Abstract
Transient receptor potential vanilloid 1 (TRPV1) is a multifunctional ion channel playing important roles in a numerous biological processes including the regulation of body temperature. Within distinct and tight chemical space of chromanyl ureas TRPV1 ligands were identified that exhibit distinctive pharmacology and a spectrum of thermoregulatory effects ranging from hypothermia to hyperthermia. The ability to manipulate these effects by subtle structural modifications of chromanyl ureas may serve as a productive approach in TRPV1 drug discovery programs addressing either side effect or desired target profiles of the compounds. Because chromanyl ureas in the TRPV1 context are generally antagonists, we verified observed partial agonist effects of a subset of compounds within that chemotype by comparing the in vitro profile of Compound 3 with known partial agonist 5′-I-RTX.
Keywords: chromanyl ureas, hyperthermia, hypothermia, TRPV1, TRPV1 antagonists, TRPV1 agonists, 5′-iodo-RTX, thermoregulation
Abbreviations: TRPV1, transient receptor potential vanilloid 1; FLIPR, fluorometric imaging plate reader; OA, osteoarthritis; Compound 1, (R)-1-(2,2-dimethyl-7-(trifluoromethyl)chroman-4-yl)-3-(3,6-dimethylisoquinolin-5-yl)urea; Compound 2, (R)-1-(2,2-dimethyl-7-(trifluoromethyl)chroman-4-yl)-3-(3-methylisoquinolin-5-yl)urea; Compound 3, (R)-1-(2,2-dimethyl-8-(trifluoromethoxy)chroman-4-yl)-3-(3-methylisoquinolin-5-yl)urea; 5′-I-RTX, 5′-iodo-resiniferatoxi
Introduction
Transient receptor potential vanilloid 1 (TRPV1) is the founding member of a 28-member TRP ion channel superfamily1 and a subclass of thermoTRP channels that exhibit thermal responses within a wide range of temperatures.2 TRPV1 is activated at temperatures exceeding 43°C but also responds to a wide range of stimuli, including exogenous capsaicin, metabolites of endogenous arachidonic acid, chemical stimuli, such as acidic media, and a variety of inflammatory agents including prostaglandins, bradykinin, and nerve growth factor.3,4 The polymodal nature of TRPV1 function along with observed reduced thermal hyperalgesia in TRPV1 knockout mice in response to inflammatory mediators5,6,7 generated significant interest in the pain research community and prompted extensive research in exploring effects of TRPV1 modulation.8 During the last 10–15 years TRPV1 agonists and antagonists have been extensively evaluated for their potential as efficacious and safe analgesics.9,10 However, first generation TRPV1 antagonists caused hyperthermia, an undesired side effect, in both preclinical species and humans.11
In the course of evaluating mechanisms underlying hyperthermia associated with TRPV1 antagonists12 we13,14 and others15 have identified a subset of TRPV1 antagonists that do not increase body temperature in rodents but still provide analgesic relief in pain models. In this communication we report on identification of a subset of TRPV1 antagonists within a distinct chemical class of chromanyl ureas14,16 that elicits hyperthermic, hypothermic or no effects on body temperature depending on subtle structural perturbations. We demonstrate that minor structural differences also change the ability of the compounds to block acid stimulation of TRPV1 and affect the functional nature of a compound as TRPV1 antagonist or agonist. Pharmacological profiles of some compounds are species-dependent and, therefore, temperature effects in humans may not be accurately predicted by observations in preclinical species.
Materials and Methods
5′-I-RTX was purchased from Sigma Aldrich. Other reagents were sourced as described.13 Functional studies were conducted on a fluorometric imaging plate reader (FLIPRTETRA) which monitored effects of capsaicin or mild acid on Ca2+ uptake into recombinant HEK293 cells expressing rat or human TRPV1 as described previously.13 Antagonism of heat activation was measured at 48°C by using a custom made device (GNF Systems, San Diego, CA) for the control of temperature. Telemeters were inserted into male Sprague-Dawley rats and temperature was monitored as detailed elsewhere.13 Pharmacokinetic parameters were monitored by procedures as described.13
Results
Compound 1, Compound 2, Compound 3, and 5′-I-RTX exhibit different pharmacological profiles against polymodal activation of recombinant rat and human TRPV1
The ability of Compound 1, Compound 2, and Compound 3 to inhibit TRPV1 activation was investigated using recombinant HEK293 cells expressing rat or human TRPV1. All three chromans as well as 5′-I-RTX were competitive with 50 nM capsaicin at both rat and human TRPV1 and elicited potent antagonism (Table 1). These compounds were also evaluated for activity on pH 5-stimulated rat and human TRPV1. Acidic responses at rat TRPV1 were blocked fully by Compound 1, partially by Compound 2, and not by Compound 3 (Fig. 1) or <11.25 μM 5′-I-RTX (Fig. 2). Both Compound 3 and 5′-I-RTX elicited partial agonism upon addition to recombinant rat TRPV1 (Fig. 3) while Compound 1 and Compound 2 did not.
Table 1.
Potencies of Compound 1, Compound 2, Compound 3, and 5′-I-RTX at rat and human TRPV1 Potencies were determined by using Ca2+ flux assays performed on the FLIPRTETRA. All compounds completely blocked capsaicin-evoked responses at rat and human TRPV1. The concentration of compounds used to determine the percentage of inhibition against acid stimulation was 11.25 μM. Results are shown as mean values and SD for at least 2–4 determinations. ND represents data not determined due to insufficient block of pH 5 activation.
| Rat |
Human |
|||||
|---|---|---|---|---|---|---|
| Compound | Capsaicin IC50 (nM) | Acid IC50 (nM) | Acid % inhibition | Capsaicin IC50 (nM) | Acid IC50 (nM) | Acid % inhibition |
| Compound 1 | 54.6 +/− 26.3 | 72.7 +/− 8.0 | 99.7 +/− 0.1 | 144.0 +/− 9.4 | 107.0 +/− 18.3 | 95.4 +/− 1.8 |
| Compound 2 | 20.9 +/− 3.1 | ND | 20.1 +/− 8.5 | 17.8 +/− 16.3 | 20.1 +/− 7.3 | 76.2 +/− 6.6 |
| Compound 3 | 429.0 +/− 162.0 | ND | −5.1 +/− 9.2 | 65.7 +/− 10.6 | ND | 35.4 +/− 2.1 |
| 5′-I-RTX | 200.0 +/− 90.3 | ND | −3.0 +/− 0.55 | 274.0 +/− 28.3 | 114.0 +/− 50.7 | 64.4 +/− 11.1 |
Figure 1.
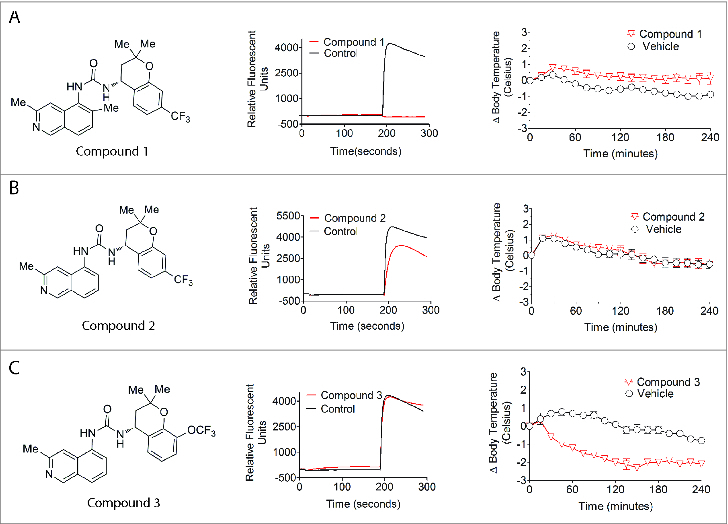
Effects of TRPV1 ligands on acid-induced Ca2+ flux into recombinant HEK293 cells expressing rat TRPV1 and on rat core body temperature. Representative Ca2+ flux responses as monitored in FLIPR traces are shown for Compound 1, Compound 2, and Compound 3 (11.25 μM) to demonstrate their effects on acid activation of rat TRPV1. The change in rat core body temperature represents mean and SEM for at least 5 determinations. (A) Compound 1 is a full acid blocker of rat TRPV1 and elicits hyperthermia in rats. (B) Compound 2 is a partial acid blocker of rat TRPV1 and does not affect core body temperature in rats. (C) Compound 3 is a partial agonist that does not block acid activation of rat TRPV1 and evokes hypothermia in rats.
Figure 2.
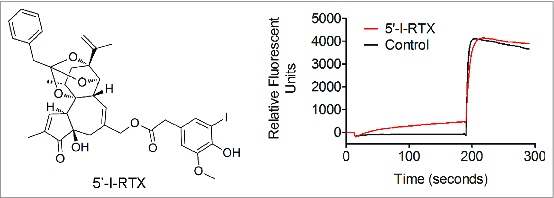
Effects of 5‘-I-RTX on acid-induced Ca2+ flux into recombinant HEK293 cells expressing rat TRPV1. The pharmacological profile of 11.25 μM 5′-I-RTX against acid (pH 5.0) activation is illustrated in a representative FLIPR trace to demonstrate that 5′-I-RTX is a partial agonist and an acid non-blocker of rat TRPV1.
Figure 3.
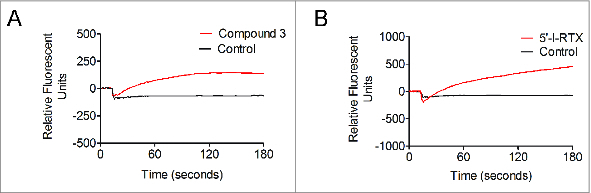
Partial agonist effects of Compound 3 and 5‘-I-RTX on recombinant rat TRPV1 expressed on HEK293 cells. Representative Ca2+ flux responses reflected in FLIPR traces for 11.25 μM Compound 3 (A) and 5′-I-RTX (B). Agonist effects were calculated based on a maximal capsaicin response.
All three chromanyl urea compounds potently blocked heat activation of human TRPV1 with IC50 values of 20 nM, 5 nM and 17 nM for Compounds 1, 2, and 3, respectively. Additionally, these compounds were selective against human “cold” receptors TRPA1 and TRPM8 exhibiting no activity up to 100 μM at TRPA1 and IC50 values of >100 μM, 30 μM, and 25 μM at TRPM8 for Compounds 1, 2 and 3, respectively.
Pharmacological profiles of Compound 1 and Compound 2 at human TRPV1 were similar to their profiles at rat TRPV1; the former fully blocked acid activation, while <11.25 μM of the latter partially blocked the channel (Fig. 4). However, in vitro data for 2 other compounds, Compound 3 and 5′-I-RTX, were distinctly different at human versus rat TRPV1. Both compounds behaved as partial blockers upon pH 5 activation of human – but not rat – TRPV1. Moreover, Compound 3 and 5′-I-RTX did not activate human TRPV1 when applied to recombinant HEK293 cells at pH 5.
Figure 4.
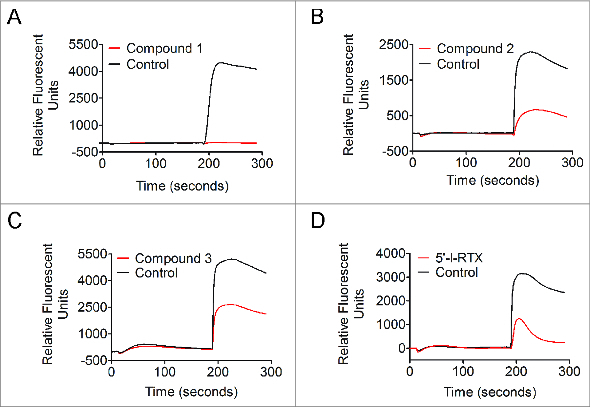
Pharmacological profiles of Compound 1, Compound 2, and Compound 3 at acid-stimulated recombinant human TRPV1 expressed on HEK293 cells. Representative Ca2+ flux responses reflected in FLIPR traces for TRPV1 ligands at 11.25 μM. (A) Compound 1, full acid blocker; (B) Compound 2, partial acid blocker; (C) Compound 3, partial acid blocker; (D) 5′-I-RTX, partial acid blocker.
Compound 1, Compound 2, and Compound 3 exhibit hyperthermic, temperature-neutral and hypothermic effects, respectively, in rats
Compound 1 administered orally at 100 µmol/kg resulted in a plasma concentration of 0.7 μg/mL, which produced a significant increase in core body temperature with a maximum effect of 1.4°C above that observed in vehicle-treated rats (Fig. 1). The same study performed with the pH 5 partial blocker Compound 2 resulted in no meaningful effect on core body temperature for the 24 hr duration of the study (Fig. 1). The peak plasma concentration of 3.1 μg/mL achieved at 1 hr post dose was ˜2-fold higher than efficacious plasma levels in the OA pain model (unpublished data). In contrast to temperature elevating Compound 1 and temperature-neutral Compound 2, the other chroman, pH 5 non-blocker Compound 3, produced hypothermia (Fig. 1). Despite achieving a drug concentration ˜31-fold higher than its efficacious plasma concentration in a rat OA model (15.5 μg/mL in the telemetry study vs. 0.5 μg/mL in the OA pain model; unpublished data), Compound 3 decreased rat body temperature 2.0°C. This hypothermic effect persisted for ˜13 hr.
Discussion
First generation TRPV1 antagonists increase body temperature in both preclinical species and humans.11 Efforts in our group to develop different series of TRPV1 antagonists led to discovery of the chromanyl urea chemotype.16 Potent compounds within this narrow structural class exhibit significantly diverse pharmacological responses at TRPV1 and thermoregulatory effects.17 Minor structural manipulations generate compounds that elicit effects in rats ranging from hypothermia to hyperthermia. Thus, Compound 1 increases body temperature 1.4°C at a relatively low plasma concentration of 0.7 μg/mL (Fig. 1). Removal of the methyl group in the isoquinoline fragment of Compound 1 generated Compound 2 that does not affect temperature in rats at concentrations 2-fold higher than efficacious plasma levels in the OA pain model (Fig. 1). On the other hand, replacing the trifluoromethyl group in Compound 2 with a trifluoromethoxy group and moving it from the 7-position to the adjacent 8-position of the chroman fragment results in Compound 3 which elicits hypothermic effects of about −2°C (Fig. 1). Temperature-elevating and temperature-neutral effects of TRPV1 antagonists correlate with the pharmacological profile of TRPV1 antagonists. Compounds that inhibit all modes of channel activation (represented, e.g., by capsaicin, heat, and acid and exemplified by Compound 1) would be expected to increase body temperature, while compounds that potently block capsaicin – but not other modes of TRPV1 activation – (e.g., Compound 2) would not be expected to affect body temperature.13,14 While these observations proved largely consistent over the course of an aggressive campaign to identify temperature-neutral TRPV1 antagonists, select compounds with distinctly different properties have been identified. For example, it was initially difficult to explain hypothermia evoked by Compound 3 (Fig. 1). According to the generally accepted in vitro/in vivo relationship for TRPV1 ligands, this compound – potent against capsaicin and weak against acid activation – would be expected to be temperature-neutral in preclinical models. Yet Compound 3 proves hypothermic in rats, an effect typically associated with TRPV1 small molecule agonists such as capsaicin. Close inspection of the FLIPR curves of Compound 3 reveals subtle activation of the channel upon application of the compound directly to HEK293 cells expressing recombinant TRPV1. This observation suggests that Compound 3 evokes partial agonism. The signal is minimal but is consistently reproducible among select hypothermic TRPV1 ligands in the chromanyl urea series. In order to probe further into this observation the behavior of 5′-I-RTX, a well documented partial agonist of TRPV1, was examined in the identical FLIPR setting. This analog of the ultrapotent TRPV1 agonist RTX was originally classified as a TRPV1 antagonist based on in vitro data generated with recombinant and native TRPV1 cells.18-20 However, profound hypothermic effects of this compound in mice21 were inconsistent with TRPV1 antagonism predicted from its in vitro behavior. This paradoxical link between TRPV1 antagonism and hypothermia triggered extensive in vitro functional studies which demonstrated that 5′-I-RTX is a partial agonist of TRPV1.22 Comparison of FLIPR profiles of 5′-I-RTX and Compound 3 indicates that both compounds elicit Ca2+ influx into recombinant rat TRPV1 cells in a qualitatively similar manner, supporting the conclusion that Compound 3 is a partial agonist of the channel (Fig. 3). Interestingly, compounds with distinct pharmacological profiles (Compound 2, Compound 3, and 5′-I-RTX) evoke different responses in human versus rat TRPV1 (Fig. 4). Compound 2, Compound 3, and 5′-I-RTX more potently inhibit acid-evoked responses at human TRPV1. There is no indication of partial agonism for latter 2 compounds. Such interspecies differences in the pharmacology of TRPV1 ligands serve as a caution in projecting thermoregulatory effects from in vitro pharmacology and preclinical models to the clinical setting.
Discovery of compounds with a wide range of pharmacological profiles within one well defined structural scaffold such as chromanyl ureas represents a useful finding for TRPV1 drug discovery and may provide an economical and effective way of identifying full antagonists, modality-specific antagonists, or partial agonists from a common structural core with favorable drug-like properties. However, despite recent advances, discovering a TRPV1 ligand with desired pharmacology remains more of an empirical than rational exercise. Nevertheless, recent advances in structural analysis of TRPV1 may provide meaningful support for more predictable structure-based drug design. In 2013 the structure of rat TRPV1 was determined by single particle electron cryo-microscopy revealing architecture of the channel.23 Further investigation of rat TRPV1-ligand complex by electron cryo-microscopy24 and molecular docking experiments with a human TRPV1 homology model25 provide useful information on restriction points of the protein defined as selectivity filter and lower gate. These data along with earlier mutagenesis studies26-29 are starting to provide insight into TRPV1 ligand binding pockets and associated conformational changes upon binding of agonists and antagonists to TRPV1.
Conclusions
In summary, slight variation of the nature or position of substituents in a structurally distinct chromanyl urea scaffold exerts significant impact on the pharmacological behavior of TRPV1 ligands and, as a consequence, produces a variety of thermoregulatory effects in rats ranging from hyperthermia to hypothermia. In vitro pharmacological profiles and thermoregulatory effects of representatives from 3 subsets of chromanyl urea TRPV1 ligands that are closely related are described. Compound 1 inhibits both capsaicin- and acid-evoked responses in rat recombinant TRPV1 and evokes hyperthermic effects in rats. Compound 2 fully blocks capsaicin activation, only partially blocks acid response, and does not evoke significant temperature effects in rats. Compound 3 has been shown to be a partial agonist and produces hypothermic effects in rats. The latter shows no agonist effects at recombinant human TRPV1. Similarly 5′-I-RTX behaves as partial agonist at recombinant rat – but not human – TRPV1. Ability to generate and characterize TRPV1 ligands with different pharmacological and thermoregulatory profiles within the same structural class of compounds may accelerate the discovery of still more effective modulators of the TRPV1 channel. Although Compounds 1–3 display favorable selectivity profiles against other thermosensory receptors, TRPA1 and TRPM8, a possibility of non-TRPV1-mediated thermal effects or lack thereof cannot be fully excluded.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Gees M, Owsianik G, Nilius B, Voets T. TRP channels. Compr Physiol 2012; 2:563-608; PMID:23728980 [DOI] [PubMed] [Google Scholar]
- 2.Vriens J, Nilius B, Voets T. Peripheral thermosensation in mammals. Nature Rev Neurosci 2014; 15:573-89; PMID:25053448; http://dx.doi.org/ 10.1038/nrn3784 [DOI] [PubMed] [Google Scholar]
- 3.Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci 2001; 24:487-517; PMID:11283319; http://dx.doi.org/ 10.1146/annurev.neuro.24.1.487 [DOI] [PubMed] [Google Scholar]
- 4.Szallasi A, Cortright DN, Blum CA, Eid SR. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat Rev Drug Discov 2007; 6:357-72; PMID:17464295; http://dx.doi.org/ 10.1038/nrd2280 [DOI] [PubMed] [Google Scholar]
- 5.Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 2000; 288:306-13; PMID:10764638; http://dx.doi.org/ 10.1126/science.288.5464.306 [DOI] [PubMed] [Google Scholar]
- 6.Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, et al.. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature 2000; 405:183-7; PMID:10821274; http://dx.doi.org/ 10.1038/35012076 [DOI] [PubMed] [Google Scholar]
- 7.Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature 2001; 411:957-62; PMID:11418861; http://dx.doi.org/ 10.1038/35082088 [DOI] [PubMed] [Google Scholar]
- 8.Patapoutian A, Tate S, Woolf CJ. Transient receptor potential channels: targeting pain at the source. Nat Rev Drug Discov 2009; 8:55-69; PMID:19116627; http://dx.doi.org/ 10.1038/nrd2757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Petrocellis L, Moriello AS. Modulation of the TRPV1 channel: Current clinical trials and recent patents with focus on neurological conditions. Recent Pat CNS Drug Discov 2013; 8: 180-204; PMID:24330123 [DOI] [PubMed] [Google Scholar]
- 10.Trevisani M, Gatti R. TRPV1 antagonists as analgesic agents. Open Pain Journal 2013; 6:108-18; http://dx.doi.org/ 10.2174/1876386301306010108 [DOI] [Google Scholar]
- 11.Gomtsyan A, Brederson J-D. Clinical and Preclinical Experience with TRPV1 Antagonists as Potential Analgesic Agents In: Szallasi A, ed. TRP Channels as Therapeutic Targets: from Basic Science to Clinical Use. Academic Press: 2015:129-45. [Google Scholar]
- 12.Garami A, Shimansky YP, Pakai E, Oliveira DL, Gavva NR, Romanovsky AA. Contributions of different modes of TRPV1 activation to TRPV1 antagonist induced hyperthermia. J Neurosci 2010; 30:1435-40; PMID:20107070; http://dx.doi.org/ 10.1523/JNEUROSCI.5150-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reilly RM, McDonald HA, Puttfarcken PS, Joshi SK, Lewis L, Pai M,. Pharmacology of modality-specific transient receptor potential vanilloid-1 antagonists that do not alter body temperature. J Pharmacol Exp Ther 2012; 342:416-28; PMID:22570364; http://dx.doi.org/ 10.1124/jpet.111.190314 [DOI] [PubMed] [Google Scholar]
- 14.Voight EA, Gomtsyan A, Daanen JF, Perner RJ, Schmidt RG, Bayburt EK. Discovery of (R)1-(7-chloro-2,2-bis(fluoromethyl)chroman-4-yl)-3-(3-methylisoquinolin-5-yl)urea (A-1165442): A temperature-neutral transient receptor potential vanilloid1 (TRPV1) antagonist with analgesic efficacy. J Med Chem 2014; 57:7412-24; PMID:25100568; http://dx.doi.org/ 10.1021/jm500916t [DOI] [PubMed] [Google Scholar]
- 15.Lehto S, Tamir R, Deng H, Klionsky L, Kuang R, Le A, Lee D, Louis JC, Magal E, Manning BH, et al.. Antihyperalgesic effects of AMG8562, a novel vanilloid receptor TRPV1 modulator that does not cause hyperthermia in rats. J Pharmacol Exp Ther 2008; 326:218-29; PMID: 18420600; http://dx.doi.org/ 10.1124/jpet.107.132233 [DOI] [PubMed] [Google Scholar]
- 16.Schmidt RG, Bayburt EK, Latshaw SP, Koenig JR, Daanen JF, McDonald HA,. Chroman and tetrahydroquinoline ureas as potent TRPV1 antagonists. Bioorg Med Chem Lett 2011; 21:1338-41; PMID:21315587; http://dx.doi.org/ 10.1016/j.bmcl.2011.01.056 [DOI] [PubMed] [Google Scholar]
- 17.Gomtsyan A, Daanen J, Kort MK, Kym PR, Voight EA, Woller KR. US Patent Application US 2013/0345255 (2013). [Google Scholar]
- 18.Seabrook GR, Sutton KG, Jarolimek W, Hollingworth GJ, Teague S, Webb J, Clark N, Boyce S, Kerby J, Ali Z, et al.. Functional properties of the high-affinity TRPV1 (VR1) vanilloid receptor antagonist (4-hydroxy-5-iodo-3-methoxyphenylacetateester) iodo-resiniferatoxin. J Pharmacol Exp Ther 2002; 303:1052-60; PMID:12438527; http://dx.doi.org/ 10.1124/jpet.102.040394 [DOI] [PubMed] [Google Scholar]
- 19.Rigoni M, Trevisani M, Gazzieri D, Nadaletto R, Tognetto M, Creminon C, Davis JB, Campi B, Amadesi S, Geppetti P, et al.. Neurogenic responses mediated by vanilloid receptor-1 (TRPV1) are blocked by the high affinity antagonist, iodoresiniferatoxin. Br J Pharmacol 2003; 138:977-85; PMID:12642400; http://dx.doi.org/ 10.1038/sj.bjp.0705110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Correll CC, Phelps PT, Anthes JC, Umland S, Greenfeder S. Cloning and pharmacological characterization of mouse TRPV1. Neurosci Lett 2004; 370:55-60; PMID:15489017; http://dx.doi.org/ 10.1016/j.neulet.2004.07.058 [DOI] [PubMed] [Google Scholar]
- 21.Dogan MD, Patel S, Rudaya AY, Steiner AA, Szekely M, Romanovsky AA. Lipopolysaccharide fever is initiated via a capsaicin-sensitive mechanism independent of the subtype-1 vanilloid receptor. Br J Pharmacol 2004; 143: 1023-32; PMID:15492017; http://dx.doi.org/ 10.1038/sj.bjp.0705977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shimizu I, Iida T, Horiuchi N, Caterina MJ. 5-Iodoresiniferatoxin evokes hypothermia in mice and is a partial transient receptor potential vanilloid 1 agonist in vitro. J Pharmacol Exp Ther 2005; 303:1052-60; PMID:15947039 [DOI] [PubMed] [Google Scholar]
- 23.Liao M, Cao E, Julius D, Cheng Y. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature 2013; 504:107-12; PMID: 24305160; http://dx.doi.org/ 10.1038/nature12822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao E, Liao M, Cheng Y, Julius D. TRPV1 structures in distinct conformations reveal activation mechanisms. Nature 2013; 504:113-18; PMID:24305161; http://dx.doi.org/ 10.1038/nature12823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng Z, Pearce LV, Xu X, Yang X, Yang P, Blumberg PM, Xiang-Qun Xie X-Q. Structural insight into tetrameric hTRPV1 from homology modeling, molecular docking, molecular dynamics simulation, virtual screening, and bioassay validations. J Chem Inf Model 2015; 55:572-88; PMID:25642729; http://dx.doi.org/ 10.1021/ci5007189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chou MZ, Mtui T, Gao YD, Kohler M, Middleton RE. Resiniferatoxin binds to the capsaicin receptor (TRPV1) near the extracellular side of the S4 transmembrane domain. Biochemistry 2004; 43:2501-11; PMID:14992587; http://dx.doi.org/ 10.1021/bi035981h [DOI] [PubMed] [Google Scholar]
- 27.Gavva NR, Klionsky L, Qu Y, Shi L, Tamir R, Edenson S, Zhang TJ, Viswanadhan VN, Toth A, Pearce LV, et al.. Molecular determinants of vanilloid sensitivity in TRPV1. J Biol Chem 2004; 279: 20283-95; PMID:14996838; http://dx.doi.org/ 10.1074/jbc.M312577200 [DOI] [PubMed] [Google Scholar]
- 28.Jordt SE, Julius D. Molecular basis for species-specific sensitivity to “hot” chili peppers. Cell 2002; 108:421-30; PMID:11853675; http://dx.doi.org/ 10.1016/S0092-8674(02)00637-2 [DOI] [PubMed] [Google Scholar]
- 29.Phillips E, Reeve A, Bevan S, McIntyre P. Identification of species-specific determinants of the action of the antagonist capsazepine and the agonist PPAHV on TRPV1. J Biol Chem 2004; 279:17165-72; PMID:14960593; http://dx.doi.org/ 10.1074/jbc.M313328200 [DOI] [PubMed] [Google Scholar]


