Abstract
Transient receptor potential ankyrin 1 (TRPA1) is a polymodal ion channel sensitive to temperature and chemical stimuli. The importance of temperature and aversive chemical detection for survival has driven the evolutionary diversity of TRPA1 sensitivity. This diversity can be observed in the various roles of TRPA1 in different species, where it is proposed to act as a temperature-insensitive chemosensor, a heat transducer, a noxious cold transducer, or a detector of low-intensity heat for prey localization. Exploring the variation of TRPA1 functions among species provides evolutionary insight into molecular mechanisms that fine-tune thermal and chemical sensitivity, and offers an opportunity to address basic principles of temperature gating in ion channels. A decade of research has yielded a number of hypotheses describing physiological roles of TRPA1, modulators of its activity, and biophysical principles of gating. This review surveys the diversity of TRPA1 adaptations across evolutionary taxa and explores possible mechanisms of TRPA1 activation.
Keywords: TRPA1, ANKTM1, ThermoTRP, Ion channels, Thermosensation
Abbreviations: ΔCp -change in heat capacity, AITC- allyl isothiocyanate, ANKTM1- ankyrin-like with transmembrane domains protein 1, AR- ankyrin repeat, ceTRPA1- Caenorhabditis elegans TRPA1, dTRPA1- Drosophila melanogaster TRPA1, EC50- half maximal effective concentration, hsTRPA1- hymenoptera-specific TRPA1, Keq- equillibrium constant, PH- pore helix, PI- phosphatidylinositol, Q10- temperature activation coefficient, S1-S6- transmembrane helices 1–6, TRP- Transient Receptor Potential, TRPA1- transient receptor potential ankyrin 1, TRPM8- transient receptor potential melastatin 8, TRPV1- transient receptor potential vanilloid 1
Introduction
The ability to sense and respond to external stimuli is vital for the success of life in a fluctuating environment. Temperature is a particularly salient variable that demands careful monitoring due to its ubiquitous, penetrating presence and effects on the very foundations of life—including the rates of chemical reactions and the stability of proteins, lipids, and other molecules. Additionally, temperature cues can be used to a variety of ends, including locating food or prey, synchronizing rhythms and activities like mating, and alerting an organism to environmental changes. It should not come as a surprise, therefore, that a number of temperature-sensing mechanisms have developed over the course of evolution. For example, prokaryotes have developed different thermosensors based on DNA, RNA, lipids, and/or proteins.1 In eukaryotes, the identification of thermosensors has predominately been limited to multipass transmembrane proteins such as the temperature sensitive Transient Receptor Potential channels (“thermoTRPs”).
In this review, we will focus on the thermosensitive properties of a particularly fascinating thermoTRP—transient receptor potential ankyrin 1 (TRPA1). As a temperature sensor, TRPA1 is intriguing, because while this ancient polymodal ion channel is found throughout vertebrates and invertebrates, it has acquired divergent functions over the course of evolution, such as temperature or chemical sensitivity, depending on the group of species under consideration. Additionally, TRPA1 took on more specialized roles in certain groups, including vipers, boas and pythons, where the thermal sensitivity of the channel has been linked to detection of infrared radiation emitted by warm-blooded prey.2 Therefore, due to the diversity of activation properties observed across species, careful analyses of ortholog differences may yield invaluable clues into the molecular mechanisms of temperature sensitivity in TRPA1 and/or other temperature sensitive proteins and ion channels. Before considering these questions, however, we will briefly discuss what is known about the channel structure and survey the broad cellular and organismal functions of TRPA1 as a detector of external and internal hazards.
TRPA1 was originally identified as a transmembrane protein in cultured human fibroblasts.3 The first study of TRPA1 function (then referred to as ankyrin-like with transmembrane domains protein 1 (ANKTM1)) determined it to be a putative sensor for noxious cold in mice.4 Soon after the description of its thermosensitivity, TRPA1 was found to be activated by a variety of chemical compounds, including mustard oil and cannabinoids (Fig. 1).5,6 Subsequent studies identified additional ligands and revealed roles in pain, inflammation, itch, mechanical sensation, oxygen regulation, and more.7-9
Figure 1.
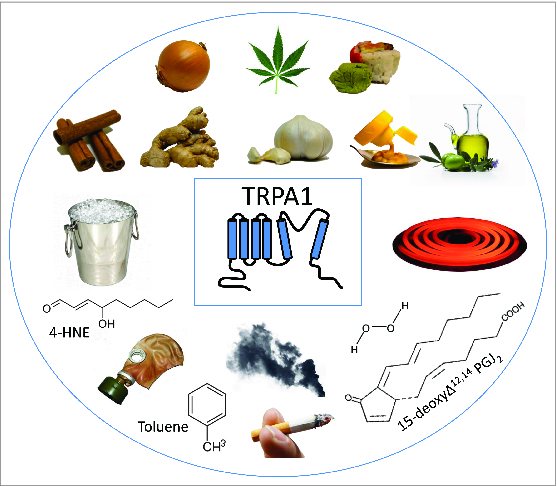
Activators of TRPA1 channel. TRPA1 depicted along with several activators of the channel. TRPA1 is sensitive to temperature and, depending on the ortholog, activates in response to either cold or heat. TRPA1 also responds to pungent plant compounds such as thiosulfinates (onion), Δ9-tetrahydrocannabinol (cannabis, marijuana), allyl isothiocyanate (AITC; wasabi, mustard), cinnamaldehyde (cinnamon), gingerol (ginger), allicin and diallyl disulfide (garlic), and oleocanthal (olive oil). Additionally, the channel can be activated by electrophiles in the environment, including tear gas, toluene, α, β-aldehydes such as acrolein and crotonaldehyde (smoke), and endogenous molecules like 4-hydroxynonenal (4-HNE), hydrogen peroxide (H2O2), and 15-deoxy-δ(12,14)-prostaglandin J2 (15-deoxyΔ12,14 PGJ2).
Similar to other TRPs, TRPA1 channels assemble as tetramers, with each subunit composed of 6 transmembrane segments (S1-S6) with a pore loop located between S5 and S6 (Fig. 2). The intracellular N- and C-termini contain many of the channel's regulatory elements. TRPA1 is named for the numerous (14–18) ankyrin repeats (ARs) on its notably long N-terminus (∼720 amino acids).3,4 ARs are 33-amino-acid-long motifs that are generally involved in protein-protein interactions and targeting to the plasma membrane.10 In TRPA1, they have been proposed to be involved in trafficking, mechanical gating and activation by thermal stimuli.10-16 Additionally, the ARs contain an EF hand-like motif that may directly interact with intracellular Ca2+, an important modulator of TRPA1 activity.17,18 However, other groups have reported that mutations to this region do not change the effects of Ca2+, suggesting that the mechanism of regulation by Ca2+requires further clarification.19 Importantly, the N-terminus also contains several conserved cysteine and lysine residues in the linker region between the ARs and the transmembrane domains, which confer TRPA1's sensitivity to electrophilic compounds.20,21 The C-terminus is much shorter but contains several sites that may contribute to the channel's activation as well.22,23 Both the N- and C-termini contain putative binding sites for metals such as zinc and cadmium, which can lead to the pain and irritation associated with heavy metal exposure.24-27 The extracellular face may also contribute to the regulation of TRPA1 and has been suggested to interact with several channel antagonists. Specifically, the extracellular loops of S1-S4 have been suggested to be involved in the binding of a tarantula toxin28 and the pore domain is implicated in inhibition by 6-methyl-5-(2-(trifluoromethyl)phenyl)-1H-indazole.29
Figure 2.
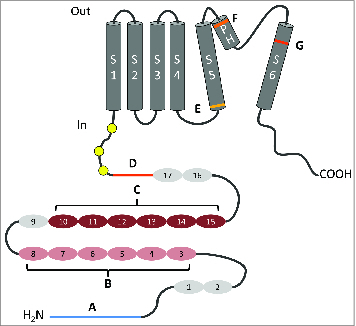
Topology diagram of functional domains of TRPA1. Topology diagram of TRPA1, highlighting domains implicated in thermosensation and electrophile-reactive cysteines (yellow circles). (A) Pre-AR N-terminal domain (blue) is suspected to suppress heat activation of dTRPA(A).83 (B) AR 3-8 (pink) contribute to heat activation of rattlesnake TRPA115; AR 6 is specifically important for heat sensitivity in mouse and fly TRPA1.16 (C) AR 10-15 (dark red) from both rattlesnake and fly TRPA1 is a portable heat sensitive module.15 (D) Only fly TRPA1 isoforms with TRP ankyrin cap (red) are activated in response to heat.91 (E) G878 in S5 (light orange) is necessary for cold activation of rat TRPA1.119 (F) Point mutation of R1073 or N1066 and I1067 in PH (orange) eliminate heat activation of fly TRPA1.137 (G) Point mutation of L1105 and I1106 in S6 (orange red) abolish heat sensitivity of fly TRPA1.137
The tissue distribution of TRPA1 in mammals facilitates its function as a detector of chemical and thermal conditions, both internally and externally. In mammals, TRPA1 is expressed primarily in the peripheral nervous system, specifically in Aδ- and C-fibers of dorsal root, trigeminal, and nodose ganglia, and displays a high degree of overlap with transient receptor potential vanilloid 1 (TRPV1)-positive cells, in line with its important role in somatic sensation and pain transduction.4,5,30-32 In addition to the peripheral nervous system, evidence for TRPA1 expression has been found in epithelial cells, melanocytes, pancreatic β cells, hair cells, cancer cells, as well as in neurons and astrocytes in the central nervous system.33-40
As a detector of potentially harmful conditions, TRPA1 is activated by noxious chemicals, including a number of electrophilic compounds. Electrophiles can react indiscriminately with electron donors abundant in the intracellular environment. Since these reactions can lead to toxic effects, such as enzyme inactivation and DNA mutations,41 it is essential to detect the presence of these potentially damaging compounds. Notably, several naturally occurring electrophiles that activate TRPA1 are produced by pungent or spicy plants (Fig. 1). These include allyl isothiocyanate (AITC; found in wasabi, mustard, and horseradish),5,6 cinnamaldehyde (cinnamon),6 and allicin and diallyl disulfide (raw garlic).42,43 Pollutants like toluene diisocyanate, hypochlorite, H2O2, and α, β-aldehydes such as acrolein and crotonaldehyde (from smoke) react with TRPA1 as well.44-48 Activation of TRPA1 can cause extreme irritation, lacrimation, coughing, bronchial contraction and depressed respiration.49 Therefore, potent electrophilic compounds (for example, 1-chloroacetophenone, dibenz[b,f][1,4]-oxazepine, 2-chlorobenzylidene malononitrile, and chloropicrin) are used as tear gases and riot control agents.45,50
Despite possessing few structural similarities, the electrophilic nature of these compounds allows them to form reversible covalent bonds with thiol groups of specific cysteine residues in the TRPA1 channel in a process confirmed by mass spectrometry.20,21,51 Detecting these compounds through their harmful reactivity is very economical compared to canonical ligand binding, which would be limited by the structural diversity of electrophilic compounds. Interestingly, TRPA1 can also be activated by other plant compounds, including oleocanthal (olive oil),52 carvacrol (oregano, marjoram),53 and Δ9-tetrahydrocannabinol (marijuana)5 independent of the reactive cysteines, suggesting the presence of alternative activation mechanisms to those used by the pungent electrophilic agonists (Fig. 1).52,54
In addition to detecting harmful substances from the external surroundings, TRPA1 monitors the internal environment and is involved in nociceptive signaling in response to the consequences of internal injury. A clear role for TRPA1 in pain transduction is established by the fact that it mediates both the formalin-induced pain response55-57 and carrageenan-induced inflammatory pain response,58,59 which are routinely used in the laboratory for behavioral studies of nociception. Furthermore, the role of TRPA1 in pain signaling is underscored by its involvement in an autosomal familial episodic pain syndrome, migraine headaches, and other human pain disorders.7,60,61
Low pH, which may occur in cases of injury-induced local acidosis or after application of weak acid, can activate the channel to alert an organism to damage.62 Similarly, the stinging sensation associated with carbonated beverages is believed to be the result of activation of TRPA1 due to intracellular acidification by carbon dioxide.63 On the other hand, deviations in pH toward more alkaline conditions also lead to activation of the TRPA1 channel and pain.64
Additionally, TRPA1 is suggested to contribute to pain and mechanical hypersensitivity induced by inflammation.65,66 Several inflammation-related factors including NO, H2O2, 4-hydroxynonenal, 15-deoxy-δ(12,14)-prostaglandin J2, and H2S activate TRPA1 directly (Fig. 1),46,55,67-74 while other factors, such as bradykinin, activate TRPA1 indirectly through downstream signaling from G-protein coupled receptors in a phospholipase C dependent manner.6,75 Similarly, indirect activation of TRPA1, this time through signaling from the Gβγ subunit, is linked to itch sensation (particularly histamine-independent itch).76-79
Clearly, chemical activation of TRPA1 plays a broad role as a detector and integrator of warning signals—both in terms of damaging external stimuli as well as internal stimuli indicative of tissue injury. For some species, such as humans and non-human primates, expression of a temperature-insensitive channel dictates that chemical sensitivity likely accounts for a major physiological role of the protein. While chemical sensitivity is indeed an ancient modality transduced by TRPA1, it is only one facet of the activation properties associated with this polymodal channel. In other species, chemical sensitivity can be downgraded in order to enhance temperature related signaling mediated by TRPA1, either by direct changes to protein sequence to render the channel less-sensitive to agonists, or by tissue-specific isoform expression strategies to dissociate signaling due to activation by temperature and chemicals (see next section).
It remains unclear whether the ancestral TRPA1 protein was particularly temperature sensitive, or at what point TRPA1 took on the role of a thermosensor. Nevertheless, the fact that many other TRPA family members are also temperature sensitive suggests that this may be an even more ancient modality transduced by the channel.80 The observation that thermosensitive TRPA1 channels are widespread with reported thresholds of activation that vary considerably across species indicates that there is evolutionary pressure on the temperature sensitivity of TRPA1, leading to multiple adjustments over time to suit specific ecological niches (Fig. 3).
Figure 3.

Interspecies differences in temperature sensitivities of TRPA1 orthologues. Temperature that evokes detectable channel activation (Tact) is listed. Common name refers to TRPA1 orthologues cloned from the following species: Clawed frog-Xenopus tropicalis; Chicken-Gallus gallus domesticus; Ratsnake-Elaphe obsoleta lindheimeri; Anole-Anolis carolinensis; Python-Python regius; Boa-Corallus hortulanus; Rattlesnake-Crotalus atrox; Fruitfly-Drosophila melanogaster; Mosquito-Anopheles gambiae; Nematode-Caenorhabditis elegans; Mouse-Mus musculus; Human-Homo sapiens. Images of ratsnake, rattlesnake, boa, mosquito and human were adapted from Wikimedia Commons (boa image by Geoff Gallice).
Species-Specific Temperature Sensitivity of TRPA1
TRPA1 in Ectotherms: a heat and chemical sensor
Insects
Soon after the original reports of cold activation of mouse TRPA1, the Drosophila melanogaster ortholog (dTRPA1) was cloned.81 Like the mouse channel, insect TRPA1 is sensitive to electrophilic compounds, albeit with a higher EC50 than mammalian orthologs.82 Interestingly, dTRPA1 expressed in Xenopus oocytes was shown to be activated not by cold, but by heat with a threshold of activation around 27–29°C (Fig. 4).81,83 Heat activation of TRPA1 was also reported for the mosquito Anopheles gambiae83-86 and the silkworm Bombyx mori.87
Figure 4.
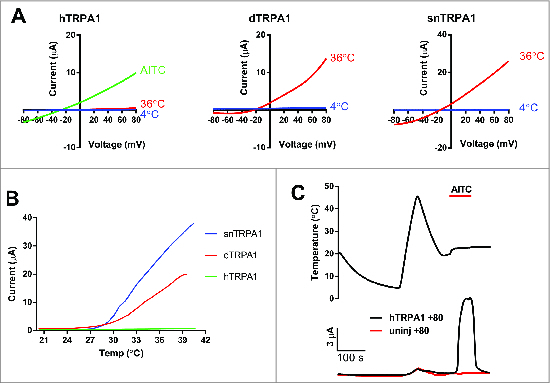
Electrophysiological analyses of TRPA1 function. (A) Exemplar 2-electrode voltage clamp recordings from Xenopus oocytes expressing human (h), Drosophila (d), or rattlesnake (sn) TRPA1. Currents were elicited by a 2s voltage ramp from −150 to +90 mV from a holding potential of −80 mV, in standard ND96 solution (final concentration, mM: 2 KCl, 96 NaCl, 20 MgCl2, 1.8 CaCl2, 5 HEPES pH 7.4) at 36°C (red), 4°C (blue), and/or in the presence of 1 mM AITC (green). (B) Exemplar temperature responses of human (h, green), Drosophila (d, red), and rattlesnake (sn, blue) TRPA1 orthologues recorded at different temperatures and measured at +80 mV as described in (A). (C) Exemplar responses of human TRPA1 (black) and uninjected oocytes (red) to cooling, heating and AITC (1mM) measured at +80mV.
In vivo experiments provided support for heat activation of dTRPA1 and demonstrated the crucial role of the protein in controlling thermotaxis and temperature preference in both larvae88 and adult flies.84 The behavioral deficits observed in mutant animals, combined with the observation that the activation threshold of dTRPA1 is conspicuously elevated just above the fly's preferred temperature of 25°C, led to the suggestion that the physiological functions of the channel include both detection and subsequent avoidance of heat and noxious chemicals.82,84,88-90
Despite the fact that dTRPA1 is a polymodal ion channel, it is important to maintain the ability to discriminate between activation by electrophilic chemicals or heat. Flies are capable of doing so through the expression of spatially localized isoforms of dTRPA1.83,91 The TRPA1(A)/(B) isoforms differ by alternative N-termini and, while both exhibit similar sensitivities to electrophilic agonists, the TRPA1(A) isoform is dramatically less thermosensitive than TRPA1(B), with temperature coefficient (Q10; a measure of the fold change in current per 10°C) values of ∼9 and ∼116, respectively. TRPA1(A) is expressed in the proboscis, where the chemosensitive neurons are located. In contrast, TRPA1(B) is predominately expressed within the head, where the thermosensors are found. Unlike in larger animals, the relatively small size of the fly means that heat can readily penetrate the body and thermosensors can be sequestered away to prevent activation by external irritants. Similarly, expressing a less thermosensitive isoform in the proboscis prevents warm temperatures from inappropriately initiating nocifensive responses such as regurgitation that are reserved for exposure to hazardous chemicals.83
Some insects use thermal cues to locate the warm-blooded animals on which they feed. For these organisms, it is crucial to be able to differentiate between the presence of aversive reactive electrophiles and the presence of attractive warm temperatures that may signal the location of the next blood meal. Therefore, hematophagous disease vectors like the typhus louse Pediculus humanus corporis and the mosquitos Anopheles gambiae, Aedes aegypti, and Culex quinquefasciatus make use of a similar isoform strategy.83
The ability of thermal signals to permeate through the body of small insects is the basis for the developing field of thermogenetics. Similar to optogenetics, which uses light to control and dissect intact neural circuits through the expression and activation of light-sensitive membrane proteins, thermogenetics seeks to use temperature signals to achieve similar control through the expression of thermosensitive proteins including the thermoTRPs.92,93 This approach is particularly relevant to model organisms like Drosophila, where implementation of optogenetics has been challenging. There have been a number of recent reports describing the successful use of ectopic expression of dTRPA1 to drive such diverse behaviors as courtship,94,95 regurgitation,83 sleep,96 walking97 and flight.98 On the other hand, a number of significant challenges remain before such a system can be effectively scaled up for use in larger mammalian model organisms like mice, including limited spatial resolution and temporal kinetics as well as a restricted thermogenetic toolkit that can function in the narrow temperature range found in mammalian tissues (which, for instance, are generally elevated above dTRPA1 activation temperatures).93
Nematodes/Hymenoptera: loss of ancestral TRPA1
In the nematode worm Caenorhabditis elegans, TRPA1 (ceTRPA1) is expressed in several tissue types, including neurons, muscle, intestine, and epithelial cells. ceTRPA1 was originally implicated in mechanosensation because mutant worms displayed deficits in foraging and other mechanosensory behaviors. Additionally, heterologous expression of ceTRPA1 in Chinese hamster ovary (CHO) cells leads to mechanically activated current.99,100 In contrast to arthropod TRPA1 channels, several studies have linked ceTRPA1 to activation by cold temperatures.101,102 Ectopic expression confers cold-sensitivity to otherwise non-responsive neurons as well as cultured cells.101 A recent study proposed that ceTRPA1 may function to detect decreases in environmental temperature and increase lifespan.102 It should be noted, however, that a molecular phylogenetic analysis of TRPA proteins suggested that ceTRPA1 does not derive from the ancestral TRPA1 and, rather than representing a true ortholog of the mammalian protein, it is more closely related to the basal TRPA proteins found in anemones and choanoflagellates.82 This may explain the differences observed between ceTRPA1 and other invertebrate TRPA1 channels, both in terms of temperature sensitivity and the fact that, unlike other TRPA1s, ceTRPA1 lacks conserved cysteine residues, therefore rendering it insensitive to electrophiles.99
In addition to Nematoda, loss of the ancient TRPA1 gene occurred in at least one order of Arthropoda as well. Hymenopterans, which includes bees, wasps, and ants, lack a true ortholog and instead express a hymenoptera specific TRPA (hsTRPA) that is believed to have evolved as a result of duplication of the Water witch gene.103,104 Intriguingly, hsTRPA displays a number of functional similarities to dTRPA1, including activation by warm temperatures and sensitivity to noxious chemicals. It was hypothesized that the duplication and neofunctionalization that gave rise to hsTRPA may have played a critical role in the development of social behavior in honey bees (Apis mellifera). Bees are known to monitor and control hive temperatures in order to provide a stable 32–36°C environment for the development of offspring.105,106 The hsTRPA activation threshold of 34°C (slightly higher than that of dTRPA1) may then serve to detect increases in temperature, thereby leading to hive cooling behavior.
Vertebrate ectotherms
The physiological function of TRPA1 as a heat and chemical sensor appears to be largely conserved in vertebrate ectotherms. The orthologous channels of frogs (western clawed frog (Xenopus tropicalis)), lizards (green anole (Anolis carolinensis)),107,108 and non-pit bearing snakes (Texas rat snake (Elaphe obsoleta lindheimeri))2 are sensitive to heat and exhibit detectable thermal activation at around 40°C, 34°C, and 37°C, respectively. However, the zebrafish (Danio rerio) is a noticeable exception to this trend. While the 2 TRPA1 paralogs from zebrafish are sensitive to electrophiles, they do not display activation by heat, nor do they seem to be involved in behavioral responses to noxious temperatures.2,109 Interestingly, there is evidence that zebrafish do not possess a true ortholog for the cold-sensitive ion channel transient receptor potential melastatin 8 (TRPM8), suggesting that multiple facets of thermosensation may be altered in these organisms.109,110
Nevertheless, for the TRPA1 orthologs that do display thermal sensitivity, activation temperatures are all above the preferred temperature ranges of these animals. This fact, combined with the channel's sensitivity to noxious chemicals, suggests that TRPA1 functions physiologically in reptiles and amphibians as a sensor of uncomfortable heat. The varying thresholds of activation may reflect adaptations to different ecological habitats. For instance, it was noted that X. tropicalis inhabits warm tropical waters and can survive aquatic temperatures above 42°C.111 Therefore, the higher activation threshold of the frog channel compared to that of the anole may reflect evolutionary pressures to adapt to warmer environments.
The apparent flexibility of TRPA1 thermal properties took on special physiological significance in at least one group of vertebrates—pit-bearing snakes.2,15 Infrared sensation in snakes is believed to have evolved independently at least 3 times in groups of species separated by millions of years of evolution. Yet surprisingly, each of these groups (boas, pythons, and pit vipers) may have evolved the capacity through modifications to the same protein. These animals all display dramatic differential expression of TRPA1 in the trigeminal neurons that innervate the pit compared to those neurons that innervate the body. There is also an expansion of the percentage of trigeminal neurons that express TRPA1, from ∼20% of trigeminal ganglia neurons in rodents5 to over 70% in pythons.2
When heterologously expressed, rattlesnake TRPA1 exhibits thermal activation with a comparatively low detectable threshold of around 27°C (Fig. 4).2 At the same time, the chemical sensitivity of the rattlesnake channel is substantially decreased, consistent with the notion that the physiological function of TRPA1 appears to have changed from somatic thermo-/chemo- sensation, to detection of infrared radiation. The locally restricted expression pattern and reduction in chemosensitivity may focus the ability to localize prey and prevent confusing or contradictory signals arising from accidental channel activation by electrophiles. It is interesting to note that this strategy is similar to the previously described isoform strategy used by some insects, including fruit flies and mosquitos, the latter of which may also rely on the same channel to locate warm animals.83
TRPA1 in endotherms
Mammals
The initial description of TRPA1 as a thermosensor came from a study using the mouse ortholog, which reported thermal activation in the sub-TRPM8 range (<17°C).4 Because this temperature range is registered as painful by humans,112 it was suggested that mammalian TRPA1 may serve as a detector of noxious cold. Since then, contradictory reports have emerged which either support,4,6,24,81,113-117 or dispute direct cold-induced activation of rodent TRPA1 channels in heterologous expression systems.36 Similarly, there is mixed opinion as to the temperature sensitivity of human TRPA1 to noxious cold. Although human TRPA1 cold sensitivity has recently been demonstrated in artificial liposomes118 this sensitivity is disputed for heterologous systems where some studies have reported a robust response,113 while others documented the absence of any cold-induced activation (Fig. 4).5,15,18,42,119 Together, this strongly suggests that—while the channel is intrinsically thermosensitive—its activity is tightly controlled in the cell.
Far from settling the issue, the generation of independent lines of TRPA1 knockout mice resulted in additional conflicting reports as to the role, or lack thereof, for TRPA1 as a temperature sensor in vivo.47,115,120-124 Due to the aforementioned overlap between TRPA1 and TRPV1 expressing neurons, ablation of TRPV1-positive cells in adult mice represents an alternative strategy to dissect the potential contributions of TRPA1 to temperature sensation in vivo. When such TRPV1-specific ablation was carried out, noxious cold sensitivity was unaffected, indicating that TRPA1 may not be directly involved in this process under normal physiological conditions.125
Obviously, temperature sensitivity of mammalian TRPA1 remains a controversial topic and, as such, has been summarized on multiple occasions.7,8,126-129 Several theories have been put forward in attempts to reconcile the divergent observations. Of particular note, one model contends that TRPA1 plays an important role in the development of cold hypersensitivity that may occur after injury.115,130-133 Other groups hold that the cold activation ascribed to TRPA1—particularly human TRPA1 in heterologous systems—is actually indirect, resulting from channel activation by cold-induced calcium influx that can occur even in cultured cells not expressing TRPA1.18 It should be noted, however, that multiple groups have shown cold activation of mammalian TRPA1 in the presence of calcium-free conditions.114,115,119,124
While inter-studies comparisons to resolve the conflicts are complicated in most cases by the non-uniform experimental conditions and channel orthologs used, a recent report emerged describing the side-by-side analysis of TRPA1 channels from humans, macaques, mice, and rats.119 Interestingly, the authors reported that, under the same experimental conditions, the rodent channels showed cold activation while the primate channels were insensitive to thermal stimuli.
One aspect of mammalian TRPA1 physiology that is more agreed upon is the evidence suggesting that TRPA1 does not play a clear role in thermoregulation. Unlike the case of other thermoTRPs like TRPM8,134 genetic or pharmacological manipulation of TRPA1 channel function in mice and rats does not result in changes to core body temperature or lead to any differences in autonomic thermoregulatory responses to cold exposure.116,135
Birds
Interestingly, it was proposed that the shift from heat sensitivity to cold sensitivity (or insensitivity) did not necessarily occur coincident with the evolution of endothermy.136 Chickens (Gallus gallus domesticus) are endothermic animals that maintain a body temperature of 41–42°C. TRPA1 cloned from birds showed a higher similarity to green anole TRPA1 (82%) than to other endotherms like mouse (65%) or human (64%). Perhaps reflecting this, the channel displays detectable activation in response to heat ramps at around 39°C. This apparent threshold can be shifted several degrees to the right if the channel is subjected to a slower ramp or preincubation at 40°C. Because this is just above the temperature of the animal's skin, the authors hypothesize that the channel may activate in response to slight elevations in temperature.
Molecular prerequisites for temperature sensitivity
The diversity of temperature responses in different orthologs lends TRPA1 as a versatile model for studying the biophysical principles of ion channel heat sensitivity. Cold activation of human TRPA1 in artificial lipid bilayers demonstrates that thermosensitivity is intrinsic to the channel.118 This finding, along with data from cell-free patches demonstrating heat and cold activation of green anole and mouse TRPA1, respectively,108,119 suggest that non-human TRPA1 orthologs are also intrinsically thermosensitive and require only the lipid bilayer to be cold- or heat-activated. Several groups used mutagenesis and chimeragenesis to identify structural modules that contribute to activation of TRPA1 by temperature. Thermodynamic and allosteric modeling of TRP channel opening provided insight into the energetics involved in temperature sensing.
Structure-function studies of TRPA1 from various species have led to the identification of several regions important for sensing temperature: the ankyrin repeats (AR) in the N-terminus, the area around the pore, including S5, PH (pore helix), and S6, and the C-terminus (Fig. 2). Identification of alternatively spliced TRPA1 isoforms from Drosophila, revealed a role for the N-terminus, both before and after ARs, in regulation of heat responses.83,91 Replacement of human AR10-15 with that from the Drosophila ortholog resulted in a thermally activated channel. Similarly, replacement of human AR3-8 or AR10-15 with those from heat-sensitive rattlesnake TRPA1 also conferred heat sensitivity.15 More strikingly, even a single point mutation in AR6 (S250N) of mouse TRPA1 lead to generation of a heat-activated channel, while a mutation at a homologous residue in the Drosophila ortholog (G176N) abolished heat responses.16 Together, these studies point toward a key role of the N-terminal ankyrin repeats in TRPA1 temperature sensitivity.
Even though the data appears to indicate that the N-terminal AR region contains a portable, heat-sensitive module across different species, the real picture is apparently far more complex. In addition to the ARs, the transmembrane core of TRPA1 is also implicated in temperature transduction. For instance, a TRPA1 human / Drosophila chimera with human N- and C- termini but Drosophila transmembrane core region remains heat sensitive, indicating that the various AR domains alone are not necessary for TRPA1 activation by temperature, and highlighting the importance of the pore-forming domain.137 Heat sensitivity of this chimera was ablated by point mutations from fly to corresponding human residues in the pore helix and S6 transmembrane domain. However, mutation of these same amino acids in human TRPA1 to corresponding fly residues, or even generation of human TRPA1 with fly pore helix and S6, did not confer heat sensitivity.137 This channel element has also been implicated in cold sensation of mammalian TRPA1. By generating a rat TRPA1 channel containing human S5 and S5-6 linker, Chen et al.119 ablated rat TRPA1 temperature sensitivity. In fact, a single point mutation of the S5 domain in TRPA1 was sufficient to eliminate rat TRPA1 cold responses.119 Finally, N-terminally truncated human TRPA1 lacking ARs remains cold-activated in artificial liposomes, further demonstrating that channel regions outside the ARs are important for thermosensitivity of TRPA1.118 Thus, a number of regions in the TRPA1 structure have been proposed to play a role in thermosensitivity, but the precise molecular mechanism for temperature activation remains unclear.
Similar to the case of TRPA1, the search for a temperature sensing mechanism in TRPV1 has also yielded a variety of results without a clear consensus, highlighting multiple regions throughout the molecule as key elements of temperature sensing and gating, including the N- and C-termini and the pore-forming region.138-144 In light of the breadth of domains implicated in thermal sensing, Clapham and Miller145 suggested that the mechanism of thermoTRP gating is a special case of that worked out for proteins in general, whereby temperature-driven conformational rearrangements can be accompanied by large changes in molar heat capacity of channel opening, Cp.146,147 Incorporation of a nonzero change in molar heat capacity (ΔCp) into calculation of the equilibrium constant (Keq) between closed and open conformations of a thermoTRP yields a nonmonotonic Keq dependence on temperature (Fig. 5). At ΔCp values around 2–5 kcal/mol*K, which is roughly equivalent to solvation of 10–20 buried hydrophobic side chains per channel subunit,148 Keqbecomes steeply dependent on temperature with Q10 around 20, i.e. in the range determined experimentally for thermo-TRPs, including different TRPA1 orthologs.2,16,83,91
Figure 5.
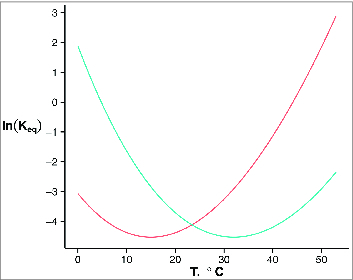
Two models predict ion channel activation by heat and cold. Equilibrium constant is nonmonotonic with temperature given positive ΔCp (here, 2kcal/mol-K) under the thermodynamics relationship: .145 Given ΔS° at temperature of minimal Keq of −9 cal/mol*K, setting minimal temperature of channel opening (T0) at 32°C results in a cold-activated channel under physiological conditions while setting T0 (blue) at 15°C results in a heat-activated channel under physiological conditions (red). A shift in T0 could account for interspecies temperature sensitivity differences of TRPA1. Allosteric gating of heat sensitive modules to the channel gate, as proposed by Jara-Oseguera and Islas,155 yields a similar nonmonotonicity without a requirement for large change in channel heat capacity.
Thus, if the closed to open transition is accompanied by changes in the solvation status of only 10–20 hydrophobic side chains, that alone could account for the steep temperature dependence. In this model, the side chains do not have to be clustered in a single structural domain, but could instead be distributed throughout the molecule. Even though this hypothesis does not preclude the existence of structurally defined temperature sensing domains in temperature-gated channels in principle, it provides an explanation to the seemingly frustrating search for a spatially defined temperature sensor in the TRPA1 structure.
Incorporation of large ΔCp values into the Keq calculation yields a U-shaped curve of temperature dependence, which predicts that temperature-gated channels are simultaneously sensitive to both heat and cold.145 This, however, has not been observed experimentally with TRPA1 or other thermo-TRPs, possibly because only one of the arms of the curve falls in a physiologically measurable range (Fig. 5). This hypothesis could explain the source of heat vs. cold sensitivity in different TRPA1 orthologs119 without invoking changes in the temperature-sensing factor ΔCp. According to the model, even if all the structural elements that determine ΔCp (i.e., the steepness of the U-curve arms) remain unaltered, modification in channel regions that determine To (the point of minimum Keq on the curve) could shift the curve on the horizontal axis and lead to an apparent reversal of temperature sensitivity from heat to cold and vice versa (Fig. 5).
The polymodal nature of many TRP channels has driven efforts to understand the interplay between temperature and other modalities like pH, voltage, and ligand binding. In studying the relationship between voltage- and temperature- activation of thermo-TRPs, some have adopted an allosteric gating model, which yields good fits to experimental data.149-154 Further development of this allosteric gating model demonstrated TRP temperature activation dependence on coupling of temperature module energy to the gate rather than an overall change in protein heat capacity.155,156 Since coupling strength is dependent on temperature, the possibility for channels activated by both heat and cold remains. Importantly, this model does not invoke large changes in protein heat capacity, providing an alternative model of TRP channel hot and cold activation.
Whether or not opening of TRPA1 or other thermo-gated ion channels is indeed accompanied by large ΔCp remains to be determined. Recently, the hypothesis of ΔCp-driven temperature sensitivity was explored through mutagenesis of the voltage-gated Shaker potassium channel, a member of the same superfamily as the TRP channels.157 Opening of Shaker is well characterized structurally, allowing for identification of the side chains that are exposed to water upon channel opening. Selective mutation to change the hydrophobicity of one or more of these amino acids led to temperature activated channel opening. Some of the constructs engineered for heat activation have Q10 values of 18.3 and 15.5—values in the range of those for thermo-TRPs. Although this study does not prove that this is the mechanism for thermosensitivity in TRPs, it provides an important demonstration of how small conformational changes that have the potential to alter protein heat capacity can contribute to temperature sensitivity, rendering the channel heat or cold sensitive.157 Interestingly, a contemporaneous study of a Shaker mutant in which the voltage sensor is decoupled from the gate revealed that the gate is intrinsically heat-sensitive.158 This finding suggests that the temperature sensor can, in principle, be confined within the gate itself and does not require allosteric coupling with a dedicated sensing domain (whether spatially confined or otherwise). To which extent findings in Shaker are applicable to other thermo-gated ion channels is unclear. For example, in contrast to Shaker, heat activates the 2-pore (K2P) potassium channel K2P2.1 (TREK-1) through the selectivity filter-based gate,159 but the gate itself lacks robust intrinsic heat sensitivity. Instead, it controls ion flow in response to allosteric commands from a sensor located elsewhere in the channel, most likely in the intracellular C-terminal domain.160 Thus it appears possible that while temperature-driven rearrangements of protein structures undoubtedly follow the same fundamental principles, the exact mechanism of temperature gating could be different in different ion channel classes or even orthologs of the same channel.
Much progress has been made in characterizing temperature activation of TRPA1. Chimeragenesis and mutagenesis have yielded multiple structural elements implicated in temperature activation. Modeling of TRP channel opening also provides insight on TRPA1 response to temperature. An allosteric coupling model integrates these modules, while the ΔCp-driven thermodynamics portray thermal activation as an event dependent on solvation or desolvation of multiple amino acid side chains which are not necessarily clustered into a structurally defined thermosensitive module. Continuing TRPA1 comparison across species and structure-function analyses will likely deepen our understanding of the critical domains involved in TRPA1 temperature activation, as well as elucidate key interspecies differences. The recent demonstration of cold sensitivity of human TRPA1 in artificial lipid bilayer has become a landmark in understanding the requirements of temperature activation of the channel, showing that this activity is intrinsic to the protein-lipid system alone.118 Although intrinsic temperature sensitivity should not be assumed for all TRPA1 orthologs, including those that are heat-sensitive, it is tempting to speculate they too will not require any component other than the bilayer to sense heat. Future work in characterizing open TRPA1 channel structure will aid not only in the process of defining the molecular basis of thermal activation of TRPA1 but—perhaps—temperature activation of thermo TRPs in general. In this regard, the recent breakthrough in solving the structure of TRPV1 by electron cryo-microscopy161,162 provides hope that the solution to the conformational changes during temperature gating in ion channels will soon be revealed.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors would like to thank Mustafa Khokha, Cheryl Laursen, Margaretha, Daniel and Sanet Blignaut for help with photography.
Funding
This work was supported by fellowships from the Beckman Foundation, Rita Allen Foundation and Alfred P. Sloan Foundation to EOG, by a Scientist Development Grant from American Heart Association (14SDG17880015) to SNB. WJL and LJH were supported by training grants from National Institute of Health (T32 HG-3198-10). EOA was supported by a training grant from National Institute of Health (T32 GM-1008-84) and a Gruber Science Fellowship. EOA is the Edward L. Tatum Fellow.
About the Authors
Elena Gracheva and Sviatoslav (Slav) Bagriantsev graduated from Moscow State University in 2001, obtained their Ph.D. from the University of Illinois at Chicago in 2006, and received postdoctoral training at University of California San Francisco. Elena and Slav joined Yale University School of Medicine in 2012 as Assistant Professors at the Department of Cellular & Molecular Physiology. Willem Laursen (B.A., Macalester College, 2011) and Lydia Hoffstaetter (B.A., Cornell University, 2012) are graduate students in Elena' s lab (Interdepartmental Neuroscience Program track), working on the molecular basis of thermosensitivity and thermoregulation in hibernating mammals. Evan Anderson (B.A., St. Olaf College, 2013) is a graduate student in Slav' s lab (Molecular Medicine, Pharmacology and Physiology track), studying the molecular basis of mechano- and thermosensitivity in the somatosensory system of vertebrates.

References
- 1.Sengupta P, Garrity P. Sensing temperature. Curr Biol 2013; 23:R304-7; PMID:23618661; http://dx.doi.org/ 10.1016/j.cub.2013.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gracheva EO, Ingolia NT, Kelly YM, Cordero-Morales JF, Hollopeter G, Chesler AT, Sanchez EE, Perez JC, Weissman JS, Julius D. Molecular basis of infrared detection by snakes. Nature 2010; 464:1006-11; PMID:20228791; http://dx.doi.org/ 10.1038/nature08943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaquemar D, Schenker T, Trueb B. An ankyrin-like protein with transmembrane domains is specifically lost after oncogenic transformation of human fibroblasts. J Biol Chem 1999; 274:7325-33; PMID:10066796; http://dx.doi.org/ 10.1074/jbc.274.11.7325 [DOI] [PubMed] [Google Scholar]
- 4.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, et al. . ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 2003; 112:819-29; PMID:12654248; http://dx.doi.org/ 10.1016/S0092-8674(03)00158-2 [DOI] [PubMed] [Google Scholar]
- 5.Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 2004; 427:260-5; PMID:14712238; http://dx.doi.org/ 10.1038/nature02282 [DOI] [PubMed] [Google Scholar]
- 6.Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 2004; 41:849-57; PMID:15046718; http://dx.doi.org/ 10.1016/S0896-6273(04)00150-3 [DOI] [PubMed] [Google Scholar]
- 7.Nilius B, Appendino G, Owsianik G. The transient receptor potential channel TRPA1: from gene to pathophysiology. Pflugers Arch 2012; 464:425-58; PMID:23001121; http://dx.doi.org/ 10.1007/s00424-012-1158-z [DOI] [PubMed] [Google Scholar]
- 8.Zygmunt PM, Hogestatt ED. Trpa1. Handb Exp Pharmacol 2014; 222:583-630; PMID:24756722; http://dx.doi.org/ 10.1007/978-3-642-54215-2_23 [DOI] [PubMed] [Google Scholar]
- 9.Julius D. TRP Channels and Pain. Annu Rev Cell Dev Biol 2013; 29:355-84; PMID:24099085 [DOI] [PubMed] [Google Scholar]
- 10.Gaudet R. A primer on ankyrin repeat function in TRP channels and beyond. Mol Biosyst 2008; 4:372-9; PMID:18414734; http://dx.doi.org/ 10.1039/b801481g [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howard J, Bechstedt S. Hypothesis: a helix of ankyrin repeats of the NOMPC-TRP ion channel is the gating spring of mechanoreceptors. Curr Biol 2004; 14:R224-6; PMID:15043829; http://dx.doi.org/ 10.1016/j.cub.2004.02.050 [DOI] [PubMed] [Google Scholar]
- 12.Lee G, Abdi K, Jiang Y, Michaely P, Bennett V, Marszalek PE. Nanospring behaviour of ankyrin repeats. Nature 2006; 440:246-9; PMID:16415852; http://dx.doi.org/ 10.1038/nature04437 [DOI] [PubMed] [Google Scholar]
- 13.Sotomayor M, Corey DP, Schulten K. In search of the hair-cell gating spring elastic properties of ankyrin and cadherin repeats. Structure 2005; 13:669-82; PMID:15837205; http://dx.doi.org/ 10.1016/j.str.2005.03.001 [DOI] [PubMed] [Google Scholar]
- 14.Nilius B, Prenen J, Owsianik G. Irritating channels: the case of TRPA1. J Physiol 2011; 589:1543-9; PMID:21078588; http://dx.doi.org/ 10.1113/jphysiol.2010.200717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cordero-Morales JF, Gracheva EO, Julius D. Cytoplasmic ankyrin repeats of transient receptor potential A1 (TRPA1) dictate sensitivity to thermal and chemical stimuli. Proc Natl Acad Sci U S A 2011; 108:E1184-91; PMID:21930928; http://dx.doi.org/ 10.1073/pnas.1114124108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jabba S, Goyal R, Sosa-Pagan JO, Moldenhauer H, Wu J, Kalmeta B, Bandell M, Latorre R, Patapoutian A, Grandl J. Directionality of temperature activation in mouse TRPA1 ion channel can be inverted by single-point mutations in ankyrin repeat six. Neuron 2014; 82:1017-31; PMID:24814535; http://dx.doi.org/ 10.1016/j.neuron.2014.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doerner JF, Gisselmann G, Hatt H, Wetzel CH. Transient receptor potential channel A1 is directly gated by calcium ions. J Biol Chem 2007; 282:13180-9; PMID:17353192; http://dx.doi.org/ 10.1074/jbc.M607849200 [DOI] [PubMed] [Google Scholar]
- 18.Zurborg S, Yurgionas B, Jira JA, Caspani O, Heppenstall PA. Direct activation of the ion channel TRPA1 by Ca2+. Nat Neurosci 2007; 10:277-9; PMID:17259981; http://dx.doi.org/ 10.1038/nn1843 [DOI] [PubMed] [Google Scholar]
- 19.Wang YY, Chang RB, Waters HN, McKemy DD, Liman ER. The nociceptor ion channel TRPA1 is potentiated and inactivated by permeating calcium ions. J Biol Chemi 2008; 283:32691-703; PMID:18775987; http://dx.doi.org/ 10.1074/jbc.M803568200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hinman A, Chuang HH, Bautista DM, Julius D. TRP channel activation by reversible covalent modification. Proc Natl Acad Sci U S A 2006; 103:19564-8; PMID:17164327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macpherson LJ, Dubin AE, Evans MJ, Marr F, Schultz PG, Cravatt BF, Patapoutian A. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature 2007; 445:541-5; PMID:17237762; http://dx.doi.org/ 10.1038/nature05544 [DOI] [PubMed] [Google Scholar]
- 22.Samad A, Sura L, Benedikt J, Ettrich R, Minofar B, Teisinger J, Vlachova V. The C-terminal basic residues contribute to the chemical- and voltage-dependent activation of TRPA1. Biochem J 2011; 433:197-204; PMID:20946100; http://dx.doi.org/ 10.1042/BJ20101256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sura L, Zima V, Marsakova L, Hynkova A, Barvik I, Vlachova V. C-terminal acidic cluster is involved in Ca2+-induced regulation of human transient receptor potential ankyrin 1 channel. J Biol Chem 2012; 287:18067-77; PMID:22461626; http://dx.doi.org/ 10.1074/jbc.M112.341859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andersson DA, Gentry C, Moss S, Bevan S. Clioquinol and pyrithione activate TRPA1 by increasing intracellular Zn2+. Proc Natl Acad Sci U S A 2009; 106:8374-9; PMID:19416844; http://dx.doi.org/ 10.1073/pnas.0812675106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu H, Bandell M, Petrus MJ, Zhu MX, Patapoutian A. Zinc activates damage-sensing TRPA1 ion channels. Nat Chem Biol 2009; 5:183-90; PMID:19202543; http://dx.doi.org/ 10.1038/nchembio.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gu Q, Lin RL. Heavy metals zinc, cadmium, and copper stimulate pulmonary sensory neurons via direct activation of TRPA1. J Appl Physiol 2010; 108:891-7; PMID:20133428; http://dx.doi.org/ 10.1152/japplphysiol.01371.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miura S, Takahashi K, Imagawa T, Uchida K, Saito S, Tominaga M, Ohta T. Involvement of TRPA1 activation in acute pain induced by cadmium in mice. Mol Pain 2013; 9:7; PMID:23448290; http://dx.doi.org/ 10.1186/1744-8069-9-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gui J, Liu B, Cao G, Lipchik AM, Perez M, Dekan Z, Mobli M, Daly NL, Alewood PF, Parker LL, et al. . A tarantula-venom peptide antagonizes the TRPA1 nociceptor ion channel by binding to the S1-S4 gating domain. Curr Biol 2014; 24:473-83; PMID:24530065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moldenhauer H, Latorre R, Grandl J. The pore-domain of TRPA1 mediates the inhibitory effect of the antagonist 6-methyl-5-(2-(trifluoromethyl)phenyl)-1H-indazole. PloS one 2014; 9:e106776; PMID:25181545; http://dx.doi.org/ 10.1371/journal.pone.0106776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kobayashi K, Fukuoka T, Obata K, Yamanaka H, Dai Y, Tokunaga A, Noguchi K. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J Comp Neurol 2005; 493:596-606; PMID:16304633; http://dx.doi.org/ 10.1002/cne.20794 [DOI] [PubMed] [Google Scholar]
- 31.Hjerling-Leffler J, Alqatari M, Ernfors P, Koltzenburg M. Emergence of functional sensory subtypes as defined by transient receptor potential channel expression. J Neurosci 2007; 27:2435-43; PMID:17344381; http://dx.doi.org/ 10.1523/JNEUROSCI.5614-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim YS, Son JY, Kim TH, Paik SK, Dai Y, Noguchi K, Ahn DK, Bae YC. Expression of transient receptor potential ankyrin 1 (TRPA1) in the rat trigeminal sensory afferents and spinal dorsal horn. J Comp Neurol 2010; 518:687-98; PMID:20034057; http://dx.doi.org/ 10.1002/cne.22238 [DOI] [PubMed] [Google Scholar]
- 33.Buch TR, Schafer EA, Demmel MT, Boekhoff I, Thiermann H, Gudermann T, Steinritz D, Schmidt A. Functional expression of the transient receptor potential channel TRPA1, a sensor for toxic lung inhalants, in pulmonary epithelial cells. Chem Biol Interact 2013; 206:462-71; PMID:23994502; http://dx.doi.org/ 10.1016/j.cbi.2013.08.012 [DOI] [PubMed] [Google Scholar]
- 34.Bellono NW, Kammel LG, Zimmerman AL, Oancea E. UV light phototransduction activates transient receptor potential A1 ion channels in human melanocytes. Proc Natl Acad Sci U S A 2013; 110:2383-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao DS, Zhong L, Hsieh TH, Abooj M, Bishnoi M, Hughes L, Premkumar LS. Expression of transient receptor potential ankyrin 1 (TRPA1) and its role in insulin release from rat pancreatic beta cells. PloS One 2012; 7:e38005; PMID: 22701540; http://dx.doi.org/ 10.1371/journal.pone.0038005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagata K, Duggan A, Kumar G, Garcia-Anoveros J. Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J Neurosci 2005; 25:4052-61; PMID:15843607; http://dx.doi.org/ 10.1523/JNEUROSCI.0013-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stokes A, Wakano C, Koblan-Huberson M, Adra CN, Fleig A, Turner H. TRPA1 is a substrate for de-ubiquitination by the tumor suppressor CYLD. Cell Signal 2006; 18:1584-94; PMID:16500080; http://dx.doi.org/ 10.1016/j.cellsig.2005.12.009 [DOI] [PubMed] [Google Scholar]
- 38.Shigetomi E, Jackson-Weaver O, Huckstepp RT, O'Dell TJ, Khakh BS. TRPA1 channels are regulators of astrocyte basal calcium levels and long-term potentiation via constitutive D-serine release. J Neurosci 2013; 33:10143-53; PMID:23761909; http://dx.doi.org/ 10.1523/JNEUROSCI.5779-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fernandes ES, Fernandes MA, Keeble JE. The functions of TRPA1 and TRPV1: moving away from sensory nerves. Br J Pharmacol 2012; 166:510-21; PMID:22233379; http://dx.doi.org/ 10.1111/j.1476-5381.2012.01851.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vennekens R, Menigoz A, Nilius B. TRPs in the Brain. Rev Physiol Biochem Pharmacol 2012; 163:27-64; PMID:23184016 [DOI] [PubMed] [Google Scholar]
- 41.Liebler DC. Protein damage by reactive electrophiles: targets and consequences. Chem Res Toxicol 2008; 21:117-28; PMID:18052106; http://dx.doi.org/ 10.1021/tx700235t [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Hogestatt ED, Julius D, Jordt SE, Zygmunt PM. Pungent products from garlic activate the sensory ion channel TRPA1. Proc Nat Acad Sci U S A 2005; 102:12248-52; PMID:16103371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Macpherson LJ, Geierstanger BH, Viswanath V, Bandell M, Eid SR, Hwang S, Patapoutian A. The pungency of garlic: activation of TRPA1 and TRPV1 in response to allicin. Curr Biol 2005; 15:929-34; PMID:15916949; http://dx.doi.org/ 10.1016/j.cub.2005.04.018 [DOI] [PubMed] [Google Scholar]
- 44.Taylor-Clark TE, Kiros F, Carr MJ, McAlexander MA. Transient receptor potential ankyrin 1 mediates toluene diisocyanate-evoked respiratory irritation. Am J Respirat Cell Mol Biol 2009; 40:756-62; PMID:19059884; http://dx.doi.org/ 10.1165/rcmb.2008-0292OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bessac BF, Sivula M, von Hehn CA, Caceres AI, Escalera J, Jordt SE. Transient receptor potential ankyrin 1 antagonists block the noxious effects of toxic industrial isocyanates and tear gases. FASEB J 2009; 23:1102-14; PMID:19036859; http://dx.doi.org/ 10.1096/fj.08-117812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Andersson DA, Gentry C, Moss S, Bevan S. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J Neurosci 2008; 28:2485-94; PMID:18322093; http://dx.doi.org/ 10.1523/JNEUROSCI.5369-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 2006; 124:1269-82; PMID:16564016; http://dx.doi.org/ 10.1016/j.cell.2006.02.023 [DOI] [PubMed] [Google Scholar]
- 48.Andre E, Campi B, Materazzi S, Trevisani M, Amadesi S, Massi D, Creminon C, Vaksman N, Nassini R, Civelli M, et al. . Cigarette smoke-induced neurogenic inflammation is mediated by alpha,beta-unsaturated aldehydes and the TRPA1 receptor in rodents. J Clin Invest 2008; 118:2574-82; PMID:18568077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bessac BF, Jordt SE. Sensory detection and responses to toxic gases: mechanisms, health effects, and countermeasures. Proc Am Thorac Soc 2010; 7:269-77; PMID:20601631; http://dx.doi.org/ 10.1513/pats.201001-004SM [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brone B, Peeters PJ, Marrannes R, Mercken M, Nuydens R, Meert T, Gijsen HJ. Tear gasses CN, CR, and CS are potent activators of the human TRPA1 receptor. Toxicol App Pharmacol 2008; 231:150-6; PMID:18501939; http://dx.doi.org/ 10.1016/j.taap.2008.04.005 [DOI] [PubMed] [Google Scholar]
- 51.Wang L, Cvetkov TL, Chance MR, Moiseenkova-Bell VY. Identification of in vivo disulfide conformation of TRPA1 ion channel. J Biol Chem 2012; 287:6169-76; PMID:22207754; http://dx.doi.org/ 10.1074/jbc.M111.329748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peyrot des Gachons C, Uchida K, Bryant B, Shima A, Sperry JB, Dankulich-Nagrudny L, Tominaga M, Smith AB 3rd, Beauchamp GK, Breslin PA. Unusual pungency from extra-virgin olive oil is attributable to restricted spatial expression of the receptor of oleocanthal. J Neurosci 2011; 31:999-1009; PMID:21248124; http://dx.doi.org/ 10.1523/JNEUROSCI.1374-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu H, Delling M, Jun JC, Clapham DE. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat Neurosci 2006; 9:628-35; PMID:16617338; http://dx.doi.org/ 10.1038/nn1692 [DOI] [PubMed] [Google Scholar]
- 54.Cavanaugh EJ, Simkin D, Kim D. Activation of transient receptor potential A1 channels by mustard oil, tetrahydrocannabinol and Ca2+ reveals different functional channel states. Neuroscience 2008; 154:1467-76; PMID:18515013; http://dx.doi.org/ 10.1016/j.neuroscience.2008.04.048 [DOI] [PubMed] [Google Scholar]
- 55.Macpherson LJ, Xiao B, Kwan KY, Petrus MJ, Dubin AE, Hwang S, Cravatt B, Corey DP, Patapoutian A. An ion channel essential for sensing chemical damage. J Neurosci 2007; 27:11412-5; PMID:17942735; http://dx.doi.org/ 10.1523/JNEUROSCI.3600-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, Hayward NJ, Chong JA, Julius D, Moran MM, et al. . TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci U S A 2007; 104:13525-30; PMID:17686976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yonemitsu T, Kuroki C, Takahashi N, Mori Y, Kanmura Y, Kashiwadani H, Ootsuka Y, Kuwaki T. TRPA1 detects environmental chemicals and induces avoidance behavior and arousal from sleep. Sci Rep 2013; 3:3100; PMID:24172941; http://dx.doi.org/ 10.1038/srep03100] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moilanen LJ, Laavola M, Kukkonen M, Korhonen R, Leppanen T, Hogestatt ED, Zygmunt PM, Nieminen RM, Moilanen E. TRPA1 contributes to the acute inflammatory response and mediates carrageenan-induced paw edema in the mouse. Sci Rep 2012; 2:380; PMID:22532928; http://dx.doi.org/ 10.1038/srep00380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bonet IJ, Fischer L, Parada CA, Tambeli CH. The role of transient receptor potential A 1 (TRPA1) in the development and maintenance of carrageenan-induced hyperalgesia. Neuropharmacology 2013; 65:206-12; PMID:23098993; http://dx.doi.org/ 10.1016/j.neuropharm.2012.09.020 [DOI] [PubMed] [Google Scholar]
- 60.Kremeyer B, Lopera F, Cox JJ, Momin A, Rugiero F, Marsh S, Woods CG, Jones NG, Paterson KJ, Fricker FR, et al. . A gain-of-function mutation in TRPA1 causes familial episodic pain syndrome. Neuron 2010; 66:671-80; PMID:20547126; http://dx.doi.org/ 10.1016/j.neuron.2010.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Benemei S, Fusi C, Trevisan G, Geppetti P. The TRPA1 channel in migraine mechanism and treatment. Br J Pharmacol 2013; 10:2552-67; PMID: 24206166; http://doi.org/ 10.1111/bph.12512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang YY, Chang RB, Allgood SD, Silver WL, Liman ER. A TRPA1-dependent mechanism for the pungent sensation of weak acids. J Gen Physiol 2011; 137:493-505; PMID:21576376; http://dx.doi.org/ 10.1085/jgp.201110615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang YY, Chang RB, Liman ER. TRPA1 is a component of the nociceptive response to CO2. J Neurosci 2010; 30:12958-63; PMID:20881114; http://dx.doi.org/ 10.1523/JNEUROSCI.2715-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fujita F, Uchida K, Moriyama T, Shima A, Shibasaki K, Inada H, Sokabe T, Tominaga M. Intracellular alkalization causes pain sensation through activation of TRPA1 in mice. J Clin Invest 2008; 118:4049-57; PMID:19033673; http://dx.doi.org/ 10.1172/JCI35957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bautista DM, Pellegrino M, Tsunozaki M. TRPA1: A gatekeeper for inflammation. Annu Rev Physiol 2013; 75:181-200; PMID:23020579; http://dx.doi.org/ 10.1146/annurev-physiol-030212-183811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Koivisto A, Chapman H, Jalava N, Korjamo T, Saarnilehto M, Lindstedt K, Pertovaara A. TRPA1: a transducer and amplifier of pain and inflammation. Basic Clin Pharmacol Toxicol 2014; 114:50-5; PMID:24102997; http://dx.doi.org/ 10.1111/bcpt.12138 [DOI] [PubMed] [Google Scholar]
- 67.Takahashi N, Mizuno Y, Kozai D, Yamamoto S, Kiyonaka S, Shibata T, Uchida K, Mori Y. Molecular characterization of TRPA1 channel activation by cysteine-reactive inflammatory mediators. Channels 2008; 2:287-98; PMID:18769139; http://dx.doi.org/ 10.4161/chan.2.4.6745 [DOI] [PubMed] [Google Scholar]
- 68.Miyamoto T, Dubin AE, Petrus MJ, Patapoutian A. TRPV1 and TRPA1 mediate peripheral nitric oxide-induced nociception in mice. PloS one 2009; 4:e7596; PMID:1989361; http://dx.doi.org/ 10.1371/journal.pone.0007596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sawada Y, Hosokawa H, Matsumura K, Kobayashi S. Activation of transient receptor potential ankyrin 1 by hydrogen peroxide. Eur J Neurosci 2008; 27:1131-42; PMID:18364033; http://dx.doi.org/ 10.1111/j.1460-9568.2008.06093.x [DOI] [PubMed] [Google Scholar]
- 70.Trevisani M, Siemens J, Materazzi S, Bautista DM, Nassini R, Campi B, Imamachi N, Andre E, Patacchini R, Cottrell GS, et al. . 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc Natl Acad Sci U S A 2007; 104:13519-24; PMID:17684094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cruz-Orengo L, Dhaka A, Heuermann RJ, Young TJ, Montana MC, Cavanaugh EJ, Kim D, Story GM. Cutaneous nociception evoked by 15-delta PGJ2 via activation of ion channel TRPA1. Mol Pain 2008; 4:30; PMID:18671867; http://dx.doi.org/ 10.1186/1744-8069-4-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Taylor-Clark TE, McAlexander MA, Nassenstein C, Sheardown SA, Wilson S, Thornton J, Carr MJ, Undem BJ. Relative contributions of TRPA1 and TRPV1 channels in the activation of vagal bronchopulmonary C-fibres by the endogenous autacoid 4-oxononenal. J Physiol 2008; 586:3447-59; PMID:18499726; http://dx.doi.org/ 10.1113/jphysiol.2008.153585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Materazzi S, Nassini R, Andre E, Campi B, Amadesi S, Trevisani M, Bunnett NW, Patacchini R, Geppetti P. Cox-dependent fatty acid metabolites cause pain through activation of the irritant receptor TRPA1. Proc Natl Acad Sci U S A 2008; 105:12045-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Streng T, Axelsson HE, Hedlund P, Andersson DA, Jordt SE, Bevan S, Andersson KE, Hogestatt ED, Zygmunt PM. Distribution and function of the hydrogen sulfide-sensitive TRPA1 ion channel in rat urinary bladder. Eur Urol 2008; 53:391-9; PMID:18031925; http://dx.doi.org/ 10.1016/j.eururo.2007.10.024 [DOI] [PubMed] [Google Scholar]
- 75.Wang S, Dai Y, Fukuoka T, Yamanaka H, Kobayashi K, Obata K, Cui X, Tominaga M, Noguchi K. Phospholipase C and protein kinase A mediate bradykinin sensitization of TRPA1: a molecular mechanism of inflammatory pain. Brain 2008; 131:1241-51; PMID:18356188; http://dx.doi.org/ 10.1093/brain/awn060 [DOI] [PubMed] [Google Scholar]
- 76.Wilson SR, Gerhold KA, Bifolck-Fisher A, Liu Q, Patel KN, Dong X, Bautista DM. TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nat Neurosci 2011; 14:595-602; PMID:21460831; http://dx.doi.org/ 10.1038/nn.2789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Oh MH, Oh SY, Lu J, Lou H, Myers AC, Zhu Z, Zheng T. TRPA1-dependent pruritus in IL-13-induced chronic atopic dermatitis. J Immunol 2013; 191:5371-82; PMID:24140646; http://dx.doi.org/ 10.4049/jimmunol.1300300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wilson SR, Nelson AM, Batia L, Morita T, Estandian D, Owens DM, Lumpkin EA, Bautista DM. The ion channel TRPA1 is required for chronic itch. J Neurosci 2013; 33:9283-94; PMID:23719797; http://dx.doi.org/ 10.1523/JNEUROSCI.5318-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wilson SR, Bautista DM. Role of transient receptor potential channels in acute and chronic itch In: Carstens E, Akiyama T, eds. Itch: Mechanisms and Treatment. Boca Raton (FL: ), 2014. [PubMed] [Google Scholar]
- 80.Panzano VC, Kang K, Garrity PA. Infrared snake eyes: TRPA1 and the thermal sensitivity of the snake pit organ. Sci Signal 2010; 3:pe22; PMID:20571127 [DOI] [PubMed] [Google Scholar]
- 81.Viswanath V, Story GM, Peier AM, Petrus MJ, Lee VM, Hwang SW, Patapoutian A, Jegla T. Opposite thermosensor in fruitfly and mouse. Nature 2003; 423:822-3; PMID:12815418; http://dx.doi.org/ 10.1038/423822a [DOI] [PubMed] [Google Scholar]
- 82.Kang K, Pulver SR, Panzano VC, Chang EC, Griffith LC, Theobald DL, Garrity PA. Analysis of Drosophila TRPA1 reveals an ancient origin for human chemical nociception. Nature 2010; 464:597-600; PMID:20237474; http://dx.doi.org/ 10.1038/nature08848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kang K, Panzano VC, Chang EC, Ni L, Dainis AM, Jenkins AM, Regna K, Muskavitch MA, Garrity PA. Modulation of TRPA1 thermal sensitivity enables sensory discrimination in Drosophila. Nature 2012; 481:76-80; PMID:22139422; http://dx.doi.org/ 10.1038/nature10715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hamada FN, Rosenzweig M, Kang K, Pulver SR, Ghezzi A, Jegla TJ, Garrity PA. An internal thermal sensor controlling temperature preference in Drosophila. Nature 2008; 454:217-20; PMID:18548007; http://dx.doi.org/ 10.1038/nature07001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang G, Qiu YT, Lu T, Kwon HW, Pitts RJ, Van Loon JJ, Takken W, Zwiebel LJ. Anopheles gambiae TRPA1 is a heat-activated channel expressed in thermosensitive sensilla of female antennae. Eur J Neurosci 2009; 30:967-74; PMID:19735290; http://dx.doi.org/ 10.1111/j.1460-9568.2009.06901.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu C, Zwiebel LJ. Molecular characterization of larval peripheral thermosensory responses of the malaria vector mosquito Anopheles gambiae. PloS One 2013; 8:e72595; PMID:23940815; http://dx.doi.org/ 10.1371/journal.pone.0072595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sato A, Sokabe T, Kashio M, Yasukochi Y, Tominaga M, Shiomi K. Embryonic thermosensitive TRPA1 determines transgenerational diapause phenotype of the silkworm, Bombyx mori. Proc Natl Acad Sci U S A 2014; 111:E1249-55; PMID:24639527; http://dx.doi.org/ 10.1073/pnas.1322134111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rosenzweig M, Brennan KM, Tayler TD, Phelps PO, Patapoutian A, Garrity PA. The Drosophila ortholog of vertebrate TRPA1 regulates thermotaxis. Gen Dev 2005; 19:419-24; PMID:15681611; http://dx.doi.org/ 10.1101/gad.1278205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kwon Y, Shim HS, Wang X, Montell C. Control of thermotactic behavior via coupling of a TRP channel to a phospholipase C signaling cascade. Nat Neurosci 2008; 11:871-3; PMID:18660806; http://dx.doi.org/ 10.1038/nn.2170 [DOI] [PubMed] [Google Scholar]
- 90.Neely GG, Keene AC, Duchek P, Chang EC, Wang QP, Aksoy YA, Rosenzweig M, Costigan M, Woolf CJ, Garrity PA, et al. . TrpA1 regulates thermal nociception in Drosophila. PloS One 2011; 6:e24343; PMID:21909389; http://dx.doi.org/ 10.1371/journal.pone.0024343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhong L, Bellemer A, Yan H, Ken H, Jessica R, Hwang RY, Pitt GS, Tracey WD. Thermosensory and nonthermosensory isoforms of Drosophila melanogaster TRPA1 reveal heat-sensor domains of a thermoTRP Channel. Cell Rep 2012; 1:43-55; PMID:22347718; http://dx.doi.org/ 10.1016/j.celrep.2011.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pulver SR, Pashkovski SL, Hornstein NJ, Garrity PA, Griffith LC. Temporal dynamics of neuronal activation by Channelrhodopsin-2 and TRPA1 determine behavioral output in Drosophila larvae. J Neurophysiol 2009; 101:3075-88; PMID:19339465; http://dx.doi.org/ 10.1152/jn.00071.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bernstein JG, Garrity PA, Boyden ES. Optogenetics and thermogenetics: technologies for controlling the activity of targeted cells within intact neural circuits. Curr Opin Neurobiol 2012; 22:61-71; PMID:22119320; http://dx.doi.org/ 10.1016/j.conb.2011.10.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kohatsu S, Koganezawa M, Yamamoto D. Female contact activates male-specific interneurons that trigger stereotypic courtship behavior in Drosophila. Neuron 2011; 69:498-508; PMID:21315260; http://dx.doi.org/ 10.1016/j.neuron.2010.12.017 [DOI] [PubMed] [Google Scholar]
- 95.von Philipsborn AC, Liu T, Yu JY, Masser C, Bidaye SS, Dickson BJ. Neuronal control of Drosophila courtship song. Neuron 2011; 69:509-22; PMID:21315261; http://dx.doi.org/ 10.1016/j.neuron.2011.01.011 [DOI] [PubMed] [Google Scholar]
- 96.Donlea JM, Thimgan MS, Suzuki Y, Gottschalk L, Shaw PJ. Inducing sleep by remote control facilitates memory consolidation in Drosophila. Science 2011; 332:1571-6; PMID:21700877; http://dx.doi.org/ 10.1126/science.1202249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bidaye SS, Machacek C, Wu Y, Dickson BJ. Neuronal control of Drosophila walking direction. Science 2014; 344:97-101; PMID:24700860; http://dx.doi.org/ 10.1126/science.1249964 [DOI] [PubMed] [Google Scholar]
- 98.Sadaf S, Hasan G. Serotonergic neurons of the Drosophila air-puff-stimulated flight circuit. J Biosci 2014; 39:575-83; PMID:25116612; http://dx.doi.org/ 10.1007/s12038-014-9449-5 [DOI] [PubMed] [Google Scholar]
- 99.Kindt KS, Viswanath V, Macpherson L, Quast K, Hu H, Patapoutian A, Schafer WR. Caenorhabditis elegans TRPA-1 functions in mechanosensation. Nat Neurosci 2007; 10:568-77; PMID:17450139; http://dx.doi.org/ 10.1038/nn1886 [DOI] [PubMed] [Google Scholar]
- 100.Xiao R, Xu XZ. Function and regulation of TRP family channels in C. elegans. Pflugers Arch 2009; 458:851-60; PMID:19421772; http://dx.doi.org/ 10.1007/s00424-009-0678-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chatzigeorgiou M, Yoo S, Watson JD, Lee WH, Spencer WC, Kindt KS, Hwang SW, Miller DM, 3rd, Treinin M, Driscoll M, et al. . Specific roles for DEG/ENaC and TRP channels in touch and thermosensation in C. elegans nociceptors. Nat Neurosci 2010; 13:861-8; PMID:20512132; http://dx.doi.org/ 10.1038/nn.2581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xiao R, Zhang B, Dong Y, Gong J, Xu T, Liu J, Xu XZ. A genetic program promotes C. elegans longevity at cold temperatures via a thermosensitive TRP channel. Cell 2013; 152:806-17; PMID:23415228; http://dx.doi.org/ 10.1016/j.cell.2013.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Matsuura H, Sokabe T, Kohno K, Tominaga M, Kadowaki T. Evolutionary conservation and changes in insect TRP channels. BMC evol biol 2009; 9:228; PMID:19740447; http://dx.doi.org/ 10.1186/1471-2148-9-228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kohno K, Sokabe T, Tominaga M, Kadowaki T. Honey bee thermal/chemical sensor, AmHsTRPA, reveals neofunctionalization and loss of transient receptor potential channel genes. J Neurosci 2010; 30:12219-29; PMID:20844118; http://dx.doi.org/ 10.1523/JNEUROSCI.2001-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Seeley TD. The Wisdom of the Hive: The Social Physiology of Honey bee Colonies Cambridge, Mass: Harvard University Press, 1995. [Google Scholar]
- 106.Tautz J, Maier S, Groh C, Rossler W, Brockmann A. Behavioral performance in adult honey bees is influenced by the temperature experienced during their pupal development. Proc Nat Acad Sci U S A 2003; 100:7343-7; PMID:12764227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Saito S, Nakatsuka K, Takahashi K, Fukuta N, Imagawa T, Ohta T, Tominaga M. Analysis of transient receptor potential ankyrin 1 (TRPA1) in frogs and lizards illuminates both nociceptive heat and chemical sensitivities and coexpression with TRP vanilloid 1 (TRPV1) in ancestral vertebrates. J Biol Chem 2012; 287:30743-54; PMID:22791718; http://dx.doi.org/ 10.1074/jbc.M112.362194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kurganov E, Zhou Y, Saito S, Tominaga M. Heat and AITC activate green anole TRPA1 in a membrane-delimited manner. Pflugers Arch 2014; 466:1873-84; PMID:24385018; http://dx.doi.org/ 10.1007/s00424-013-1420-z [DOI] [PubMed] [Google Scholar]
- 109.Prober DA, Zimmerman S, Myers BR, McDermott BM Jr., Kim SH, Caron S, Rihel J, Solnica-Krezel L, Julius D, Hudspeth AJ, et al. . Zebrafish TRPA1 channels are required for chemosensation but not for thermosensation or mechanosensory hair cell function. J Neurosci 2008; 28:10102-10; PMID:18829968; http://dx.doi.org/ 10.1523/JNEUROSCI.2740-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Saito S, Shingai R. Evolution of thermoTRP ion channel homologs in vertebrates. Physiol Genomics 2006; 27:219-30; PMID:16926268; http://dx.doi.org/ 10.1152/physiolgenomics.00322.2005 [DOI] [PubMed] [Google Scholar]
- 111.Ohkita M, Saito S, Imagawa T, Takahashi K, Tominaga M, Ohta T. Molecular cloning and functional characterization of Xenopus tropicalis frog transient receptor potential vanilloid 1 reveal its functional evolution for heat, acid, and capsaicin sensitivities in terrestrial vertebrates. J Biol Chem 2012; 287:2388-97; PMID:22130664; http://dx.doi.org/ 10.1074/jbc.M111.305698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Davis KD, Pope GE. Noxious cold evokes multiple sensations with distinct time courses. Pain 2002; 98:179-85; PMID:12098630; http://dx.doi.org/ 10.1016/S0304-3959(02)00043-X [DOI] [PubMed] [Google Scholar]
- 113.Klionsky L, Tamir R, Gao B, Wang W, Immke DC, Nishimura N, Gavva NR. Species-specific pharmacology of Trichloro(sulfanyl)ethyl benzamides as transient receptor potential ankyrin 1 (TRPA1) antagonists. Mol Pain 2007; 3:39; PMID:18086308; http://dx.doi.org/ 10.1186/1744-8069-3-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sawada Y, Hosokawa H, Hori A, Matsumura K, Kobayashi S. Cold sensitivity of recombinant TRPA1 channels. Brain Res 2007; 1160:39-46; PMID:17588549; http://dx.doi.org/ 10.1016/j.brainres.2007.05.047 [DOI] [PubMed] [Google Scholar]
- 115. del Camino D, Murphy S, Heiry M, Barrett LB, Earley TJ, Cook CA, Petrus MJ, Zhao M, D'Amours M, Deering N, et al. . TRPA1 contributes to cold hypersensitivity. J Neurosci 2010; 30:15165-74; PMID:21068322; http://dx.doi.org/ 10.1523/JNEUROSCI.2580-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.de Oliveira C, Garami A, Lehto SG, Pakai E, Tekus V, Pohoczky K, Youngblood BD, Wang W, Kort ME, Kym PR, et al. . Transient receptor potential channel ankyrin-1 is not a cold sensor for autonomic thermoregulation in rodents. J Neurosci 2014; 34:4445-52; PMID:24671991; http://dx.doi.org/ 10.1523/JNEUROSCI.5387-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen J, Zhang XF, Kort ME, Huth JR, Sun C, Miesbauer LJ, Cassar SC, Neelands T, Scott VE, Moreland RB, et al. . Molecular determinants of species-specific activation or blockade of TRPA1 channels. J Neurosci 2008; 28:5063-71; PMID:18463259; http://dx.doi.org/ 10.1523/JNEUROSCI.0047-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Moparthi L, Survery S, Kreir M, Simonsen C, Kjellbom P, Hogestatt ED, Johanson U, Zygmunt PM. Human TRPA1 is intrinsically cold- and chemosensitive with and without its N-terminal ankyrin repeat domain. Proc Natl Acad Sci U S A 2014; 111:16901-6; PMID:25389312; http://dx.doi.org/ 10.1073/pnas.1412689111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen J, Kang D, Xu J, Lake M, Hogan JO, Sun C, Walter K, Yao B, Kim D. Species differences and molecular determinant of TRPA1 cold sensitivity. Nat Commun 2013; 4:2501; PMID:24071625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, Corey DP. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron 2006; 50:277-89; PMID:16630838; http://dx.doi.org/ 10.1016/j.neuron.2006.03.042 [DOI] [PubMed] [Google Scholar]
- 121.Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 2007; 448:204-8; PMID:17538622; http://dx.doi.org/ 10.1038/nature05910 [DOI] [PubMed] [Google Scholar]
- 122.Fajardo O, Meseguer V, Belmonte C, Viana F. TRPA1 channels mediate cold temperature sensing in mammalian vagal sensory neurons: pharmacological and genetic evidence. J Neurosci 2008; 28:7863-75; PMID:18667618; http://dx.doi.org/ 10.1523/JNEUROSCI.1696-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Knowlton WM, Bifolck-Fisher A, Bautista DM, McKemy DD. TRPM8, but not TRPA1, is required for neural and behavioral responses to acute noxious cold temperatures and cold-mimetics in vivo. Pain 2010; 150:340-50; PMID:20542379; http://dx.doi.org/ 10.1016/j.pain.2010.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Karashima Y, Talavera K, Everaerts W, Janssens A, Kwan KY, Vennekens R, Nilius B, Voets T. TRPA1 acts as a cold sensor in vitro and in vivo. Proc Natl Acad Sci U S A 2009; 106:1273-8; PMID:19144922; http://dx.doi.org/ 10.1073/pnas.0808487106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pogorzala LA, Mishra SK, Hoon MA. The cellular code for mammalian thermosensation. J Neurosci 2013; 33:5533-41; PMID:23536068; http://dx.doi.org/ 10.1523/JNEUROSCI.5788-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.McKemy DD. How cold is it? TRPM8 and TRPA1 in the molecular logic of cold sensation. Mol Pain 2005; 1:16; PMID:15847696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.McKemy DD. The molecular and cellular basis of cold sensation. ACS Chem Neurosci 2013; 4:238-47; PMID:23421674; http://dx.doi.org/ 10.1021/cn300193h [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kwan KY, Corey DP. Burning cold: involvement of TRPA1 in noxious cold sensation. J Gen Physiol 2009; 133:251-6; PMID:19237590; http://dx.doi.org/ 10.1085/jgp.200810146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Caspani O, Heppenstall PA. TRPA1 and cold transduction: an unresolved issue? J Gen Physiol 2009; 133:245-9; PMID:19237589; http://dx.doi.org/ 10.1085/jgp.200810136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Obata K, Katsura H, Mizushima T, Yamanaka H, Kobayashi K, Dai Y, Fukuoka T, Tokunaga A, Tominaga M, Noguchi K. TRPA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury. J Clin Invest 2005; 115:2393-401; PMID:16110328; http://dx.doi.org/ 10.1172/JCI25437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dai Y, Wang S, Tominaga M, Yamamoto S, Fukuoka T, Higashi T, Kobayashi K, Obata K, Yamanaka H, Noguchi K. Sensitization of TRPA1 by PAR2 contributes to the sensation of inflammatory pain. J Clin Invest 2007; 117:1979-87; PMID:17571167; http://dx.doi.org/ 10.1172/JCI30951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. da Costa DS, Meotti FC, Andrade EL, Leal PC, Motta EM, Calixto JB. The involvement of the transient receptor potential A1 (TRPA1) in the maintenance of mechanical and cold hyperalgesia in persistent inflammation. Pain 2010; 148:431-7; PMID:20056530; http://dx.doi.org/ 10.1016/j.pain.2009.12.002 [DOI] [PubMed] [Google Scholar]
- 133.Gentry C, Stoakley N, Andersson DA, Bevan S. The roles of iPLA2, TRPM8 and TRPA1 in chemically induced cold hypersensitivity. Mol Pain 2010; 6:4; PMID:20092626; http://dx.doi.org/ 10.1186/1744-8069-6-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Almeida MC, Hew-Butler T, Soriano RN, Rao S, Wang W, Wang J, Tamayo N, Oliveira DL, Nucci TB, Aryal P, et al. . Pharmacological blockade of the cold receptor TRPM8 attenuates autonomic and behavioral cold defenses and decreases deep body temperature. J Neurosci 2012; 32:2086-99; PMID:22323721; http://dx.doi.org/ 10.1523/JNEUROSCI.5606-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chen J, Joshi SK, DiDomenico S, Perner RJ, Mikusa JP, Gauvin DM, Segreti JA, Han P, Zhang XF, Niforatos W, et al. . Selective blockade of TRPA1 channel attenuates pathological pain without altering noxious cold sensation or body temperature regulation. Pain 2011; 152:1165-72; PMID:21402443; http://dx.doi.org/ 10.1016/j.pain.2011.01.049 [DOI] [PubMed] [Google Scholar]
- 136.Saito S, Banzawa N, Fukuta N, Saito CT, Takahashi K, Imagawa T, Ohta T, Tominaga M. Heat and noxious chemical sensor, chicken TRPA1, as a target of bird repellents and identification of its structural determinants by multispecies functional comparison. Mol Biol Evol 2014; 31:708-22; PMID:24398321; http://dx.doi.org/ 10.1093/molbev/msu001 [DOI] [PubMed] [Google Scholar]
- 137.Wang H, Schupp M, Zurborg S, Heppenstall PA. Residues in the pore region of Drosophila transient receptor potential A1 dictate sensitivity to thermal stimuli. J Physiol 2013; 591:185-201; PMID:23027824; http://dx.doi.org/ 10.1113/jphysiol.2012.242842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Vlachova V, Teisinger J, Susankova K, Lyfenko A, Ettrich R, Vyklicky L. Functional role of C-terminal cytoplasmic tail of rat vanilloid receptor 1. J Neurosci 2003; 23:1340-50; PMID:12598622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Brauchi S, Orta G, Salazar M, Rosenmann E, Latorre R. A hot-sensing cold receptor: C-terminal domain determines thermosensation in transient receptor potential channels. J Neurosci 2006; 26:4835-40; PMID:16672657; http://dx.doi.org/ 10.1523/JNEUROSCI.5080-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Grandl J, Kim SE, Uzzell V, Bursulaya B, Petrus M, Bandell M, Patapoutian A. Temperature-induced opening of TRPV1 ion channel is stabilized by the pore domain. Nat Neurosci 2010; 13:708-14; PMID:20414199; http://dx.doi.org/ 10.1038/nn.2552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yang F, Cui Y, Wang K, Zheng J. Thermosensitive TRP channel pore turret is part of the temperature activation pathway. Proc Natl Acad S U S A 2010; 107:7083-8; PMID:20351268; http://dx.doi.org/ 10.1073/pnas.1000357107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yao J, Liu B, Qin F. Pore turret of thermal TRP channels is not essential for temperature sensing. Proc Nat Acad Sci U S A 2010; 107:E125; author reply E6-7; PMID:20660307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yao J, Liu B, Qin F. Modular thermal sensors in temperature-gated transient receptor potential (TRP) channels. Proce Nat Acad Sci U S A 2011; 108:11109-14; PMID:21690353; http://dx.doi.org/ 10.1073/pnas.1105196108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cui Y, Yang F, Cao X, Yarov-Yarovoy V, Wang K, Zheng J. Selective disruption of high sensitivity heat activation but not capsaicin activation of TRPV1 channels by pore turret mutations. J Gen Physiol 2012; 139:273-83; PMID:22412190; http://dx.doi.org/ 10.1085/jgp.201110724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Clapham DE, Miller C. A thermodynamic framework for understanding temperature sensing by transient receptor potential (TRP) channels. Proc Natl Acad Sci U S A 2011; 108:19492-7; PMID:22109551; http://dx.doi.org/ 10.1073/pnas.1117485108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wintrode PL, Makhatadze GI, Privalov PL. Thermodynamics of ubiquitin unfolding. Proteins 1994; 18:246-53; PMID:8202465; http://dx.doi.org/ 10.1002/prot.340180305 [DOI] [PubMed] [Google Scholar]
- 147.Makhatadze GI, Clore GM, Gronenborn AM, Privalov PL. Thermodynamics of unfolding of the all beta-sheet protein interleukin-1 beta. Biochemistry 1994; 33:9327-32; PMID:8049234; http://dx.doi.org/ 10.1021/bi00197a037 [DOI] [PubMed] [Google Scholar]
- 148.Makhatadze GI, Lopez MM, Privalov PL. Heat capacities of protein functional groups. Biophys Chem 1997; 64:93-101; PMID:17029831; http://dx.doi.org/ 10.1016/S0301-4622(96)02234-X [DOI] [PubMed] [Google Scholar]
- 149.Liu B, Hui K, Qin F. Thermodynamics of heat activation of single capsaicin ion channels VR1. Biophys J 2003; 85:2988-3006; PMID:14581201; http://dx.doi.org/ 10.1016/S0006-3495(03)74719-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Brauchi S, Orio P, Latorre R. Clues to understanding cold sensation: thermodynamics and electrophysiological analysis of the cold receptor TRPM8. Proc Natl Acad Sci U S A 2004; 101:15494-9; PMID:15492228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Voets T, Droogmans G, Wissenbach U, Janssens A, Flockerzi V, Nilius B. The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature 2004; 430:748-54; PMID:15306801; http://dx.doi.org/ 10.1038/nature02732 [DOI] [PubMed] [Google Scholar]
- 152.Matta JA, Ahern GP. Voltage is a partial activator of rat thermosensitive TRP channels. J Physiol 2007; 585:469-82; PMID:17932142; http://dx.doi.org/ 10.1113/jphysiol.2007.144287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yao J, Liu B, Qin F. Kinetic and energetic analysis of thermally activated TRPV1 channels. Biophys J 2010; 99:1743-53; PMID:20858418; http://dx.doi.org/ 10.1016/j.bpj.2010.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Salazar M, Moldenhauer H, Baez-Nieto D. Could an allosteric gating model explain the role of TRPA1 in cold hypersensitivity? J Neurosci 2011; 31:5554-6; PMID:21490194; http://dx.doi.org/ 10.1523/JNEUROSCI.6775-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jara-Oseguera A, Islas LD. The role of allosteric coupling on thermal activation of thermo-TRP channels. Biophys J 2013; 104:2160-9; PMID:23708356; http://dx.doi.org/ 10.1016/j.bpj.2013.03.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Qin F. Demystifying thermal channels: driving a channel both forwards and backwards with a single gear? Biophys J 2013; 104:2118-20; PMID:23708350; http://dx.doi.org/ 10.1016/j.bpj.2013.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chowdhury S, Jarecki Brian W, Chanda B. A molecular framework for temperature-dependent gating of ion channels. Cell 2014; 158:1148-58; PMID:25156949; http://dx.doi.org/ 10.1016/j.cell.2014.07.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yang F, Zheng J. High temperature sensitivity is intrinsic to voltage-gated potassium channels. eLife 2014; 3:e03255; PMID:25030910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bagriantsev SN, Peyronnet R, Clark KA, Honore E, Minor DL, Jr. Multiple modalities converge on a common gate to control K2P channel function. EMBO J 2011; 30:3594-606; PMID:21765396; http://dx.doi.org/10.1038/emboj.2011.230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bagriantsev SN, Clark KA, Minor DL, Jr. Metabolic and thermal stimuli control K(2P)2.1 (TREK-1) through modular sensory and gating domains. EMBO J 2012; 31:3297-308; PMID:22728824; http://dx.doi.org/10.1038/emboj.2012.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cao E, Liao M, Cheng Y, Julius D. TRPV1 structures in distinct conformations reveal activation mechanisms. Nature 2013; 504:113-8; PMID:24305161; http://dx.doi.org/ 10.1038/nature12823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Liao M, Cao E, Julius D, Cheng Y. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature 2013; 504:107-12; PMID:24305160; http://dx.doi.org/ 10.1038/nature12822 [DOI] [PMC free article] [PubMed] [Google Scholar]


