Abstract
Winter is coming. Some animals successfully cope with the hostility of this season by hibernating. But how do hibernators survive the procoagulant state of months of immobility at very low body temperatures, with strongly decreased blood flow and increased blood viscosity? Changing the coagulation system seems crucial for preventing thromboembolic complications.
Keywords: coagulation, hemostasis, hibernation, hypothermia, margination, metabolism, platelet, temperature, thrombocy-topenia, thrombosis
Abbreviations
- 5′-AMP
5′-adenosine monophosphate
- H2S
hydrogen sulfide
Once disclosed, the adaptive mechanisms in the coagulation system of hibernators may be hijacked to prevent thromboembolism in non-hibernators including humans. The mechanisms demonstrate a large overlap with the effects of cooling (in non-hibernators). Studying coagulation in natural hibernation and in suspended animation in non-hibernators, may unveil ways to enable cold storage of platelets for transfusion, improve the use of therapeutic hypothermia and optimize anticoagulative strategies during immobility.
While drastic changes in many physiological systems have been reported in hibernators, our recent study explored the rapid adaptations in the coagulation system.1 Key to hibernation is the dramatic drop in metabolic rate–and subsequent reduction of body temperature–to save energy. During hibernation, phases with metabolic suppression (called torpor) are interspersed by shorter periods of arousal, when metabolism and body temperature restore to normal. Deep hibernation typically features torpor phases of 4–14 d and arousals lasting up to 1 day. Alternatively, depending on ambient temperature and animal size, daily hibernation with torpor phases lasting a couple of hours may be energetically more favorable. In addition to natural hibernation, ‘hibernation’ may be induced by the administration of compounds inducing reversible metabolic suppression. The research of this pharmacologically induced ‘hibernation’ was boosted by the discovery that H2S reversibly suppresses metabolism inducing a state of suspended animation2 and further stimulated by the discovery of the action of 5′-AMP (5′-adenosine monophosphate) in torpid (hibernating) mice.3
Given the full preservation of organ structure and function, mechanisms of hibernation (and perhaps also of suspended animation) may be beneficial in the medical setting to protect cellular and organ function, thus improving therapeutic hypothermia in pediatric and adult patient care, organ transplantation, cardiopulmonary bypass, stroke, cancer radiotherapy, cardiac arrest, hemorrhagic shock and malignant fever. It is however quite uncertain that hibernation may be induced in humans, as it presumably requires additional adaptations in physiology beyond metabolic suppression. Unraveling the mechanisms of such additional adaptations in hibernation seems warranted to fully exploit opportunities in the medical setting.
The coagulation system is bound to undergo major changes during hibernation. According to Virchow's postulates, hibernation represents a procoagulant state due to the prolonged immobility, decreased heart and ventilation rate, low blood flow, increased blood viscosity and repetitive cycles of cooling and rewarming (i.e. from 37°C to 8°C). Despite this gloomy prospect, no signs of thromboembolism are detected in hibernators. In contrast, in a human bedridden patient, such a dire situation surely leads to potential lethal complications including venous thrombosis and pulmonary embolism (Fig. 1). One of the key adaptations in the coagulation system of hibernators is a fully reversible state of thrombocytopenia (low blood platelet count).1 Platelets form along with coagulation factors the primary respectively secondary pathway of the coagulation system. Balancing the coagulant state depends on blood constituents, the vessel wall and endothelial integrity, and hemodynamic stability.
Figure 1.
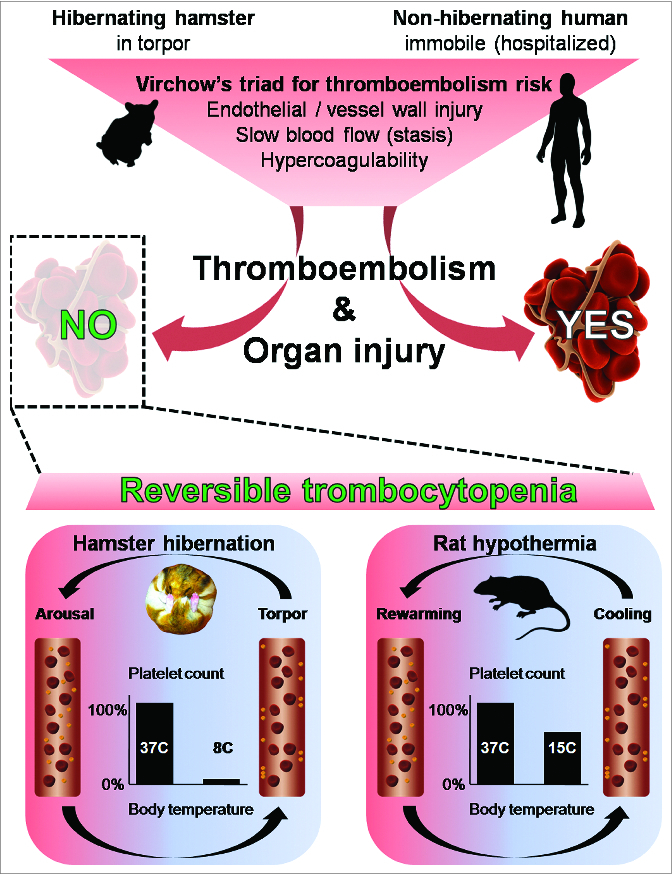
Following Virchow's triad, animals in hibernation are at high risk of thromboembolism and subsequent organ injury, similar to hospitalized humans, subjected to a period of prolonged immobility. Consequently, bedridden patients sooner or later develop thrombosis and embolism with organ injury, whereas hibernating mammals (such as the hamster) do not. Unraveling why they do not suffer thromboembolism, we show that during the repetitive phases of torpor there is a reversible decrease (by 96%) in circulating platelet count (thrombocytopenia). The thrombocytopenia is likely due to margination of platelets to the vessel wall, as depicted in the blood vessel during torpor, which rapidly reverses upon phases of arousal. Similarly, but to a lesser extent, thrombocytopenia can be induced in a non-hibernating mammal by forced hypothermia, also this low platelet count is reversible upon rewarming. Disclosing the shared underlying mechanism in hibernating and non-hibernating species can help us improve anticoagulant control for humans at risk of thromboembolism and organ injury.
To disclose how hibernating mammals survive their procoagulant hibernation period, we analyzed their platelets. We reveal that during torpor almost all platelets (96%) disappear from the hamster's circulation. However, the function of the few circulating platelets seems fully preserved, as measured by in vitro platelet activatibility. Remarkably, upon arousal, platelets are rapidly reintroduced in the circulation to normal levels. This swift recovery of platelet counts upon arousal advocates a storage-and-release mechanism rather than de novo synthesis.
Reversible clearance of platelets could either be due to sequestration in the spleen, or reversible adherence to vessels by means of margination. Employing splenectomy before or during torpor, we demonstrated that the spleen neither serves an essential role in induction nor in recovery of thrombocytopenia during hibernation. Hence, margination of platelets seems the prevalent mechanism to deplete circulating platelets. However, it is unclear whether margination during torpor is induced by the mechanism causing hibernation, or induced indirectly, e.g. by lowering of body temperature. Previously, employing forced cooling of hamster, we demonstrated that the decrease in circulating lymphocytes in torpor is solely driven by storage in response to the lowering of body temperature, inducing a drop in plasma levels of shingosine-1-phosphate.4
The situation seems similar for platelets (albeit independent of sphingosine-1-phosphate). Forced cooling of hamster to 8°C induced thrombocytopenia, which is rapidly reversible upon rewarming. In addition, forced cooling of mouse (which may display daily hibernation upon food restriction) and rat (a non-hibernator) show a decrease in platelet count, generating a decrease of 28% at 20°C and 35% at 15°C, respectively, which was reversed upon rewarming.1 However, 5′-AMP hypothermia decreased circulating lymphocyte but not platelet count. As 5′-AMP decreases platelet function, it is conceivable that patent platelet function, as found during torpor and forced hypothermia, is relevant for low body temperature induced thrombocytopenia.
Our study hints at a shared mechanism between non-hibernating and hibernating mammals depending on low body temperature induced reversible hypocoagulability consisting of storage of functional platelets, most likely by margination.
Modulating platelet function by storage seems an apt strategy in hibernation, as platelets play a key role in blood clotting. In addition, platelets mediate a.o. the immune system, wound healing, tissue repair and angiogenesis. Hence platelets are thought to be involved in a myriad of diseases, in which their role ranges from hero to villain. Therefore, the body temperature dependent storage of platelets induces a hypocoagulability during the potential hypercoagulant state of torpor and aids in preventing organ injury, ensuring full revitalization upon arousal. Thus, surviving the hostile winter through hibernation does not only depend on mechanisms lowering metabolism, but also on physiological adaptations rooted in lowering of body temperature.
Apparently representing a common phenomenon across species,1,5,6 hypothermia-induced thrombocytopenia may be exploited to reversibly inhibit hemostatic function and/or to prevent organ injury. Particularly, cooling during suspended animation may additionally invoke suspended coagulation, which may alleviate thromboembolic complications and organ (ischemia/reperfusion) injury, e.g. in major surgery and organ transplantation. Moreover, platelets from hibernators withstand cold storage ex vivo and can readily be infused without aggregation.5 Further insight into the mechanism preventing platelet function change and aggregation by the cold may enable cold storage of platelets for transfusion, improving logistics, shelf life and eliminating the risk of bacterial contamination during preservation at room temperature.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. de Vrij EL, et al. PloS One 2014; 9:e93218; PMID:24722364; http://dx.doi.org/ 10.1371/journal.pone.0093218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Blackstone E, et al. Science (New York, NY) 2005; 308:518; http://dx.doi.org/ 10.1126/science.1108581 [DOI] [PubMed] [Google Scholar]
- 3. Zhang J, et al. Nature 2006; 439:340-3; PMID:16421573; http://dx.doi.org/ 10.1038/nature04368 [DOI] [PubMed] [Google Scholar]
- 4. Bouma HR, et al. Proc Natl Acad Sci U S A 2011; 108:2052-7; PMID:21245336; http://dx.doi.org/ 10.1073/pnas.1008823108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cooper ST, et al. Am J Physiol Regul, Integr Comp Physiol 2012; 302:R1202-8; PMID:22492817; http://dx.doi.org/ 10.1152/ajpregu.00018.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bouma HR, et al. Vet Immunol Immunopathol 2010; 136:319-23; PMID:20399508; http://dx.doi.org/ 10.1016/j.vetimm.2010.03.016 [DOI] [PubMed] [Google Scholar]


