Abstract
Long-term heat acclimation appears to improve tolerance to hypoxic insults in various tissues, including brain, providing a promising avenue to improve functional outcomes following cerebrovascular events. Glutamate discharge is implicated in dysfunction following hypoxic stress and thus, targeting glutamate receptors with heat acclimation could improve cognitive outcomes following hypoxic injury.
Keywords: concussion, stroke, brain, cross-tolerance, NMDA
Abbreviations
- α-amino-3-hydroxy-5-methyl-4-isoazole-propionic acid receptor
(AMPA-R)
- N-methyl-D-aspartate receptor
(NMDA-R)
- traumatic brain injury
(TBI)
‘Cross tolerance’ describes the transfer of cytoprotective effects of stress adaptation to enhanced tolerance to novel stressors. For instance, a heat acclimated phenotype confers protective benefits to hypoxic stress in some tissues. The study by Yacobi et al.1 in this issue of Temperature investigated the effects of heat acclimation on hypoxic injury in the brain. The brain, particularly the hippocampus and frontal cortex, is the most sensitive tissue to ischemic or hypoxic insults. Brain damage associated with hypoxia is thought to be predominantly caused by a massive glutamate discharge and resultant large calcium influx, leading to inhibition of protein synthesis, increased free radical formation, lactic acidosis, and ultimately, cell death. Hypoxia-induced changes in the properties and expression of glutamate-gated calcium channels are thought to contribute to the magnitude of calcium influx and damage. Yacobi and colleagues examined the regulation of glutamate receptors in the brain following long-and short-term heat acclimation, including the resultant glutamate receptor response and cognitive impairment associated with acute hypoxic injury. In this study, rats exposed to long-term (34°C for 30 days) but not short-term (2 days) heat acclimation exhibited marked changes in glutamate receptors, both under resting normoxic conditions and following hypoxic insult. Furthermore, long-term heat acclimated rats performed better than control or short-term heat acclimated rats in sensory-motor behavioral testing post-hypoxic insult. The findings demonstrate, for the first time, that long-term heat acclimation provides protection from acute hypoxic injury by altering the structure of the types of glutamate receptors most associated with hypoxic damage.
Glutamate is the most abundant neurotransmitter in the human brain and glutamate receptors are implicated in a number of neurological and neurodegenerative conditions. Changes in receptor expression, as observed following heat acclimation, may have far-reaching impacts on health and cognitive function. In addition, the findings have direct implications for functional recovery from common hypoxic injuries to the brain, such as those associated with stroke.
The two primary types of glutamate receptors associated with hypoxic insult are N-methyl-D-aspartate receptor (NMDA-R) and the α-amino-3-hydroxy-5-methyl-4-isoazole-propionic acid receptor (AMPA-R). Both type of receptors are composed of subunits that can vary across different sub-types of the receptors, creating differences in excitability and calcium permeability. In particular, NMDA-R made with the GluN1/GluN2A subunits has 4-fold higher calcium permeability as compared with receptors composed of GluN1/GluN2B. As displayed in Figure 1, Yacobi et al.1 observed a decrease in total NMDA-R expression following long-term heat acclimation, as well as an increase in the ratio of GluN2B/GluN2A receptor subunits in the hippocampus and frontal cortex. Additionally, heat acclimation prevented the changes in NMDA-R and AMPA-R subunit expression that were induced by hypoxic injury in non-acclimated animals. Functionally, this could reduce the magnitude of calcium influx in response to the glutamate discharge associated with hypoxic injury. Indeed, the authors theorize that this change in receptor expression in heat-acclimated animals decreased calcium influx, facilitating the enhanced brain cell survival and attenuating the impairment in sensory-motor behavioral tests noted following the hypoxic insult.
Figure 1.
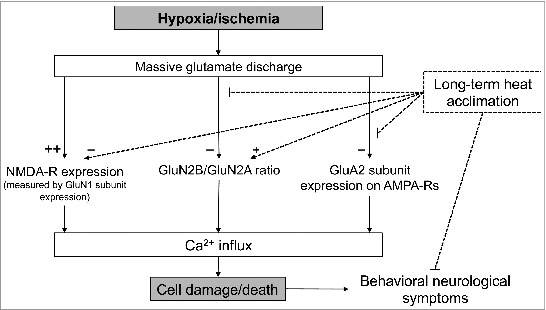
Schematic of the current theory on how hypoxia/ischemia facilitates cell death in the brain. Yacobi et al.1 demonstrated that long-term heat acclimation can reduce glutamate-mediated calcium influx and improve outcomes following hypoxic injury by altering the expression of NMDA and AMPA receptor subunits.
The key findings of Yacobi et al.1 indicate that hypoxic tolerance of brain tissue, particularly the hippocampal and frontal cortex neurons, can be improved by long-term heat acclimation. The change in expression and composition of glutamate receptors, particularly down-regulation of NMDA receptors, has wide-ranging impacts on surviving hypoxic insults. In addition to having clear implications for the hypoxic stress of cerebrovascular events such as stroke, these results may provide a cytoprotective means for minimizing cognitive dysfunction following concussion and other traumatic brain injuries (TBI). Similar to hypoxic injury, the neurological detriments associated with concussion are facilitated by massive glutamate discharge and calcium imbalance. Interestingly, NMDA receptor blockade in animals 30 minutes prior to trauma results in better functional outcomes post-TBI compared to saline-treatment,2 suggesting similar pathophysiology between TBI and hypoxic insult. In addition, heat acclimation attenuates apoptosis in brain cells following TBI,3 providing another mechanism by which heat acclimation can maintain cognitive function in individuals who have suffered cerebral trauma.
These results further enhance our knowledge of the potential for heat acclimation cross tolerance to improve cell survivability to a variety of insults. In animals, cross-tolerance has previously been examined in the heart, where enhanced survival of cardiac myocytes following ischemia-reperfusion injury was noted in animals treated with a 30-day passive heat acclimation.4 This initial work suggested that survival and functional outcomes in individuals who suffered from myocardial infarction would be improved if the patients were heat acclimated prior to injury. While these studies have led to interest in heat acclimation in humans for heat-hypoxia tolerance and exercise performance benefits,5,6 the potential for heat acclimation to improve health outcomes following hypoxic/ischemic events in humans requires more attention. And, thanks to the elegant body of work that has been put forth by Horowitz and colleagues over recent years, there are now clear indications for the use of heat acclimation to enhance both neuro-and cardio-protection in individuals susceptible to myocardial infarction, stroke, and TBIs such as concussion.
In addition to heat acclimation providing cytoprotection against acute stressors, this work highlights the potential for heat acclimation to improve health under non-stressed conditions. Yacobi et al.1 demonstrated beneficial changes in glutamate receptors under basal non-stressed conditions. Likely, heat acclimation is capable of inducing cellular changes throughout the body that, in addition to protecting against catastrophic events such as heart attack and stroke, can slow disease progression in general. Unfortunately, the physiological benefits of long-term heat acclimation in humans have thus far received very little attention. As such, the cardiovascular and neurological health benefits of long-term heat acclimation, as well as the cellular pathways and mechanisms of these protective benefits, represent a fertile area for future research. In particular, extending these findings to humans may provide a therapeutic means to enhance cardiovascular and cognitive health through long-term heat acclimation in individuals susceptible to or suffering from a range of cardiovascular and/or neurological diseases.
Disclosure of Potential Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Yacobi A, et al. Temperature 2014; 1: 57-65; http://dx.doi.org/ 10.4161/temp.29719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewén A, et al. Acta Neurochir (Wien) 1999; 141:193–202; PMID:10189503; http://dx.doi.org/ 10.1007/s007010050286 [DOI] [PubMed] [Google Scholar]
- 3.Umschwief G, et al. J Cereb Blood Flow Metab 2009; 30:616–627; PMID:19904288; http://dx.doi.org/ 10.1038/jcbfm.2009.234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maloyan A, et al. Physiol Genomics 2005; 23:79–88; PMID:16046617; http://dx.doi.org/ 10.1152/physiolgenomics.00279.2004 [DOI] [PubMed] [Google Scholar]
- 5.Heled Y, et al. Aviat Space Environ Med 2012; 83:649–653; PMID:22779306; http://dx.doi.org/ 10.3357/ASEM.3241.2012 [DOI] [PubMed] [Google Scholar]
- 6.White AC, et al. Int J Sports Med 2014; 35:975–81; PMID:24816886; http://dx.doi.org/ 10.1055/s-0034-1368724 [DOI] [PubMed] [Google Scholar]


