Abstract
Temperatures above and below what is generally regarded as “comfortable” for the human being have long been known to induce various airway symptoms, especially in combination with exercise in cold climate with temperatures below 0°C, which is naturally since exercise is followed by enhanced ventilation and thus greater amounts of inhaled cold air. The aim was to highlight the knowledge we have today on symptoms from the airways (here also including the eyes) arisen from various temperatures; the mechanisms, the pathophysiology and their clinical significance. The most common eye and airway conditions related to temperature changes are dry eye disease, rhinitis, laryngeal dysfunction, asthma, chronic obstructive pulmonary disease and chronic cough. Transient receptor potential (TRP) ion channels are probably involved in all temperature induced airway symptoms but via different pathways, which are now beginning to be mapped out. In asthma, the most persuasive hypothesis today is that cold-induced asthmatic bronchoconstriction is induced by dehydration of the airway mucosa, from which it follows that provocations with osmotic stimuli like hypertonic saline and mannitol can be used as a surrogate for exercise provocation as well as dry air inhalation. In chronic unexplained cough there seems to be a direct influence of cold air on the TRP ion channels followed by coughing and increased cough sensitivity to inhaled capsaicin. Revelations in the last decades of the ability of several airway TRP ion channels to sense and react to ambient air temperature have opened new windows for the understanding of the pathogenesis in a diversity of airway reactions appearing in many common respiratory diseases.
Keywords: asthma, capsaicin, chemical sensitivity, cold air, cough, cold air-induced dyspnea, rhinitis, sensory hyperreactivity
Abbreviations: COPD, chronic obstructive pulmonary disease; EID, exercise induced dyspnea; e-NANC, non-adrenergic non-cholinergic; TRP, transient receptor potential; TRPA1, transient receptor potential ankyrin 1; TRPM8, transient receptor potential melastin 8; TRPV1, transient receptor potential vanilloid 1
Introduction
The transient receptor potential (TRP) ion channels are the sensors giving the human being vital information on environmental temperature, and thereby the possibility to react with alarm to both noxious heat and cold, and to escape from them. Beyond these essential and necessary functions, temperature stimulation of TRP channels may contribute to unwanted reactions and symptoms.1 For the airways, there are few reports of negative influences of temperature above normal ambient degrees, although noxious heat is likely to be followed by airway injury. Luckily, human beings are seldom in contact with ambient air temperature in the noxious range. On the other hand, the first identified TRP channel, the transient receptor ion channel vanilloid 1 (TRPV1), is triggered by noxious heat, capsaicin and low pH.2 Capsaicin has for decades been used in airway research to provoke cough, as when inhaled, it is considered to mirror the airway sensory nerve reactivity. Somewhat to the contrary, inhalation of cold air has long been known to provoke various airway symptoms, especially in combination with exercise, which is logical, since exercise is followed by increased respiratory rate and thus greater amounts of inhaled cold air. TRP channels are widely expressed in the airways,3–5 and the growing knowledge of the role of these receptors in sensing and reacting to environmental stimuli is followed by greater focus on these channels as targets for respiratory disease.6–10 It seems likely that the transient receptor potential melastin 8 (TRPM8) and the transient receptor potential ankyrin 1 (TRPA1), known to react to low temperature, are involved in airway symptoms induced by cold air. However, TRP channels belonging to the vanilloid (V) group, like those sensing osmotic stimuli (TRPV3 and TRPV4) and the before mentioned TRPV1 (also known to function as “integrator” of a wide field of sensory stimuli) may influence temperature induced airway symptoms.11 There are a number of recognized airway diseases and symptoms related to cold temperature, and these may affect both the upper and lower airways and may include the eyes. Table 1 gives an overview of TRP channels and temperatures related to various airway symptoms.
Table 1.
Relation between TRP ion channels, airway symptoms and temperature
| TRP ion channels | Airway symptoms | Temperature °C | Related stimuli |
|---|---|---|---|
| TRPV1 | Cough, rhinitis and dyspnea induced from “noxious” stimuli | ≥42° | Capsaicin |
| pH ≤ 5.9 | |||
| “Noxious” stimuli | |||
| Ethanol | |||
| Vanilloides | |||
| Pain | |||
| TRPV3 TRPV4 | Asthma, cough, dry eyes | 22–40° | Osmolarity Mechanical stimuli |
| TRPM8 TRPM4 | Inhalation of menthol reduces cough | 8–22° | “Cool” stimuli |
| Menthol | |||
| TRPA1 | Asthma, cough, laryngeal obstruction, rhinitis | <8° and below | Mustard oil |
| Garlic | |||
| “Noxious” stimuli? | |||
| Pain |
Upper Airways and Eyes
Dry eye disease
There is a close connection between the responses to environmental airborne stimuli affecting the eyes and to those affecting the upper airways; an example of this connection is the allergic reactions labeled as rhino-conjunctivitis. Expression of TRPV isotypes 1, 2, and 3 were detected in human corneal endothelial cells,12 and also TRPM8 has been identified in the eyes as a cold- and menthol-activated ion channel in peripheral sensory neurons, where it plays an important role in cold temperature detection.13 Dry eye disease is a multifactorial disorder affecting the composition and volume of tears. It causes ocular surface dryness, cooling, and hyperosmolarity and may lead to corneal epithelium damage and reduced visual performance. Recent findings indicate that elevations of tear osmolarity found in this disease predominantly excite cold thermoreceptors, indicating that dryness sensations experienced by these patients can be due to an augmented activity of corneal cold thermoreceptors.14
Rhinitis
It is common knowledge that the nose may be runny in cold weather and that such problems increase with aging.15 TRPV1 is widely expressed in the nasal mucosa, suggesting that capsaicin can directly influence the epithelial secretory and other functions via TRPV1, followed by activation of the sensory neurons.3 In both allergic and non-allergic rhinitis a non-specific nasal hyperreactivity can be diagnosed with cold dry air provocations.16,17 Such findings have given rise to hopes of targeting TRPV1 in the development of new therapeutic agents,6 though in a clinical trial a TRPV1 inhibitor did not prevent cold dry air–elicited symptoms in non-allergic rhinitis.18 Other studies report TRPV1 overexpression in patients with idiopathic rhinitis, and topical capsaicin treatment reduced nasal hyperreactivity and nasal symptoms, the capsaicin action interpreted as ablating the TRPV1 nociceptive signaling pathway in the nasal mucosa.19,20 The TRPM8, known to be triggered by cold temperatures, is abundant in the nasal sub-epithelium21 and in idiopathic rhinitis Van Gerven et al found reduced expression of TRPM8, after capsaicin treatment, a finding that may have contributed to improvement of nasal hyperreactivity.19
Laryngeal dysfunction
Reports of respiratory difficulties caused by exercise-induced laryngeal obstructions are increasing in frequency.22 Laryngeal obstruction like vocal cord dysfunction, often occurring in young women, especially elite athletes, can masquerade as asthma and also coexist with asthma.23 Since both these conditions are characterized by exercise-induced dyspnea (EID), differential diagnosis is problematic and may be followed by incorrect information and medication. Non-specific stimuli like cold and dry air are known to be among the most common triggers in the newly established laryngeal hypersensitivity syndrome.24,25 Studies of the expression of TRP channels in the human larynx are sparse, but at least in rat, TRPV1 seems to play an important role in the nociception of the laryngeal innervation,26 and it is logical to hypothesize that the laryngeal reaction to exercise and cold air is dependent on TRP signaling.
Lower Airways
Asthma
Bronchial asthma as a disease is characterized by an underlying inflammation that in recent decades has been the main target for pharmacological treatment and the focus of intensive research activity.27,28 However, the link between the inflammatory cascade of asthma, causing bronchial hyperreactivity, and the axon reflex of bronchoconstriction remains unclear.29 Increasing evidence points to a potential role for members of the TRP family of cation channels on airway sensory non-adrenergic non-cholinergic (e-NANC) nerves in the development of bronchial hyperreactivity and several features of asthmatic disease.27,30,31 The nature of the neurotransmitters involved in e-NANC bronchodilation and bronchoconstriction is still under debate, but the most likely candidates are vasoactive intestinal peptide and nitric oxide.29
EID due to bronchoconstriction is regarded as a cardinal feature of asthma and is a measure of bronchial hyperreactivity, as most asthmatic patients develop breathing problems after physical exertion, especially in a cold temperature.27 A number of different mechanisms have been suggested as potential causes of cold air- and hyperventilation-provoked airflow limitation.32,33 The most persuasive hypothesis today is that cold-induced asthmatic bronchoconstriction depends on dehydration of the airway mucosa, from which it follows that provocations with osmotic stimuli like hypertonic saline and mannitol can be used as a surrogate for exercise provocation as well as dry air inhalation.34 Cold air is by definition dry and the dehydration of the airways following exercise and hyperventilation, especially in cold climate, results in release of mediators followed by bronchoconstriction. The likely source of these mediators is the mast cell, but the exact mechanism by which dehydration and hyperosmolarity trigger the mast cell is not known. Alterations in the osmolarity of the media surrounding the cell will lead to changes in cell volume as water (and solute) leaves or enters the cell to maintain osmotic balance. Accumulating evidence indicates that osmotic activation of some TRP channels may occur via one of 2 mechanisms: (1) swelling-induced activation whose downstream metabolites then activate TRP channels, or (2) post-synaptic density protein supporting proteins such as ezrin/radixin/moesin that act as the mediators between some TRP channels and F-actin cytoskeleton.35 On the other hand, some studies support a reflex mechanism from the upper to the lower airways with quickly induced bronchoconstriction after nasal cold air stimulation,36 and cold air provocations of the face in healthy subjects and asthmatics have been shown to induce decreased of lung function.34 These studies were performed before the identification of TRPV1, and the authors hypothesized vagal and trigeminal reflexes to be the underlying mechanism. The role of the cold sensitive TRPM8 channel in the normal and diseased airway is relatively unexplored, but the findings of TRPM8 channels in a subset of nasal trigeminal afferent neurons that do not coincidently express TRPA1 or TRPV1 indicate the possibility of a reflex initiated from the nose.37
Some studies have found increased capsaicin cough sensitivity in asthma patients,38,39 while others have failed to detect this phenomenon,40–43 and the cough sensitivity seemed to “normalize” when the asthmatic inflammation was treated with inhaled corticosteroids. Further, a recent study reported overexpression of TRPV1 in asthma, especially in patients with a severe condition.44
Chronic Obstructive Pulmonary Disease (COPD)
It is a common clinical experience that patients with COPD complain of airway symptoms provoked by environmental factors like cold temperature, although few studies can confirm such connections.45 In contrast to asthma, COPD is characterized by airflow limitation that is not fully reversible and is usually progressive, comprising a heterogeneous group of airway diseases and types of inflammation. Cough reflex sensitivity to capsaicin in patients with COPD has been recorded in only a few studies with small groups of patients and was reported to be normal or increased,39,46,47 and so far, only sparse reports have focused on TRP channels in COPD disease, though the interest seems rising.8,10 One study suggests that TRPA1 is involved in the neurogenic inflammation in COPD and contributes to the progression of the disease.48
Chronic cough
Coughing is a common clinical problem and is followed by reduced quality of life.49-51 When it persists for more than 2 months, it is regarded as chronic, though the definition of “chronic cough” varies in the literature.52 In a cross-sectional, population-based epidemiologic study the overall prevalence of self-reported chronic cough was 6.3%, and cold air sensitivity was related to cough with statistical significance.53 The pathophysiology behind coughing is fairly well understood in a number of conditions, but it is not always possible to attribute a persistent cough to an airway disorder or any other medical disorder. Cough can be a sign of many disorders, but there remains a group of patients with no obvious explanation for their coughing; these patients are often described as presenting with chronic idiopathic cough.54,55
A subgroup of patients with airway complaints who report cough and other airway symptoms from environmental irritants and cold air have increased capsaicin cough sensitivity levels.41 As this capsaicin-induced cough can be blocked by inhaled lidocaine, it has been suggested that sensory nerves are involved in the pathophysiology.56 Consequently, previously was proposed a mechanism to increase sensitivity of the afferent sensory nerves in such patients and further suggesting using the term sensory hyper-reactivity (SHR) to identify this mechanism.57 The prevalence of SHR, often misdiagnosed as asthma or allergy, is estimated to be 6% in the Swedish population.58 The meeting with SHR patients is often problematic for the physician and the risk for wrong diagnosis and medication is evident. Figure 1 illustrates the airway reaction in asthmatic hyperresponsiveness with wheeze and bronchial narrowing and in SHR where the airway symptoms can be copared to hyperalgesia or neuropathic pain. In many chronic cough patients where the cough is labeled idiopathic or unexplained, there is a history of sensitivity to environmental irritants such as chemicals and perfumed products as well as increased capsaicin cough sensitivity.51,59
Figure 1.
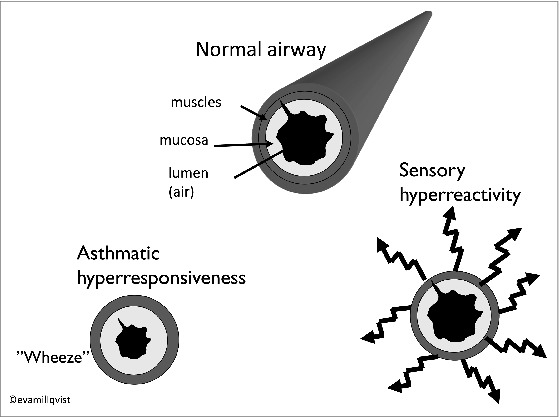
Airway reaction in asthmatic hyperresponsiveness with wheeze and bronchial narrowing and in SHR where the airway symptoms can be compared to hyperalgesia.
The augmented capsaicin sensitivity is a general characterization of chronic cough,60 and when, in such patients, capsaicin inhalation was preceded by a standardized exercise provocation in cold air, it was followed by coughing and increased capsaicin cough sensitivity.61 On the other hand, pre-inhalation of menthol, known to stimulate the cold sensitive receptor TRPM8, reduced cough sensitivity both in healthy subjects and in patients with chronic cough,62–64 and when menthol was administered in the nose cough parameters like capsaicin cough sensitivity were significantly modulated while the reactions were opposed following application of a TRPA1 agonist.37 The findings may provide scientific support for the common practice of using menthol as a reliever for variant airway discomfort. The use of menthol in different cigarette brands could be questioned, since it could conceal the natural irritation following smoking.65
A new paradigm, the “cough hypersensitivity syndrome,” has recently been developed. It includes both patients with general cough hypersensitivity toward, for example, cold air, environmental irritants, and those having cough in combination with symptoms that may indicate a reflux disease.66,67 In a new report there was a high degree of agreement among opinion leaders to the idea that cough hypersensitivity underlies the etiology of chronic cough in the majority of patients.68 Biopsy studies have shown increased expression of TRPV1 in chronic cough patients,69,70 and great interest has been focused on the possibility of blocking this receptor, sometimes called the “cough receptor.” Following this pathway, a TRPV1 antagonist was developed, and a recent study reported improvement of capsaicin cough sensitivity, although not in cough symptoms of patients with refractory chronic cough.71 On the other hand, capsaicin powder taken orally decreased capsaicin cough sensitivity and cough symptoms in chronic cough patients indicating a desensitization of the TRPV1.72
Summary
Revelations in the last decades of the ability of several airway TRP ion channels to sense and react to ambient air temperature have opened new windows for the understating of the pathogenesis in a diversity of airway reactions appearing in many common respiratory diseases. This understanding should lead to development of new drugs targeting cough and other symptoms, which today are followed by reduced health and quality of life.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Ma Q. Labeled lines meet and talk: population coding of somatic sensations. J Clin Investigat 2010; 120: 3773-8; PMID:21041959; http://dx.doi.org/ 10.1172/JCI43426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 1997; 389:816-24; PMID:9349813; http://dx.doi.org/ 10.1038/39807 [DOI] [PubMed] [Google Scholar]
- 3.Seki N, Shirasaki H, Kikuchi M, Sakamoto T, Watanabe N, Himi T. Expression and localization of TRPV1 in human nasal mucosa. Rhinology 2006; 44:128-34; PMID:16792172 [PubMed] [Google Scholar]
- 4.Guibert C, Ducret T, Savineau JP. Expression and physiological roles of TRP channels in smooth muscle cells. Adv Exp Med Biol 2011; 704:687-706; PMID:21290322; http://dx.doi.org/ 10.1007/978-94-007-0265-3_36 [DOI] [PubMed] [Google Scholar]
- 5.Banner KH, Igney F, Poll C. TRP channels: emerging targets for respiratory disease. Pharmacol Ther 2011; 130:371-84; PMID:21420429; http://dx.doi.org/ 10.1016/j.pharmthera.2011.03.005 [DOI] [PubMed] [Google Scholar]
- 6.Abbott-Banner K, Poll C, Verkuyl JM. Targeting TRP channels in airway disorders. Curr Topics Med Chem 2013; 13:310-21; PMID:23506455; http://dx.doi.org/ 10.2174/1568026611313030008 [DOI] [PubMed] [Google Scholar]
- 7.Jia Y, Lee LY. Role of TRPV receptors in respiratory diseases. Biochim Biophys Acta 2007; 1772:915-27; PMID:17346945; http://dx.doi.org/ 10.1016/j.bbadis.2007.01.013 [DOI] [PubMed] [Google Scholar]
- 8.Kaneko Y, Szallasi A. Transient receptor potential (TRP) channels: a clinical perspective. Br J Pharmacol 2014; 171:2474-507; PMID:24102319; http://dx.doi.org/ 10.1111/bph.12414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khairatkar-Joshi N, Szallasi A. TRPV1 antagonists: the challenges for therapeutic targeting. Trends Mol Med 2009; 15:14-22; PMID:19097938; http://dx.doi.org/ 10.1016/j.molmed.2008.11.004 [DOI] [PubMed] [Google Scholar]
- 10.Grace MS, Baxter M, Dubuis E, Birrell MA, Belvisi MG. Transient receptor potential (TRP) channels in the airway: role in airway disease. Br J Pharmacol 2014; 171:2593-607; PMID:24286227; http://dx.doi.org/ 10.1111/bph.12538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Planells-Cases R, Valente P, Ferrer-Montiel A, Qin F, Szallasi A. Complex regulation of TRPV1 and related thermo-TRPs: implications for therapeutic intervention. Adv Exp Med Biol 2011; 704:491-515; PMID:21290313; http://dx.doi.org/ 10.1007/978-94-007-0265-3_27 [DOI] [PubMed] [Google Scholar]
- 12.Mergler S, Valtink M, Coulson-Thomas VJ, Lindemann D, Reinach PS, Engelmann K, Pleyer U. TRPV channels mediate temperature-sensing in human corneal endothelial cells. Exp Eye Res 2010; 90:758-70; PMID:20338165; http://dx.doi.org/ 10.1016/j.exer.2010.03.010 [DOI] [PubMed] [Google Scholar]
- 13.Almaraz L, Manenschijn JA, de la Pena E, Viana F. Trpm8. Handbook Exp Pharmacol 2014; 222:547-79; PMID:24756721; http://dx.doi.org/ 10.1007/978-3-642-54215-2_22 [DOI] [PubMed] [Google Scholar]
- 14.Parra A, Gonzalez-Gonzalez O, Gallar J, Belmonte C. Tear fluid hyperosmolality increases nerve impulse activity of cold thermoreceptor endings of the cornea. Pain 2014; 155:1481-91; PMID:24785271; http://dx.doi.org/ 10.1016/j.pain.2014.04.025 [DOI] [PubMed] [Google Scholar]
- 15.Kim YH, Jang TY. Subjective cold hyper-responsiveness grade reflects age- and duration-related increase of nonspecific nasal hyperreactivity. Auris, Nasus, Larynx 2013; 40:184-8; PMID:22938731; http://dx.doi.org/ 10.1016/j.anl.2012.07.015 [DOI] [PubMed] [Google Scholar]
- 16.Kim YH, Jang TY. Nasal provocation test using allergen extract versus cold dry air provocation test: which and when? Am J Rhinol Allergy 2013; 27:113-7; PMID:23562199; http://dx.doi.org/ 10.2500/ajra.2013.27.3870 [DOI] [PubMed] [Google Scholar]
- 17.Van Gerven L, Boeckxstaens G, Jorissen M, Fokkens W, Hellings PW. Short-time cold dry air exposure: a useful diagnostic tool for nasal hyperresponsiveness. Laryngoscope 2012; 122:2615-20; PMID:22865676; http://dx.doi.org/ 10.1002/lary.23495 [DOI] [PubMed] [Google Scholar]
- 18.Murdoch RD, Bareille P, Denyer J, Newlands A, Bentley J, Smart K, Yarnall K, Patel D. TRPV1 inhibition does not prevent cold dry air-elicited symptoms in non-allergic rhinitis. Int J Clin Pharmacol Therap 2014; 52:267-76; PMID:24472402; http://dx.doi.org/ 10.5414/CP202013 [DOI] [PubMed] [Google Scholar]
- 19.Van Gerven L, Alpizar YA, Wouters MM, Hox V, Hauben E, Jorissen M, Boeckxstaens G, Talavera K, Hellings PW. Capsaicin treatment reduces nasal hyperreactivity and transient receptor potential cation channel subfamily V, receptor 1 (TRPV1) overexpression in patients with idiopathic rhinitis. J Allergy Clin Immunol 2014; 133:1332-9; PMID:24139494; http://dx.doi.org/ 10.1016/j.jaci.2013.08.026 [DOI] [PubMed] [Google Scholar]
- 20.Van Rijswijk JB, Boeke EL, Keizer JM, Mulder PG, Blom HM, Fokkens WJ. Intranasal capsaicin reduces nasal hyperreactivity in idiopathic rhinitis: a double-blind randomized application regimen study. Allergy 2003; 58:754-61; PMID:12859554; http://dx.doi.org/ 10.1034/j.1398-9995.2003.00203.x [DOI] [PubMed] [Google Scholar]
- 21.Keh SM, Facer P, Yehia A, Sandhu G, Saleh HA, Anand P. The menthol and cold sensation receptor TRPM8 in normal human nasal mucosa and rhinitis. Rhinology 2011; 49:453-7; PMID:21991571 [DOI] [PubMed] [Google Scholar]
- 22.Christensen PM, Thomsen SF, Rasmussen N, Backer V. Exercise-induced laryngeal obstructions: prevalence and symptoms in the general public. Eur Arch Oto-Rhino-Laryngol: Off J Eur Federat Oto-Rhino-Laryngol Soc 2011; 268:1313-9; http://dx.doi.org/ 10.1007/s00405-011-1612-0 [DOI] [PubMed] [Google Scholar]
- 23.Newman KB, Mason UG 3rd, Schmaling KB. Clinical features of vocal cord dysfunction. Am J Resp Crit Care Med 1995; 152:1382-6; PMID:7551399; http://dx.doi.org/ 10.1164/ajrccm.152.4.7551399 [DOI] [PubMed] [Google Scholar]
- 24.Vertigan AE, Bone SL, Gibson PG. Laryngeal sensory dysfunction in laryngeal hypersensitivity syndrome. Respirology 2013; 18:948-56; PMID:23617471 [DOI] [PubMed] [Google Scholar]
- 25.Gibson PG, Simpson JL, Ryan NM, Vertigan AE. Mechanisms of cough. Curr Opin Allergy Clin Immunol 2014; 14:55-61; PMID:24345788; http://dx.doi.org/ 10.1097/ACI.0000000000000027 [DOI] [PubMed] [Google Scholar]
- 26.Uno T, Koike S, Bamba H, Hirota R, Hisa Y. Capsaicin receptor expression in rat laryngeal innervation. Ann Otol, Rhinol, Laryngol 2004; 113:356-8; PMID:15174761; http://dx.doi.org/ 10.1177/000348940411300503 [DOI] [PubMed] [Google Scholar]
- 27.National Institutes of Health Global initiative for asthma (GINA). Glob Strat Asthma Manage Prevent. 2004. (updated 2007). NIH publication number 02-3659 Available at: http://www.ginasthma.org [Google Scholar]
- 28.Hanania NA. Targeting airway inflammation in asthma: current and future therapies. Chest 2008; 133:989-98; PMID:18398119; http://dx.doi.org/ 10.1378/chest.07-0829 [DOI] [PubMed] [Google Scholar]
- 29.Veres TZ, Rochlitzer S, Braun A. The role of neuro-immune cross-talk in the regulation of inflammation and remodelling in asthma. Pharmacol Ther 2009; 122:203-14; PMID:19292991; http://dx.doi.org/ 10.1016/j.pharmthera.2009.02.007 [DOI] [PubMed] [Google Scholar]
- 30.Bessac BF, Jordt SE. Breathtaking TRP channels: TRPA1 and TRPV1 in airway chemosensation and reflex control. Physiol (Bethesda) 2008; 23:360-70; PMID:19074743; http://dx.doi.org/ 10.1152/physiol.00026.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colsoul B, Nilius B, Vennekens R. On the putative role of transient receptor potential cation channels in asthma. Clin Exp Allergy: J Br Soc Allergy Clin Immunol 2009; 39:1456-66; PMID:19624522; http://dx.doi.org/ 10.1111/j.1365-2222.2009.03315.x [DOI] [PubMed] [Google Scholar]
- 32.Anderson SD, Daviskas E. The mechanism of exercise-induced asthma J Allergy Clin Immunol 2000; 106:453-9; PMID:10984363; http://dx.doi.org/ 10.1067/mai.2000.109822 [DOI] [PubMed] [Google Scholar]
- 33.Kotaru C, Hejal RB, Finigan JH, Coreno AJ, Skowronski ME, Brianas L, McFadden ER Jr. Desiccation and hypertonicity of the airway surface fluid and thermally induced asthma. J Appl Physiol 2003; 94:227-33; PMID:12391050 [DOI] [PubMed] [Google Scholar]
- 34.Anderson SD. How does exercise cause asthma attacks? Curr Opin Allergy Clin Immunol 2006; 6:37-42; PMID:16505610; http://dx.doi.org/ 10.1097/01.all.0000199797.02423.78 [DOI] [PubMed] [Google Scholar]
- 35.Jin M, Berrout J, O'Neil RG. Regulation of TRP channels by osmomechanical stress In: Zhu MX, ed. TRP Channels; Boca Raton, FL: CRC Press; 2011; Chap. 16; PMID:24681560.10565485 [PubMed] [Google Scholar]
- 36.Millqvist E. Effect of nasal air temperature on lung function. Allergy 1999; 54 Suppl 57: 106-11; PMID:10565485; http://dx.doi.org/ 10.1111/j.1398-9995.1999.tb04411.x [DOI] [PubMed] [Google Scholar]
- 37.Buday T, Brozmanova M, Biringerova Z, Gavliakova S, Poliacek I, Calkovsky V, Shetthalli MV, Plevkova J. Modulation of cough response by sensory inputs from the nose - role of trigeminal TRPA1 versus TRPM8 channels. Cough 2012; 8:11; PMID:23199233; http://dx.doi.org/ 10.1186/1745-9974-8-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dicpinigaitis PV. Capsaicin responsiveness in asthma and COPD. Thorax 2001; 56:162; PMID:11245103; http://dx.doi.org/ 10.1136/thorax.56.2.161b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doherty MJ, Mister R, Pearson MG, Calverley PM. Capsaicin responsiveness and cough in asthma and chronic obstructive pulmonary disease. Thorax 2000; 55:643-9; PMID:10899239; http://dx.doi.org/ 10.1136/thorax.55.8.643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fujimura M, Kamio Y, Hashimoto T, Matsuda T. Airway cough sensitivity to inhaled capsaicin and bronchial responsiveness to methacholine in asthmatic and bronchitic subjects. Respirology 1998; 3:267-72; PMID:10201054; http://dx.doi.org/ 10.1111/j.1440-1843.1998.tb00133.x [DOI] [PubMed] [Google Scholar]
- 41.Millqvist E, Bende M, Löwhagen O. Sensory hyperreactivity - a possible mechanism underlying cough and asthma-like symptoms. Allergy 1998; 53:1208-12; PMID:9930599; http://dx.doi.org/ 10.1111/j.1398-9995.1998.tb03843.x [DOI] [PubMed] [Google Scholar]
- 42.Ternesten-Hasseus E, Farbrot A, Löwhagen O, Millqvist E. Sensitivity to methacholine and capsaicin in patients with unclear respiratory symptoms. Allergy 2002; 57:501-7; PMID:12028115; http://dx.doi.org/ 10.1034/j.1398-9995.2002.23380.x [DOI] [PubMed] [Google Scholar]
- 43.Di Franco A, Dente FL, Giannini D, Vagaggini B, Conti I, Macchioni P, Scuotri L, Taccola M, Bacci E, Paggiaro PL. Effects of inhaled corticosteroids on cough threshold in patients with bronchial asthma. Pulmonary Pharmacol Therap 2001; 14:35-40; PMID:11162417; http://dx.doi.org/ 10.1006/pupt.2000.0264 [DOI] [PubMed] [Google Scholar]
- 44.McGarvey LP, Butler CA, Stokesberry S, Polley L, McQuaid S, Abdullah H, Ashraf S, McGahon MK, Curtis TM, Arron J, et al.. Increased expression of bronchial epithelial transient receptor potential vanilloid 1 channels in patients with severe asthma. J Allergy Clin Immunol 2014; 133:704-12 e704; PMID:24210884; http://dx.doi.org/ 10.1016/j.jaci.2013.09.016 [DOI] [PubMed] [Google Scholar]
- 45.Cawley D, Billings J, Oliver D, Kendall M, Pinnock H. Potential triggers for the holistic assessment of people with severe chronic obstructive pulmonary disease: analysis of multiperspective, serial qualitative interviews. BMJ Support Palliative Care 2014; PMID:24681560; http://dx.doi.org/ 10.1136/bmjspcare-2013-000629 [DOI] [PubMed] [Google Scholar]
- 46.Wong CH, Morice AH. Cough threshold in patients with chronic obstructive pulmonary disease. Thorax 1999; 54:62-4; PMID:10343635; http://dx.doi.org/ 10.1136/thx.54.1.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Terada K, Muro S, Ohara T, Haruna A, Marumo S, Kudo M, Ogawa E, Hoshino Y, Hirai T, Niimi A, et al.. Cough-reflex sensitivity to inhaled capsaicin in COPD associated with increased exacerbation frequency. Respirology 2009; 14:1151-5; PMID:19761536; http://dx.doi.org/ 10.1111/j.1440-1843.2009.01620.x [DOI] [PubMed] [Google Scholar]
- 48.Andre E, Campi B, Materazzi S, Trevisani M, Amadesi S, Massi D, Creminon C, Vaksman N, Nassini R, Civelli M, et al.. Cigarette smoke-induced neurogenic inflammation is mediated by alpha,beta-unsaturated aldehydes and the TRPA1 receptor in rodents. J Clin Investigat 2008; 118:2574-82; PMID:18568077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Young EC, Smith JA. Quality of life in patients with chronic cough. Ther Adv Respir Dis 2010; 4:49-55; PMID:20051447; http://dx.doi.org/ 10.1177/1753465809358249 [DOI] [PubMed] [Google Scholar]
- 50.Yousaf N, Lee KK, Jayaraman B, Pavord ID, Birring SS. The assessment of quality of life in acute cough with the Leicester Cough Questionnaire (LCQ-acute). Cough 2011; 7:4; PMID:21767404; http://dx.doi.org/ 10.1186/1745-9974-7-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ternesten-Hasseus E, Larsson S, Millqvist E. Symptoms induced by environmental irritants and health-related quality of life in patients with chronic cough - A cross-sectional study. Cough 2011; 7:6; PMID:21981855; http://dx.doi.org/ 10.1186/1745-9974-7-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morice AH, Fontana GA, Sovijarvi AR, Pistolesi M, Chung KF, Widdicombe J, O'Connell F, Geppetti P, Gronke L, De Jongste J, et al.. The diagnosis and management of chronic cough. Eur Resp J 2004; 24:481-92; PMID:15358710; http://dx.doi.org/ 10.1183/09031936.04.00027804 [DOI] [PubMed] [Google Scholar]
- 53.Bende M, Millqvist E. Prevalence of chronic cough in relation to upper and lower airway symptoms; the Skovde population-based study. Front Physiol 2012; 3:251; PMID:22934008; http://dx.doi.org/ 10.3389/fphys.2012.00251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haque RA, Usmani OS, Barnes PJ. Chronic idiopathic cough: a discrete clinical entity? Chest 2005; 127:1710-3; PMID:15888850; http://dx.doi.org/ 10.1378/chest.127.5.1710 [DOI] [PubMed] [Google Scholar]
- 55.McGarvey L, McKeagney P, Polley L, Macmahon J, Costello RW. Are there clinical features of a sensitized cough reflex? Pulmonary Pharmacol Therap 2008; 22:59-64; PMID:19049891; http://dx.doi.org/ 10.1016/j.pupt.2008.11.003 [DOI] [PubMed] [Google Scholar]
- 56.Millqvist E. Cough provocation with capsaicin is an objective way to test sensory hyperreactivity in patients with asthma-like symptoms. Allergy 2000; 55:546-50; PMID:10858985; http://dx.doi.org/ 10.1111/j.1398-9995.2000.all2513.x [DOI] [PubMed] [Google Scholar]
- 57.Millqvist E. The airway sensory hyperreactivity syndrome. Pulmonary Pharmacol Therap 2011; 24:263-6; PMID:20937402; http://dx.doi.org/ 10.1016/j.pupt.2010.10.001 [DOI] [PubMed] [Google Scholar]
- 58.Johansson A, Millqvist E, Nordin S, Bende M. Relationship between self-reported odor intolerance and sensitivity to inhaled capsaicin: proposed definition of airway sensory hyperreactivity and estimation of its prevalence. Chest 2006; 129:1623-28; PMID:16778284; http://dx.doi.org/ 10.1378/chest.129.6.1623 [DOI] [PubMed] [Google Scholar]
- 59.Ternesten-Hasseus E, Larsson C, Larsson S, Millqvist E. Capsaicin sensitivity in patients with chronic cough - results from a cross-sectional study. Cough 2013; 9:5; PMID:23448366; http://dx.doi.org/ 10.1186/1745-9974-9-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chung KF, Pavord ID. Prevalence, pathogenesis, and causes of chronic cough. Lancet 2008; 371:1364-74; PMID:18424325; http://dx.doi.org/ 10.1016/S0140-6736(08)60595-4 [DOI] [PubMed] [Google Scholar]
- 61.Ternesten-Hasseus E, Johansson EL, Bende M, Millqvist E. Dyspnea from exercise in cold air is not always asthma. J Asthma: Off J Assoc Care Asthma 2008; 45:705-9; PMID:18951264; http://dx.doi.org/ 10.1080/02770900802207287 [DOI] [PubMed] [Google Scholar]
- 62.Wise PM, Breslin PA, Dalton P. Sweet taste and menthol increase cough reflex thresholds. Pulmonary Pharmacol Therap 2012; 25:236-41; PMID:22465565; http://dx.doi.org/ 10.1016/j.pupt.2012.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Millqvist E, Ternesten-Hasseus E, Bende M. Inhalation of menthol reduces capsaicin cough sensitivity and influences inspiratory flows in chronic cough. Resp Med 2013; 107:433-8; PMID:23266255; http://dx.doi.org/ 10.1016/j.rmed.2012.11.017 [DOI] [PubMed] [Google Scholar]
- 64.Plevkova J, Kollarik M, Poliacek I, Brozmanova M, Surdenikova L, Tatar M, Mori N, Canning BJ. The role of trigeminal nasal TRPM8-expressing afferent neurons in the antitussive effects of menthol. J Appl Physiol 2013; 115:268-74; PMID:23640596; http://dx.doi.org/ 10.1152/japplphysiol.01144.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Anderson SJ. Menthol cigarettes and smoking cessation behaviour: a review of tobacco industry documents. Tob Control 2011; 20 Suppl 2: ii49-56; PMID:21504932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chung KF. Chronic ‘cough hypersensitivity syndrome’: a more precise label for chronic cough. Pulmonary Pharmacol Therap 2011; 24:267-71; PMID:21292019; http://dx.doi.org/ 10.1016/j.pupt.2011.01.012 [DOI] [PubMed] [Google Scholar]
- 67.Morice AH, Faruqi S, Wright CE, Thompson R, Bland JM. Cough hypersensitivity syndrome: a distinct clinical entity. Lung 2011; 189:73-9; PMID:21240613; http://dx.doi.org/ 10.1007/s00408-010-9272-1 [DOI] [PubMed] [Google Scholar]
- 68.Morice A, Millqvist E, Belvisi M, Bieksiene K, Birring SS, Chung KF, Dal Negro RW, Dicpinigaitis P, Kantar A, McGarvey LP, et al.. Expert opinion on the cough hypersensitivity syndrome in respiratory medicine. Eur Resp J 2014; 44:1132-48; PMID:25142479; http://dx.doi.org/ 10.1183/09031936.00218613; [Epub ahead of print [DOI] [PubMed] [Google Scholar]
- 69.Groneberg DA, Niimi A, Dinh QT, Cosio B, Hew M, Fischer A, Chung KF. Increased expression of transient receptor potential vanilloid-1 in airway nerves of chronic cough. Am J Resp Crit Care Med 2004; 170:1276-80; PMID:15447941; http://dx.doi.org/ 10.1164/rccm.200402-174OC [DOI] [PubMed] [Google Scholar]
- 70.Mitchell JE, Campbell AP, New NE, Sadofsky LR, Kastelik JA, Mulrennan SA, Compton SJ, Morice AH. Expression and characterization of the intracellular vanilloid receptor (TRPV1) in bronchi from patients with chronic cough. Exp Lung Res 2005; 31:295-306; PMID:15962710; http://dx.doi.org/ 10.1080/01902140590918803 [DOI] [PubMed] [Google Scholar]
- 71.Khalid S, Murdoch R, Newlands A, Smart K, Kelsall A, Holt K, Dockry R, Woodcock A, Smith JA. Transient receptor potential vanilloid 1 (TRPV1) antagonism in patients with refractory chronic cough: a double-blind randomized controlled trial. J Allergy Clin Immunol 2014; 134:56-62; PMID:24666696; http://dx.doi.org/ 10.1016/j.jaci.2014.01.038 [DOI] [PubMed] [Google Scholar]
- 72.Ternesten-Hasseus E, Johansson EL, Millqvist E. Cough reduction using capsaicin. Resp Med 2015; 109:27-37; PMID:25468411; http://dx.doi.org/ 10.1016/j.rmed.2014.11.001 [DOI] [PubMed] [Google Scholar]


