) counterparts.47 Muscle sympathetic nerve activity during passive cold increased in older but not young adults,48 and limb muscle mass appeared to account for the greatest variance in elderly response to mild cold.49 Thermal sensitivity also decreases with age, with greater range in thresholds for thermal detection; many factors may contribute to this impaired thermal perception, including decreased thermoreceptor density within the skin, skin blood flow, and conduction velocity.50 Despite these clear age-related differences, research into aging effects on extremity responses to the cold is sparse, with older individuals demonstrating delayed CIVD onset and reduced amplitude compared to young adults.51 Given the decreased physiological and perceptual response to cold stress with aging, it is highly likely that older individuals may be at greater risk for cold injuries, necessitating a greater focus on research in this population.
Perceptual Responses
While local cold exposure is consistently rated as uncomfortable, the magnitude of this thermal discomfort is inconsistent. This is likely related to methodological differences across studies, as local skin temperature and surface area exposed both alter pain perception. A cold pressor test involving hand immersion into 0–10°C water is often used in psychology experiments to induce pain in adults and children.52 Not surprisingly, hand immersion in 5°C water led to greater discomfort than with 8, 10, or 15°C, though no differences were observed across the 3 warmer conditions (see Fig. 2).53 Interestingly, thermal sensation was similar across all 4 water temperatures, such that the difference in discomfort at 5°C was possibly driven by nociceptive rather than thermal stimuli. In laboratory studies, thermal discomfort typically inversely tracks extremity temperature, with an initial rapid increase in discomfort during the early drop in extremity temperature, followed by a relative stabilization. Surprisingly, most laboratory CIVD studies, even ones that specifically tested neuromuscular function at the peak of finger CIVD response,54 record thermal perception at set time periods rather than during fluctuations in extremity temperature. Thus, while a priori reasoning should suggest that CIVD should decrease thermal discomfort, the actual perceptual effects of CIVD episodes are not clear. No heart rate or blood pressure rise occurs with only index finger immersion in 5°C; however, significant increases in blood pressure and heart rate were observed with both hand and hand plus forearm immersion.55 This contrasting response is likely due to a greater amount of surface area exposed leading to a greater sympathetic drive and resultant cardiovascular response. Unfortunately, this latter study did not measure perceptual responses, so the dose-response relationship across different immersion protocols remains unclear.
Figure 2.
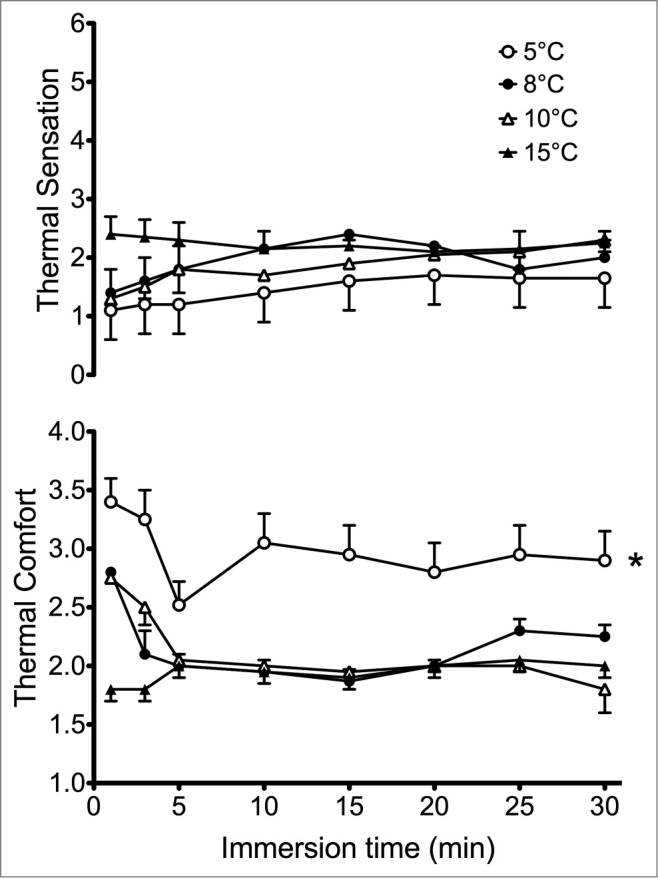
Thermal sensation (top) and thermal discomfort (bottom) during whole-hand immersion into 5, 8, 10, and 15°C water. * Thermal discomfort was significantly higher during the entire 30 min of 5°C immersion for 5°C, with no differences among 8, 10, and 15°C. In contrast, all temperatures elicited the same thermal sensation. Adapted from Mekjavić et al., Appl Physiol Nutr Metab 2013; 38:14–20. Used with permission.
Sudden whole-body cold water immersion (CWI) may be seen as a more severe analog to local cold exposure of the peripheries, due to both the larger surface area exposed and the greater survival threat from submersion and drowning. Cold (2°C) water immersion or dousing is recommended as the safest first aid method for recovery from exertional heat illness.56-58 However, in a normothermic situation, a series of studies on CWI clearly demonstrates the powerful effects of anxiety and psychological interventions.59-62 Greater acute anxiety, elicited by deceiving individuals to impending immersion into colder water than planned, significantly increased the cold shock responses of tachycardia and hyperventilation compared to correct immersion temperature information.61 Interestingly, despite overall habituation of the cold shock response with CWI training, this anxiety effect was present both before and after training, implying that psychological cues can overwhelm and negate any physiological adaptation. In turn, a broad-spectrum psychological skills training program specifically focused on cold response, even in the absence of any CWI training, attenuated cold shock response,59 though there was no additive effect above and beyond CWI training.60 From a practical perspective, even repeated immersions into thermoneutral water were effective in reducing anxiety, albeit with no physiological effect on cold shock response.62 Based on these studies, psychological interventions clearly can play a major role in overall tolerance and management of local cold exposure, regardless of any physiological adaptation.
A frequent though not universal trend with long-term adaptation to local cooling is a decrease in pain or subjective thermal discomfort.25 Especially in lab-based acclimation studies, this perceptual habituation is often the primary or only adaptive response, with minimal evidence of physiological adaptation (see Fig. 3).27,28 Alterations in sympathetic outflow due to reduction in anxiety or psychological stress over time may contribute to CIVD adaptation, as the repeated immersions should result in a reduced sympathetic outflow over time. The reduction in pain sensation may be caused by less sensory input, but is more likely caused by central nervous inhibition of the afferent sensory input.63 While perceptual habituation improves comfort in the cold, some have postulated that this discrepancy between physiological and perceptual response may be a negative adaptation that impedes appropriate behavioral response and increases the risk of cold injuries.27,64 However, others have suggested that the stress of cold exposure causes an elevation in sympathetic activity, resulting in enhanced vasoconstrictor tone and negative adaptations to local cold acclimation.31 Only one study measured blood markers of sympathetic outflow and found no changes in catecholamines over the acclimation protocol.27 As this study also observed no changes in CIVD response, the potential role for these factors in any changes in finger thermal responses to repeated cold exposure remains inconclusive. The relative change in sympathetic/parasympathetic drive may be estimated using heart-rate variability measurements during repeated cold immersions of the hands, and initial work suggests that CIVD may be driven by sympathetic vasoconstrictor withdrawal.65
Figure 3.
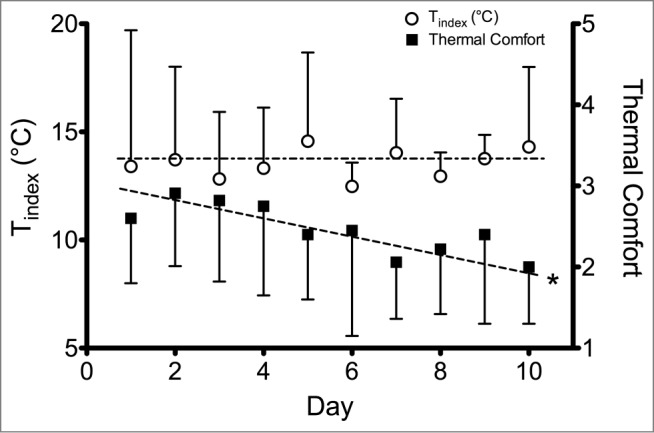
Index finger temperature and thermal comfort after 30 min of cold-water immersion of the whole hand in 8°C water over 10 d of acclimation. Index finger temperature remained similar but thermal comfort steadily improved throughout acclimation. Adapted from Geurts et al., Appl Physiol Nutr Metab 2006; 31:110–7. Used with permission.
Hypoxia
Beyond direct cold exposure, hypoxia and/or altitude exposure has been utilized as a tool to investigate both whole-body thermoregulation and CIVD. This integration has a practical context, as cold and altitude are common co-stressors in mountaineering expeditions. Even moderate altitude may exacerbate the risk of cold injuries, with both a high mean incidence rate of ∼37% per year and the majority occurring at moderate (2,800 – 3,960 m) altitudes.35 Exertional fatigue and fuel depletion has both been proposed66-68 and discounted69 as a factor in altering vasoconstrictory and shivering threshold temperatures or in developing hypothermia. One unexplored epidemiological question is the effects of heavy physical exertion on vascular conductance and risks for developing cold injuries during expeditions. Understanding the effect of hypoxia on thermal sensation and comfort during cold stress is important, as perceptual cues are critical for appropriate behavioral thermoregulatory responses to avoid cold injuries, but the literature is both limited and contradictory. Acute normobaric hypoxia (inspired fraction of oxygen: FIO2 = 0.14) did not alter either the lower or upper thresholds for thermal comfort when whole-body skin temperature was manipulated using a water-perfused suit,70 and breathing FIO2 = 0.115 did not alter preferred hand temperature.71 While this suggests that thermal perception is not altered by hypoxia, contradictory findings exist from the same research group, with a decrease in the toes’ sensitivity to cold during acute hypoxia (FIO2 = 0.14).72
Acute exposure to altitude in the field initially reduces CIVD response in lowland natives.73,74 This impairment was initially assumed to be directly attributable to hypoxia, as it was observed in native lowlanders directly airlifted to 3,500 m.74 A series of studies explored the separate and combined effects of hypoxia and cold on both the cutaneous vasculature and also whole-body thermoregulation. In a normothermic environment, isocapnic hypoxia, but not matching controlled hyperventilation, increased cutaneous vascular conductance at the forearm, suggesting a direct vasodilatory effect from hypoxia.75 This hypoxia-induced vasodilatation was maintained above normoxic levels despite brief 10 min whole-body skin cooling using a water-perfused suit, suggesting that cold-induced vasoconstrictor stimulus does not overwhelm the vasodilatory effect of hypoxia.76 While this may suggest a greater propensity for hypothermia due to higher peripheral blood flow and convective heat loss in hypoxia, the interaction between hypoxia and cold on vascular conductance may be dependent on the intensity of cold exposure. Prolonged cold exposure from whole-body immersion in 23°C for 30 min, followed by 10°C for 45 min was tested with normoxia and poikilocapnic hypoxia to 80% arterial saturation.77 While hypoxia induced vasodilatation before cold exposure, the intensity of vasoconstriction was higher during cold exposure with hypoxia compared to normoxia, and likely driven by a decrease in active vasodilatation.78 Parallel to this increased vasoconstriction, core cooling rates did not differ between normoxia and hypoxia.77 Thus, a sufficiently strong cold stimulus may overwhelm any hypoxic vasodilatory effect, and this may extend to local cold exposure and CIVD. Keramidis et al.79 reported that acute normobaric hypoxia (FIO2 = 0.14) prolonged vasoconstriction and decreased the rate of rewarming following 8°C hand immersion, but had no effect on any CIVD parameter during immersion compared to a control trial breathing normoxia (FIO2 = 0.21).
In contrast to brief exposure to hypoxia, prolonged altitude exposure progressively increases extremity blood flow. CIVD response initially decreased upon altitude exposure in 5 lowland natives;73 this response gradually improved over 45 d at altitude, but importantly did not recover back to sea level baseline. The effect of prolonged living or experience at altitude on CIVD is unclear. Mathew et al.74 found that high altitude natives had CIVD responses that were similar to those of lowland natives at sea level, while lowlanders living at altitude had intermediate responses at altitude compared to unacclimatized lowlanders and highland natives. In contrast, experienced Alpinists had a trend toward improved CIVD responses compared to non-Alpinist controls in both the hands and feet;80 furthermore, CIVD responses in these Alpinists systematically improved in the hands and especially in the feet following a 3-week Himalayan expedition.80 As typical with cross-sectional studies based on specific occupational or recreational groups, the contribution of self-selection can serve as a confounder.25,65,81-83 Whole body thermal status is known to be an important determinant for CIVD prevalence and intensity, with an inverse relationship between CIVD responses and body temperature, along with the lack of any observed CIVD below a threshold body temperature. Therefore, without more detailed nutritional and physiological tracking, overall differences in thermal balance or confounding factors such as altered nutrition or metabolism present during field or mountaineering studies make altitude and cold acclimatization studies difficult to interpret.
Recent studies have attempted to isolate the effects of prolonged hypoxic acclimatization on CIVD response while controlling confounders such as ambient pressure, exercise, and nutrition. Amon et al.84 attempted to isolate the effects of hypoxia on 8°C hand immersion before and following a live-high train-low regimen. While no changes in CIVD parameters were observed in a control group at either normoxia or hypoxia following 28 d of aerobic training, sleeping at 2,800 – 3,400 m elicited increased frequency and amplitude of CIVD along with higher finger skin temperature at both normoxia and hypoxia. In contrast, 10 d of confinement in normobaric hypoxia (FIO2 = 0.14) did not alter CIVD response to 8°C hand immersion, but reduced rewarming rates in normoxia conditions;85 in this same study, the experimental group performing exercise training while acclimatizing in normobaric hypoxia reported no CIVD changes.
Overall, the disparate findings suggest that the effects of acute and short-term hypoxia exposure, along with the mechanisms underlying improved extremity blood flow in experienced mountaineers or altitude natives, remains unclear. Hypoxia seems to hold much promise for advancing CIVD research due to its ability to alter peripheral blood flow and possibly thermal perception, with or without whole-body cold stress. Further responses common to both hypoxia and cold stress are likely to be alterations in autonomic nervous system activity. Thus, hypoxia may be used both separately and in conjunction with cold to test particular mechanisms of CIVD, such as by using levels of hypoxia to elicit specific changes to sympathetic neural drive that may be more easily controlled than by using whole body cold exposure or pharmacological agents.
Physical Fitness
While peripheral blood flow is strongly dependent upon overall body thermal status, which may be influenced by exposure to environmental and/or exercise-induced heat loads, the effect of chronic exercise training and improved physical fitness on CIVD is lacking. Aerobic fitness may improve the reactivity of the cutaneous vasculature, with greater vasodilatory responsiveness in both glabrous and non-glabrous skin in a group of competitive cyclists compared to sedentary controls.86 Endurance-trained older males had less reduction in the rapid vasodilator response to local heating than sedentary older males,87 while 48 weeks of endurance training in post-menopausal women improved their cutaneous microvascular reactivity.88 These data suggest a protective effect from exercise on skin blood flow control, and this may be due to improved endothelial health89 and specifically enhanced production or sensitivity to nitric oxide (NO) with increased fitness.90,91 Moriya and Nakagawa92 reported a significant positive correlation between maximal aerobic capacity, reported in absolute values of L·min-1, and higher finger temperature during 0°C immersion in relatively fit Japanese women born in the northern island of Hokkaido; however, these relationships disappeared when the same correlation was done using relative aerobic fitness, suggesting that body size or composition (data not reported) also played a role.
Acute exercise improved CIVD responses,93 with greater frequency of toe CIVD upon foot immersion immediately post-exercise compared to no exercise. This improvement was largely attributed to the 0.6°C increase in core temperature with exercise, as the prevalence and magnitude of CIVD responses have a strong direct relationship with overall body temperature81,82 or heat content.65,83 However, a protocol involving repeated exercise sessions eliciting ∼0.5°C core temperature rises during the course of hand immersion had no impact on improved CIVD responses.28 Therefore, while a fundamental determinant of the rate of heat acclimation is the magnitude and duration of regular heat exposure,94 a small additional thermal stress during exercise – stimulating increased blood volume and increased peripheral blood flow – does not appear to benefit local cold exposure.
Habitual physical activity, especially in the cold, has been assumed to benefit response and tolerance to local cold exposure. This comes from population studies involving particular ethnic groups such as polar natives95-97 and occupational groups such as fish filleters.98 Laboratory studies focusing on physical fitness effects have typically used aerobic capacity as the benchmark measure. The quality of this relationship is arguable, as high aerobic capacity by itself – whether achieved via training or natural genetics - prolonged exercise heat tolerance.99 No analogous study on the effects of physical fitness vs. aerobic capacity on cold tolerance has been performed. Following twenty aerobic training sessions over 4 weeks, through which maximal aerobic capacity increased by 13% in 18 moderately-fit males, thermal responses to hand immersion in 8°C was improved, with greater CIVD frequency, higher average skin temperature, and decreased pain sensation.100 Contrasting this beneficial effect of improved fitness, Keramidis et al.101 also tested the other extreme of local cold response with prolonged inactivity, using a 35-day bedrest model that decreased peak oxygen uptake by 18% (see Fig. 4). With foot immersion into 8°C, average skin temperature decreased 0.8°C post-bedrest, peaking at 1.6°C decrease in the big toe. However, with a negative-pressure vacuum challenge countermeasure – which maintains local vascular flow – regularly applied to the other leg, the decrement in foot thermal responses was negated. The combined studies100,101 suggest that aerobic fitness moderates vasoconstrictor tone with local cold exposure, such that low physical fitness may be a predictor of local cold injuries. At the same time, the effectiveness of the negative-pressure countermeasure in attenuating the thermal impairment also suggests that the mechanism may be a local maintenance of blood flow or vascular sensitivity rather than a systemic change in sympathetic drive.
Figure 4.
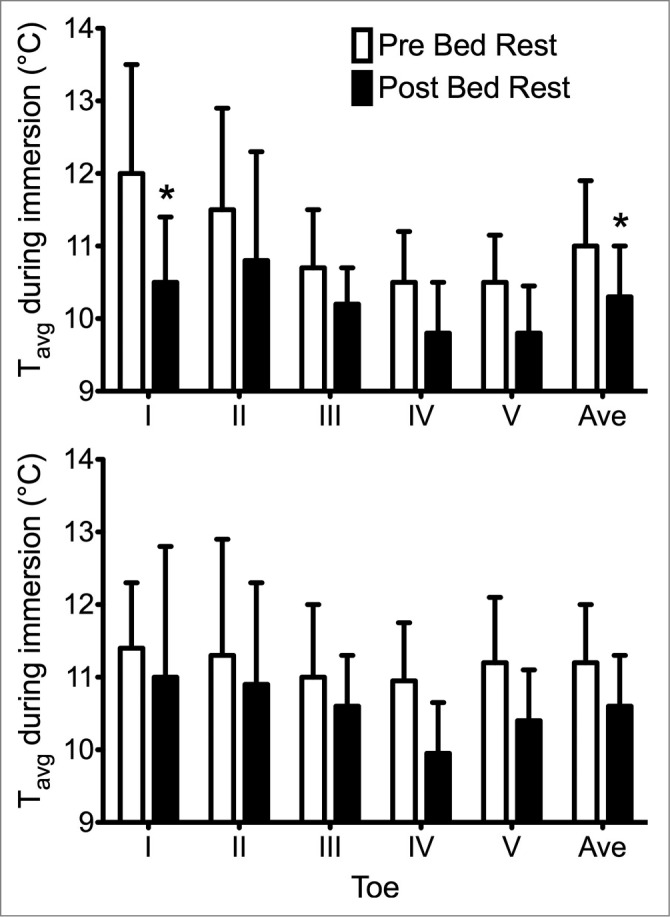
Average temperature (Tavg) in the 5 toes of the detrained foot (top) and vascular countermeasure foot (bottom) during the 30-min cold-water immersion performed pre- (open bars) and post-35 d horizontal bed rest (dark bars). *Significant decrease in average and big toe temperatures after bed rest in the detrained foot, but no differences were seen in the foot with the sub-atmospheric vascular pressure countermeasure throughout bedrest. Adapted from Keramidas et al., Appl Physiol Nutr Metab 2014; 39:369–74. Used with permission.
While it is tempting to conclude that physical fitness provides a beneficial local cryoprotective effect, a confounding and unexplored issue is the potential interactions between physical fitness, altered body composition, whole-body cold tolerance, and local thermal responses. Specifically, while physical fitness and aerobic capacity provides a clear advantage in reduced thermoregulatory strain and increased exercise-heat tolerance,99 the typical reduced body fatness and subcutaneous fat thickness with increasing fitness may predispose an individual to more rapid whole-body cooling. As CIVD is well-known to be highly dependent on overall body thermal status, the systemic changes from physical training may reduce whole-body cold tolerance and offset any local adaptations. Finally, it must be kept in mind that enhanced peripheral blood flow and vascular reactivity may be counterproductive to overall thermal balance by increasing blood flow and convective heat loss through the peripheries and extremities during cold exposure.64
Muscle Function
Survival in the cold is often dependent on being able to perform critical emergency tasks, such as operating an escape mechanism (e.g., unclipping a harness or turning an exit handle), holding onto a lifeline, opening a flare package, or even as mundane an act as closing a zipper. Performance of such actions quickly deteriorate with even brief cold exposure (10°C immersion of the hands and forearms),5 with Daanen102 developing a regression for expected deterioration in manual and hand dexterity along with grip strength based on the Wind Chill Equivalent Temperature and the square root of exposure time. Therefore, along with potential for protection from freezing and non-freezing cold injuries, another major underlying reason for the continued interest in CIVD research is its possible benefit for maintaining or improving manual dexterity and muscle function during cold exposure.
While a logical argument, data over the past decade suggests that muscle function is only minimally affected over the CIVD cycle. One counterargument is that, for CIVD to be useful for manual function, there should be increased blood flow and warming beyond the immediate local region of the fingers being measured. However, as seen in the Methods and Standardization section of this review, the relatively recent shift toward whole-hand/feet immersion and multiple measurement sites demonstrate both a wide spatial heterogeneity in thermal response, such as a 1.5–10°C gradient between the proximal and distal phalanges of a finger with hand exposure to 0°C air, and also a strong localization of CIVD to the distal portions of the fingers.103 In particular, Geurts et al.54 reported a sustained decrease in temperature of the first dorsal interosseous muscle at the same time as CIVD occurred in the index finger.
Investigating neuromuscular function of the whole hand with moderate to prolonged local cold exposure, focusing on the first dorsal interosseous muscle – important in index finger abduction and gripping – can provide more insight into the relevance of CIVD to manual function. The overall consensus from these studies was that cooling the first dorsal interosseous muscle through 30 min of local hand immersion in 8°C impaired the contractile characteristics during evoked twitches, including delayed time to peak tension, delayed half-relaxation time, and reduced peak twitch force.26-28,54,104 These impairments were not restored or improved by 2–3 weeks of 8°C hand immersion acclimation, along with minimal changes27,28 and even blunted26 CIVD response. Importantly, Geurts et al.54 reported that the nadir and peaks of CIVD at the index fingertip did not correlate with any parallel changes with first dorsal interosseous temperature, which continued to fall throughout immersion. Furthermore, neuromuscular testing specifically timed at the nadir and apex of CIVD did not elicit any difference in contractile characteristics, with both timepoints equally impaired compared to baseline (see Fig. 5). Thus, current data suggests that CIVD is highly localized to the distal portions of the digits, and may not be relevant for maintaining manual performance.
Figure 5.
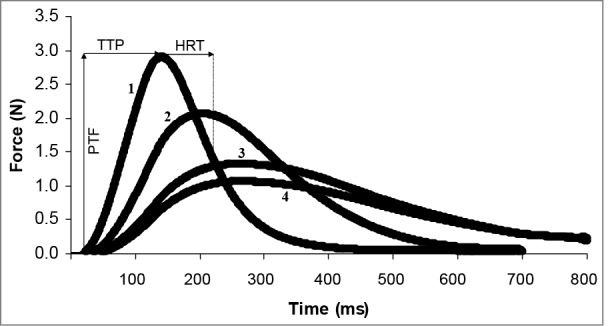
An example of evoked twitch force of the first dorsal interosseous muscle in one subject at 4 different stages during cold water immersion of the hand. (1) Initial [right index finger temperature (Tif) = 29.5°C, and skin temperature above the first dorsal interosseus (Tfdi) = 31.5°C], (2) nadir cold-induced vasodilatation (CIVD) (Tif = 9.5°C, Tfdi = 17.2°C), (3) apex CIVD (Tif = 13.3°C, Tfdi = 14.8°C), (4) final (Tif = 12.1°C, Tfdi = 14.1°C). Reprinted from Geurts et al., Eur J Appl Physiol; 2005; 93:524–9. Used with permission.
Along with muscle contractile characteristics, another important issue with manual function in the cold is changes to motor control due to the decreased or otherwise altered tactile sensitivity with digit cooling.105 Cooling the hands and forearms also caused systematic underestimation of handgrip force in both males and females,106 possibly as a result of suppressed proprioceptive activity. Compared to overall muscle changes, temporary digit cooling with the topical anesthetic ethyl chloride resulted in much higher grip forces both while stationary and at the apex and nadir of a vertical movement.107,108 However, the anticipatory and temporal coupling pattern of grip and load forces was maintained despite the greater force requirement. A follow up study by Cheung et al.109 reported similar observations with actual hand cooling to < 8°C local skin temperature, and this held whether core temperature was normothermic or hypo- or hyperthermic by 0.5°C. Overall, these findings suggest that grip control is ingrained in robust central motor patterns and not affected despite the requirement for greater force, though the motor control of more complex aspects of manual dexterity in the cold remain unexplored. Further, when learning new complex manual tasks, sensorimotor adaptation to cold appears critical, as performance time for rifle and radio tasks were slower for soldiers who trained only in warm conditions compared to those trained in cold, with additional improvements in those first trained in warm followed by cold conditions.110 Motor control impairments can also exist due to lower limb cooling, with a progressive decrease in dynamic balance with greater portions of the lower limb exposed to cold.111 The neuromuscular or tactile sensitivity effects of toe cooling, and their potential contribution to this altered lower limb motor control and balance, remains unknown.
Neural Mechanisms and Mediators
Skin blood flow during cold and heat exposure is a complex interplay between whole-body and local control, through both neural and non-neural regulation.112 With cooling, the initial vasoconstriction appears largely driven by systemic sympathetic activity.113-115 This was supported by leg immersion up to the knees in 12°C eliciting skin temperature decreases in non-cooled areas such as the thigh, palm, and fingers.116 The degree of systemic sympathetic activity can be altered with repeated local acclimation. For example, heart rate and blood pressure, along with initial skin temperature decrease, were reduced in locally acclimated individuals compared to controls,117 suggested a reduced sympathetic tone with local acclimation. In contrast, skin temperature in non-immersed areas were reduced in acclimated individuals, and this was supported by Mekjavić et al.31 reporting impaired thermal responses in both the hand used for repeated cold immersion acclimation along with the contralateral hand. These data therefore suggests an overall enhancement of sympathetic activity and vasoconstrictor tone with acclimation. Overall, these data suggest whole body sympathetic activity modulating responses to local cold exposure rather than a local mechanism.
While sympathetic activity may be an important driver in vasomotor tone with cooling, the direct role of sympathetic activity in initiating CIVD is equivocal. Based on analysis of CIVD occurrences in the finger over differing core temperatures along with heart rate variability analysis, Flouris and Cheung65 concluded that CIVD was central in origin from sympathetic withdrawal, and that its primary purpose may be as a whole-body heat loss mechanism.65,83 This proposal was based on CIVD occurring consistently only above a threshold mean body temperature regardless of extremity temperature, along with CIVD ending when body temperature decreased below this threshold.83 Brandstrom et al.118 compared heart rate variability in military special forces members to a local cold provocation, and reported a higher power in both the low and high frequency components in normal rewarmers compared to slow rewarmers. Both groups had reduced heart rates and altered heart rate variability parameters upon cold provocation following 15 months of winter military training. Together, such indirect studies of the autonomic nervous system suggest their role in controlling peripheral blood flow and vasodilatation with local cold exposure.
Direct examination – such as by nerve injury models – are impossible to perform in humans except for post-injury examinations. Peripheral sympathectomy in rats resulted in cold intolerance and mechanical hypersensitivity but did not affect CIVD response in the hind paws, suggesting that the sympathetic system does not play a major role in the initiation or regulation of CIVD.119 Also in the rat hind limb, Kusters et al.120 reported a trend toward reduced CIVD responses with sciatic nerve transection and repair, which then gradually improved with nerve regeneration beyond 35 d The majority of 107 human patients surveyed with median and/or ulnar nerve injury reported substantial cold intolerance121 that was greater than non-injured controls.122 In 4 of 12 patients with median or ulnar nerve injury, CIVD responses to local digit cooling were absent in the injured hand but present in the non-injured hand; 6 patients, however, exhibited CIVD responses in the injured hand despite perceptual cold intolerance.123 Basal blood flow increased with neural blockade at the finger tip, and even more with local skin anesthesia; furthermore, during local cooling, vasoconstriction was maintained with nerve blockade at the finger base, but was inhibited by local anesthesia.124 These animal and clinical studies imply that CIVD regulation may not be dominated by sympathetic activity, but rather that vascular tone at the finger skin is strongly dependent on local reflexes. Future research will need to separate and investigate the relative contributions of central versus local regulation of the CIVD response.
Vasomotor tone may then be modified by a range of local or systemically derived non-adrenergic mediators. Chief among the candidates for local regulators of skin blood flow during cold stress has been NO, a potent vasodilator.125 The vasodilatory role of NO in skin blood flow control112,125 has led to its exploration as a stimulus for regulating CIVD. While it is unclear whether exhaled NO is representative or a direct result of vascular production,126 exhaled NO during cold (−10°C) running exercise was less than in temperate conditions both during127 and after128 exercise. Using in vitro microvascular endothelial cell cultures, Binti MD Isa et al.129 tested the effects of temperature changes along with shear stress on endothelial NO synthase (eNOS) activation. Cold (4°C) temperature by itself did not affect eNOS activation over 1 h of exposure; cold combined with shear stress did not affect eNOS over 15 and 30 min, but significantly decreased its activation at 60 min. Hodges et al.130 showed that local skin cooling reduced NOS activity and NO handling downstream of NO at the smooth muscle in non-glabrous skin. Furthermore, sublingual glycerol trinitrate, an endothelial-independent NO donor, improved toe temperature and blood flow during cold immersion of the foot along with the rate of rewarming in cold-sensitive individuals.131 This suggests that NO may play a role in regulating microvascular circulation in the cold, especially in the maintenance of vasoconstriction and inhibition of vasodilatation. However, further work is required to confirm whether there is a direct relationship between local NO activity at the extremities and the sudden vasodilation associated with CIVD.
Another hormonal mediator with cold exposure is endothelin-1, a vasoconstrictor peptide. In a rat model, prolonged cold exposure of up to 5 weeks resulted in an elevated level of endothelin-1 along with enhanced receptor expression in the heart, mesenteric arteries, and kidneys.132 In humans, endothelin-1 was measured by Nakamura et al.133 in control individuals, along with individuals with Raynauds and also vibration-induced white finger. Endothelin-1 levels were elevated rapidly upon finger cold immersion in both control and Raynauds individuals, but was much higher in Raynauds and it remained elevated even after immersion. However, there was no correlation between endothelin-1 levels and incidences of CIVD, suggesting that, while endothelin-1 is highly related to sympathetic hyperactivity, it is not directly contributing to the opening of peripheral blood vessels eliciting CIVD.133
The role of changes in neurotransmitter activity or sensitivity in extremity cold response is difficult to investigate, largely due to methodological hurdles. One issue is the difficulty in continuous blood sampling over the frequent time points required to detect rapid CIVD response. A second hurdle is the tenuous extrapolation from systemic blood samples to local action at the skin or specifically the extremities. Related to this difficulty of local manipulations and sampling, while intradermal microdialysis has been employed to selectively block sympathetic activity or neurotransmitters such as NO at the forearm and thigh skin surface, this cannot be used directly at the site of CIVD activity at the fingers or toes. Overall, the difficulty of such research is seen in that only one study exists concerning local mediating factors and CIVD response.27 This study investigated NO and endothelin-1 response to cold acclimation was performed before and after 2 weeks of daily 30 min hand immersions in 8°C, with no observed changes in either venous endothelin-1 nor NO levels. Apart from the logistical and sampling difficulties, the lack of overall local thermal acclimation in CIVD responses further precluded any firm conclusions.
Methods & Standardization
One of the issues plaguing CIVD research has been the relative lack of consistency in its definition and testing, making it hard to compare findings across studies or to build a consensus on overall effects. Since the initial report by Lewis,15 the dominant CIVD protocol has involved single-finger immersion into cold to near-freezing water. Such a protocol makes practical sense, due to the relative ease of maintaining near-freezing water temperatures with snow or ice in the field, the relative difficulty in setting up toe or foot immersion, along with the ability to test multiple individuals simultaneously when using a single data channel per subject. For example, Daanen and van der Struijs36 used response time and magnitude from single-finger immersion in ice water for 30 min to tabulate a Resistance Index of Frostbite (RIF)134 in 206 Dutch marines before Arctic exercises, and found that the 11 soldiers who experienced cold injuries had lower RIF than the non-injured. Such a study highlights both the simplicity of CIVD research and also the limitations of such a logistically simple field study design. Arguably, a more complex protocol, requiring more sensors per subject, longer immersions, larger basins for whole-limb immersion or thermal control with equipment besides snow/ice, would make such field studies difficult. The same logistical arguments also extend to mountaineering studies in the field. Another simple methodological issue is that the presence or absence of appropriate and consistent water circulation – important due to its effect on convective heat loss – is often not reported in the literature.
Irrespective of field constraints, the scientific validity of single-finger testing or measurement, especially when such data may be extrapolated to overall risks for cold injuries across the entire hand or feet, is highly questionable.135 Practically, it is rare that only a single digit is exposed to cold in field settings. Further, such extrapolation assumes that CIVD responses are homogenous across all fingers, and also that responses from the fingers can be directly translated to responses at the feet. For example, Purkayastha et al.136 immersed the whole hand in 4°C water, yet measured only the index finger. Chen et al.103 reported a wide spatial heterogeneity of responses across the hand, and this has been supported by further studies over the past decade. Hand immersion in 8°C, measured at the nailbed of each digit, elicited a wide range of responses across digits, including: different magnitudes of CIVD among fingers, CIVD in some but not all fingers, and asynchronous timing of CIVD onset.29,53,135 Reynolds et al.30 reported similar spatial heterogeneity with toe response to whole-foot immersion. Overall, toe CIVD responses were either absent135 or greatly reduced30 compared to hand immersion, highlighting the disparity in responses across the hands and feet and the impracticality of extrapolation across body regions.
Standardization in CIVD quantification has improved since the definition of many parameters by Daanen.16 These include parameters such as minimum/mean/maximum temperatures, onset time, amplitude, and peak time. However a systemic problem in CIVD research is defining a consistent measurement site and threshold magnitude for CIVD. O’Brien137 performed a reliability study over 5 separate single-finger immersions, and reported an overall greater reproducibility at the nail bed compared to the pad of the middle finger. Despite this recommendation, studies have continued to use digit pad as a measurement site, citing this location as rich in both blood flow and arteriovenous anastomoses (AVA) presence.31 Further, due to the high variability in toe response measured at the nail bed,30 the toe pad has been maintained for some studies.29 A large proportion of studies define CIVD with a 0.5°C threshold,31,53,84,93,100,137 while others opt for a more conservative 1.0°C,29,30,135 and even 4.0°C83 threshold. Not surprisingly, differences in these most basic sites and definition of CIVD can strongly impact conclusions and hamper comparisons across studies; standardization is a fundamental requirement to continued progress.
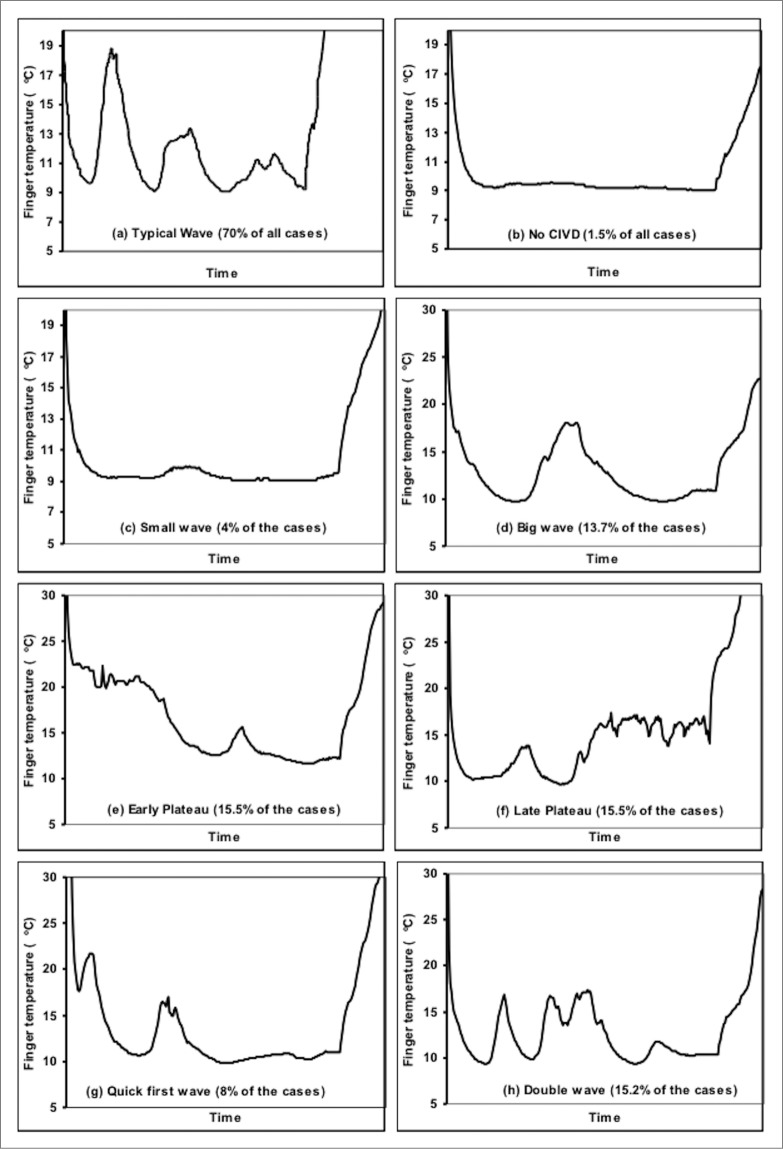
Another important change in studies within this century has been increasing recognition that CIVD can form a multiplicity of responses, rather than the traditional sharply defined and cyclical pattern stylized in Figure 1. Instead, many types of CIVD responses can occur30,31,53,135 as outlined in Figure 6. These responses include: no waves, traditional cyclical waves, small (1–2°C) waves, single large wave, early plateau, late plateau, quick first wave (occurring during the initial drop in skin temperature with immersion), and double wave (CIVD initiating during decreasing skin temperature from a prior CIVD). Interestingly, Cheung and Mekjavić135 reported instances of asynchronous CIVD over different fingers of the same hand during immersion, suggesting either a local mechanism of action or else temporally offset responses to a central vasodilatory drive. Such disparate responses, all of which fit within the traditional parameter of a threshold rise in skin temperature, further complicate quantification of CIVD. For example, a sustained plateau response would only be quantified as a single CIVD response and may be concluded as relatively ineffective compared to many frequent small cyclic CIVD waves (i.e. low vs. high CIVD frequency), yet it may be arguably more beneficial for tissue integrity due to greater blood flow.
Figure 6.
Representative finger skin temperature responses and frequency of occurrence during cold water (8°C) immersion derived from 9 participants and 5 fingers over 13 immersions of the right hand. Reprinted from Mekjavić et al., Eur J Appl Physiol; 2008; 104:193–9. Used with permission.
Modeling
Cold injuries continue to be a challenge in modern military operations138,139 and many other occupations.3,4 Ultimately, a primary goal of research in CIVD is to be able to predict an individual's response to cold exposure, and to model the effects on performance and injury risk. This can be broken down into 2 components: 1) an a priori prediction for who may be at risk for cold injury, and 2) a model for thermal response during cold exposure.
Risk Prediction
The goal of risk prediction can be to understand who may be at greatest risk for developing cold injuries, and to decrease those risks by either removing those individuals from danger beforehand, or else to plan preventative or care policies. Some information may be gleaned from epidemiological or cross-sectional population studies highlighted earlier in this review, demonstrating that racial differences exist in cold injury prevalence. Another tool may be screening tests. A direct screening test of a brief 30 min single-finger immersion in 0°C water has been proposed by Daanen and van der Struijs.36 The study tested 190 Netherlands Marines before Arctic deployment, and classified their CIVD responses (minimum and mean temperature along with time of CIVD onset) according to the RIF. The 11 Marines who developed cold injuries in the Daanen and van der Struijs36 cohort had a lower/worse RIF (5.2 ± 1.6) compared to the non-injured (7.0 ± 1.6), suggesting the possible utility of this simple finger immersion screening tool for both hand and foot prediction. The same study observed higher RIF in Caucasians compared to a combined other ethnicities, along with a higher RIF in smokers compared to non-smokers. While hand or finger water immersion is the dominant mode of CIVD testing, foot cold injuries are at least as common as hand injuries.11 As highlighted by the catalog of injuries in the 11 afflicted,36 approximately equal cases occurred in the hands and feet, suggesting that cold water immersion of the finger can predict cold air response of the feet. Such a correlation was indeed reported between CIVD responses in the fingers during hand immersion in 5°C water and toes with foot exposure to −18°C air.140 However, other studies report no correlation, with moderate prevalence of finger CIVD with 8°C hand immersion but no cases of toe CIVD during 8°C foot immersion.135 Therefore, further studies are needed to clearly prove or develop predictive models extrapolating across body regions.
Cold Exposure Modeling
Cold temperatures can rapidly decrease finger temperature and manual performance within seconds of exposure.5 Another modeling focus has therefore been to quantify the changes in extremity temperature and manual performance based on such parameters as ambient conditions and duration of exposure. Numerous mathematical models for finger temperature and CIVD prediction have been developed and refined over the past 15 years,141,142 with major controllers incorporating body/core temperature, finger skin temperature, and rate of initial finger cooling. In contrast to modeling skin temperature, Carrillo et al.143 developed a regression equation to predict finger blood flow based on core and finger temperatures, and a similar approach of modeling limb blood flow and heat generation based on skin temperature have been proposed.144 In terms of direct application toward changes in manual performance – finger dexterity, hand dexterity, and grip strength – Daanen102 found that Wind Chill Equivalent Temperature and the square root of exposure duration were the strongest predictors, providing a potentially easily measured model for use in the field.
The development of mathematical models are dependent upon quality data from human studies differentiating and validating such inputs. Across a range of core and local extremity temperatures, a very strong core temperature dominance in extremity blood flow regulation existed in both the hands and feet, along with consistently lower blood flow in all conditions in the foot compared to the hand.145 This supports the importance of maintaining a warm core for maximizing local blood flow82 and the greater prevalence and risk of cold injuries in the foot.11 Flouris and Cheung146 found mean body temperature and the rate of body heat storage, rather than rectal or tympanic temperature, to be most strongly associated with finger blood flow during whole body heating and cooling. Using whole-body cold exposure, Vanggaard et al.147 demonstrated an initial, robust defense in finger temperature, which then dropped sharply upon reaching a relatively narrow range of 32.1–35.8°C. This threshold finger temperature was more distinct than the minimal change in core temperature or thermal sensation, or the wide range of mean skin temperature, supporting the importance of incorporating actual finger temperature measurement into models. A database of finger contact with different metallic and non-metallic materials across a wide range of cold temperatures from +2 to −40°C has also been used to establish time for finger skin temperature to reach pain (15°C), numbness (7°C), and frostbite risk (0°C)148 These non-linear contact times are presented in Figure 7. Overall, such specific testing of particular driving inputs and conditions remain critical in improving occupational exposure guidelines.
Figure 7.
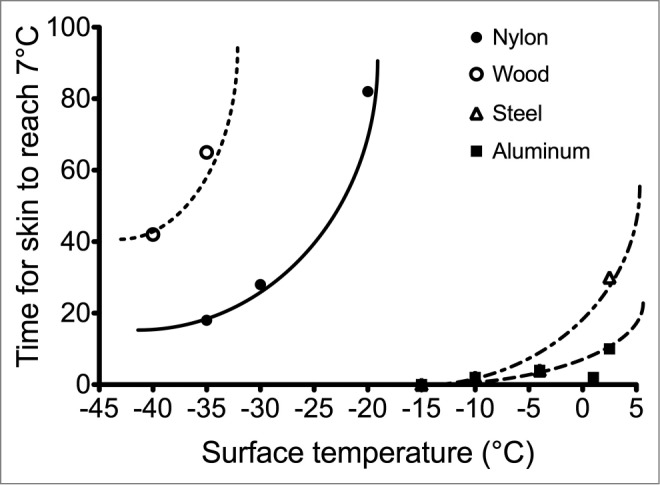
Observed (symbols) and predicted (lines) lower quartiles of the time for the fingertips to reach a contact temperature of 7°C for wood, nylon, steel, and aluminum. Adapted from Geng et al., Ann Occup Hyg; 2006; 50:851–62. Used with permission.
Future Research
Future research into the thermal responses of the extremities require further studies directly investigating the specific potential mechanisms for CIVD. While the present review has highlighted recent literature on central and peripheral neural control along with mediating factors, research focused specifically on understanding how CIVD is initiated and regulated lags far behind those of skin blood flow control as a whole. This latter field112 has primarily used the forearm and, less frequently, the leg as study sites due to their relatively large surface area, but CIVD is only rarely studied or reported at the forearm.18 Specifically, iontophoresis may be a useful method of introducing specific neurotransmitters to the fingertips to study local microvascular control.124
Research into the thermal responses of the extremities to cold exposure is both simple and complicated. It is simple because of the relative ease of instrumentation with skin temperature measurement, which are unobstrusive enough to permit field studies. However, while a fundamental measure, skin temperature provides minimal mechanistic insight into blood flow control. The dominant variable supplementing skin temperature has been cutaneous blood flow, measured using laser-Doppler probes or strain-gauge plethysmography, and these have provided important mechanistic insights. However, besides being currently restricted to laboratory settings due to technological limitations, these measures can be easily affected by movement, making them difficult to use during exposure or dynamic testing. Improvements in imaging for both skin temperature and blood flow has also permitted a more holistic measure of overall extremity thermal and hemodynamics than the point measures from wired probes, and are more practical for field testing.149,150 Relatively newer techniques for quantifying microcirculation or muscular physiology, such as sidestream dark field imaging,151 near-infrared spectroscopy,152 and laser-speckle contrast imaging153 are emerging as tools potentially relevant to finger blood flow control. However, to date sidestream dark field imaging was developed for microcirculation in the sublingual and gastrointestinal tissues, and it has yet to be validated for measuring cutaneous tissue. Near-infrared spectroscopy can provide information on tissue oxygenation, but the probes currently remain too large for use in the extremities. The study of extremity blood flow is likely to experience an exciting growth due to new technological developments, and Daly and Leahy154 provides an excellent survey of existing and emerging technologies for blood flow measurement.
In terms of expanding the scope of extremity cold exposure research, a fruitful avenue appears to be integration with research into chronic diseases such as diabetes and hypertension. These fields, along with aging discussed earlier, already have large bodies of literature examining skin blood flow control alterations and their underlying mechanisms. For example, one of the main long-term risks with diabetes is peripheral neuropathies and potential amputations due to impaired circulation. Incorporating such information can further enrich existing work on peripheral cold exposure by providing mechanistic models or alternate experimental platforms. Apart from mechanistic cross-pollination, there is a strong clinical imperative to protect these vulnerable populations from risks of cold injuries.
Biography
Stephen S Cheung, PhD, is a Professor and Canada Research Chair in Environmental Ergonomics within the Department of Kinesiology at Brock University, Canada. His research includes mechanistic studies on neuromuscular function with thermal and hypoxic stress, thermal and blood flow responses of the hands to cold exposure, and hydration impact on exercise capacity. His applied research includes minimizing heat strain with protective clothing for the Toronto Fire Service and Canadian Forces along with improving survival modeling for Search & Rescue. He has authored the textbook “Advanced Environmental Exercise Physiology” and co-authored “Cutting-Edge Cycling” on cycling science.

Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
SS Cheung is supported by a Canada Research Chair.
About the Author
Stephen S Cheung, PhD, is a Professor and Canada Research Chair in Environmental Ergonomics within the Department of Kinesiology at Brock University, Canada. His research includes mechanistic studies on neuromuscular function with thermal and hypoxic stress, thermal and blood flow responses of the hands to cold exposure, and hydration impact on exercise capacity. His applied research includes minimizing heat strain with protective clothing for the Toronto Fire Service and Canadian Forces along with improving survival modelling for Search & Rescue. He has authored the textbook “Advanced Environmental Exercise Physiology” and co-authored “Cutting-Edge Cycling” on cycling science.
References
- 1. Taylor NAS, Machado-Moreira CA, van den Heuvel AMJ, Caldwell JN. Hands and feet: physiological insulators, radiators and evaporators. Eur J Appl Physiol 2014; 114:2037-60; PMID:25011493; http://dx.doi.org/ 10.1007/s00421-014-2940-8 [DOI] [PubMed] [Google Scholar]
- 2. Romanovsky AA. Skin temperature: its role in thermoregulation. Acta Physiol 2014; 210:498-507; PMID:24716231; http://dx.doi.org/ 10.1111/apha.12231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Oksa J, Hosio S, Mäkinen T, Lindholm H, Rintamäki H, Rissanen S, Latvala J, Vaara K, Oksa P. Muscular, cardiorespiratory and thermal strain of mast and pole workers. Ergonomics 2014; 57:669-78; PMID:24655301; http://dx.doi.org/ 10.1080/00140139.2014.895854 [DOI] [PubMed] [Google Scholar]
- 4. Bridgman SA. Thermal status of antarctic divers. Aviat Space Environ Med 1990; 61:795-801; PMID:2241743. [PubMed] [Google Scholar]
- 5. Cheung SS, Montie DL, White MD, Behm D. Changes in manual dexterity following short-term hand and forearm immersion in 10°C water. Aviat Space Environ Med 2003; 74:990-3; PMID:14503680. [PubMed] [Google Scholar]
- 6. Brajkovic D, Ducharme MB. Finger dexterity, skin temperature, and blood flow during auxiliary heating in the cold. J Appl Physiol 2003; 95:758-70; PMID:12730145. [DOI] [PubMed] [Google Scholar]
- 7. Flouris AD, Cheung SS, Fowles JR, Kruisselbrink LD, Westwood DA, Carrillo AE, Murphy RJ. Influence of body heat content on hand function during prolonged cold exposures. J Appl Physiol 2006; 101:802-8; PMID:16709657; http://dx.doi.org/ 10.1152/japplphysiol.00197.2006 [DOI] [PubMed] [Google Scholar]
- 8. Golden F, Tipton M. Essentials of Sea Survival Champaign, IL: Human Kinetics, 2002. [Google Scholar]
- 9. Imray CH, Richards P, Greeves J, Castellani JW. Nonfreezing cold-induced injuries. J R Army Med Corps 2011; 157:79-84; PMID:21465916; http://dx.doi.org/ 10.1136/jramc-157-01-14 [DOI] [PubMed] [Google Scholar]
- 10. Golant A, Nord RM, Paksima N, Posner MA. Cold exposure injuries to the extremities. J Am Acad Orthop Surg 2008; 16:704-15; PMID:19056919. [DOI] [PubMed] [Google Scholar]
- 11. Valnicek SM, Chasmar LR, Clapson JB. Frostbite in the prairies: a 12-year review. Plast Reconstr Surg 1993; 92:633-41; PMID:8356126; http://dx.doi.org/ 10.1097/00006534-199309001-00012 [DOI] [PubMed] [Google Scholar]
- 12. Hamlet MP. Human Cold Injuries. In: Pandolf KB, Sawka MN, Gonzalez RR, ed. Human Performance Physiology and Environmental Medicine in Terrestrial Extremes. Indianapolis: Benchmark Press, 1988:435-466. [Google Scholar]
- 13. Wilson O, Goldman RF. Role of air temperature and wind in the time necessary for a finger to freeze. J Appl Physiol 1970; 29:658-64; PMID:5474858. [DOI] [PubMed] [Google Scholar]
- 14. Castellani JW, Young AJ, Ducharme MB, Giesbrecht GG, Glickman E, Sallis RE. American college of sports medicine position stand: prevention of cold injuries during exercise. Med Sci Sports Exerc 2006; 38:2012-29; PMID:17095937; http://dx.doi.org/ 10.1249/01.mss.0000218122.59968.eb [DOI] [PubMed] [Google Scholar]
- 15. Lewis T. Observations upon the reactions of the vessels of the human skin to cold. Heart 1930; 15:177-208. [Google Scholar]
- 16. Daanen HA. Finger cold-induced vasodilation: a review. Eur J Appl Physiol 2003; 89:411-26; PMID:12712346; http://dx.doi.org/ 10.1007/s00421-003-0818-2 [DOI] [PubMed] [Google Scholar]
- 17. Brajkovic D, Ducharme MB. Facial cold-induced vasodilation and skin temperature during exposure to cold wind. Eur J Appl Physiol 2006; 96:711-21; PMID:16450168; http://dx.doi.org/ 10.1007/s00421-005-0115-3 [DOI] [PubMed] [Google Scholar]
- 18. Ducharme MB, VanHelder WP, Radomski MW. Cyclic intramuscular temperature fluctuations in the human forearm during cold-water immersion. Eur J Appl Physiol Occup Physiol 1991; 63:188-93; PMID:1761006; http://dx.doi.org/ 10.1007/BF00233846 [DOI] [PubMed] [Google Scholar]
- 19. Fox RH, Wyatt HT. Cold-induced vasodilatation in various areas of the body surface of man. J Physiol 1962; 162:289-97; PMID:13894393; http://dx.doi.org/ 10.1113/jphysiol.1962.sp006933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Spealman CR. Effect of ambient air temperature and of hand temperature on blood flow in hands. Am J Physiol 1945; 145:218-22; PMID:21009263. [DOI] [PubMed] [Google Scholar]
- 21. Bergersen TK. A search for arteriovenous anastomoses in human skin using ultrasound doppler. Acta Physiol Scand 1993; 147:195-201; PMID:8475746; http://dx.doi.org/ 10.1111/j.1748-1716.1993.tb09489.x [DOI] [PubMed] [Google Scholar]
- 22. Bergersen TK, Eriksen M, Walloe L. Effect of local warming on hand and finger artery blood velocities. Am J Physiol 1995; 269:R325-30; PMID:7653653. [DOI] [PubMed] [Google Scholar]
- 23. Bergersen TK, Eriksen M, Walloe L. Local constriction of arteriovenous anastomoses in the cooled finger. Am J Physiol 1997; 273:R880-6.; PMID:9321863. [DOI] [PubMed] [Google Scholar]
- 24. Bergersen TK, Hisdal J, Walloe L. Perfusion of the human finger during cold-induced vasodilatation. Am J Physiol 1999; 276:R731-7; PMID:10070133. [DOI] [PubMed] [Google Scholar]
- 25. Cheung SS, Daanen HA. Dynamic adaptation of the peripheral circulation to cold exposure. Microcirculation 2012; 19:65-77; PMID:21851473; http://dx.doi.org/ 10.1111/j.1549-8719.2011.00126.x [DOI] [PubMed] [Google Scholar]
- 26. Geurts CL, Sleivert GG, Cheung SS. Local cold acclimation of the hand impairs thermal responses of the finger without improving hand neuromuscular function. Acta Physiol Scand 2005; 183:117-24; PMID:15654925; http://dx.doi.org/ 10.1111/j.1365-201X.2004.01374.x [DOI] [PubMed] [Google Scholar]
- 27. Geurts CL, Sleivert GG, Cheung SS. Central and peripheral factors in thermal, neuromuscular, and perceptual adaptation of the hand to repeated cold exposures. Appl Physiol Nutr Metab 2006; 31:110-7; PMID:16604128; http://dx.doi.org/ 10.1139/h05-007 [DOI] [PubMed] [Google Scholar]
- 28. Geurts CL, Sleivert GG, Cheung SS. Local cold acclimation during exercise and its effect on neuromuscular function of the hand. Appl Physiol Nutr Metab 2006; 31:717-25; PMID:17213886; http://dx.doi.org/ 10.1139/h06-076 [DOI] [PubMed] [Google Scholar]
- 29. Daanen HAM, Koedam J, Cheung SS. Trainability of cold induced vasodilatation in fingers and toes. Eur J Appl Physiol 2012; 112:2595-601; PMID:22081047; http://dx.doi.org/ 10.1007/s00421-011-2233-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Reynolds LF, Mekjavic IB, Cheung SS. Cold-induced vasodilatation in the foot is not homogenous or trainable over repeated cold exposure. Eur J Appl Physiol 2007; 102:73-8; PMID:17891413; http://dx.doi.org/ 10.1007/s00421-007-0566-9 [DOI] [PubMed] [Google Scholar]
- 31. Mekjavić IB, Dobnikar U, Kounalakis SN, Musizza B, Cheung SS. The trainability and contralateral response of cold-induced vasodilatation in the fingers following repeated cold exposure. Eur J Appl Physiol 2008; 104:193-9; PMID:18408950; http://dx.doi.org/ 10.1007/s00421-008-0727-5 [DOI] [PubMed] [Google Scholar]
- 32. Wakabayashi H, Wijayanto T, Kuroki H, Lee J-Y, Tochihara Y. The effect of repeated mild cold water immersions on the adaptation of the vasomotor responses. Int J Biometeorol 2012; 56:631-7; PMID:21695574; http://dx.doi.org/ 10.1007/s00484-011-0462-1 [DOI] [PubMed] [Google Scholar]
- 33. Ingram BJ, Raymond TJ. Recognition and treatment of freezing and nonfreezing cold injuries. Curr Sports Med Rep 2013; 12:125-30; PMID:23478565; http://dx.doi.org/ 10.1249/JSR.0b013e3182877454 [DOI] [PubMed] [Google Scholar]
- 34. Firth PG, Zheng H, Windsor JS, Sutherland AI, Imray CH, Moore GW, Semple JL, Roach RC, Salisbury RA. Mortality on mount everest, 1921-2006: descriptive study. BMJ 2008; 337:a2654; PMID:19074222; http://dx.doi.org/ 10.1136/bmj.a2654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Harirchi I, Arvin A, Vash JH, Zafarmand V. Frostbite: incidence and predisposing factors in mountaineers. Br J Sports Med 2005; 39:898-901; PMID:16306495; http://dx.doi.org/ 10.1136/bjsm.2004.016097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Daanen HA, van der Struijs NR. Resistance index of frostbite as a predictor of cold injury in arctic operations. Aviat Space Environ Med 2005; 76:1119-22; PMID:16370261. [PubMed] [Google Scholar]
- 37. Davey M, Eglin C, House J, Tipton M. The contribution of blood flow to the skin temperature responses during a cold sensitivity test. Eur J Appl Physiol 2013; 113:2411-7; PMID:23760737; http://dx.doi.org/ 10.1007/s00421-013-2678-8 [DOI] [PubMed] [Google Scholar]
- 38. Morrison SA, Gorjanc J, Mekjavic IB. Mount everest and makalu cold injury amputation: 40 years on. High Alt Med Biol 2014; 15:78-83; PMID:24559468; http://dx.doi.org/ 10.1089/ham.2013.1069 [DOI] [PubMed] [Google Scholar]
- 39. Maley MJ, Eglin CM, House JR, Tipton MJ. The effect of ethnicity on the vascular responses to cold exposure of the extremities. Eur J Appl Physiol 2014; 114:2369-79; PMID:25081130; http://dx.doi.org/ 10.1007/s00421-014-2962-2 [DOI] [PubMed] [Google Scholar]
- 40. Reimann M, Hamer M, Schlaich MP, Malan NT, Ruediger H, Ziemssen T, Malan L. Greater cardiovascular reactivity to a cold stimulus is due to higher cold pain perception in black africans: the sympathetic activity and ambulatory blood pressure in africans (SABPA) study. J Hypertens 2012; 30:2416-24; PMID:23111622; http://dx.doi.org/ 10.1097/HJH.0b013e328358faf7 [DOI] [PubMed] [Google Scholar]
- 41. Lee J-Y, Bakri I, Matsuo A, Tochihara Y. Cold-induced vasodilation and vasoconstriction in the finger of tropical and temperate indigenes. J Therm Biol 2013; 38:70-8; http://dx.doi.org/ 10.1016/j.jtherbio.2012.11.004 [DOI] [Google Scholar]
- 42. Burgess JE, Macfarlane F. Retrospective analysis of the ethnic origins of male British army soldiers with peripheral cold weather injury. J R Army Med Corps 2009; 155:11-5; PMID:19817081; http://dx.doi.org/ 10.1136/jramc-155-01-04 [DOI] [PubMed] [Google Scholar]
- 43. DeGroot DW, Castellani JW, Williams JO, Amoroso PJ. Epidemiology of US army cold weather injuries, 1980-1999. Aviat Space Environ Med 2003; 74:564-70; PMID:12751587 [PubMed] [Google Scholar]
- 44. Jay O, Havenith G. Finger skin cooling on contact with cold materials: a comparison between male and female responses during short-term exposures. Eur J Appl Physiol 2004; 91:373-81; PMID:14605896; http://dx.doi.org/ 10.1007/s00421-003-0986-0 [DOI] [PubMed] [Google Scholar]
- 45. Jay O, Havenith G. Finger skin cooling on contact with cold materials: an investigation of male and female responses during short-term exposures with a view on hand and finger size. Eur J Appl Physiol 2004; 93:1-8; PMID:15205959; http://dx.doi.org/ 10.1007/s00421-004-1146-x [DOI] [PubMed] [Google Scholar]
- 46. Lunt H, Tipton M. Differences in conductive foot cooling: a comparison between males and females. Eur J Appl Physiol 2014; 114:2635-44; PMID:25173096; http://dx.doi.org/ 10.1007/s00421-014-2988-5 [DOI] [PubMed] [Google Scholar]
- 47. Degroot DW, Kenney WL. Impaired defense of core temperature in aged humans during mild cold stress. Am J Physiol Regul Integr Comp Physiol 2007; 292:R103-8; PMID:17197640; http://dx.doi.org/ 10.1152/ajpregu.00074.2006 [DOI] [PubMed] [Google Scholar]
- 48. Greaney JL, Stanhewicz AE, Kenney WL, Alexander LM. Muscle sympathetic nerve activity during cold stress and isometric exercise in healthy older adults. J Appl Physiol 2014; 117:648-57; PMID:25103970; http://dx.doi.org/ 10.1152/japplphysiol.00516.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. DeGroot DW, Havenith G, Kenney WL. Responses to mild cold stress are predicted by different individual characteristics in young and older subjects. J Appl Physiol 2006; 101:1607-15; PMID:16888045; http://dx.doi.org/ 10.1152/japplphysiol.00717.2006 [DOI] [PubMed] [Google Scholar]
- 50. Guergova S, Dufour A. Thermal sensitivity in the elderly: a review. Ageing Res Rev 2011; 10:80-92; PMID:20685262; http://dx.doi.org/ 10.1016/j.arr.2010.04.009 [DOI] [PubMed] [Google Scholar]
- 51. Sawada S. Cold-induced vasodilatation response of finger skin blood vessels in older men observed by using a modified local cold tolerance test. Ind Health 1996; 34:51-6; PMID:8707622; http://dx.doi.org/ 10.2486/indhealth.34.51 [DOI] [PubMed] [Google Scholar]
- 52. Birnie KA, Parker JA, Chambers CT. Relevance of water temperature, apparatus, and age to children's pain during the cold pressor task. Pain Pract 2014;; PMID:25385307; http://dx.doi.org/ 10.1111/papr.12257 [DOI] [PubMed] [Google Scholar]
- 53. Mekjavic IB, Dobnikar U, Kounalakis SN. Cold-induced vasodilatation response in the fingers at 4 different water temperatures. Appl Physiol Nutr Metab 2013; 38:14-20; PMID:23368823; http://dx.doi.org/ 10.1139/apnm-2012-0118 [DOI] [PubMed] [Google Scholar]
- 54. Geurts CL, Sleivert GG, Cheung SS. Effect of cold-induced vasodilatation in the index finger on temperature and contractile characteristics of the first dorsal interosseus muscle during cold-water immersion. Eur J Appl Physiol 2005; 93:524-9; PMID:15605281; http://dx.doi.org/ 10.1007/s00421-004-1254-7 [DOI] [PubMed] [Google Scholar]
- 55. Sendowski I, Savourey G, Besnard Y, Bittel J. Cold induced vasodilatation and cardiovascular responses in humans during cold water immersion of various upper limb areas. Eur J Appl Physiol Occup Physiol 1997; 75:471-7; PMID:9202941; http://dx.doi.org/ 10.1007/s004210050191 [DOI] [PubMed] [Google Scholar]
- 56. Proulx CI, Ducharme MB, Kenny GP. Effect of water temperature on cooling efficiency during hyperthermia in humans. J Appl Physiol 2003; 94:1317-23; PMID:12626467. [DOI] [PubMed] [Google Scholar]
- 57. Proulx CI, Ducharme MB, Kenny GP. Safe cooling limits from exercise-induced hyperthermia. Eur J Appl Physiol 2006; 96:434-45; PMID:16341523; http://dx.doi.org/ 10.1007/s00421-005-0063-y [DOI] [PubMed] [Google Scholar]
- 58. Casa DJ, McDermott BP, Lee EC, Yeargin SW, Armstrong LE, Maresh CM. Cold water immersion: the gold standard for exertional heatstroke treatment. Exerc Sport Sci Rev 2007; 35:141-9; PMID:17620933; http://dx.doi.org/ 10.1097/jes.0b013e3180a02bec [DOI] [PubMed] [Google Scholar]
- 59. Barwood MJ, Dalzell J, Datta AK, Thelwell RC, Tipton MJ. Breath-hold performance during cold water immersion: effects of psychological skills training. Aviat Space Environ Med 2006; 77:1136-42; PMID:17086766 [PubMed] [Google Scholar]
- 60. Barwood MJ, Datta AK, Thelwell RC, Tipton MJ. Breath-hold time during cold water immersion: effects of habituation with psychological training. Aviat Space Environ Med 2007; 78:1029-34; PMID:18018434; http://dx.doi.org/ 10.3357/ASEM.2100.2007 [DOI] [PubMed] [Google Scholar]
- 61. Barwood MJ, Corbett J, Green R, Smith T, Tomlin P, Weir-Blankenstein L, Tipton MJ. Acute anxiety increases the magnitude of the cold shock response before and after habituation. Eur J Appl Physiol 2013; 113:681-9; PMID:22918558; http://dx.doi.org/ 10.1007/s00421-012-2473-y [DOI] [PubMed] [Google Scholar]
- 62. Barwood MJ, Corbett J, Wagstaff CRD. Habituation of the cold shock response may include a significant perceptual component. Aviat Space Environ Med 2014; 85:167-71; PMID:24597161; http://dx.doi.org/ 10.3357/ASEM.3759.2014 [DOI] [PubMed] [Google Scholar]
- 63. Makinen TM. Different types of cold adaptation in humans. Front Biosci (Schol Ed) 2010; 2:1047-67; PMID:20515840; http://dx.doi.org/ 10.2741/S117 [DOI] [PubMed] [Google Scholar]
- 64. Fox WC, Hall C, Hall E, Kolkhorst F, Lockette W. Cardiovascular baroreceptors mediate susceptibility to hypothermia. Aviat Space Environ Med 2003; 74:132-7; PMID:12602444 [PubMed] [Google Scholar]
- 65. Flouris AD, Cheung SS. Influence of thermal balance on cold-induced vasodilation. J Appl Physiol 2009; 106:1264-71; PMID:19213938; http://dx.doi.org/ 10.1152/japplphysiol.91426.2008 [DOI] [PubMed] [Google Scholar]
- 66. Passias TC, Meneilly GS, Mekjavic IB. Effect of hypoglycemia on thermoregulatory responses. J Appl Physiol 1996; 80:1021-32; PMID:8964720. [DOI] [PubMed] [Google Scholar]
- 67. Young AJ, Castellani JW. Exertion-induced fatigue and thermoregulation in the cold. comp biochem physiol. Part A, Mol Integrat Physiol 2001; 128:769-76; PMID:11282320; http://dx.doi.org/ 10.1016/S1095-6433(01)00282-3 [DOI] [PubMed] [Google Scholar]
- 68. Young AJ, Castellani JW. Exertional fatigue and cold exposure: mechanisms of hiker's hypothermia. Appl Physiol Nutr Metab 2007; 32:793-8; PMID:17622297; http://dx.doi.org/ 10.1139/H07-041 [DOI] [PubMed] [Google Scholar]
- 69. Tikuisis P, Ducharme MB, Moroz D, Jacobs I. Physiological responses of exercised-fatigued individuals exposed to wet-cold conditions. J Appl Physiol 1999; 86:1319-28; PMID:10194218 [DOI] [PubMed] [Google Scholar]
- 70. Golja P, Kacin A, Tipton MJ, Mekjavic IB. Moderate hypoxia does not affect the zone of thermal comfort in humans. Eur J Appl Physiol 2005; 93:708-13; PMID:15666176; http://dx.doi.org/ 10.1007/s00421-004-1306-z [DOI] [PubMed] [Google Scholar]
- 71. Golja P, Mekjavic IB. Effect of hypoxia on preferred hand temperature. Aviat Space Environ Med 2003; 74:522-6; PMID:12751580 [PubMed] [Google Scholar]
- 72. Golja P, Kacin A, Tipton MJ, Eiken O, Mekjavic IB. Hypoxia increases the cutaneous threshold for the sensation of cold. Eur J Appl Physiol 2004; 92:62-8; PMID:14991327; http://dx.doi.org/ 10.1007/s00421-004-1058-9 [DOI] [PubMed] [Google Scholar]
- 73. Daanen HA, van Ruiten HJ. Cold-induced peripheral vasodilation at high altitudes–a field study. High Alt Med Biol 2000; 1:323-9; PMID:11256468; http://dx.doi.org/ 10.1089/15270290050502390 [DOI] [PubMed] [Google Scholar]
- 74. Mathew L, Purkayastha SS, Selvamurthy W, Malhotra MS. Cold-induced vasodilatation and peripheral blood flow under local cold stress in man at altitude. Aviat Space Environ Med 1977; 48:497-500; PMID:869833 [PubMed] [Google Scholar]
- 75. Simmons GH, Minson CT, Cracowski JL, Halliwill JR. Systemic hypoxia causes cutaneous vasodilation in healthy humans. J Appl Physiol 2007; 103:608-15; PMID:17510298; http://dx.doi.org/ 10.1152/japplphysiol.01443.2006 [DOI] [PubMed] [Google Scholar]
- 76. Simmons GH, Fieger SM, Minson CT, Halliwill JR. Hypoxic cutaneous vasodilation is sustained during brief cold stress and is not affected by changes in CO2. J Appl Physiol 2010; 108:788-92; PMID:20167671; http://dx.doi.org/ 10.1152/jappl-physiol.01221.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Simmons GH, Barrett-O'Keefe Z, Minson CT, Halliwill JR. Cutaneous vascular and core temperature responses to sustained cold exposure in hypoxia. Exp Physiol 2011; PMID:21705404; http://dx.doi.org/ 10.1113/expphysiol.2011.059147 [DOI] [PubMed] [Google Scholar]
- 78. Simmons GH, Fieger SM, Wong BJ, Minson CT, Halliwill JR. No effect of systemic isocapnic hypoxia on α-adrenergic vasoconstrictor responsiveness in human skin. Acta Physiol (Oxf) 2011; 201:339-47; PMID:20946237 [DOI] [PubMed] [Google Scholar]
- 79. Keramidas ME, Kölegård R, Mekjavic IB, Eiken O. Acute effects of normobaric hypoxia on hand-temperature responses during and after local cold stress. High Alt Med Biol 2014; 15:183-91; PMID:24666109; http://dx.doi.org/ 10.1089/ham.2013.1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Felicijan A, Golja P, Milcinski M, Cheung SS, Mekjavic IB. Enhancement of cold-induced vasodilatation following acclimatization to altitude. Eur J Appl Physiol 2008; 104:201-6; PMID:18408951; http://dx.doi.org/ 10.1007/s00421-008-0720-z [DOI] [PubMed] [Google Scholar]
- 81. Daanen HA, Van de Linde FJ, Romet TT, Ducharme MB. The effect of body temperature on the hunting response of the middle finger skin temperature. Eur J Appl Physiol Occup Physiol 1997; 76:538-43; PMID:9404866; http://dx.doi.org/ 10.1007/s004210050287 [DOI] [PubMed] [Google Scholar]
- 82. Daanen HA, Ducharme MB. Finger cold-induced vasodilation during mild hypothermia, hyperthermia and at thermoneutrality. Aviat Space Environ Med 1999; 70:1206-10; PMID:10596776 [PubMed] [Google Scholar]
- 83. Flouris AD, Westwood DA, Mekjavic IB, Cheung SS. Effect of body temperature on cold induced vasodilation. Eur J Appl Physiol 2008; 104:491-9; PMID:18568361; http://dx.doi.org/ 10.1007/s00421-008-0798-3 [DOI] [PubMed] [Google Scholar]
- 84. Amon M, Keramidas ME, Kounalakis SN, Mekjavic IB. The effect of a sleep high-train low regimen on the finger cold-induced vasodilation response. High Alt Med Biol 2012; 13:32-9; PMID:22429230; http://dx.doi.org/ 10.1089/ham.2011.1044 [DOI] [PubMed] [Google Scholar]
- 85. Keramidas ME, Kölegård R, Mekjavic IB, Eiken O. Hand temperature responses to local cooling after a 10-day confinement to normobaric hypoxia with and without exercise. Scand J Med Sci Sports 2014; 15:183-91; PMID:25039992; http://dx.doi.org/ 10.1111/sms.12291 [DOI] [PubMed] [Google Scholar]
- 86. Lenasi H, Strucl M. Effect of regular physical training on cutaneous microvascular reactivity. Med Sci Sports Exerc 2004; 36:606-12; PMID:15064588; http://dx.doi.org/ 10.1249/01.MSS.0000121948.86377.51 [DOI] [PubMed] [Google Scholar]
- 87. Tew GA, Saxton JM, Klonizakis M, Moss J, Ruddock AD, Hodges GJ. Aging and aerobic fitness affect the contribution of noradrenergic sympathetic nerves to the rapid cutaneous vasodilator response to local heating. J Appl Physiol 2011; 110:1264-70; PMID:21330615; http://dx.doi.org/ 10.1152/japplphysiol.01423.2010 [DOI] [PubMed] [Google Scholar]
- 88. Tew GA, George KP, Cable NT, Hodges GJ. Endurance exercise training enhances cutaneous microvascular reactivity in post-menopausal women. Microvasc Res 2012; 83:223-8; PMID:21945654; http://dx.doi.org/ 10.1016/j.mvr.2011.09.002 [DOI] [PubMed] [Google Scholar]
- 89. Hodges GJ, Sharp L, Clements RE, Goldspink DF, George KP, Cable NT. Influence of age, sex, and aerobic capacity on forearm and skin blood flow and vascular conductance. Eur J Appl Physiol 2010; 109:1009-15; PMID:20354719; http://dx.doi.org/ 10.1007/s00421-010-1441-7 [DOI] [PubMed] [Google Scholar]
- 90. Simmons GH, Wong BJ, Holowatz LA, Kenney WL. Changes in the control of skin blood flow with exercise training: where do cutaneous vascular adaptations fit in? Exp Physiol 2011; 96:822-8; PMID:21602295; http://dx.doi.org/ 10.1113/expphysiol.2010.056176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Tew GA, Saxton JM, Hodges GJ. Exercise training and the control of skin blood flow in older adults. J Nutr Health Aging 2012; 16:237-41; PMID:22456779; http://dx.doi.org/ 10.1007/s12603-011-0156-8 [DOI] [PubMed] [Google Scholar]
- 92. Moriya K, Nakagawa K. Cold-induced vasodilatation of finger and maximal oxygen consumption of young female athletes born in hokkaido. Int J Biometeorol 1990; 34:15-9; PMID:2361774; http://dx.doi.org/ 10.1007/BF01045814 [DOI] [PubMed] [Google Scholar]
- 93. Dobnikar U, Kounalakis SN, Mekjavic IB. The effect of exercise-induced elevation in core temperature on cold-induced vasodilatation response in toes. Eur J Appl Physiol 2009; 106:457-64; PMID:19319561; http://dx.doi.org/ 10.1007/s00421-009-1035-4 [DOI] [PubMed] [Google Scholar]
- 94. Armstrong LE, Maresh CM. The induction and decay of heat acclimatisation in trained athletes. Sports Med 1991; 12:302-12; PMID:1763248; http://dx.doi.org/ 10.2165/00007256-199112050-00003 [DOI] [PubMed] [Google Scholar]
- 95. Miller LK, Irving L. Local reactions to air cooling in an Eskimo population. J Appl Physiol 1962; 17:449-55; PMID:14474063 [DOI] [PubMed] [Google Scholar]
- 96. Krog J, Folkow B, Fox RH, Andersen KL. Hand circulation in the cold of lapps and north norwegian fisherman. J Appl Physiol 1960; 15:654-8; PMID:14412101 [DOI] [PubMed] [Google Scholar]
- 97. Brown GM, Semple RE, Lennox CS, Bird GS, Baugh CW. Response to cold of eskimos of the eastern canadian arctic. J Appl Physiol 1963; 18:970-4; PMID:14063271 [DOI] [PubMed] [Google Scholar]
- 98. Nelms JD, Soper DJ. Cold vasodilatation and cold acclimatization in the hands of british fish filleters. J Appl Physiol 1962; 17:444-8; PMID:14478882 [DOI] [PubMed] [Google Scholar]
- 99. Selkirk GA, McLellan TM. Influence of aerobic fitness and body fatness on tolerance to uncompensable heat stress. J Appl Physiol 2001; 91:2055-63.; PMID:11641344 [DOI] [PubMed] [Google Scholar]
- 100. Keramidas ME, Musizza B, Kounalakis SN, Mekjavic IB. Enhancement of the finger cold-induced vasodilation response with exercise training. Eur J Appl Physiol 2010; 109:133-40; PMID:20135142; http://dx.doi.org/ 10.1007/s00421-010-1374-1 [DOI] [PubMed] [Google Scholar]
- 101. Keramidas ME, Kölegård R, Eiken O, Mekjavic IB. Prolonged physical inactivity leads to a drop in toe skin temperature during local cold stress. Appl Physiol Nutr Metab 2014; 39:369-74; PMID:24552380; http://dx.doi.org/ 10.1139/apnm-2013-0315 [DOI] [PubMed] [Google Scholar]
- 102. Daanen HA. Manual performance deterioration in the cold estimated using the wind chill equivalent temperature. Ind Health 2009; 47:262-70; PMID:19531912; http://dx.doi.org/ 10.2486/indhealth.47.262 [DOI] [PubMed] [Google Scholar]
- 103. Chen F, Liu ZY, Holmer I. Hand and finger skin temperatures in convective and contact cold exposure. Eur J Appl Physiol Occup Physiol 1996; 72:372-9; PMID:8851908; http://dx.doi.org/ 10.1007/BF00599699 [DOI] [PubMed] [Google Scholar]
- 104. Geurts C, Sleivert GG, Cheung SS. Temperature effects on the contractile characteristics and sub-maximal voluntary isometric force production of the first dorsal interosseus muscle. Eur J Appl Physiol 2004; 91:41-5; PMID:14508690; http://dx.doi.org/ 10.1007/s00421-003-0938-8 [DOI] [PubMed] [Google Scholar]
- 105. Provins KA, Morton R. Tactile discrimination and skin temperature. J Appl Physiol 1960; 15:155-60; PMID:14435120. [DOI] [PubMed] [Google Scholar]
- 106. Cheng C-C, Shih Y-C, Tsai Y-J, Chi C-F. The influence of cooling forearm/hand and gender on estimation of handgrip strength. Ergonomics 2014; 57:1499-511; PMID:25030838; http://dx.doi.org/ 10.1080/00140139.2014.934298 [DOI] [PubMed] [Google Scholar]
- 107. Nowak DA, Hermsdorfer J, Glasauer S, Philipp J, Meyer L, Mai N. The effects of digital anaesthesia on predictive grip force adjustments during vertical movements of a grasped object. European J Neuroscience 2001; 14:756-62; PMID:11556900; http://dx.doi.org/ 10.1046/j.0953-816x.2001.01697.x [DOI] [PubMed] [Google Scholar]
- 108. Nowak DA, Hermsdorfer J. Digit cooling influences grasp efficiency during manipulative tasks. Eur J Appl Physiol Occup Physiol 2003; 89:127-33; PMID:12665975; http://dx.doi.org/ 10.1007/s00421-002-0759-1 [DOI] [PubMed] [Google Scholar]
- 109. Cheung SS, Reynolds LF, Macdonald MA, Tweedie CL, Urquhart RL, Westwood DA. Effects of local and core body temperature on grip force modulation during movement-induced load force fluctuations. Eur J Appl Physiol 2008; 103:59-69; PMID:18205008; http://dx.doi.org/ 10.1007/s00421-008-0671-4 [DOI] [PubMed] [Google Scholar]
- 110. Oksa J, Rintamäki H, Mäkinen T. The effect of training of military skills on performance in cold environment. Mil Med 2006; 171:757-61; PMID:16933818. [DOI] [PubMed] [Google Scholar]
- 111. Montgomery RE, Hartley GL, Tyler CJ, Cheung SS. Effect of segmental, localized lower limb cooling on dynamic balance. Med Sci Sports Exerc 2015; 47:66-73; PMID:24870570; http://dx.doi.org/ 10.1249/MSS.0000000000000379 [DOI] [PubMed] [Google Scholar]
- 112. Johnson JM, Minson CT, Kellogg DL. Cutaneous vasodilator and vasoconstrictor mechanisms in temperature regulation. Compr Physiol 2014; 33-89; PMID:24692134; http://dx.doi.org/ 10.1002/cphy.c130015 [DOI] [PubMed] [Google Scholar]
- 113. Johnson JM, Yen TC, Zhao K, Kosiba WA. Sympathetic, sensory, and nonneuronal contributions to the cutaneous vasoconstrictor response to local cooling. Am J Physiol Heart Circ Physiol 2005; 288:H1573-9; PMID:15576441; http://dx.doi.org/ 10.1152/ajpheart.00849.2004 [DOI] [PubMed] [Google Scholar]
- 114. Pérgola PE, Kellogg DL, Johnson JM, Kosiba WA, Solomon DE. Role of sympathetic nerves in the vascular effects of local temperature in human forearm skin. Am J Physiol 1993; 265:H785-92; PMID:8214111 [DOI] [PubMed] [Google Scholar]
- 115. Pérgola PE, Johnson JM, Kellogg DL, Kosiba WA. Control of skin blood flow by whole body and local skin cooling in exercising humans. Am J Physiol 1996; 270:H208-15; PMID:8769753 [DOI] [PubMed] [Google Scholar]
- 116. Janský L, Vávra V, Janský P, Kunc P, Knížková I, Jandová D, Slováček K. Skin temperature changes in humans induced by local peripheral cooling. J Therm Biol 2003; 28:429-37; PMID:16343045; http://dx.doi.org/ 10.1016/S0306-4565(03)00028-716343045 [DOI] [Google Scholar]
- 117. Janský L, Matousková E, Vávra V, Vybíral S, Janský P, Jandová D, Knízková I, Kunc P. Thermal, cardiac and adrenergic responses to repeated local cooling. Physiol Res 2006; 55:543-9; PMID:16343045 [DOI] [PubMed] [Google Scholar]
- 118. Brändström H, Wiklund U, Karlsson M, Ängquist K-A, Grip H, Haney M. Autonomic nerve system responses for normal and slow rewarmers after hand cold provocation: effects of long-term cold climate training. Int Arch Occup Environ Health 2013; 86:357-65; PMID:22526086; http://dx.doi.org/ 10.1007/s00420-012-0767-3 [DOI] [PubMed] [Google Scholar]
- 119. Smits ES, Duraku LS, Niehof SP, Daanen HAM, Hovius SER, Selles RW, Walbeehm ET. Cold-induced vasodilatation in cold-intolerant rats after nerve injury. J Plast Reconstr Aesthet Surg 2013; 66:1279-86; PMID:23660280; http://dx.doi.org/ 10.1016/j.bjps.2013.04.004 [DOI] [PubMed] [Google Scholar]
- 120. Kusters FJG, Walbeehm ET, Niehof SP. Neural influence on cold induced vasodilatation using a new set-up for bilateral measurement in the rat hind limb. J Neurosci Methods 2010; 193:100-5; PMID:20709101; http://dx.doi.org/ 10.1016/j.jneumeth.2010.08.007 [DOI] [PubMed] [Google Scholar]
- 121. Ruijs AC, Jaquet JB, van Riel WG, Daanen HA, Hovius SE. Cold intolerance following median and ulnar nerve injuries: prognosis and predictors. J Hand Surg 2007; 32:434-9; PMID:17482322; http://dx.doi.org/ 10.1016/j.jhsb.2007.02.012 [DOI] [PubMed] [Google Scholar]
- 122. Ruijs AC, Niehof SP, Selles RW, Jaquet JB, Daanen HA, Hovius SE. Digital rewarming patterns after median and ulnar nerve injury. J Hand Surg 2009; 34:54-64; PMID:1912173; http://dx.doi.org/ 10.1016/j.jhsa.2008.09.014 [DOI] [PubMed] [Google Scholar]
- 123. Ruijs ACJ, Niehof SP, Hovius SER, Selles RW. Cold-induced vasodilatation following traumatic median or ulnar nerve injury. J Hand Surg 2011; 36:986-93; PMID:21514740; http://dx.doi.org/ 10.1016/j.jhsa.2011.02.009 [DOI] [PubMed] [Google Scholar]
- 124. Lindblad LE, Ekenvall L, Klingstedt C. Neural regulation of vascular tone and cold induced vasoconstriction in human finger skin. J Auton Nerv Syst 1990; 30:169-73; PMID:2370420; http://dx.doi.org/ 10.1016/0165-1838(90)90141-5 [DOI] [PubMed] [Google Scholar]
- 125. Johnson JM, Kellogg DLJ. Local thermal control of the human cutaneous circulation. J Appl Physiol 2010; 109:1229-38; PMID:20522732; http://dx.doi.org/ 10.1152/japplphysiol.00407.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Croix CM, Wetter TJ, Pegelow DF, Meyer KC, Dempsey JA. Assessment of nitric oxide formation during exercise. Am J Respir Crit Care Med 1999; 159:1125-33; PMID:10194156; http://dx.doi.org/ 10.1164/ajrccm.159.4.9806144 [DOI] [PubMed] [Google Scholar]
- 127. Therminarias A, Oddou MF, Favre-Juvin A, Flore P, Delaire M. Bronchial obstruction and exhaled nitric oxide response during exercise in cold air. Eur Resp J 1998; 12:1040-5; PMID:9863994; http://dx.doi.org/ 10.1183/09031936.98.12051040 [DOI] [PubMed] [Google Scholar]
- 128. Stensrud T, Stang J, Thorsen E, Bråten V. Exhaled nitric oxide concentration in the period of 60 min after submaximal exercise in the cold. Clin Physiol Funct Imaging 2014; PMID:25302764; http://dx.doi.org/ 10.1111/cpf.12196 [DOI] [PubMed] [Google Scholar]
- 129. Binti Md Isa K, Kawasaki N, Ueyama K, Sumii T, Kudo S. Effects of cold exposure and shear stress on endothelial nitric oxide synthase activation. Biochem Biophys Res Commun 2011; 412:318-22; PMID:21820412; http://dx.doi.org/ 10.1016/j.bbrc.2011.07.092 [DOI] [PubMed] [Google Scholar]
- 130. Hodges GJ, Zhao K, Kosiba WA, Johnson JM. The involvement of nitric oxide in the cutaneous vasoconstrictor response to local cooling in humans. J Physiol 2006; 574:849-57; PMID:16728451; http://dx.doi.org/ 10.1113/jphysiol.2006.109884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Hope K, Eglin C, Golden F, Tipton M. Sublingual glyceryl trinitrate and the peripheral thermal responses in normal and cold-sensitive individuals. Microvasc Res 2014; 91:84-9; PMID:24280630; http://dx.doi.org/ 10.1016/j.mvr.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 132. Chen G-F, Sun Z. Effects of chronic cold exposure on the endothelin system. J Appl Physiol 2006; 100:1719-26; PMID:16384835; http://dx.doi.org/ 10.1152/japplphysiol.01407.2005 [DOI] [PubMed] [Google Scholar]
- 133. Nakamura H, Matsuzaki I, Hatta K, Nagase H, Nobokuni Y, Kambayashi Y, Ogino K. Blood endothelin-1 and cold-induced vasodilation in patients with primary Raynauld's phenomenon and workers with vibration-induced white finger. Int Angiol 2003; 22:243-9; PMID:14612851; http://dx.doi.org/ 10.1007/s00547-004-0999-5 [DOI] [PubMed] [Google Scholar]
- 134. Yoshimura H, Iida T. Studies on the reactivity of skin vessels to extreme cold part 1. a point test on the resistance against frostbite. Japan J Physiol 1950; 1:147-59; PMID:14927254; http://dx.doi.org/ 10.2170/jjphysiol.1.147 [DOI] [PubMed] [Google Scholar]
- 135. Cheung SS, Mekjavic IB. Cold-induced vasodilatation is not homogenous or generalizable across the hand and feet. Eur J Appl Physiol 2007; 99:701-5; PMID:17219169; http://dx.doi.org/ 10.1007/s00421-006-0383-6 [DOI] [PubMed] [Google Scholar]
- 136. Purkayastha SS, Selvamurthy W, Ilavazhagan G. Peripheral vascular response to local cold stress of tropical men during sojourn in the arctic cold region. Japan J Physiol 1992; 42:877-89; PMID:1297856; http://dx.doi.org/ 10.2170/jjphysiol.42.877 [DOI] [PubMed] [Google Scholar]
- 137. O'Brien C. Reproducibility of the cold-induced vasodilation response in the human finger. J Appl Physiol 2005; 98:1334-40; PMID:15579576; http://dx.doi.org/ 10.1152/japplphysiol.00859.2004 [DOI] [PubMed] [Google Scholar]
- 138. Nindl BC, Castellani JW, Warr BJ, Sharp MA, Henning PC, Spiering BA, Scofield DE. Physiological employment standards III: physiological challenges and consequences encountered during international military deployments. Eur J Appl Physiol 2013; 113:2655-72; PMID:23430237; http://dx.doi.org/ 10.1007/s00421-013-2591-1 [DOI] [PubMed] [Google Scholar]
- 139. Imray CHE, Oakley E. Cold still kills: cold-related illnesses in military practice freezing and non-freezing cold injury. J R Army Med Corps 2005; 151:218-22; PMID:16548337; http://dx.doi.org/ 10.1136/jramc-151-04-02 [DOI] [PubMed] [Google Scholar]
- 140. Van der Struijs NR, Van Es EM, Raymann RJ, Daanen HA. Finger and toe temperatures on exposure to cold water and cold air. Aviat Space Environ Med 2008; 79:941-6; PMID:18856183; http://dx.doi.org/ 10.3357/ASEM.2258.2008 [DOI] [PubMed] [Google Scholar]
- 141. Xu X, Tikuisis P. Thermoregulatory modeling for cold stress. Compr Physiol 2014; 4:1057-81; PMID:24944030; http://dx.doi.org/ 10.1002/cphy.c130047 [DOI] [PubMed] [Google Scholar]
- 142. Rida M, Karaki W, Ghaddar N, Ghali K, Hoballah J. A new mathematical model to simulate AVA cold-induced vasodilation reaction to local cooling. Int J Biometeorol 2014; 58:1905-18; PMID:24448777; http://dx.doi.org/ 10.1007/s00484-014-0792-x [DOI] [PubMed] [Google Scholar]
- 143. Carrillo AE, Cheung SS, Flouris AD. A novel model to predict cutaneous finger blood flow via finger and rectal temperatures. Microcirculation 2011; 18:670-6; PMID:21951311; http://dx.doi.org/ 10.1111/j.1549-8719.2011.00136.x [DOI] [PubMed] [Google Scholar]
- 144. Upreti SR, Jeje AA. A noninvasive technique to determine peripheral blood flow and heat generation in a human limb. Chem Eng Sci 2004; 59:4415-23; http://dx.doi.org/ 10.1016/j.ces.2004.07.003 [DOI] [Google Scholar]
- 145. Caldwell JN, Matsuda-Nakamura M, Taylor NAS. Three-dimensional interactions of mean body and local skin temperatures in the control of hand and foot blood flows. Eur J Appl Physiol 2014; 114:1679-89; PMID:24819447; http://dx.doi.org/ 10.1007/s00421-014-2894-x [DOI] [PubMed] [Google Scholar]
- 146. Flouris AD, Cheung SS. Thermal basis of finger blood flow adaptations during abrupt perturbations in thermal homeostasis. Microcirculation 2011; 18:56-62; PMID:21166926; http://dx.doi.org/ 10.1111/j.1549-8719.2010.00068.x [DOI] [PubMed] [Google Scholar]
- 147. Vanggaard L, Kuklane K, Holmer I, Smolander J. Thermal responses to whole-body cooling in air with special reference to arteriovenous anastomoses in fingers. Clin Physiol Funct Imaging 2012; 32:463-9; PMID:23031067; http://dx.doi.org/ 10.1111/j.1475-097X.2012.01151.x [DOI] [PubMed] [Google Scholar]
- 148. Geng Q, Holmer I, Hartog DE, Havenith G, Jay O, Malchaire J, Piette A, Rintamaki H, Rissanen S. Temperature limit values for touching cold surfaces with the fingertip. Ann Occup Hyg 2006; 50:851-62; PMID:16777911; http://dx.doi.org/ 10.1093/annhyg/mel030 [DOI] [PubMed] [Google Scholar]
- 149. Buzanello MR, Moro ARP. Slaughterhouse workers exposed to cold: proposal of reference thermography values for hands. Work 2012; 41 1:2876-81; PMID:22317155; http://dx.doi.org/ 10.3233/WOR-2012-0537-2876 [DOI] [PubMed] [Google Scholar]
- 150. Ruijs AC, Jaquet JB, Brandsma M, Daanen HA, Hovius SE. Application of infrared thermography for the analysis of rewarming in patients with cold intolerance. Scand J Plast Reconstr Surg Hand Surg 2008; 42:206-10; PMID:18763198; http://dx.doi.org/ 10.1080/02844310802033943 [DOI] [PubMed] [Google Scholar]
- 151. Treu CM, Lupi O, Bottino DA, Bouskela E. Sidestream dark field imaging: the evolution of real-time visualization of cutaneous microcirculation and its potential application in dermatology. Arch Dermatol Res 2011; 303:69-78; PMID:20972572; http://dx.doi.org/ 10.1007/s00403-010-1087-7 [DOI] [PubMed] [Google Scholar]
- 152. Krite Svanberg E, Wollmer P, Andersson-Engels S, Åkeson J. Physiological influence of basic perturbations assessed by non-invasive optical techniques in humans. Appl Physiol Nutr Metab 2011; 36:946-57; PMID:22111558; http://dx.doi.org/ 10.1139/h11-119 [DOI] [PubMed] [Google Scholar]
- 153. Tew GA, Klonizakis M, Crank H, Briers JD, Hodges GJ. Comparison of laser speckle contrast imaging with laser doppler for assessing microvascular function. Microvasc Res 2011; 82:326-32; PMID:21803051; http://dx.doi.org/ 10.1016/j.mvr.2011.07.007 [DOI] [PubMed] [Google Scholar]
- 154. Daly SM, Leahy MJ. 'Go with the flow': a review of methods and advancements in blood flow imaging. J Biophotonics 2013; 6:217-55; PMID:22711377; http://dx.doi.org/ 10.1002/jbio.201200071 [DOI] [PubMed] [Google Scholar]